1. Introduction
Glaucoma is a neurodegenerative disease of the eye and is the major cause of irreversible vision loss. It has been projected to affect approximately 60 million people by 2020, translating to more than 8.4 million cases of irreversible blindness worldwide [1]. The underlying pathological mechanism associated with disease progression is still under investigation, but evidence from both human and experimental models suggest that elevated intraocular pressure (IOP) obstructs retrograde and anterograde axonal transport of neurotrophins in retinal ganglion cells (RGCs) axons at the level
of the optic nerve head (ONH) [2]. Genome-wide association studies (GWAS) and other genomic analyses have successfully identified over 70 single nucleotide polymorphism (SNPs) associated with primary open angle glaucoma (POAG) [3]. The molecular basis of cell signalling pathways and genetic risk factors can be studied together to understand their contribution to this complex disease and could provide cell signalling and gene regulatory based screening tests. In this review, we summarize the recent advances in the neurotrophin signalling pathways and genomics associations that are identified in glaucoma (Fig. 1). Deprivation of nerve growth factor (NGF) and other neurotrophic factors contributes to disease progression. A better understanding of the gene linked molecular events is necessary to produce novel therapeutic targeted treatment, which may give hope to preserve vision.
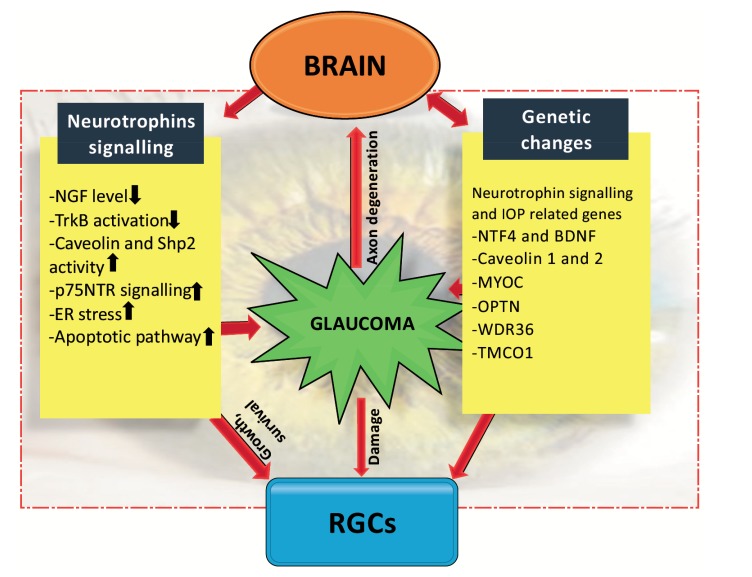
Fig. (1).
Schematic representation from brain to eye highlighting association of neurotrophins and genes in glaucoma (NGF, nerve growth factor; TrkB, tropomyosin receptor kinase B; Shp2, SH2 domain containing protein tyrosine phosphatase; p75NTR, p75 neurotrophin receptor; ER, endoplasmic reticulum; IOP, intraocular pressure; NTF4, neurotrophin factor 4; BDNF, brain derived neurotrophic factor; MYOC, myocilin; OPTN, optineurin; WDR36, WD repeat domain 36; TMCO1, transmembrane and coiled-coil domains 1).
2. Neurotrophin role in RGCS
Neurotrophins play a key role in neuronal cell survival. In healthy or normal conditions, RGCs can receive neurotrophin support from Muller cells [4] or retrograde axonal transport from the brain directly. This has been visualized using live-cell imaging in vitro [5]. Human and mouse adult RGCs not only express Trk family receptors but also abundantly express p75NTR receptor during retinal development [6]. The p75NTR receptor is predominantly expressed in Müller glial cells [6, 7]. The immature form of neurotrophin, such as pro-NGF binding to p75NTR leads to its activation and programmed cell death via tumor necrosis factor-alpha (TNFα) production in glial cells [8]. NGF binding to the p75NTR receptor in the absence of TrkA has been suggested to induce apoptosis in developing RGCs [9]. Lack of neurotrophin support has been suggested as a reason for RGCs degeneration and death in glaucoma [10]. A significant question is where do neurotrophins originate, and how do neurotrophins function upon engaging receptors on RGCs? Differential activation of neurotrophin receptors can occur when neurotrophins act at the axonal terminal or at the neuronal cell body [11, 12]. Neurotrophins are also shown to transport retrogradely from the superior colliculus (SC) in the central nervous system (CNS). Retrograde transportation of neurotrophin take place from SC to RGCs cell bodies. Studies in rats demonstrated that acute IOP elevation inhibits the retrograde transport of brain-derived neurotrophic factor (BDNF) from SC to the RGCs soma. Deprivation of BDNF to RGCs contributes to the loss of visual signal [11]. NGF also diffuses to presynaptic axon terminals of neurons such as the cholinergic neurons where it binds its receptor TrkA, bringing its dimerization, phosphorylation and the formation of an extended complex of proteins involved in signalling pathways. These complexes are then internalized in part by clathrin-coated membranes and moved to early endosomes that are then retrogradely transported from axons to the cell body through a dynein-microtubule based mechanism. When the signalling endosome reaches the cell body, the signal is communicated to the cytoplasm and the nucleus, triggering gene expression. Lysosomes promote the degradation of this signalling complex [13]. This model proposed for NGF may well apply for other neurotrophins. Many studies have led to the conclusion that BDNF is also locally synthesized in the retina. The RGCs and neighbouring amacrine cells produce BDNF that may act in a paracrine fashion. The RGCs themselves act in autocrine fashion and these neurons have been shown to survive in absence of additional BDNF from the brain and are able to express it in culture [14, 15]. Other neurotrophic factors such as ciliary neurotrophic factor (CNTF) and glial cell-line derived neurotrophic factor (GDNF) have also been reported to be involved in RGCs maintenance and survival [16, 17].
3. Neurotrophins deprivation theory in glaucoma
Neurotrophins are transported to the retina by retrograde manner. They are responsible for regulation of neuronal growth, function and survival. The long-range retrograde neurotrophin transportation is probably facilitated via endosome signalling [18]. Neurotrophins bind to the Trk receptors at axon terminals which is then taken up by cell body retrogradely [19]. In glaucomatous conditions, due to high IOP the retrograde transport is thought to be blocked at the ONH region and RGCs fail to receive BDNF and TrkB support for survival [11, 20]. This deprivation causes an alteration of axoplasmic transport of neurotrophins from target neurons in the SC and lateral geniculate nucleus in the brain [21]. RGCs seem to be especially dependent upon BDNF, NT-4, CNTF, and GDNF [12, 22-24]. Pease and colleagues (2000) demonstrated that experimental glaucoma induces abnormal TrkB axonal distribution, focal accumulation of TrkB and BDNF, increased levels of TrkB in ganglion cell layer (GCL), and increased TrkB in glia [20]. The ability of RGCs to survive in culture with the lack of exogenous BDNF has also been demonstrated [15] suggesting that the deprivation of extrinsic BDNF because of retrograde transport interruption is not the only reason for RGCs death in glaucoma. This is important because BDNF/ TrkB downstream signalling is involved in apoptosis inhibition and has been shown to support survival of retinal explants and cultures. It has also been shown to increase the rate of axonal elongation but only when it is present in the axonal terminals [25-27]. The presence of TrkB and BDNF in the all sub-populations of the RGCs has been demonstrated in the retina [14]. It is believed that neurotrophin deprivation in neurons exerts stress and initiates apoptotic pathways with in the cell through activated JNK which stimulates expression of BH-3-containing proteins that facilitate the actions of a proapoptotic proteins (BAX) causing mitochondrial dysfunction [28, 29]. In summary, neurotrophin deprivation induces cell death and auxiliary therapy could therefore be a potential approach in glaucoma treatment.
4. BDNF-TRKB signalling
There is evidence for the protective role of BDNF in maintaining the survival of RGCs under conditionally induced apoptosis like optic nerve injury or glaucoma [30]. In regenerating RGCs neurons, BDNF initiates neuritogenesis [31], and can repair damaged optic nerve and RGCs [30, 32]. Our recent studies have established the role of BDNF impairment in structural, functional and molecular neurodegenerative changes in the inner retina that are exacerbated with age and in glaucoma in BDNF+/− mice [33]. TrkB activation leads to an enhanced PI3K/Akt and Erk1/2 signalling in the RGCs and Erk1/2 appears to be responsible for promoting the survival of RGCs [34, 35]. Previous studies have shown that BDNF mediated TrkB signalling modulates glycogen synthase kinase 3β (GSK3β) activity in retinoic acid differentiated neuroblastoma, SH-SY5Y cells [36]. BDNF binds to the TrkB receptor to initiate multiple signalling cascades as discussed above, including PI3K activation which in turn leads to the stimulation of Ser/Thr kinase Akt [37]. Akt is a major upstream regulator of GSK3β and regulates GSK3β signalling by its phosphorylation at Ser9, thereby inactivating it [38]. The active form of GSK3β (GSK3β phosphorylation) inhibits collapsin response mediator protein-2 (CRMP-2) binding to tubulin and promotes microtubule dynamics along with regulating axonal transport and outgrowth in neurons. In order to understand the mechanism of BDNF/ TrkB signalling in RGCs, we have demonstrated that activation of the BDNF-TrkB signalling in PC-12 cell lines regulate GSK3β activity which is involved in cellular survival, cell fate determination and proliferation [39]. BDNF knockdown in PC-12 cell lines resulted in marked downregulation of GSK3β phosphorylation [39]. In addition, ONH of BDNF+/− mice illustrated reduced GSK3β phosphorylation with age [39]. Further studies to stimulate BDNF-TrkB signalling in RGCs neuroprotection may lead to development of effective treatment therapies for glaucoma.
5. Glial BDNF-TRKB signalling
BDNF-TrkB signalling promotes cell survival by the activation of pro-survival pathways [8, 40]. Notably, the expression of TrkB is relatively higher in inner retinal cells like Müller glial cells and RGCs as compared to the photoreceptor cells [8, 41, 42]. This suggests that the neuroprotective roles of BDNF-TrkB signalling in the inner and outer retina is different. Indeed, Kimura et al. (2016), reported a direct neuroprotective action of glia on photoreceptor cells. BDNF treated Muller glia cells in vitro show upregulated CNTF levels, while upregulation of basic fibroblast growth factor by glia protects the photoreceptor cells undergoing damage [43]. BDNF-TrkB signalling in glial cells produces neuroprotective effects. Two different TrkB knockout mice models were used to confirm the positive role of BDNF-TrkB signalling in RGC survival. These models were TrkBflox/flox mice crossed with GFAP-Cre mice to produce TrkBGFAP KO mice in which TrkB is selectively deleted from Muller glial cells, and TrkBc-kit KO mice that have TrkB deleted only from the RGCs. In both of these mice models, a similar degree of neuronal damage from glutamate neurotoxicity was observed. Likewise, apoptosis in the photoreceptor cells induced by methylnitrosourea (MNU), was augmented in TrkBGFAP KO mice. In addition, an optic nerve damage model demonstrated that TrkBGFAP KO mice had a higher percentage of RGC loss in comparison to the wild-type mice [42, 43]. TrkB.T1 is the truncated isoform of TrkB receptor and BDNF binding to this truncated isoform in Muller cells demonstrated protection of photoreceptor cells against light-induced damage [44]. Localization of BDNF and TrkB along with other adaptor protein like Src homology 2 domain containing (Shc) and phospholipase Cγ1 (PLC-γ1) in the endosomes potentially makes it possible for TrkB to be transported within the cell [45]. This could be a mechanism of BDNF-TrkB translocation in the optic nerve axons. Taken altogether, these studies highlight the neuroprotective role of glial BDNF-TrkB signalling in retinal cells by activating the downstream pro-survival cascades.
6. Pharmacological modulation of TRKB
Even though BDNF is accepted to be neuroprotective in RGCs, its impact on RGC survival has been observed to be transient. While BDNF at first enhances RGC survival, it loses its protective effect over time [46]. On repeated TrkB activation by higher doses or multiple applications, the pathway becomes desensitized to BDNF. Considering different caveats of BDNF, for example, its short half-life, cross-reactions associated with the prolonged use or expanded doses, and difficulty of delivery, the clinical prospect of utilizing BDNF for neuroprotective therapy has been disappointing [47].
There are not many natural or synthetic compounds that can act as an agonist to the TrkB receptor and mimic the function of BDNF with minimal side effects. For example, TrkB agonist antibody 29D7 enhanced RGCs survival in culture in a dose-dependent manner and enhanced the cAMP elevation, consistent with prior observations on the ability of cAMP to enhance the RGC response to BDNF for the survival and axon growth [48]. Hua et al. (2010) also demonstrated the ability of antibody 29D7 to enhance RGCs survival and regeneration in vivo after intravitreal injection of 29D7 antibody. The density of surviving RGCs was increased by single antibody 29D7 injection but to a lesser degree than in the BDNF-treated retinas [48].
We and others have shown that naturally occurring flavonoid, 7,8-dihydroxyflavone (7,8-DHF) has potential to activate the TrkB receptor extracellular domain (ECD) triggering conformational changes in the receptor and stimulating phosphorylation of the TrkB receptor intracellular domain (ICD) which is critical for the tyrosine kinase activity of the TrkB receptor and it’s downstream signalling events [49-51]. The binding of 7,8-DHF and BDNF to TrkB receptor is shown to be on two different sites. 7,8-DHF binds specifically to the ECD of the TrkB receptor and induces TrkB phosphorylation at Tyr515, Try706 and Tyr816 in ICD in neurons. In neuronal lysates, the BDNF-triggered phosphorylation activity of TrkB and in return TrkB was highly ubiquitinated [52, 53]. In contrast, 7,8-DHF rapidly elicited TrkB phosphorylation in the retinal ganglion cells [54]. Remarkably, no TrkB ubiquitination was detected in 7,8-DHF induced TrkB receptor activation [55]. Another small molecule that mimics the neurotrophic activity of BDNF is deoxygedunin which is a tetranortriterpenoid present in the Indian neem tree (Azadirachta indica) [56]. Deoxygedunin also binds to the ECD of TrkB and was shown to elicit strong TrkB activation in hippocampal neurons of rat. Deoxygedunin is also found to stimulate downstream signalling activation of both Erk1/2 and Akt pathways in neuronal culture and prevent neurons from undergoing apoptosis [55, 57].
7. TRKB regulation by endogenous phosphatase SHP2
Protein tyrosine phosphatase (PTP) is the Src homology 2 (SH2) domain containing phosphatase Shp2 [58]. It is expressed ubiquitously and plays a key role in several cellular signalling pathways affiliated with cell growth, differentiation, mitotic cycle, metabolic control, transcription regulation and cell migration, along with positive or negative regulatory role in TrkB receptor signalling and neural fate in retina [59-62]. Shp2 binds to tyrosine kinase receptors like platelet-derived growth factor receptor (PDGFR) and epidermal growth factor receptor (EGFR) via its SH2 domains directly or indirectly [63]. In addition, Shp2 has been found in the receptor complexes in which the Jak/Tyk family of receptor-associated tyrosine kinases phosphorylate tyrosine residues in response to stimulation with CNTF, leukemia inhibitory factor, oncostatin M and interleukin-6 [64]. Shp2 acts either as an adaptor protein [65] or as a modulator of tyrosine phosphorylation [63] in these signalling complexes. It has also been shown that Shp2 acts as a mediator of BDNF-activated signalling [66]. The presence of BDNF in cerebral cortical neurons is suggested to promote the phosphorylation of Shp2 [54, 67, 68]. Although, Shp2 enhances the survival effect of BDNF in these neurons [69], it also acts as a negative regulator of BDNF-activated signalling during neuronal excitotoxicity [66, 70]. Cai et al. (2011) demonstrated that Shp2 is essential for the initiation of retinal neurogenesis but is not crucial for tissue differentiation [71]. However, Shp2 deletion in embryonic stages resulted in retinal degenerative changes including optic nerve dystrophy in mice, further explaining its role in retinal development. Also, Shp2 mediated tyrosine dephosphorylation is reported in Semaphorin-4D (Sema4D) induced axonal repulsion in RGCs and hippocampus neurons [72, 73]. Shp2 has been shown to be predominantly expressed in the inner retina including ganglion cell layer (GCL), although it also regulates photoreceptor differentiation and is suggested to protect the outer retina indirectly through Muller glial cell involvement [71, 74]. Studies in our group have demonstrated interaction of TrkB with Shp2 is increased in the RGCs by 2-3 fold in ON axotomy and microbead injected glaucoma animal models. Further, primary culture of rat RGCs showed high TrkB and Shp2 interaction when subjected to glutamate excitotoxic and hydrogen peroxide (H2O2) induced oxidative stress conditions [75]. Interestingly, increased dephosphorylation of TrkB was observed in the RGC stress models in vivo. Not much is known about how Shp2 regulation of TrkB activity affects the RGC survival in healthy and disease conditions. Therefore, Shp2 may serve as a potential target to explore and understand the mechanisms of RGC death. It also offers an opportunity to explore the potential for Shp2 gene therapy as a therapeutic target in glaucoma conditions. Caveolins (Cav) are integral membrane adaptor proteins that form the principal components of the omega-shaped caveolar structures in the plasma membrane. The various form of cav are caveolin-1 (cav1), caveolin-2 (cav2), and caveolin-3 (cav3). These variants differ with respect to tissue distribution and their specific function. Origin of ancestry is similar for cav1 and cav2 and is expressed in smooth muscle cells, neuronal cells, endothelial cells, adipocytes and fibroblast in abundance [76, 77]. Cav3 expression is predominantly limited to muscle cells [76], but has also been identified in astroglial cells, vegetative ganglion neurons etc [78, 79]. Cav1 alterations have been linked to retinal pathologies including diabetic retinopathy and glaucoma [80, 81] but its role in retinal neuroprotection is still unknown. Recently, Reagan and colleagues (2016) demonstrated the neuroprotective role of cav1 in the retina. Cav1 is involved in recruiting circulating leukocytes during inflammation and these cytokines activate the JAK/STAT pathway, which downregulates apoptotic factors to prevent retinal neuronal death [82, 83].
