Abstract
Background:
Primary open-angle glaucoma (POAG) is a multifactorial pathology
involving a variety of pathogenic mechanisms, including oxidative/nitrosative stress. This latter is the consequence of the imbalance between excessive formation and insufficient protection against reactive oxygen/nitrogen species.
Objective:
Our main goal is to gather molecular information to better managing pathologic variants that may determine the individual susceptibility to oxidative/nitrosative stress (OS/NS) and POAG.
Method:
An extensive search of the scientific literature was conducted using PUBMED, the Web of Science, the Cochrane Library, and other references on the topic of POAG and OS/NS from human and animal model studies published between 2010 and 2017. Finally, 152 works containing relevant information that may help understanding the role of antioxidants, essential fatty acids, natural compounds and other similar strategies for counteracting OS/NS in POAG were considered.
Results:
A wide variety of studies have proven that antioxidants, among them vitamins B3, C and E, Coenzyme Q10 or melatonin, ω-3/ω-6 fatty acids and other natural compounds (such as coffee, green tea, bear bile, gingko biloba, coleus, tropical fruits, etc.,) may help regulating the intraocular pressure as well as protecting the retinal neurons against OS/NS in POAG.
Conclusion:
Based on the impact of antioxidants and ω-3/ω-6 fatty acids at the molecular level in the glaucomatous anterior and posterior eye segments, further studies are needed by integrating all issues involved in glaucoma pathogenesis, endogenous and exogenous risk factors and their interactions that will allow us to reach newer effective biotherapies for preventing glaucomatous irreversible blindness.
Keywords: Glaucoma, oxidative stress, nitrosative stress, antioxidants, essential fatty acids, natural compounds
1. INTRODUCTION
Glaucoma is the leading cause of visual impairment and irreversible blindness worldwide. Throughout the clinical course, the disease is characterized by a progressive damage and death of the retinal ganglion cells (RGCs) and optic fibers [1]. Neurodegeneration process extends beyond the retina and optic nerve into the visual pathway [2]. Among the pathogenic mechanisms of glaucoma oxidative stress (OS) [3], inflammation [4], excitotoxicity [5], vascular impairment and hypoxia [6], glial dysfunction [7] and altered axonal transport [8] are the most recognized processes.
Glaucoma-related cell death occurs by means of apoptosis, and apoptosis is triggered by oxidative stress via (a) mitochondrial damage, (b) inflammation, (c) endothelial dysregulation and dysfunction, and (d) hypoxia. It has been recently reported that proteomics of the aqueous humor is significantly altered in primary open-angle glaucoma (POAG) as a result of oxidation-induced trabecular damage [9]. Those proteins whose aqueous humor levels are increased in this disease are biomarkers of trabecular meshwork damage. Their diffusion from the anterior to the posterior eye segments may be pivotal in the cascade of events triggering apoptosis in the innermost retinal layers.
Current POAG treatment is quite limited. In spite of lowering the intraocular pressure (IOP) the process continues to worsen, and in most cases, it is impossible to delay glaucoma progression. It is imperative to better identify the pathogenic mechanisms [10], to discover IOP-independent risk factors as well as to improve diagnostic techniques (including the multimodal imaging) and to develop more effective therapies.
Hopefully, the issue is moving ahead [11]. New therapeutic strategies are arising, including the rationale use of antioxidants and essential fatty acids [12], distinct neurotrophins [13], adenosine receptor antagonists [14], Rho family GTPases inhibitors [15], as well as sophisticated stem cell/gene therapies [16-18].
Taking into consideration that OS has been largely involved in POAG pathogenesis the scientific literature has evaluated the role of antioxidants in glaucoma with controversial results. Some authors described that a diet rich in antioxidant-containing fruits and vegetables was capable to protect the eyes against glaucoma development and progression [19]. Micronutrient supplementation enhances antioxidant defense and healthy eyes and subsequently may help preventing or delaying glaucoma progression, as previously reported [20-22]. Nevertheless, other authors did not find any benefit from the oral supplementation with antioxidants in POAG patients through two-year of follow-up [21]. Till today, applicability of the nutritional and oral supplementation with antioxidants and essential fatty acids upon the glaucoma practice is to a greater extent controversial. A better explanation of the pros and cons of this type of adjunctive interventions in POAG therapy is required for optimal utilization of these means.
Adapted from the Guidelines of the European Glaucoma Society [31] [10] and from van der Valk et al [34] [12]. IOP: intraocular pressure. *Drugs are listed based on the year of introduction in the market. ** Without benzalkonium chloride. (+) Bimatoprost 0.03%.
In this review, we will take a look to the current concepts in oxidative/nitrosative stress, the role of reactive oxygen species (ROS)/reactive nitrogen species (RNS) and its downstream effectors in the initiation and progression of POAG, and the experimental models and clinical trials with antioxidants, essential fatty acids, natural compounds and other substances than can counteract the generation of ROS/NOS in glaucoma. Our main goal is to provide the readers a comprehensive document that assists in achieving their personal challenges for better understanding glaucoma pathogenesis and therapy.
2. CURRENT CONCEPTS IN GLAUCOMA THERAPEUTICS
Glaucoma is characterized by the slow, progressive degeneration of the RGCs and optic nerve axons. The glaucoma-induced visual loss is irreversible, underscoring the importance of early diagnosis and treatment [24-26]. Among the recognized risk factors for glaucoma, the main is the elevated IOP [27, 28]. Reduction of IOP is today the only proven method to treat glaucoma [29], as reflected in several multicenter clinical trials that have shown the benefit of IOP loweing in preventing glaucoma, as well as slowing disease progression. [26, 28, 30-32]. Treatment options for glaucoma include medications, laser therapy and incisional surgery, with an extraordinary development of microsurgical devices that have arisen through the past decade [33, 34]. For many years hypotensive medical therapy has traditionally been the modality of glaucoma treatment. Up today still remains the gold standard for the onset of glaucoma therapy and even for managing the different stages of disease. Currently,there are two approaches for effective medical IOP reduction: by decreasing the aqueous humor production with the use of β-blockers (inhibition of the beta-mediated stimulation of Na+/K+-ATPase), carbonic anhydrase inhibitors (CAI) and/or sympathomimetic drugs (activation of the α-mediated inhibition of Na+/K+-ATPase), and by increasing the outflow aqueous humor, with the use of cholinergic/parasympathomimetic drugs and prostaglandin analogues (PGAs) (trabecular meshwork: TM), sympathomimetic drugs (uveoscleral outflow) and/or PGAs (uveoscleral outflow) [35-37].
However, some controversy exists as to the degree of IOP reduction that can be achieved with any of these hypotensive drugs (Table 1). The results of a meta-analysis showed that the highest IOP reduction ranged from 33% (bimatoprost) to 17% (brinzolamide) [38]. According to the European Gaucoma Society (EGS) guidelines, PGA, β-blockers, α-2 agonists, and topical CAI are first choice agents [35]. In general, PGAs are the first-line of medical therapy, for being most effective in lowering IOP [39]. Other hypotensive eye drops are used as second-line agents or in cases of intolerance or side-effects to PGAs [40]. Some other new hypotensive agents are actually being investigated (Table 2) [41-44].
Table 1.
Overview of the main features of some of the most prescribed topical anti-glaucoma medications available in the market.
| Generic Name* | % IOP Reduction from Baseline |
Year of
Introduction |
Instillation
Frequency |
Preservative Free | Wash-out Time | |
|---|---|---|---|---|---|---|
| Peak | Trough | |||||
| Pilocarpine | 25 | 20 | 1875 | 3-4 times daily | No | 1 week |
| Timolol | 27 | 26 | 1978 | 1-2 times daily | Yes/ No | 2-5 weeks |
| Dorzolamide | 20 | 17 | 1994 | 2-3 times daily | Yes | 1 week |
| Brimonidine | 25 | 18 | 1996 | 2-3 times daily | No | 1-3 weeks |
| Latanoprost | 31 | 28 | 1996 | Once daily | No/ Yes | 4-6 weeks |
| Brinzolamide | 20 | 17 | 1998 | 2-3 times daily | No | 1 week |
| Unoprostone | 20 | 15 | 2000 | 2 times daily | No | 4-6 weeks |
| Travoprost | 31 | 29 | 2001 | Once daily | No ** | 4-6 weeks |
| Bimatoprost (+) | 33 | 28 | 2001 | Once daily | No/ Yes | 4-6 weeks |
| Tafluprost | 27 | 20 | 2008 | Once daily | Yes | 4-6 weeks |
Table 2.
Update on the new strategies for glaucoma therapy based on the distinct pathogenic mechanisms.
| Mechanisms | Substance | Effect |
|---|---|---|
|
Renin-Angiotensin System ACE inhibitors |
Enalaprilat, Fosinopril, Perindopril, Ramiprilat, | Ocular hypotensive effect |
| Calcium Channels Ion channel blockers/inhibitors | Betaxolol, Diltiazem, flunarizine, Iganidipine, lomerizine, Nifedipine, Nimodipine, Nilvadipine Verapamil, Nimodipine, | Improving ocular blood perfusion, neuroprotection and IOP lowering. |
| Cell kinases Cell cyclin-dependent kinase inhibitor | Roscovitine | Cell contraction-relaxation in trabecular meshwork |
| Cell kinases (Rho family) | AMA0076 AR-13324 K-115 PG324 Y-39983 RKI-983 H-1152 |
Modulating signal transduction pathways and actin cytoskeleton function and cell motility of trabecular meshwork, canal of Schelmm and ciliary muscle cells |
| Aqueous humor homeostasis. PGAs EP2 receptor agonist | SAR366234 | EP2 receptor agonist. Lowering IOP |
| Aqueous humor homeostasis PGAs Dual receptor affinity for FP and EP3 receptors, | ONO 9054 | Lower and more sustained IOP reduction |
| Energy supplier Adenosine A2a receptor agonist | OPA-6566 | Increase aqueous outflow facility by shrinking TM cell volume |
| Aqueous humor homeosthasis TM cells contractility Actin modulator | Latrunculin B (INS115644) marine macrolide | Improves TM outflow facility by inhibiting the assembly of actin microfilaments in cell cytoplasm |
Abbreviations: ACE: angiotensin converting enzyme; IOP: Intraocular pressure; PGAs: prostaglandin analogs; EP2: prostaglandin E2; EP3: prostaglandin E3; FP: prostaglandin F; TM: trabecular meshwork.
Glaucoma, a long-lasting disease, requires reinforcing the treatment regimen by controlling the compliance with the prescribed medication. When medical treatment does not achieve adequate IOP reduction, laser or incisional surgeries are then indicated. Types of laser surgery used to treat glaucoma include argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT) [45-47]. Both techniques induce biological changes in the TM resulting in increased aqueous outflow and IOP lowering. However, in the majority of patients, the reduced IOP effect decreases gradually over time with a failure rate of about 10% per year [47]. Most common surgical procedures to IOP lowering include trabeculectomy and nonpenetrating deep sclerectomy [48]. Alternatives to these procedures have recently been introduced, such as the micro-invasive glaucoma surgery (MIGS) and others that are currently under research [49-51]. It has to be considered that some new surgical devices and maneuvers are succesfully been used at the time of cataract surgery which constitutes itself an effective procedure for glaucoma treatment.
Clearly, elevated IOP plays a major role in glaucomatous RGCs damage, but therapeutic control of IOP in many patients is not sufficient to improve the visual function and to arrest disease progression [52]. Besides, glaucomatous changes have been observed in individuals with normal IOP. This suggests a critical role of other factors and mechanisms in the initiation/progression of glaucomatous changes. Since visual damage in glaucoma is ultimately induced by the visual field loss resulting from optic nerve degeneration, the possibility remains that neuroprotective/neuroregeneration/neuromodulation strategies could be excellent efficacious means to slow or even stop glaucoma progression [53]. Among others, possible future antiglaucoma therapeutic options are: glutamate excitotoxicity inhibitors, nitric oxide synthase inhibitors, blood flow enhancers, calcium/sodium channel blockers, neurotrophins, antioxidants, vasotonic agents, genetic modulators (micro RNAs), gene therapy, as well as regenerative stem cell therapy. Some of them are reflected in the Table 2 [2, 16, 53, 54].
