Abstract
Background:
At present intraocular pressure (IOP) lowering therapies are the only approach to treat glaucoma. Neuroprotective strategies to protect the retinal ganglion cells (RGC) from apoptosis are lacking to date. Substantial amount of research concerning the role of the immune system in glaucoma has been performed in the recent years. This review aims to analyse changes found in the peripheral immune system, as well as selected local changes of retina immune cells in the glaucomatous retina.
Methods:
By dividing the immune system into the innate and the adaptive immune system, a systematic literature research was performed to find recent approaches concerning the modulation of the immune system in the context of glaucoma. Also ClinicalTrials.gov was assessed to identify studies with a translational context.
Results:
We found that some aspects of the immune system, such as changes in antibody levels, changes in toll like receptor signalling, T cells and retinal microglial cells, experience more research activity than other areas such as changes in dendritic cells or macrophages. Briefly, results from clinical studies revealed altered immunoreactivities against retinal and optic nerve antigens in sera and aqueous humor of glaucoma patients and point toward an autoimmune involvement in glaucomatous neurodegeneration and RGC death. IgG accumulations along with plasma cells were found localised in human glaucomatous retinae in a pro-inflammatory environment possibly maintained by microglia. Animal studies show that antibodies (e.g. anti- heat shock protein 60 and anti-myelin basic protein) elevated in glaucoma patients provoke autoaggressive RGC loss and are associated with IgG depositions and increased microglial cells. Also, studies addressing changes in T lymphocytes, macrophages but also local immune responses in the retina have been performed and also hold promising results.
Conclusions:
This recapitulation of recent literature demonstrates that the immune system definitely plays a role in the pathogenesis of glaucoma. Multiple changes in the peripheral innate as well as adaptive immune system have been detected and give room for further research concerning valuable therapeutic targets. We conclude that there still is a great need to bring together the results derived from basic research analysing different aspects of the immune system in glaucoma to understand the immune context of the disease. Furthermore local immune changes in the retina of glaucoma patients still leave room for further therapeutic targets
Keywords: Immunomodulation, B cells, T cells, glaucoma, neuroprotection, immune system
1. INTRODUCTION
Glaucoma is a group of multifactorial, neurodegenerative diseases and one of the leading causes for blindness worldwide. Approximately 111.8 million people will be affected by the disease in the year 2040 [1]. The main risk factor for developing glaucoma is an elevated intraocular pressure (IOP), followed by advanced age [2]. Although other factors such as mitochondrial dysfunction [3], endoplasmic reticulum stress [4] and oxidative stress [5] are involved in glaucomatous apoptosis of retinal ganglion cells (RGC), the elevated IOP up to date is the only target that can be addressed therapeutically, either through IOP lowering eye drops or with a surgical approach. Substantial amount of research concerning the role of the immune system in glaucoma has been performed in the recent years. This review aims to analyse changes found in the peripheral immune system, as well as selected local changes of retina immune cells in the glaucomatous retina. Very briefly, the immune system can be divided into the innate and the adaptive immune system. One of the main functions the innate immune system inherits is to prevent infections and diseases induced by potential pathogenic agents. In comparison to the adaptive immune system which is antigen specific, the innate immune system reacts to none-specific antigens. The innate immune system includes anatomical barriers such as the skin or mucosal surfaces which ensure a physical barrier against viruses, bacteria, parasites as well as and other foreign particles. The innate immune system furthermore includes antibacterial proteins and several phagocytic cells that recognize conserved features of pathogens and can be activated within hours. Cells belonging to the innate immune system include granulocytes, monocytes and macrophages, dendritic cells as well as innate lymphoid cells. Cells such as eosinophils, neutrophils or mast cells belong to the group of the granulocytes. Next to being responsible for pathogen defence, the innate immune system also initiates and directs the adaptive immune response [6, 7]. Antigen-specific lymphocytes are the main cellular component of the adaptive immune system. In comparison to the innate immune system, the adaptive immune system requires up to 7 days to be activated. In this review, we will focus on the main cellular components of the innate as well as adaptive immune system in context with possible modulations for glaucoma therapy.
1.1. Macrophages
Macrophages belong to the innate immune system. One of their main functions is the phagocytosis of microorganisms and the subsequent induction of T cell mediated immune responses. They are also important for tissue repair under non-infectious inflammatory situations and can discriminate self from non-self [8]. Classically activated macrophages produce pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF), IL-12, and IL-18 and have microbicide functions. In contrast, alternatively activated macrophages secrete IL-10 and IL-12 and are involved in tissue remodeling and allergic diseases and also play a role in modulating tumor environments [9]. Furthermore, macrophage derived IL-6 as well as IL-12 can induce antibody production and activate CD4 T cells. Additionally macrophages characterised by the cluster of differentiation (CD) marker 169 are involved in maintaining immunological tolerance [8]. Macrophage research concerning glaucoma is fairly sparse. Mostly macrophages from the classically activated type (M1 type (CD68+, CD163-)) as well as dendritic cells can be found in the healthy human scleral stroma and episcleral tissue [10]. Retinal macrophages are involved in different pathologies such as laser induced anterior ischemic optic neuropathy (AION) [11] and are also increased in human retinae of patients with age related macular degeneration (AMD), where they play a role in angiogenesis [12]. Studies analysing the trabecular meshwork of patients with POAG as well as primary acute angle closure glaucoma found macrophages in this tissue by means of scanning electron microscopy [13]. Furthermore, using an optic nerve crush model in rats and applying Zymosan intravitreally RGC axonal regeneration could be provoked. Zymosan is a macrophage activator. The idea for using Zymosan was fed by the fact that lens injury is capable of inducing RGC regeneration by macrophage infiltration. Macrophages are thought to possess parts of their neuroregenerative effects via Growth Associated Protein 43. Zymosan can induce inflammatory signals in macrophages through the Toll-like receptors TLR2 and TLR6 [14]. Still the authors state that a finer definition of the protective active factors of the macrophages is needed in order to bring forward a therapeutic approach [15].
1.2. Dendritic Cells in Glaucoma
Dendritic cells (DCs) are antigen-presenting cells that play pivotal roles in the initiation of the adaptive immune response [16]. Regarding glaucoma there are few studies showing an involvement of this cell type in glaucoma. Dendritic cells can be characterised by several cluster of differentiation (CD) markers such as CD141, CD8a, CD103 or CD11b+ as well as by the chemokine receptor Xcr1 [17]. As described in detail by Vu Manh et al. in 2015, cell surface as well as functional analyses should be performed to define DCs [18]. Lehmann et al. found DCs in the quiescent retina of a transgenic CD11c-DTR (diphtheria toxin receptor) mouse line. CD11c is a frequent marker used for murine DCs. The cells were detected in the peripapillary region but also the peripheral as well as the far peripheral retina. An increase of DCs was detected after performing an optic nerve crush. Furthermore, the CD11 positive dendritic cells showed an upregulation of MHC Class II as a result of dendritic cell activation. Moreover, an increase in DC was detected in the contralateral eye [19]. Other studies working with DBA/2J (D2) mice showed an involvement of bone marrow derived immune cells in pigmentary glaucoma. DBA/J2 mice develop a form of pigmentary glaucoma which is caused by mutations of the Gpnmb and Tyrp1 genes. Gpnmb (Transmembrane glycoprotein NMB) is also expressed in some types of dendritic cells. The authors hypothesised that dendritic cells with a Gpnmb mutation and therefore lacking Gpnmb, cannot prevent pigment dispersion triggered by bone marrow derived cells. They assume that this possibly is due to the fact that the DC with the mutation can alter ocular immune tolerance [20]. To the best of our knowledge, clinical trials using dendritic cells as therapeutic approach have not been performed for glaucoma so far but an interesting phase 1B, single group assignment, open label treatment study applying tolerogenic dendritic cells loaded with myelin peptides in patients suffering from optic myelitis in multiple sclerosis is being performed (ClinicalTrials.gov Identifier: NCT02283671). Tolerogenic DCs e.g. have the capability of inducing regulatory T cells as well as influencing autoimmunity [21].
1.3. Toll-like Receptor Signalling in Glaucoma
Toll-like receptors (TLR) are part of the innate immune system and belong to the pattern recognition receptors. They recognise different structures such as saccharides, peptidoglycans, nucleic acids and lipoproteins. In response an adaptive immune response can be triggered which is mediated by proinflammatory cytokines. TLR can be detected on several immune cells such as T cells, B cells, macrophages or dendritic cells. There they can be found not only on the cell surface (TLR2, TLR2, TLR4, TLR5 and TLR6) but also in the endosomes (TLR3, TLR7, TLR8 and TLR9). Next to recognising bacterial lipopolysaccharides or viral double- or single-stranded RNA, TLR activation can be involved in autoimmunity by activation through self-components. One ligand that is non-pathogenic and can be recognised by TLRs are the heat shock proteins (HSP) [22]. A study performed by Luo et al. analysed the occurrence of TLRs in human glaucoma donor eyes and furthermore was able to detect that HSPs and oxidative stress can stimulate immune activity through glial TLR signaling in rat retinal microglia and astrocytes in vitro. Massspectrometric analysis showed an increase of TLR2, TLR4 isoform A, TLR7, TLR8 isoform 1 and TLR10 precursor in the retinae of glaucoma donor eyes. Immunohistochemical analysis of rat retinal microglia and astrocytes in vitro demonstrated that TLR2, TLR3 and TLR4 were detected on both cell types, although TLR3 was predominantly found on astrocytes whereas TLR2 and TLR4 were predominantly detected in microglial cells. Incubating the cells with HSP60, -70 and subsequent H2O2 stress provoked an increase in TLR expression of the cells. This was accompanied by an upregulation of TNF-α secretion [23]. A study aiming to unravel the potential role of TLR4, the inflammasome as well as IL-1β in IOP induced RGC death was performed using animal models of acute glaucoma. By increasing IOP up to levels of 110mmHg, retinal ischaemia was induced. TLR4 was specifically chosen for this study as previous studies had been able to demonstrate that TLR4 deficient mice were protected against ischaemic brain damage. The authors were able to detect TLR4 involvement in IOP induced RGC death. TLR4 mRNA was significantly increased, as well as TLR4 protein levels. Using TLR4 knockout mice RGC death was significantly decreased. Furthermore upregulation of caspase 8 was demonstrated. By inhibiting caspase 8 through intravitreal injections of an inhibitor, RGC death was reduced, as well as microglial activation. Caspase 8 was not increased after IOP elevation in TLR4 knockout mice. The authors conclude that suppression of the TLR4/caspase 8 and IL-1β axis could be a potential therapeutic treatment strategy for glaucoma [24]. A study looking at the oral microbiome of glaucoma patients found increased oral bacterial loads in glaucoma patients. Therefore the effect of bacterial lipopolysaccharide (LPS) on glaucoma pathology was studied in 2 different glaucoma animal models. Using DBA/2J and a microbead-induced IOP glaucoma model more optic nerve damage was detected in mice with additional subcutaneous LPS injections. Myd88 gene upregulation was detected in the animals. Myd88 is a downstream target of TLR4, which itself is the main receptor for LPS. Furthermore higher levels of complement factor C1q were detected in eyes with larger optic nerve damage. By partially inhibiting TLR4 with Naloxone RGC damage could be reduced in DBA2/J mice. Additionally the authors could detect increased numbers of CD11b+ cells in the optic nerve prelaminar region of LPS treated animals indication activated microglial cells. The authors conclude that bacterial activity could contribute in glaucoma pathophysiology by TLR4 as well as microglial activation [25]. A study of blood derived leukocytes from Japanese glaucoma patients also could reveal that TLR4 polymorphisms were correlated to NTG as well as POAG or pseudoexfoliation glaucoma [26]. Contrary results were demonstrated for TLR4 polymorphisms in South Korean NTG patients [27]. This also was true for Saudi Arabian POAG patients [28].
1.4. T Lymphocytes
Although changes in T lymphocytes have been detected frequently for other neurodegenerative diseases with an immune component, such as Parkinson’s disease (PD) as well as Alzheimer’s disease (AD), there are only few studies analysing T lymphocytes in the serum of glaucoma patients. One study performed by Yang et al. looked at the changes of different T lymphocyte groups in glaucoma patients. They detected a non-significant increase in CD3+/CD4+ T-lymphocytes in POAG patients as well as a significant increase in CD3+/CD8+ T-lymphocytes in POAG and normal tension glaucoma (NTG) patients. An increase of the soluble interleukin-2 (IL-2) receptor which is a marker for T lymphocyte activation was found in POAG and NTG patients. The soluble IL-2 receptor plays an important role in infectious, autoimmune diseases as well as metastatic solid tumours [29, 30]. Using an experimental autoimmune glaucoma (EAG) model, Kuehn et al. conclude that possibly peripheral changes in lymphocytes of animal glaucoma models could be epiphenomenal rather than causative for RGC loss. Lewis rats were immunized either with bovine optic nerve homogenate (ONA) or purified S100 applied intraperitoneally in order to induce RGC loss. The authors were able to detect a small increase of CD3+ T lymphocytes in the inner retinal layers of animals immunized with ONA. Staining against CD4 was performed but only could reveal very marginal amounts of CD3+/CD4+ T Helper cells. Therefore the type of T cells infiltrating the retinal ganglion cell layer in the ONA immunised animals remains elusive. None of the experimental groups showed T cell infiltration of the optic nerve. Additionally a slight, but significant reduction of the T cell population of the spleen in ONA animals was demonstrated. Analysing the results the authors conclude that systemic changes of T cell populations in glaucoma especially in this model are most likely a secondary, epiphenomenal effect. This conclusion is in conformity to other published results in glaucoma animal models showing delayed changes of spleen lymphocytes in comparison to the early microglial changes in the retina of the animals [31].
In contrast a study published by Gramlich et al. showed that T lymphocytes could play a crucial role in glaucomatous RGC loss. Using an adoptive lymphocyte transfer approach, splenocytes from two hereditary glaucoma models (B6.Sh3pxd2bnee and B6SJL;Tg-MYOCY437H mice) were transferred to either C57BL/6J or B6:SJL mice. Specifically either CD3+ or CD19+ cells were transferred. CD3+ cells represented T lymphocytes whereas CD 19+ cells represented B lymphocytes. RGC and optic nerve analysis was performed. 4 months after cell transfer a significant reduction of RGCs could be observed in mice receiving splenocytes from either of the glaucoma donor mice. Further analysis showed that the transferred T lymphocytes had a larger impact on RGC loss than the transferred B lymphocytes. Analysing the recipient’s retina, small amounts of T and B lymphocytes were detected in all animals regardless of receiving healthy or glaucoma splenocytes. The optic nerve did not show any lymphocyte infiltration. Although it is possible to draw some tentative comparisons to findings in the retinae of human glaucoma samples, the authors cannot rule out, that the detected changes rather are provoked by the genetic modification of the used models. Furthermore, other questions such as the mechanism of RGC loss in this model are speculated on but in the end remain unanswered. The authors cannot conclude but also cannot completely rule out that the detected RGC damage is caused directly by T lymphocytes infiltrating the retina [32]. T lymphocytes can migrate through the intact blood retina barrier in a non-inflamed site [33]. Furthermore, increased amount of activated T cell trafficking due to physiological breakdown of the blood retina barrier during ageing has been demonstrated [34].