Cav1 is also shown to interact with toll-like receptors (TLRs) in the retina that recognize and respond to pathogenic stimuli and initiate pro-inflammatory cytokine responses. Cav1 associates with TLRs and regulates TLR signalling [84]. Recently, we have shown molecular evidence that Shp2-TrkB interaction in RGCs is mediated through cav. Under ON axotomy and glaucomatous stress cav1 and cav3 undergo hyperphosphorylation and interact with Shp2 in the RGCs [75]. Cav may bind to the SH2 domain of Shp2 and thus diminish the auto-inhibition of Shp2 and enhance its phosphatase activity [85]. There might be the possibility of recruitment of Shp2 by cav acting as a signalling platform and increase its proximity to TrkB receptor, thereby increasing dephosphorylation of TrkB and influencing downstream signalling (Fig. 2). Cav1−/− mice show an enhanced Erk1/2 phosphorylation in the hippocampus [86]. Consistent with Cav-Shp2 interactions in the RGCs, previous studies also that cav1 undergoes hyper-phosphorylation in oxidative and osmotic cellular stress in fibroblast cultures [87, 88]. Trabecular Meshwork (TM) cell lines from POAG patients treated with dexamethasone show a decline in cav1 phosphorylation [89], implicating that cav phosphorylation is affected by glaucoma in the RGCs. Cav is also reported to play a critical in Shp2 activation in astrocytes, retinal homeostasis, preservation of blood retinal barrier permeability and outer retinal functions [90-92].
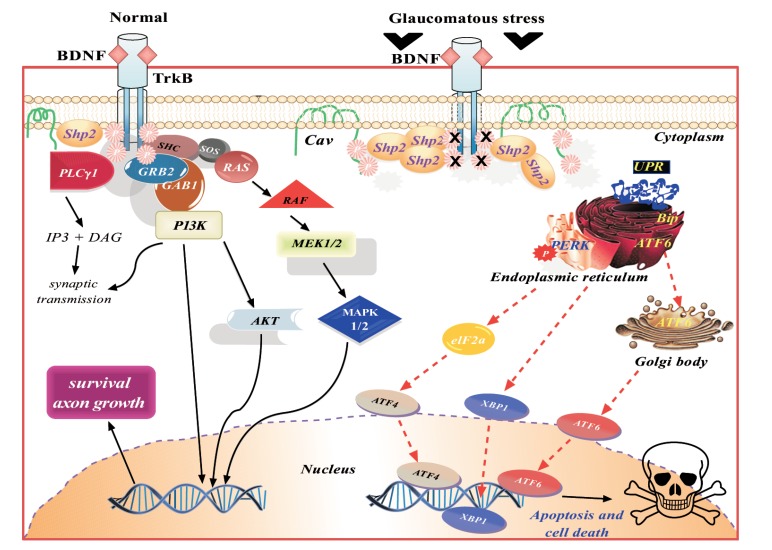
Fig. (2).
Schematic representation of the BDNF/ TrkB signalling in normal and glaucomatous conditions. The downstream cellular effects of BDNF-TrkB complex in normal condition promote neuronal survival. Under glaucomatous stress, the downstream survival pathway ceases and induces ER stress and promote cell apoptosis.
8. Apoptosis in RGCS
Ganglion cells undergo apoptosis in glaucoma leading to progressive loss of vision - the loss of RGCs is the principal endpoint in experimental glaucoma [93]. This mechanism of cell death is controlled by gene modulation.
Pro-apoptotic factors can arise in the retina through various mechanisms such as ischemia, inflammation, excitotoxicity, oxidative stress and hyperglycemia [50, 94-98]. Recent evidence suggests that abnormal protein aggregation induced stress may lead to an unfolded protein response in the endoplasmic reticulum (ER) [99]. Furthermore, some of the ER stress signalling proteins control cell fate either by activating pro-apoptotic, Bcl2 or anti-apoptotic Bax molecules in response to cell burden [100].
9. ER stress and unfolded protein response
Intracellular synthesized protein is further processed by the ER. Protein folding, maturation, and trafficking are some of the important functions of this organelle. The folded membranes of ER help the newly synthesized proteins to undergo posttranslational modification and correctly fold in their three-dimensional (3D) conformations. However, only mature (correctly folded) proteins can be taken up by the Golgi bodies for further targeting. ER is the major intracellular organelle that senses environmental changes, cellular stresses, coordinates signalling pathways and controls cell function/ survival [101]. Different pathological and physiological conditions, nutrient scarcity, change in redox status and viral infection can influence the ER ability to facilitate protein folding, resulting in unfolded or misfolded protein accumulation in the ER lumen and consequently increased ER stress. The unfolded proteins form insoluble protein aggregates that are toxic to the cell. This has been reported in neurodegenerative diseases like glaucoma, Parkinson’s disease and Alzheimer’s disease [102-105]. Within the cell, an adaptive mechanism is activated that comprises various intracellular signalling pathways to remove toxic proteins, termed the unfolded protein response (UPR). UPR alleviates ER stress by three different strategies; (1) through ER-related molecular chaperons to stimulate re-folding of the folding proteins; (2) inhibit mRNA translation so that no more generation of unfolded proteins take place and (3) stimulate the retrograde transport of the misfolded proteins from the ER lumen into the cytosol for ubiquitination by activation of ER-associated protein degradation (ERAD) [106].
Recent research has shown that the ER stress is associated with neuronal cell death in neurodegenerative diseases like glaucoma [107]. In an animal model of chronic glaucoma and acute ON traumatic injury, an increase in ER stress proteins in the RGCs was observed [108, 109]. Intravitreal injection of N-methyl-D-aspartate (NMDA) given in ischemic insult animal model resulted in increased ER stress protein expression in the inner retinal cells like RGCs, amacrine and microglia. Activation of Bip, ATF4, and CHOP by tunicamycin or NMDA-induced apoptosis in mouse primary RGCs also suggested an imperative role of ER stress in neuronal cell death in the retina [110]. An antipsychotic drug, Valproate demonstrated neuroprotective actions in the ischemic retina from ER stress-induced apoptosis by inhibiting histone deacetylase activity [111]. Neuronal ER stress is emerging as a promising therapeutic target for glaucoma and potentially for other neurodegeneration diseases.
10. Signalling pathways of ER stress-associated apoptosis
Cell death often occurs if ER stress is chronic and the protein burden on ER is unable to fold the protein accurately and perform necessary cellular function. The three-major proteins involved in UPR are (1) protein kinase RNA (PKR)-like ER kinase (PERK), (2) ATF6 and (3) IRE1α. Under normal biological conditions, BiP, an ER local chaperon, binds PERK and ATF6 which keeps these ER stress proteins idle. Upon aggregation of ER misfolded proteins, BiP is set free from these complexes and helps in folding of accumulated proteins [112]. The function of PERK is to regulate mRNA translation and to prevent the entry of newly formed unfolded protein to the ER compartment already experiencing stress. PERK phosphorylates the elongation initiation factor 2α (eIF2α) and nuclear erythroid 2 p45-related factor 2 (NRF2). Phosphorylated eIF2α stops polypeptide synthesis and phosphorylated NRF2 induces oxidative stress through cell reinforcement genes such as heme oxygenase 1 (HO-1) [113-115]. Another transcription factor, ATF6 is taken up by the Golgi apparatus upon UPR and cleaved into luminal domain by serine protease site-1, whereas site-2 protease cleaves the N-terminal domain. The separated N-terminal domain of ATF6 then moves into the nucleus and forms a complex with ATF/cAMP response elements (CRE) and ER stress-response elements (ERSE-1). The ATF6-CRE-ERSE-1 complex initiates transcription of BiP, Grp94 and CHOP [116]. IRE1α promotes apoptosis by stimulating apoptotic-signalling kinase-1 (ASK1) and downstream activation of JNK and p38 MAPK [117]. Anti-apoptotic marker, Bcl-2 expression is downregulated and Bim upregulated on p38 MAPK activation (Fig. 2).
11. Genes associated with glaucoma
A genetic association of POAG has been recognised for decades with a family history commonly identified, although the non-mendelian nature of inheritance indicates a multifactorial etiology that may again vary in different ethnicities [118]. Variants in myocilin (MYOC) [119], optineurin (OPTN) [120], WD repeat domain 36 (WDR36) [121], and neurotrophin 4 (NTF4) [122] have previously been reported as showing an association with POAG. Genome-wide association studies (GWAS) have recognized new loci in POAG such as caveolin 1 or 2 (Cav1/Cav2) [123], transmembrane and coiled-coil domain 1 (TMCO1) [124], ankyrin repeat and SOCS-box containing 10 (ASB 10), susceptible loci- TXNRD2, ATXN2, and FOXC1 [125]. New gene associations with glaucoma continue to be discovered in larger cohorts of different ethnicities although there remains a need to identify whether statistical differences observed in these studies are translatable into clinical significance. Molecular mechanisms to identify the potential roles of these genes will be a significant step forward to understand the glaucoma pathophysiology.
11.1. NTF4 and BDNF
Pasutto et al. (2009) demonstrated the association of six-heterozygous variants in NT-4 (C7_Y, E48_K, A88_V, R90_H, R206_W and R206_Q) with POAG in European cohort [126]. In contrast to this study, subjects with European ancestry from the south-eastern United States showed no association between coding variants in NTF4 gene and POAG, moreover, variants R206_W and A88_V were also reported in the control individuals [127]. Interestingly, Rao et al. (2010) showed that these variants in NTF4 were not associated in POAG in an Indian population [128]. A single missense mutation, L113_S in one Chinese patient was found to alter the NT-4 protein structure and disrupt the binding and activity of the TrkB receptor but this could be a rare case of NTF4 variant in POAG [129]. The composite nature of the disease phenotype and enormous genetic heterogeneity suggests that NTF4 mutation alone is not sufficient to explain glaucoma pathogenesis. A recent study in 167 Polish patients and 193 healthy individuals showed no significant association between G196_A allele polymorphism of the BDNF gene and POAG [130] although dysregulation of BDNF at the biochemical level has been implicated in the disease pathology.
11.2. Caveolin 1 and 2
Genome-wide association studies (GWAS) resulted in discovery of variations in caveolin gene (cav1/ cav2) locus that is associated with POAG [131]. The study on an Icelandic cohort, found that a variant rs4236601_A in cav1 and cav2 gene loci on chromosome 7q31 was associated with POAG. They also performed replication studies in multiple cohorts from Europe and East Asian ancestry. Further studies on this SNP (rs4236601) in Iowa, USA involving 545 POAG patients and 297 control subjects detected no association of the disease with this variation [132]. However, Wiggs et al. further confirmed the association of cav1 and cav2 SNP with POAG in another cohort of Caucasian US population [123]. Both cav1 and cav2 were reported to express in glaucoma TM cell lines [89]. The recent study in cav1-/- (cav1 knockout) mice suggested a possible reduced aqueous humour drainage outflow as demonstrated by slightly higher IOP in these animals [133].
11.3. MYOC
MYOC gene was identified as the first gene associated with POAG and found at locus GLC1A on chromosome 1q23 [134]. The MYOC gene was first studied in vitro in TM as response of glucocorticoid-induced gene (TIGR) while studying the effects of dexamethasone on TM cell cultures [135]. This gene encodes myocilin protein and intracellular accumulation of its mutant leads to a misfolded form that increases the IOP [136]. Recently, a mutation C1456_T in MYOC gene was identified in a Chinese family. Beta-1, 4-galactosyltransferase 3 (B4GALT3) gene also showed mutation at G322_A in this POAG family [137]. In a transgenic mouse model of POAG, MYOC mutation Y437_H shows a significant increase in IOP with abnormal extracellular matrix accumulation that potentially reduces aqueous outflow [136]. Likewise, MYOC mutant Q368_X and Y437_H were shown to activate the IL-1/NF-kB pathway activity in culture TM-1 cell line [138]. A meta-analysis within Caucasian populations suggested a genetic association between MYOC polymorphism and POAG [139].
11.4. OPTN
Optinuerin (OPTN) was shown to have protective role in glaucoma progression in response to TM stress [104]. Recent studies on OPTN mutation demonstrated that overexpression of E50_K mutation triggers the apoptotic factors and impaired the mitochondrial dynamics (fusion and fission) in retina of aged E50_K-tg mice as well as in the primary cultured RGCs [141]. The aged transgenic mice with overexpression of E50_K OPTN demonstrated diffused retinal layers with thinner retina. In contrast, low expression of E50_K OPTN in aged transgenic mice did not lead to changes in retinal layer thickness [142]. Clinical examination of subjects with glaucoma with E50_K OPTN mutation was studied in caucasian families. It was examined subjects with E50_K mutation at a younger age had more advanced optic disc cupping and smaller neuroretinal rim area and were found to have NTG features that appeared to be more severe than that in control subjects with NTG without this mutation [143].
11.5. WDR36
The alterations in WDR36 alone are not sufficient to cause POAG, notwithstanding, the relationship of WDR36 sequence variants with more severe disease in affected individuals [144]. WDR36 encodes protein thought to be involved with T-cell activation and proliferation and in ribosomal RNA processing [145]. The loss of WDR36 in zebrafish line with viral insert wdr36hi3630aTg showed reduction in WDR36 protein and was found to activate p53 stress response pathway without alteration in pro-apoptotic gene, bax [121]. In contrast, variant of WDR36 D658_G mutation in Australian families was reported to be a neutral variant for glaucoma [146]. These studies were also supported by WDR36 mutation variant (N355_S, A449T, R529_Q and D658_G) may be only rare disease-causing gene in glaucoma in German population [147]. In Japanese populations, WDR36 was not reported as a major contributing factor to POAG [148]. At the molecular level, expression studies in yeast indicated that WDR36 sequence variants can produce changes in cellular phenotype [149].
11.6. TMCO1
TMCO1 is highly expressed in the human TM, ciliary body and retina [150]. The location of this gene is upstream to the MYOC gene at chromosome 1q24.1. The function of TMCO1 is to encode transmembrane coiled domain protein that localize in the Golgi apparatus, ER or mitochondria in different cell types and this protein has been suggested to be possibly involved in RGCs apoptosis [151]. Recent studies in the Han Chinese population identified the SNP rs4656461 and rs7555523 in TMCO1 to be associated with POAG [152]. In addition, a study in a Pakistani cohort (total 268 POAG patients) confirmed that the SNP rs4656461 in TMCO1 was highly associated with POAG [153]. Furthermore, black South African and Saudi cohorts showed no association of the TMCO1 gene variants with POAG [124, 154]. GWAS studies and meta-analysis of >6,000 subjects of European ancestry identified a significant association of TMCO1 variants with IOP changes (SNP rs7518099) [155]. Additional studies are needed to identify the effect of these polymorphisms and mutations in populations with different ethnicities and to match for age and gender differences.
12. Therapeutic prospects in glaucoma
This section outlines various therapeutic prospects to protect the retina in glaucoma by targeting neurotrophin signalling, apoptotic pathways and by modulating ER stress response.
