Abstract
Background:
Emerging neuroprotective strategies are being explored to preserve the retina from degeneration, that occurs in eye pathologies like glaucoma, diabetic retinopathy, age-related macular degeneration, and retinitis pigmentosa. Incidentally, neuroprotection of retina is a defending mechanism designed to prevent or delay neuronal cell death, and to maintain neural function following an initial insult, thus avoiding loss of vision.
Methods:
Numerous studies have investigated potential neuroprotective properties of plant-derived phytocannabinoids, as well as of their endogenous counterparts collectively termed endocannabinoids (eCBs), in several degenerative diseases of the retina. eCBs are a group of neuromodulators that, mainly by activating G protein-coupled type-1 and type-2 cannabinoid (CB1 and CB2) receptors, trigger multiple signal transduction cascades that modulate central and peripheral cell functions. A fine balance between biosynthetic and degrading enzymes that control the right concentration of eCBs has been shown to provide neuroprotection in traumatic, ischemic, inflammatory and neurotoxic damage of the brain.
Results:
Since the existence of eCBs and their binding receptors was documented in the retina of numerous species (from fishes to primates), their involvement in the visual processing has been demonstrated, more recently with a focus on retinal neurodegeneration and neuroprotection.
Conclusion:
The aim of this review is to present a modern view of the endocannabinoid system, in order to discuss in a better perspective available data from preclinical studies on the use of eCBs as new neuroprotective agents, potentially useful to prevent glaucoma and retinal neurodegenerative diseases.
Keywords: Neuroprotection, glaucoma, retinal diseases, retinal ganglion cells, endocannabinoids, phytocannabinoids
1. INTRODUCTION: GLAUCOMA AND RETINAL NEURODEGENERATION
Glaucoma comprises a group of eye disorders that can lead to progressive and/or irreversible blindness. It affects the elderly but is becoming more widespread also among younger people and even children [1, 2]. Glaucoma is generally caused by increased intraocular pressure (IOP), although other factors are involved such as progressive damage of retinal ganglion cells (RGCs), known as “the messengers of retina”, leading to optic nerve degeneration [3-5]. These conditions cause distinct visual field defects, and eventually complete vision loss [6]. In turn, apoptotic death of RGCs in
glaucoma is due to different defects in the connection between central nervous system (CNS) and retina, including faults of reactive glia, synaptic connectivity and axonal transport, neurotrophic factor deprivation, pro-apoptotic signaling activation of neurotransmitters and neuromodulators, as well as excitotoxicity and oxidative stress [7, 8]. Besides glaucoma, RGC neurodegeneration occurs in several other ocular pathologies such as diabetic retinopathy (DR), age-related macular degeneration (AMD) and some inherited retinal disorders as well as in Alzheimer’s disease and Parkinson’s disease, where the retina appears to be an early site of damage [9-11]. Yet, signs of pigmentary retinopathy and degeneration of retinal nerve fibers have been identified in another form of neurodegenerative disorder known as autosomal dominant cerebellar ataxias [12, 13]. Other areas potentially affected are retinal microvessels, in DR [14], and retinal pigment epithelium (RPE) and photoreceptors, together with vascular and RGC damages, in AMD [15, 16].
So far the most effective intervention used to block glaucoma progression is the administration of drugs capable of lowering IOP, although many patients have IOP within the normal range and disease progression can continue even when IOP is effectively lowered [17, 18]. Moreover, glaucomatous damage is not limited to the eye, but it also involves central visual pathways and vascular diseases of the CNS [19]. Indeed, neurodegeneration in glaucoma shares many pathway components with other retinal and non-retinal neurodegenerative diseases, so that an innovative therapeutic approach is now to keep RGCs and photoreceptors alive to avoid irreversible damage of optic nerve, as well as synaptic connectivity and retinal microvascular alterations [20, 21]. Interestingly, the five most common classes of drugs used topically to lower IOP (α2-agonists, β-antagonists/blockers, prostaglandin analogs, carbonic anhydrase and cholinergic agents) possess an indirect neuroprotective action on the retina and/or optic nerve, by triggering mechanisms that include neuronal, glial and vascular pathways [22-24]. On the other hand, many potential biochemical pathways are activated in a receptor-dependent or -independent manner by several natural and synthetic compounds, that directly provide neuroprotection: antioxidants, N-methyl-D-aspartate (NMDA) receptor antagonists, calcium channel blockers, acetylcholinesterase inhibitors like galantamine, acetylsalicylic acid, Ginkgo biloba extracts, resveratrol, fish oil and ω-3 (n-3) fatty acids, stem cells, as well as neurotrophic factors such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell-line derived neurotrophic factor (GDNF) and nerve growth factor (NGF) [25-34]. To this list other natural compounds can be added, namely phytocannabinoids (pCBs) and endogenous cannabinoids (eCBs), based on independent studies that documented their neuroprotective effects in ocular tissues [35-44]. In this review, we summarize the main outcomes of preclinical studies that support the potential benefits of pCBs and eCBs as new neuroprotective agents, potentially useful to prevent, slow down or even cure glaucoma and retinal neurodegenerative diseases.
2. PHYTOCANNABINOIDS AND ENDOCANNABINOIDS: SYNTHESIS AND PRODUCTION
The pCBs family is best represented by the active ingredient of cannabis (Cannabis sativa or Cannabis indica), Δ9-tetrahydrocannabinol (THC). Yet, it should be recalled that cannabis contains more than 480 different compounds, of which ~65 have been identified as pCBs [45]. The latter are terpenophenolic substances, that include also abundant (e.g., cannabidiol [CBD]) or minor (e.g., cannabidivarin [CBDV] and Δ9-tetrahydrocannabivarin [THCV]) THC-like molecules able to interact with G protein-coupled type-1 and type-2 cannabinoid receptors (CB1 and CB2), that are the most relevant eCB-binding targets within the so-called “endocannabinoid system (ECS)” [46]. CB1 and CB2 are present in the CNS (apparently CB2 only upon (a)biotic insults) and at the periphery [47-49]. Both receptors modulate various signal transduction pathways, such as inhibition of cAMP production, activation of pERK and G protein-coupled inward rectifying K+-channels (GIRKs), and recruitment of β-arrestin [50]. CB1 and CB2 are activated by the two most active eCBs, N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) [51]. AEA and 2-AG also bind to other receptors, like GPR55 [52], transient receptor potential vanilloid 1 (TRPV1) ion channel [53], and nuclear peroxisome proliferator-activated receptors (PPAR) α and γ [54]. Also pCBs may engage ECS receptors, as well as metabolic enzymes of eCBs [45, 46]. Among them, N-acyl-phosphatidylethanolamine-selective phospholipase D (NAPE-PLD) and diacylglycerol lipases (DAGL) α and β synthesize AEA and 2-AG, respectively, whereas fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) degrade them, respectively [55-57]. ECS is involved throughout the body in several physiological and pathological processes, visual processing included [35-37, 41, 58].
3. PRESENCE OF ECS ELEMENTS IN RETINA AND OCULAR TISSUES
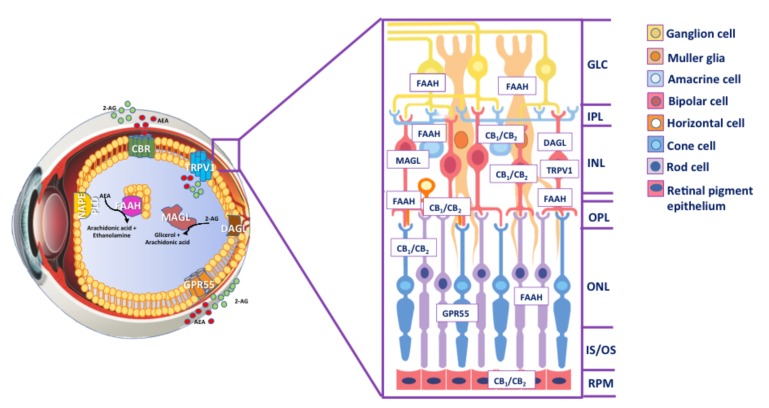
The presence of the major ECS elements has been demonstrated in ocular tissues of different species, from fishes to primates [35-37, 41, 44, 59-61]. Most studies on ECS localization have been focused on retina, that represents the key area of visual information processing [37, 41, 43, 62-65]. In particular, the presence of CB1 and CB2 was investigated in the different layers of retina (inner and outer plexiform layer, INL and ONL respectively) as well as in amacrine, RGCs, photoreceptors, rod bipolar, horizontal, retinal pigment epithelial cells by using different methodological approaches, such as immunohistochemistry, mRNA and protein analysis, and radioligand binding studies [62, 64, 66-69]. Incidentally, CB1 was also found in brain areas like thalamus and cortex, known to influence visual output [70-72], as well as in trabecular meshwork, Schlemm’s canal and ciliary body, where also CB2 is expressed [66, 73-75]. Numerous studies demonstrated the presence of TRPV1 and other TRP subunits at mRNA and protein level in mammalian and non-mammalian retina, and in a variety of neuronal and glial cells of this area; yet, results remained controversial, possibly because of the use of different antibodies and staining protocols [35, 63, 76-81]. Interestingly, TRPV1 may play a major functional role in the inner retina, since it was not detected in photoreceptors and bipolar cells [63]. As for GPR55, its presence has been documented only in the inner segments of rod photoreceptors of monkey retina, suggesting a function role in scotopic vision [65, 69]. Indeed, retinal function has been assessed by several flash electroretinogram (ERG) measurements in the presence of selective antagonists of CB1 and CB2, suggesting the involvement of eCB signaling in the modulation of retinal response. ECS is also involved in neurotransmission within the retina. Indeed, by acting on ionic currents and electrical potentials, it may modulate the release of several neurotransmitters such as dopamine, noradrenaline, GABA and glutamate, that control synaptic activity in retinal ganglion cells and consequently modulate visual response [82-87]. In addition, AEA, 2-AG and their congener N-palmitoylethanolamine (PEA) have been measured by gas chromatography-mass spectrometry in human ocular tissues, demonstrating an overall higher content of 2-AG compared to AEA in human retina, and a content change upon retinal degenerative diseases [75, 88, 89]. 2-AG and AEA levels are high in retina with DR and age-related macular degeneration [89], whereas glaucoma patients have reduced levels of 2-AG and PEA without changes in AEA in the same patients [88]. Furthermore, FAAH expression is remarkable in different layers of retina, from OPL to GCL (ganglion cell layer) in rats, zebrafishes, gold fishes, monkeys and humans [64, 90, 91], and FAAH activity can be measured in the same species and particularly in mice and rats, where it is higher in rods, bipolar cells, horizontal cells, amacrine cells, Muller cells and ganglion cells. Moreover, NAPE-PLD was identified in the retina of rodents and other mammals [90], and recently the presence of DAGL and MAGL mRNAs was documented in rat retina [41], extending previous data on their localization during postnatal development [62]. More specifically, DAGL was found to be expressed in the postsynaptic terminals of cone bipolar cells, whereas MAGL in the IPL and OPL [92]. Localization of ECS components in retina is schematically depicted in Fig. (1).