A study published by Wax et al. aimed to analyse the role of Fas-ligand (FasL) in the interactions between activated T cells and RGC. Previous studies had been able to show the involvement of FasL which belongs to the TNF family in the apoptotic elimination of T cells in the brain and the eye. Lewis rats were immunised with recombinant heat shock proteins (HSPs) and RGC decrease could be detected. By labelling against T cell receptor αβ (TCRαβ) T cell infiltration into the retina was demonstrated for a transient amount of time. Subsequently the authors analysed whether CD3+ T cells isolated from rats after HSP60 or HSP27 immunization where capable of inducing RGC apoptosis in vitro. This was true for the conditioned medium gathered from HSP60 immunised T cells. The analysis of the medium was able to reveal higher levels of sFasL [35].
Studies looking at specific T cell subgroups in glaucoma, such as T-Helper cells or regulatory T cells have also been performed.
1.5. T-Helper Cells and their Relevant Cytokines
Depending on their cytokine secretion CD 4 + T cells can be divided into 2 sets of T Helper cells, namely T-Helper 1 (Th1) and T-Helper 2 (Th2) cells. Th1 cells are predominantly important for pathogens control and macrophage activation, whereas Th2 cells are known to stimulate antibody production through B cells [36]. Furthermore Th2 cells seem to be beneficial in the context of CNS lesions. Studies using a glaucoma rat model hypothesized that Th1/Th2 cytokine imbalance plays a role in glaucomatous neurodegeneration. Th1 cells secret cytokines such as interferon- gamma (IFN-γ), IL-2, IL-12, and tumour necrosis factor (TNF)-α whereas Th2 cells secret cytokines necessary for B cell activation such as IL-4, IL-5, IL-6, IL-10 and IL-13. Wong et al. analysed iris material from POAG as well as chronic angle closure glaucoma (CACG) patients undergoing trabeculectomy as well as iris material from non-glaucomatous donor eyes. Th1 cell cytokines, Th2 cell cytokines as well as the Th3 cell cytokine transforming growth factor beta (TGF-β) were examined in the iris material. The Th1 cell cytokine IL-2 as well as the Th3 cytokine TGF-β were significantly increased in both glaucomatous materials whereas the Th2 cell cytokine IL-6 was significantly decreased in the iris of POAG patients. IFN-γ was significantly increased in the iris material of CACG patients whereas other cytokines were either not detectable or did not show significant changes between the groups. The authors hypothesize that this cytokine imbalance leads to changes in the immune microenvironment and plays an important role in glaucomatous optic nerve damage [37]. Another study immunohistochemically analysing the trabecular meshwork of a small number of glaucoma patients in comparison to non-glaucoma donors, shows increased expression levels of the pro-inflammatory cytokines IL-6, IL-1β, TNF-α as well as TGF-β1 in the trabecular meshwork of POAG patients. Using an electron microscopic approach, the authors found TGF-β1 in the extracellular region of the cribriform trabecular meshwork. TGF- β1 can induce fibrosis which then can lead to remodelling of the extracellular matrix and elevated IOP. Furthermore TGF- β1 can increase trabecular meshwork rigidity by promoting differentiation of fibroblasts to myoblasts. TGF- β1 also induces the expression of pro-inflammatory IL-6. Therefore the authors conclude that topical and systemic anti-inflammatory treatment should be administered in addition to IOP reducing therapy. By dampening the pro-inflammatory cytokine production a slow increase in IOP due to fibrotic and inflammatory changes of the trabecular meshwork could be reduced [38]. A recent meta-analysis performed by Agarwal et al. detected elevated levels of TGF-β2 in the aqueous humour of POAG patients. TGF-β2 is supposed to be the predominant TGF-β form in the eye and acts as an immunosuppressant. Furthermore it is involved in the synthesis of extracellular matrix components and therefore possibly responsible for IOP increase [39]. Recently a phase I clinical trial using an antisense nucleotide targeting TGF-β2 called ISTH0036 is being performed (Isarna Therapeutics, Phase I Dose Escalation Study to Investigate the Safety of ISTH0036 in Subjects With Glaucoma Undergoing Trabeculectomy, https://clinicaltrials.gov/ct2/show/NCT02406833). Antisense nucleotides cleave or disable mRNA by selectively binding cognate mRNA sequences. The agent used in this study aims to reduce scaring after trabeculectomy. Furthermore it is supposed to reduce trabecular meshwork transformation. A first interims analysis of the nine treated patients did not show any safety concerns, further data analyses are still outstanding (https://www.isarna-therapeutics.com/news-events/press-releases/2016/). Furthermore a randomized, double-masked, multi-centre, placebo-controlled trial Phase III Study aiming to prevent scaring after trabeculectomy has been performed. Within this study the human, monoclonal immunoglobulin G4 antibody CAT-152 (lerdelimumab) targeted against TGF-β2 was injected subconjunctivaly shortly before as well as after trabeculectomy. Treatment success was determined as primary outcome measure and was defined by an unmedicated IOP <17mmHg 6 and 12 months after surgery. Unfortunately the results failed to show a difference in the rate of success in patients receiving CAT-152, nevertheless there were no safety concerns [40].
Another neuroprotective therapeutic option with the trade name Copaxone® and the active ingredient Glatiramer (GA) targets T cell responses and has been tested in animal glaucoma models. GA is a synthetic mixture of four amino acid copolymers (L-alanine, L-lysine, L-glutamic acid and L-tyrosine). It was discovered during studies actually aiming to induce experimental autoimmune encephalomyelitis (EAE). These amino acids were chosen analogue to the autoantigen myelin basic protein (MPB) in multiple sclerosis (MS). Instead of inducing EAE the amino acid composition showed protection against EAE [41]. This finding eventually resulted in an approved therapy against MS under the name Copaxone® which is still widely used [42]. The mode of action of GA is not fully understood. Next to the widely accepted shift of the T cell response from a pro-inflammatory to a non-inflammatory response induced by Copaxone® other modes of action have been reviewed in detail recently [41]. Using an optic nerve crush model Kipnis et al. were able to demonstrate that neuroprotection could be triggered either by adjuvant active immunisation with Copaxone (Cop-1) or through the adoptive transfer of Cop-1 reactive T- cells. The rats were injected with Cop-1 reactive T cells on the day of the injury or alternatively immunised with Cop-1 immediately after the optic nerve crush and subsequently fed with Cop-1 for 5 days. Both groups showed protective effects on the nerve fibres of the optic nerve. The authors conclude that both treatment options lead to post-traumatic neuroprotection by boosting the autoimmune T cell response which is induced by the optic nerve crush without inducing any other side effects [43]. Protective autoimmunity is a mechanism found in non-pathogen derived CNS damage and has the function to induce a protective, rather than a destructive self-immune response. Optic nerve crush models show T cell infiltration at the site of lesion. As stated in her review discussing the possibility of a vaccination for glaucoma, M. Schwartz concludes that vaccination with specific antigens only addresses one part of the problem and does not involve other important factors contributing to glaucoma. Furthermore this treatment option cannot prevent the disease but rather is meant to halt its progression [44]. Further studies also showed the neuroprotective effect of Cop-1 immunisation on retinal ganglion cells in various other animal glaucoma models such as an IOP elevation model through photocoagulation of the scleral veins [45, 46]. A clinical randomized, double blind phase III trial aiming to analyse the neuroprotective effect of Cop-1 in acute primary angle closure glaucoma patients with the Singapore Eye Research Institute as lead sponsor was registered at clinical trials in 2013 (ClinicalTrials.gov Identifier: NCT01936129). The aim was to analyse the effect of 20mg Cop-1 administered subcutaneously no later than 7 days after the attack and 24 h after presentation at the hospital in addition to standard therapy. Furthermore another injection was to follow one week later. The study aimed to assess the neuroprotective effect of Cop-1 in reducing functional and structural damage after acute primary angle closure (APAC). The primary outcome measure was the point-wise linear regression in the visual fields and the aim had been to include 196 patients in this trial. To the best of our knowledge, no results have been published concerning this study so far.
1.6. Regulatory T Cells in Glaucoma
Not many studies analysing the changes in T cells and especially regulatory T cells (Tregs) have been performed in glaucoma research. Tregs belong to the subset of CD4+ T cells and maintain self-tolerance by suppressing autoimmunity. They play a major role in maintaining immune homeostasis. Changes in levels of regulatory T cell levels have been detected for several autoimmune diseases such as systemic lupus erythematosus (SLE), and MS although studies show contrary results concerning the levels of regulatory T cells in these diseases. A recent study showed that the level of regulatory T cells was unchanged in SLE patients but elevated levels of activated effector T phenotypes (CD4+/CD25+/++/FOXP3+, CD4+/CD25+/CD45RA-/CD45RO+) were recorded [47]. Other groups in contrast conclude that changes of regulatory T cell levels (CD4+/CD25+/Foxp3+) could be a good indicator for the activity of SLE and showed reduced numbers in active SLE patients [48]. Reduced levels of CD4+/CD25+ activated T lymphocytes were also detected in patients with Parkinson’s disease [49].
Regulatory T cells were originally characterized on the basis of constitutive CD4 and CD25 surface expression. The transcription factor forkheadbox P3 (FoxP3) was then detected to be linage specific for regulatory T cells and more recently CD127 is used as specific regulatory T lymphocyte marker [50]. We performed a study analysing the levels of CD4+/CD25+ T lymphocytes in glaucoma patients. CD4+ cells were also evaluated in both groups. Using peripheral blood samples we found that POAG patients and healthy controls had the same amount of CD4+ peripheral T lymphocytes but that POAG patients showed a significant higher percentage of CD4+/CD25+ regulatory T cells (8.45% in POAG patients in comparison to 5.49% in healthy controls; p<0.01) [51]. A previous study also analysing this T cell population in glaucoma patients in contrast could not find any differences in this cell population, which could be due to the fact that very small cell numbers were analysed [29].
1.7. Cytotoxic T Cells in Glaucoma
The analysis of conjunctival biopsies from POAG patients in comparison to non-glaucoma patients lead to the assumption that T cells are involved in post-operative failure of deep-sclerectomy. POAG patients showed higher amounts of plasma cells, T Helper cells as well as CD8+ cytotoxic T cells [52]. The pre-operative treatment of the patients is not stated in the study. All patients had received IOP lowering topical medication containing topical benzalkonium chloride (BAK) but it is unclear whether the patients received additional Dexamethasone eye drops before surgery. Pre-operative Dexamethasone eye drops, which can be used before surgery could possibly have a positive effect of the number of cytotoxic T cells [53, 54]. Furthermore Yang et al. detected higher concentrations of CD8+ cytotoxic T cells in NTG patients [29]. Cytotoxic T cells have importance functions in host defence against cytosolic pathogens, are capable of eliminating other cells by inducing apoptosis and are antigen specific [55]. They also release cytokines, especially IFN-γ, TNF-α, and TNF-β. As far as we can judge, these cells have so far not been specifically addressed in clinical or animal studies concerning glaucoma.
1.8. B Lymphocytes and Antibodies
Changes of antibody levels in the serum of glaucoma patients have been demonstrated showing not only enhanced, but also decreased antibody levels. Using western blot analysis complex autoantibodies were detected not only in the serum of POAG but also of NTG patients [56]. Other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and Myasthenia gravis also show changes in autoantibody levels [57]. Autoantibody changes such as elevated levels of antibodies against alpha-fodrin, glutathione-S-transferase, spectrin [58-60] and Hsp70 [61] and decreased levels of antibodies against αB Crystalline and Vimentin [61] were detected in the serum of glaucoma patients. Equivalent changes in the autoantibody levels could also be detected in the aqueous humour of the patients. Using a microarray approach elevated level of antibodies against HSP70, α-fodrin as well as lower levels of GFAP, ubiquitin or β-l Crystalline could be detected not only in the serum but also the aqueous humour of POAG patients. The intra-individual analysis showed that most of the detected antibodies (70%) were found with corresponding changes in the serum as well as the aqueous humour. Only a few of the analysed antibodies showed oppositional levels in the serum in comparison to the aqueous humour. Antibodies only elevated in the aqueous humour were targeted against e.g. fibronectin (in POAG and control patients) and γ-Crystalline in POAG patients. Further information concerning possible functions of autoantibodies differently regulated in the aqueous humour of patients as well as healthy subjects still has to be obtained [62]. Although changes in the levels of autoantibodies have been detected in several studies it still is not clear whether these are epiphenomenal or causative. A study analysing autoantibody reactivities in the serum of patients after an acute angle-closure glaucoma with IOPs > 40mmHg showed that antibodies against HSP27, tubulin-tyrosine ligase-like protein 12 and neuron-specific enolase (NSE) showed an increasing linear trend over time (up to week 12) (p<0.05). The authors conclude that the antibody changes detected in this study are due to IOP elevation and the effect on the retinal ganglion cells. Nevertheless this still cannot answer the question whether the antibodies themselves lead to a glaucomatous damage in humans. This only has been demonstrated in animal models without previous IOP elevation [63].
The effect of several antibodies differently regulated in glaucoma patients on retinal ganglion cells has been analysed. Tezel et al. found that HSP27 antibodies induced apoptosis in human retinal ganglion cells in vitro. Furthermore, they were able to show that apoptosis of the cells was accompanied by the internalization of the antibodies. The authors concluded that the antibody internalisation was HSP27 specific rather than driven via an Fc receptor or a TNF receptor. The authors could show that HSP27 antibodies led to degradation of actin in the cells in a caspase dependent manner. Cell loss was mostly found in the retinal ganglion cell layer [64].