13. Can neurotrophin therapy scale down RGC damage in glaucoma?
Neurotropic factor supplementation has been shown to protect the RGCs in ocular hypertension (OHT) animal models via intravitreal injection of human recombinant BDNF [156]. The disadvantage of using BDNF is that it requires repeated intravitreal injections to achieve a recognizable neuroprotective effect [157]. Ko and colleagues have shown an increase in RGC protection after four intravitreal injections of BDNF therapy. BDNF enhances and prolongs RGCs survival both in vivo [30, 158, 159] and in vitro [5, 25] after optic nerve injury and axotomy. However, TrkB expression in RGCs is down-regulated after axotomy, making the cells less sensitive to the neuroprotective actions of BDNF. TrkB gene transfer to RGCs coupled with intravitreal BDNF administration significantly increased RGC survival after axotomy [160]. The neuroprotective effects of BDNF can be enhanced by associative administration of other growth factors, for example, FGF2 and NT-3 [161, 162]. FGF2 administrated after ON injury increased BDNF and TrkB expression in RGCs [161].
Several other growth factors also have protective effects on the RGCs. Recombinant CNTF intravitreal injections exhibit neuroprotective effects in OHT animal model. A single intravitreal injection of low dose CNTF (2 μg CNTF) exerted a neuroprotective effect with 15% less RGC loss in 6 weeks [16]. This effect correlated with upregulation of STAT3 in cells within the RGC layer and the inner nuclear layer [163]. However, partial neuroprotective effect on the RGCs upon exogenous administration of CNTF was reported in ocular hypertension model [164]. While short-term advantageous effects have been shown at times for single intraocular administrations of NGF, long-term neuroprotection in glaucoma patients likely requires a sustained NGF supply.
Gene therapy techniques could be applied to neurotrophin factor supplementation to meet the requirements for sustained delivery in glaucoma. By using viral vectors to increase endogenous retinal production of select NGF(s), it might be possible to reduce RGC damage over a long period. For example, adenovirus (AdV) vector mediated over-expression of BDNF in Müller glial cells prolonged the survival of RGCs in a rat model of optic nerve transection [165]. In another approach, repeated intravitreal injection of AdV vector for GDNF delivery prior to optic nerve axotomy lead to 125%-fold increase in RGC survival at 14 days post axotomy [166]. However, the efficacy of AdV-mediated gene transfer is limited by its relatively short duration of expression and by the fact that AdV triggers significant inflammatory reactions. AdV has also been shown to be highly immunogenic in clinical trials [167]. Alternatively, adeno-associated virus (AAV), could be used and it has been shown that these vectors do not integrate into the host genome. AAV-FGF2 transduction of the retina protected RGCs in models of optic nerve crush and excitotoxicity [168]. Martin and colleagues (2003) used AAV to transduce retinal cells in the laser-induced OHT model of glaucoma [169]. They used a hybrid promoter of cytomegalovirus (CMV), chicken β-actin (CAG) enhancer and the woodchuck hepatitis post-transcriptional regulatory element (WPRE) to deliver BDNF gene incorporated in an AAV viral vector. The promoter could efficiently transduce more than 70,000 cells within the RGC layer per eye. A single intravitreal injection of AAV-BDNF gene therapy reduced the axonal loss in the ON from 52.3% to 32.3% four weeks after the laser induced experimental model of glaucoma in rodents. Likewise, CNTF gene therapy also promoted long term survival and regeneration of rat RGCs after 7 weeks of ON crush [170]. Using a similar vector, Leaver and colleagues (2006) showed increase in RGC protection in retinas injected with CNTF compared to viral vector containing no CNTF. However, a combination of CNTF-BDNF had no significant improvement in RGC axon survival in laser induced rat glaucoma model and the reason for lack of improved effect was not clear [16]. Recent advances in recombinant AAV engineering have led to the development of vectors that are less susceptible to ubiquitin-mediated degradation and can be used for expression modulation over a long period of time [171].
14. Targeting neurotrophin receptors in glaucoma
NGF signalling pathways are often subject to complex regulation and accordingly increase of neurotrophic factor levels does not always benefit neuronal survival. In addition to promoting cell survival via Trk family receptor, NGF can bind to p75NTR receptor and trigger pro-apoptotic signals. Indeed, it has been suggested that a cause of failure of NGF in glaucoma may be related to over-activation of p75NTR due to administration of a selective Trk family agonist [172]. In that capacity, design of modified receptor-defined ligands as compared to naturally occurring neurotrophic factors, is being investigated as an approach to achieve greater neuroprotection. Lebrun-Julien et al. (2009) demonstrated that combined TrkA activation and p75NTR inhibition in p75NTR null mice was protective for survival of RGCs following optic nerve injury [173]. This study indicated that the deleterious effects of p75NTR in Muller cells may regulate neuronal death by emancipating neurotoxins or indirectly by diminishing their sympathetic functions. Also, p75NTR activation could attenuate the beneficial effects of BDNF delivery and inhibition of p75NTR was shown to unmask a potent neuroprotective effect of NGF [173]. BDNF combined with CNS-specific leucine –rich repeat protein LINGO-1 (LINGO-1 negatively regulates p75NTR signalling) antagonists have been found to promote long-term RGC survival in a laser-induced OHT rat model [174]. Fu et al. demonstrated that LINGO-1-TrkB receptor complex adversely controls TrkB activation in the retina after OHT injury. Antibody-1A7 or soluble LINGO-1 (LINGO-1-Fc) commonly used as LINGO-1 antagonist, protected RGCs from death by activating the BDNF-TrkB signalling pathway in animal models of OHT [175]. Alternatively, novel ligands which specifically activate TrkB and not p75NTR might be of interest. A monoclonal antibody and a natural flavonoid, 7,8-DHF which specifically activates TrkB have shown RGC neuroprotection in the optic nerve transection and OHT animal models [48, 50]. In the light of these outcomes, it is likely that selectively activating multiple Trk receptors while blocking p75NTR using natural/synthetic receptor ligands may exert more robust neuroprotective effect than just delivering the neurotrophic factors alone.
Our group has demonstrated that Shp2 interacts with the TrkB receptor in RGCs and negatively affects its action in ON transection and chronically elevated IOP rodent models. The Shp2-TrkB binding is suggested to be mediated through the adapter protein, caveolin. Under stress conditions, caveolin isoforms 1 and 3 undergo hyperphosphorylation in RGCs and further bind to Shp2 phosphatase. The increase in phosphatase activity of Shp2 in glaucomatous stress reduces TrkB activity [75]. These proteins may be good candidates for targeted gene therapy studies in glaucoma. Pernet et al., (2005) used AAV to transduce RGCs with genes encoding MEK1, the upstream activator of Erk1/2. MEK1 activation induced in vivo phosphorylation of Erk1/2 in RGC bodies and axons. A single injection of AAV encoding MEK1 gene increased RGC survival at 2 weeks after axotomy [176]. In addition, we have also shown the neuroprotective effect of sphingosine-1-phosphate analogue fingolimod (FTY720) in a chronic OHT animal model. Administration of FTY720 reduced the loss of electrophysiological responses and protected against GCL and ON loss. It was observed that the fingolimod effects were associated with increased activation of Akt and Erk1/2. SIPR receptor expression was found to be increased in the RGCs in experimental glaucomatous condition [177].
15. Cross-talk of BDNF with VEGF
Vascular endothelial growth factor (VEGF) plays an important role both in physiological and pathological angiogenesis. BDNF signalling is identified as potential proangiogenic factor and stimulates VEGF expression through PI3K pathway for promotion of endothelial cell survival in neuroblastoma cells [178, 179]. Similarly, VEGF, an angiogenic factor, was also shown to stimulate axonal outgrowth from dorsal root ganglia by acting as a neurotrophic factor [180]. Apart from its angiogenesis function, VEGF can also stimulate non-vascular cells such as Tenon’s fibroblasts, a target for antifibrotic treatment in glaucoma filtration surgery [181]. VEGF is usually released after retinal ischemia and can further spread through the aqueous humor to the anterior segment of the eye. The latter results in neovascularization of the iris and angle, causing secondary closed angle glaucoma [182]. Inhibition of VEGF signalling remains an important focus for the treatment of retinal and choroid neovascularization and in retina edema. VEGF binds to both of its receptor forms VEGFR1 and VEGFR2. VEGFR1 may be important in development by sequestering VEGF, preventing its interaction with VEGFR2, which is responsible for endothelial cell mitogenesis, survival and permeability [183]. Therapeutically targeting VEGF has been successful with intravitreal injections of VEGF neutralising antibodies preventing vision loss and salvaging visual activity in age-related macular degeneration (AMD) patients [184]. Ranizumab therapy has been shown to protect against retinal thickness loss and it improved visual acuity in patients with diabetic macular edema [185]. VEGF also has neurogenic and neuroprotective effects [186]. RGCs overexpressing VEGF in transgenic mice were protected from axotomy-induced degeneration [187]. It will be interesting to determine how neurotrophin factor signalling overlaps with angiogenic factors and how manipulating one pathway would affect the other [49].
16. Inhibiting apoptotic pathway in the RGCS in glaucoma
Apoptosis is a programmed cell death that has essential roles in physiological processes but also is a feature in the pathophysiology of many diseases. Dkhissi et al. (1999) observed DNA fragmentation using the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) method in the GCL and INL in an avian glaucoma-like disorder model [188]. The apoptosis in RGCs was also studied in experimental glaucoma animal models by activation of the tumor suppressor protein, p53, which functions to activate the proapoptotic pathways [189]. Considering these observations, it was suggested that anti-apoptotic molecules may preserve the phenotype damage of ON in glaucoma. Etanercept, TNF-α inhibitor, when given intraperitoneally, blocked the TNF-α activity in experimental OHT produced due to activation of microglia and reduced axonal degeneration and loss of RGCs [190]. Clinically, a new therapy was investigated in glaucoma using TNF-α antagonist as suppressors of inflammation [190]. Furthermore, intravitreal injection of second-generation tetracycline drug, minocycline, has been shown to exhibit neuroprotection in experimental glaucoma and optic nerve transection animal models. Minocycline increased the expression of antiapoptotic gene Bcl-2 and at the same time decreased the expression of apoptotic gene Bax [191]. Also, TNF-α, inhibitor of apoptosis protein (IAP1) and Gadd45α was upregulated in retinas with optic nerve transection [191]. Calcineurin inhibitor, FK506 when administered orally, showed RGC and ON protection from apoptosis by decreasing Bad dephosphorylation and inhibiting mitochondrial cytochrome c release under experimentally induced high IOP [192]. In addition, agents that alter the expression of endogenous anti-apoptotic proteins can also be employed for neuroprotection function in injured ON. For instance the use of brimonidine, an α2 adrenergic receptor agonist decreases the level of Bcl-2 and also reduces mitochondrial-dependent apoptosis in RGCs of post-mortem eyes from glaucoma individuals [193]. Similarly, suppressing downstream signalling of pro-apoptotic pathways may induce RGC neuroprotection. Indeed C-terminal binding protein 2 (CtBP2) was recently shown to be neuroprotective in CNS injury in DBA/2J mice. Lentivirus mediated overexpression of CtBP2 in cultured RGCs subjected to L-glutamate-induced apoptosis showed a decrease in expression of Bax and caspase-3 [194]. Cobalt chloride induced hypoxia in primary rat RGCs treated with GABA receptor agonist baclofen, prevented the RGC apoptosis through Akt activation [195].
17. Targeting ER stress marker proteins in the RGCS in glaucoma
Several studies have focussed on utilizing the therapeutic application of synthetic chaperons to subdue ER retention of the misfolded proteins, minimizing their generation and to amend the ER folding capacity. UPR is triggered in ON axotomy resulting in RGCs death. RGCs shown to have upregulated CHOP in ON injury and promote neuronal apoptosis. CHOP knockout (KO) mice demonstrated increased RGCs survival by 24% after two weeks of ON axotomy [196]. Alternatively, the overexpression of XBP-1 in RGCs was carried out using intravitreal AAV injection in WT and CHOP KO mice [196]. AAV viral vector expressing XBP-1, showed considerably increased RGCs survival in both WT and transgenic mice. RGC survival was increased by 64% in animals overexpressing XBP-1 whereas AAV-GFP control mice demonstrated only 20% RGC survival. RGCs survival was increased to 82% in transgenic mice (CHOP KO mice) overexpressing XBP-1 following ON crush injury compared to the wild type animals. Altogether, this experiment suggested an opposite action of XBP-1 and CHOP in controlling RGCs survival and apoptosis after ON injury [106, 109]. Recently, Nakano et al. (2006) designed two novel compounds KUS121 and KUS187 and treated PC12 cells with these two substances. Both compounds induced mitrochondrial respiratory chain complex III and V inhibition by reducing cellular ATP levels. CHOP expression was reduced in cultured PC12 cells upon treatment with these KUSs inhibitors. In vivo neuroprotective effects on RGCs were also demonstrated by KUSs inhibitor treatment in mice in the ON axotomy model as well as experimentally increased IOP model. The rescue of RGCs in ON axotomy and high IOP was associated with low expression levels of Grp78 or Bip [197]. RGC death was also induced by the activation of chemokines (CXCL10) and chemokines receptors such as CXCR3. Experimentally induced ischemia and retinal stress modulate the expression of CXCL10 and CXCR3. Upregulation of chemokines and its receptor increases the expression of Grp78, CHOP and ATF4 in RGCs [198]. However, when the mice were treated with ER stress blockers 4-pheylybutyric acid and taurousodeoxycholic [199], the chemokines expression was attenuated by 61% and 43% respectively. Moreover, in CXCR3 KO mice, the ER stress inhibitor did not transform the expression of ER stress markers, suggesting that the downstream event of CXCR3 activation is not associated with ER stress [198]. It may therefore be essential to comprehend the dual biological functions of UPR signalling both in survival and apoptosis before exploring therapeutic application focusing ER stress.
18. Building bridges between neuro-trophin pathway and genetics research in glaucoma
The BDNF-TrkB signalling pathway has been implicated as a mediator for survival, development and synaptic plasticity of neurodegenerative neurons in POAG [200]. BDNF and NGF serum levels are reduced in the early and moderate glaucoma stages [201]. Mutation in OPTN has been observed in POAG and amyotrophic lateral sclerosis (ALS) and its deficiency leads to a reduced secretion of neurotrophic factors [202]. OPTN in association with Tank binding kinase 1 (TBK1) gene are involved in neuroinflammation and autophagy [203]. These two genes, OPTN and TBK1, are important components of the pathway required for removal of pathological ribonucleoprotein inclusions [203]. Depletion or dysregulation of OPTN and TBK1 may lead to protein aggregates which is one of the main characteristics associated with loss of neurons in neurodegenerative disorders like ALS, Parkinson’s, Huntington’s and Alzheimer’s diseases [204] (Fig. 3).
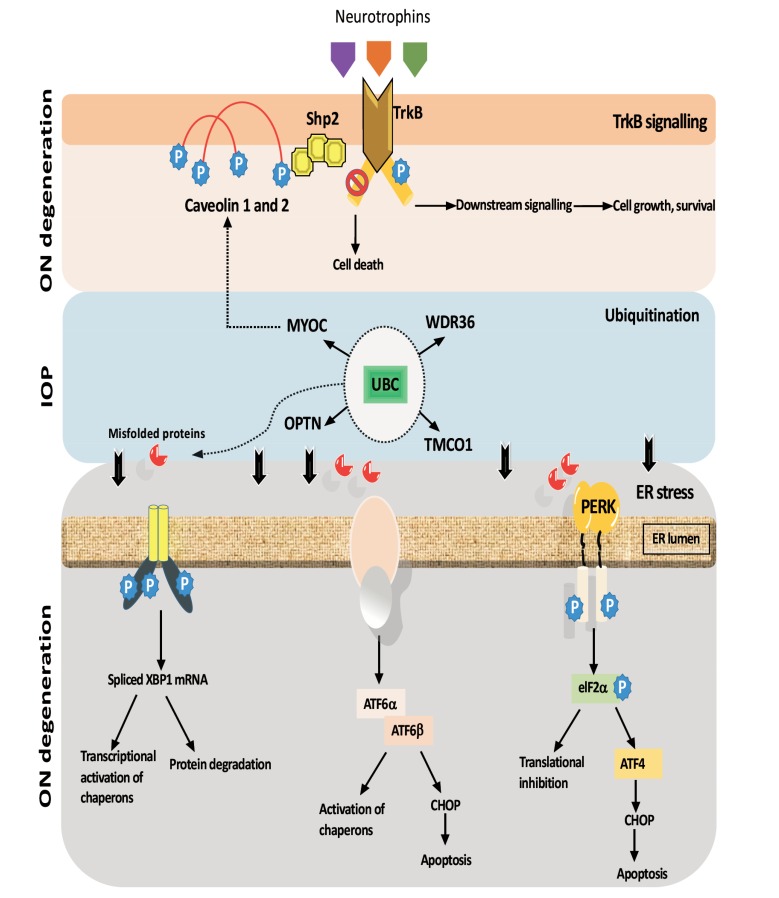
Fig. (3).