Fig. (1).
Inside the eye. Schematic representation of the human eye with an overview of ECS distribution. AEA is manly synthesized by NAPE-PLD, whereas DAGL is the most important enzyme for the biosynthesis of 2-AG. AEA and 2-AG signalling pathways are terminated by enzymatic hydrolysis, mediated primarily by the serine hydrolases FAAH and MAGL, respectively. In the cross-section, the presence of ECS element in different layers of the retina is shown. Abbreviations: AEA, anandamide; 2-AG, 2-arachidonoylglycerol; NAPE-PLD, N-arachidonoylphosphatidylethanolamine-specific phospholipase D; DAGL, diacylglycerol lipase; FAAH; fatty acid amide hydrolase; MAGL, monoacylglycerol lipase; CBR, cannabinoid receptors; GPR55, G protein-coupled receptor 55; TRPV1, transient receptor potential vanilloid type 1; GLC, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS/OS photoreceptor layer; RPE, retinal pigment epithelium.
4. PHYTOCANNABINOIDS, IOP REDUCTION AND RETINAL PROTECTION
The first evidence for a positive role of pCBs in retina protection dates back to the ‘70s, when smoking marijuana was found to lower IOP in a small number of subjects [93]. Then, several experimental findings demonstrated that oral or intravenous administration of THC to human subjects with glaucoma reduces IOP, though with development of tolerance and significant education of systemic blood pressure and tachycardia [94-97]. Instead, single sublingual administration of THC temporarily reduced IOP in a well-tolerated manner in most patients, whereas 40 mg of CBD, a non-psychotropic pCB, produced a transient increase of IOP [98]. In this context, the use of animal models pretreated with the CB1 antagonist SR141716A allowed to demonstrate that THC and other pCBs can lower IOP by directly activating ocular CB1 [99], and also by modulating production and drainage of aqueous humor [99, 100]. However, independent studies have shown that N-arachidonoylglycine (NAGly) and abnormal cannabidiol (Abn-CBD), two agonists of GPR18 (a recently deorphanized G protein-coupled receptor related to eCB-binding targets), are able to reduce IOP in a murine model of disease [101]. Many additional preclinical and clinical studies have interrogated the effects of THC and CBD on IOP modulation, establishing beneficial effects in patients with glaucoma although it is just a relief of symptoms and, moreover, tolerance, short duration of these compounds as well as peripheral and CNS side-effects did not allow their use in ophthalmic clinic [98, 102-107]. More recently, scientific interest towards these compounds has been focused on their neuroprotective action that results in a greater long-term efficacy in treating glaucoma and retinal neurodegenerative diseases. In this context, different animal models of ocular diseases have represented suitable tools to dissect the mechanisms by which pCBs and/or eCBs can exert neuroprotective effects: NMDA-induced retinal cell degeneration, AMPA (amino-3hydroxy-5-methyl-4-isoxazole-propionic acid)-induced or light-induced transient ischemia, IOP-reperfusion (glaucoma) and the streptozotocin (STZ)-induced diabetic retinopathy [35, 38, 39, 41, 43, 105, 108-110].
Incidentally, it should be noted that an excessive extracellular glutamate stimulates NMDA receptors involved in retinal neuronal cell death, an event that is common to glaucoma, retinal ischemia, and diabetic retinopathy. For instance, in a NMDA excitotoxic rat model, THC and CBD were found to protect the retina in a CB1/CB2-independent manner, by decreasing peroyxnitrite levels and oxidative stress-related substances in neurons of the INL and GCL [108]. Consistently, independent studies underlined the antioxidant capacity of THC (and other pCBs) as a key feature to provide retinal neuroprotection (for a comprehensive review see [44]). In DR, retinal vascular dysfunction is associated to enhanced production of inflammatory mediators, such as vascular endothelial growth factor (VEGF) and cytokines (tumor necrosis factor TNF-α and inteleukin-6) [111, 112]. Additionally, adenosine and its receptors have been shown to possess anti-inflammatory properties in DR [113], where also CBD blocks retinal inflammation by equilibrating nucleoside transporter and A2A adenosine receptor, and suppresses lipopolysaccharide-induced TNF-α release [114, 115]. Moreover, an increase in peroxynitrite correlates with accelerated retinal endothelial damage, breakdown of the blood-retinal barrier (BRB), and accelerated neuronal cell death in experimental models of diabetes, inflammation, and neurotoxicity [108]. Yet, CBD treatment reduced neurotoxicity, inflammation, and BRB breakdown in diabetic animals through activities that may involve inhibition of p38 MAP kinase [109]. The best investigated effects of THC and CBD on retinal neurodegenerative diseases are summarized in Table 1.
Table 1.
Main effects of THC and CBD on retinal neurodegenerative diseases.
| Source of Model | Target | Overall Effect | Phatology | References |
|---|---|---|---|---|
| Human | Eye | IOP reduction | Glaucoma | Hepler and Frank 1971; Flom et al., 1975; Purnell and Gregg, 1975; Cooler and Gregg 1977; Flach et al., 2002; Tomida et al., 2006 |
| Cat, Rat | Eye | IOP reduction | Glaucoma | Colasanti 1990 |
| Mouse | Anterior Eye | IOP reduction | Glaucoma | Caldwell et al., 2013 |
| Dog | Eye | IOP reduction | Glaucoma | Fischer et al., 2013 |
| Rabbit | Cornea | IOP reduction | Glaucoma | Hingorani et al., 2012 |
| Rat | Retinal Ganglion Cells | Cell protection | Glaucoma | El-Remessy et al., 2003 Crandall et al., 2007 |
| Rat | Retinal Neuronal Cells | Cell protection | Diabetic retinopathy | El-Remessy et al., 2006 |
| Chick | Retinal Section | Cell protection | Diabetic retinopathy and glaucoma | Araujoa et al., 2017 |
5. ENDOCANNABINOIDS AND RETINAL PROTECTION
eCBs show neuroprotective effects in different models of retinal neurodegeneration [35, 37-39,110]. Retinal ischemia models, induced by chemicals or acute elevation of IOP, affect the viability of a variety of amacrine, rod bipolar and RGC cells and lead to increased glutamate levels and activation of ionotropic glutamate (NMDA and AMPA) receptors. Consequently, intracellular calcium ions and NOS activity increase, resulting in glutamate-mediated excitotoxic retinal cell death [116, 117]. In that context, AEA produces a neuroprotective effect against retinal cell death induced by high IOP, through engagement of CB1 and TRPV1 [35]. In particular, blocking AEA degradation with the specific FAAH inhibitor URB587, or mimicking this effect by using the non-hydrolysable analogue of AEA, methanandamide, confers retinal neuroprotection against high IOP-induced cell death [35]. Moreover, CB1 was reported to reduce IOP via the β-adrenergic system, through inhibition of norepinephrine release [118]. In a rat model of optic nerve axotomy, URB587 promotes retinal ganglion cell neuroprotection through CB1, and its efficacy declines with age and is associated to a significant increase in AEA levels. In parallel, a decrease in the AEA congener N-arachidonoyl-glycine is observed in young (but not in aged) animals, and 2-AG levels are not affected [38]. Furthermore, AEA and the synthetic cannabinoids HU-210 and MetAEA, injected intravitreally, protect retinal amacrine cells from AMPA excitotoxicity via a mechanism involving CB1 and the PI3K/Akt and/or MEK/ERK1/2 signaling pathways [110]. Otherwise it has been shown that deletion of CB1 or treatment of diabetic mice with CB1 antagonist SR141716 prevented retinal cell death in a mouse model of DR, as well as in human primary retinal endothelial cells (HREC) exposed to high glucose, by reducing MAPK activation, oxidative stress and inflammatory signaling [119]. Also oral PEA given for three months seems to reduce IOP in ocular hypertensive patients [120], possibly by increasing AEA content, that is reduced in glaucomatous eyes [99], through inhibition of its degradation [121]. 2-AG was found to lower IOP in a concentration- and CB1-dependent manner [122, 123], and indeed in a murine model of disease MAGL blockade can lower IOP by raising endogenous eCB levels [123] and consequently providing indirect neuroprotection. Interestingly, several studies reported that TRPV1 plays a major role as a mediator of RGC function and survival [124-126]. In line with this, in an inducible mouse model of glaucoma both genetic (knock-outs) and pharmacological (antagonists) blockade of TRPV1 accelerate RGC degeneration upon exposure to elevated IOP [125]. Moreover, in vivo TRPV1 expression increases in monkey and human RGCs in response to elevated IOP, thus supporting enhanced excitability. Such an enhancement is likely mediated by Ca2+ currents, since activation of TRPV1 in RGCs increases intracellular Ca2+ in isolated RGCs [124, 126]. In addition to promoting RGC excitability during retinal stress, TRPV1 seems to mediate the release of neuroprotective cytokines, such as interleukin (IL) 6, from glial cells [124]. Instead, in adult retinal explants both genetic and pharmacological blockade of TRPV1 improved RGC survival upon exposure to elevated hydrostatic pressure, as did chelation of extracellular Ca2+ [124]. Activation of TRPV1 was found to protect retinal neurons in vivo from injury induced by intravitreal NMDA in rats [127]. Indeed, treatment with the TRPV1 antagonist capsazepine almost completely erased the protective effect of the TRPV1 agonist capsaicin in the same model [127]. Other studies investigated the involvement of eCB-binding receptors in cell death induced by ischemia in an avascular (chick) retina model where oxygen and glucose deprivation (OGD) was induced. They failed to demonstrate an involvement of CB1 and CB2 in driving cell death at the early stages of ischemia [39], despite several studies showing that these receptors have a protective role against this type of damage [110, 128-130]. Probably, such a discrepancy depends on the different models used (AMPA toxicity, ischemia/reperfusion