We analysed the effect of the serum of glaucoma patients as well as the effect of several antibodies on retinal ganglion cells. We found that the antibodies in the serum of glaucoma patients rather than an applied elevated hydrostatic pressure showed a larger effect on the protein expression profiles of neuroretinal cells [65]. In our subsequent studies we aimed to take a closer look at the effects of specific antibodies found in lower concentrations in glaucoma patients. These autoantibodies belong to the natural autoimmune system. Natural autoantibodies are self-reactive antibodies also detected in healthy humans without causing diseases. They are considered as regulatory factors [66, 67]. Up-regulated autoantibodies can be auto-aggressive and therefore lead to pathogenic conditions [68]. Lower autoantibody concentrations can also be detected in other neurodegenerative diseases such as in Alzheimer’s disease [69]. We hypothesise that lower antibody levels lead to changes in their regulatory function, and therefore lead to a higher vulnerability of retinal ganglion cells in in glaucoma patients.
The effect of γ-Synuclein, 14-3-3 and GFAP antibodies, which are all detected in lower concentrations in glaucoma patients, on neuroretinal cells as well as on RGCs of an adult retinal organ culture was analysed. γ-Synuclein is involved in neurodegenerative and ocular diseases, is highly expressed in RGCs. Changes in localisation of the protein can be detected in the optic nerve head and retina of glaucoma patients in comparison to healthy controls [70, 71]. Using the neuroretinal cell line RGC5 we found that γ-Synuclein antibodies have protective effects on cells stressed with glutamate as well as H202. We were aware of the fact that RGC5 cells are subject of discussion and do not represent a pure RGC line. A review on this cell line nevertheless stated that the majority of the published articles characterising this cell line showed Brn3 and Thy 1 staining. Additionally nestin expression was detected in RGC5 cells showing their neuronal origin. Therefore the authors concluded that RGC5 cells can act as retinal cell line of neuronal origin and can be used in order to follow up initial hypotheses [72]. Significant increased cell viability of up to 15% (p<0.05) when preincubating the cells with 0.05 - 5 µg/ml γ-Synuclein antibodies and additional stressing with H2O2 in comparison to the control cells only treated with H2O2 were detected. Furthermore significantly decreased ROS-level of up to −12% (p = 0.010) were demonstrated. The antibodies also had a protective effect on cells stressed with glutamate (viability increase of up to 14%). The mass-spectrometric analysis showed changes of the intrinsic apoptotic pathway, such as a significant down-regulation of BAX, VDAC 1/2/3 and S100A4 and an up-regulation of BIRC6. These proteins all are involved in the regulation of the mitochondrial apoptosis pathways and were regulated in an anti-apoptotic manner [73]. We could also detect protective effects of the 14-3-3 as well as the GFAP antibodies on neuroretinal cells. 14-3-3 antibodies provoked an increased in cell viability of 22% (p < 0.01) in a concentration of 10 μg/ml and additional stress with H2O2 as well as a significantly decreased ROS-production of 31% (p < 0.01). An involvement of the intrinsic apoptotic pathway such as significant down-regulation of BAX, PRFA1, VDAC 1/2/3 and S100A4 as well as significant up-regulation of BIRC6 and ERK1 were demonstrated [74]. The GFAP antibodies showed protective effects on H202 stressed cells (increased viability and lower ROS-levels of up to 9%). Due to the mass-spectrometric results we assume that the effect is due to changes provoked in the actin cytoskeletal signalling pathway. This pathway also is involved in apoptotic processes [75]. Furthermore we could detect an internalization of 14-3-3 antibodies and γ-Synuclein antibodies, the GFAP antibody was not internalised but rather showed a cross reactivity with and binding to ERP57 on the cell membrane of the cells.
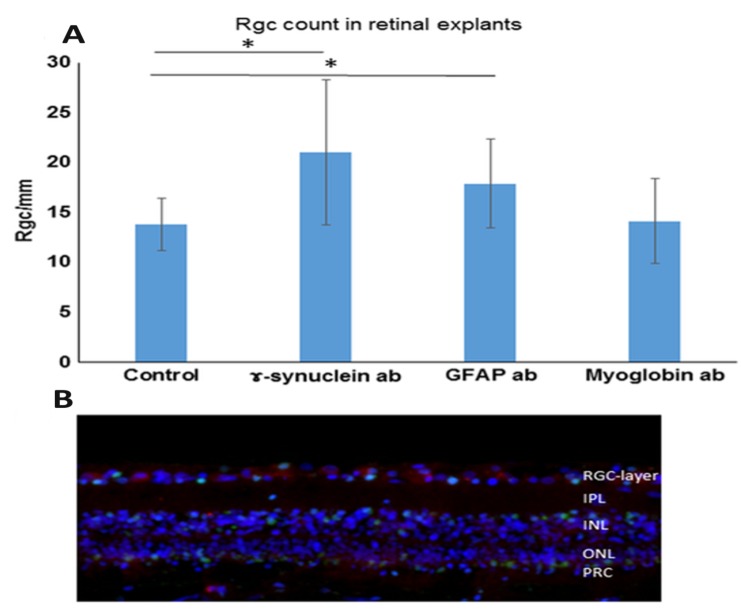
In a recent study we analysed the effect of these antibodies on primary RGCs in a porcine adult retinal explant organ culture. We could detect a significant increase in survival of RGC/mm in γ-Synuclein antibody (21.0 RGC/mm) as well as GFAP antibody (17.9 RGC/mm) treated retinal explants in comparison to the untreated controls (13.8 RGC/mm), see Fig. (1). The mass-spectrometric analysis of the retinae incubated with the protective antibodies showed an involvement of the ER stress pathway. Calreticulin, calnexin, glucose-related protein 94 (Grp94), HSP 70 kDa 2, binding immunoglobulin protein (BiP), HSP 60 kDa 1 and reticulon 1 were down-regulated in GFAP incubated cells. Higher levels of Cdc42 as well as a down-regulation of reticulon 4 were detected in γ-Synuclein antibody treated cells. Both groups showed an elevated expression of glutamine synthetase. This was detected in the mass-spectrometric analysis and confirmed by immunohistochemical staining. In addition we found a shift of the glutamine synthetase within the Müller cells towards the retinal ganglion cell layer of the antibody treated explants. We therefore conclude that the antibodies not only have direct effects on RGC but also possess their protective functions on RGC indirectly via the Müller cells [76]. Animal models found a correlation between systemic IgG autoantibody reactivities and the axonal optic nerve damage. Intermittent IOP elevations in Long Evans rats imitating IOP fluctuations observed in glaucoma patients were performed as well as measurements of autoantibody levels and optic nerve damage [77]. Depending on the grade of axonal damage in the animals changes in the immunoreactivity against glutathione-S-transferase, spectrin, and transferrin were detected. Additionally the effect of Belimumab, an inhibitor of the B lymphocytes activating component of the tumor necrosis factor family (BAFF), was investigated in this model. Belimumab leads to decreased B lymphocyte survival or even to complete B lymphocyte depletion and therefore results in decreased antibody production. In humans it is especially successful in treatment of systemic lupus erythematosus. Although total IgG serum concentration was lowered by 29% this had very little effect on axon or RGC survival. It furthermore could not provoke microglia deactivation or a reduction of IgG depositions in the retina. The authors show a reduction of IgG in the animals treated with Belimumab and therefore claim to show the bioavailability of the drug in rats. Therefore the authors conclude that autoantibodies only have a minor impact on the induction of neurodegenerative processes, at least in this experimental animal glaucoma model [77, 78].
Fig. (1).
Effect of abs on the number of RGCs. Retinal explants were cultivated with control medium without additional abs (control retinae) or with medium with additional 0.5 μg/mL γ-Synuclein abs or 1 μg/mL glial fibrillary acidic protein (GFAP) abs for 24 h. Furthermore, retinal explants were also incubated with medium with added anti-myoglobin abs serving as an isotype-matched control ab (0.5 μg/mL anti-myoglobin abs). The retinae were fixed, embedded and then prepared for immunohistological staining. The cells were counted manually and quantified using ImageJ and subsequent statistics were performed. *p < 0.05 (error bars are standard deviation). (A) Quantification of RGC/mm: We were able to detect significantly increased RGC numbers in both the retinae incubated with GFAP abs as well as retinae incubated with γ-Synuclein abs. The RGC number in GFAP ab-treated retinal explants was 20.1 RGC/mm. The RGC number in γ-Synuclein ab-treated retinal explants: 20.8 RGC/mm. The control retinae counted up to 13.8 RGC/mm and the retinae incubated with the control antibody (anti-myoglobin antibody) showed 14.15 RGC/mm. (B) Example image of retinal explant incubated with γ-Synuclein abs for 24 h. Immunohistological staining was performed in which the nuclei are shown in blue (DAPI staining), the RGC in red (Brn3a staining) and apoptotic cells in green (TUNEL staining) (modified after: [76], published in Bell, K.; Wilding, C.; et al. Neuroprotective effects of antibodies on retinal ganglion cells in an adolescent retina organ culture. J Neurochem, 2016, 139(2), 256-269./permission number: 4081430398238).
Nevertheless these results do not interfere with the assumption that antibodies are involved in the pathogenesis of glaucoma. An analysis of retinae derived from post-mortem glaucoma patients in comparison to non-glaucoma controls showed significant changes in the amounts of retinal IgG deposits as well as the occurrence of plasma cells in the retinae of glaucoma patients. IgG deposits were determined immunohistochemically using a fluorescein isothiocyanate labeled anti-rat IgG antibody. Using 5 glaucoma retinae and 5 control retinae IgG accumulations were detected in the RGC layer. In comparison to non-glaucoma eyes IgG deposits in the RGC layer were twice as high in glaucoma tissue (9.4±1.9 IgG deposits per 100 cells vs. 5.0±0.5 IgG deposits per 100 cells). Furthermore CD27+ cells were detected in the glaucomatous retinae. Some of these cells could be identified as plasma cells (CD27+/IgG+). CD3+ T cells could also be detected in some of the glaucomatous tissues and activated microglial cells were found to cluster nearby larger IgG deposits in glaucoma tissue. In comparison microglia cell had a ramified, quiescent appearance in the non-glaucoma tissue [79]. Furthermore non-glaucoma retinae did not display any CD3+ or CD 27+ cells [80].
1.9. Immunisation Induced Glaucoma Animal Models
Studies exploring the autoimmune aspects of glaucomatous neurodegeneration aimed to analyse whether elevated antibody reactions can induce autoaggressive RGC loss. An experimental autoimmune glaucoma animal (EAG) model was developed in which Lewis rats were immunised with proteins known to be elevated in glaucoma patients. The rats developed antibodies against the applied antigens such as heat shock protein 27 (HSP27) [81], HSP60 [81, 82] or myelin basic protein (MBP) [83] which resulted in glaucomatous damage and distinct RGC loss.
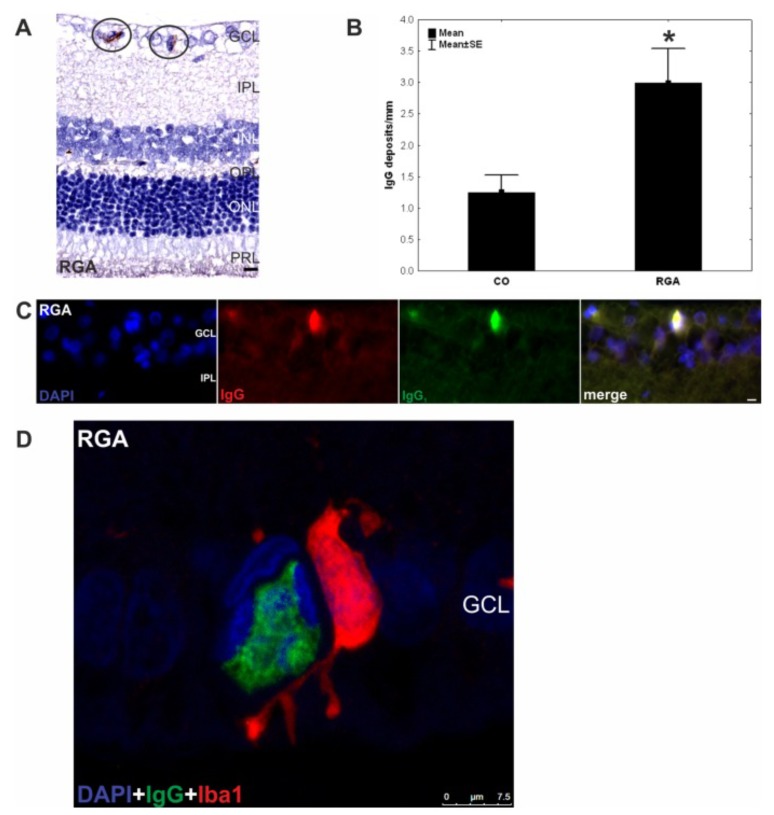
Subsequent EAG studies focused on the pathomechanism of RGC loss induced by immunisation with retinal and optic nerve associated antigens. RGC loss in the EAG model depended on the specificity of the antigen and was IOP-independent [84, 85]. Immunisation with non-neuronal antigens such as the non-glaucoma associated antigen keratin resulted in immunologic response against keratin but failed to show RGC loss [84]. In contrast rats immunised with retinal ganglion cell layer homogenate or homogenates of optic nerve associated antigens showed a significant reduction of RGCs as well as IgG deposits in the ganglion cell layer (Fig. 2A-C) [86-88]. Additionally complex autoantibody alterations were detected in the immunised animals [81, 82]. Using the serum from different time points the occurrence of autoreactive IgG antibodies against retina or optic nerve antigens in the EAG model were analysed. Long-term alterations of these autoreactive antibody patterns with a time-dependent increasing severity were detected [81, 82, 84, 85]. Moreover a significant demyelination of the optic nerve and a strong microglial involvement was detected after immunisation. These were often accompanied by IgG accumulations or appeared in co-localization with IgG-deposition (Fig. 2D) [84, 86].
Fig. (2).
IgG deposits and microglia in retina of EAG animals six weeks after immunization with RGC layer homogenate (RGA). A) IgG deposits were observed in retina cross-sections (n = 10 eyes/group) of RGA animals immunized with RGA layer homogenate in an equal volume of incomplete Freund’s adjuvant plus pertussis toxin (circled). B) counts revealed a significantly higher number of these deposits in the RGA group in relation to control animals injected with NaCl in Freund’s adjuvant and pertussis toxin. (p = 0.02). C) DAPI, IgG, and IgG1 staining in a representative retina cross-section of a RGA immunized animal. Co-localization of IgG and IgG1 is evident in the merged rightmost picture. The majority of the detected deposits were of the IgG1 subtype. D) Confocal image of the retina of an RGA animal. A colocalisation of the IgG deposit and an Iba1+ microglia can be seen. Values are mean+/-SE. Abbreviations: GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, OPL = outer plexiform layer, ONL = outer nuclear layer, PRL = photoreceptor layer. (*, p<0.05; scale bars in A and C: 10 mm and in D:7.5 mm) (published by S. C. Joachim et al; PLoS One. 2012; 7(7): e40616. doi:10.1371/journal.pone.0040616) [86] Concerning the copy right license: Copyright notice: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited).
