1. INTRODUCTION
Cognition is the mental process of acquiring, understanding, and storing knowledge through senses, experience, and thought. The proper operation of the cognitive process requires the structural and functional interaction of neurons with their surrounding vasculature, i.e., a constant and appropriate supply of oxygen, glucose, and other nutrients for the activity demands, as well as removal of a variety of bio-metabolites. The close correlation between the functional integrity of neurons and their vascular support is mainly due to the fact that the brain does not have much energy reserve but constantly consumes a lot of energy (2% of total body mass needing 15% cardiac output, and consuming 25% of body oxygen and 25% total body glucose [1]). A reduced blood flow, no matter acutely or chronically, would certainly
jeopardize the cognitive process. A subset of stroke survivors, for instance, exhibits vascular cognitive impairment (VCI; 62.6% at 3 months post-stroke [2]) and a third of stroke patients would develop to dementia between 1 and 3 years post-stroke [3, 4], especially in those whose blood circulation in the hippocampal and the prefrontal areas is dramatically affected.
Dementia is derived from the Latin word, demens, or dement, meaning ‘out of one’s mind’. Dementia, one of the most disabling health problems, affects the quality of life of affected and their caregivers. Because of brain’s high energy consumption, it is not surprising that vascular pathology has a central role in cognitive deterioration [5] in almost all types of memory disorders and has a worldwide incidence of approximately 1 in 20 people over the age of 65 [6]. Vascular dementia (VaD) is currently the second leading form of dementia (20% [7]), following Alzheimer’s disease (AD: 50%–75%), and followed by dementia with Lewy bodies (5%), and frontotemporal dementia (5%). Patients with VaD often show memory loss and dysfunctions of attention and task execution, such as slowed thinking, disorientation, diminished abilities of planning, reasoning, and judgement, and reduced capacities for problem solving [8, 9]. Activities of daily living (ADL) are also impaired in VaD patients.
This review focuses on potential neuropharmacology of VCI and VaD, especially of those recent preclinical and clinical studies related to rescuing cognitive functions from ischemic injuries, while the underlying pathological mechanisms [10] are not discussed in detail. Not covered in detail are also the clinical coexistence of VaD and AD [11, 12] and the complicated nature that vascular pathology may unmask preexisting pathology underlying AD and other memory disorders, or significantly deteriorate cognitive impairments [13-16]. About 40% of AD patients also have some forms of vascular cognitive impairment and dementia (VCID) [17-19]. On the other hand, amyloid pathology contributes to small vessel disease and reduces blood flow through vasoconstriction [20, 21]. VCD and VaD are basically discussed as one type of memory disorders, although they actually encompass diverse subtypes and differ in clinical presentation. These are all important issues in the pathology of memory disorders. The readers are, however, referred to other excellent reviews [5, 7, 22-26].
2. VASCULAR COGNITIVE IMPAIRMENT AND DEMENTIA
Like AD, VaD has its early stage changes, the VCI, in which patients exhibit cognitive decline, but can still maintain independent living. The term VCI was first proposed by Sachdev in 1999 [27] to describe cognitive deficits of vascular origin and later used by O'Brien et al. [28] to refer to all forms of mild to severe cognitive impairment associated with and presumed to be caused by cerebrovascular disease. The VCI is sometime still used in the literature as an umbrella term to describe the entire spectrum of vascular cognitive dysfunction [29], ranging from mild impairment in cognition to dementia. In keeping with the terms widely used in vascular cognitive deficits, VCID here refers to a spectrum of cognitive decline, caused by or associated with vascular pathology, including mild impairment in cognition: VCI; and dementia: VaD. The VCI is defined as cognitive deficits in at least one cognitive domain without impaired ADL [23, 30]. The VaD, on the other hand, has impairment in two or more areas of cognitive domain, and has impaired ADL. The VCID is diagnosed by a combination of medical history, assessment of cognitive function (including ADL) and behavioral symptoms, and neuroimaging techniques. Whilst various clinically and frequently used diagnostic criteria of VaD are available [31-33]: such as NINDS-AIREN [34], DSM-IV [35], and ADDTC [36], it is still difficult in developing a unified criterion for VaD diagnosis. Generally speaking, four cognitive domains are commonly screened: executive/activation (planning and attention), language (comprehension and/or expression), memory (learning and recalling), and visuospatial skills (ability to deal with nonverbal, graphic or geographic information). Vascular evidence includes the presence of cardiovascular risk factors (such as uncontrolled hypertension, diabetes, cerebrovascular hypoperfusion, obesity, stroke, dyslipidemia, recurrent stroke, and tobacco smoking), and magnetic resonance imaging (MRI) scanning of cerebral insults (showing diffuse white matter hyperintensities, WMH, in periventricular and deep white matter regions [37]). Blood vessels and circulation may also be damaged by other factors, such as brain radiation therapy [38] and substance abuse. The genetic basis of VCID has been described, including some monogenic disorders [39], but remains less well-defined for sporadic VCID. Study of VCID genetics meta-analysis has found an association of APOE epsilon alleles; ɛ4, with susceptibility for VCID [40]. Recurrent stroke is considered as a risk factor, probably because it increases the volume of brain injury, decreases the cardiovascular reserve, and would at least double the rates of post-stroke dementia than the first-ever stroke [41]. In the aging population, frailty syndrome has also been found to be a short-term predictor of developing overall dementia and, in particular, VCID [42]. Brain imaging, especially MRI [43], can track cerebrovascular pathology, including WMH (leukoaraiosis), small subcortical infarcts, and microbleeds [43-48].