and acute ischemia). In a cellular model of AMD the expression of CB1 is upregulated and its pharmacological blockade and/or inhibition of CB1 with small interfering RNA (siRNA) can ameliorate H2O2-induced retinal oxidative stress and production of superoxide dismutase (SOD), thus preventing RPE cell death through PI3K/Akt signaling pathway [131]. In the pathogenesis of AMD and in other retinal diseases, also photoreceptors play a pivotal role, because they represent the main actors in phototransduction. Light-damaged animal models have been widely used to investigate the mechanisms of neuroretinal dysfunction in several ocular diseases, including human AMD [132, 133]. In line with this, our group provided the first evidence that bright continuous light (BCL) selectively affects ECS gene and protein expression in the albino rat retina, where only CB1 and CB2 levels were increased [41]. Similarly, accumulated evidence showed that CB1/CB2 levels are elevated in pathological retinal conditions, sometimes in association with oxidative stress [37, 131]. Of note, the other major components of retinal ECS were not modulated by BCL [41], including TRPV1 that plays a role in retinal death induced during IOP-related disease [39]. Remarkably, the selective blockage of both CB1 and CB2 was able to reduce light damage-induced photoreceptor death, thus preserving morphology and visual function, with a major involvement of CB2 compared to CB1 [41]. Consistently with these data, an upregulation of CB1 expression was demonstrated in a light-induced photoreceptor damage model, both in vitro and in vivo, particularly in the photoreceptor outer segment layer [43]. Here, the CB1 antagonist rimonabant effectively and
potently blocked neuronal damage, tissue loss, and functional impairment via suppression of oxidative stress and inflammation [43]. In this context, it has been shown that photoreceptor death can be reduced in several animal models of neurodegeneration, by using both neuroprotectants [134] and antioxidants [135], and remarkably saffron [136]. Experimental studies demonstrated that saffron (Crocus sativus), given as a dietary supplement, counteracts the effects of BCL exposure in the albino rat retina, preserving both morphology and function [137]. Then, a pilot clinical trial conducted on AMD patients provided the first evidence of a therapeutic benefit of saffron treatment [138], also over time [139] and in patients carrying genetic defects [140]. Multiple actions of saffron have been suggested, including modulation of gene expression in animal models of retinal degeneration [141]. In keeping with this notion, recently we demonstrated that saffron down-regulates gene and protein expression of CB1 and CB2 in an animal model of retinal degeneration induced by light exposure [41]. Taking into account that some retinal pathologies are associated with a decrease in the amplitude of the electroretinographic waves, the measurement of b-wave of the electroretinogram is considered a solid indicator of inner retina functionality. In rats with retinal damage the b-wave amplitude was modulated by saffron or CB1 and CB2 antagonists in quite a similar manner, suggesting that these molecules could trigger the same mechanism, or else that saffron might directly impinge on CB1/CB2-dependent signal transduction to afford retinal protection [41]. In line with these data, CB1 and CB2 were found to modulate the electroretinographic waves in vervet monkey [65]. In particular, under photopic conditions blockade of CB2 increased the amplitude of the b-wave above the standard flash intensity value, whereas under scotopic conditions blockade of either CB1 or CB2 increased only the amplitude of the b-wave irrespective of flash intensity [65], suggesting a role of both receptors in vision and retinal protection. Recently, a novel mechanism underlying a CB1-mediated increase in RGC intrinsic excitability through AMPK-dependent inhibition of the Na+-K+-2Cl− co-transporter 1 (NKCC1) has been proposed [142]. CB1 activation markedly improved visual contrast sensitivity under low-light conditions [142], whereas the role of CB2 in intraocular pressure, aqueous humor outflow and ocular inflammatory pathologies remains unclear [143-145]. For example, activation of CB2 has anti-inflammatory effects on the retina in a chronic experimental model of autoimmune uveoretinitis, associated with inhibition of leukocyte trafficking in vivo and reduction of inflammatory mediators in vitro [146]. Modulation of ocular CB2 in a model of endotoxin-induced uveitis by the synthetic agonist HU-308 attenuates leukocyte-endothelial cell adhesion in the iridial microvasculature and reduces release of pro-inflammatory mediators (TNF-α, IL-1β, IL-6, INF-γ, CCL5 and CXCL2), but also of transcription factors NF-κβ and AP-1 [147], which enhance transcription of pro-inflammatory genes [148, 149]. In addition, CB2 activation reduces leukocyte adhesion and improves capillary perfusion in the iridial microvasculature during systemic inflammation induced by lipopolysaccharide [150]. The main functions of ECS in retinal neurodegenerative diseases are summarized in Table 2.
Table 2.
Main effects of ECS on retinal neuroprotection.
| Animal/Human/Cell Model | Target | Molecular Effect | Effect on eCB-Binding Receptors | Overall Effect | References |
|---|---|---|---|---|---|
| Retinal ischemia mice model | Retinal Ganglion Cells | FAAH inhibition | ↓ CB1, TRPV1 | IOP reduction | Nucci et al., 2007 |
| Knockout mice (-/-) for β1 AR, β2 AR, CB1, or CB2 | Anterior Eye | NE release Inhibition | ↑ CB1 | IOP reduction | Hudson et al., 2011 |
| Rat model of axotomy | Retinal Ganglion Cells | FAAH inhibition | ↑ CB1 | Cell protection | Slusar et al., 2013 |
| AMPA excitotoxicity animal model | Amacrine Cells | PI3K/AKT and MEK/ ERK1/2 signalling pathway |
↑ CB1 | Cell protection | Kokona et al., 2015 |
| Ocular hypertensive subjects | Vascular Endothelium | Inhibition of AEA degradation (?) | Receptor- independent |
IOP reduction | Strobbe et al., 2013 |
| Knockout (-/-) mice for CB1, CB2, or MAGL | Nonpigmented Ciliary Epithelium | MAGL blockage | ↑ CB1 | IOP reduction | Miller et al., 2016 |
| Knockout (-/-) mice for TRPV1 | Retinal Ganglion Cells | Enhanced excitability by Ca2+ efflux | ↑ TRPV1 | Cell protection | Sappington et al., 2015 |
| Streptozotocin-induced diabetics rat model Human retinal endothelial cell | Retinal Endothelial Cells | Suppression of oxidative stress and inflammation | ↑ CB1 | Cell protection | El-Remessy et al., 2008 |
| Animal/Human/Cell Model | Target | Molecular Effect | Effect on eCB-binding Receptors | Overall Effect | References |
| NMDA excitotoxicity rat model | Retinal Ganglion Cells | Activation of CGRP and tachykinin NK1 receptors | ↑ TRPV1 | Cell protection | Sakamoto et al., 2014 |
| Xenopus leavis | Retinal Ganglion Cells | Enhanced excitability by chloride channel current | ↑ CB1 | Visual response protection | Miracourt et al., 2016 |
| Light-induced damage mice model | Murine Retinal Cone Cells | Suppression of oxidative stress and inflammation | ↑ CB1 | Photoreceptor protection | Inamura et al., 2017 |
| Human retinal pigmental epithelial cells | Retinal Pigment Epithelium | Downregulation oxidative stress | ↑ CB1 | Cell protection | Wei et al., 2013 |
| Light-induced photo- receptor damage rat model |
Retinal Section | Mediated by saffron | ↓ CB1, CB2 | Photoreceptor protection | Maccarone et al., 2016 |
6. TOPICAL ADMINISTRATION OF CANNABINOIDS FOR RETINAL DISORDERS
Natural and synthetic cannabinoids are highly lipophilic molecules with a low aqueous solubility, which limits their application by topical administration, consequently it is very challenging determine the appropriate route for delivering these compounds. Various formulation strategies have been used to overcome this obstacle, including the use of surfactants and cyclodextrins [151, 152]. Actually, in the case of THC the use of light mineral oil, as a vehicle, improved the aqueous solubility and transcorneal permeability, but caused irritation to the human eye [153]. Also different microemulsions, such as submicron emulsion of THC, and cyclodextrins (macrocyclic oligosaccharides) have been shown to improve the corneal penetration of cannabinoids [143, 151]. Recently, prodrug strategy represents a new approach to development of molecules with high solubility, permeability and absorption in ocular drug delivery [154]. Incidentally, prodrugs are pharmacologically inactive molecules which must undergo chemical or enzymatic transformations within the body before exerting their pharmacological or therapeutic effect [154]. In this context, phosphate ester prodrugs of three cannabinoids (arachidonylethanolamide, R-methanandamide and noladin ether) have been synthesized and their physicochemical properties have been studied, showing a significantly enhanced aqueous solubility compared to their parent drugs with adequate chemical stability in buffer solutions [155]. Similarly, hemisuccinate (THC-HS) and hemiglutarate (THC-HG) ester prodrugs and WIN 55-212-2 (WIN), a synthetic cannabinoid, as well as prodrug-ion-pair complexes with l-arginine or tromethamine have been used in rabbit cornea demonstrating an improved solubility and permeability of an ion-pair complex of THC-HG [106]. In addition, use of surfactants led to a significant improvement in the aqueous solubility of THC-HG [152]. A recent study demonstrated that combination of THC with mono and di-valine esters (THC-Val and THC-Val-Val) and amino acid (valine)-dicarboxylic acid (hemisuccinate) ester (THC-Val-HS) improve the penetration of THC into the anterior segment of the eye following topical application [156]. Lowering IOP is still the primary target of these prodrugs in the glaucoma treatment, although as discussed here, neuroprotective drugs represent a promising next-generation therapy that could employ the strategy of prodrugs to enhance the solubility and ocular penetration of eCBs.