Downstream consequences of neurotrophin signalling pathway affecting gene function and ER stress marker is possibly implicated in progression of RGCs axon damage in glaucoma.
Degradation of aggregated and misfolded proteins is the critical pathway by which protein cellular homeostasis is regulated in POAG. Ubiquitin-proteosome system (UPS) is responsible for the elimination of misfolded or damaged proteins (Fig. 3). RGCs and retinal pigment epithelium are rich in ubiquitin [205]. The mutated form of OPTN (E50_K) that causes glaucoma leads to malfunction of UPS [206] and overexpression of wild type OPTN results in increased apoptosis in PC12 and RGC5 cells [207]. On the other hand, when PC12 cells transfected with wild-type and mutated MYOC (P37_L) plasmid they showed inhibition in neurite outgrowth and number [207]. When myocilin is overexpressed or mutated, the UPS function is compromised, and autophagy is induced [208]. In human trabecular meshwork line TM-1 mutant MYOC activate the IL-1/NF-κB inflammatory stress response and the glaucoma marker SELE, whereas the wild-type MYOC has anti-inflammatory activity [138]. Mutations in OPTN are associated with NTG while mutations in MYOC induce blockage of aqueous humor outflow [119], suggesting that alteration of the UPS system is implicated in both NTG and POAG.
It will be important to integrate the role of system biology and functional genomics to discover the exact role of these cellular pathways. Glaucoma is clearly a complex disease with at least 70 loci identified so far which highlights variance in POAG phenotype. There is a lack of functional validation and detailed analysis of the in vivo function of these genes. More experimental data based on the animal models may help clarify this in the near future.
CONCLUSION
The detrimental effects of glaucoma can be prevented to a certain extent if effective treatment can be provided at the appropriate time. This disease involves optic nerve atrophy and loss of RGCs and can occur even under normal IOP conditions. The fate of RGCs is programmed by complex interplay of several molecular signalling pathways that are functioning simultaneously. RGC death can be triggered by many acute and chronic insults such as neurotrophic factor deprivation, synaptic dysfunction, protein-protein interaction, gene dysregulation, neuronal stress due to ischemia/ hypoxia and excitotoxicity, activation of apoptotic pathways and UPR under ER stress. Simulating neuroprotective endogenous mechanisms has the potential to significantly transform the equilibrium between the pro/apoptotic and the pro/survival pathways, leading to a delay in RGCs death with beneficial effects in glaucoma.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
This work was supported by funds from Ophthalmic Research Institute of Australia (ORIA, 9201400700), National Health and Medical Research Council (NHMRC, 1084767), Hillcrest foundation and Macquarie University.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Davis B.M., Crawley L., Pahlitzsch M., Javaid F., Cordeiro M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016;132(6):807–826. doi: 10.1007/s00401-016-1609-2. [http://dx.doi.org/10.1007/s00401-016-1609-2]. [PMID: 27544758]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stowell C., Burgoyne C.F., Tamm E.R., Ethier C.R., Lasker I.I.A., Glaucomatous Neurodegeneration P. Biomechanical aspects of axonal damage in glaucoma: A brief review. Exp. Eye Res. 2017;157:13–19. doi: 10.1016/j.exer.2017.02.005. [http://dx.doi.org/10.1016/j.exer.2017.02.005]. [PMID: 28223180]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Amero K., Kondkar A.A., Chalam K.V. An updated review on the genetics of primary open angle glaucoma. Int. J. Mol. Sci. 2015;16(12):28886–28911. doi: 10.3390/ijms161226135. [http://dx.doi.org/10.3390/ ijms161226135]. [PMID: 26690118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki M., Tanaka T., Sakai Y., Fukuchi T., Abe H., Nawa H., Takei N. Müller cells as a source of brain-derived neurotrophic factor in the retina: noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Müller cells. Neurochem. Res. 2005;30(9):1163–1170. doi: 10.1007/s11064-005-7936-7. [http://dx.doi.org/10.1007/s11064-005-7936-7]. [PMID: 16292510]. [DOI] [PubMed] [Google Scholar]
- 5.Takihara Y., Inatani M., Hayashi H., Adachi N., Iwao K., Inoue T., Iwao M., Tanihara H. Dynamic imaging of axonal transport in living retinal ganglion cells in vitro. Invest. Ophthalmol. Vis. Sci. 2011;52(6):3039–3045. doi: 10.1167/iovs.10-6435. [http://dx.doi.org/10.1167/iovs.10-6435]. [PMID: 21310905]. [DOI] [PubMed] [Google Scholar]
- 6.Lebrun-Julien F., Bertrand M.J., De Backer O., Stellwagen D., Morales C.R., Di Polo A., Barker P.A. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc. Natl. Acad. Sci. USA. 2010;107(8):3817–3822. doi: 10.1073/pnas.0909276107. [http://dx.doi.org/10.1073/pnas. 0909276107]. [PMID: 20133718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibáñez C.F., Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35(7):431–440. doi: 10.1016/j.tins.2012.03.007. [http://dx.doi.org/10.1016/ j.tins.2012.03.007]. [PMID: 22503537]. [DOI] [PubMed] [Google Scholar]
- 8.Kimura A., Namekata K., Guo X., Harada C., Harada T. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. Int. J. Mol. Sci. 2016;17(9):E1584. doi: 10.3390/ijms17091584. [http://dx.doi. org/10.3390/ijms17091584]. [PMID: 27657046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada C., Harada T., Nakamura K., Sakai Y., Tanaka K., Parada L.F. Effect of p75NTR on the regulation of naturally occurring cell death and retinal ganglion cell number in the mouse eye. Dev. Biol. 2006;290(1):57–65. doi: 10.1016/j.ydbio.2005.08.051. [http://dx.doi.org/10.1016/j.ydbio. 2005.08.051]. [PMID: 16343477]. [DOI] [PubMed] [Google Scholar]
- 10.Dekeyster E., Geeraerts E., Buyens T., Van den Haute C., Baekelandt V., De Groef L., Salinas-Navarro M., Moons L. Tackling glaucoma from within the brain: An unfortunate interplay of BDNF and TrkB. PLoS One. 2015;10(11):e0142067. doi: 10.1371/journal.pone.0142067. [http://dx. doi.org/10.1371/journal.pone.0142067]. [PMID: 26560713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley H.A., McKinnon S.J., Zack D.J., Pease M.E., Kerrigan-Baumrind L.A., Kerrigan D.F., Mitchell R.S. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest. Ophthalmol. Vis. Sci. 2000;41(11):3460–3466. [PMID: 11006239]. [PubMed] [Google Scholar]
- 12.Feng L., Chen H., Yi J., Troy J.B., Zhang H.F., Liu X. Long-term protection of retinal ganglion cells and visual function by brain-derived neurotrophic factor in mice with ocular hypertension. Invest. Ophthalmol. Vis. Sci. 2016;57(8):3793–3802. doi: 10.1167/iovs.16-19825. [http://dx. doi.org/10.1167/iovs.16-19825]. [PMID: 27421068]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber L.A., Teis D. Lysosomal signaling in control of degradation pathways. Curr. Opin. Cell Biol. 2016;39:8–14. doi: 10.1016/j.ceb.2016.01.006. [http://dx. doi.org/10.1016/j.ceb.2016.01.006]. [PMID: 26827287]. [DOI] [PubMed] [Google Scholar]
- 14.Vecino E., García-Crespo D., García M., Martinez-Millán L., Sharma S.C., Carrascal E. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vision Res. 2002;42(2):151–157. doi: 10.1016/s0042-6989(01)00251-6. [http://dx.doi.org/10.1016/S0042-6989(01)00251-6]. [PMID: 11809469]. [DOI] [PubMed] [Google Scholar]
- 15.García M., Forster V., Hicks D., Vecino E. In vivo expression of neurotrophins and neurotrophin receptors is conserved in adult porcine retina in vitro. Invest. Ophthalmol. Vis. Sci. 2003;44(10):4532–4541. doi: 10.1167/iovs.03-0419. [http://dx.doi.org/10.1167/iovs.03-0419]. [PMID: 14507902]. [DOI] [PubMed] [Google Scholar]
- 16.Pease M.E., Zack D.J., Berlinicke C., Bloom K., Cone F., Wang Y., Klein R.L., Hauswirth W.W., Quigley H.A. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2009;50(5):2194–2200. doi: 10.1167/iovs.08-3013. [http://dx. doi.org/10.1167/iovs.08-3013]. [PMID: 19060281]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauck S.M., Kinkl N., Deeg C.A., Swiatek-de Lange M., Schöffmann S., Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol. Cell. Biol. 2006;26(7):2746–2757. doi: 10.1128/MCB.26.7.2746-2757.2006. [http://dx.doi.org/10.1128/MCB.26.7.2746-2757.2006]. [PMID: 16537917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosker K.E., Courchesne S.L., Segal R.A. Action in the axon: generation and transport of signaling endosomes. Curr. Opin. Neurobiol. 2008;18(3):270–275. doi: 10.1016/j.conb.2008.08.005. [http://dx.doi.org/10.1016/j.conb. 2008.08.005]. [PMID: 18778772]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascaño M., Richmond A., Borden P., Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J. Neurosci. 2009;29(37):11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.1542-09.2009]. [PMID: 19759314]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pease M.E., McKinnon S.J., Quigley H.A., Kerrigan-Baumrind L.A., Zack D.J. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2000;41(3):764–774. [PMID: 10711692]. [PubMed] [Google Scholar]
- 21.Tanaka H., Ito Y., Nakamura S., Shimazawa M., Hara H. Involvement of brain-derived neurotrophic factor in time-dependent neurodegeneration in the murine superior colliculus after intravitreal injection of N-methyl-D-aspartate. Mol. Vis. 2009;15:662–669. [PMID: 19347051]. [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y.T., Hsieh T., Forbes M.E., Johnson J.E., Frost D.O. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J. Neurosci. 1998;18(6):2097–2107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [http://dx.doi.org/10.1523/JNEUROSCI.18-06-02097.1998]. [PMID: 9482796]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen R., Tao W., Li Y., Sieving P.A. CNTF and retina. Prog. Retin. Eye Res. 2012;31(2):136–151. doi: 10.1016/j.preteyeres.2011.11.005. [http://dx.doi.org/10.1016/j. preteyeres.2011.11.005]. [PMID: 22182585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Q., Wang J., Matheson C.R., Urich J.L. Glial cell line-derived neurotrophic factor (GDNF) promotes the survival of axotomized retinal ganglion cells in adult rats: comparison to and combination with brain-derived neurotrophic factor (BDNF). J. Neurobiol. 1999;38(3):382–390. doi: 10.1002/(sici)1097-4695(19990215)38:3<382::aid-neu7>3.0.co;2-5. [http://dx.doi.org/10.1002/(SICI) 1097-4695(19990215)38:3<382:AID-NEU7>3.0.CO;2-5]. [PMID: 10022580]. [DOI] [PubMed] [Google Scholar]
- 25.Thanos S., Bähr M., Barde Y.A., Vanselow J. Survival and axonal elongation of adult rat retinal ganglion cells. Eur. J. Neurosci. 1989;1(1):19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [http://dx.doi.org/10.1111/j.1460-9568.1989.tb00770.x]. [PMID: 12106170]. [DOI] [PubMed] [Google Scholar]
- 26.Frade J.M., Bovolenta P., Martínez-Morales J.R., Arribas A., Barbas J.A., Rodríguez-Tébar A. Control of early cell death by BDNF in the chick retina. Development. 1997;124(17):3313–3320. doi: 10.1242/dev.124.17.3313. [PMID: 9310326]. [DOI] [PubMed] [Google Scholar]
- 27.de Rezende Corrêa G., Soares V.H., de Araújo-Martins L., Dos Santos A.A., Giestal-de-Araujo E. Ouabain and BDNF crosstalk on ganglion cell survival in mixed retinal cell cultures. Cell. Mol. Neurobiol. 2015;35(5):651–660. doi: 10.1007/s10571-015-0160-3. [http://dx.doi.org/10.1007/s10571-015-0160-3]. [PMID: 25651946]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virdee K., Bannister A.J., Hunt S.P., Tolkovsky A.M. Comparison between the timing of JNK activation, c-Jun phosphorylation, and onset of death commitment in sympathetic neurones. J. Neurochem. 1997;69(2):550–561. doi: 10.1046/j.1471-4159.1997.69020550.x. [http://dx.doi.org/10.1046/j.1471-4159.1997.69020550.x]. [PMID: 9231712]. [DOI] [PubMed] [Google Scholar]
- 29.Tsuruta F., Sunayama J., Mori Y., Hattori S., Shimizu S., Tsujimoto Y., Yoshioka K., Masuyama N., Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23(8):1889–1899. doi: 10.1038/sj.emboj.7600194. [http://dx.doi.org/ 10.1038/sj.emboj.7600194]. [PMID: 15071501]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour-Robaey S., Clarke D.B., Wang Y.C., Bray G.M., Aguayo A.J. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. USA. 1994;91(5):1632–1636. doi: 10.1073/pnas.91.5.1632. [http://dx.doi.org/10.1073/pnas.91.5.1632]. [PMID: 8127857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen-Cory S., Fraser S.E. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995;378(6553):192–196. doi: 10.1038/378192a0. [http://dx.doi.org/10.1038/378192a0]. [PMID: 7477323]. [DOI] [PubMed] [Google Scholar]
- 32.Chen H., Weber A.J. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest. Ophthalmol. Vis. Sci. 2001;42(5):966–974. [PMID: 11274073]. [PubMed] [Google Scholar]
- 33.Gupta V., You Y., Li J., Gupta V., Golzan M., Klistorner A., van den Buuse M., Graham S. BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochim. Biophys. Acta. 2014;1842(9):1567–1578. doi: 10.1016/j.bbadis.2014.05.026. [http://dx.doi.org/10.1016/j.bbadis.2014.05.026]. [PMID: 24942931]. [DOI] [PubMed] [Google Scholar]
- 34.Cheng L., Sapieha P., Kittlerova P., Hauswirth W.W., Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J. Neurosci. 2002;22(10):3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [http://dx.doi.org/10.1523/JNEUROSCI.22-10-03977.2002]. [PMID: 12019317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajala A., Gupta V.K., Anderson R.E., Rajala R.V. Light activation of the insulin receptor regulates mitochondrial hexokinase. A possible mechanism of retinal neuroprotection. Mitochondrion. 2013;13(6):566–576. doi: 10.1016/j.mito.2013.08.005. [http://dx.doi.org/10.1016/j.mito.2013.08.005]. [PMID: 23993956]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mai L., Jope R.S., Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J. Neurochem. 2002;82(1):75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [http://dx.doi.org/10.1046/j.1471-4159.2002.00939.x]. [PMID: 12091467]. [DOI] [PubMed] [Google Scholar]