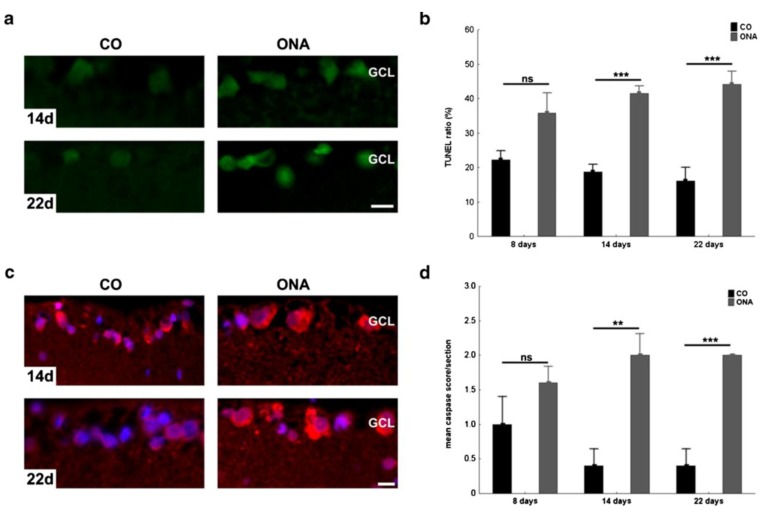
Apoptosis is the predominant form of RGC death in the normotensive EAG model. Significantly higher numbers of TUNEL-(terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling technique)-positive cells were observed in retinal cross sections from day 14 to day 22 after immunisation (Fig. 3A+B) [87]. Additionally caspase 3 immunoreactivity in EAG retinae was significantly increased (Fig. 3C+D). Caspase 3 is a key mediator of apoptosis and activates caspase-activated DNase which cleaves DNA in fragments typical for apoptotic cells [89]. Interestingly IgG deposits were often found in close vicinity of apoptotic cells in the ganglion cell layer [87].
Fig. (3).
Apoptotic cells and caspase 3 activity in EAG model. A) Exemplary TUNEL stained retina cross-sections of control (CO) and animals injected with optic nerve antigen homogenate (ONA) in Freund’s adjuvant and pertussis toxin, 14 and 22 days after immunization (n=4–5 eyes/group). The control group (CO) received 1 ml sodium chloride instead of the homogenate. More TUNEL+ cells were observed on ONA retinas. B) No difference was noted in the number of TUNEL+ cells/section at 8 days (p =0.09). At 14 (p =0.0002) and 22 days (p =0.0002) significantly more TUNEL+ cells were present in the ONA group. C) Caspase 3 immunoreactivity of retinas of control (CO) and ONA immunized animals at 14 and 22 days (n =5 eyes/group). D) Distribution of the caspase score assigned to CO and ONA animals 8, 14, and 22 days after immunization. Similar scores were seen in both groups at 8 days (p =0.23). The caspase 3 rate significantly increased in the ONA group at 14 (p =0.004) and 22 days (p =0.0007). GCL ganglion cell layer. Scale bar 10 μm; ns not significant; **p <0.01; ***p <0.001. (modified after [87]; Published in Joachim, S.C., Mondon, C., Gramlich, O.W. et al. J Mol Neurosci (2014) 52: 216. doi:10.1007/s12031-013-0125-2/permission number 4081271209841).
1.10. Caspases as Potential Therapeutic Targets in the Treatment of Glaucoma
Cysteine aspartyl-specific proteases (Caspases) are a family of endoproteases involved in extrinsic and intrinsic apoptotic pathways. They are key mediators of apoptosis and inflammation. Activation of pro-apoptotic caspases which include caspases 2, 3 and 6–10 [90] results in the generation of a signalling cascade permitting controlled cell death [91, 92]. Intrinsic apoptosis is also known as mitochondrial apoptosis as it depends on factors released from mitochondria and is activated by a wide range of cellular stress factors. Cytochrome C released by the mitochondria due to DNA damage or reactive oxygen species activates caspase 9, resulting in initiation of apoptosis by cleaving and thereby activating executioner caspases 3, 6, and 7. In contrast extrinsic apoptosis is triggered by extracellular stimuli delivered in form of ligands binding to death receptors. Members of the TNF superfamily, like TNF receptor-1 (TNFR1), CD95 (also called Fas and APO-1), death receptor 3 (DR3), TNF-related apoptosis-inducing ligand receptor-1 (TRAIL-R1; also called DR4), and TRAIL-R2 (also called DR5 in humans) belong to the death receptors. Ligand binding activates caspase 8 which also leads to executioner caspase signalling and cell death [91, 92].
Evidence from humans [93, 94] as well as animal glaucoma models [24, 87, 88, 95-97] supports the hypothesis that caspase activation could be responsible for progressive glaucomatous neurodegeneration. This includes TUNEL -positive cells in the ganglion cell layer in post-mortem human cases with glaucoma [93, 94] and enhanced immunohistochemical staining of Fas receptor and caspase 3 in optic nerve axons of post-mortem glaucoma patients [98] Fas receptors are Endogenous apoptosis inhibitor proteins (IAPs) and were found in higher concentrations after IOP elevation in experimental glaucoma and provide neuroprotective potential. Some IAPs furthermore are known to inhibit specific caspases [99]. Unfortunately increased expression of IAPs returned to baseline at an earlier time point than the concurrently expressed proapoptotic genes, leading to cell death [100]. This raises the question whether targeted caspase inhibition could be a potential therapeutic target for glaucoma management. Caspase 7 knock-out in mice significantly protected against optic nerve crush induced RGC loss indicating that blocking specific caspases may be neuroprotective. This was demonstrated by a significantly reduced RGC loss in the caspase 7 knock-out mice after optic nerve crush [101]. Other studies providing intravitreal injections of siRNA to knockdown caspase-2 after optic nerve crush in adult rats also showed enhanced RGC survival [102] but failed to demonstrate RGC axon regeneration [103].
Caspases also are discussed as potential therapeutic targets for several neurodegenerative disorders like Alzheimer’s Disease (AD) [104], Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS) [105]. Ona et al. demonstrated delayed disease progression and mortality in a mouse model of HD through intracerebroventricular administration of a caspase inhibitor [106]. A similar approach performed by Li et al. with an animal model of familial ALS examined the effect of a broad caspase inhibition in transgenic mice expressing mutant human copper/zinc superoxide dismutase 1 (SOD1). The administration of pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp fluoromethyl-ketone (Z-VADfmk) delayed disease onset and mortality [107]. Other promising neuro-protective results were achieved using a newer caspase inhibitor named Quinolyl-valyl-O-methylaspartyl-(-2, 6-difluorophenoxy)-methyl ketone (Q-VD-OPh) in PD, HD and stroke animal models [108, 109]. Numerous novel approaches inhibiting caspase activity are being evaluated and several therapeutics have progressed to clinical testing [110]. However, a recently published study from Ni et al. raises concerns about the safety of currently ongoing clinical trials using caspase inhibitors [111]. They found that blocking apoptosis can trigger alternative necrotic cell death. The authors detected that although in vitro-induced apoptosis was completely blocked by caspase inhibitor ZVAD-fmk at 24 hours the hepatocytes still died by necrosis at 48 hours. These in vitro findings could be confirmed in vivo using an endotoxin-induced liver injury animal model. Inhibition of caspases also protected mice against apoptosis at an early time point but this protection was lost after prolonged treatment through a switch from apoptosis to necrosis [111].
1.11. Microglia as Therapeutic Target in Glaucoma
As briefly stated above the EAG model neuronal loss is paralleled by an early microglial response [88]. Findings from Rutar et al. provide evidence that microglia are responsible for the local propagation of complement in the retina [112]. Using a light-induced model of progressive retinal degeneration the study showed that microglia/macrophages synthesise C3 after retinal damage and implicates their recruitment during the local activation of complement in the retina [112]. Further data demonstrates that recruited microglia/monocytes contribute to activation of complement through local expression of C3 mRNA also in the aging retina [113].
Microglia are key players in neuroinflammation which is an immune response involving diverse mechanisms of different immune-related cells upon an insult as ischemia, infection, loss of tissue homeostasis, trauma or other events unpoising CNS homeostasis and also is a key process in glaucoma [114]. Microglial activation, also called microgliosis, is a frequent feature of glaucoma animal models, indicating the induction of neuroinflammation in neural tissue [77, 86, 115]. Microgliosis can be detected in humans as well as in animal models of glaucoma, however it remains unclear whether microglia activation is damaging or beneficial for the diseases progress. While the phenotype M1 microglia secretes many pro-inflammatory cytokines as Tumour necrosis factor-α (TNF-α), IL-23, IL-1β, IL-6, IL-18 and IL-12 leading to neurotoxic neuronal death, the phenotype M2 microglia secretes Transforming growth factor-β, IL-4, IL-10 and IL-13, inducing neuroprotective neuronal survival [116].
The microglial response within the visual pathway in experimental glaucoma in a model of translimbal laser photocoagulation of the trabecular meshwork in Sprague Dawley rats has been described in detail by Ebneter et al. Activated microglia, characterised by an amoeboid phenotype and increased in expression, were identified in the retina, the optic nerve and the optic tract. Microglial activation remained stable even after decrease of IOP to baseline levels. Major histocompatibility complex I and II (MHC I/II) surface proteins were localized to microglia and persistently upregulated in optic nerves. It is interesting to note that activated microglia were not only found in the ipsilateral optic nerve, but also contralaterally, probably due to 5-10% of RGC axons projecting to the ipsilateral half of the brain instead of crossing the optic chiasm [117]. The phenomenon of the actually not involved partner eye showing similar differences as the involved eye has also been shown in other studies [118, 119] and can also be demonstrated for human unilateral glaucoma, in which also the fellow eye has shown to be challenged by significant loss of retinal nerve fibre layer thickness over time. This can be detected by spectral-domain optical coherence tomography [120].
Several experimental therapeutic options have been able to show significant “side-effects”/additive effects on microglial response without actually aiming to target these cells. Sprague Dawley rats with ocular hypertension induced by laser photocoagulation were additionally administered with caffeine, an antagonist of adenosine receptors (A2AR), which was added to the drinking water. Caffeine is supposed to inherit anti-inflammatory qualities in the CNS by dampening microglial neuroinflammation. This treatment was able to lower IOP and prevent retinal microglia-mediated neuroinflammatory responses. It additionally decreased RGCs loss [121]. Another rat glaucoma model with induced high IOP through episcleral vein occlusion showed increased survival of central RGCs as well as reduced activation of microglia. This was achieved through intravitreal injections of an antagonist to the A2A receptor named ZM241385, a modulator of microglial proliferation, cyclooxygenase-2 and inflammatory mediator synthesis and release. The authors state that the amount of proinflammatory cytokines released by microglia was dramatically lowered after application of A2A receptor antagonist [122]. Other studies using a BAX-/- mouse transgenic optic nerve crush model showed a dramatic reduction of RGC death accompanied with reduced microglia activation. Bcl-2-associated X (BAX) protein mediates intrinsic apoptosis by opening of mitochondrial voltage-dependent anion channels to release cytochrome c, finally leading to neurodegeneration. In this setting the authors tested intravitreal injections of NMDA to induce RGC loss and found elevated expression of microglia markers. This shows that microglial activation was not dependent on BAX proteins [123]. In another rat glaucoma model with IOP increase via angle laser photocoagulation the animals were treated with a daily intraperitoneal injection of triptolide, an active ingredient of Celastraceae Tripterygium known for its anti-inflammatory and immunosuppressive properties. This treatment led to increased RGC survival accompanied by decreased amounts of microglia in the retina [124]. The above described studies show neuroprotective agents, which do not actively aim for microglial cells but possess increased RGC survival and also show effects on microglia. It still remains unknown whether RGC survival remains higher due to reduced microglial activation and subsequent possible release of anti-inflammatory cytokines or if resting microglia lower their release of pro-inflammatory cytokines which then could lead to a decrease of the overall level of apoptotic RGCs.
Studies directly targeting microglial cells have also been performed. Activated microglia are known to upregulate major histocompatibility complex (MHC) class II RT1B chain expression which is important for the immune response against infections and can be found on professional antigen-presenting cells after optic nerve degeneration. Thus, a group of Sprague-Dawley rats expressing only low amounts of MHC II RT1B showed lower axonal degeneration, RGC loss and microgliosis after IOP induction by trabecular meshwork laser treatment [125]. In another study aiming to target the microglial cells, high-dose irradiation of the head in DBA/2J mouse, a genetic glaucoma model, was performed from 5 to 8 weeks of age as this is still a time point prior to microglial activation in this model. The irradiation led to a reduced proliferation and activation of microglia and also resulted in reduced optic nerve damage with improved structural integrity and anterograde transport function as well as lower extent of retinal neurodegeneration. Nevertheless, it is well known that radiation retinopathy along with adverse effects on retinal vessels can result as a complication of irradiating the retina [126]. A major focus has been set on research based on minocycline, a semisynthetic tetracycline derivative with anti-inflammatory properties, which inhibits microglia activation. A description of research in animal models is summarized in [127]. Evidence shows that deactivation of retinal microglia by minocycline results in reduced neurodegeneration at early stages of the disease. This is probably performed by addressing two targets: attenuating the innate and adaptive immunity and blocking of apoptotic processes. Clinical studies are being performed to detect a therapeutic benefit of oral minocycline as a microglia inhibitor in the treatment of branch retinal vein occlusions or central retinal vein occlusions (ClinicalTrials.gov Identifier: NCT01468831 and ClinicalTrials.gov Identifier: NCT01468844). But so far, no studies are being conducted looking at this therapeutic target in humans with glaucoma. In conclusion there are a variety of glaucoma animal models based on different techniques inducing a glaucomatous-like injury which show that reduction of the extent of neurodegeneration also resulted in reduction of microglia activation and/or vice versa. Only a limited number of studies targeting microglia itself as therapeutic target have been published in the last years. The most promising agent seems to be minocycline, which was not only successfully applied in glaucoma research, but also in PD, AD, MS and ALS [128]. Considering the close relationship of microgliosis, neuroinflammation and neurodegeneration, it is essential to pursue this therapeutic approach in the future to investigate the full potential of microglia in glaucoma animal models on one side, but also to target them in clinical glaucoma to finally bridge the gap between animal research and improved patient therapy.