CONCLUSION
The neuroprotective effects of pCBs and eCBs on retinal neurodegenerative diseases discussed here appear to engage different mechanisms, including IOP reduction, RGCs and photoreceptor preservation through anti- inflammatory and antioxidant actions. Interestingly, some of them are mediated by CB1 whereas others go through non-CB receptors, especially TRPV1, and/or additional neuromodulatory systems. Therefore, CB1 and TRPV1 agonists or antagonists, and inhibitors of distinct metabolic enzymes, like FAAH and MAGL, that extend the duration of action of eCBs, could have therapeutic potential for the treatment of glaucoma and other ocular pathologies like DR, AMD and retinal disorders associated to Alzheimer’s disease and Parkinson’s disease. In addition, drugs targeting CB2 may be valuable therapeutics for ocular inflammation [146, 147, 150]. As discussed in this review, several preclinical studies underline anti-inflammatory and neuroprotective effects of CBD, a pCB devoid of unwanted psychoactive side effects. Given that CBD in combination with THC has been approved for the treatment of inflammation, pain, and spasticity associated with multiple sclerosis in humans [157, 158], it could be tested also to treat degenerative eye diseases such as glaucoma and DR. On a final note, new combined therapies where cannabinergic agents are associated with antioxidant molecules, possibly in innovative prodrug formulations that allow “smart” delivery to a distinct area of the body, could provide better efficacy to prevent neurodegeneration and death of retinal cells. For instance, topical use of CB1 and CB2 antagonists, in combination with saffron supplement in the diet, might hold potential to prevent, slow down or even cure retinal neurodegenerative processes.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bourne R.R., Taylor H.R., Flaxman S.R., Keeffe J., Leasher J., Naidoo K., Pesudovs K., White R.A., Wong T.Y., Resnikoff S., Jonas J.B. Number of People Blind or Visually Impaired by Glaucoma Worldwide and in World Regions 1990 - 2010: A Meta-Analysis. PLoS One. 2016;11(10):e0162229. doi: 10.1371/journal.pone.0162229. [http://dx.doi.org/ 10.1371/journal.pone.0162229]. [PMID: 27764086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlmann-Noor A., Tailor V., Bunce C., Abou-Rayyah Y., Adams G., Brookes J., Khaw P.T., Papadopoulos M. Quality of life and functional vision in children with glau-coma. 2017. http://dx.doi.org/10 [DOI] [PMC free article] [PubMed]
- 3.Quigley H.A. Proportion of those with open-angle glaucoma who become blind. Ophthalmology. 1999;106(11):2039–2041. doi: 10.1016/S0161-6420(99)90516-X. [http:// dx.doi.org/10.1016/S0161-6420(99)90516-X]. [PMID: 10571331]. [DOI] [PubMed] [Google Scholar]
- 4.Morrone L.A., Rombolà L., Corasaniti M.T., Bagetta G., Nucci C., Russo R. Natural compounds and retinal ganglion cell neuroprotection. 2015. [DOI] [PubMed] [Google Scholar]
- 5.Russo R., Varano G.P., Adornetto A., Nucci C., Corasaniti M.T., Bagetta G., Morrone L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016;787:134–142. doi: 10.1016/j.ejphar.2016.03.064. [http://dx.doi.org/10.1016/j.ejphar. 2016.03.064]. [PMID: 27044433]. [DOI] [PubMed] [Google Scholar]
- 6.Davis B.M., Crawley L., Pahlitzsch M., Javaid F., Cordeiro M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016;132(6):807–826. doi: 10.1007/s00401-016-1609-2. [http://dx.doi.org/10.1007/s00401-016-1609-2]. [PMID: 27544758]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L., Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [http://dx. doi.org/10.1016/j.preteyeres.2011.11.002]. [PMID: 22155051]. [DOI] [PubMed] [Google Scholar]
- 8.Levkovitch-Verbin H. 2015. [Google Scholar]
- 9.Almasieh M., Catrinescu M-M., Binan L., Costantino S., Levin L.A. Axonal degeneration in retinal ganglion cells is associated with a membrane polarity-sensitive redox process. J. Neurosci. 2017;37(14):3824–3839. doi: 10.1523/JNEUROSCI.3882-16.2017. [http://dx.doi.org/10.1523/JNEUROSCI. 3882-16.2017]. [PMID: 28275163]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Haan J., Verbraak F.D., Visser P.J., Bouwman F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimers Dement. (Amst.) 2017;6:162–170. doi: 10.1016/j.dadm.2016.12.014. [http://dx. doi.org/10.1016/j.dadm.2016.12.014]. [PMID: 28275698]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekker M.S., Janssen S., Seppi K., Poewe W., De Vries N.M., Theelen T., Nonnekes J., Bloem B.R. Ocular and visual disorders in Parkinson's Disease: Common but frequently overlooked. . Parkinsonism Relat. Disord. 2017. [DOI] [PubMed]
- 12.Pula J.H., Gomez C.M., Kattah J.C. Ophthalmologic features of the common spinocerebellar ataxias. Curr. Opin. Ophthalmol. 2010;21(6):447–453. doi: 10.1097/ICU.0b013e32833eaf71. [http://dx.doi.org/10.1097/ICU.0b013e32833 eaf71]. [PMID: 20811282]. [DOI] [PubMed] [Google Scholar]
- 13.Stricker S., Oberwahrenbrock T., Zimmermann H., Schroeter J., Endres M., Brandt A.U., Paul F. Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS One. 2011;6(7):e23024. doi: 10.1371/journal.pone.0023024. [http://dx.doi.org/10.1371/journal.pone.0023024]. [PMID: 21829579]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quigley H.A. The contribution of the sclera and lamina cribrosa to the pathogenesis of glaucoma: Diagnostic and treatment implications. Prog. Brain Res. 2015; 220 :59–86. doi: 10.1016/bs.pbr.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kinnunen K., Petrovski G., Moe M.C., Berta A., Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90(4):299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [http://dx.doi.org/10.1111/j.1755-3768.2011.02179.x]. [PMID: 22112056]. [DOI] [PubMed] [Google Scholar]
- 16.Cachafeiro M., Bemelmans A.P., Samardzija M., Afanasieva T., Pournaras J.A., Grimm C., Kostic C., Philippe S., Wenzel A., Arsenijevic Y. Hyperactivation of retina by light in mice leads to photoreceptor cell death mediated by VEGF and retinal pigment epithelium permeability. Cell Death Dis. 2013;4:e781. doi: 10.1038/cddis.2013.303. [http://dx.doi.org/10.1038/cddis.2013.303]. [PMID: 23990021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiose Y., Kitazawa Y., Tsukahara S., Akamatsu T., Mizokami K., Futa R., Katsushima H., Kosaki H. Epidemiology of glaucoma in Japan--a nationwide glaucoma survey. Jpn. J. Ophthalmol. 1991;35(2):133–155. [PMID: 1779484]. [PubMed] [Google Scholar]
- 18.Anderson D.R., Drance S.M., Schulzer M. Factors that predict the benefit of lowering intraocular pressure in normal tension glaucoma. Am. J. Ophthalmol. 2003;136(5):820–829. doi: 10.1016/s0002-9394(03)00478-1. [http://dx.doi. org/10.1016/S0002-9394(03)00478-1]. [PMID: 14597032]. [DOI] [PubMed] [Google Scholar]
- 19.Masuzzo A., Dinet V., Cavanagh C., Mascarelli F., Krantic S. Amyloidosis in Retinal Neurodegenerative Diseases. Front. Neurol. 2016;7:127. doi: 10.3389/fneur.2016.00127. [http://dx.doi.org/10.3389/fneur.2016.00127]. [PMID: 27551275]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin L.A., Crowe M.E., Quigley H.A. 2016.
- 21.Nucci C., Russo R., Martucci A., Giannini C., Garaci F., Floris R., Bagetta G., Morrone L.A. New strategies for neuroprotection in glaucoma, a disease that affects the central nervous system. Eur. J. Pharmacol. 2016;787:119–126. doi: 10.1016/j.ejphar.2016.04.030. [http://dx.doi.org/10.1016/ j.ejphar.2016.04.030]. [PMID: 27089818]. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer N., Lamparter J., Gericke A., Grus F.H., Hoffmann E.M., Wahl J. Neuroprotection of medical IOP-lowering therapy. Cell Tissue Res. 2013;353(2):245–251. doi: 10.1007/s00441-013-1671-1. [http://dx.doi.org/10.1007/ s00441-013-1671-1]. [PMID: 23836043]. [DOI] [PubMed] [Google Scholar]
- 23.Tamm E.R., Grehn F., Pfeiffer N. Neuroprotection in glaucoma. Cell Tissue Res. 2013;353(2):201–203. doi: 10.1007/s00441-013-1672-0. [http://dx.doi.org/10.1007/ s00441-013-1672-0]. [PMID: 23812823]. [DOI] [PubMed] [Google Scholar]
- 24.Tamm E. R., Ethier C.R., Dowling J.E, Downs C., Ellisman M.H., Fisher S., Fortune B., Fruttiger M., Jakobs T., Lewis G., Mitchell C.H., Morrison J., Sharma S.C., Sigal I., Sofroniew M., Wang L., Wiggs J., Wu S., Masland R.H. Biological aspects of axonal damage in glaucoma: A brief review. 2017. http://dx.doi.org/10 [DOI] [PMC free article] [PubMed]
- 25.De Castro D.K., Punjabi O.S., Bostrom A.G., Stamper R.L., Lietman T.M., Ray K., Lin S.C. Effect of statin drugs and aspirin on progression in open-angle glaucoma suspects using confocal scanning laser ophthalmoscopy. Clin. Experiment. Ophthalmol. 2007;35(6):506–513. doi: 10.1111/j.1442-9071.2007.01529.x. [http://dx.doi.org/10.1111/j.1442-9071.2007. 01529.x]. [PMID: 17760631]. [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarieh M., Flammer J. Is there more to glaucoma treatment than lowering IOP? Surv. Ophthalmol. 2007;52(2) Suppl. 2:S174–S179. doi: 10.1016/j.survophthal.2007.08.013. [http://dx.doi.org/10.1016/j.survophthal.2007.08.013]. [PMID: 17998043]. [DOI] [PubMed] [Google Scholar]
- 27.Dahlmann-Noor A.H., Vijay S., Limb G.A., Khaw P.T. Strategies for optic nerve rescue and regeneration in glaucoma and other optic neuropathies. Drug Discov. Today. 2010;15(7-8):287–299. doi: 10.1016/j.drudis.2010.02.007. [http://dx.doi.org/10.1016/j.drudis.2010.02.007]. [PMID: 20197108]. [DOI] [PubMed] [Google Scholar]
- 28.Unsicker K. Neurotrophic molecules in the treatment of neurodegenerative disease with focus on the retina: status and perspectives. Cell Tissue Res. 2013;353(2):205–218. doi: 10.1007/s00441-013-1585-y. [http://dx.doi.org/10. 1007/s00441-013-1585-y]. [PMID: 23463189]. [DOI] [PubMed] [Google Scholar]