3. OXIDATIVE AND NITROSATIVE STRESS IN GLAUCOMA RESEARCH AND THERAPEUTICS
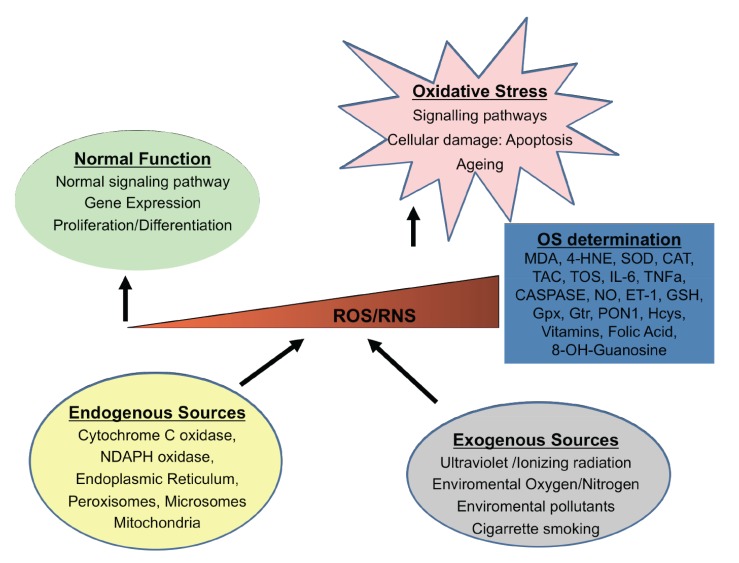
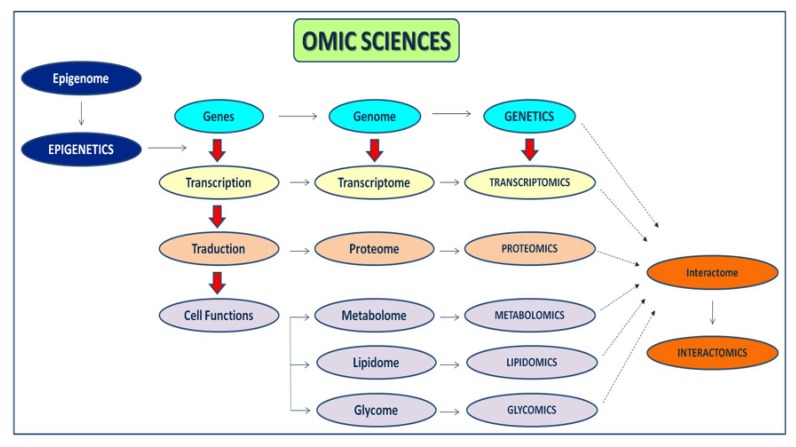
During the past three decades, major research has demonstrated the mechanisms by which the OS, an imbalance in the redox status of pro-oxidant/antioxidant reactions in cells, can cause cellular damage by peroxidation of lipids, proteins, carbohydrates, nucleic acids and bases. However, the exact signalling pathways regulated by ROS are not clear. There are both endogenous-exogenous ROS sources. Inside the cells, mitochondria, endoplasmic reticulum, and peroxysomes can generate ROS (Fig. 1). Also, some enzymes produce ROS from their reaction cycles [55]. External conditions can also generate ROS [56]. In addition to OS, the NS produced by the augmented synthesis of RNS can also induce cell damage and death [57]. The RNS are produced from nitric oxide (NO), by reacting with the superoxide anion, generating peroxynitrite and other highly reactive molecules. The OS/NS has been implicated in ophthalmic processes [58]. Searching for new therapeutic glaucoma strategies a big amount of information emerged from experimental investigations, involving the pathogenic mechanisms, risk factors and potential endogenous-exogenous processes involving the disease. This section looks into the current knowledge on the role of OS/NS stress and the possible pharmacological approaches for glaucoma treatment. As said before, OS is the result of the imbalance between the ROS/RNS formation and the counteracting activity of antioxidant defenses. Regarding glaucoma pathogenesis many questions remain a mystery, but a better knowledge of the cellular and molecular events occurring in the initiation and progression can help improve patient management [59-62]. It has been widely demonstrated that altered redox processes are linked to inflammation [4, 7, 63], apoptosis [63], excitotoxicity [5, 64], vascular disorders and/or glial dysfunction [6, 7, 65, 66]. In this context, it has been recently reported some presumptive biomarkers of all these pathogenic mechanisms and clinical endpoints (including the progression rates) that are fundamental to help managing the affected individuals [67-73] (Table 3). In spite of this, there is a lack of global standardization in this type of studies (design, sample size, analytical techniques, statistics, reproducibility etc.) to improve proper identification of glaucoma biomarkers. In this context, the new disciplines named “OMICs” have arisen in the past years to integrate large amounts of data pertaining to the analysis of specific entire sets of molecules present in a cell, tissue, organ, or the complete organism, with a broad range of biomedical applications (Fig. 2).
Fig. (1).
Endogenous and exogenous sources capable to produce cellular response to reactive oxygen/nitrogen species (ROS/RNS). The level of reactive species is regulated by different antioxidant defense mechanisms. When there are low/normal levels of ROS/RNS the physiological functions are maintained. However when an excess of them exists, an oxidative stress occurs. (MDA: malondialdehyde; 4-HNE: 4 hydroxynonenal; SOD: superoxide dismutase; CAT: catalase; TAC: total antioxidant capacity; IL-6: interleuquin-6; TNFa: tumor necrosis factor alpha; NO: nitric oxide; ET-1: endothelin-1; GSH: glutathione; Gpx: glutathione peroxidase; Gtr: glutathione S-transferase; Hcys: homocysteine).
Table 3.
Reactive oxygen and nitrogen species.
| Reactive Oxygen Species (ROS) | Reactive Nitrogen Species (RNS) |
|---|---|
| Singlet oxygen – 1O2 | Nitric oxide – NO· |
| Superoxide anion – O2·- | Nitric dioxide – NO2· |
| Hydroxyl radical – OH·- | Peroxynitrite – ONOO·- |
| Hydrogen peroxide – H2O2 | |
| Hydroperoxyl radical – ROOH· |
Fig. (2).
Flowchart showing the most relevant OMIC sciences.
Moreover, the above endogenous processes can be aggravated by the interaction of various exogenous agents, such as ultraviolet radiation, toxic substances, cigarrette smoking and pollutants [74] (see the Fig. 1). Moreover, animal and in vitro models have helped researchers to deep into the knowledge of the mechanisms that affect different cellular types involved in glaucoma and that will be explain in the next sections.
Biomarkers and surrogate end points of OS and its downstream effectors have been extensively studied. Different molecules have been assayed to evaluate the OS in glaucomatous patients and animal/in vitro models using several ophthalmic samples (aqueous humor, vitreous body, human tears). These molecules can be classified into two groups: pro-oxidants, and antioxidants, as exposed below (Table 4).
Table 4.
Oxidative stress-related studies in glaucoma.
| Study | Sample | Significant Findings | Refs. |
|---|---|---|---|
| OS | Aqueous humor (human) | Higher oxidative status and lower antioxidant activity in POAG |
Zanón-Moreno et al. 2008 [61] |
| Biomarkers of OS, immune response and apoptosis | ROS, immune inflammatory response mediators, and apoptogenic molecules are engaged in glaucoma disease |
Pinazo-Durán et al. 2013 [69] |
|
| Polymorphisms in several genes with biomarkers in POAG | Plasma (human) | Novel association: rs737723 polymorphism (SEC14L2/TAP) from GPX4 and higher POAG risk |
Zanón-Moreno et al. 2013 [91] |
| Association of TAC with type and severity | Plasma (humor) | TAC levels are lower in glaucoma (more so in POAG and PEG than PACG) |
Mousa et al. 2015 [92] |
| OS markers evaluation | Aqueous humor (human) | Increased oxidative stress may play a role in the pathogenesis of both POAG and PACG |
Goyal et al. 2014 [87] |
| Association of clinical indices of PACG with TAC | Plasma (human) | The authors demonstrated an inverse correlation of TAC level with IOP |
Abu-Amero at al. 2014 [93] |
| Evaluation of GS, NOS, SOD and GST | Aqueous humor (human) | GS, NOS2, SOD and GST may be useful oxidative markers in glaucoma |
Bagnis et al. 2012 [89] |
| OS and antioxidants | Systematic Review and Meta-Analysis | The increase of some antioxidant markers could be a protective response of the eye against oxidative stress. |
Benoist dÁzy et al. 2016 [75] |
| OS markers | Blood and aqueous humor (human) | Oxidative stress and decreased antioxidant defenses are involved in POAG. |
Nucci et al. 2013 [76] |
| OS | Serum (human) | Involvement of oxidative stress in PACG. Identify ischemia-modified albumin as a new biomarker to asses oxidative stress in PACG |
Chang et al. 2011 [77] |
| OS markers (MDA, 8-OHdG and PON1) | Blood samples (human) |
Higher MDA and 8-OHdG levels may be correlated with decreased PON1 activity in POAG |
Mumcu et al. 2016 [82] |
| Apoptosis markers (PARP1 and OGG1) | RNA from Blood (human) | Results suggest that oxidative stress-induced DNA damage is associated with POAG. This increase could be by the decreased expression of DNA repair enzymes |
Mohanty et al. 2017 [83] |
| NS | Aqueous humor (human) | Involvement of NO in POAG |
Zanón-Moreno et al. 2008 [85] |
| Biochemical indicators of lipid peroxidation | Serum and tear (human) | In POAG there are: free radical oxidation, suppression of antioxidant system defense and endothelial dysfunction |
Openkova et al. 2013 [86] |
| OS | Red Blood Cells (human) | An oxidative disorder was observed in POAG: CAT and GPX upregulation and higher MDA levels |
Rokicki et al. 2016 [90] |
Abbreviations: OS: Oxidative stress; NS: Nitrosative stress; POAG: primary open-angle glaucoma; ROS: reactive oxygen species; GPX: glutathione peroxidase; TAC: total antioxydant capacity; PEG: pseudoexfoliation glaucoma; PACG: primary angle closure glaucoma; IOP: intraocular pressure; GS: glutamine synthase; NOS: nitric oxide synthase; SOD: superoxide dismutase; GST: glutathione transferase; MDA: malondialdehyde; 8-OHdG: 8-hydroxy-2' –deoxyguanosine; PON: paraoxonase PARP1: Poly(ADP-ribose) polymerase; OGG1: 8-Oxoguanine DNA glycosylase NO: nitric oxide.
3.1. Pro-oxidant Biomarkers
Malondialdehyde (MDA) is the most studied lipid peroxidation by product in human samples and animal models. Many studies have shown significantly higher MDA levels in glaucomatous subjects vs controls. Benoist d'Azy et al. [75] carried out a meta-analysis reporting higher serum and aqueous humor MDA levels in POAG patiens. Nucci et al., [76] and Chang et al., [77] found increased MDA levels in serum and/or aqueous humor of POAG patients as compared to the controls. Our group also reported higher MDA concentration in human aqueous humor of glaucomatous individuals, as compared to a comparative group of patients operated from cataracts [3, 58, 61, 69, 78, 79]. Other OS by products have also been identified in glaucomatous eyes, such as 4-hydroxynonenal (4-HNE) or 8-hydroxy-2′-deoxyguanosine (8-OHdG). Regarding these latter metabolites, 4-HNE (or 4-hydroxy-2-nonenal; C9H16O2, is a lipid peroxidation cell by product and it can be found at higher concentration during oxidative stress processes. In fact, 4-HNE plays a key role in cell signal transduction. The 8-OHdG (or 8-oxo-7,8-dihydro-2' -deoxyguanosine) is one of the most abundant forms of free radical-induced oxidative damage to both the nuclear and mitochondrial DNA. It has been widely utilized as a biomarker for oxidative stress. Regarding the role of these metabolites in glaucoma, Malone et al. [80] studied the dose and time-dependent effects of 4-HNE on the viability of primary cultures of human ONH astrocytes, concluding that 4-HNE is neurotoxic and that astrocytes itself can counteract these effects, protecting the ONH from glaucomatous injury. Also Chang et al., [77] evaluated the serum 4-HNE concentration POAG patients, reporting noticeably higher levels in glaucoma samples than in the healthy ones (with no statistically significant differences). The 8-OHdG is also a marker of oxidative DNA damage and its concentration increases with age in a variety of mammalian tissues [81]. Recent studies have shown significantly higher 8-OHdG levels in POAG individuals vs controls [82, 83]. The nitrosative cell damage has also been studied in relation to glaucoma. Luthra et al., [84] demonstrated that nitrotyrosine (NT) can be considered a marker for peroxynitrite-mediated oxidative injury in glaucoma. Our research group carried out a case-control study in aqueous humor of POAG patients and a comparative group of cataract subjects, with significantly increased NO concentration in the glaucomatous patients [85]. Openkova et al., [86] found similar results, reporting an NO increase and its metabolites in blood serum and lacrimal fluid of POAG patients. These data support NO as a potential biomarker for identifying those individuals at risk of glaucoma progression and visual loss.