VCID is a heterogeneous disease, differing in the underlying pathology, vessel sizes, location, severity, and duration. Upon reduced blood flow, the brain structures that are most vulnerable to hypoxia and energy shortage are always hit the first and damaged the most. The injuries could be acute, such as major stroke or heart failure [49], or chronic. The common feature [50] is that the hypoperfusion-induced VCID starts when the insults impair neuronal functions in the hippocampus, the prefrontal cortical-basal ganglia networks, and probably also the periventricular white matter. The hippocampi in the mammals are among the most vulnerable to hypoxic damage due to their high activity-demanded energy cost. Like AD and other memory disorders, the core problem with VaD is an impaired ability to form synapses and/or communicate through synaptic connections in the hippocampus and the cortex upon cognitive demands [51]. Such impairment usually occurs way before any significant loss of their principal neurons. The neuronal count, for instance, in the hippocampal CA1 subregion has been found not to differ in VaD patients from that of control subjects [52]. The observation means that a significant loss in the number of neurons is not a necessary mechanism for VaD to occur. On the other hand, severe or long-lasting cerebrovascular pathology can certainly cause neuronal death through necrosis and apoptosis. Cerebral white matter locates deep in the brain and has limited blood supply and poor collateral blood flow. Reduced blood flow to cerebral white matter leads to damage from demyelination, due to hypoxic injury to oligiodendrocytes [53]. There are reports that activation of NF-κB in astrocytes may be involved in white matter demyelination [54]. The involvement of white matter damage in VCID as indicated by WMH is weakened by a general lack of a good correlation between the severity of WMH and cognitive decline [55], suggesting that WMT might not be a direct phenomenon determining the cognitive functions.
Diabetes mellitus increases the long-term risk of dementia by a factor of 2. The type II diabetes is a risk factor for atherosclerosis and small vessel disease [56] and increases progression of cognitive impairment to VaD [57, 58]. The consequence of insulin resistance is cellular energy defect, high plasma lipids, and hypertension [59], negatively correlated with verbal cognitive performance [60]. Others found that poorly controlled and long-standing diabetes has VaD prevalence as high as 40% [61].
One issue cannot be ignored in vascular integrity is the impact of aging [1, 62, 63]. Dementia is uncommon in people younger than 65 years of age. Aging brings with depletion of cerebrovascular reserve, blood-brain barrier (BBB) breakdown (initially in the hippocampus [64]), a decreased number of capillaries, thickened fibrotic basal membrane, reduced cerebral perfusion, and an enhanced vulnerability to ischemic injury. All point to a reduced blood perfusion to the brain tissues and an increased cell vulnerability to ischemic injury.
VCI and VaD are generally viewed as the functional consequences of reduced blood flow to the brain, i.e. a disorder of hypoperfusion-induced pathogenesis [65]. Reduced supplies of oxygen and nutrition impair operation of synaptic activities and mitochondrial function, which exacerbates neuronal damage [66, 67]. However, cerebrovascular disease and ischemic hypoperfusion are usually accompanied by BBB damage, including the BBB in the hippocampus [64, 68]. The BBB protects the brain environment from various molecules in the peripheral circulation and its dysfunction may play a significant role in the pathogenesis of VaD [69, 70]. The potential involvement of BBB function in the VCID pathogenesis means that its effective therapeutic strategy may require a functional restoration of the BBB.