1.12. Complement System as Therapeutic Target in Glaucoma
Next to showing changes in the microglial activation, studies in animal models of ocular hypertension observed a comparable upregulation of complement components C1q, C3 and membrane-attack complex (MAC) in the retina following the development of ocular hypertension (OHT) [129, 130]. In general the complement system plays an important role in immune responses and inflammatory processes and is a cascade of three separate pathways: 1) the classical pathway, which is triggered by direct binding of complement component C1q to the pathogen surface, can also be activated during an adaptive immune response by the binding of C1q to antibody:antigen complexes and is thus linking the effector mechanisms of innate and adaptive immunity [131]. Additionally to activation by immune complexes, C1q also may be activated by apoptotic and necrotic cells and by acute phase proteins such as C-reactive protein [132]. 2) The mannan-binding lectin pathway (MB-lectin pathway) which is triggered by mannan-binding lectin, a serum protein that can bind mannose-containing carbohydrates on bacteria and viruses; and 3) the alternative pathway, which is triggered directly by binding to the surface of a pathogen [131]. All three pathways of complement activation involve a series of cleavage reactions that generate the protease C3 convertase. The latter cleaves the C3 protein into active fragments C3a and C3b. C3a is a peptide mediator of proinflammatory signalling, while C3b can induce the cleavage of the C5 protein to C5a and C5b. C5a is a small peptide that acts as mediator of inflammation, while the large C5b fragment initiates formation of a MAC, which induces cell lysis by forming a hydrophilic pore in the cell membrane. Other complement factors in the MAC are C6, C7, C8 and C9 [131]. Complement activation plays a complex role in neurodegenerative disorders in the CNS such as AD, PD, dementia with Lewy bodies, Huntington's and prion diseases [133].
Accordingly, Jha et al., 2011 demonstrated that complement mediated apoptosis lead to the loss of RGCs in chronic ocular hypertension model of glaucoma and that a depletion of the complement system reduced the loss of RGC accompanied by decreased expression of GFAP, active caspase-8, active caspase-9 and reduced MAC deposition [134]. These results provide evidence that complement mediated apoptosis plays a pivotal role in the loss of RGCs in chronic ocular hypertension model of glaucoma [134]. This also could be demonstrated by altered levels of C3 in other studies of elevated IOP rat retinal glaucoma models [134, 135]. Complement activation has also been demonstrated independently from IOP in the EAG model. A significant increase of complement protein C3, MAC and lectin pathway-associated mannose-serine-protease-2 (MASP2) was observed in the retinas and the optic nerves of an EAG model 7 days after immunization with bovine optic nerve homogenate antigen [136]. MASP2 is believed to be the principal serine protease involved in the activation of the complement cascade. Like C1s in the classical pathway, MASP2 cleaves the complement components C4 and C2 to form the C3 convertase C4b2a, a common step in the activation of both the lectin and classical pathway [137]. These results let assume that immunization led to an activation of the complement system via the lectin pathway in retinas and optic nerves independent from elevated IOP [137]. Kühn et al compared human eyes with elevated intraocular pressure (IOP) and healthy retinal tissue as well as retinal tissue from a rat model of OHT induced by laser cauterisation of the trabecular meshwork and episcleral veins. They reported elevated transcript levels for C1q and C3 in retinae subjected to OHT, both in the animal model as well as in human eyes [129]. Stevens et al. also reported that in a mouse model of glaucoma, C1q becomes upregulated and synaptically relocalised in the adult retina early in the disease and may be involved in synapse elimination during neurodegeneration [138]. Concordantly, another study found an upregulation of C1q mRNA and C1q protein in the retina of two commonly used glaucoma models (in the DBA/2 mouse and the monkey) and in some human glaucomatous eyes [139]. Furthermore, proteomic analysis of human retinal samples obtained from donor eyes with or without glaucoma detected upregulation of various complement components and down-regulation of complement inhibitors in glaucoma retinae [140]. However, analysing human glaucomatous donor eyes via microarray approach we found that the cumulative level of complement proteins in the retina did not differ between glaucoma and healthy subjects in our analysis. Our results also give hint to the alternative pathway in complement activation due to the increased level of C3 in glaucoma. Looking at individual patients data the complement proteins tended to be slightly increased in single glaucoma samples and we assume a possible activation of the complement system in individual glaucoma cases, rather than in every glaucoma patient [79].
Anyway, disruption of the complement cascade in C3 deficient mice delayed RGC death following retinal ischemia-reperfusion [141]. Furthermore, DBA/2J mice deficient in C1qa were protected from glaucomatous RGC loss [142] and also deficiency of complement component C5 ameliorated glaucoma in DBA/2J mice compared with C5-sufficient DBA/2J mice [143]. As also stated above, Jha et al. reported that drug mediated complement depletion in chronic OHT model of glaucoma reduced the loss of RGC and the deposition of MAC (C5b-9) [134]. Taken together, these results suggest that complement based therapies could be very promising. Although huge effort is being done regarding the role of the complement cascade in glaucoma, many challenges remain with regard to the development of therapies targeting complement. Interventions in this complex cascade have not only to consider the role of complement in immune surveillance, but also its contribution to other biological functions like for example cell homeostasis, tissue development and repair, or the resolution of inflammation by promoting safe clearance of apoptotic cells and immune complexes [144]. Currently, there are a number of complement-targeting compounds in clinical and pre-clinical trials for retinal diseases, predominately for the management of AMD [145]. Additionally, a variety of ongoing clinical trials currently evaluate different classes of complement inhibitors in numerous diseases and will provide information about the safety and efficacy of distinct strategies for complement targeting, [145] potentially opening new opportunities for glaucoma therapy.
CONCLUSION
This recapitulation of recent literature demonstrates that the immune system definitely plays a role in the pathogenesis of glaucoma. Multiple changes not only in the peripheral innate immune system, but also in the peripheral adaptive immune system have been detected and give room for further research concerning valuable therapeutic targets. Some clinical trials targeting specific cytokines or mechanisms detected through more basic research are already being performed. Nevertheless, there is still a great need to bring together the results derived from the studies analysing different aspects of the immune system in glaucoma to understand the immune context of the disease. Furthermore, local immune changes in the retina of glaucoma patients still leave room for further therapeutic options and clinical studies e.g. targeting microglial cells in other ophthalmologic diseases such as CRVO could help bringing forward this need.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [http://dx.doi.org/10. 1016/j.ophtha.2014.05.013]. [PMID: 24974815]. [DOI] [PubMed] [Google Scholar]
- 2.Actis A.G., Versino E., Brogliatti B., Rolle T. Risk factors for primary open angle glaucoma (POAG) progression: A study ruled in torino. Open Ophthalmol. J. 2016;10:129–139. doi: 10.2174/1874364101610010129. [http://dx.doi. org/10.2174/1874364101610010129]. [PMID: 27347249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Bergen N.J., Crowston J.G., Craig J.E., Burdon K.P., Kearns L.S., Sharma S., Hewitt A.W., Mackey D.A., Trounce I.A. Measurement of systemic mitochondrial function in advanced primary open-angle glaucoma and leber hereditary optic neuropathy. PLoS One. 2015;10(10):e0140919. doi: 10.1371/journal.pone.0140919. [http://dx.doi.org/10. 1371/journal.pone.0140919]. [PMID: 26496696]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doh S.H., Kim J.H., Lee K.M., Park H.Y., Park C.K. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010;1308:158–166. doi: 10.1016/j.brainres.2009.10.025. [http:// dx.doi.org/10.1016/j.brainres.2009.10.025]. [PMID: 19853589]. [DOI] [PubMed] [Google Scholar]
- 5.Benoist d’Azy C., Pereira B., Chiambaretta F., Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: A systematic review and meta-analysis. PLoS One. 2016;11(12):e0166915. doi: 10.1371/journal.pone.0166915. [http://dx.doi.org/10.1371/journal.pone.0166915]. [PMID: 27907028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts B. Innate Immunity. 4th ed. New York: Garland Science; 2002. Molecular Biology of the Cell. [Google Scholar]
- 7.Pinoli M., Marino F., Cosentino M. Dopaminergic regulation of innate immunity: A review. J. Neuroimmune Pharmacol. 2017;12(4):602–623. doi: 10.1007/s11481-017-9749-2. [http://dx.doi.org/10.1007/s11481-017-9749-2]. [PMID: 28578466]. [DOI] [PubMed] [Google Scholar]
- 8.Chávez-Galán L., Olleros M.L., Vesin D., Garcia I. Much more than M1 and M2 macrophages, there are also CD169(+) and TCR(+) macrophages. Front. Immunol. 2015;6:263. doi: 10.3389/fimmu.2015.00263. [PMID: 26074923]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hume D.A. The many alternative faces of macrophage activation. Front. Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [http://dx.doi.org/10.3389/fimmu. 2015.00370]. [PMID: 26257737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlereth S.L., Kremers S., Schrödl F., Cursiefen C., Heindl L.M. Characterization of antigen-presenting macrophages and dendritic cells in the healthy human sclera. Invest. Ophthalmol. Vis. Sci. 2016;57(11):4878–4885. doi: 10.1167/iovs.15-18552. [http://dx.doi.org/10.1167/iovs. 15-18552]. [PMID: 27654414]. [DOI] [PubMed] [Google Scholar]
- 11.Kokona D., Häner N.U., Ebneter A., Zinkernagel M.S. Imaging of macrophage dynamics with optical coherence tomography in anterior ischemic optic neuropathy. Exp. Eye Res. 2017;154:159–167. doi: 10.1016/j.exer.2016.11.020. [http://dx.doi.org/10.1016/j.exer.2016.11.020]. [PMID: 27914988]. [DOI] [PubMed] [Google Scholar]
- 12.Chen M., Lechner J., Zhao J., Toth L., Hogg R., Silvestri G., Kissenpfennig A., Chakravarthy U., Xu H. STAT3 activation in circulating monocytes contributes to neovascular age-related macular degeneration. Curr. Mol. Med. 2016;16(4):412–423. doi: 10.2174/1566524016666160324130031. [http://dx.doi.org/10.2174/1566524016666160324130031]. [PMID: 27009107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sihota R., Goyal A., Kaur J., Gupta V., Nag T.C. Scanning electron microscopy of the trabecular meshwork: understanding the pathogenesis of primary angle closure glaucoma. Indian J. Ophthalmol. 2012;60(3):183–188. doi: 10.4103/0301-4738.95868. [http://dx.doi.org/10.4103/0301-4738.95868]. [PMID: 22569378]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Underhill D.M. Macrophage recognition of zymosan particles. J. Endotoxin Res. 2003;9(3):176–180. doi: 10.1179/096805103125001586. [http://dx.doi.org/10.1177/ 09680519030090030601]. [PMID: 12831459]. [DOI] [PubMed] [Google Scholar]
- 15.Yin Y., Cui Q., Li Y., Irwin N., Fischer D., Harvey A.R., Benowitz L.I. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci. 2003;23(6):2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [http://dx.doi.org/ 10.1523/JNEUROSCI.23-06-02284.2003]. [PMID: 12657687]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipscomb M.F., Masten B.J. Dendritic cells: immune regulators in health and disease. Physiol. Rev. 2002;82(1):97–130. doi: 10.1152/physrev.00023.2001. [http://dx. doi.org/10.1152/physrev.00023.2001]. [PMID: 11773610]. [DOI] [PubMed] [Google Scholar]
- 17.Dalod M., Chelbi R., Malissen B., Lawrence T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014;33(10):1104–1116. doi: 10.1002/embj.201488027. [http://dx.doi.org/10.1002/embj.201488027]. [PMID: 24737868]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vu Manh T.P., Bertho N., Hosmalin A., Schwartz-Cornil I., Dalod M. Investigating evolutionary conservation of dendritic cell subset identity and functions. Front. Immunol. 2015;6:260. doi: 10.3389/fimmu.2015.00260. [http://dx.doi.org/10.3389/fimmu.2015.00260]. [PMID: 26082777]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann U., Heuss N.D., McPherson S.W., Roehrich H., Gregerson D.S. Dendritic cells are early responders to retinal injury. Neurobiol. Dis. 2010;40(1):177–184. doi: 10.1016/j.nbd.2010.05.022. [http://dx.doi.org/10.1016/ j.nbd.2010.05.022]. [PMID: 20580926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mo J.S., Anderson M.G., Gregory M., Smith R.S., Savinova O.V., Serreze D.V., Ksander B.R., Streilein J.W., John S.W. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J. Exp. Med. 2003;197(10):1335–1344. doi: 10.1084/jem.20022041. [http://dx.doi.org/10.1084/jem. 20022041]. [PMID: 12756269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [http:// dx.doi.org/10.1146/annurev.immunol.21.120601.141040]. [PMID: 12615891]. [DOI] [PubMed] [Google Scholar]