3.2. Antioxidant Biomarkers
Among the antioxidant molecules the endogenous-exogenous sources of these compounds must be considered. Endogenous antioxidants are mainly enzymes with antioxidant activity, such as superoxide dismutase (SOD), catalase (CAT), and both glutathione peroxidase (GPx) and S- transferase (GS-T). Also, a wide spectrum of non-enzymatic molecules (glutathione: GSH, coenzyme Q-10; CoQ-10 and co-factors) are enclosed within this type of antioxidants. Exogenous antioxidants are mainly the vitamins E and C, β-carotene and flavonoids, as well as minerals involved in the corresponding redox reactions (selenium, copper, zinc, manganese and iron). Goyal et al. [87] found increased SOD and GPx activity and decreased vitamin C and E in POAG patients. In this context, melatonin (a pineal hormone) has a potent antioxidant function acting as effective free radical scavenger. It has been shown that metalotin is capable to protect the ocular tissues from oxidative stress, and ultimately metatolin analogs have been proposed as promising candidates for glaucoma theraphy [88].
Bagnis et al. [89] observed a reduction in the expression of SOD and GS-T in the aqueous humor of POAG patients vs controls. Rokicki et al., [90] showed significant increased GPx and CAT activities in red blood cells of POAG patients vs controls. Our group analysed the concentration of vitamins C and E and GPx in serum of POAG patients. Significantly lower levels of these antioxidants were seen in the glaucomatous eyes vs the controls [91]. Independently of the source and type of antioxidant, one of the most generalized biomarkers is the total antioxidant capacity (TAC), reflecting the complete antioxidant status in biological samples. In this regard, our group incorporated the TAC determination throughout distinct glaucoma studies, concluding that TAC was significantly reduced in glaucomatous patients [3, 58, 51, 69, 76, 78]. Also Mousa et al. [92] and Abu-Amero et al., [93] found similar results by comparing the TAC activity in different glaucoma types, as well as in relation to glaucoma severity. Regarding the NS Yokota et al., [94] reported that molecular hydrogen importantly reduces the cellular peroxynitrite concentration, suggesting that it may be a useful and effective molecule to develop new therapeutic drugs for glaucoma. Moreover, it has been sugeested that lower systemic antioxidant capacity measured by ferric-reducing activity is associated with more severe visual field damage in POAG, partly explaining its roles in IOP elevation [95].
For summarizing, the antioxidants counteract the ROS/RNS generation, constituting the first natural barrier to fight against oxidative attack in glaucoma disease. Specific animal models and clinical trials in this topic will be discussed in the following sections.
4. EXPERIMENTAL GLAUCOMA MODELS OF ANTIOXIDANTS, ESSENTIAL FATTY ACIDS AND OTHER NATURAL PRODUCTS
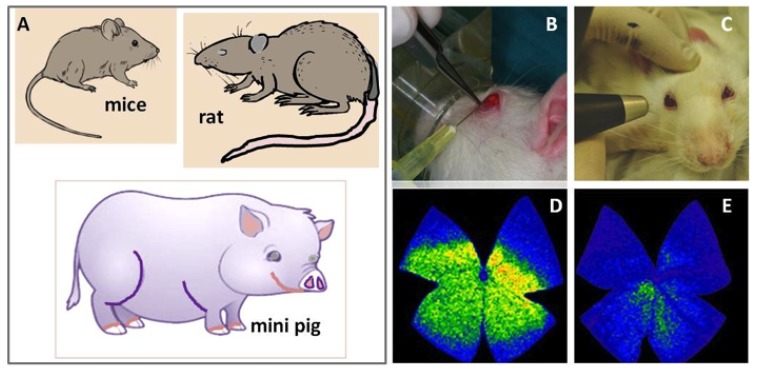
Animal models are extremely useful to avoid interaction of confounding factors that are impossible to control in humans. With these models of elevated IOP, unique and powerful tools to further explore the pathogenic mechanisms of glaucomatous RGC and optic fiber damage and death have been getting into the scientific landscape through the latter decades. Experimental glaucoma can be induced by a variety of techniques with the main goal of elevating the IOP, leading to RGCs and axonal damage (Fig. 3). The most utilized experimental glaucoma models have been set up in rats and rabbits by means of the cauterization of the limbal veins [96, 97], the hyaluronic acid intracameral seriate injections (see the Fig. 3) [98, 99] the microbead/viscoelastic intracameral injection [100], and the magnetic latex microspheres (Polybead) [101]. Other researchers have used transgenic mice, such as the mouse glaucoma model DBA/2J (D2), which spontaneously develops elevated IOP [102]. Other species have also been used for excellent glaucoma models [103]. It has been demonstrated that OS leads to lipid peroxidation [104, 105] as well as to conformational changes of proteins [106] and nucleic acids damage with the activation of apoptotic signals causing RGCs death [107]. In this scenario, several authors that have supported the role of OS in glaucoma pathogenesis, have also investigated the protective effects of antioxidants, omega 3 fatty acids and a wide variety of natural compounds in experimental models of glaucoma, with the aim of offering alternative/adjunctive means for preventing the irreversible loss of RGCs and optic fibers in glaucoma. The main contributions are set out below.
Fig. (3).
Experimental glaucoma models. A) The most usual animal models utilized in glaucoma research have been set up in mice, rat and mini pig. B) Experimental rat model of chronic glaucoma by seriate intracameral injections of sodium hyaluronate in the left eye vs balanced salt solution injection in the sham operated right eye [92]. C) rat ocular tonometry. D) Whole mount retinal staining of the RGCs in the non glaucomatous rat eye, and E) showing the significant decrease in RGCs density in the induced glaucoma eye (left).
4.1. Antioxidants
From a theoretical viewpoint ROS/NOS inhibition, together with the strengthening of antioxidant defences can help improve cell health and survival. In a rat glaucoma model, vitamin E-deficiency induced greater lipid peroxidation and subsequently increased RGCs death as compared to normal feed rats [108] Moreover, other researchers have developed a contact lens with extra-vitamin E loading, demonstrating the effectivity of this technique for providing antihypertensive combination therapy (timolol + dorzolamide) by contact lenses, [109]. Mice genetically predisposed to glaucoma receiving vitamin B3 in the drinking water showed
signs compatible with disease prevention. In this mice glaucoma model, vitamin B3 averted early signs of glaucoma in young mice, as well as halted further glaucoma development in aged mice [110]. Xu et al., [111] demonstrated that vit C can help managing glaucoma progression. Ammar et al., [112] using primary porcine TM cells, examined the effect of the pretreatment with resveratrol, urate, ascorbate, reduced glutathione (rGSH), or ρ-coumarate after H2O2 exposition, concluding that these antioxidants protected TM cells from H2O2-induced damage. Coenzyme Q10 (CoQ10), a cofactor of the electron transport chain, is known to protect neurons against oxidative injury. Major function of CoQ10 is to stabilize the mitochondrial membrane potential, supporting ATP as well as to inhibit ROS formation. Nucci et al., [113], Russo et al., [114] and Nakajima et al., [115] among others, reported that CoQ10 was capable to prevent RGCs damage from elevated IOP. Moreover, in a mouse glaucoma model supplemented with CoQ10, Lee et al., [116] reported a significant glutamate excitotoxicity inhibition. Analyzing peroxide stress assays in human TM cells, Famili et al., [117] observed that samples receiving pre- and co-treatment of ethyl piruvate (a pyruvic acid ethyl ester that is utilized as a food flavoring compound with anti-inflammatory properties) showed significantly higher TM cell survival respect the non treated samples. The effects of tempol, a multifunctional antioxidant were analyzed in a rat glaucoma model. Data showed that interleukins (IL) -1, -2, interferon-γ, tumor necrosis factor-α, and NF-κB significantly descreased in the glaucoma tempol-treated eyes [99]. By means of the transgenic mouse model of glaucoma, DBA/2J (D2), and “in vitro” RGC cultures, Kim et al., [102] reported that OS enhanced mitochondrial damage and loss by increasing dynamin-related protein 1 (Drp1) in the retina of DBA/2J mice. The authors concluded that inhibiting Drp1 substantial rescue of the RGCs and optic axons by preserving mitochondrial function and integrity was seen. All these experiments have been summarized in the Table 5. Moreover, it has recently reported the hypotensive effects of melatonin as well as of 5-MCA-NAT, a putative melatonin MT3 receptor agonist, which effectively reduced IOP in a glaucoma model [118]. Also Yang et al, [119] using Tempol in a SOD1(-/-) mice model, showed that proinflammatory cytokines such as the IL-1, IL-2, IFN-γ, and TNF-α, exhibited more than 2-fold decreased titers in the Tempol-treated ocular hypertensive eyes. Evenmore, antioxidant treatment also resulted in a prominent decrease in NF-κB activation in the ocular hypertensive retina and optic nerve.
Table 5.
Animal models on the effects of antioxidants, essential fatty acids and natural compounds in glaucoma.
| Study (Assayed Substance) | Animal Model | References |
|---|---|---|
| Antioxidants: Vit E | Rat | Ku et al. 2010 [108] |
| Antioxidants: Vit E | Mouse | Hsu et al. 2015 [109] |
| Antioxidants: Vit C | Pig | Xu et al., 2014 [111] |
| Antioxidants: Resveratrol, Urate, Ascorbate, GSH, P-Coumarate | Pig | Amman et al,. 2012 [112] |
| Antioxidants: Co Q-10 | Rat |
Nucci et al., 2007 [113] Russo et al., 2008 [114] Nakajima et al., 2008 [115] |
| Antioxidants: Co Q-10 | Mouse | Lee et al. 2014 [116] |
| Antioxidants: Tempol | Rat | Yang et al., 2016 [100] |
| Essential Fatty Acids: ω-3 | Rat | Nguyen et al., 2007 [121] |
| Essential Fatty Acids: ω-3, Vit A | Rat | Schnebelen et al., 2009 [124] |
| Essential Fatty Acids: ω-3/ ω-6 | Rat | Huang et al., 2011 [125] |
| Natural compounds: epigallocatechin (green tea) | Rat | Osborne 2008 [128] |
| Natural compounds: gallocatechin and epigallocatechin (dark green tea) | Rat | Chu et al., 2010 [129] |
| Natural compounds: forskolin, homotaurine, L-carnosine | Rat | Russo et al., 2015 [130] |
| Natural compounds: intravitreal (AAPH) | Mouse | Yokohama et al., 2014 [131] |
| Natural compounds: Brimonidine | Rat | Lee et al., 2012 [132] |
| Natural compounds: TUDCA | Rat | Boatright et al., 2006 [113] |
| Natural compounds: Saffranal (saffron) and bear bile | Rat | Fernández-Sánchez et al., 2011 [134], 2005 [135] |
| Natural compounds: Ginkho Biloba | Rat | Eckert et al., 2005 [136] Cybulska-Heinrich et al, 2012 [137] |
| Antioxidant: Melatonin and 5-MCA-NAT | Mouse | Martínez-Águila et al., 2016 [118] |
| Antioxidant: Tempol | Mouse | Yang et al., 2016 [119] |
Abbreviations: Vit: vitamin; GSH: glutathione; Co Q-10: Coenzyme Q-10; ω-3 / ω-6: omega-3/omega-6 fatty acids; AAPH: 2,2'-azobis (2-amidinopropane) dihydrochloride; TUDCA: Taurousodeoxycholic acid; 5-MCA-NAT: 5-Methoxycarbonylamino-N-Acetyltryptamine.
4.2. Essential Fatty Acids
Essential fatty acids, omega (ω)-6 and ω-3 are important in biomedical research because their anti-inflammatory, anti-angiogenic, antithrombotic, hypolipidemic, and vasodilatory functions [120] It has also been suggested that excessive availability of ω-6 fatty acids or a dysbalance ω-3/ω-6 ratio may induce inflammatory, metabolic, cardiovascular, cerebrovascular, or autoimmune diseases, as well as cancer, neurodegenerative disorders or ageing. Studies involving essential fatty acids in experimental glaucoma model are less abundant. Nguyen et al., [121] demonstrated that dietary ω-3 reduces IOP with age in response to an increased outflow facility, resulting from an increase in docosanoids availability in rats. In relation to the beneficial effects of ω-3/ω-6 fatty acids on peroxide-mediated OS responses, Tourtas et al., [122] studied changes of mitochondrial activity, proliferation, heat shock proteins, extracellular matrix components, and inflammatory markers in hTM cells, concluding that ω-3 appears to be beneficial respect to prophylactic intake for glaucoma prevention. Inman et al., [123] demonstrated that the dietary administration of the antioxidant α-lipoic acid (ALA) to DBA/2J mice resulted in a significant decreased in RGCs death and dysfunction that was measured in specific genes and proteins related to OS. In the same research line, Schebelen et al., [124] reported that in a rat glaucoma model the dietary intervention with both ω-6 and ω-3 is better than only single supplements for protecting against the retinal damage induced by IOP elevation. In addition, Huang et al., [125] published that oral supplementation with cod liver oil containing high doses of DHA to rats exerts a protective effect against glaucomatous damage. In this context, to better understand the tolerability of DHA eye injections, Dolz-Marco et al., [126] indicated that intravitreal DHA is safe in the albino rabbit model up to the maximum tolerated dose of 25 µg/50 µl. These data can be extrapolated to treatment, alone or in combination, of different retinal diseases including glaucoma.