3. conventional therapeutics for vas-cular cognitive impairment and dementia
For functional recovery, VCID patients would need both anti-dementia therapeutics and therapeutics for the underlying cerebrovascular problems. The current management strategies for VCID patients include the symptomatic treatment of VCI and VaD, management of risk factors, and non-pharmacological approaches aimed at preventing VCI progression to VaD.
With regards to anti-dementia therapeutics, there are no specific drugs approved for VCID treatment. The cholinesterase inhibitors, donepezil, galantamine, and rivastigmine, and the NMDA (the N-methyl-D-aspartate receptor) antagonist memantine, the only medications currently licensed for the AD treatment, have been found to show some cognitive improvements in VCID [5]. The main problem with these agents as anti-dementia drugs is that they produce small, short-lived improvements in mild to moderate cases and marginal benefits in severe cases [71]. The weak benefits are nevertheless overwhelmed by their adverse effects [72]. There are also reports that the benefits of galantamine [73] and memantine [5] on VCI are uncertain and that memantine may cause or contribute to delirium in VCID patients [74].
Emerging evidence, however, suggests that anti-dementia therapeutics may be developed through synaptic pharmacology. Like synaptic damage in AD brains, synaptic damage and/or synaptic loss appear underling a critical part of VCID. The VCID pharmacology is thus to restore the synaptic functions and brain’s ability to process memory-related information through synaptic interconnections upon cognitive demands. There are various signaling pathways that can be facilitated to enhance synaptic functions and brains’ ability to repair and maintain its proper synaptic connections. Bryostatin-1, a relatively selective protein kinase Cε activator, for instance, can rescue synaptogenesis and cognitive function after global cerebral ischemia [75, 76]. PKCε is predominantly expressed in the brain [77], is anti-apoptotic [78], and is suppressed in neurons after cerebral ischemia [79]. The therapeutic effects are highly correlated and consistent with increased expression and activity, in the hippocampus and related cortex, of brain derived neurotrophic factor (BDNF). BDNF is especially enriched in the hippocampus and cortex and play an essential role in synaptic functions and processing of memory-related information in the brain [80]. The therapeutic benefits of neurotrophic enhancers, such as bryostatin-1, to cognitive functions in patients with VCID, however, remain to be evaluated. Other potential therapeutic mechanisms include producing an antiapoptotic impact [74, 75], preserving ventricular gap junction protein connexin signaling [81], reducing the damage due to cerebral reperfusion after ischemia [82], phosphorylating the mitochondrial K+ATP channel, and increasing synaptosomal mitochondrial respiration [83, 84]. It remains to be studied which mechanism(s) are involved in the therapeutic impact.
An effective therapy for VCID patients needs directly address the problem of hypoperfusion and provide therapeutic improvement in cerebrovascular circulation. This involves a life-time management of cardiovascular risk factors, such as hypertension [26] and diabetes, and acute treatment with thrombolytic therapy in ischemic stroke. A recent meta-analysis reveals renin-angiotension system-targeting antihypertensive drugs produce remarkable efficacy on reducing the incidence of VCID [85]. Beneficial impacts to VCID could also be achieved through enhancing cellular integrity or signaling pathway in cognition. Citicoline, an essential intermediate in the biosynthesis of structural phospholipids in cell membranes, has been reported to exhibit neuroprotective activity [86-88], although its clinical benefits in ischemic stroke and traumatic brain injury remain uncertain [89]. Benefit effects of changing life styles and activity (diet, exercise, and environmental enrichment [90, 91]) on VCI and VaD have been reported. Some of the strategies may involve both, producing an anti-dementia impact and reducing the cerebrovascular burdens. Repetitive transcranial magnetic stimulation has been reported to have neuroprotective effects against VaD in rats [92]. Along this line are also exercise and environmental