- 37.Patapoutian A., Reichardt L.F. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7. [http://dx.doi.org/10.1016/S0959-4388(00)00208-7]. [PMID: 11399424]. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y.S., Long N., Pigino G., Brady S.T., Lazarov O. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3β, neurotrophin-3 and CREB signaling. PLoS One. 2013;8(5):e64460. doi: 10.1371/journal.pone.0064460. [http://dx.doi.org/10.1371/journal.pone.0064460]. [PMID: 23700479]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta V., Chitranshi N., You Y., Gupta V., Klistorner A., Graham S. Brain derived neurotrophic factor is involved in the regulation of glycogen synthase kinase 3β (GSK3β) signalling. Biochem. Biophys. Res. Commun. 2014;454(3):381–386. doi: 10.1016/j.bbrc.2014.10.087. [http://dx.doi. org/10.1016/j.bbrc.2014.10.087]. [PMID: 25451258]. [DOI] [PubMed] [Google Scholar]
- 40.Liu X., Grishanin R.N., Tolwani R.J., Rentería R.C., Xu B., Reichardt L.F., Copenhagen D.R. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J. Neurosci. 2007;27(27):7256–7267. doi: 10.1523/JNEUROSCI.0779-07.2007. [http://dx.doi.org/10.1523/JNEUROSCI.0779-07.2007]. [PMID: 17611278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Q., Tang L.S., Hu B., So K.F., Yip H.K. Expression of trkA, trkB, and trkC in injured and regenerating retinal ganglion cells of adult rats. Invest. Ophthalmol. Vis. Sci. 2002;43(6):1954–1964. [PMID: 12037005]. [PubMed] [Google Scholar]
- 42.Harada C., Guo X., Namekata K., Kimura A., Nakamura K., Tanaka K., Parada L.F., Harada T. Glia- and neuron-specific functions of TrkB signalling during retinal degeneration and regeneration. Nat. Commun. 2011;2:189. doi: 10.1038/ncomms1190. [http://dx.doi.org/10.1038/ ncomms1190]. [PMID: 21304518]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada T., Harada C., Kohsaka S., Wada E., Yoshida K., Ohno S., Mamada H., Tanaka K., Parada L.F., Wada K. Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J. Neurosci. 2002;22(21):9228–9236. doi: 10.1523/JNEUROSCI.22-21-09228.2002. [http://dx.doi.org/10.1523/JNEUROSCI.22-21-09228.2002]. [PMID: 12417648]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z.Z., Zhu L.Q., Eide F.F. Critical role of TrkB and brain-derived neurotrophic factor in the differentiation and survival of retinal pigment epithelium. J. Neurosci. 1997;17(22):8749–8755. doi: 10.1523/JNEUROSCI.17-22-08749.1997. [http://dx.doi.org/10.1523/JNEUROSCI.17-22-08749.1997]. [PMID: 9348344]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [http://dx.doi. org/10.1146/annurev.biochem.72.121801.161629]. [PMID: 12676795]. [DOI] [PubMed] [Google Scholar]
- 46.Klöcker N., Cellerino A., Bähr M. Free radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells In vivo. J. Neurosci. 1998;18(3):1038–1046. doi: 10.1523/JNEUROSCI.18-03-01038.1998. [http://dx.doi.org/10.1523/JNEUROSCI.18-03-01038.1998]. [PMID: 9437024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang S.W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [http://dx.doi.org/10.1073/pnas.0913572107]. [PMID: 20133810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y., Cho S., Goldberg J.L. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2010;51(3):1747–1754. doi: 10.1167/iovs.09-4450. [http://dx.doi.org/10.1167/iovs.09-4450]. [PMID: 19875669]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chitranshi N., Gupta V., Kumar S., Graham S.L. Exploring the Molecular Interactions of 7,8-Dihydroxyflavone and Its Derivatives with TrkB and VEGFR2 Proteins. Int. J. Mol. Sci. 2015;16(9):21087–21108. doi: 10.3390/ijms160921087. [http://dx.doi.org/10.3390/ijms160921087]. [PMID: 26404256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta V.K., You Y., Li J.C., Klistorner A., Graham S.L. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J. Mol. Neurosci. 2013;49(1):96–104. doi: 10.1007/s12031-012-9899-x. [http://dx.doi.org/10.1007/s12031-012-9899-x]. [PMID: 23054592]. [DOI] [PubMed] [Google Scholar]
- 51.Dheer Y., Chitranshi N., Gupta V., Abbasi M., Mirzaei M., You Y., Chung R., Graham S.L., Gupta V. Bexarotene modulates retinoid-X-receptor expression and is protective against neurotoxic endoplasmic reticulum stress response and apoptotic pathway activation. Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1041-9. [http://dx.doi.org/10. 1007/s12035-018-1041-9]. [PMID: 29637440]. [DOI] [PubMed] [Google Scholar]
- 52.Makkerh J.P., Ceni C., Auld D.S., Vaillancourt F., Dorval G., Barker P.A. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6(10):936–941. doi: 10.1038/sj.embor.7400503. [http://dx.doi.org/ 10.1038/sj.embor.7400503]. [PMID: 16113645]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arévalo J.C., Waite J., Rajagopal R., Beyna M., Chen Z.Y., Lee F.S., Chao M.V. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50(4):549–559. doi: 10.1016/j.neuron.2006.03.044. [http://dx.doi.org/10.1016/j.neuron.2006.03.044]. [PMID: 16701206]. [DOI] [PubMed] [Google Scholar]
- 54.Gupta V.K., You Y., Gupta V.B., Klistorner A., Graham S.L., Trk B. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013;14(5):10122–10142. doi: 10.3390/ijms140510122. [http://dx.doi.org/10.3390/ijms140510122]. [PMID: 23670594]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X., Obianyo O., Chan C.B., Huang J., Xue S., Yang J.J., Zeng F., Goodman M., Ye K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J. Biol. Chem. 2014;289(40):27571–27584. doi: 10.1074/jbc.M114.562561. [http://dx.doi.org/ 10.1074/jbc.M114.562561]. [PMID: 25143381]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu C., Chan C.B., Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl. Neurodegener. 2016;5:2. doi: 10.1186/s40035-015-0048-7. [http://dx.doi. org/10.1186/s40035-015-0048-7]. [PMID: 26740873]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang S.W., Liu X., Chan C.B., France S.A., Sayeed I., Tang W., Lin X., Xiao G., Andero R., Chang Q., Ressler K.J., Ye K. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS One. 2010;5(7):e11528. doi: 10.1371/journal.pone.0011528. [http://dx.doi.org/10.1371/ journal.pone.0011528]. [PMID: 20644624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol. Rev. 2009;228(1):342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [http://dx.doi.org/10.1111/j.1600-065X.2008.00760.x]. [PMID: 19290938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsang Y.H., Han X., Man W.Y., Lee N., Poon R.Y. Novel functions of the phosphatase SHP2 in the DNA replication and damage checkpoints. PLoS One. 2012;7(11):e49943. doi: 10.1371/journal.pone.0049943. [http://dx. doi.org/10.1371/journal.pone.0049943]. [PMID: 23189174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neel B.G., Gu H., Pao L. The 'Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003;28(6):284–293. doi: 10.1016/S0968-0004(03)00091-4. [http://dx.doi.org/10.1016/S0968-0004 (03)00091-4]. [PMID: 12826400]. [DOI] [PubMed] [Google Scholar]
- 61.Kontaridis M.I., Yang W., Bence K.K., Cullen D., Wang B., Bodyak N., Ke Q., Hinek A., Kang P.M., Liao R., Neel B.G. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation. 2008;117(11):1423–1435. doi: 10.1161/CIRCULATIONAHA.107.728865. [http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.728865]. [PMID: 18316486]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbasi M., Gupta V., Chitranshi N., You Y., Dheer Y. ; Mirzaei M., Graham S.L. Regulation of brain-derived neurotrophic factor and growth factor signaling pathways by tyrosine phosphatase Shp2 in the retina: A brief review. Front. Cell. Neurosci. 2018;12:85. doi: 10.3389/fncel.2018.00085. [http://dx.doi.org/10.3389/fncel.2018.00085]. [PMID: 29636665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agazie Y.M., Hayman M.J. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol. Cell. Biol. 2003;23(21):7875–7886. doi: 10.1128/MCB.23.21.7875-7886.2003. [http://dx.doi.org/10.1128/MCB. 23.21.7875-7886.2003]. [PMID: 14560030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaper F., Gendo C., Eck M., Schmitz J., Grimm C., Anhuf D., Kerr I.M., Heinrich P.C. Activation of the protein tyrosine phosphatase SHP2 via the interleukin-6 signal transducing receptor protein gp130 requires tyrosine kinase Jak1 and limits acute-phase protein expression. Biochem. J. 1998;335(Pt 3):557–565. doi: 10.1042/bj3350557. [http:// dx.doi.org/10.1042/bj3350557]. [PMID: 9794795]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanafusa H., Torii S., Yasunaga T., Matsumoto K., Nishida E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J. Biol. Chem. 2004;279(22):22992–22995. doi: 10.1074/jbc.M312498200. [http://dx.doi.org/10.1074/jbc.M312498200]. [PMID: 15031289]. [DOI] [PubMed] [Google Scholar]
- 66.Rusanescu G., Yang W., Bai A., Neel B.G., Feig L.A. Tyrosine phosphatase SHP-2 is a mediator of activity-dependent neuronal excitotoxicity. EMBO J. 2005;24(2):305–314. doi: 10.1038/sj.emboj.7600522. [http://dx.doi.org/ 10.1038/sj.emboj.7600522]. [PMID: 15650750]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Easton J.B., Moody N.M., Zhu X., Middlemas D.S. Brain-derived neurotrophic factor induces phosphorylation of fibroblast growth factor receptor substrate 2. J. Biol. Chem. 1999;274(16):11321–11327. doi: 10.1074/jbc.274.16.11321. [http://dx.doi.org/10.1074/jbc.274.16.11321]. [PMID: 10196222]. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L., Talebian A., Meakin S.O. The signaling adapter, FRS2, facilitates neuronal branching in primary cortical neurons via both Grb2- and Shp2-dependent mechanisms. J. Mol. Neurosci. 2015;55(3):663–677. doi: 10.1007/s12031-014-0406-4. [http://dx.doi.org/10.1007/s12031-014-0406-4]. [PMID: 25159185]. [DOI] [PubMed] [Google Scholar]
- 69.Araki T., Yamada M., Ohnishi H., Sano S., Uetsuki T., Hatanaka H. Shp-2 specifically regulates several tyrosine-phosphorylated proteins in brain-derived neurotrophic factor signaling in cultured cerebral cortical neurons. J. Neurochem. 2000;74(2):659–668. doi: 10.1046/j.1471-4159.2000.740659.x. [http://dx.doi.org/10.1046/j.1471-4159.2000.740659.x]. [PMID: 10646517]. [DOI] [PubMed] [Google Scholar]
- 70.Kim H.J., Han A.M., Shim J.H., Yoon H.H., Kwon H., Kwon Y.K. Shp2 is involved in neuronal differentiation of hippocampal precursor cells. Arch. Pharm. Res. 2007;30(6):750–754. doi: 10.1007/BF02977638. [http:// dx.doi.org/10.1007/BF02977638]. [PMID: 17679554]. [DOI] [PubMed] [Google Scholar]
- 71.Cai Z., Simons D.L., Fu X.Y., Feng G.S., Wu S.M., Zhang X. Loss of Shp2-mediated mitogen-activated protein kinase signaling in Muller glial cells results in retinal degeneration. Mol. Cell. Biol. 2011;31(14):2973–2983. doi: 10.1128/MCB.05054-11. [http://dx.doi.org/10.1128/MCB.05054-11]. [PMID: 21576358]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kruger R.P., Aurandt J., Guan K.L. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 2005;6(10):789–800. doi: 10.1038/nrm1740. [http://dx.doi.org/10.1038/nrm1740]. [PMID: 16314868]. [DOI] [PubMed] [Google Scholar]
- 73.Fuchikawa T., Nakamura F., Fukuda N., Takei K., Goshima Y. Protein tyrosine phosphatase SHP2 is involved in Semaphorin 4D-induced axon repulsion. Biochem. Biophys. Res. Commun. 2009;385(1):6–10. doi: 10.1016/j.bbrc.2009.05.024. [http://dx.doi.org/10.1016/j.bbrc.2009.05.024]. [PMID: 19433062]. [DOI] [PubMed] [Google Scholar]
- 74.Pinzon-Guzman C., Xing T., Zhang S.S., Barnstable C.J. Regulation of rod photoreceptor differentiation by STAT3 is controlled by a tyrosine phosphatase. J. Mol. Neurosci. 2015;55(1):152–159. doi: 10.1007/s12031-014-0397-1. [http://dx.doi.org/10.1007/s12031-014-0397-1]. [PMID: 25108518]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta V.K., You Y., Klistorner A., Graham S.L. Shp-2 regulates the TrkB receptor activity in the retinal ganglion cells under glaucomatous stress. Biochim. Biophys. Acta. 2012;1822(11):1643–1649. doi: 10.1016/j.bbadis.2012.07.016. [http://dx.doi.org/10.1016/j.bbadis.2012.07.016]. [PMID: 22878065]. [DOI] [PubMed] [Google Scholar]
- 76.Okamoto T., Schlegel A., Scherer P.E., Lisanti M.P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998;273(10):5419–5422. doi: 10.1074/jbc.273.10.5419. [http://dx.doi.org/10.1074/jbc.273.10.5419]. [PMID: 9488658]. [DOI] [PubMed] [Google Scholar]
- 77.Galbiati F., Volonte D., Gil O., Zanazzi G., Salzer J.L., Sargiacomo M., Scherer P.E., Engelman J.A., Schlegel A., Parenti M., Okamoto T., Lisanti M.P. Expression of caveolin-1 and -2 in differentiating PC12 cells and dorsal root ganglion neurons: caveolin-2 is up-regulated in response to cell injury. Proc. Natl. Acad. Sci. USA. 1998;95(17):10257–10262. doi: 10.1073/pnas.95.17.10257. [http://dx.doi.org/10.1073/pnas. 95.17.10257]. [PMID: 9707634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishiyama K., Trapp B.D., Ikezu T., Ransohoff R.M., Tomita T., Iwatsubo T., Kanazawa I., Hsiao K.K., Lisanti M.P., Okamoto T. Caveolin-3 upregulation activates beta-secretase-mediated cleavage of the amyloid precursor protein in Alzheimer’s disease. J. Neurosci. 1999;19(15):6538–6548. doi: 10.1523/JNEUROSCI.19-15-06538.1999. [http://dx.doi.org/10.1523/ JNEUROSCI.19-15-06538.1999]. [PMID: 10414982]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiss A.L., Turi A., Müller N., Kántor O., Botos E. Caveolae and caveolin isoforms in rat peritoneal macrophages. Micron. 2002;33(1):75–93. doi: 10.1016/s0968-4328(00)00100-1. [http://dx.doi.org/10.1016/S0968-4328(00)00100-1]. [PMID: 11473817]. [DOI] [PubMed] [Google Scholar]
- 80.Klaassen I., Van Noorden C.J., Schlingemann R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [http://dx.doi.org/10.1016/j.preteyeres.2013. 02.001]. [PMID: 23416119]. [DOI] [PubMed] [Google Scholar]
- 81.Thorleifsson G., Walters G.B., Hewitt A.W., Masson G., Helgason A., DeWan A., Sigurdsson A., Jonasdottir A., Gudjonsson S.A., Magnusson K.P., Stefansson H., Lam D.S., Tam P.O., Gudmundsdottir G.J., Southgate L., Burdon K.P., Gottfredsdottir M.S., Aldred M.A., Mitchell P., St Clair D., Collier D.A., Tang N., Sveinsson O., Macgregor S., Martin N.G., Cree A.J., Gibson J., Macleod A., Jacob A., Ennis S., Young T.L., Chan J.C., Karwatowski W.S., Hammond C.J., Thordarson K., Zhang M., Wadelius C., Lotery A.J., Trembath R.C., Pang C.P., Hoh J., Craig J.E., Kong A., Mackey D.A., Jonasson F., Thorsteinsdottir U., Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010;42(10):906–909. doi: 10.1038/ng.661. [http://dx.doi.org/10.1038/ng.661]. [PMID: 20835238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reagan A., Gu X., Hauck S.M., Ash J.D., Cao G., Thompson T.C., Elliott M.H. Retinal caveolin-1 modulates neuroprotective signaling. Adv. Exp. Med. Biol. 2016;854:411–418. doi: 10.1007/978-3-319-17121-0_54. [http://dx.doi. org/10.1007/978-3-319-17121-0_54]. [PMID: 26427439]. [DOI] [PubMed] [Google Scholar]