4.3. Natural Substances
Natural substances including polyphenolic flavonoids present in coffee, green tea, dark chocolate or wine, anthocyanosides present in bilberries or blueberries, as well as in raspberries, blackberries, peppers, greens, grapes or tropical fruits, and other similar compounds may possess antioxidant activity, as suggested before [127]. Osborne et al., [128] in rat studies with oral administration of epigallocatechin demonstrated a reduced light-induced retinal neuronal death, suggesting that this substance can be useful for preventing retinal neurons damage and death. In fact, Chu et al., [129] in experiments with rats that drank green tea, reported that the retina absorbed the highest levels of gallocatechin, and the aqueous humor better absorbed epigallocatechin. These data confirmed that green tea consumption could protect our eyes against oxidative attack. Forskolin (Coleus) is an extract from the roots of this plant that traditionally has been utilized in ayurvedic practices. Russo et al., [130] intravitreally administered forskolin, homotaurine, and L-carnosine to adult male Wistar rats in which the IOP was acutely increased. These authors reported a significant neuroprotective RGCs effect associated with reduced calpain activity upregulation of phosphoinositide 3-kinase (PI3K)/Akt pathway, and inhibition of glycogen synthase kinase-3β (GSK-3β). In the same atmosphere, Yokoyama et al., [131] analyzed the role of calpain in C57BL/6 mice model of OS-induced RGC damage by means of intravitreal administration of 2,2'-azobis (2-amidinopropane) dihydrochloride (AAPH). Data showed that AAPH administration was an effective model for inducing OS in the RGCs. The authors also demonstrated that the inhibition of the calpain pathway significantly protected the RGCs after AAPH administration. By using brimonidine, an alpha 2-adrenergic agonist receptor, Lee et al., [132] demonstrated that administration of this product to rats contributes to RGCs neuroprotection. A series of natural products have been investigated in animal models of retinal degeneration. Boatright et al., [133] and Fernández-Sánchez et al., [134] evaluated the effects of a systemic injection of tauroursodeoxycholic acid (TUDCA), primary constituent of bear bile, in mouse models of retinal degeneration. Data showed that TUDCA is a substance able to protect the retinal cells from degeneration. Fernández-Sánchez et al., [135] reported amelioration of the induced retinal degenerative damage in P23H rats by the bear bile and by saffranal, a constituent of saffron, a plant native to the eastern Mediterranean regions (widely used to color foods and as a cooking spice) [135]. The antioxidant effects of Ginkgo biloba could be due to its poly-phenolic flavonoids contents, which might protect the tissues against OS injury. It has been reported that the organic acids of Ginkgo biloba extract may be the responsible for its antioxidant, anti-inflammatory, antiproliferative and antiallergic properties, among others. Several authors have cloncluded that Ginkgo Biloba may theoretically prevent oxidative stress in the mitochondria and thereby protect the glaucomatous RGCs [136, 137].
5. CLINICAL GLAUCOMA TRIALS OF ANTI-OXIDANTS, ESSENTIAL FATTY ACIDS AND OTHER NATURAL PRODUCTS
Some oral antioxidants, such as extract of Ginkgo biloba (EGB), anthocyanins, vitamins, saffron and essential fatty acids, have been studied in different clinical trials in POAG, and normotensive glaucoma (NTG). The clinical effects of these supplementations have been estimated in terms of IOP decrease or progression of distinct parameters in automated perimetry, optical coherence tomography (OCT) or electroretinogram (ERG) and ocular blood flow (Table 6). The ECB (containing flavonoids), has been tested mainly on NTG but also recently on POAG. Quaranta et al [138] showed that oral EGB supplementation improved preexisting perimetric indices (mean deviation -MD- and pattern standard deviation -PSD-) without IOP changes in NTG patients. Later on, it was demonstrated that intake of EGB (80 mg, 2 times daily) or bilberry anthocyanins (60 mg, twice a day) during almost 2 years was associated with MD improvement
Table 6.
Results of clinical trials with antioxidants, essential fatty acids and natural compounds in glaucoma patients.
|
Oral Antioxidant
Studied |
Sample Size | Outcomes in Treated Patients |
Follow-up
(Type of Study) |
Reference |
|---|---|---|---|---|
| ω-3 PUFAs | 40 ocular hypertensive patients (2 groups: treated vs placebo) |
Improvent of global perimetric indices (blue/yellow automated perimetry) | 3 months (prospective) |
Cellini et al., 1999 [148] |
| Epigallocatechin-gallate | 18 ocular hypertensive patients and 18 POAG patients | Improvements in ERG. Automated perimetry did not show changes. | 3 months (prospective) |
Falsini et al., 2009 [147] |
| Two AREDS-based antioxidant formulas (antioxidants + minerals) with/without ω-3 PUFAs |
117 POAG patients (2 groups according to supplementation with/without ω-3 PUFAs, and a control group | No significant differences between perimetric global índices, peripapillary RNFL or macular GCC at the beginning and at the end of follow-up. | 2 years (prospective) |
Garcia-Medina et al., 2015 [21] |
| Extract of Ginkgo biloba | 35 NTG patients (2 groups: treated vs placebo) |
No effect on automated perimetry or contrast sensitivity | 4-8 weeks-of washout- 4 weeks (prospective, crossover study) | Guo et al. 2014 [142] |
| Extract of saffron | 34 POAG patients (2 groups: treated vs placebo) | IOP decrease | 4 weeks (prospective) |
Jabbarpoor Bonyadi et al., 2014 [146] |
| Extract of Ginkgo biloba | 42 NTG patients | Improvement of perimetric global indices with no changes in IOP | 12 years (4 years observation + 8 years treatment) (retrospective) | Lee et al., 2013 [140] |
| Combination of forskolin, homotaurine, carnosine, folic acid, vitamins (B1, B1, B6) and magnesium | 22 POAG patients (2 groups: treated vs not treated) | Lower IOP and better ERG parameters and foveal sensitivity | 1 year (prospective) |
Mutolo et al., 2016 [20] |
| Extract of blackcurrant anthocyanins | 38 POAG patients (2 groups: treated vs placebo) | Improvement of MD and increase of ocular blood flow | 2 years (prospective) |
Ohguro et al., 2012 [144] |
| Extract of blackcurrant anthocyanins | 21 POAG patients (2 groups: treated vs placebo) | Lower IOP | 2 years (prospective) |
Ohguro et al., 2013 [145] |
| Extract of blackcurrant anthocyanins | 12 healthy subjects (2 groups: treated vs placebo) | Lower IOP | 4 weeks-2 weeks of washout-4 weeks (prospective, crossover study) | Ohguro et al.,2013 [145] |
| Extract of Ginkgo biloba | 30 NTG patients (2 groups: treated vs placebo) | Increase of peripapillary retinal blood flow | 4 weeks (prospective) |
Park et al., 2011 [141] |
| Extract of Ginkgo biloba | 27 NTG patients (2 groups: treated vs not treated) | Improvement of perimetric indices without IOP changes | 4 weeks (prospective) |
Quaranta et al,. 2003 [138] |
| Extract of Ginkgo biloba | 40 POAG patients (2 groups: treated vs placebo) | Improvement of perimetric global indices, slower decrease of superior and inferior peripapillary RNFL. No changes in IOP | 6 months (prospective) |
Sari et al., 2016 [143] |
| Extract of Ginkgo biloba or bilberry anthocyanins | 332 NTG patients (3 groups according to supplementation) |
Improvement of MD in both supplemented groups. Better visual acuity with anthocyanins |
Almost 2 years (retrospective) |
Shim et al,. 2012 [139] |
Abbreviations: AREDS, age-related eye disease study; ERG, electroretinogram; IOP, intraocular pressure; GGC, ganglion cell complex; MD, mean deviation (automated perimetry); NTG, normotensive glaucoma; POAG, primary open-angle glaucoma; ω-3: omega-3 fatty acids; PUFAs: polyunsaturated fatty acids; RNFL, retinal nerve fiber layer.
in NTG patients. In addition, patients supplemented with bilberry anthocyanins exhibited a better visual acuity [139]. Another trial concluded that NTG patients with preexisting perimetric progression during a 4-year period showed attenuation of this progression in relation to EGB intake (80 mg, 2 times daily) during additional 8 years of follow-up [140]. Moreover, 80 mg of EGB (twice daily) during 4 weeks has proved to improve peripapillary retinal blood flow in NTG patients [141]. In contrast with these results, a more recent work did not find any improvement of MD in NTG patients after supplementation with EGB (40 mg 3 times daily) in the short term (two periods of 4 weeks) [142]. The EGB has also been administrated during 6 months (40 mg 2 times daily) to POAG patients, and the results showed a significant correltion with the improvement of perimetric global indices and a slower decrease of peripapillary retinal nerve fiber layer (RNFL) thickness (superior and inferior quadrants) [143]. Additionally, other supplementations have also demonstrated positive effects in glaucoma patients. Extract of black currant anthocyanins (EBCA) during two years (50 mg per day) was related to smaller deterioration of MD and augmentation of ocular blood flow [144]. Same authors also reported that EBCA lowered the IOP both in healthy and POAG subjects [145]. Similarly, oral intake of extract of saffron (30 mg per day) for 4 weeks has been found to diminish IOP in POAG patients [146]. In addition, the 3-month intake of epigallocatechin-gallate, have showed to improve ERG parameters but not perimetric indices in hypertensive and POAG patients [147]. Otherwise, two randomized, controlled trials have been performed using different combinations of antioxidants and minerals in POAG patients. Our investigational team did not find differences between global perimetric indices, peripapillary RFNL thickness and macular ganglion cell complex thickness among the groups taking antioxidant supplements with or without ω-3 fatty acids at the beginning and at the end of 2 years of follow-up [21]. However, in a previous study Cellini et al., found that oral supplementation with high doses of ω-3 fatty acids for three months in hypertensive patients were related to amelioration of blue/yellow, perimetric global indices [148]. Mutolo et al. [20] recently concluded that the intake of a combination of forskolin, homotaurine, carnosine, folic acid, vitamins (B1, B2, B6) and magnesium during 12 months (with results quarterly checked) was associated to IOP decrease and improvement of ERG (at 6, 9 and 12 months) and foveal sensitivity obtained by frequency doubling perimetry (at 12 months).
Besides, as far as we know, some other clinical trials relating antioxidant supplementation and ω-3/ ω-6 fatty acids have been completed. However, their results have not been already published [149-152].