- 22.Farrugia M., Baron B. The role of toll-like receptors in autoimmune diseases through failure of the self-recognition mechanism. Int. J. Inflamm. 2017;2017:8391230. doi: 10.1155/2017/8391230. [http://dx.doi.org/10.1155/ 2017/8391230]. [PMID: 28553556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo C., Yang X., Kain A.D., Powell D.W., Kuehn M.H., Tezel G. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest. Ophthalmol. Vis. Sci. 2010;51(11):5697–5707. doi: 10.1167/iovs.10-5407. [http://dx.doi.org/10.1167/ iovs.10-5407]. [PMID: 20538986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi W., Li F., Chen H., Wang Y., Zhu Y., Yang X., Zhu J., Wu F., Ouyang H., Ge J., Weinreb R.N., Zhang K., Zhuo Y. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. USA. 2014;111(30):11181–11186. doi: 10.1073/pnas.1402819111. [http://dx.doi.org/10.1073/pnas. 1402819111]. [PMID: 25024200]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astafurov K., Elhawy E., Ren L., Dong C.Q., Igboin C., Hyman L., Griffen A., Mittag T., Danias J. Oral microbiome link to neurodegeneration in glaucoma. PLoS One. 2014;9(9):e104416. doi: 10.1371/journal.pone.0104416. [http://dx.doi.org/10.1371/journal.pone.0104416]. [PMID: 25180891]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano Y. Association of Toll-like receptor 4 gene polymorphisms in Japanese subjects with primary open-angle, normal-tension, and exfoliation glaucoma. Am. J. Ophthalmol. 2012;154(5):825–832. doi: 10.1016/j.ajo.2012.03.050. [http://dx.doi.org/10.1016/j.ajo.2012.03.050]. [DOI] [PubMed] [Google Scholar]
- 27.Suh W., Kim S., Ki C.S., Kee C. Toll-like receptor 4 gene polymorphisms do not associate with normal tension glaucoma in a Korean population. Mol. Vis. 2011;17:2343–2348. [PMID: 21921986]. [PMC free article] [PubMed] [Google Scholar]
- 28.Mousa A., Kondkar A.A., Al-Obeidan S.A., Azad T.A., Sultan T., Osman E.A., Abu-Amero K.K. Lack of association between polymorphism rs4986791 in TLR4 and primary open-angle glaucoma in a saudi cohort. Genet. Test. Mol. Biomarkers. 2016;20(9):556–559. doi: 10.1089/gtmb.2016.0095. [http://dx.doi.org/10.1089/gtmb.2016.0095]. [PMID: 27526043]. [DOI] [PubMed] [Google Scholar]
- 29.Yang J., Patil R.V., Yu H., Gordon M., Wax M.B. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am. J. Ophthalmol. 2001;131(4):421–426. doi: 10.1016/s0002-9394(00)00862-x. [http://dx.doi.org/10.1016/S0002-9394(00)00862-X]. [PMID: 11292402]. [DOI] [PubMed] [Google Scholar]
- 30.Mariotti S., Barbesino G., Caturegli P., Marinò M., Manetti L., Fugazzola L., Pacini F., Pinchera A. Serum soluble interleukin 2 (IL-2) receptor (sIL-2R) in differentiated thyroid carcinoma. J. Endocrinol. Invest. 1994;17(11):861–867. doi: 10.1007/BF03347792. [http://dx.doi.org/10.1007/ BF03347792]. [PMID: 7745234]. [DOI] [PubMed] [Google Scholar]
- 31.Kuehn S., Stellbogen M., Noristani R., Peters M., Dick H.B., Joachim S.C. Systemic ocular antigen immunization leads only to a minor secondary immune response. J. Neuroimmunol. 2016;293:114–122. doi: 10.1016/j.jneuroim.2016.02.017. [http://dx.doi.org/10.1016/j.jneuroim.2016.02.017]. [PMID: 27049571]. [DOI] [PubMed] [Google Scholar]
- 32.Gramlich O.W., Ding Q.J., Zhu W., Cook A., Anderson M.G., Kuehn M.H. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients. Acta Neuropathol. Commun. 2015;3:56. doi: 10.1186/s40478-015-0234-y. [http://dx.doi.org/10.1186/ s40478-015-0234-y]. [PMID: 26374513]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H., Manivannan A., Liversidge J., Sharp P.F., Forrester J.V., Crane I.J. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J. Neuroimmunol. 2003;142(1-2):47–57. doi: 10.1016/s0165-5728(03)00258-3. [http://dx.doi.org/10.1016/S0165-5728(03)00258-3]. [PMID: 14512163]. [DOI] [PubMed] [Google Scholar]
- 34.Chan-Ling T., Hughes S., Baxter L., Rosinova E., McGregor I., Morcos Y., van Nieuwenhuyzen P., Hu P. Inflammation and breakdown of the blood-retinal barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation. 2007;14(1):63–76. doi: 10.1080/10739680601073451. [http://dx.doi.org/10.1080/10739680601073451]. [PMID: 17365662]. [DOI] [PubMed] [Google Scholar]
- 35.Wax M.B., Tezel G., Yang J., Peng G., Patil R.V., Agarwal N., Sappington R.M., Calkins D.J. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J. Neurosci. 2008;28(46):12085–12096. doi: 10.1523/JNEUROSCI.3200-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.3200-08.2008]. [PMID: 19005073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrix S., Nitsch R. The role of T helper cells in neuroprotection and regeneration. J. Neuroimmunol. 2007;184(1-2):100–112. doi: 10.1016/j.jneuroim.2006.11.019. [http: //dx.doi.org/10.1016/j.jneuroim.2006.11.019]. [PMID: 17198734]. [DOI] [PubMed] [Google Scholar]
- 37.Wong M., Huang P., Li W., Li Y., Zhang S.S., Zhang C. T-helper1/T-helper2 cytokine imbalance in the iris of patients with glaucoma. PLoS One. 2015;10(3):e0122184. doi: 10.1371/journal.pone.0122184. [http://dx.doi. org/10.1371/journal.pone.0122184]. [PMID: 25811482]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taurone S., Ripandelli G., Pacella E., Bianchi E., Plateroti A.M., De Vito S., Plateroti P., Grippaudo F.R., Cavallotti C., Artico M. Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: immunohistochemical profile of a number of inflammatory cytokines. Mol. Med. Rep. 2015;11(2):1384–1390. doi: 10.3892/mmr.2014.2772. [http://dx.doi.org/10.3892/mmr.2014.2772]. [PMID: 25351602]. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal P., Daher A.M., Agarwal R. Aqueous humor TGF-β2 levels in patients with open-angle glaucoma: A meta-analysis. Mol. Vis. 2015;21:612–620. [PMID: 26019480]. [PMC free article] [PubMed] [Google Scholar]
- 40.Khaw P., Grehn F., Holló G., Overton B., Wilson R., Vogel R., Smith Z. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114(10):1822–1830. doi: 10.1016/j.ophtha.2007.03.050. [http://dx.doi.org/10.1016/ j.ophtha.2007.03.050]. [PMID: 17908591]. [DOI] [PubMed] [Google Scholar]
- 41.Aharoni R. Immunomodulation neuroprotection and remyelination - the fundamental therapeutic effects of glatiramer acetate: a critical review. J. Autoimmun. 2014;54:81–92. doi: 10.1016/j.jaut.2014.05.005. [http://dx.doi.org/10. 1016/j.jaut.2014.05.005]. [PMID: 24934599]. [DOI] [PubMed] [Google Scholar]
- 42.Steinman L., Shoenfeld Y. From defining antigens to new therapies in multiple sclerosis: honoring the contributions of Ruth Arnon and Michael Sela. J. Autoimmun. 2014;54:1–7. doi: 10.1016/j.jaut.2014.08.001. [http://dx.doi. org/10.1016/j.jaut.2014.08.001]. [PMID: 25308417]. [DOI] [PubMed] [Google Scholar]
- 43.Kipnis J., Yoles E., Porat Z., Cohen A., Mor F., Sela M., Cohen I.R., Schwartz M. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proc. Natl. Acad. Sci. USA. 2000;97(13):7446–7451. doi: 10.1073/pnas.97.13.7446. [http://dx.doi.org/10.1073/pnas.97.13.7446]. [PMID: 10861010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz M. Vaccination for glaucoma: dream or reality? Brain Res. Bull. 2004;62(6):481–484. doi: 10.1016/S0361-9230(03)00073-X. [http://dx.doi.org/10.1016/S0361-9230(03)00073-X]. [PMID: 15036561]. [DOI] [PubMed] [Google Scholar]
- 45.Fu W.C., Jiang Y., Zhang L. Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model. Asian Pac. J. Trop. Med. 2014;7(4):317–320. doi: 10.1016/S1995-7645(14)60047-X. [http://dx.doi.org/10.1016/S1995-7645(14)60047-X]. [PMID: 24507684]. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X., Xia X.B., Xiong S.Q. Neuro-protection of retinal stem cells transplantation combined with copolymer-1 immunization in a rat model of glaucoma. Mol. Cell. Neurosci. 2013;54:1–8. doi: 10.1016/j.mcn.2012.12.001. [http://dx.doi.org/10.1016/j.mcn.2012.12.001]. [PMID: 23246669]. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt A., Rieger C.C., Venigalla R.K., Éliás S., Max R., Lorenz H.M., Gröne H.J., Krammer P.H., Kuhn A. Analysis of FOXP3+ regulatory T cell subpopulations in peripheral blood and tissue of patients with systemic lupus erythematosus. Immunol. Res. 2017;65(2):551–563. doi: 10.1007/s12026-017-8904-4. [http://dx.doi.org/10.1007/s12026-017-8904-4]. [PMID: 28224362]. [DOI] [PubMed] [Google Scholar]
- 48.Żabińska M., Krajewska M., Kościelska-Kasprzak K., Jakuszko K., Bartoszek D., Myszka M., Klinger M. CD4(+)CD25(+) CD127(-) and CD4(+)CD25(+)Foxp3(+) regulatory t cell subsets in mediating autoimmune reactivity in systemic lupus erythematosus patients. Arch. Immunol. Ther. Exp. (Warsz.) 2016;64(5):399–407. doi: 10.1007/s00005-016-0399-5. [http://dx.doi.org/10.1007/s00005-016-0399-5]. [PMID: 27156107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocha N.P. Reduced activated T lymphocytes (CD4+CD25+) and plasma levels of cytokines in parkinson’s disease. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0404-y. [PMID: 28176275]. [DOI] [PubMed] [Google Scholar]
- 50.Dasgupta A., Saxena R. Regulatory T cells: a review. Natl. Med. J. India. 2012;25(6):341–351. [PMID: 23998865]. [PubMed] [Google Scholar]
- 51.Bell K. Elevated Regulatory T Cell Levels in Glaucoma Patients in Comparison to Healthy Controls. Curr. Eye Res. 2016;•••:1–6. doi: 10.1080/02713683.2016.1205629. [PMID: 27723363]. [DOI] [PubMed] [Google Scholar]
- 52.Helin M., Rönkkö S., Puustjärvi T., Teräsvirta M., Ollikainen M., Uusitalo H. Conjunctival inflammatory cells and their predictive role for deep sclerectomy in primary open-angle glaucoma and exfoliation glaucoma. J. Glaucoma. 2011;20(3):172–178. doi: 10.1097/IJG.0b013e3181d9ccb0. [http:// dx.doi.org/10.1097/IJG.0b013e3181d9ccb0]. [PMID: 20577105]. [DOI] [PubMed] [Google Scholar]
- 53.Chucair-Elliott A.J., Carr M.M., Carr D.J.J. Long-term consequences of topical dexamethasone treatment during acute corneal HSV-1 infection on the immune system. J. Leukoc. Biol. 2017;101(5):1253–1261. doi: 10.1189/jlb.4A1116-459R. [http://dx.doi.org/10.1189/jlb.4A1116-459R]. [PMID: 28115476]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theiss-Suennemann J., Jörß K., Messmann J.J., Reichardt S.D., Montes-Cobos E., Lühder F., Tuckermann J.P. AWolff, H.; Dressel, R.; Gröne, H.J.; Strauß, G.; Reichardt, H.M. Glucocorticoids attenuate acute graft-versus-host disease by suppressing the cytotoxic capacity of CD8(+) T cells. J. Pathol. 2015;235(4):646–655. doi: 10.1002/path.4475. [http://dx.doi.org/10.1002/path.4475]. [PMID: 25358639]. [DOI] [PubMed] [Google Scholar]
- 55.Janeway C.A., Jr . 2001. [Google Scholar]
- 56.Grus F.H., Joachim S.C., Hoffmann E.M., Pfeiffer N. Complex autoantibody repertoires in patients with glaucoma. Mol. Vis. 2004;10:132–137. [PMID: 14990890]. [PubMed] [Google Scholar]
- 57.Diamond B., Huerta P.T., Mina-Osorio P., Kowal C., Volpe B.T. Losing your nerves? Maybe it’s the antibodies. Nat. Rev. Immunol. 2009;9(6):449–456. doi: 10.1038/nri2529. [http://dx.doi.org/10.1038/nri2529]. [PMID: 19424277]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J., Tezel G., Patil R.V., Romano C., Wax M.B. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 2001;42(6):1273–1276. [PMID: 11328739]. [PubMed] [Google Scholar]
- 59.Grus F.H., Joachim S.C., Bruns K., Lackner K.J., Pfeiffer N., Wax M.B. Serum autoantibodies to alpha-fodrin are present in glaucoma patients from Germany and the United States. Invest. Ophthalmol. Vis. Sci. 2006;47(3):968–976. doi: 10.1167/iovs.05-0685. [http://dx.doi.org/10. 1167/iovs.05-0685]. [PMID: 16505031]. [DOI] [PubMed] [Google Scholar]
- 60.Joachim S.C., Reichelt J., Berneiser S., Pfeiffer N., Grus F.H. Sera of glaucoma patients show autoantibodies against myelin basic protein and complex autoantibody profiles against human optic nerve antigens. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246(4):573–580. doi: 10.1007/s00417-007-0737-8. [http://dx.doi.org/10.1007/s00417-007-0737-8]. [PMID: 18193265]. [DOI] [PubMed] [Google Scholar]
- 61.Joachim S.C., Bruns K., Lackner K.J., Pfeiffer N., Grus F.H. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr. Eye Res. 2007;32(6):501–509. doi: 10.1080/02713680701375183. [http://dx.doi.org/10.1080/ 02713680701375183]. [PMID: 17612966]. [DOI] [PubMed] [Google Scholar]
- 62.Boehm N., Wolters D., Thiel U., Lossbrand U., Wiegel N., Pfeiffer N., Grus F.H. New insights into autoantibody profiles from immune privileged sites in the eye: a glaucoma study. Brain Behav. Immun. 2012;26(1):96–102. doi: 10.1016/j.bbi.2011.07.241. [http://dx.doi.org/10.1016/j. bbi.2011.07.241]. [PMID: 21843631]. [DOI] [PubMed] [Google Scholar]