- 29.Quaranta L., Riva I., Floriani I. Ginkgo biloba extract improves visual field damage in some patients affected by normal-tension glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55(4):2417. doi: 10.1167/iovs.14-13942. [http://dx.doi.org/10.1167/iovs.14-13942]. [PMID: 24736415]. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Amero K.K., Kondkar A.A., Chalam K.V. Resveratrol and Ophthalmic Diseases. Nutrients. 2016;8(4):200. doi: 10.3390/nu8040200. [http://dx.doi. org/10.3390/nu8040200]. [PMID: 27058553]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura A., Namekata K., Guo X., Harada C., Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int. J. Mol. Sci. 2016;17(9):1584. doi: 10.3390/ijms17091584. [http://dx.doi. org/10.3390/ijms17091584]. [PMID: 27657046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura A., Namekata K., Guo X., Harada C., Harada T. Dock3-NMDA receptor interaction as a target for glaucoma therapy. Histol. Histopathol. 2017;32(3):215–221. doi: 10.14670/HH-11-820. [PMID: 27615513]. [DOI] [PubMed] [Google Scholar]
- 33.Garg A., Yang J., Lee W., Tsang S.H. Stem cell therapies in retinal disorders. Cells. 2017;6(1):4. doi: 10.3390/cells6010004. [http://dx.doi.org/10.3390/ cells6010004]. [PMID: 28157165]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holan V., Hermankova B., Kossl J. Perspectives of stem cell-based therapy for age-related retinal degenerative diseases. Cell Transplant. 2017;26(9):1538–1541. doi: 10.1177/0963689717721227. [http://dx.doi.org/10.1177/ 0963689717721227]. [PMID: 29113466]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nucci C., Gasperi V., Tartaglione R., Cerulli A., Terrinoni A., Bari M., De Simone C., Agrò A.F., Morrone L.A., Corasaniti M.T., Bagetta G., Maccarrone M. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Invest. Ophthalmol. Vis. Sci. 2007;48(7):2997–3004. doi: 10.1167/iovs.06-1355. [http://dx.doi.org/10.1167/iovs.06-1355]. [PMID: 17591864]. [DOI] [PubMed] [Google Scholar]
- 36.Nucci C., Bari M., Spanò A., Corasaniti M., Bagetta G., Maccarrone M., Morrone L.A. 2008. [DOI] [PubMed] [Google Scholar]
- 37.Yazulla S. Endocannabinoids in the retina: from marijuana to neuroprotection. Prog. Retin. Eye Res. 2008;27(5):501–526. doi: 10.1016/j.preteyeres.2008.07.002. [http:// dx.doi.org/10.1016/j.preteyeres.2008.07.002]. [PMID: 18725316]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slusar J.E., Cairns E.A., Szczesniak A-M., Bradshaw H.B., Di Polo A., Kelly M.E. The fatty acid amide hydrolase inhibitor, URB597, promotes retinal ganglion cell neuroprotection in a rat model of optic nerve axotomy. Neuropharmacology. 2013;72:116–125. doi: 10.1016/j.neuropharm.2013.04.018. [http://dx.doi.org/10.1016/j.neuropharm.2013.04.018]. [PMID: 23643752]. [DOI] [PubMed] [Google Scholar]
- 39.Araújo D.S.M., Miya-Coreixas V.S., Pandolfo P., Calaza K.C. Cannabinoid receptors and TRPA1 on neuroprotection in a model of retinal ischemia. Exp. Eye Res. 2017;154:116–125. doi: 10.1016/j.exer.2016.11.015. [http://dx. doi.org/10.1016/j.exer.2016.11.015]. [PMID: 27876485]. [DOI] [PubMed] [Google Scholar]
- 40.Kokona D., Georgiou P.C., Kounenidakis M., Kiagiadaki F., Thermos K. Endogenous and synthetic cannabinoids as therapeutics in retinal disease. Neural Plast. 2016;2016:8373020. doi: 10.1155/2016/8373020. [http:// dx.doi.org/10.1155/2016/8373020]. [PMID: 26881135]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maccarone R., Rapino C., Zerti D., di Tommaso M., Battista N., Di Marco S., Bisti S., Maccarrone M. Modulation of type-1 and type-2 cannabinoid receptors by saffron in a rat model of retinal neurodegeneration. PLoS One. 2016;11(11):e0166827. doi: 10.1371/journal.pone.0166827. [http:// dx.doi.org/10.1371/journal.pone.0166827]. [PMID: 27861558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwitzer T., Schwan R., Angioi-Duprez K., Giersch A., Laprevote V. The endocannabinoid system in the retina: From physiology to practical and therapeutic applications. Neural Plast. 2016;2016:2916732. doi: 10.1155/2016/2916732. [http://dx.doi.org/10.1155/2016/2916732]. [PMID: 26881099]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imamura T., Tsuruma K., Inoue Y., Otsuka T., Ohno Y., Ogami S., Yamane S., Shimazawa M., Hara H. Rimonabant, a selective cannabinoid1 receptor antagonist, protects against light-induced retinal degeneration in vitro and in vivo. Eur. J. Pharmacol. 2017;803:78–83. doi: 10.1016/j.ejphar.2017.03.018. [http://dx.doi.org/10.1016/j.ejphar.2017. 03.018]. [PMID: 28315677]. [DOI] [PubMed] [Google Scholar]
- 44.Panahi Y., Manayi A., Nikan M., Vazirian M. The arguments for and against cannabinoids application in glaucomatous retinopathy. Biomed. Pharmacother. 2017;86:620–627. doi: 10.1016/j.biopha.2016.11.106. [http://dx.doi.org/10. 1016/j.biopha.2016.11.106]. [PMID: 28027538]. [DOI] [PubMed] [Google Scholar]
- 45.Morales P., Hurst D.P., Reggio P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [http://dx.doi.org/10.1007/978-3-319-45541-9_4]. [PMID: 28120232]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Marzo V., Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12(4):692–698. doi: 10.1007/s13311-015-0374-6. [http://dx.doi.org/10.1007/s13311-015-0374-6]. [PMID: 26271952]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-Ruiz J., Romero J., Velasco G., Tolón R.M., Ramos J.A., Guzmán M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol. Sci. 2007;28(1):39–45. doi: 10.1016/j.tips.2006.11.001. [http://dx.doi.org/10.1016/j.tips.2006.11.001]. [PMID: 17141334]. [DOI] [PubMed] [Google Scholar]
- 48.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., Sharkey K.A., Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36(5):277–296. doi: 10.1016/j.tips.2015.02.008. [http://dx.doi.org/10.1016/j.tips.2015.02.008]. [PMID: 25796370]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bisogno T., Oddi S., Piccoli A., Fazio D., Maccarrone M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016;111:721–730. doi: 10.1016/j.phrs.2016.07.021. [http://dx.doi.org/10.1016/j.phrs.2016.07.021]. [PMID: 27450295]. [DOI] [PubMed] [Google Scholar]
- 50.Pertwee R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010;17(14):1360–1381. doi: 10.2174/092986710790980050. [http://dx.doi.org/10.2174/092986710790980050]. [PMID: 20166927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fezza F., Bari M., Florio R., Talamonti E., Feole M., Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19(11):17078–17106. doi: 10.3390/molecules191117078. [http://dx.doi.org/ 10.3390/molecules191117078]. [PMID: 25347455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009;30(3):156–163. doi: 10.1016/j.tips.2008.12.004. [http://dx.doi.org/10.1016/j.tips. 2008.12.004]. [PMID: 19233486]. [DOI] [PubMed] [Google Scholar]
- 53.Di Marzo V., De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010;17(14):1430–1449. doi: 10.2174/092986710790980078. [http://dx.doi.org/10. 2174/092986710790980078]. [PMID: 20166923]. [DOI] [PubMed] [Google Scholar]
- 54.Pistis M., Melis M. From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr. Med. Chem. 2010;17(14):1450–1467. doi: 10.2174/092986710790980014. [http://dx.doi.org/10.2174/092986710790980014]. [PMID: 20166922]. [DOI] [PubMed] [Google Scholar]
- 55.Ahn K., McKinney M.K., Cravatt B.F. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 2008;108(5):1687–1707. doi: 10.1021/cr0782067. [http://dx.doi.org/10.1021/cr0782067]. [PMID: 18429637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [PMID: 18481028]. [DOI] [PubMed] [Google Scholar]
- 57.Ueda N., Tsuboi K., Uyama T., Ohnishi T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors. 2011;37(1):1–7. doi: 10.1002/biof.131. [http://dx.doi.org/10.1002/biof.131]. [PMID: 21328621]. [DOI] [PubMed] [Google Scholar]
- 58.Maccarrone M. Need for methods to investigate endocannabinoid signaling. Methods Mol. Biol. 2016;1412:1–8. doi: 10.1007/978-1-4939-3539-0_1. [http://dx.doi.org/ 10.1007/978-1-4939-3539-0_1]. [PMID: 27245886]. [DOI] [PubMed] [Google Scholar]
- 59.Lograno M.D., Romano M.R. Cannabinoid agonists induce contractile responses through Gi/o-dependent activation of phospholipase C in the bovine ciliary muscle. Eur. J. Pharmacol. 2004;494(1):55–62. doi: 10.1016/j.ejphar.2004.04.039. [http://dx.doi.org/10.1016/j.ejphar.2004.04.039]. [PMID: 15194451]. [DOI] [PubMed] [Google Scholar]
- 60.Cottone E., Pomatto V., Cerri F., Campantico E., Mackie K., Delpero M., Guastalla A., Dati C., Bovolin P., Franzoni M.F. Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol. Biochem. 2013;39(5):1287–1296. doi: 10.1007/s10695-013-9783-9. [http://dx.doi.org/10.1007/s10695-013-9783-9]. [PMID: 23504102]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouchard J-F., Casanova C., Cécyre B., Redmond W.J. Expression and function of the endocannabinoid system in the retina and the visual brain. Neural Plast. 2016;2016:9247057. doi: 10.1155/2016/9247057. [http://dx. doi.org/10.1155/2016/9247057]. [PMID: 26839718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cécyre B., Monette M., Beudjekian L., Casanova C., Bouchard J.F. Localization of diacylglycerol lipase alpha and monoacylglycerol lipase during postnatal development of the rat retina. Front. Neuroanat. 2014;8:150. doi: 10.3389/fnana.2014.00150. [PMID: 25565975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryskamp D.A., Redmon S., Jo A.O., Križaj D. TRPV1 and endocannabinoids: Emerging molecular signals that modulate mammalian vision. Cells. 2014;3(3):914–938. doi: 10.3390/cells3030914. [http://dx.doi.org/ 10.3390/cells3030914]. [PMID: 25222270]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouskila J., Burke M.W., Zabouri N., Casanova C., Ptito M., Bouchard J-F. Expression and localization of the cannabinoid receptor type 1 and the enzyme fatty acid amide hydrolase in the retina of vervet monkeys. Neuroscience. 2012;202:117–130. doi: 10.1016/j.neuroscience.2011.11.041. [http:// dx.doi.org/10.1016/j.neuroscience.2011.11.041]. [PMID: 22142900]. [DOI] [PubMed] [Google Scholar]