CONCLUSION
A wide variety of studies in human and experimental animals have proven that antioxidants, ω-3/ω-6 fatty acids and some other natural compounds help regulating IOP as well as protecting the RGCs against OS/NS in glaucoma. The question arises as it is posible to incorporate these substances for glaucoma therapy. Based on the impact of antioxidants and ω-3/ω-6 fatty acids at the molecular level in the glaucomatous anterior and posterior eye segments, ongoing research have to ultimately decipher whether or not sustainable differences can exists in taking or not these supplements for glaucoma progression in order to prevent optic atrophy and irreversible blindness.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Saeedi O., Ashraf H., Slade E.P., Medoff D.R., Li L., Friedman D.S., Kreyenbuhl J. Trends in prevalence of diagnosed ocular disease and utilization of eye care services in american veterans. Am. J. Ophthalmol. 2017;173:70–75. doi: 10.1016/j.ajo.2016.09.030. [http://dx.doi.org/10.1016/j.ajo. 2016.09.030]. [PMID: 27702620]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nucci C., Russo R., Martucci A., Giannini C., Garaci F., Floris R., Bagetta G., Morrone L.A. New strategies for neuroprotection in glaucoma, a disease that affects the central nervous system. Eur. J. Pharmacol. 2016;787:119–126. doi: 10.1016/j.ejphar.2016.04.030. [http://dx.doi.org/10.1016/j. ejphar.2016.04.030]. [PMID: 27089818]. [DOI] [PubMed] [Google Scholar]
- 3.Pinazo-Durán M.D., Zanón-Moreno V., Gallego-Pinazo R., García-Medina J.J. Oxidative stress and mitochondrial failure in the pathogenesis of glaucoma neurodegeneration. 2015. [DOI] [PubMed] [Google Scholar]
- 4.Russo R., Varano G.P., Adornetto A., Nucci C., Corasaniti M.T., Bagetta G., Morrone L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016;787:134–142. doi: 10.1016/j.ejphar.2016.03.064. [http://dx.doi.org/10.1016/j.ejphar. 2016.03.064]. [PMID: 27044433]. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L., Chen G., Li J., Fu Y., Mavlyutov T.A., Yao A., Nickells R.W., Gong S., Guo L.W. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J. Control. Release. 2017;247:153–166. doi: 10.1016/j.jconrel.2016.12.038. [http://dx.doi. org/10.1016/j.jconrel.2016.12.038]. [PMID: 28063892]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flammer J., Konieczka K. Retinal venous pressure: the role of endothelin. EPMA J. 2015;6:21. doi: 10.1186/s13167-015-0043-1. [http://dx.doi.org/10.1186/ s13167-015-0043-1]. [PMID: 26504500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas B., Gallego B.I., Ramírez A.I., Salazar J.J., de Hoz R., Valiente-Soriano F.J., Avilés-Trigueros M., Villegas-Perez M.P., Vidal-Sanz M., Triviño A., Ramírez J.M. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J. Neuroinflam. 2014;11:133. doi: 10.1186/1742-2094-11-133. [http://dx.doi.org/10.1186/1742-2094-11-133]. [PMID: 25064005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleesattel D., Crish S.D., Inman D.M. Decreased energy capacity and increased autophagic activity in optic nerve axons with defective anterograde transport. Invest. Ophthalmol. Vis. Sci. 2015;56(13):8215–8227. doi: 10.1167/iovs.15-17885. [http://dx.doi.org/10.1167/iovs.15-17885]. [PMID: 26720474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saccà S.C., Gandolfi S., Bagnis A., Manni G., Damonte G. Traverso. C.E., Izzotti, A. From DNA damage to functional changes of the trabecular meshwork in aging and glaucoma. Ageing Res. Rev. 2016;29:6–41. doi: 10.1016/j.arr.2016.05.012. [http://dx.doi.org/10.1016/j.arr.2016.05.012]. [DOI] [PubMed] [Google Scholar]
- 10.Wachtl J., Töteberg-Harms M., Frimmel S., Kniestedt C.A. 2017. [DOI] [PubMed]
- 11.Kimura A., Namekata K., Guo X., Noro T., Harada C., Harada T. Targeting oxidative stress for treatment of glaucoma and optic neuritis. Oxid. Med. Cell. Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. [http://dx. doi.org/10.1155/2017/2817252]. [PMID: 28270908]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gusev A.N., Krasnogorskaya V.N. Antioxidants in complex treatment of far-advanced open-angle glaucoma. Vestn. Oftalmol. 2016;132(1):63–67. doi: 10.17116/oftalma2016132163-67. [http://dx.doi.org/10.17116/oftalma2016132163-67]. [PMID: 27030437]. [DOI] [PubMed] [Google Scholar]
- 13.Dekeyster E., Geeraerts E., Buyens T., Van den Haute C., Baekelandt V., De Groef L., Salinas-Navarro M., Moons L. Tackling glaucoma from within the brain: An unfortunate interplay of BDNF and TrkB. PLoS One. 2015;10(11):e0142067. doi: 10.1371/journal.pone.0142067. [http://dx.doi. org/10.1371/journal.pone.0142067]. [PMID: 26560713]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvao J., Elvas F., Martins T., Cordeiro M.F., Ambrósio A.F., Santiago A.R. Adenosine A3 receptor activation is neuroprotective against retinal neurodegeneration. Exp. Eye Res. 2015;140:65–74. doi: 10.1016/j.exer.2015.08.009. [http://dx.doi.org/10.1016/j.exer.2015.08.009]. [PMID: 26297614]. [DOI] [PubMed] [Google Scholar]
- 15.Defert O., Boland S. Rho kinase inhibitors: a patent review (2014 - 2016). Expert Opin. Ther. Pat. 2017;27(4):507–515. doi: 10.1080/13543776.2017.1272579. [http://dx. doi.org/10.1080/13543776.2017.1272579]. [PMID: 28048944]. [DOI] [PubMed] [Google Scholar]
- 16.Khatib T.Z., Martin K.R. Protecting retinal ganglion cells. Eye (Lond.) 2017;•••:13. doi: 10.1038/eye.2016.299. [http://dx.doi.org/10.1038/eye.2016.299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabral T., DiCarlo J.E., Justus S., Sengillo J.D., Xu Y., Tsang S.H. CRISPR applications in ophthalmologic genome surgery. Curr. Opin. Ophthalmol. 2017;28(3):252–259. doi: 10.1097/ICU.0000000000000359. [http://dx.doi.org/ 10.1097/ICU.0000000000000359]. [PMID: 28141764]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengillo J.D., Justus S., Cabral T., Tsang S.H. Correction of monogenic and common retinal disorders with gene therapy. Genes (Basel) 2017;8(2):E53. doi: 10.3390/genes8020053. [http://dx.doi.org/10.3390/genes8020053]. [PMID: 28134823]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grover A.K., Samson S.E. Antioxidants and vision health: facts and fiction. Mol. Cell. Biochem. 2014;388(1-2):173–183. doi: 10.1007/s11010-013-1908-z. [http:// dx.doi.org/10.1007/s11010-013-1908-z]. [PMID: 24311110]. [DOI] [PubMed] [Google Scholar]
- 20.Mutolo M.G., Albanese G., Rusciano D., Pescosolido N. Oral Administration of Forskolin, Homotaurine, Carnosine, and Folic Acid in Patients with Primary Open Angle Glaucoma: Changes in Intraocular Pressure, Pattern Electroretinogram Amplitude, and Foveal Sensitivity. J. Ocul. Pharmacol. Ther. 2016;32(3):178–183. doi: 10.1089/jop.2015.0121. [http://dx.doi.org/10.1089/jop.2015.0121]. [PMID: 26771282]. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Medina J.J., Garcia-Medina M., Garrido-Fernandez P., Galvan-Espinosa J., Garcia-Maturana C., Zanon-Moreno V., Pinazo-Duran M.D. A two-year follow-up of oral antioxidant supplementation in primary open-angle glaucoma: an open-label, randomized, controlled trial. Acta Ophthalmol. 2015;93(6):546–554. doi: 10.1111/aos.12629. [http://dx.doi.org/10.1111/aos.12629]. [PMID: 25545196]. [DOI] [PubMed] [Google Scholar]
- 22.Kang J.H., Wu J., Cho E., Ogata S., Jacques P., Taylor A., Chiu C.J., Wiggs J.L., Seddon J.M., Hankinson S.E., Schaumberg D.A., Pasquale L.R. Contribution of the Nurses’ Health Study to the Epidemiology of Cataract, Age-Related Macular Degeneration, and Glaucoma. Am. J. Public Health. 2016;106(9):1684–1689. doi: 10.2105/AJPH.2016.303317. [http://dx.doi.org/10.2105/AJPH.2016.303317]. [PMID: 27459452]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park L.K., Friso S., Choi S.W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012;71(1):75–83. doi: 10.1017/S0029665111003302. [http://dx.doi.org/10.1017/S0029665111003302]. [PMID: 22051144]. [DOI] [PubMed] [Google Scholar]
- 24.Friedman D.S., Wilson M.R., Liebmann J.M., Fechtner R.D., Weinreb R.N. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am. J. Ophthalmol. 2004;138(3) Suppl.:S19–S31. doi: 10.1016/j.ajo.2004.04.058. [http://dx.doi.org/10.1016/ j.ajo.2004.04.058]. [PMID: 15364049]. [DOI] [PubMed] [Google Scholar]
- 25.Kass M.A., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.L., Miller J.P., Parrish R.K., II, Wilson M.R., Gordon M.O. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. [http://dx.doi.org/10.1001/ archopht.120.6.701]. [PMID: 12049574]. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb R.N., Khaw P.T. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [http://dx.doi.org/10.1016/S0140-6736(04)16257-0]. [PMID: 15158634]. [DOI] [PubMed] [Google Scholar]
- 27.Spry P.G., Sparrow J.M., Diamond J.P., Harris H.S. Risk factors for progressive visual field loss in primary open angle glaucoma. Eye (Lond.) 2005;19(6):643–651. doi: 10.1038/sj.eye.6701605. [http://dx.doi.org/10.1038/ sj.eye.6701605]. [PMID: 15192695]. [DOI] [PubMed] [Google Scholar]
- 28.Heijl A., Leske M.C., Bengtsson B., Hyman L., Bengtsson B., Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [http://dx.doi.org/10.1001/ archopht.120.10.1268]. [PMID: 12365904]. [DOI] [PubMed] [Google Scholar]
- 29.Boland M.V., Ervin A.M., Friedman D.S., Jampel H.D., Hawkins B.S., Vollenweider D., Chelladurai Y., Ward D., Suarez-Cuervo C., Robinson K.A. Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013;158(4):271–279. doi: 10.7326/0003-4819-158-4-201302190-00008. [http://dx.doi.org/10.7326/0003-4819-158-4-201302190-00008]. [PMID: 23420235]. [DOI] [PubMed] [Google Scholar]
- 30.The Advanced Glaucoma Intervention Study (AGIS) 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [http://dx. doi.org/10.1016/S0002-9394(00)00538-9]. [PMID: 11024415]. [DOI] [PubMed] [Google Scholar]
- 31.Lichter P.R., Musch D.C., Gillespie B.W., Guire K.E., Janz N.K., Wren P.A., Mills R.P. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [http://dx.doi.org/10.1016/S0161-6420 (01)00873-9]. [PMID: 11713061]. [DOI] [PubMed] [Google Scholar]
- 32.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998;126(4):487–497. doi: 10.1016/s0002-9394(98)00223-2. [http://dx.doi.org/10.1016/S0002-9394(98)00223-2]. [PMID: 9780093]. [DOI] [PubMed] [Google Scholar]
- 33.European Glaucoma Society . Terminology and Guidelines for Glaucoma. 4th ed. Savona, Italy: Dogma; 2014. [Google Scholar]
- 34.American Academy of Ophthalmology Primary open-angle glaucoma, preferred practice pattern. 2015 www.aao.org/ppp
- 35.Martinez Garcia A., Benitez-del-Castillo J. 2011. Medical treatment in chronic open-angle glaucoma: an update. [Google Scholar]
- 36.Katz A., Tal D.M., Heller D., Habeck M., Ben Zeev E., Rabah B., Bar K.Y., Marcovich A.L., Karlish S.J. Digoxin derivatives with selectivity for the α2β3 isoform of Na,K-ATPase potently reduce intraocular pressure. Proc. Natl. Acad. Sci. USA. 2015;112(44):13723–13728. doi: 10.1073/pnas.1514569112. [http://dx.doi.org/10.1073/pnas.1514569112]. [PMID: 26483500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidl D., Schmetterer L., Garhöfer G., Popa-Cherecheanu A. Pharmacotherapy of glaucoma. J. Ocul. Pharmacol. Ther. 2015;31(2):63–77. doi: 10.1089/jop.2014.0067. [http://dx.doi.org/10.1089/jop.2014.0067]. [PMID: 25587905]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Valk R., Webers C.A., Schouten J.S., Zeegers M.P., Hendrikse F., Prins M.H. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112(7):1177–1185. doi: 10.1016/j.ophtha.2005.01.042. [http://dx. doi.org/10.1016/j.ophtha.2005.01.042]. [PMID: 15921747]. [DOI] [PubMed] [Google Scholar]