- 83.Chucair-Elliott A.J., Elliott M.H., Wang J., Moiseyev G.P., Ma J.X., Politi L.E., Rotstein N.P., Akira S., Uematsu S., Ash J.D. Leukemia inhibitory factor coordinates the down-regulation of the visual cycle in the retina and retinal-pigmented epithelium. J. Biol. Chem. 2012;287(29):24092–24102. doi: 10.1074/jbc.M112.378240. [http://dx.doi.org/10.1074/ jbc.M112.378240]. [PMID: 22645143]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiao H., Zhang Y., Yan Z., Wang Z.G., Liu G., Minshall R.D., Malik A.B., Hu G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J. Immunol. 2013;191(12):6191–6199. doi: 10.4049/jimmunol.1300873. [http://dx.doi.org/10.4049/ jimmunol.1300873]. [PMID: 24244013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pawson T., Gish G.D., Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001;11(12):504–511. doi: 10.1016/s0962-8924(01)02154-7. [http://dx.doi.org/10.1016/S0962-8924(01)02154-7]. [PMID: 11719057]. [DOI] [PubMed] [Google Scholar]
- 86.Takayasu Y., Takeuchi K., Kumari R., Bennett M.V., Zukin R.S., Francesconi A. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proc. Natl. Acad. Sci. USA. 2010;107(50):21778–21783. doi: 10.1073/pnas.1015553107. [http://dx.doi.org/10.1073/pnas.1015553107]. [PMID: 21098662]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Volonté D., Galbiati F., Pestell R.G., Lisanti M.P. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr(14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J. Biol. Chem. 2001;276(11):8094–8103. doi: 10.1074/jbc.M009245200. [http://dx.doi.org/10.1074/jbc.M009245200]. [PMID: 11094059]. [DOI] [PubMed] [Google Scholar]
- 88.Sanguinetti A.R., Cao H., Corley Mastick C. Fyn is required for oxidative- and hyperosmotic-stress-induced tyrosine phosphorylation of caveolin-1. Biochem. J. 2003;376(Pt 1):159–168. doi: 10.1042/BJ20030336. [http:// dx.doi.org/10.1042/bj20030336]. [PMID: 12921535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Surgucheva I., Surguchov A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol. Vis. 2011;17:2878–2888. [PMID: 22128235]. [PMC free article] [PubMed] [Google Scholar]
- 90.Jo A., Park H., Lee S.H., Ahn S.H., Kim H.J., Park E.M., Choi Y.H. SHP-2 binds to caveolin-1 and regulates Src activity via competitive inhibition of CSK in response to H2O2 in astrocytes. PLoS One. 2014;9(3):e91582. doi: 10.1371/journal.pone.0091582. [http://dx.doi.org/10.1371/journal.pone. 0091582]. [PMID: 24632723]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li X., McClellan M.E., Tanito M., Garteiser P., Towner R., Bissig D., Berkowitz B.A., Fliesler S.J., Woodruff M.L., Fain G.L., Birch D.G., Khan M.S., Ash J.D., Elliott M.H. Loss of caveolin-1 impairs retinal function due to disturbance of subretinal microenvironment. J. Biol. Chem. 2012;287(20):16424–16434. doi: 10.1074/jbc.M112.353763. [http://dx.doi.org/10.1074/jbc.M112.353763]. [PMID: 22451674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian X.F., Xia X.B., Xu H.Z., Xiong S.Q., Jiang J. Caveolin-1 expression regulates blood-retinal barrier permeability and retinal neovascularization in oxygen-induced retinopathy. Clin. Experiment. Ophthalmol. 2012;40(1):e58–e66. doi: 10.1111/j.1442-9071.2011.02656.x. [http://dx.doi.org/10.1111/ j.1442-9071.2011.02656.x]. [PMID: 21794046]. [DOI] [PubMed] [Google Scholar]
- 93.Vrabec J.P., Levin L.A. The neurobiology of cell death in glaucoma. Eye (Lond.) 2007;21(Suppl. 1):S11–S14. doi: 10.1038/sj.eye.6702880. [http://dx.doi.org/ 10.1038/sj.eye.6702880]. [PMID: 18157171]. [DOI] [PubMed] [Google Scholar]
- 94.Osborne N.N., Casson R.J., Wood J.P., Chidlow G., Graham M., Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004;23(1):91–147. doi: 10.1016/j.preteyeres.2003.12.001. [http://dx.doi.org/10.1016/j.preteyeres.2003.12.001]. [PMID: 14766318]. [DOI] [PubMed] [Google Scholar]
- 95.Barot M., Gokulgandhi M.R., Mitra A.K. Mitochondrial dysfunction in retinal diseases. Curr. Eye Res. 2011;36(12):1069–1077. doi: 10.3109/02713683.2011.607536. [http://dx.doi.org/10.3109/02713683.2011.607536]. [PMID: 21978133]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., Shen D., Wang V.M., Yu C.R., Wang R.X., Tuo J., Chan C.C. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis. 2012;17(11):1144–1155. doi: 10.1007/s10495-012-0750-1. [http://dx.doi.org/10.1007/s10495-012-0750-1]. [PMID: 22911474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ientile R., Macaione V., Teletta M., Pedale S., Torre V., Macaione S. Apoptosis and necrosis occurring in excitotoxic cell death in isolated chick embryo retina. J. Neurochem. 2001;79(1):71–78. doi: 10.1046/j.1471-4159.2001.00532.x. [http://dx.doi.org/10.1046/j.1471-4159.2001.00532.x]. [PMID: 11595759]. [DOI] [PubMed] [Google Scholar]
- 98.Feenstra D.J., Yego E.C., Mohr S. Modes of retinal cell death in diabetic retinopathy. J. Clin. Exp. Ophthalmol. 2013;4(5):298. doi: 10.4172/2155-9570.1000298. [PMID: 24672740]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jing G., Wang J.J., Zhang S.X. ER stress and apoptosis: a new mechanism for retinal cell death. Exp. Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [http://dx.doi.org/10.1155/2012/589589]. [PMID: 22216020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X., Olberding K.E., White C., Li C. Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ. 2011;18(1):38–47. doi: 10.1038/cdd.2010.68. [http://dx.doi.org/10.1038/cdd.2010.68]. [PMID: 20539308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwarz D.S., Blower M.D. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016;73(1):79–94. doi: 10.1007/s00018-015-2052-6. [http://dx.doi.org/10.1007/s00018-015-2052-6]. [PMID: 26433683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cornejo V.H., Hetz C. The unfolded protein response in Alzheimer’s disease. Semin. Immunopathol. 2013;35(3):277–292. doi: 10.1007/s00281-013-0373-9. [http://dx.doi.org/10.1007/s00281-013-0373-9]. [PMID: 23609500]. [DOI] [PubMed] [Google Scholar]
- 103.Varma D., Sen D. Role of the unfolded protein response in the pathogenesis of Parkinson’s disease. Acta Neurobiol. Exp. (Warsz.) 2015;75(1):1–26. [PMID: 25856519]. [PubMed] [Google Scholar]
- 104.Anholt R.R., Carbone M.A. A molecular mechanism for glaucoma: endoplasmic reticulum stress and the unfolded protein response. Trends Mol. Med. 2013;19(10):586–593. doi: 10.1016/j.molmed.2013.06.005. [http://dx.doi. org/10.1016/j.molmed.2013.06.005]. [PMID: 23876925]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mirzaei M., Gupta V.B., Chick J.M., Greco T.M., Wu Y., Chitranshi N., Wall R.V., Hone E., Deng L., Dheer Y., Abbasi M., Rezaeian M., Braidy N., You Y., Salekdeh G.H., Haynes P.A., Molloy M.P., Martins R., Cristea I.M., Gygi S.P., Graham S.L., Gupta V.K. Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci. Rep. 2017;7(1):12685. doi: 10.1038/s41598-017-12858-7. [http://dx.doi.org/10. 1038/s41598-017-12858-7]. [PMID: 28978942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bravo R., Parra V., Gatica D., Rodriguez A.E., Torrealba N., Paredes F., Wang Z.V., Zorzano A., Hill J.A., Jaimovich E., Quest A.F., Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. [http://dx.doi.org/10.1016/ B978-0-12-407704-1.00005-1]. [PMID: 23317820]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peters J.C., Bhattacharya S., Clark A.F., Zode G.S. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest. Ophthalmol. Vis. Sci. 2015;56(6):3860–3868. doi: 10.1167/iovs.14-16220. [http://dx.doi.org/10.1167/iovs.14-16220]. [PMID: 26066753]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doh S.H., Kim J.H., Lee K.M., Park H.Y., Park C.K. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010;1308:158–166. doi: 10.1016/j.brainres.2009.10.025. [http:// dx.doi.org/10.1016/j.brainres.2009.10.025]. [PMID: 19853589]. [DOI] [PubMed] [Google Scholar]
- 109.Hu Y. Axon injury induced endoplasmic reticulum stress and neurodegeneration. Neural Regen. Res. 2016;11(10):1557–1559. doi: 10.4103/1673-5374.193225. [http://dx.doi.org/10.4103/1673-5374.193225]. [PMID: 27904477]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang M.J., Ryoo H.D. Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc. Natl. Acad. Sci. USA. 2009;106(40):17043–17048. doi: 10.1073/pnas.0905566106. [http://dx.doi. org/10.1073/pnas.0905566106]. [PMID: 19805114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Z., Tong N., Gong Y., Qiu Q., Yin L., Lv X., Wu X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci. Lett. 2011;504(2):88–92. doi: 10.1016/j.neulet.2011.09.003. [http://dx.doi.org/10.1016/j.neulet.2011. 09.003]. [PMID: 21939735]. [DOI] [PubMed] [Google Scholar]
- 112.Gardner B.M., Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333(6051):1891–1894. doi: 10.1126/science.1209126. [http://dx.doi.org/10.1126/science. 1209126]. [PMID: 21852455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marciniak S.J., Garcia-Bonilla L., Hu J., Harding H.P., Ron D. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J. Cell Biol. 2006;172(2):201–209. doi: 10.1083/jcb.200508099. [http://dx.doi.org/10.1083/jcb.200508099]. [PMID: 16418533]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [http://dx.doi.org/10.1128/MCB.23.20.7198-7209.2003]. [PMID: 14517290]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chitranshi N., Dheer Y., Gupta V., Abbasi M., Mirzaei M., You Y., Chung R., Graham S.L., Gupta V. PTPN11 induces endoplasmic stress and apoptosis in SH-SY5Y cells. Neuroscience. 2017;364:175–189. doi: 10.1016/j.neuroscience.2017.09.028. [http://dx.doi.org/10.1016/j.neuroscience. 2017.09.028]. [PMID: 28947394]. [DOI] [PubMed] [Google Scholar]
- 116.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [http://dx.doi. org/10.1016/j.bbamcr.2013.06.028]. [PMID: 23850759]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y., Yamada E., Zong H., Pessin J.E. Fyn activation of mTORC1 stimulates the IRE1α-JNK pathway, leading to cell death. J. Biol. Chem. 2015;290(41):24772–24783. doi: 10.1074/jbc.M115.687020. [http://dx.doi. org/10.1074/jbc.M115.687020]. [PMID: 26306048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mabuchi F., Sakurada Y., Kashiwagi K., Yamagata Z., Iijima H., Tsukahara S. Involvement of genetic variants associated with primary open-angle glaucoma in pathogenic mechanisms and family history of glaucoma. Am. J. Ophthalmol. 2015;159(3):437–444. doi: 10.1016/j.ajo.2014.11.023. [http://dx.doi.org/10.1016/j.ajo.2014.11.023]. [DOI] [PubMed] [Google Scholar]
- 119.Kim B.S., Savinova O.V., Reedy M.V., Martin J., Lun Y., Gan L., Smith R.S., Tomarev S.I., John S.W., Johnson R.L. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol. Cell. Biol. 2001;21(22):7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [http://dx.doi.org/10.1128/MCB.21.22. 7707-7713.2001]. [PMID: 11604506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sarfarazi M., Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol. Clin. North Am. 2003;16(4):529–541. doi: 10.1016/s0896-1549(03)00061-0. [http:// dx.doi.org/10.1016/S0896-1549(03)00061-0]. [PMID: 14740994]. [DOI] [PubMed] [Google Scholar]
- 121.Skarie J.M., Link B.A. The primary open-angle glaucoma gene WDR36 functions in ribosomal RNA processing and interacts with the p53 stress-response pathway. Hum. Mol. Genet. 2008;17(16):2474–2485. doi: 10.1093/hmg/ddn147. [http://dx.doi.org/10.1093/hmg/ddn147]. [PMID: 18469340]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen L.J., Ng T.K., Fan A.H., Leung D.Y., Zhang M., Wang N., Zheng Y., Liang X.Y., Chiang S.W., Tam P.O., Pang C.P. Evaluation of NTF4 as a causative gene for primary open-angle glaucoma. Mol. Vis. 2012;18:1763–1772. [PMID: 22815630]. [PMC free article] [PubMed] [Google Scholar]
- 123.Wiggs J.L., Kang J.H., Yaspan B.L., Mirel D.B., Laurie C., Crenshaw A., Brodeur W., Gogarten S., Olson L.M., Abdrabou W., DelBono E., Loomis S., Haines J.L., Pasquale L.R., Consortium G. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum. Mol. Genet. 2011;20(23):4707–4713. doi: 10.1093/hmg/ddr382. [http://dx.doi.org/ 10.1093/hmg/ddr382]. [PMID: 21873608]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kondkar A.A., Mousa A., Azad T.A., Sultan T., Alawad A., Altuwaijri S., Al-Obeidan S.A., Abu-Amero K.K. Polymorphism rs7555523 in transmembrane and coiled-coil domain 1 (TMCO1) is not a risk factor for primary open angle glaucoma in a Saudi cohort. J. Negat. Results Biomed. 2016;15(1):17. doi: 10.1186/s12952-016-0060-1. [http://dx.doi.org/ 10.1186/s12952-016-0060-1]. [PMID: 27687253]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bailey J.N., Loomis S.J., Kang J.H., Allingham R.R., Gharahkhani P., Khor C.C., Burdon K.P., Aschard H., Chasman D.I., Igo R.P., Jr, Hysi P.G., Glastonbury C.A., Ashley-Koch A., Brilliant M., Brown A.A., Budenz D.L., Buil A., Cheng C.Y., Choi H., Christen W.G., Curhan G., De Vivo I., Fingert J.H., Foster P.J., Fuchs C., Gaasterland D., Gaasterland T., Hewitt A.W., Hu F., Hunter D.J., Khawaja A.P., Lee R.K., Li Z., Lichter P.R., Mackey D.A., McGuffin P., Mitchell P., Moroi S.E., Perera S.A., Pepper K.W., Qi Q., Realini T., Richards J.E., Ridker P.M., Rimm E., Ritch R., Ritchie M., Schuman J.S., Scott W.K., Singh K., Sit A.J., Song Y.E., Tamimi R.M., Topouzis F., Viswanathan A.C., Verma S.S., Vollrath D., Wang J.J., Weisschuh N., Wissinger B., Wollstein G., Wong T.Y., Yaspan B.L., Zack D.J., Zhang K., Study E.N., Weinreb R.N., Pericak-Vance M.A., Small K., Hammond C.J., Aung T., Liu Y., Vithana E.N., MacGregor S., Craig J.E., Kraft P., Howell G., Hauser M.A., Pasquale L.R., Haines J.L., Wiggs J.L., Wiggs J.L. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016;48(2):189–194. doi: 10.1038/ng.3482. [http://dx.doi.org/10. 1038/ng.3482]. [PMID: 26752265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pasutto F., Matsumoto T., Mardin C.Y., Sticht H., Brandstätter J.H., Michels-Rautenstrauss K., Weisschuh N., Gramer E., Ramdas W.D., van Koolwijk L.M., Klaver C.C., Vingerling J.R., Weber B.H., Kruse F.E., Rautenstrauss B., Barde Y.A., Reis A. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am. J. Hum. Genet. 2009;85(4):447–456. doi: 10.1016/j.ajhg.2009.08.016. [http://dx.doi.org/10.1016/j.ajhg.2009.08.016]. [PMID: 19765683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Y., Liu W., Crooks K., Schmidt S., Allingham R.R. ; Hauser M.A. No evidence of association of heterozygous NTF4 mutations in patients with primary open-angle glaucoma. Am. J. Hum. Genet. 2010;86(3):498–499. doi: 10.1016/j.ajhg.2009.11.018. [http://dx.doi.org/10.1016/ j.ajhg.2009.11.018]. [PMID: 20215012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao K.N., Kaur I., Parikh R.S., Mandal A.K., Chandrasekhar G., Thomas R., Chakrabarti S. Variations in NTF4, VAV2, and VAV3 genes are not involved with primary open-angle and primary angle-closure glaucomas in an indian population. Invest. Ophthalmol. Vis. Sci. 2010;51(10):4937–4941. doi: 10.1167/iovs.10-5553. [http://dx.doi.org/10. 1167/iovs.10-5553]. [PMID: 20463313]. [DOI] [PubMed] [Google Scholar]