- 65.Bouskila J., Javadi P., Elkrief L., Casanova C., Bouchard J-F., Ptito M. A comparative analysis of the endocannabinoid system in the retina of mice, tree shrews, and monkeys. Neural Plast. 2016;2016:3127658. doi: 10.1155/2016/3127658. [http://dx.doi.org/10.1155/2016/3127658]. [PMID: 26977322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Straiker A., Stella N., Piomelli D., Mackie K., Karten H.J., Maguire G. Cannabinoid CB1 receptors and ligands in vertebrate retina: localization and function of an endogenous signaling system. Proc. Natl. Acad. Sci. USA. 1999;96(25):14565–14570. doi: 10.1073/pnas.96.25.14565. [http://dx.doi.org/10.1073/pnas.96.25.14565]. [PMID: 10588745]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yazulla S., Studholme K.M., McIntosh H.H., Deutsch D.G. Immunocytochemical localization of cannabinoid CB1 receptor and fatty acid amide hydrolase in rat retina. J. Comp. Neurol. 1999;415(1):80–90. doi: 10.1002/(sici)1096-9861(19991206)415:1<80::aid-cne6>3.0.co;2-h. [http://dx.doi.org/10.1002/(SICI)1096-9861(19991206) 415:1<80:AID-CNE6>3.0.CO;2-H]. [PMID: 10540359]. [DOI] [PubMed] [Google Scholar]
- 68.López E.M., Tagliaferro P., Onaivi E.S., López-Costa J.J. Distribution of CB2 cannabinoid receptor in adult rat retina. Synapse. 2011;65(5):388–392. doi: 10.1002/syn.20856. [http://dx.doi.org/10.1002/syn.20856]. [PMID: 20803619]. [DOI] [PubMed] [Google Scholar]
- 69.Bouskila J., Javadi P., Casanova C., Ptito M., Bouchard J-F. Müller cells express the cannabinoid CB2 receptor in the vervet monkey retina. J. Comp. Neurol. 2013;521(11):2399–2415. doi: 10.1002/cne.23333. [http://dx.doi.org/10.1002/cne.23333]. [PMID: 23630038]. [DOI] [PubMed] [Google Scholar]
- 70.Dasilva M.A., Grieve K.L., Cudeiro J., Rivadulla C. Endocannabinoid CB1 receptors modulate visual output from the thalamus. Psychopharmacology (Berl.) 2012;219(3):835–845. doi: 10.1007/s00213-011-2412-3. [http://dx. doi.org/10.1007/s00213-011-2412-3]. [PMID: 21773721]. [DOI] [PubMed] [Google Scholar]
- 71.Ohiorhenuan I.E., Mechler F., Purpura K.P., Schmid A.M., Hu Q., Victor J.D. Cannabinoid neuromodulation in the adult early visual cortex. PLoS One. 2014;9(2):e87362. doi: 10.1371/journal.pone.0087362. [http://dx.doi.org/10. 1371/journal.pone.0087362]. [PMID: 24586271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Javadi P., Bouskila J., Bouchard J-F., Ptito M. The endocannabinoid system within the dorsal lateral geniculate nucleus of the vervet monkey. Neuroscience. 2015;288:135–144. doi: 10.1016/j.neuroscience.2014.12.029. [http://dx.doi. org/10.1016/j.neuroscience.2014.12.029]. [PMID: 25575947]. [DOI] [PubMed] [Google Scholar]
- 73.Porcella A., Casellas P., Gessa G.L., Pani L. Cannabinoid receptor CB1 mRNA is highly expressed in the rat ciliary body: implications for the antiglaucoma properties of marihuana. Brain Res. Mol. Brain Res. 1998;58(1-2):240–245. doi: 10.1016/s0169-328x(98)00105-3. [http://dx.doi.org/10.1016/ S0169-328X(98)00105-3]. [PMID: 9685662]. [DOI] [PubMed] [Google Scholar]
- 74.Porcella A., Maxia C., Gessa G.L., Pani L. The human eye expresses high levels of CB1 cannabinoid receptor mRNA and protein. Eur. J. Neurosci. 2000;12(3):1123–1127. doi: 10.1046/j.1460-9568.2000.01027.x. [http://dx.doi.org/ 10.1046/j.1460-9568.2000.01027.x]. [PMID: 10762343]. [DOI] [PubMed] [Google Scholar]
- 75.Stamer W.D., Golightly S.F., Hosohata Y., Ryan E.P., Porter A.C., Varga E., Noecker R.J., Felder C.C., Yamamura H.I. Cannabinoid CB(1) receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur. J. Pharmacol. 2001;431(3):277–286. doi: 10.1016/s0014-2999(01)01438-8. [http://dx.doi.org/ 10.1016/S0014-2999(01)01438-8]. [PMID: 11730719]. [DOI] [PubMed] [Google Scholar]
- 76.Zimov S., Yazulla S. Localization of vanilloid receptor 1 (TRPV1/VR1)-like immunoreactivity in goldfish and zebrafish retinas: restriction to photoreceptor synaptic ribbons. J. Neurocytol. 2004;33(4):441–452. doi: 10.1023/B:NEUR.0000046574.72380.e8. [http://dx.doi.org/10.1023/B:NEUR. 0000046574.72380.e8]. [PMID: 15520529]. [DOI] [PubMed] [Google Scholar]
- 77.Sappington R.M., Sidorova T., Long D.J., Calkins D.J. TRPV1: contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Invest. Ophthalmol. Vis. Sci. 2009;50(2):717–728. doi: 10.1167/iovs.08-2321. [http://dx.doi.org/10. 1167/iovs.08-2321]. [PMID: 18952924]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonelli M., Martins D.O., Kihara A.H., Britto L.R. Ontogenetic expression of the vanilloid receptors TRPV1 and TRPV2 in the rat retina. Int. J. Dev. Neurosci. 2009;27(7):709–718. doi: 10.1016/j.ijdevneu.2009.07.003. [http://dx.doi. org/10.1016/j.ijdevneu.2009.07.003]. [PMID: 19619635]. [DOI] [PubMed] [Google Scholar]
- 79.Leonelli M., Martins D.O., Britto L.R. TRPV1 receptors are involved in protein nitration and Müller cell reaction in the acutely axotomized rat retina. Exp. Eye Res. 2010;91(5):755–768. doi: 10.1016/j.exer.2010.08.026. [http://dx.doi.org/10.1016/j.exer.2010.08.026]. [PMID: 20826152]. [DOI] [PubMed] [Google Scholar]
- 80.Ryskamp D.A., Witkovsky P., Barabas P., Huang W., Koehler C., Akimov N.P., Lee S.H., Chauhan S., Xing W., Rentería R.C., Liedtke W., Krizaj D. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J. Neurosci. 2011;31(19):7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.0359-11.2011]. [PMID: 21562271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martínez-García M.C., Martínez T., Pañeda C., Gallego P., Jimenez A.I., Merayo J. Differential expression and localization of transient receptor potential vanilloid 1 in rabbit and human eyes. Histol. Histopathol. 2013;28(11):1507–1516. doi: 10.14670/HH-28.1507. [PMID: 23709255]. [DOI] [PubMed] [Google Scholar]
- 82.Yazulla S., Studholme K.M., McIntosh H.H., Fan S-F. Cannabinoid receptors on goldfish retinal bipolar cells: electron-microscope immunocytochemistry and whole-cell recordings. Vis. Neurosci. 2000;17(3):391–401. doi: 10.1017/s0952523800173079. [http://dx.doi.org/10.1017/S0952523800173079]. [PMID: 10910107]. [DOI] [PubMed] [Google Scholar]
- 83.Fan S-F., Yazulla S. Biphasic modulation of voltage-dependent currents of retinal cones by cannabinoid CB1 receptor agonist WIN 55212-2. Vis. Neurosci. 2003;20(2):177–188. doi: 10.1017/s095252380320208x. [http://dx.doi.org/ 10.1017/S095252380320208X]. [PMID: 12916739]. [DOI] [PubMed] [Google Scholar]
- 84.Fan S-F., Yazulla S. Inhibitory interaction of cannabinoid CB1 receptor and dopamine D2 receptor agonists on voltage-gated currents of goldfish cones. Vis. Neurosci. 2004;21(1):69–77. doi: 10.1017/s0952523804041070. [http:// dx.doi.org/10.1017/S0952523804041070]. [PMID: 15137583]. [DOI] [PubMed] [Google Scholar]
- 85.Fan S-F., Yazulla S. Retrograde endocannabinoid inhibition of goldfish retinal cones is mediated by 2-arachidonoyl glycerol. Vis. Neurosci. 2007;24(3):257–267. doi: 10.1017/S095252380707006X. [http://dx.doi.org/10.1017/ S095252380707006X]. [PMID: 17592669]. [DOI] [PubMed] [Google Scholar]
- 86.Straiker A., Sullivan J.M. Cannabinoid receptor activation differentially modulates ion channels in photoreceptors of the tiger salamander. J. Neurophysiol. 2003;89(5):2647–2654. doi: 10.1152/jn.00268.2002. [http://dx.doi. org/10.1152/jn.00268.2002]. [PMID: 12740409]. [DOI] [PubMed] [Google Scholar]
- 87.Lalonde M.R., Jollimore C.A., Stevens K., Barnes S., Kelly M.E. Cannabinoid receptor-mediated inhibition of calcium signaling in rat retinal ganglion cells. Mol. Vis. 2006;12:1160–1166. [PMID: 17093402]. [PubMed] [Google Scholar]
- 88.Chen J., Matias I., Dinh T., Lu T., Venezia S., Nieves A., Woodward D.F., Di Marzo V. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem. Biophys. Res. Commun. 2005;330(4):1062–1067. doi: 10.1016/j.bbrc.2005.03.095. [http://dx.doi.org/10. 1016/j.bbrc.2005.03.095]. [PMID: 15823551]. [DOI] [PubMed] [Google Scholar]
- 89.Matias I., Wang J.W., Moriello A.S., Nieves A., Woodward D.F., Di Marzo V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75(6):413–418. doi: 10.1016/j.plefa.2006.08.002. [http://dx.doi.org/10. 1016/j.plefa.2006.08.002]. [PMID: 17011761]. [DOI] [PubMed] [Google Scholar]
- 90.Bisogno T., Delton-Vandenbroucke I., Milone A., Lagarde M., Di Marzo V. Biosynthesis and inactivation of N-arachidonoylethanol-amine (anandamide) and N-docosahexaenoylethanolamine in bovine retina. Arch. Biochem. Biophys. 1999;370(2):300–307. doi: 10.1006/abbi.1999.1410. [http://dx.doi.org/10.1006/abbi.1999.1410]. [PMID: 10577359]. [DOI] [PubMed] [Google Scholar]
- 91.Glaser S.T., Deutsch D.G., Studholme K.M., Zimov S., Yazulla S. Endocannabinoids in the intact retina: 3 H-anandamide uptake, fatty acid amide hydrolase immunoreactivity and hydrolysis of anandamide. Vis. Neurosci. 2005;22(6):693–705. doi: 10.1017/S0952523805226020. [http://dx.doi. org/10.1017/S0952523805226020]. [PMID: 16469181]. [DOI] [PubMed] [Google Scholar]
- 92.Hu S.S-Y., Arnold A., Hutchens J.M., Radicke J., Cravatt B.F., Wager-Miller J., Mackie K., Straiker A. Architecture of cannabinoid signaling in mouse retina. J. Comp. Neurol. 2010;518(18):3848–3866. doi: 10.1002/cne.22429. [http://dx.doi.org/10.1002/cne.22429]. [PMID: 20653038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hepler R.S., Frank I.R. Marihuana smoking and intraocular pressure. JAMA. 1971;217(10):1392. [http://dx.doi.org/10.1001/jama. 1971.03190100074024]. [PMID: 5109652]. [PubMed] [Google Scholar]
- 94.Flom M.C., Adams A.J., Jones R.T. Marijuana smoking and reduced pressure in human eyes: drug action or epiphenomenon? Invest. Ophthalmol. 1975;14(1):52–55. [PMID: 1089090]. [PubMed] [Google Scholar]
- 95.Cooler P., Gregg J.M. Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. South. Med. J. 1977;70(8):951–954. doi: 10.1097/00007611-197708000-00016. [http://dx.doi.org/10.1097/00007611-197708000-00016]. [PMID: 329423]. [DOI] [PubMed] [Google Scholar]