- 39.Li T., Lindsley K., Rouse B., Hong H., Shi Q., Friedman D.S., Wormald R., Dickersin K. Comparative effectiveness of first-line medications for primary open-angle glaucoma: A systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129–140. doi: 10.1016/j.ophtha.2015.09.005. [http://dx.doi.org/10.1016/j.ophtha.2015.09.005]. [PMID: 26526633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart W.C., Konstas A.G., Nelson L.A., Kruft B. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115(7):1117–1122.e1. doi: 10.1016/j.ophtha.2007.10.004. [http://dx.doi.org/10.1016/j.ophtha.2007.10.004]. [PMID: 18082886]. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald M., Payne S.C., Bartlett C.A., Evill L., Harvey A.R., Dunlop S.A. Secondary retinal ganglion cell death and the neuroprotective effects of the calcium channel blocker lomerizine. Invest. Ophthalmol. Vis. Sci. 2009;50(11):5456–5462. doi: 10.1167/iovs.09-3717. [http://dx.doi.org/ 10.1167/iovs.09-3717]. [PMID: 19474405]. [DOI] [PubMed] [Google Scholar]
- 42.Kasai H., Imamura T., Tsuruma K., Takahashi Y., Kurasawa T., Hirata H., Shimazawa M., Hara H. Effects of roscovitine, a cell cyclin [correction of cycling]-dependent kinase inhibitor, on intraocular pressure of rabbit and retinal ganglion cell damage. Neurosci. Lett. 2013;535:95–99. doi: 10.1016/j.neulet.2012.12.025. [http://dx.doi.org/10.1016/j.neulet. 2012.12.025]. [PMID: 23274706]. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K., Zhang L., Weinreb R.N. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 2012;11(7):541–559. doi: 10.1038/nrd3745. [http://dx.doi.org/ 10.1038/nrd3745]. [PMID: 22699774]. [DOI] [PubMed] [Google Scholar]
- 44.Cholkar K., Trinh H.M., Pal D., Mitra A.K. Discovery of novel inhibitors for the treatment of glaucoma. Expert Opin. Drug Discov. 2015;10(3):293–313. doi: 10.1517/17460441.2015.1000857. [http://dx.doi.org/10.1517/17460441. 2015.1000857]. [PMID: 25575654]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samples J.R., Singh K., Lin S.C., Francis B.A., Hodapp E., Jampel H.D., Smith S.D. Laser trabeculoplasty for open-angle glaucoma: a report by the american academy of ophthalmology. Ophthalmology. 2011;118(11):2296–2302. doi: 10.1016/j.ophtha.2011.04.037. [http://dx.doi.org/10. 1016/j.ophtha.2011.04.037]. [PMID: 21849211]. [DOI] [PubMed] [Google Scholar]
- 46.Realini T. Selective laser trabeculoplasty for the management of open-angle glaucoma in St. Lucia. JAMA Ophthalmol. 2013;131(3):321–327. doi: 10.1001/jamaophthalmol.2013.1706. [http://dx.doi.org/10.1001/jamaophthalmol.2013. 1706]. [PMID: 23348420]. [DOI] [PubMed] [Google Scholar]
- 47.Bovell A.M., Damji K.F., Hodge W.G., Rock W.J., Buhrmann R.R., Pan Y.I. Long term effects on the lowering of intraocular pressure: selective laser or argon laser trabeculoplasty? Can. J. Ophthalmol. 2011;46(5):408–413. doi: 10.1016/j.jcjo.2011.07.016. [http://dx.doi.org/10.1016/ j.jcjo.2011.07.016]. [PMID: 21995983]. [DOI] [PubMed] [Google Scholar]
- 48.Landers J., Martin K., Sarkies N., Bourne R., Watson P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119(4):694–702. doi: 10.1016/j.ophtha.2011.09.043. [http://dx.doi. org/10.1016/j.ophtha.2011.09.043]. [PMID: 22196977]. [DOI] [PubMed] [Google Scholar]
- 49.Francis B.A., Singh K., Lin S.C., Hodapp E., Jampel H.D., Samples J.R., Smith S.D. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(7):1466–1480. doi: 10.1016/j.ophtha.2011.03.028. [PMID: 21724045]. [DOI] [PubMed] [Google Scholar]
- 50.Ayyala R.S., Chaudhry A.L., Okogbaa C.B., Zurakowski D. Comparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months’ follow-up. Ophthalmology. 2011;118(12):2427–2433. doi: 10.1016/j.ophtha.2011.05.021. [http://dx.doi.org/10.1016/j.ophtha.2011.05. 021]. [PMID: 21856008]. [DOI] [PubMed] [Google Scholar]
- 51.Rulli E., Biagioli E., Riva I., Gambirasio G., De Simone I., Floriani I., Quaranta L. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–1582. doi: 10.1001/jamaophthalmol.2013.5059. [http://dx. doi.org/10.1001/jamaophthalmol.2013.5059]. [PMID: 24158640]. [DOI] [PubMed] [Google Scholar]
- 52.Rossetti L., Marchetti I., Orzalesi N., Scorpiglione N., Torri V., Liberati A. Randomized clinical trials on medical treatment of glaucoma. Are they appropriate to guide clinical practice? Arch. Ophthalmol. 1993;111(1):96–103. doi: 10.1001/archopht.1993.01090010100034. [http://dx.doi.org/10.1001/ archopht.1993.01090010100034]. [PMID: 8424732]. [DOI] [PubMed] [Google Scholar]
- 53.Chang E.E., Goldberg J.L. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119(5):979–986. doi: 10.1016/j.ophtha.2011.11.003. [http://dx.doi.org/10.1016/j.ophtha.2011.11.003]. [PMID: 22349567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doozandeh A., Yazdani S. Neuroprotection in Glaucoma. J. Ophthalmic Vis. Res. 2016;11(2):209–220. doi: 10.4103/2008-322X.183923. [http://dx.doi.org/10.4103/ 2008-322X.183923]. [PMID: 27413504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [http://dx.doi.org/10.1038/ nrm3801]. [PMID: 24854789]. [DOI] [PubMed] [Google Scholar]
- 56.Aseervatham G.S., Sivasudha T., Jeyadevi R., Arul Ananth D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans--an overview. Environ. Sci. Pollut. Res. Int. 2013;20(7):4356–4369. doi: 10.1007/s11356-013-1748-0. [http://dx.doi.org/10.1007/s11356-013-1748-0]. [PMID: 23636598]. [DOI] [PubMed] [Google Scholar]
- 57.Mozos I., Luca C.T. Crosstalk between oxidative and nitrosative stress and arterial stiffness. Curr. Vasc. Pharmacol. 2017;15(5):446–456. doi: 10.2174/1570161115666170201115428. [http://dx.doi.org/10.2174/1570161115666170201115428]. [PMID: 28155616]. [DOI] [PubMed] [Google Scholar]
- 58.Pinazo-Durán M.D., Gallego-Pinazo R., García-Medina J.J., Zanón-Moreno V., Nucci C., Dolz-Marco R., Martínez-Castillo S., Galbis-Estrada C., Marco-Ramírez C., López-Gálvez M.I., Galarreta D.J., Díaz-Llópis M. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging. 2014;9:637–652. doi: 10.2147/CIA.S52662. [http://dx.doi.org/10.2147/CIA.S52662]. [PMID: 24748782]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreira S.M., Lerner S.F., Brunzini R., Evelson P.A., Llesuy S.F. Oxidative stress markers in aqueous humor of glaucoma patients. Am. J. Ophthalmol. 2004;137(1):62–69. doi: 10.1016/s0002-9394(03)00788-8. [http://dx.doi.org/ 10.1016/S0002-9394(03)00788-8]. [PMID: 14700645]. [DOI] [PubMed] [Google Scholar]
- 60.Izzotti A., Bagnis A., Saccà S.C. The role of oxidative stress in glaucoma. Mutat. Res. 2006;612(2):105–114. doi: 10.1016/j.mrrev.2005.11.001. [http://dx.doi.org/ 10.1016/j.mrrev.2005.11.001]. [PMID: 16413223]. [DOI] [PubMed] [Google Scholar]
- 61.Zanón-Moreno V., Marco-Ventura P., Lleó-Perez A., Pons-Vazquez S., García-Medina J.J., Vinuesa-Silva I., Moreno-Nadal M.A., Pinazo-Durán M.D. Oxidative stress in primary open-angle glaucoma. J. Glaucoma. 2008;17(4):263–268. doi: 10.1097/IJG.0b013e31815c3a7f. [http://dx.doi.org/ 10.1097/IJG.0b013e31815c3a7f]. [PMID: 18552610]. [DOI] [PubMed] [Google Scholar]
- 62.Saccà S.C., Pulliero A., Izzotti A. The dysfunction of the trabecular meshwork during glaucoma course. J. Cell. Physiol. 2015;230(3):510–525. doi: 10.1002/jcp.24826. [http://dx.doi.org/10.1002/jcp.24826]. [PMID: 25216121]. [DOI] [PubMed] [Google Scholar]
- 63.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931. [http://dx.doi.org/10.1155/ 2016/5698931]. [PMID: 26881031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen D., Alavi M.V., Kim K-Y., Kang T., Scott R.T., Noh Y.H., Lindsey J.D., Wissinger B., Ellisman M.H., Weinreb R.N., Perkins G.A., Ju W.K. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2011;2:e240. doi: 10.1038/cddis.2011.117. [http://dx.doi.org/10.1038/cddis.2011. 117]. [PMID: 22158479]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016;2016:3164734. doi: 10.1155/2016/3164734. [http://dx.doi.org/10.1155/2016/3164734]. [PMID: 26881021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goyal A., Srivastava A., Sihota R., Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014;39(8):823–829. doi: 10.3109/02713683.2011.556299. [http://dx.doi.org/10.3109/02713683.2011. 556299]. [PMID: 24912005]. [DOI] [PubMed] [Google Scholar]
- 67.Ohira S., Inoue T., Shobayashi K., Iwao K., Fukushima M., Tanihara H. Simultaneous increase in multiple proinflammatory cytokines in the aqueous humor in neovascular glaucoma with and without intravitreal bevacizumab injection. Invest. Ophthalmol. Vis. Sci. 2015;56(6):3541–3548. doi: 10.1167/iovs.14-15918. [http://dx.doi.org/10.1167/iovs.14-15918]. [PMID: 26030108]. [DOI] [PubMed] [Google Scholar]
- 68.Babizhayev M.A., Yegorov Y.E. Senescent phenotype of trabecular meshwork cells displays biomarkers in primary open-angle glaucoma. Curr. Mol. Med. 2011;11(7):528–552. doi: 10.2174/156652411800615126. [http://dx. doi.org/10.2174/156652411800615126]. [PMID: 21707516]. [DOI] [PubMed] [Google Scholar]
- 69.Pinazo-Durán M.D., Zanón-Moreno V., García-Medina J.J., Gallego-Pinazo R. Evaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucoma. Curr. Opin. Pharmacol. 2013;13(1):98–107. doi: 10.1016/j.coph.2012.10.007. [http://dx. doi.org/10.1016/j.coph.2012.10.007]. [PMID: 23142105]. [DOI] [PubMed] [Google Scholar]
- 70.Agnifili L., Pieragostino D., Mastropasqua A., Fasanella V., Brescia L., Tosi G.M., Sacchetta P., Mastropasqua L. Molecular biomarkers in primary open-angle glaucoma: from noninvasive to invasive. 2015. [DOI] [PubMed] [Google Scholar]
- 71.Bhattacharya S.K., Lee R.K., Grus F.H. Molecular biomarkers in glaucoma. Invest. Ophthalmol. Vis. Sci. 2013;54(1):121–131. doi: 10.1167/iovs.12-11067. [http://dx.doi.org/10.1167/iovs.12-11067]. [PMID: 23297392]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knepper P.A., Samples J.R., Yue B.Y. Biomarkers of primary open-angle glaucoma. Expert Rev. Ophthalmol. 2010;5(6):731–742. doi: 10.1586/EOP.10.73. [http://dx.doi.org/10.1586/eop.10.73]. [PMID: 26435732]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J., Wang S., Zhong W., Yang B., Sun L., Zheng Y. Oxidative stress in the trabecular meshwork. Int. J. Mol. Med. 2016;38(4):995–1002. doi: 10.3892/ijmm.2016.2714. [Review]. [Review]. [http://dx.doi.org/10. 3892/ijmm.2016.2714]. [PMID: 27572245]. [DOI] [PubMed] [Google Scholar]
- 74.Peluso I., Palmery M., Pérez-Jiménez J., Drummen G. Biomarkers of oxidative stress in experimental models and human studies with nutraceuticals: Measurement, interpretation, and significance. Oxid. Med. Cell. Longev. 2016;2016:6159810. doi: 10.1155/2016/6159810. [http://dx.doi.org/ 10.1155/2016/6159810]. [PMID: 26925193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benoist d’Azy C., Pereira B., Chiambaretta F., Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: A systematic review and meta-analysis. PLoS One. 2016;11(12):e0166915. doi: 10.1371/journal.pone.0166915. [http://dx.doi.org/10.1371/journal.pone.0166915]. [PMID: 27907028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nucci C., Di Pierro D., Varesi C., Ciuffoletti E., Russo R., Gentile R., Cedrone C., Pinazo-Durán M.D., Coletta M., Mancino R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013;19:1841–1846. [PMC free article] [PubMed] [Google Scholar]