- 129.Vithana E.N., Nongpiur M.E., Venkataraman D., Chan S.H., Mavinahalli J., Aung T. Identification of a novel mutation in the NTF4 gene that causes primary open-angle glaucoma in a Chinese population. Mol. Vis. 2010;16:1640–1645. [PMID: 20806036]. [PMC free article] [PubMed] [Google Scholar]
- 130.Nowak A., Szaflik J.P., Gacek M., Przybylowska-Sygut K., Kamińska A., Szaflik J., Majsterek I. BDNF and HSP gene polymorphisms and their influence on the progression of primary open-angle glaucoma in a Polish population. Arch. Med. Sci. 2014;10(6):1206–1213. doi: 10.5114/aoms.2014.45089. [http://dx.doi.org/10.5114/aoms.2014.45089]. [PMID: 25624860]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thorleifsson G., Walters G.B., Hewitt A.W., Masson G., Helgason A., DeWan A., Sigurdsson A., Jonasdottir A., Gudjonsson S.A., Magnusson K.P., Stefansson H., Lam D.S.C., Tam P.O.S., Gudmundsdottir G.J., Southgate L., Burdon K.P., Gottfredsdottir M.S., Aldred M.A., Mitchell P., St Clair D., Collier D.A., Tang N., Sveinsson O., Macgregor S., Martin N.G., Cree A.J., Gibson J., Macleod A., Jacob A., Ennis S., Young T.L., Chan J.C.N., Karwatowski W.S.S., Hammond C.J., Thordarson K., Zhang M., Wadelius C., Lotery A.J., Trembath R.C., Pang C.P., Hoh J., Craig J.E., Kong A., Mackey D.A., Jonasson F., Thorsteinsdottir U., Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010;42(10):906–909. doi: 10.1038/ng.661. [http://dx.doi.org/10. 1038/ng.661]. [PMID: 20835238]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuehn M.H., Wang K., Roos B., Stone E.M., Kwon Y.H., Alward W.L., Mullins R.F., Fingert J.H. Chromosome 7q31 POAG locus: ocular expression of caveolins and lack of association with POAG in a US cohort. Mol. Vis. 2011;17:430–435. [PMID: 21321670]. [PMC free article] [PubMed] [Google Scholar]
- 133.Elliott M.H., Ashpole N.E., Gu X., Herrnberger L., McClellan M.E., Griffith G.L., Reagan A.M., Boyce T.M., Tanito M., Tamm E.R., Stamer W.D. Caveolin-1 modulates intraocular pressure: implications for caveolae mechanoprotection in glaucoma. Sci. Rep. 2016;6:37127. doi: 10.1038/srep37127. [http://dx.doi.org/10.1038/srep37127]. [PMID: 27841369]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Green C.M., Kearns L.S., Wu J., Barbour J.M., Wilkinson R.M., Ring M.A., Craig J.E., Wong T.L., Hewitt A.W., Mackey D.A. How significant is a family history of glaucoma? Experience from the glaucoma inheritance study in tasmania. Clin. Experiment. Ophthalmol. 2007;35(9):793–799. doi: 10.1111/j.1442-9071.2007.01612.x. [http://dx.doi.org/10.1111/ j.1442-9071.2007.01612.x]. [PMID: 18173405]. [DOI] [PubMed] [Google Scholar]
- 135.Polansky J.R., Fauss D.J., Chen P., Chen H., Lütjen-Drecoll E., Johnson D., Kurtz R.M., Ma Z.D., Bloom E., Nguyen T.D. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211(3):126–139. doi: 10.1159/000310780. [http://dx.doi.org/10.1159/ 000310780]. [PMID: 9176893]. [DOI] [PubMed] [Google Scholar]
- 136.Kasetti R.B., Phan T.N., Millar J.C., Zode G.S. Expression of mutant myocilin induces abnormal intracellular accumulation of selected extracellular matrix proteins in the trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2016;57(14):6058–6069. doi: 10.1167/iovs.16-19610. [http://dx. doi.org/10.1167/iovs.16-19610]. [PMID: 27820874]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liao R.F., Zhong Z.L., Ye M.J., Han L.Y., Ye D.Q., Chen J.J. Identification of mutations in myocilin and beta-1,4-galactosyl-transferase 3 genes in a chinese family with primary open-angle glaucoma. Chin. Med. J. (Engl.) 2016;129(23):2810–2815. doi: 10.4103/0366-6999.194641. [http:// dx.doi.org/10.4103/0366-6999.194641]. [PMID: 27900994]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Itakura T., Peters D.M., Fini M.E. Glaucomatous MYOC mutations activate the IL-1/NF-κB inflammatory stress response and the glaucoma marker SELE in trabecular meshwork cells. Mol. Vis. 2015;21:1071–1084. [PMID: 26396484]. [PMC free article] [PubMed] [Google Scholar]
- 139.Guo H., Li M., Wang Z., Liu Q., Wu X. Association of MYOC and APOE promoter polymorphisms and primary open-angle glaucoma: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8(2):2052–2064. [PMID: 25932136]. [PMC free article] [PubMed] [Google Scholar]
- 140.Kroeber M., Ohlmann A., Russell P., Tamm E.R. Transgenic studies on the role of optineurin in the mouse eye. Exp. Eye Res. 2006;82(6):1075–1085. doi: 10.1016/j.exer.2005.11.004. [http://dx.doi.org/10.1016/j.exer.2005.11. 004]. [PMID: 16442524]. [DOI] [PubMed] [Google Scholar]
- 141.Shim M.S., Takihara Y., Kim K.Y., Iwata T., Yue B.Y., Inatani M., Weinreb R.N., Perkins G.A., Ju W.K. Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration. Sci. Rep. 2016;6:33830. doi: 10.1038/srep33830. [http://dx.doi.org/10.1038/srep33830]. [PMID: 27654856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tseng H.C., Riday T.T., McKee C., Braine C.E., Bomze H., Barak I., Marean-Reardon C., John S.W., Philpot B.D., Ehlers M.D. Visual impairment in an optineurin mouse model of primary open-angle glaucoma. Neurobiol. Aging. 2015;36(6):2201–2212. doi: 10.1016/j.neurobiolaging.2015.02.012. [http://dx.doi.org/10.1016/j.neurobiolaging.2015.02.012]. [PMID: 25818176]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aung T., Rezaie T., Okada K., Viswanathan A.C., Child A.H., Brice G., Bhattacharya S.S., Lehmann O.J., Sarfarazi M., Hitchings R.A. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Invest. Ophthalmol. Vis. Sci. 2005;46(8):2816–2822. doi: 10.1167/iovs.04-1133. [http://dx.doi.org/10.1167/iovs. 04-1133]. [PMID: 16043855]. [DOI] [PubMed] [Google Scholar]
- 144.Hauser M.A., Allingham R.R., Linkroum K., Wang J., LaRocque-Abramson K., Figueiredo D., Santiago-Turla C., del Bono E.A., Haines J.L., Pericak-Vance M.A., Wiggs J.L. Distribution of WDR36 DNA sequence variants in patients with primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 2006;47(6):2542–2546. doi: 10.1167/iovs.05-1476. [http://dx.doi.org/10.1167/iovs.05-1476]. [PMID: 16723468]. [DOI] [PubMed] [Google Scholar]
- 145.Mao M., Biery M.C., Kobayashi S.V., Ward T., Schimmack G., Burchard J., Schelter J.M., Dai H., He Y.D., Linsley P.S. T lymphocyte activation gene identification by coregulated expression on DNA microarrays. Genomics. 2004;83(6):989–999. doi: 10.1016/j.ygeno.2003.12.019. [http://dx.doi.org/10.1016/j.ygeno.2003.12.019]. [PMID: 15177553]. [DOI] [PubMed] [Google Scholar]
- 146.Hewitt A.W., Dimasi D.P., Mackey D.A., Craig J.E. A Glaucoma Case-control Study of the WDR36 Gene D658G sequence variant. Am. J. Ophthalmol. 2006;142(2):324–325. doi: 10.1016/j.ajo.2006.02.041. [http://dx.doi. org/10.1016/j.ajo.2006.02.041]. [PMID: 16876519]. [DOI] [PubMed] [Google Scholar]
- 147.Weisschuh N., Wolf C., Wissinger B., Gramer E. Variations in the WDR36 gene in German patients with normal tension glaucoma. Mol. Vis. 2007;13:724–729. [PMID: 17563723]. [PMC free article] [PubMed] [Google Scholar]
- 148.Miyazawa A., Fuse N., Mengkegale M., Ryu M., Seimiya M., Wada Y., Nishida K. Association between primary open-angle glaucoma and WDR36 DNA sequence variants in Japanese. Mol. Vis. 2007;13:1912–1919. [PMID: 17960130]. [PubMed] [Google Scholar]
- 149.Footz T.K., Johnson J.L., Dubois S., Boivin N., Raymond V., Walter M.A. Glaucoma-associated WDR36 variants encode functional defects in a yeast model system. Hum. Mol. Genet. 2009;18(7):1276–1287. doi: 10.1093/hmg/ddp027. [http://dx.doi.org/10.1093/hmg/ddp027]. [PMID: 19150991]. [DOI] [PubMed] [Google Scholar]
- 150.van Koolwijk L.M., Ramdas W.D., Ikram M.K., Jansonius N.M., Pasutto F., Hysi P.G., Macgregor S., Janssen S.F., Hewitt A.W., Viswanathan A.C., ten Brink J.B., Hosseini S.M., Amin N., Despriet D.D., Willemse-Assink J.J., Kramer R., Rivadeneira F., Struchalin M., Aulchenko Y.S., Weisschuh N., Zenkel M., Mardin C.Y., Gramer E., Welge-Lüssen U., Montgomery G.W., Carbonaro F., Young T.L., Bellenguez C., McGuffin P., Foster P.J., Topouzis F., Mitchell P., Wang J.J., Wong T.Y., Czudowska M.A., Hofman A., Uitterlinden A.G., Wolfs R.C., de Jong P.T., Oostra B.A., Paterson A.D., Mackey D.A., Bergen A.A., Reis A., Hammond C.J., Vingerling J.R., Lemij H.G., Klaver C.C., van Duijn C.M., Klaver C.C., van Duijn C.M. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8(5):e1002611. doi: 10.1371/journal.pgen.1002611. [http://dx.doi.org/10.1371/journal.pgen.1002611]. [PMID: 22570627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Burdon K.P., Macgregor S., Hewitt A.W., Sharma S., Chidlow G., Mills R.A., Danoy P., Casson R., Viswanathan A.C., Liu J.Z., Landers J., Henders A.K., Wood J., Souzeau E., Crawford A., Leo P., Wang J.J., Rochtchina E., Nyholt D.R., Martin N.G., Montgomery G.W., Mitchell P., Brown M.A., Mackey D.A., Craig J.E. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 2011;43(6):574–578. doi: 10.1038/ng.824. [http://dx.doi.org/10.1038/ng.824]. [PMID: 21532571]. [DOI] [PubMed] [Google Scholar]
- 152.Chen Y., Hughes G., Chen X., Qian S., Cao W., Wang L., Wang M., Sun X. Genetic variants associated with different risks for high tension glaucoma and normal tension glaucoma in a chinese population. Invest. Ophthalmol. Vis. Sci. 2015;56(4):2595–2600. doi: 10.1167/iovs.14-16269. [http://dx.doi.org/10.1167/iovs.14-16269]. [PMID: 25711633]. [DOI] [PubMed] [Google Scholar]
- 153.Micheal S., Ayub H., Khan M.I., Bakker B., Schoenmaker-Koller F.E., Ali M., Akhtar F., Khan W.A., Qamar R., den Hollander A.I. Association of known common genetic variants with primary open angle, primary angle closure, and pseudoexfoliation glaucoma in Pakistani cohorts. Mol. Vis. 2014;20:1471–1479. [PMID: 25489222]. [PMC free article] [PubMed] [Google Scholar]
- 154.Williams S.E., Carmichael T.R., Allingham R.R., Hauser M., Ramsay M. The genetics of POAG in black South Africans: a candidate gene association study. Sci. Rep. 2015;5:8378. doi: 10.1038/srep08378. [http://dx. doi.org/10.1038/srep08378]. [PMID: 25669751]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ozel A.B., Moroi S.E., Reed D.M., Nika M., Schmidt C.M., Akbari S., Scott K., Rozsa F., Pawar H., Musch D.C., Lichter P.R., Gaasterland D., Branham K., Gilbert J., Garnai S.J., Chen W., Othman M., Heckenlively J., Swaroop A., Abecasis G., Friedman D.S., Zack D., Ashley-Koch A., Ulmer M., Kang J.H., Liu Y., Yaspan B.L., Haines J., Allingham R.R., Hauser M.A., Pasquale L., Wiggs J., Richards J.E., Li J.Z., Li J.Z. Genome-wide association study and meta-analysis of intraocular pressure. Hum. Genet. 2014;133(1):41–57. doi: 10.1007/s00439-013-1349-5. [http://dx.doi.org/10.1007/ s00439-013-1349-5]. [PMID: 24002674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Domenici L., Origlia N., Falsini B., Cerri E., Barloscio D., Fabiani C., Sansò M., Giovannini L. Rescue of retinal function by BDNF in a mouse model of glaucoma. PLoS One. 2014;9(12):e115579. doi: 10.1371/journal.pone.0115579. [http://dx.doi.org/10.1371/journal.pone.0115579]. [PMID: 25536045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ko M.L., Hu D.N., Ritch R., Sharma S.C. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2000;41(10):2967–2971. [PMID: 10967052]. [PubMed] [Google Scholar]
- 158.Mey J., Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602(2):304–317. doi: 10.1016/0006-8993(93)90695-j. [http://dx.doi.org/ 10.1016/0006-8993(93)90695-J]. [PMID: 8448673]. [DOI] [PubMed] [Google Scholar]
- 159.Pernet V., Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129(Pt 4):1014–1026. doi: 10.1093/brain/awl015. [http://dx.doi.org/10.1093/brain/awl015]. [PMID: 16418178]. [DOI] [PubMed] [Google Scholar]
- 160.Pollock G.S., Robichon R., Boyd K.A., Kerkel K.A., Kramer M., Lyles J., Ambalavanar R., Khan A., Kaplan D.R., Williams R.W., Frost D.O. TrkB receptor signaling regulates developmental death dynamics, but not final number, of retinal ganglion cells. J. Neurosci. 2003;23(31):10137–10145. doi: 10.1523/JNEUROSCI.23-31-10137.2003. [http://dx.doi.org/10.1523/ JNEUROSCI.23-31-10137.2003]. [PMID: 14602830]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blanco R.E., Soto I., Duprey-Díaz M., Blagburn J.M. Up-regulation of brain-derived neurotrophic factor by application of fibroblast growth factor-2 to the cut optic nerve is important for long-term survival of retinal ganglion cells. J. Neurosci. Res. 2008;86(15):3382–3392. doi: 10.1002/jnr.21793. [http://dx.doi.org/10.1002/jnr.21793]. [PMID: 18655198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [http://dx.doi.org/10.1038/nrn3379]. [PMID: 23254191]. [DOI] [PubMed] [Google Scholar]
- 163.Bok D., Yasumura D., Matthes M.T., Ruiz A., Duncan J.L., Chappelow A.V., Zolutukhin S., Hauswirth W., LaVail M.M. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp. Eye Res. 2002;74(6):719–735. doi: 10.1006/exer.2002.1176. [http://dx.doi.org/10.1006/exer.2002.1176]. [PMID: 12126945]. [DOI] [PubMed] [Google Scholar]
- 164.Ji J.Z., Elyaman W., Yip H.K., Lee V.W., Yick L.W., Hugon J., So K.F. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur. J. Neurosci. 2004;19(2):265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [http://dx.doi.org/10.1111/j.0953-816X.2003.03107.x]. [PMID: 14725620]. [DOI] [PubMed] [Google Scholar]
- 165.Di Polo A., Aigner L.J., Dunn R.J., Bray G.M., Aguayo A.J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. USA. 1998;95(7):3978–3983. doi: 10.1073/pnas.95.7.3978. [http://dx.doi.org/10.1073/pnas.95.7.3978]. [PMID: 9520478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schmeer C., Straten G., Kügler S., Gravel C., Bähr M., Isenmann S. Dose-dependent rescue of axotomized rat retinal ganglion cells by adenovirus-mediated expression of glial cell-line derived neurotrophic factor in vivo. Eur. J. Neurosci. 2002;15(4):637–643. doi: 10.1046/j.1460-9568.2002.01893.x. [http://dx.doi.org/10.1046/j.1460-9568.2002.01893.x]. [PMID: 11886444]. [DOI] [PubMed] [Google Scholar]