- 96.Flach A.J. Delta-9-tetrahydrocannabinol (THC) in the treatment of end-stage open-angle glaucoma. Trans. Am. Ophthalmol. Soc. 2002;100:215–222. [PMID: 12545695]. [PMC free article] [PubMed] [Google Scholar]
- 97.Zobor D., Strasser T., Zobor G., Schober F., Messias A., Strauss O., Batra A., Zrenner E. Ophthalmological assessment of cannabis-induced persisting perception disorder: is there a direct retinal effect? Doc. Ophthalmol. 2015;130(2):121–130. doi: 10.1007/s10633-015-9481-2. [http://dx. doi.org/10.1007/s10633-015-9481-2]. [PMID: 25612939]. [DOI] [PubMed] [Google Scholar]
- 98.Tomida I., Azuara-Blanco A., House H., Flint M., Pertwee R.G., Robson P.J. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J. Glaucoma. 2006;15(5):349–353. doi: 10.1097/01.ijg.0000212260.04488.60. [http://dx.doi.org/10.1097/01.ijg.0000212260.04488.60]. [PMID: 16988594]. [DOI] [PubMed] [Google Scholar]
- 99.Chien F.Y., Wang R.F., Mittag T.W., Podos S.M. Effect of WIN 55212-2, a cannabinoid receptor agonist, on aqueous humor dynamics in monkeys. Arch. Ophthalmol. 2003;121(1):87–90. doi: 10.1001/archopht.121.1.87. [http://dx.doi.org/10.1001/archopht.121.1.87]. [PMID: 12523891]. [DOI] [PubMed] [Google Scholar]
- 100.Njie Y.F., Kumar A., Qiao Z., Zhong L., Song Z-H. Noladin ether acts on trabecular meshwork cannabinoid (CB1) receptors to enhance aqueous humor outflow facility. Invest. Ophthalmol. Vis. Sci. 2006;47(5):1999–2005. doi: 10.1167/iovs.05-0729. [http://dx.doi.org/10.1167/iovs.05-0729]. [PMID: 16639008]. [DOI] [PubMed] [Google Scholar]
- 101.Caldwell M.D., Hu S.S., Viswanathan S., Bradshaw H., Kelly M.E., Straiker A.A. GPR18-based signalling system regulates IOP in murine eye. Br. J. Pharmacol. 2013;169(4):834–843. doi: 10.1111/bph.12136. [http://dx.doi.org/10.1111/bph.12136]. [PMID: 23461720]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Purnell W.D., Gregg J.M. Delta(9)-tetrahydrocannabinol, euphoria and intraocular pressure in man. Ann. Ophthalmol. 1975;7(7):921–923. [PMID: 1147519]. [PubMed] [Google Scholar]
- 103.Colasanti B.K. A comparison of the ocular and central effects of delta 9-tetrahydrocannabinol and cannabigerol. J. Ocul. Pharmacol. 1990;6(4):259–269. doi: 10.1089/jop.1990.6.259. [http://dx.doi.org/10.1089/jop.1990. 6.259]. [PMID: 1965836]. [DOI] [PubMed] [Google Scholar]
- 104.Järvinen T., Pate D.W., Laine K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002;95(2):203–220. doi: 10.1016/s0163-7258(02)00259-0. [http://dx. doi.org/10.1016/S0163-7258(02)00259-0]. [PMID: 12182967]. [DOI] [PubMed] [Google Scholar]
- 105.Crandall J., Matragoon S., Khalifa Y.M., Borlongan C., Tsai N-T., Caldwell R.B., Liou G.I. Neuroprotective and intraocular pressure-lowering effects of (-)Δ9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007;39(2):69–75. doi: 10.1159/000099240. [http://dx.doi. org/10.1159/000099240]. [PMID: 17284931]. [DOI] [PubMed] [Google Scholar]
- 106.Hingorani T., Gul W., Elsohly M., Repka M.A., Majumdar S. Effect of ion pairing on in vitro transcorneal permeability of a Δ(9) -tetrahydrocannabinol prodrug: potential in glaucoma therapy. J. Pharm. Sci. 2012;101(2):616–626. doi: 10.1002/jps.22791. [http://dx.doi.org/10.1002/ jps.22791]. [PMID: 21989812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fischer K.M., Ward D.A., Hendrix D.V.H. Effects of a topically applied 2% delta-9-tetrahydrocannabinol ophthalmic solution on intraocular pressure and aqueous humor flow rate in clinically normal dogs. Am. J. Vet. Res. 2013;74(2):275–280. doi: 10.2460/ajvr.74.2.275. [http://dx.doi. org/10.2460/ajvr.74.2.275]. [PMID: 23363354]. [DOI] [PubMed] [Google Scholar]
- 108.El-Remessy A.B., Khalil I.E., Matragoon S., Abou-Mohamed G., Tsai N-J., Roon P., Caldwell R.B., Caldwell R.W., Green K., Liou G.I. Neuroprotective effect of (-)Δ9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am. J. Pathol. 2003;163(5):1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [http://dx.doi.org/10.1016/S0002-9440(10)63558-4]. [PMID: 14578199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Remessy A.B., Al-Shabrawey M., Khalifa Y., Tsai N-T., Caldwell R.B., Liou G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006;168(1):235–244. doi: 10.2353/ajpath.2006.050500. [http://dx.doi.org/10.2353/ ajpath.2006.050500]. [PMID: 16400026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kokona D., Thermos K. Synthetic and endogenous cannabinoids protect retinal neurons from AMPA excitotoxicity in vivo, via activation of CB1 receptors: Involvement of PI3K/Akt and MEK/ERK signaling pathways. Exp. Eye Res. 2015;136:45–58. doi: 10.1016/j.exer.2015.05.007. [http://dx.doi.org/10.1016/j.exer.2015.05.007]. [PMID: 25989217]. [DOI] [PubMed] [Google Scholar]
- 111.Palenski T.L., Sorenson C.M., Sheibani N. Inflammatory cytokine-specific alterations in retinal endothelial cell function. Microvasc. Res. 2013;89:57–69. doi: 10.1016/j.mvr.2013.06.007. [http://dx.doi.org/10.1016/j.mvr. 2013.06.007]. [PMID: 23806781]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nalini M., Raghavulu B.V., Annapurna A., Avinash P., Chandi V., Swathi N. Correlation of various serum biomarkers with the severity of diabetic retinopathy. 2017. [DOI] [PubMed]
- 113.Liou G.I., Ahmad S., Naime M., Fatteh N., Ibrahim A.S. Role of adenosine in diabetic retinopathy. J. Ocul. Biol. Dis. Infor. 2011;4(1-2):19–24. doi: 10.1007/s12177-011-9067-5. [http://dx.doi.org/10.1007/s12177-011-9067-5]. [PMID: 23308298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carrier E.J., Auchampach J.A., Hillard C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA. 2006;103(20):7895–7900. doi: 10.1073/pnas.0511232103. [http://dx.doi.org/10.1073/pnas. 0511232103]. [PMID: 16672367]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liou G.I., Auchampach J.A., Hillard C.J., Zhu G., Yousufzai B., Mian S., Khan S., Khalifa Y. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest. Ophthalmol. Vis. Sci. 2008;49(12):5526–5531. doi: 10.1167/iovs.08-2196. [http://dx.doi.org/10.1167/iovs.08-2196]. [PMID: 18641283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dreyer E.B., Grosskreutz C.L. Excitatory mechanisms in retinal ganglion cell death in primary open angle glaucoma (POAG). Clin. Neurosci. 1997;4(5):270–273. [PMID: 9292254]. [PubMed] [Google Scholar]
- 117.Nucci C., Tartaglione R., Rombolà L., Morrone L.A., Fazzi E., Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005;26(5):935–941. doi: 10.1016/j.neuro.2005.06.002. [http://dx.doi.org/10.1016/j.neuro.2005.06.002]. [PMID: 16126273]. [DOI] [PubMed] [Google Scholar]
- 118.Hudson B.D., Beazley M., Szczesniak A-M., Straiker A., Kelly M.E.M. Indirect sympatholytic actions at β-adrenoceptors account for the ocular hypotensive actions of cannabinoid receptor agonists. J. Pharmacol. Exp. Ther. 2011;339(3):757–767. doi: 10.1124/jpet.111.185769. [http://dx.doi. org/10.1124/jpet.111.185769]. [PMID: 21885619]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El-Remessy A.B., Rajesh M., Mukhopadhyay P., Horváth B., Patel V., Al-Gayyar M.M., Pillai B.A., Pacher P. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia. 2011;54(6):1567–1578. doi: 10.1007/s00125-011-2061-4. [http://dx.doi.org/ 10.1007/s00125-011-2061-4]. [PMID: 21373835]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strobbe E., Cellini M., Campos E.C. Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: a randomized, placebo-controlled cross-over study. Invest. Ophthalmol. Vis. Sci. 2013;54(2):968–973. doi: 10.1167/iovs.12-10899. [http://dx.doi.org/10. 1167/iovs.12-10899]. [PMID: 23307959]. [DOI] [PubMed] [Google Scholar]
- 121.Di Marzo V., De Petrocellis L., Bisogno T. Endocannabinoids Part I: molecular basis of endocannabinoid formation, action and inactivation and development of selective inhibitors. Expert Opin. Ther. Targets. 2001;5(2):241–265. doi: 10.1517/14728222.5.2.241. [http://dx.doi.org/10.1517/ 14728222.5.2.241]. [PMID: 15992179]. [DOI] [PubMed] [Google Scholar]
- 122.Laine K., Järvinen K., Pate D.W., Urtti A., Järvinen T. Effect of the enzyme inhibitor, phenylmethylsulfonyl fluoride, on the IOP profiles of topical anandamides. Invest. Ophthalmol. Vis. Sci. 2002;43(2):393–397. [PMID: 11818382]. [PubMed] [Google Scholar]
- 123.Miller S., Leishman E., Hu S.S., Elghouche A., Daily L., Murataeva N., Bradshaw H., Straiker A. Harnessing the Endocannabinoid 2-Arachidonoylglycerol to Lower Intraocular Pressure in a Murine Model. Invest. Ophthalmol. Vis. Sci. 2016;57(7):3287–3296. doi: 10.1167/iovs.16-19356. [http://dx.doi.org/10.1167/iovs.16-19356]. [PMID: 27333182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sappington R.M., Sidorova T., Ward N.J., Chakravarthy R., Ho K.W., Calkins D.J. Activation of transient receptor potential vanilloid-1 (TRPV1) influences how retinal ganglion cell neurons respond to pressure-related stress. Channels (Austin) 2015;9(2):102–113. doi: 10.1080/19336950.2015.1009272. [http://dx.doi.org/10.1080/19336950.2015.1009272]. [PMID: 25713995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ward N.J., Ho K.W., Lambert W.S., Weitlauf C., Calkins D.J. Absence of transient receptor potential vanilloid-1 accelerates stress-induced axonopathy in the optic projection. J. Neurosci. 2014;34(9):3161–3170. doi: 10.1523/JNEUROSCI.4089-13.2014. [http://dx.doi.org/10.1523/JNEUROSCI. 4089-13.2014]. [PMID: 24573275]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weitlauf C., Ward N.J., Lambert W.S., Sidorova T.N., Ho K.W., Sappington R.M., Calkins D.J. Short-term increases in transient receptor potential vanilloid-1 mediate stress-induced enhancement of neuronal excitation. J. Neurosci. 2014;34(46):15369–15381. doi: 10.1523/JNEUROSCI.3424-14.2014. [http://dx.doi.org/10.1523/JNEUROSCI.3424-14.2014]. [PMID: 25392504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakamoto K., Kuroki T., Okuno Y., Sekiya H., Watanabe A., Sagawa T., Ito H., Mizuta A., Mori A., Nakahara T., Ishii K. Activation of the TRPV1 channel attenuates N-methyl-D-aspartic acid-induced neuronal injury in the rat retina. Eur. J. Pharmacol. 2014;733:13–22. doi: 10.1016/j.ejphar.2014.03.035. [http://dx.doi.org/10.1016/j.ejphar.2014.03.035]. [PMID: 24704373]. [DOI] [PubMed] [Google Scholar]