- 77.Chang D., Sha Q., Zhang X., Liu P., Rong S., Han T., Liu P., Pan H. The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS One. 2011;6(11):e27218. doi: 10.1371/journal.pone.0027218. [http://dx.doi.org/10.1371/journal.pone.0027218]. [PMID: 22096540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hernández-Martínez F.J., Piñas-García P., Lleó-Pérez A.V., Zanón-Moreno V.C., Bendala-Tufanisco E., García-Medina J.J., Vinuesa-Silva I., Pinazo-Durán M.D. Biomarcadores de peroxidación lipídica en el humor acuoso de pacientes con glaucoma primario de ángulo abierto. Arch. Soc. Esp. Oftalmol. 2016;91(8):357–362. doi: 10.1016/j.oftal.2016.01.031. [http://dx.doi.org/10.1016/j.oftal.2016.01.031]. [PMID: 26944209]. [DOI] [PubMed] [Google Scholar]
- 79.Guo X., Dason E.S., Zanon-Moreno V., Jiang Q., Nahirnyj A., Chan D., Flanagan J.G., Sivak J.M. PGC-1α signaling coordinates susceptibility to metabolic and oxidative injury in the inner retina. Am. J. Pathol. 2014;184(4):1017–1029. doi: 10.1016/j.ajpath.2013.12.012. [http://dx.doi.org/ 10.1016/j.ajpath.2013.12.012]. [PMID: 24508229]. [DOI] [PubMed] [Google Scholar]
- 80.Malone P.E., Hernandez M.R. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp. Eye Res. 2007;84(3):444–454. doi: 10.1016/j.exer.2006.10.020. [http://dx. doi.org/10.1016/j.exer.2006.10.020]. [PMID: 17173895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nie B., Gan W., Shi F., Hu G.X., Chen L.G., Hayakawa H., Sekiguchi M., Cai J.P. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid. Med. Cell. Longev. 2013;2013:303181. doi: 10.1155/2013/303181. [http://dx.doi.org/10.1155/ 2013/303181]. [PMID: 23738036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mumcu U.Y., Kocer I., Ates O., Alp H.H. Decreased paraoxonase1 activity and increased malondialdehyde and oxidative DNA damage levels in primary open angle glaucoma. Int. J. Ophthalmol. 2016;9(10):1518–1520. doi: 10.18240/ijo.2016.10.24. [PMID: 27803873]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohanty K., Dada R., Dada T. Oxidative DNA damage and reduced expression of DNA repair genes: Role in primary open angle glaucoma (POAG). Ophthalmic Genet. 2017;38(5):446–450. doi: 10.1080/13816810.2016.1261904. [http://dx.doi.org/10.1080/13816810.2016.1261904]. [PMID: 28129013]. [DOI] [PubMed] [Google Scholar]
- 84.Luthra A., Gupta N., Kaufman P.L., Weinreb R.N., Yücel Y.H. Oxidative injury by peroxynitrite in neural and vascular tissue of the lateral geniculate nucleus in experimental glaucoma. Exp. Eye Res. 2005;80(1):43–49. doi: 10.1016/j.exer.2004.08.016. [http://dx.doi.org/10.1016/j.exer.2004. 08.016]. [PMID: 15652525]. [DOI] [PubMed] [Google Scholar]
- 85.Zanón-Moreno V., Pons S., Gallego-Pinazo R., García-Medina J., Vinuesa I., Vila Bou V., Pinazo-Durán M.D. Arch. Soc. Esp. Oftalmol. 2008;83(6):365–372. doi: 10.4321/s0365-66912008000600006. [Involvement of nitric oxide and other molecules with redox potential in primary open angle glaucoma]. [PMID: 18521769]. [DOI] [PubMed] [Google Scholar]
- 86.Openkova Y.Y., Korobeiynikova E.N., Rykin V.S., Vinkova G.A. Klin. Lab. Diagn. 2013;5(5):8–11. [The analysis of status of biochemical indicators in blood serum and lacrimal fluid in patients with primary open-angle glaucoma]. [PMID: 24006637]. [PubMed] [Google Scholar]
- 87.Goyal A., Srivastava A., Sihota R., Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014;39(8):823–829. doi: 10.3109/02713683.2011.556299. [http://dx.doi.org/10.3109/02713683.2011. 556299]. [PMID: 24912005]. [DOI] [PubMed] [Google Scholar]
- 88.Lundmark P.O., Pandi-Perumal S.R., Srinivasan V., Cardinali D.P., Rosenstein R.E. Melatonin in the eye: implications for glaucoma. Exp. Eye Res. 2007;84(6):1021–1030. doi: 10.1016/j.exer.2006.10.018. [http://dx.doi.org/ 10.1016/j.exer.2006.10.018]. [PMID: 17174303]. [DOI] [PubMed] [Google Scholar]
- 89.Bagnis A., Izzotti A., Centofanti M., Saccà S.C. Aqueous humor oxidative stress proteomic levels in primary open angle glaucoma. Exp. Eye Res. 2012;103:55–62. doi: 10.1016/j.exer.2012.07.011. [http://dx.doi.org/10.1016/j.exer. 2012.07.011]. [PMID: 22974818]. [DOI] [PubMed] [Google Scholar]
- 90.Rokicki W., Zalejska-Fiolka J., Pojda-Wilczek D., Kabiesz A., Majewski W. Oxidative stress in the red blood cells of patients with primary open-angle glaucoma. Clin. Hemorheol. Microcirc. 2016;62(4):369–378. doi: 10.3233/CH-152029. [http://dx.doi.org/10.3233/CH-152029]. [PMID: 26890101]. [DOI] [PubMed] [Google Scholar]
- 91.Zanón-Moreno V., Asensio-Marquez E.M., Ciancotti-Oliver L., Garcia-Medina J.J., Sanz P., Ortega-Azorín C., Pinazo-Durán M.D., Ordovás J.M., Corella D. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Mol. Vis. 2013;19:231–242. [PMID: 23401652]. [PMC free article] [PubMed] [Google Scholar]
- 92.Mousa A., Kondkar A.A., Al-Obeidan S.A., Azad T.A., Sultan T., Osman E., Abu-Amero K.K. Association of total antioxidants level with glaucoma type and severity. Saudi Med. J. 2015;36(6):671–677. doi: 10.15537/smj.2015.6.10697. [http://dx.doi.org/10.15537/smj.2015.6.10697]. [PMID: 25987108]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abu-Amero K.K., Azad T.A., Mousa A., Osman E.A., Sultan T., Al-Obeidan S.A. Total antioxidant level is correlated with intra-ocular pressure in patients with primary angle closure glaucoma. BMC Res. Notes. 2014;7:163. doi: 10.1186/1756-0500-7-163. [http://dx.doi.org/10.1186/ 1756-0500-7-163]. [PMID: 24646376]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yokota T., Kamimura N., Igarashi T., Takahashi H., Ohta S., Oharazawa H. Protective effect of molecular hydrogen against oxidative stress caused by peroxynitrite derived from nitric oxide in rat retina. Clin. Experiment. Ophthalmol. 2015;43(6):568–577. doi: 10.1111/ceo.12525. [http://dx.doi.org/10.1111/ceo.12525]. [PMID: 25801048]. [DOI] [PubMed] [Google Scholar]
- 95.Tanito M., Kaidzu S., Takai Y., Ohira A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016;6:25792. doi: 10.1038/srep25792. [http://dx.doi.org/10.1038/ srep25792]. [PMID: 27165400]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Danias J., Shen F., Kavalarakis M., Chen B., Goldblum D., Lee K., Zamora M.F., Su Y., Brodie S.E., Podos S.M., Mittag T. Characterization of retinal damage in the episcleral vein cauterization rat glaucoma model. Exp. Eye Res. 2006;82(2):219–228. doi: 10.1016/j.exer.2005.06.013. [http://dx.doi.org/10.1016/j.exer.2005.06.013]. [PMID: 16109406]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Can N., Catak O., Turgut B., Demir T., Ilhan N., Kuloglu T., Ozercan I.H. Neuroprotective and antioxidant effects of ghrelin in an experimental glaucoma model. Drug Des. Devel. Ther. 2015;9:2819–2829. doi: 10.2147/DDDT.S83067. [PMID: 26082612]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mayordomo-Febrer A., López-Murcia M., Morales-Tatay J.M., Monleón-Salvado D., Pinazo-Durán M.D. Metabolomics of the aqueous humor in the rat glaucoma model induced by a series of intracamerular sodium hyaluronate injection. Exp. Eye Res. 2015;131:84–92. doi: 10.1016/j.exer.2014.11.012. [http://dx.doi.org/10.1016/j.exer.2014.11.012]. [PMID: 25479046]. [DOI] [PubMed] [Google Scholar]
- 99.Pirhan D., Yüksel N., Emre E., Cengiz A., Kürşat Y.D. Riluzole- and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr. Eye Res. 2016;41(1):59–69. doi: 10.3109/02713683.2015.1004719. [http://dx.doi.org/10.3109/02713683.2015.1004719]. [PMID: 25658983]. [DOI] [PubMed] [Google Scholar]
- 100.Yang X., Hondur G., Tezel G. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2016;57(4):2344–2354. doi: 10.1167/iovs.16-19153. [http://dx.doi.org/10.1167/iovs.16-19153]. [PMID: 27127934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samsel P.A., Kisiswa L., Erichsen J.T., Cross S.D., Morgan J.E. A novel method for the induction of experimental glaucoma using magnetic microspheres. Invest. Ophthalmol. Vis. Sci. 2011;52(3):1671–1675. doi: 10.1167/iovs.09-3921. [http://dx.doi.org/10.1167/iovs.09-3921]. [PMID: 20926815]. [DOI] [PubMed] [Google Scholar]
- 102.Kim K.Y., Perkins G.A., Shim M.S., Bushong E., Alcasid N., Ju S., Ellisman M.H., Weinreb R.N., Ju W.K. DRP1 inhibition rescues retinal ganglion cells and their axons by preserving mitochondrial integrity in a mouse model of glaucoma. Cell Death Dis. 2015;6:e1839. doi: 10.1038/cddis.2015.180. [http://dx.doi.org/10.1038/cddis.2015.180]. [PMID: 26247724]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ko M.L., Peng P.H., Ma M.C., Ritch R., Chen C.F. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic. Biol. Med. 2005;39(3):365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [http://dx.doi.org/10.1016/j.freeradbiomed.2005.03.025]. [PMID: 15993335]. [DOI] [PubMed] [Google Scholar]
- 104.Calandrella N., Scarsella G., Pescosolido N., Risuleo G. Degenerative and apoptotic events at retinal and optic nerve level after experimental induction of ocular hypertension. Mol. Cell. Biochem. 2007;301(1-2):155–163. doi: 10.1007/s11010-006-9407-0. [http://dx.doi.org/10.1007/s11010-006-9407-0]. [PMID: 17242991]. [DOI] [PubMed] [Google Scholar]
- 105.Calandrella N., De Seta C., Scarsella G., Risuleo G. Carnitine reduces the lipo-per-oxidative damage of the mem-brane and apoptosis after induction of cell stress in experi-mental glaucoma. 2010. [DOI] [PMC free article] [PubMed]
- 106.Tezel G., Yang X., Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest. Ophthalmol. Vis. Sci. 2005;46(9):3177–3187. doi: 10.1167/iovs.05-0208. [http://dx.doi.org/10.1167/iovs.05-0208]. [PMID: 16123417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L., Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [http://dx. doi.org/10.1016/j.preteyeres.2011.11.002]. [PMID: 22155051]. [DOI] [PubMed] [Google Scholar]
- 108.Ko M.L., Peng P.H., Hsu S.Y., Chen C.F. Dietary deficiency of vitamin E aggravates retinal ganglion cell death in experimental glaucoma of rats. Curr. Eye Res. 2010;35(9):842–849. doi: 10.3109/02713683.2010.489728. [http://dx. doi.org/10.3109/02713683.2010.489728]. [PMID: 20795867]. [DOI] [PubMed] [Google Scholar]
- 109.Hsu K.H., Carbia B.E., Plummer C., Chauhan A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015;94:312–321. doi: 10.1016/j.ejpb.2015.06.001. [http://dx.doi. org/10.1016/j.ejpb.2015.06.001]. [PMID: 26071799]. [DOI] [PubMed] [Google Scholar]
- 110.Williams P.A., Harder J.M., Foxworth N.E., Cochran K.E., Philip V.M., Porciatti V., Smithies O., John S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355(6326):756–760. doi: 10.1126/science.aal0092. [http://dx.doi.org/ 10.1126/science.aal0092]. [PMID: 28209901]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu P., Lin Y., Porter K., Liton P.B. Ascorbic acid modulation of iron homeostasis and lysosomal function in trabecular meshwork cells. J. Ocul. Pharmacol. Ther. 2014;30(2-3):246–253. doi: 10.1089/jop.2013.0183. [http://dx. doi.org/10.1089/jop.2013.0183]. [PMID: 24552277]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ammar D.A., Hamweyah K.M., Kahook M.Y. Antioxidants protect trabecular meshwork cells from hydrogen peroxide-induced cell death. Transl. Vis. Sci. Technol. 2012;1(1):4. doi: 10.1167/tvst.1.1.4. [eCollection]. [http://dx.doi.org/10.1167/tvst.1.1.4]. [PMID: 24049700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nucci C., Tartaglione R., Cerulli A., Mancino R., Spanò A., Cavaliere F., Rombolà L., Bagetta G., Corasaniti M.T., Morrone L.A. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int. Rev. Neurobiol. 2007;82:397–406. doi: 10.1016/S0074-7742(07)82022-8. [http://dx.doi.org/10.1016/S0074-7742(07)82022-8]. [PMID: 17678974]. [DOI] [PubMed] [Google Scholar]