- 63.Lorenz K. Course of serum autoantibodies in patients after acute angle-closure glaucoma attack. Clin. Experiment. Ophthalmol. 2017;45:280–287. doi: 10.1111/ceo.12864. [DOI] [PubMed] [Google Scholar]
- 64.Tezel G., Wax M.B. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J. Neurosci. 2000;20(10):3552–3562. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [http://dx.doi.org/10.1523/JNEUROSCI.20-10-03552. 2000]. [PMID: 10804196]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bell K., Funke S., Pfeiffer N., Grus F.H. Serum and antibodies of glaucoma patients lead to changes in the proteome, especially cell regulatory proteins, in retinal cells. PLoS One. 2012;7(10):e46910. doi: 10.1371/journal.pone.0046910. [http://dx.doi.org/10.1371/journal.pone.0046910]. [PMID: 23071659]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bayry J., Misra N., Dasgupta S., Lacroix-Desmazes S., Kazatchkine M.D., Kaveri S.V. Natural autoantibodies: immune homeostasis and therapeutic intervention. Expert Rev. Clin. Immunol. 2005;1(2):213–222. doi: 10.1586/1744666X.1.2.213. [http://dx.doi.org/10.1586/1744666X.1.2.213]. [PMID: 20476935]. [DOI] [PubMed] [Google Scholar]
- 67.Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol. Today. 1991;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [PMID: 1715166]. [DOI] [PubMed] [Google Scholar]
- 68.Meriggioli M.N. Myasthenia gravis with anti-acetylcholine receptor antibodies. Front Neurol. Neurosci. 2009;26:94–108. doi: 10.1159/000212371. [http://dx.doi.org/10.1159/000212371]. [PMID: 19349707]. [DOI] [PubMed] [Google Scholar]
- 69.Brettschneider S., Morgenthaler N.G., Teipel S.J., Fischer-Schulz C., Bürger K., Dodel R., Du Y., Möller H.J., Bergmann A., Hampel H. Decreased serum amyloid beta(1-42) autoantibody levels in Alzheimer’s disease, determined by a newly developed immuno-precipitation assay with radiolabeled amyloid beta(1-42) peptide. Biol. Psychiatry. 2005;57(7):813–816. doi: 10.1016/j.biopsych.2004.12.008. [http://dx.doi.org/ 10.1016/j.biopsych.2004.12.008]. [PMID: 15820240]. [DOI] [PubMed] [Google Scholar]
- 70.Surgucheva I., McMahan B., Ahmed F., Tomarev S., Wax M.B., Surguchov A. Synucleins in glaucoma: implication of gamma-synuclein in glaucomatous alterations in the optic nerve. J. Neurosci. Res. 2002;68(1):97–106. doi: 10.1002/jnr.10198. [http://dx.doi.org/10.1002/jnr. 10198]. [PMID: 11933054]. [DOI] [PubMed] [Google Scholar]
- 71.Maurage C.A., Ruchoux M.M., de Vos R., Surguchov A., Destee A. Retinal involvement in dementia with Lewy bodies: a clue to hallucinations? Ann. Neurol. 2003;54(4):542–547. doi: 10.1002/ana.10730. [http://dx.doi.org/10.1002/ana.10730]. [PMID: 14520672]. [DOI] [PubMed] [Google Scholar]
- 72.Sippl C., Tamm E.R. What is the nature of the RGC-5 cell line? Adv. Exp. Med. Biol. 2014;801:145–154. doi: 10.1007/978-1-4614-3209-8_19. [http://dx.doi.org/ 10.1007/978-1-4614-3209-8_19]. [PMID: 24664692]. [DOI] [PubMed] [Google Scholar]
- 73.Wilding C., Bell K., Beck S., Funke S., Pfeiffer N., Grus F.H. γ-Synuclein antibodies have neuroprotective potential on neuroretinal cells via proteins of the mitochondrial apoptosis pathway. PLoS One. 2014;9(3):e90737. doi: 10.1371/journal.pone.0090737. [http://dx.doi.org/10.1371/journal.pone. 0090737]. [PMID: 24595072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bell K., Wilding C., Funke S., Pfeiffer N., Grus F.H. Protective effect of 14-3-3 antibodies on stressed neuroretinal cells via the mitochondrial apoptosis pathway. BMC Ophthalmol. 2015;15:64. doi: 10.1186/s12886-015-0044-9. [http://dx.doi.org/10.1186/s12886-015-0044-9]. [PMID: 26115916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilding C., Bell K., Funke S., Beck S., Pfeiffer N., Grus F.H. GFAP antibodies show protective effect on oxidatively stressed neuroretinal cells via interaction with ERP57. J. Pharmacol. Sci. 2015;127(3):298–304. doi: 10.1016/j.jphs.2014.12.019. [http://dx.doi.org/10.1016/j.jphs.2014.12. 019]. [PMID: 25837926]. [DOI] [PubMed] [Google Scholar]
- 76.Bell K., Wilding C., Funke S., Perumal N., Beck S., Wolters D., Holz-Müller J., Pfeiffer N., Grus F.H. Neuroprotective effects of antibodies on retinal ganglion cells in an adolescent retina organ culture. J. Neurochem. 2016;139(2):256–269. doi: 10.1111/jnc.13765. [http://dx.doi.org/ 10.1111/jnc.13765]. [PMID: 27507598]. [DOI] [PubMed] [Google Scholar]
- 77.Gramlich O.W., Teister J., Neumann M., Tao X., Beck S., von Pein H.D., Pfeiffer N., Grus F.H. Immune response after intermittent minimally invasive intraocular pressure elevations in an experimental animal model of glaucoma. J. Neuroinflammation. 2016;13(1):82. doi: 10.1186/s12974-016-0542-6. [http://dx.doi.org/10.1186/s12974-016-0542-6]. [PMID: 27090083]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moisini I., Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin. Exp. Immunol. 2009;158(2):155–163. doi: 10.1111/j.1365-2249.2009.04007.x. [http://dx.doi.org/10.1111/j.1365-2249.2009.04007.x]. [PMID: 19737141]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gramlich O.W., Beck S., von Thun Und Hohenstein-Blaul N., Boehm N., Ziegler A., Vetter J.M., Pfeiffer N., Grus F.H. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS One. 2013;8(2):e57557. doi: 10.1371/journal.pone.0057557. [http:// dx.doi.org/10.1371/journal.pone.0057557]. [PMID: 23451242]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang P., Das P.K., Kijlstra A. Localization and characterization of immunocompetent cells in the human retina. Ocul. Immunol. Inflamm. 2000;8(3):149–157. [http://dx.doi.org/10.1076/0927-3948 (200009)831-KFT149]. [PMID: 11120576]. [PubMed] [Google Scholar]
- 81.Joachim S.C., Grus F.H., Kraft D., White-Farrar K., Barnes G., Barbeck M., Ghanaati S., Cao S., Li B., Wax M.B. Complex antibody profile changes in an experimental autoimmune glaucoma animal model. Invest. Ophthalmol. Vis. Sci. 2009;50(10):4734–4742. doi: 10.1167/iovs.08-3144. [http://dx.doi.org/10.1167/iovs.08-3144]. [PMID: 19458332]. [DOI] [PubMed] [Google Scholar]
- 82.Joachim S.C., Wax M.B., Seidel P., Pfeiffer N., Grus F.H. Enhanced characterization of serum autoantibody reactivity following HSP 60 immunization in a rat model of experimental autoimmune glaucoma. Curr. Eye Res. 2010;35(10):900–908. doi: 10.3109/02713683.2010.495829. [http://dx.doi. org/10.3109/02713683.2010.495829]. [PMID: 20858111]. [DOI] [PubMed] [Google Scholar]
- 83.Gramlich O.W., Joachim S.C., Gottschling P.F., Laspas P., Cuny C.S., Pfeiffer N., Grus F.H. Ophthalmopathology in rats with MBP-induced experimental autoimmune encephalomyelitis. Graefes Arch. Clin. Exp. Ophthalmol. 2011;249(7):1009–1020. doi: 10.1007/s00417-011-1633-9. [http://dx.doi.org/10.1007/s00417-011-1633-9]. [PMID: 21344308]. [DOI] [PubMed] [Google Scholar]
- 84.Laspas P., Gramlich O.W., Müller H.D., Cuny C.S., Gottschling P.F., Pfeiffer N., Dick H.B., Joachim S.C., Grus F.H. Autoreactive antibodies and loss of retinal ganglion cells in rats induced by immunization with ocular antigens. Invest. Ophthalmol. Vis. Sci. 2011;52(12):8835–8848. doi: 10.1167/iovs.10-6889. [http://dx.doi.org/10.1167/iovs.10-6889]. [PMID: 22003114]. [DOI] [PubMed] [Google Scholar]
- 85.Joachim S.C., Reinehr S., Kuehn S., Laspas P., Gramlich O.W., Kuehn M., Tischoff I., von Pein H.D., Dick H.B., Grus F.H. Immune response against ocular tissues after immunization with optic nerve antigens in a model of autoimmune glaucoma. Mol. Vis. 2013;19:1804–1814. [PMID: 23946635]. [PMC free article] [PubMed] [Google Scholar]
- 86.Joachim S.C., Gramlich O.W., Laspas P., Schmid H., Beck S., von Pein H.D., Dick H.B., Pfeiffer N., Grus F.H. Retinal ganglion cell loss is accompanied by antibody depositions and increased levels of microglia after immunization with retinal antigens. PLoS One. 2012;7(7):e40616. doi: 10.1371/journal.pone.0040616. [http://dx.doi.org/10.1371/ journal.pone.0040616]. [PMID: 22848388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joachim S.C., Mondon C., Gramlich O.W., Grus F.H., Dick H.B. Apoptotic retinal ganglion cell death in an autoimmune glaucoma model is accompanied by antibody depositions. J. Mol. Neurosci. 2014;52(2):216–224. doi: 10.1007/s12031-013-0125-2. [http://dx.doi.org/10.1007/s12031-013-0125-2]. [PMID: 24091788]. [DOI] [PubMed] [Google Scholar]
- 88.Noristani R., Kuehn S., Stute G., Reinehr S., Stellbogen M., Dick H.B., Joachim S.C. Retinal and optic nerve damage is associated with early glial responses in an experimental autoimmune glaucoma model. J. Mol. Neurosci. 2016;58(4):470–482. doi: 10.1007/s12031-015-0707-2. [http:// dx.doi.org/10.1007/s12031-015-0707-2]. [PMID: 26746422]. [DOI] [PubMed] [Google Scholar]
- 89.Wolf B.B., Schuler M., Echeverri F., Green D.R. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 1999;274(43):30651–30656. doi: 10.1074/jbc.274.43.30651. [http://dx. doi.org/10.1074/jbc.274.43.30651]. [PMID: 10521451]. [DOI] [PubMed] [Google Scholar]
- 90.Thornberry N.A., Rano T.A., Peterson E.P., Rasper D.M., Timkey T., Garcia-Calvo M., Houtzager V.M., Nordstrom P.A., Roy S., Vaillancourt J.P., Chapman K.T., Nicholson D.W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997;272(29):17907–17911. doi: 10.1074/jbc.272.29.17907. [http://dx.doi.org/10.1074/jbc.272.29.17907]. [PMID: 9218414]. [DOI] [PubMed] [Google Scholar]
- 91.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5(4):a008656. doi: 10.1101/cshperspect.a008656. [http://dx.doi.org/10.1101/cshperspect.a008656]. [PMID: 23545416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015;7(4):a026716. doi: 10.1101/cshperspect.a026716. [http://dx.doi.org/10.1101/cshperspect.a026716]. [PMID: 25833847]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kerrigan L.A., Zack D.J., Quigley H.A., Smith S.D., Pease M.E. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch. Ophthalmol. 1997;115(8):1031–1035. doi: 10.1001/archopht.1997.01100160201010. [http://dx. doi.org/10.1001/archopht.1997.01100160201010]. [PMID: 9258226]. [DOI] [PubMed] [Google Scholar]
- 94.Wax M.B., Tezel G., Edward P.D. Clinical and ocular histopathological findings in a patient with normal-pressure glaucoma. Arch. Ophthalmol. 1998;116(8):993–1001. doi: 10.1001/archopht.116.8.993. [http://dx.doi.org/ 10.1001/archopht.116.8.993]. [PMID: 9715678]. [DOI] [PubMed] [Google Scholar]
- 95.Huang W., Dobberfuhl A., Filippopoulos T., Ingelsson M., Fileta J.B., Poulin N.R., Grosskreutz C.L. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am. J. Pathol. 2005;167(3):673–681. doi: 10.1016/S0002-9440(10)62042-1. [http://dx.doi. org/10.1016/S0002-9440(10)62042-1]. [PMID: 16127148]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hänninen V.A., Pantcheva M.B., Freeman E.E., Poulin N.R., Grosskreutz C.L. Activation of caspase 9 in a rat model of experimental glaucoma. Curr. Eye Res. 2002;25(6):389–395. doi: 10.1076/ceyr.25.6.389.14233. [http://dx. doi.org/10.1076/ceyr.25.6.389.14233]. [PMID: 12789547]. [DOI] [PubMed] [Google Scholar]
- 97.Guo L., Moss S.E., Alexander R.A., Ali R.R., Fitzke F.W., Cordeiro M.F. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci. 2005;46(1):175–182. doi: 10.1167/iovs.04-0832. [http://dx.doi.org/10.1167/iovs.04-0832]. [PMID: 15623771]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zalewska R., Zalewski B., Reszec J., Mariak Z., Zimnoch L., Proniewska-Skretek E. The expressions of Fas and caspase-3 in human glaucomatous optic nerve axons. Med. Sci. Monit. 2008;14(12):BR274–BR278. [PMID: 19043361]. [PubMed] [Google Scholar]
- 99.Deveraux Q.L., Stennicke H.R., Salvesen G.S., Reed J.C. Endogenous inhibitors of caspases. J. Clin. Immunol. 1999;19(6):388–398. doi: 10.1023/a:1020502800208. [http://dx.doi.org/10.1023/A:1020502800208]. [PMID: 10634212]. [DOI] [PubMed] [Google Scholar]
- 100.Levkovitch-Verbin H., Dardik R., Vander S., Nisgav Y., Kalev-Landoy M., Melamed S. Experimental glaucoma and optic nerve transection induce simultaneous upregulation of proapoptotic and prosurvival genes. Invest. Ophthalmol. Vis. Sci. 2006;47(6):2491–2497. doi: 10.1167/iovs.05-0996. [http://dx.doi.org/10.1167/iovs.05-0996]. [PMID: 16723461]. [DOI] [PubMed] [Google Scholar]
- 101.Choudhury S., Liu Y., Clark A.F., Pang I.H. Caspase-7: a critical mediator of optic nerve injury-induced retinal ganglion cell death. Mol. Neurodegener. 2015;10:40. doi: 10.1186/s13024-015-0039-2. [http://dx.doi.org/10.1186/ s13024-015-0039-2]. [PMID: 26306916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahmed Z., Kalinski H., Berry M., Almasieh M., Ashush H., Slager N., Brafman A., Spivak I., Prasad N., Mett I., Shalom E., Alpert E., Di Polo A., Feinstein E., Logan A. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [http://dx.doi.org/10.1038/cddis.2011.54]. [PMID: 21677688]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vigneswara V., Berry M., Logan A., Ahmed Z. Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PLoS One. 2012;7(12):e53473. doi: 10.1371/journal.pone.0053473. [http://dx.doi.org/10.1371/journal.pone.0053473]. [PMID: 23285297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jana K., Banerjee B., Caspases P.K.P. A Potential Therapeutic Targets in the Treatment of Alzheimer’s Disea. Transl. Med. (Sunnyvale) 2013;S2(006) [Google Scholar]