- 128.Nagayama T., Sinor A.D., Simon R.P., Chen J., Graham S.H., Jin K., Greenberg D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999;19(8):2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [http://dx.doi.org/10.1523/ JNEUROSCI.19-08-02987.1999]. [PMID: 10191316]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parmentier-Batteur S., Jin K., Mao X.O., Xie L., Greenberg D.A. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J. Neurosci. 2002;22(22):9771–9775. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [http://dx. doi.org/10.1523/JNEUROSCI.22-22-09771.2002]. [PMID: 12427832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang M., Martin B.R., Adler M.W., Razdan R.K., Ganea D., Tuma R.F. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152(3):753–760. doi: 10.1016/j.neuroscience.2008.01.022. [http://dx.doi.org/ 10.1016/j.neuroscience.2008.01.022]. [PMID: 18304750]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei Y., Wang X., Zhao F., Zhao P.Q., Kang X.L. Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury. Mol. Vis. 2013;19:357–366. [PMID: 23441106]. [PMC free article] [PubMed] [Google Scholar]
- 132.Contín M.A., Arietti M.M., Benedetto M.M., Bussi C., Guido M.E. Photoreceptor damage induced by low-intensity light: model of retinal degeneration in mammals. Mol. Vis. 2013;19:1614–1625. [PMID: 23901245]. [PMC free article] [PubMed] [Google Scholar]
- 133.Niwa M., Aoki H., Hirata A., Tomita H., Green P.G., Hara A. Retinal cell degeneration in animal models. Int. J. Mol. Sci. 2016;17(1):E110. doi: 10.3390/ijms17010110. [http://dx.doi.org/10.3390/ijms17010110]. [PMID: 26784179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strettoi E., Gargini C., Novelli E., Sala G., Piano I., Gasco P., Ghidoni R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 2010;107(43):18706–18711. doi: 10.1073/pnas.1007644107. [http://dx.doi.org/10.1073/pnas.1007644107]. [PMID: 20937879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Komeima K., Rogers B.S., Campochiaro P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007;213(3):809–815. doi: 10.1002/jcp.21152. [http://dx.doi.org/10.1002/ jcp.21152]. [PMID: 17520694]. [DOI] [PubMed] [Google Scholar]
- 136.Bisti S., Maccarone R., Falsini B. Saffron and retina: neuroprotection and pharmacokinetics. Vis. Neurosci. 2014;31(4-5):355–361. doi: 10.1017/S0952523814000108. [http://dx.doi.org/10.1017/S0952523814000108]. [PMID: 24819927]. [DOI] [PubMed] [Google Scholar]
- 137.Maccarone R., Di Marco S., Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest. Ophthalmol. Vis. Sci. 2008;49(3):1254–1261. doi: 10.1167/iovs.07-0438. [http://dx.doi.org/10.1167/iovs.07-0438]. [PMID: 18326756]. [DOI] [PubMed] [Google Scholar]
- 138.Falsini B., Piccardi M., Minnella A., Savastano C., Capoluongo E., Fadda A., Balestrazzi E., Maccarone R., Bisti S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2010;51(12):6118–6124. doi: 10.1167/iovs.09-4995. [http://dx.doi.org/10.1167/iovs.09-4995]. [PMID: 20688744]. [DOI] [PubMed] [Google Scholar]
- 139.Piccardi M., Marangoni D., Minnella A.M., Savastano M.C., Valentini P., Ambrosio L., Capoluongo E., Maccarone R., Bisti S., Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012;2012:429124. doi: 10.1155/2012/429124. [http://dx.doi.org/10.1155/2012/ 429124]. [PMID: 22852021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marangoni D., Falsini B., Piccardi M., Ambrosio L., Minnella A.M., Savastano M.C., Bisti S., Maccarone R., Fadda A., Mello E., Concolino P., Capoluongo E. Functional effect of Saffron supplementation and risk genotypes in early age-related macular degeneration: a preliminary report. J. Transl. Med. 2013;11:228. doi: 10.1186/1479-5876-11-228. [http://dx.doi.org/10.1186/1479-5876-11-228]. [PMID: 24067115]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Natoli R., Zhu Y., Valter K., Bisti S., Eells J., Stone J. Gene and noncoding RNA regulation underlying photoreceptor protection: microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol. Vis. 2010;16:1801–1822. [PMID: 20844572]. [PMC free article] [PubMed] [Google Scholar]
- 142.Miraucourt L.S., Tsui J., Gobert D., Desjardins J.F., Schohl A., Sild M., Spratt P., Castonguay A., De Koninck Y., Marsh-Armstrong N., Wiseman P.W., Ruthazer E.S. Endocannabinoid signaling enhances visual responses through modulation of intracellular chloride levels in retinal ganglion cells. eLife. 2016;5:e15932. doi: 10.7554/eLife.15932. [http://dx.doi.org/10.7554/eLife.15932]. [PMID: 27501334]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tomida I., Pertwee R.G., Azuara-Blanco A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004;88(5):708–713. doi: 10.1136/bjo.2003.032250. [http://dx. doi.org/10.1136/bjo.2003.032250]. [PMID: 15090428]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhong L., Geng L., Njie Y., Feng W., Song Z.H. CB2 cannabinoid receptors in trabecular meshwork cells mediate JWH015-induced enhancement of aqueous humor outflow facility. Invest. Ophthalmol. Vis. Sci. 2005;46(6):1988–1992. doi: 10.1167/iovs.04-0651. [http://dx.doi.org/ 10.1167/iovs.04-0651]. [PMID: 15914613]. [DOI] [PubMed] [Google Scholar]
- 145.Szczesniak A.M., Maor Y., Robertson H., Hung O., Kelly M.E. Nonpsychotropic cannabinoids, abnormal cannabidiol and canabigerol-dimethyl heptyl, act at novel cannabinoid receptors to reduce intraocular pressure. J. Ocul. Pharmacol. Ther. 2011;27(5):427–435. doi: 10.1089/jop.2011.0041. [http://dx.doi.org/10.1089/jop.2011.0041]. [PMID: 21770780]. [DOI] [PubMed] [Google Scholar]
- 146.Xu H., Cheng C.L., Chen M., Manivannan A., Cabay L., Pertwee R.G., Coutts A., Forrester J.V. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007;82(3):532–541. doi: 10.1189/jlb.0307159. [http://dx.doi.org/10.1189/jlb.0307159]. [PMID: 17537989]. [DOI] [PubMed] [Google Scholar]
- 147.Toguri J.T., Lehmann C., Laprairie R.B., Szczesniak A.M., Zhou J., Denovan-Wright E.M., Kelly M.E. Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis. Br. J. Pharmacol. 2014;171(6):1448–1461. doi: 10.1111/bph.12545. [http:// dx.doi.org/10.1111/bph.12545]. [PMID: 24308861]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson G.L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [http://dx.doi.org/10.1126/science. 1072682]. [PMID: 12471242]. [DOI] [PubMed] [Google Scholar]
- 149.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [http://dx.doi.org/10.1038/nri1391]. [PMID: 15229469]. [DOI] [PubMed] [Google Scholar]
- 150.Toguri J.T., Moxsom R., Szczesniak A.M., Zhou J., Kelly M.E., Lehmann C. Cannabinoid 2 receptor activation reduces leukocyte adhesion and improves capillary perfusion in the iridial microvasculature during systemic inflammation. Clin. Hemorheol. Microcirc. 2015;61(2):237–249. doi: 10.3233/CH-151996. [http://dx.doi.org/10.3233/CH-151996]. [PMID: 26410875]. [DOI] [PubMed] [Google Scholar]
- 151.Hippalgaonkar K., Gul W., ElSohly M.A., Repka M.A., Majumdar S. Enhanced solubility, stability, and transcorneal permeability of δ-8-tetrahydrocannabinol in the presence of cyclodextrins. AAPS PharmSciTech. 2011;12(2):723–731. doi: 10.1208/s12249-011-9639-5. [http://dx.doi.org/10.1208/ s12249-011-9639-5]. [PMID: 21637944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hingorani T., Adelli G.R., Punyamurthula N., Gul W., Elsohly M.A., Repka M.A., Majumdar S. Ocular disposition of the hemiglutarate ester prodrug of ∆9-Tetrahydrocannabinol from various ophthalmic formulations. Pharm. Res. 2013;30(8):2146–2156. doi: 10.1007/s11095-013-1072-x. [http://dx.doi.org/10.1007/s11095-013-1072-x]. [PMID: 23737345]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Green K., Roth M. Ocular effects of topical administration of delta 9-tetrahydrocannabinol in man. Arch. Ophthalmol. 1982;100(2):265–267. doi: 10.1001/archopht.1982.01030030267006. [http://dx.doi.org/10.1001/archopht.1982.01030030267006]. [PMID: 6279061]. [DOI] [PubMed] [Google Scholar]
- 154.Barot M., Bagui M., Gokulgandhi M.R., Mitra A.K. Prodrug strategies in ocular drug delivery. Med. Chem. 2012;8(4):753–768. doi: 10.2174/157340612801216283. [http://dx.doi.org/10.2174/157340612801216283]. [PMID: 22530907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Juntunen J., Vepsäläinen J., Niemi R., Laine K., Järvinen T. Synthesis, in vitro evaluation, and intraocular pressure effects of water-soluble prodrugs of endocannabinoid noladin ether. J. Med. Chem. 2003;46(23):5083–5086. doi: 10.1021/jm030877j. [http://dx.doi.org/10.1021/ jm030877j]. [PMID: 14584958]. [DOI] [PubMed] [Google Scholar]
- 156.Adelli G.R., Bhagav P., Taskar P., Hingorani T., Pettaway S., Gul W., ElSohly M.A., Repka M.A., Majumdar S. Development of a Δ9-tetrahydrocannabinol amino acid-dicarboxylate prodrug with improved ocular bioavailability. Invest. Ophthalmol. Vis. Sci. 2017;58(4):2167–2179. doi: 10.1167/iovs.16-20757. [http://dx.doi.org/10.1167/iovs.16-20757]. [PMID: 28399267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barnes M.P. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin. Pharmacother. 2006;7(5):607–615. doi: 10.1517/14656566.7.5.607. [http://dx.doi.org/10.1517/14656566.7.5.607]. [PMID: 16553576]. [DOI] [PubMed] [Google Scholar]
- 158.Maccarrone M., Maldonado R., Casas M., Henze T., Centonze D. Cannabinoids therapeutic use: what is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert Rev. Clin. Pharmacol. 2017;10(4):443–455. doi: 10.1080/17512433.2017.1292849. [http://dx.doi.org/10.1080/17512433.2017.1292849]. [PMID: 28276775]. [DOI] [PubMed] [Google Scholar]