- 114.Russo R., Cavaliere F., Rombolà L., Gliozzi M., Cerulli A., Nucci C., Fazzi E., Bagetta G., Corasaniti M.T., Morrone L.A. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. 2008. [DOI] [PubMed] [Google Scholar]
- 115.Nakajima Y., Inokuchi Y., Nishi M., Shimazawa M., Otsubo K., Hara H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008;1226:226–233. doi: 10.1016/j.brainres.2008.06.026. [http:// dx.doi.org/10.1016/j.brainres.2008.06.026]. [PMID: 18598676]. [DOI] [PubMed] [Google Scholar]
- 116.Lee D., Shim M.S., Kim K.Y., Noh Y.H., Kim H., Kim S.Y., Weinreb R.N., Ju W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55(2):993–1005. doi: 10.1167/iovs.13-12564. [http://dx.doi.org/10.1167/iovs.13-12564]. [PMID: 24458150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Famili A., Ammar D.A., Kahook M.Y. Ethyl pyruvate treatment mitigates oxidative stress damage in cultured trabecular meshwork cells. Mol. Vis. 2013;19:1304–1309. [PMID: 23805037]. [PMC free article] [PubMed] [Google Scholar]
- 118.Martínez-Águila A., Fonseca B., Pérez de Lara M.J., Pintor J. Effect of Melatonin and 5-Methoxycarbonylamino-N-Acetyltryptamine on the Intraocular Pressure of Normal and Glaucomatous Mice. J. Pharmacol. Exp. Ther. 2016;357(2):293–299. doi: 10.1124/jpet.115.231456. [http://dx.doi.org/ 10.1124/jpet.115.231456]. [PMID: 26941171]. [DOI] [PubMed] [Google Scholar]
- 119.Yang X., Hondur G., Tezel G. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2016;57(4):2344–2354. doi: 10.1167/iovs.16-19153. [http://dx.doi.org/10.1167/iovs.16-19153]. [PMID: 27127934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.San G.J.P., Chew E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. [http://dx.doi.org/10.1016/j. preteyeres.2004.06.002]. [PMID: 15555528]. [DOI] [PubMed] [Google Scholar]
- 121.Nguyen C.T.O., Bui B.V., Sinclair A.J., Vingrys A.J. Dietary omega 3 fatty acids decrease intraocular pressure with age by increasing aqueous outflow. Invest. Ophthalmol. Vis. Sci. 2007;48(2):756–762. doi: 10.1167/iovs.06-0585. [http://dx.doi.org/10.1167/iovs.06-0585]. [PMID: 17251475]. [DOI] [PubMed] [Google Scholar]
- 122.Tourtas T., Birke M.T., Kruse F.E., Welge-Lüssen U.C., Birke K. Preventive effects of omega-3 and omega-6 Fatty acids on peroxide mediated oxidative stress responses in primary human trabecular meshwork cells. PLoS One. 2012;7(2):e31340. doi: 10.1371/journal.pone.0031340. [http://dx. doi.org/10.1371/journal.pone.0031340]. [PMID: 22319624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Inman D.M., Lambert W.S., Calkins D.J., Horner P.J. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One. 2013;8(6):e65389. doi: 10.1371/journal.pone.0065389. [http:// dx.doi.org/10.1371/journal.pone.0065389]. [PMID: 23755225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schnebelen C., Pasquis B., Salinas-Navarro M., Joffre C., Creuzot-Garcher C.P., Vidal-Sanz M., Bron A.M., Bretillon L., Acar N. A dietary combination of omega-3 and omega-6 polyunsaturated fatty acids is more efficient than single supplementations in the prevention of retinal damage induced by elevation of intraocular pressure in rats. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247(9):1191–1203. doi: 10.1007/s00417-009-1094-6. [http://dx.doi.org/10.1007/s00417-009-1094-6]. [PMID: 19437028]. [DOI] [PubMed] [Google Scholar]
- 125.Huang W.B., Fan Q., Zhang X.L. Cod liver oil: a potential protective supplement for human glaucoma. Int. J. Ophthalmol. 2011;4(6):648–651. doi: 10.3980/j.issn.2222-3959.2011.06.15. [PMID: 22553738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dolz-Marco R., Gallego-Pinazo R., Pinazo-Durán M.D., Pons-Vázquez S., Domingo-Pedro J.C., Díaz-Llopis M. Intravitreal docosahexaenoic acid in a rabbit model: preclinical safety assessment. PLoS One. 2014;9(5):e96872. doi: 10.1371/journal.pone.0096872. [http://dx.doi.org/10.1371/ journal.pone.0096872]. [PMID: 24809445]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.García-Medina J.J., Vinuesa-Silva I., Zanón-Moreno V., Santos-Bueso E., Pinazo-Durán M.D. What to eat and drink in glaucoma? Evidence from human studies. Arch. Soc. Esp. Oftalmol. 2014;89(3):89–91. doi: 10.1016/j.oftal.2014.02.017. [PMID: 24661734]. [DOI] [PubMed] [Google Scholar]
- 128.Osborne N.N. 2008. [Google Scholar]
- 129.Chu K.O., Chan K.P., Wang C.C., Chu C.Y., Li W.Y., Choy K.W., Rogers M.S., Pang C.P. Green tea catechins and their oxidative protection in the rat eye. J. Agric. Food Chem. 2010;58(3):1523–1534. doi: 10.1021/jf9032602. [http://dx.doi.org/10.1021/jf9032602]. [PMID: 20085274]. [DOI] [PubMed] [Google Scholar]
- 130.Russo R., Adornetto A., Cavaliere F., Varano G.P., Rusciano D., Morrone L.A., Corasaniti M.T., Bagetta G., Nucci C. Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Mol. Vis. 2015;21:718–729. [PMID: 26167113]. [PMC free article] [PubMed] [Google Scholar]
- 131.Yokoyama Y., Maruyama K., Yamamoto K., Omodaka K., Yasuda M., Himori N., Ryu M., Nishiguchi K.M., Nakazawa T. The role of calpain in an in vivo model of oxidative stress-induced retinal ganglion cell damage. Biochem. Biophys. Res. Commun. 2014;451(4):510–515. doi: 10.1016/j.bbrc.2014.08.009. [http://dx.doi.org/10.1016/j.bbrc.2014.08. 009]. [PMID: 25111816]. [DOI] [PubMed] [Google Scholar]
- 132.Lee D., Kim K.Y., Noh Y.H., Chai S., Lindsey J.D., Ellisman M.H., Weinreb R.N., Ju W.K. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. 2012;7(10):e47098. doi: 10.1371/journal.pone.0047098. [http://dx.doi.org/10.1371/journal.pone.0047098]. [PMID: 23056591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boatright J.H., Moring A.G., McElroy C., Phillips M.J., Do V.T., Chang B., Hawes N.L., Boyd A.P., Sidney S.S., Stewart R.E., Minear S.C., Chaudhury R., Ciavatta V.T., Rodrigues C.M., Steer C.J., Nickerson J.M., Pardue M.T. Tool from ancient pharmacopoeia prevents vision loss. Mol. Vis. 2006;12:1706–1714. [PMID: 17213800]. [PubMed] [Google Scholar]
- 134.Fernández-Sánchez L., Lax P., Pinilla I., Martín-Nieto J., Cuenca N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest. Ophthalmol. Vis. Sci. 2011;52(8):4998–5008. doi: 10.1167/iovs.11-7496. [http://dx.doi.org/10.1167/iovs.11-7496]. [PMID: 21508111]. [DOI] [PubMed] [Google Scholar]
- 135.Fernández-Sánchez L., Lax P., Noailles A., Angulo A., Maneu V., Cuenca N. Natural compounds from saffron and bear bile prevent vision loss and retinal degeneration. Molecules. 2015;20(8):13875–13893. doi: 10.3390/molecules200813875. [http://dx.doi.org/10.3390/molecules200813875]. [PMID: 26263962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eckert A., Keil U., Scherping I., Hauptmann S., Müller W.E. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann. N. Y. Acad. Sci. 2005;1056:474–485. doi: 10.1196/annals.1352.023. [http://dx.doi.org/ 10.1196/annals.1352.023]. [PMID: 16387710]. [DOI] [PubMed] [Google Scholar]
- 137.Cybulska-Heinrich A.K., Mozaffarieh M., Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol. Vis. 2012;18:390–402. [PMID: 22355250]. [PMC free article] [PubMed] [Google Scholar]
- 138.Quaranta L., Bettelli S., Uva M.G., Semeraro F., Turano R., Gandolfo E. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110(2):359–362. doi: 10.1016/S0161-6420(02)01745-1. [http://dx.doi.org/10.1016/S0161-6420(02)01745-1]. [PMID: 12578781]. [DOI] [PubMed] [Google Scholar]
- 139.Shim S.H., Kim J.M., Choi C.Y., Kim C.Y., Park K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food. 2012;15(9):818–823. doi: 10.1089/jmf.2012.2241. [http://dx.doi.org/10.1089/jmf.2012.2241]. [PMID: 22870951]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee J., Sohn S.W., Kee C. Effect of Ginkgo biloba extract on visual field progression in normal tension glaucoma. J. Glaucoma. 2013;22(9):780–784. doi: 10.1097/IJG.0b013e3182595075. [http://dx.doi.org/10.1097/IJG.0b013e3182595075]. [PMID: 22595937]. [DOI] [PubMed] [Google Scholar]
- 141.Park J.W., Kwon H.J., Chung W.S., Kim C.Y., Seong G.J. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J. Ophthalmol. 2011;25(5):323–328. doi: 10.3341/kjo.2011.25.5.323. [http://dx.doi.org/10.3341/kjo.2011.25.5. 323]. [PMID: 21976939]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guo X., Kong X., Huang R., Jin L., Ding X., He M., Liu X., Patel M.C., Congdon N.G. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: a randomized, crossover clinical trial. Invest. Ophthalmol. Vis. Sci. 2014;55(1):110–116. doi: 10.1167/iovs.13-13168. [http://dx.doi.org/10.1167/ iovs.13-13168]. [PMID: 24282229]. [DOI] [PubMed] [Google Scholar]
- 143.Sari M.D., Sihotang A.D., Lelo A. Ginkgo biloba extract effect on oxidative stress marker malonildialdehyde, redox enzyme gluthation peroxidase, visual field damage, and retinal nerve fiber layer thickness in primary open angle glaucoma. Int. J. Pharm. Tech. Res. 2016;9:158–166. [Google Scholar]
- 144.Ohguro H., Ohguro I., Katai M., Tanaka S. Two-year randomized, placebo-controlled study of black currant anthocyanins on visual field in glaucoma. Ophthalmologica. 2012;228(1):26–35. doi: 10.1159/000335961. [http://dx.doi.org/10.1159/000335961]. [PMID: 22377796]. [DOI] [PubMed] [Google Scholar]
- 145.Ohguro H., Ohguro I., Yagi S. Effects of black currant anthocyanins on intraocular pressure in healthy volunteers and patients with glaucoma. J. Ocul. Pharmacol. Ther. 2013;29(1):61–67. doi: 10.1089/jop.2012.0071. [http://dx.doi.org/10.1089/jop.2012.0071]. [PMID: 23046438]. [DOI] [PubMed] [Google Scholar]
- 146.Jabbarpoor Bonyadi M.H., Yazdani S., Saadat S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: a pilot study. BMC Complement. Altern. Med. 2014;14:399. doi: 10.1186/1472-6882-14-399. [http://dx.doi.org/10.1186/1472-6882-14-399]. [PMID: 25319729]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Falsini B., Marangoni D., Salgarello T., Stifano G., Montrone L., Di Landro S., Guccione L., Balestrazzi E., Colotto A. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247(9):1223–1233. doi: 10.1007/s00417-009-1064-z. [http://dx.doi.org/10.1007/s00417-009-1064-z]. [PMID: 19290537]. [DOI] [PubMed] [Google Scholar]
- 148.Cellini M., Rossi A., Moretti M. The use of polyunsaturated fatty acids in ocular hypertension. A study with blue on-yellow perimetry. Acta Ophthalmol. Scand. Suppl. 1999;77:54–55. [http://dx. doi.org/10.1111/j.1600-0420.1999.tb01152.x]. [Google Scholar]
- 149.Impact of Oral Versatile Antioxidants on Glaucoma Progression https://clinicaltrials.gov/ct2/show/
- 150.Safety and Efficacy of Anti-Oxidants and Anti-inflammatory Agents in Glaucoma and Diabetic Retinopathy https://clinicaltrials. gov/ct2/show/NCT02984813
- 151.The Effects of an Antioxidant Formulation on Ocular Blood Flow, NCT01930487. https://clinicaltrials.gov/ct2/show/
- 152.Ginkgo Biloba and Ocular Blood Flow in Primary Open-angle Glaucoma, NCT02376114. https://clinicaltrials. gov/ ct2/show/NCT02376114