- 105.Khan S., Ahmad K., Alshammari E.M., Adnan M., Baig M.H., Lohani M., Somvanshi P., Haque S. Implication of caspase-3 as a common therapeutic target for multineurodegenerative disorders and its inhibition using nonpeptidyl natural compounds. BioMed Res. Int. 2015;2015:379817. doi: 10.1155/2015/379817. [http://dx.doi.org/10.1155/2015/379817]. [PMID: 26064904]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ona V.O., Li M., Vonsattel J.P., Andrews L.J., Khan S.Q., Chung W.M., Frey A.S., Menon A.S., Li X.J., Stieg P.E., Yuan J., Penney J.B., Young A.B., Cha J.H., Friedlander R.M. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature. 1999;399(6733):263–267. doi: 10.1038/20446. [http://dx. doi.org/10.1038/20446]. [PMID: 10353249]. [DOI] [PubMed] [Google Scholar]
- 107.Li M., Ona V.O., Guégan C., Chen M., Jackson-Lewis V., Andrews L.J., Olszewski A.J., Stieg P.E., Lee J.P., Przedborski S., Friedlander R.M. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288(5464):335–339. doi: 10.1126/science.288.5464.335. [http://dx.doi.org/10.1126/science.288.5464.335]. [PMID: 10764647]. [DOI] [PubMed] [Google Scholar]
- 108.Braun J.S., Prass K., Dirnagl U., Meisel A., Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp. Neurol. 2007;206(2):183–191. doi: 10.1016/j.expneurol.2007.03.032. [http://dx.doi.org/10.1016/j.expneurol.2007.03. 032]. [PMID: 17585906]. [DOI] [PubMed] [Google Scholar]
- 109.Yang L., Sugama S., Mischak R.P., Kiaei M., Bizat N., Brouillet E., Joh T.H., Beal M.F. A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol. Dis. 2004;17(2):250–259. doi: 10.1016/j.nbd.2004.07.021. [http://dx.doi.org/ 10.1016/j.nbd.2004.07.021]. [PMID: 15474362]. [DOI] [PubMed] [Google Scholar]
- 110.Fischer U., Schulze-Osthoff K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005;12(Suppl. 1):942–961. doi: 10.1038/sj.cdd.4401556. [http://dx.doi.org/10.1038/sj.cdd.4401556]. [PMID: 15665817]. [DOI] [PubMed] [Google Scholar]
- 111.Ni H.M., McGill M.R., Chao X., Woolbright B.L., Jaeschke H., Ding W.X. Caspase inhibition prevents tumor necrosis factor-α-induced apoptosis and promotes necrotic cell death in mouse hepatocytes in vivo and in vitro. Am. J. Pathol. 2016;186(10):2623–2636. doi: 10.1016/j.ajpath.2016.06.009. [http://dx.doi.org/10.1016/j.ajpath.2016.06.009]. [PMID: 27616656]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rutar M., Natoli R., Kozulin P., Valter K., Gatenby P., Provis J.M. Analysis of complement expression in light-induced retinal degeneration: synthesis and deposition of C3 by microglia/ macrophages is associated with focal photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2011;52(8):5347–5358. doi: 10.1167/iovs.10-7119. [http://dx. doi.org/10.1167/iovs.10-7119]. [PMID: 21571681]. [DOI] [PubMed] [Google Scholar]
- 113.Rutar M., Valter K., Natoli R., Provis J.M. Synthesis and propagation of complement C3 by microglia/monocytes in the aging retina. PLoS One. 2014;9(4):e93343. doi: 10.1371/journal.pone.0093343. [http://dx.doi.org/10.1371/ journal.pone.0093343]. [PMID: 24705166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu H., Chen M., Forrester J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28(5):348–368. doi: 10.1016/j.preteyeres.2009.06.001. [http://dx.doi. org/10.1016/j.preteyeres.2009.06.001]. [PMID: 19560552]. [DOI] [PubMed] [Google Scholar]
- 115.Bosco A., Steele M.R., Vetter M.L. Early microglia activation in a mouse model of chronic glaucoma. J. Comp. Neurol. 2011;519(4):599–620. doi: 10.1002/cne.22516. [http://dx.doi.org/10.1002/cne.22516]. [PMID: 21246546]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Du L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0245-0. [PMID: 27830532]. [DOI] [PubMed] [Google Scholar]
- 117.Ebneter A., Casson R.J., Wood J.P., Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest. Ophthalmol. Vis. Sci. 2010;51(12):6448–6460. doi: 10.1167/iovs.10-5284. [http://dx. doi.org/10.1167/iovs.10-5284]. [PMID: 20688732]. [DOI] [PubMed] [Google Scholar]
- 118.Gallego B.I., Salazar J.J., de Hoz R., Rojas B., Ramírez A.I., Salinas-Navarro M., Ortín-Martínez A., Valiente-Soriano F.J., Avilés-Trigueros M., Villegas-Perez M.P., Vidal-Sanz M., Triviño A., Ramírez J.M. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflammation. 2012;9:92. doi: 10.1186/1742-2094-9-92. [http:// dx.doi.org/10.1186/1742-2094-9-92]. [PMID: 22583833]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rojas B., Gallego B.I., Ramírez A.I., Salazar J.J., de Hoz R., Valiente-Soriano F.J., Avilés-Trigueros M., Villegas-Perez M.P., Vidal-Sanz M., Triviño A., Ramírez J.M. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J. Neuroinflam. 2014;11:133. doi: 10.1186/1742-2094-11-133. [http://dx.doi.org/10.1186/1742-2094-11-133]. [PMID: 25064005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu T., Tatham A.J., Gracitelli C.P., Zangwill L.M., Weinreb R.N., Medeiros F.A. Rates of retinal nerve fiber layer loss in contralateral eyes of glaucoma patients with unilateral progression by conventional methods. Ophthalmology. 2015;122(11):2243–2251. doi: 10.1016/j.ophtha.2015.07.027. [http://dx.doi.org/10.1016/j.ophtha.2015.07.027]. [PMID: 26383993]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Madeira M.H., Ortin-Martinez A., Nadal-Nícolas F., Ambrósio A.F., Vidal-Sanz M., Agudo-Barriuso M., Santiago A.R. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci. Rep. 2016;6:27532. doi: 10.1038/srep27532. [http://dx.doi.org/10.1038/srep27532]. [PMID: 27270337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X., Huang P., Wang J., Yang Z., Huang S., Luo X., Qi J., Shen X., Zhong Y. The effect of A2A receptor antagonist on microglial activation in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2016;57(3):776–786. doi: 10.1167/iovs.15-18024. [http://dx.doi.org/10.1167/iovs.15-18024]. [PMID: 26934133]. [DOI] [PubMed] [Google Scholar]
- 123.Mac Nair C.E., Schlamp C.L., Montgomery A.D., Shestopalov V.I., Nickells R.W. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflammation. 2016;13(1):93. doi: 10.1186/s12974-016-0558-y. [http://dx.doi.org/10.1186/s12974-016-0558-y]. [PMID: 27126275]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang F., Wang D., Wu L., Li Y. Protective effects of triptolide on retinal ganglion cells in a rat model of chronic glaucoma. Drug Des. Devel. Ther. 2015;9:6095–6107. doi: 10.2147/DDDT.S92022. [http://dx.doi.org/10.2147/ DDDT.S92022]. [PMID: 26604697]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chidlow G., Ebneter A., Wood J.P., Casson R.J. Evidence supporting an association between expression of major histocompatibility complex II by microglia and optic nerve degeneration during experimental glaucoma. J. Glaucoma. 2016;25(8):681–691. doi: 10.1097/IJG.0000000000000447. [http:// dx.doi.org/10.1097/IJG.0000000000000447]. [PMID: 27253969]. [DOI] [PubMed] [Google Scholar]
- 126.Bosco A., Crish S.D., Steele M.R., Romero C.O., Inman D.M., Horner P.J., Calkins D.J., Vetter M.L. Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS One. 2012;7(8):e43602. doi: 10.1371/journal.pone.0043602. [http://dx.doi.org/10.1371/journal.pone. 0043602]. [PMID: 22952717]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Russo R., Varano G.P., Adornetto A., Nucci C., Corasaniti M.T., Bagetta G., Morrone L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016;787:134–142. doi: 10.1016/j.ejphar.2016.03.064. [http://dx.doi.org/10.1016/j.ejphar. 2016.03.064]. [PMID: 27044433]. [DOI] [PubMed] [Google Scholar]
- 128.Plane J.M., Shen Y., Pleasure D.E., Deng W. Prospects for minocycline neuroprotection. Arch. Neurol. 2010;67(12):1442–1448. doi: 10.1001/archneurol.2010.191. [http://dx.doi.org/10.1001/archneurol.2010.191]. [PMID: 20697034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuehn M.H., Kim C.Y., Ostojic J., Bellin M., Alward W.L., Stone E.M., Sakaguchi D.S., Grozdanic S.D., Kwon Y.H. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp. Eye Res. 2006;83(3):620–628. doi: 10.1016/j.exer.2006.03.002. [http://dx.doi.org/10.1016/j.exer.2006.03.002]. [PMID: 16677633]. [DOI] [PubMed] [Google Scholar]
- 130.Becker S., Reinehr S., Dick H.B., Joachim S.C. Ophthalmologe. 2015;112(1):41–48. doi: 10.1007/s00347-014-3100-6. [Complement activation after induction of ocular hypertension in an animal model]. [http://dx.doi.org/10. 1007/s00347-014-3100-6]. [PMID: 24942221]. [DOI] [PubMed] [Google Scholar]
- 131.Janeway C.J. Immunobiology: The Immune System in Health and Disease. The complement system and innate immunity. Vol. 5th ed. New York: Garland Science; 2001. [Google Scholar]
- 132.Nauta A.J., Daha M.R., van Kooten C., Roos A. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24(3):148–154. doi: 10.1016/s1471-4906(03)00030-9. [http://dx.doi.org/10. 1016/S1471-4906(03)00030-9]. [PMID: 12615211]. [DOI] [PubMed] [Google Scholar]
- 133.Bonifati D.M., Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol. Immunol. 2007;44(5):999–1010. doi: 10.1016/j.molimm.2006.03.007. [http://dx.doi.org/10.1016/j.molimm.2006.03.007]. [PMID: 16698083]. [DOI] [PubMed] [Google Scholar]
- 134.Jha P., Banda H., Tytarenko R., Bora P.S., Bora N.S. Complement mediated apoptosis leads to the loss of retinal ganglion cells in animal model of glaucoma. Mol. Immunol. 2011;48(15-16):2151–2158. doi: 10.1016/j.molimm.2011.07.012. [http://dx.doi.org/10.1016/j.molimm.2011.07.012]. [PMID: 21821293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmed F., Brown K.M., Stephan D.A., Morrison J.C., Johnson E.C., Tomarev S.I. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2004;45(4):1247–1258. doi: 10.1167/iovs.03-1123. [http://dx.doi.org/10.1167/iovs.03-1123]. [PMID: 15037594]. [DOI] [PubMed] [Google Scholar]
- 136.Reinehr S., Reinhard J., Gandej M., Kuehn S., Noristani R., Faissner A., Dick H.B., Joachim S.C. Simultaneous complement response via lectin pathway in retina and optic nerve in an experimental autoimmune glaucoma model. Front. Cell. Neurosci. 2016;10:140. doi: 10.3389/fncel.2016.00140. [http://dx.doi.org/10.3389/fncel.2016.00140]. [PMID: 27313510]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tegla C.A., Cudrici C., Patel S., Trippe R., III, Rus V., Niculescu F., Rus H. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol. Res. 2011;51(1):45–60. doi: 10.1007/s12026-011-8239-5. [http://dx.doi.org/10.1007/s12026-011-8239-5]. [PMID: 21850539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., Sher A., Litke A.M., Lambris J.D., Smith S.J., John S.W., Barres B.A. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [http://dx.doi.org/10.1016/j.cell.2007.10.036]. [PMID: 18083105]. [DOI] [PubMed] [Google Scholar]
- 139.Stasi K., Nagel D., Yang X., Wang R.F., Ren L., Podos S.M., Mittag T., Danias J. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 2006;47(3):1024–1029. doi: 10.1167/iovs.05-0830. [http://dx. doi.org/10.1167/iovs.05-0830]. [PMID: 16505037]. [DOI] [PubMed] [Google Scholar]
- 140.Tezel G., Yang X., Luo C., Kain A.D., Powell D.W., Kuehn M.H., Kaplan H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Invest. Ophthalmol. Vis. Sci. 2010;51(10):5071–5082. doi: 10.1167/iovs.10-5289. [http://dx.doi.org/10.1167/iovs.10-5289]. [PMID: 20484586]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuehn M.H., Kim C.Y., Jiang B., Dumitrescu A.V., Kwon Y.H. Disruption of the complement cascade delays retinal ganglion cell death following retinal ischemia-reperfusion. Exp. Eye Res. 2008;87(2):89–95. doi: 10.1016/j.exer.2008.04.012. [http://dx.doi.org/10.1016/j.exer.2008.04.012]. [PMID: 18572163]. [DOI] [PubMed] [Google Scholar]
- 142.Howell G.R., Macalinao D.G., Sousa G.L., Walden M., Soto I., Kneeland S.C., Barbay J.M., King B.L., Marchant J.K., Hibbs M., Stevens B., Barres B.A., Clark A.F., Libby R.T., John S.W. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 2011;121(4):1429–1444. doi: 10.1172/JCI44646. [http://dx.doi.org/10.1172/JCI44646]. [PMID: 21383504]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Howell G.R., Soto I., Ryan M., Graham L.C., Smith R.S., John S.W. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J. Neuroinflam. 2013;10:76. doi: 10.1186/1742-2094-10-76. [http://dx. doi.org/10.1186/1742-2094-10-76]. [PMID: 23806181]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [http://dx.doi.org/10.1038/ni. 1923]. [PMID: 20720586]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu H., Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016;787:94–104. doi: 10.1016/j.ejphar.2016.03.001. [http://dx.doi.org/10.1016/j.ejphar. 2016.03.001]. [PMID: 26948311]. [DOI] [PMC free article] [PubMed] [Google Scholar]