- 167.Raper S.E., Chirmule N., Lee F.S., Wivel N.A., Bagg A., Gao G.P., Wilson J.M., Batshaw M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80(1-2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [http://dx.doi.org/10.1016/j.ymgme.2003.08.016]. [PMID: 14567964]. [DOI] [PubMed] [Google Scholar]
- 168.Sapieha P.S., Peltier M., Rendahl K.G., Manning W.C., Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol. Cell. Neurosci. 2003;24(3):656–672. doi: 10.1016/s1044-7431(03)00228-8. [http://dx.doi.org/10.1016/ S1044-7431(03)00228-8]. [PMID: 14664816]. [DOI] [PubMed] [Google Scholar]
- 169.Martin K.R., Quigley H.A., Zack D.J., Levkovitch-Verbin H., Kielczewski J., Valenta D., Baumrind L., Pease M.E., Klein R.L., Hauswirth W.W. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 2003;44(10):4357–4365. doi: 10.1167/iovs.02-1332. [http://dx.doi.org/10.1167/iovs.02-1332]. [PMID: 14507880]. [DOI] [PubMed] [Google Scholar]
- 170.Leaver S.G., Cui Q., Plant G.W., Arulpragasam A., Hisheh S., Verhaagen J., Harvey A.R. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13(18):1328–1341. doi: 10.1038/sj.gt.3302791. [http://dx.doi. org/10.1038/sj.gt.3302791]. [PMID: 16708079]. [DOI] [PubMed] [Google Scholar]
- 171.Yan Z., Sun X., Evans I.A., Tyler S.R., Song Y., Liu X., Sui H., Engelhardt J.F. Postentry processing of recombinant adeno-associated virus type 1 and transduction of the ferret lung are altered by a factor in airway secretions. Hum. Gene Ther. 2013;24(9):786–796. doi: 10.1089/hum.2013.137. [http://dx.doi.org/10.1089/hum.2013.137]. [PMID: 23948055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lad S.P., Peterson D.A., Bradshaw R.A., Neet K.E. Individual and combined effects of TrkA and p75NTR nerve growth factor receptors. A role for the high affinity receptor site. J. Biol. Chem. 2003;278(27):24808–24817. doi: 10.1074/jbc.M212270200. [http://dx.doi.org/10.1074/jbc. M212270200]. [PMID: 12702729]. [DOI] [PubMed] [Google Scholar]
- 173.Lebrun-Julien F., Morquette B., Douillette A., Saragovi H.U., Di Polo A. Inhibition of p75(NTR) in glia potentiates TrkA-mediated survival of injured retinal ganglion cells. Mol. Cell. Neurosci. 2009;40(4):410–420. doi: 10.1016/j.mcn.2008.12.005. [http://dx.doi.org/10.1016/j.mcn.2008.12.005]. [PMID: 19146958]. [DOI] [PubMed] [Google Scholar]
- 174.Fu Q.L., Li X., Yip H.K., Shao Z., Wu W., Mi S., So K.F. Combined effect of brain-derived neurotrophic factor and LINGO-1 fusion protein on long-term survival of retinal ganglion cells in chronic glaucoma. Neuroscience. 2009;162(2):375–382. doi: 10.1016/j.neuroscience.2009.04.075. [http:// dx.doi.org/10.1016/j.neuroscience.2009.04.075]. [PMID: 19422885]. [DOI] [PubMed] [Google Scholar]
- 175.Fu Q.L., Hu B., Li X., Shao Z., Shi J.B., Wu W., So K.F., Mi S. LINGO-1 negatively regulates TrkB phosphorylation after ocular hypertension. Eur. J. Neurosci. 2010;31(6):1091–1097. doi: 10.1111/j.1460-9568.2010.07127.x. [http:// dx.doi.org/10.1111/j.1460-9568.2010.07127.x]. [PMID: 20377621]. [DOI] [PubMed] [Google Scholar]
- 176.Pernet V., Hauswirth W.W., Di Polo A. Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. J. Neurochem. 2005;93(1):72–83. doi: 10.1111/j.1471-4159.2005.03002.x. [http://dx.doi.org/10.1111/j.1471-4159. 2005.03002.x]. [PMID: 15773907]. [DOI] [PubMed] [Google Scholar]
- 177.You Y., Gupta V.K., Li J.C., Al-Adawy N., Klistorner A., Graham S.L. FTY720 protects retinal ganglion cells in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55(5):3060–3066. doi: 10.1167/iovs.13-13262. [http://dx.doi.org/10.1167/iovs.13-13262]. [PMID: 24744204]. [DOI] [PubMed] [Google Scholar]
- 178.Nakamura K., Martin K.C., Jackson J.K., Beppu K., Woo C.W., Thiele C.J. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66(8):4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [http://dx.doi.org/10.1158/0008-5472.CAN-05-2789]. [PMID: 16618748]. [DOI] [PubMed] [Google Scholar]
- 179.Zhang L., Hu Y., Sun C.Y., Li J., Guo T., Huang J., Chu Z.B. Lentiviral shRNA silencing of BDNF inhibits in vivo multiple myeloma growth and angiogenesis via down-regulated stroma-derived VEGF expression in the bone marrow milieu. Cancer Sci. 2010;101(5):1117–1124. doi: 10.1111/j.1349-7006.2010.01515.x. [http://dx.doi.org/10.1111/j.1349-7006.2010. 01515.x]. [PMID: 20331634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sondell M., Sundler F., Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [http://dx.doi.org/10.1046/j.0953-816X.2000.01326.x]. [PMID: 11122336]. [DOI] [PubMed] [Google Scholar]
- 181.Fischer C.V., Mans V., Horn M., Naxer S., Klettner A. [DOI] [PubMed]; van Oterendorp C. The antiproliferative effect of bevacizumab on human tenon fibroblasts is not mediated by vascular endothelial growth factor inhibition. Invest. Ophthalmol. Vis. Sci. 2016;57(11):4970–4977. doi: 10.1167/iovs.16-19938. [http://dx.doi.org/10.1167/iovs.16-19938]. [PMID: 27654424]. [DOI] [PubMed] [Google Scholar]
- 182.Simha A., Braganza A., Abraham L., Samuel P., Lindsley K. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst. Rev. 2013;(10):CD007920. doi: 10.1002/14651858.CD007920.pub2. [PMID: 24089293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holmes K., Roberts O.L., Thomas A.M., Cross M.J. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 2007;19(10):2003–2012. doi: 10.1016/j.cellsig.2007.05.013. [http://dx.doi.org/10.1016/j.cellsig.2007.05.013]. [PMID: 17658244]. [DOI] [PubMed] [Google Scholar]
- 184.Martin D.F., Maguire M.G., Fine S.L., Ying G.S., Jaffe G.J., Grunwald J.E., Toth C., Redford M., Ferris F.L., III Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [http://dx.doi.org/10.1016/j.ophtha.2012.03.053]. [PMID: 22555112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bressler S.B., Qin H., Beck R.W., Chalam K.V., Kim J.E., Melia M., Wells J.A., III Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch. Ophthalmol. 2012;130(9):1153–1161. doi: 10.1001/archophthalmol.2012.1107. [http://dx.doi.org/10.1001/archophthalmol. 2012.1107]. [PMID: 22965591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Storkebaum E., Carmeliet P. VEGF: a critical player in neurodegeneration. J. Clin. Invest. 2004;113(1):14–18. doi: 10.1172/JCI200420682. [http://dx.doi. org/10.1172/JCI20682]. [PMID: 14702101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kilic U., Kilic E., Järve A., Guo Z., Spudich A., Bieber K., Barzena U., Bassetti C.L., Marti H.H., Hermann D.M. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J. Neurosci. 2006;26(48):12439–12446. doi: 10.1523/JNEUROSCI.0434-06.2006. [http://dx.doi.org/10.1523/ JNEUROSCI.0434-06.2006]. [PMID: 17135405]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dkhissi O., Chanut E., Wasowicz M., Savoldelli M., Nguyen-Legros J., Minvielle F., Versaux-Botteri C. Retinal TUNEL-positive cells and high glutamate levels in vitreous humor of mutant quail with a glaucoma-like disorder. Invest. Ophthalmol. Vis. Sci. 1999;40(5):990–995. [PMID: 10102297]. [PubMed] [Google Scholar]
- 189.Nickells R.W. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv. Ophthalmol. 1999;43(Suppl. 1):S151–S161. doi: 10.1016/s0039-6257(99)00029-6. [http://dx.doi.org/10. 1016/S0039-6257(99)00029-6]. [PMID: 10416758]. [DOI] [PubMed] [Google Scholar]
- 190.Roh M., Zhang Y., Murakami Y., Thanos A., Lee S.C., Vavvas D.G., Benowitz L.I., Miller J.W. Etanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7(7):e40065. doi: 10.1371/journal.pone.0040065. [http://dx.doi.org/10.1371/journal.pone.0040065]. [PMID: 22802951]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Levkovitch-Verbin H., Waserzoog Y., Vander S., Makarovsky D., Ilia P. Minocycline mechanism of neuroprotection involves the Bcl-2 gene family in optic nerve transection. Int. J. Neurosci. 2014;124(10):755–761. doi: 10.3109/00207454.2013.878340. [http://dx.doi.org/10.3109/00207454.2013. 878340]. [PMID: 24410139]. [DOI] [PubMed] [Google Scholar]
- 192.Huang W., Fileta J.B., Dobberfuhl A., Filippopolous T., Guo Y., Kwon G., Grosskreutz C.L. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc. Natl. Acad. Sci. USA. 2005;102(34):12242–12247. doi: 10.1073/pnas.0505138102. [http://dx.doi.org/ 10.1073/pnas.0505138102]. [PMID: 16103353]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tatton W.G., Chalmers-Redman R.M., Tatton N.A. Apoptosis and anti-apoptosis signalling in glaucomatous retinopathy. Eur. J. Ophthalmol. 2001;11(Suppl. 2):S12–S22. [PMID: 11592526]. [PubMed] [Google Scholar]
- 194.Wang W., Zhang G., Gu H., Liu Y., Lao J., Li K., Guan H. Role of CtBP2 in the Apoptosis of Retinal Ganglion Cells. Cell. Mol. Neurobiol. 2015;35(5):633–640. doi: 10.1007/s10571-015-0158-x. [http://dx.doi.org/10.1007/ s10571-015-0158-x]. [PMID: 25627828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fu P., Wu Q., Hu J., Li T., Gao F. Baclofen protects primary rat retinal ganglion cells from chemical hypoxia-induced apoptosis through the Akt and PERK pathways. Front. Cell. Neurosci. 2016;10:255. doi: 10.3389/fncel.2016.00255. [http://dx.doi.org/10.3389/fncel.2016.00255]. [PMID: 27867349]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hu Y., Park K.K., Yang L., Wei X., Yang Q., Cho K.S., Thielen P., Lee A.H., Cartoni R., Glimcher L.H., Chen D.F., He Z. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012;73(3):445–452. doi: 10.1016/j.neuron.2011.11.026. [http://dx.doi.org/10.1016/j.neuron.2011.11. 026]. [PMID: 22325198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nakano N., Ikeda H.O., Hasegawa T., Muraoka Y., Iwai S., Tsuruyama T., Nakano M., Fuchigami T., Shudo T., Kakizuka A., Yoshimura N. Neuroprotective effects of VCP modulators in mouse models of glaucoma. Heliyon. 2016;2(4):e00096. doi: 10.1016/j.heliyon.2016.e00096. [http:// dx.doi.org/10.1016/j.heliyon.2016.e00096]. [PMID: 27441270]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ha Y., Liu H., Xu Z., Yokota H., Narayanan S.P., Lemtalsi T., Smith S.B., Caldwell R.W., Caldwell R.B., Zhang W. Endoplasmic reticulum stress-regulated CXCR3 pathway mediates inflammation and neuronal injury in acute glaucoma. Cell Death Dis. 2015;6:e1900. doi: 10.1038/cddis.2015.281. [http://dx.doi.org/10.1038/cddis.2015.281]. [PMID: 26448323]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O., Görgün C.Z., Hotamisligil G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [http://dx.doi.org/10.1126/science.1128294]. [PMID: 16931765]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tejeda G.S., Díaz-Guerra M. Integral Characterization of Defective BDNF/TrkB Signalling in Neurological and Psychiatric Disorders Leads the Way to New Therapies. Int. J. Mol. Sci. 2017;18(2):E268. doi: 10.3390/ijms18020268. [http://dx.doi.org/10.3390/ijms18020268]. [PMID: 28134845]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oddone F., Roberti G., Micera A., Busanello A., Bonini S., Quaranta L., Agnifili L., Manni G. Exploring serum levels of brain derived neurotrophic factor and nerve growth factor across glaucoma stages. PLoS One. 2017;12(1):e0168565. doi: 10.1371/journal.pone.0168565. [http:// dx.doi.org/10.1371/journal.pone.0168565]. [PMID: 28068360]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sippl C., Bosserhoff A.K., Fischer D., Tamm E.R. Depletion of optineurin in RGC-5 cells derived from retinal neurons causes apoptosis and reduces the secretion of neurotrophins. Exp. Eye Res. 2011;93(5):669–680. doi: 10.1016/j.exer.2011.08.011. [http://dx.doi.org/10.1016/j.exer.2011. 08.011]. [PMID: 21896272]. [DOI] [PubMed] [Google Scholar]
- 203.Cirulli E.T., Lasseigne B.N., Petrovski S., Sapp P.C., Dion P.A., Leblond C.S., Couthouis J., Lu Y.F., Wang Q., Krueger B.J., Ren Z., Keebler J., Han Y., Levy S.E., Boone B.E., Wimbish J.R., Waite L.L., Jones A.L., Carulli J.P., Day-Williams A.G., Staropoli J.F., Xin W.W., Chesi A., Raphael A.R., McKenna-Yasek D., Cady J., Vianney de Jong J.M., Kenna K.P., Smith B.N., Topp S., Miller J., Gkazi A., Al-Chalabi A., van den Berg L.H., Veldink J., Silani V., Ticozzi N., Shaw C.E., Baloh R.H., Appel S., Simpson E., Lagier-Tourenne C., Pulst S.M., Gibson S., Trojanowski J.Q., Elman L., McCluskey L., Grossman M., Shneider N.A., Chung W.K., Ravits J.M., Glass J.D., Sims K.B., Van Deerlin V.M., Maniatis T., Hayes S.D., Ordureau A., Swarup S., Landers J., Baas F., Allen A.S., Bedlack R.S., Harper J.W., Gitler A.D., Rouleau G.A., Brown R., Harms M.B., Cooper G.M., Harris T., Myers R.M., Goldstein D.B., Goldstein D.B. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347(6229):1436–1441. doi: 10.1126/science.aaa3650. [http://dx.doi.org/10.1126/science.aaa3650]. [PMID: 25700176]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Korac J., Schaeffer V., Kovacevic I., Clement A.M., Jungblut B., Behl C., Terzic J., Dikic I. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J. Cell Sci. 2013;126(Pt 2):580–592. doi: 10.1242/jcs.114926. [http://dx.doi.org/10.1242/jcs. 114926]. [PMID: 23178947]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ha T., Moon K.H., Dai L., Hatakeyama J., Yoon K., Park H.S., Kong Y.Y., Shimamura K., Kim J.W. The retinal pigment epithelium is a notch signaling niche in the mouse retina. Cell Reports. 2017;19(2):351–363. doi: 10.1016/j.celrep.2017.03.040. [http://dx.doi.org/10.1016/j.celrep.2017.03. 040]. [PMID: 28402857]. [DOI] [PubMed] [Google Scholar]
- 206.Shen X., Ying H., Qiu Y., Park J.S., Shyam R., Chi Z.L., Iwata T., Yue B.Y. Processing of optineurin in neuronal cells. J. Biol. Chem. 2011;286(5):3618–3629. doi: 10.1074/jbc.M110.175810. [http://dx.doi.org/10.1074/ jbc.M110.175810]. [PMID: 21059646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Koga T., Shen X., Park J.S., Qiu Y., Park B.C., Shyam R., Yue B.Y. Differential effects of myocilin and optineurin, two glaucoma genes, on neurite outgrowth. Am. J. Pathol. 2010;176(1):343–352. doi: 10.2353/ajpath.2010.090194. [http://dx.doi.org/10.2353/ajpath.2010.090194]. [PMID: 19959812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qiu Y., Shen X., Shyam R., Yue B.Y., Ying H. Cellular processing of myocilin. PLoS One. 2014;9(4):e92845. doi: 10.1371/journal.pone.0092845. [http://dx. doi.org/10.1371/journal.pone.0092845]. [PMID: 24732711]. [DOI] [PMC free article] [PubMed] [Google Scholar]