enrichment. In rats after 2-VO chronic cerebral hypoperfusion, involuntary treadmill exercise for 4 weeks has been found to reduce cognitive decline by enhancing neurogenesis and increasing BDNF expression [93]. A similar effect, attenuating 2-VO-induced vascular neurocognitive deficits, has also been observed in involuntary running (360 m per day for 2 weeks) in rats [94]. Exercise increases histone acetylation, stimulates DNA demethylation in BDNF promoter IV, and elevates levels of activated methyl-CpG-binding protein 2, a molecule important for BDNF gene transcription regulation, as well as BDNF mRNA and protein in the rat hippocampus [95]. There are reports that repetitive transcranial magnetic stimulation (5 days/week for 4 week, starting one week after establishing the hypoperfusion) increases hippocampal expression of BDNF [96] and vascular endothelial growth factor and attenuates VaD in 2-VO rat models [97]. The effects of exercise on VaD might be sex-dependent, at least in some experimental animals. A cognitive rehabilitation paradigm, which is effective in male rats, has been found to lack efficacy in female rats [98]. In patients with mild VaD, anodal transcranial direct current stimulation of the left dorsolateral prefrontal cortex has been found to produce additional effects to cognitive training on visual short-term memory, verbal working memory, and executive control [99]. Involuntary exercise evoked with functional electric stimulation has often been used in stroke rehabilitation [100]. Several recent meta-analyses have, however, examined effects of cognitive rehabilitation and found no statistical long-term therapeutic benefits in patients with post-stroke VCID [101]. Another interesting phenomenon is the pre- and post-conditioning [49, 102]. The pre-conditioning, for example, activates the protein kinase Cε isoforms in many tissues and is likely to facilitate synaptic re-modeling and synaptogenesis. To replace lost cells due to stroke or vascular pathology, stem cells might also be a valuable option [103] to consider in the future.
4. prevENTAtive pharmacology for vas-cular cognitive impairment and dementia
VCID might be prevented through two types of interventions. The first one is an early identification and medical treatment of cardiovascular conditions [104]. The risk factors for developing VCID can be divided into non-modifiable and modifiable ones. The non-modifiable risk factors include age, sex, ethnicity, family history, and genes [5, 105]. Some of these currently non-modifiable risk factors might become modifiable ones in the future through human efforts. Modifiable risk factors include hypertension, diabetes, dyslipidemia, atrial fibrillation, obesity, smoking, low education, and physical inactivity. An effective control of cardiovascular risk factors prevents VCID and may be more effective than current pharmacological treatment for VCID [106, 107]. Hypertension, for instance, is known to cause damage to the cerebral tissues, resulting in leukoaraiosis, or lesions in the periventricular and subcortical white matter regions [108]. Evidence has been presented that maintaining ideal cardiovascular health at middle age is related to better cognition in later life [109]. Antihypertensive medication, thus, in patients with hypertension has been shown to have preventative effects on cognitive deficits in later life [110, 111]. On the other hand, the causative connection between hypertension and dementia is complicated and not fully understood [82, 112]. One is not expected to find effectiveness of treating hypertension to prevent or slow cognitive decline for those hypertensive patients whose cerebral circulation is not severely impaired by the raised blood pressure to compromise their cognitive functions. It remains to be studied whether lowing cholesterol benefits patients with VCI and VaD. A recent Cochrane Database Review has indicated that statins do not prevent cognitive decline when given in late life to those people at high risk for vascular disease [113].
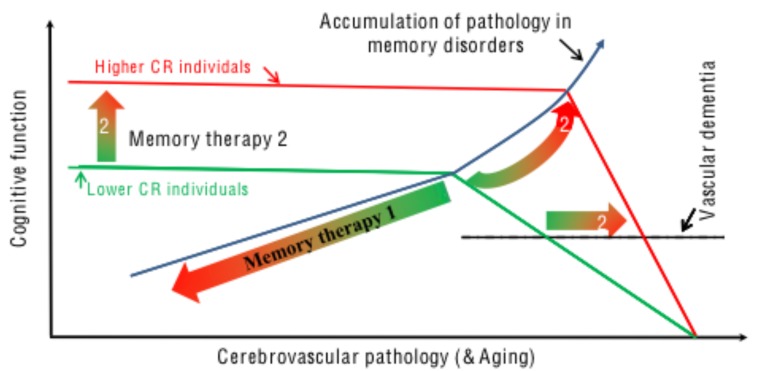
Another potentially important strategy for preventative pharmacology against VCID is to build cerebral reserve pharmacologically (Fig. 1). The concept of cerebral reserve describes differences between individuals in their ability to compensate age-related brain pathology. Thus, if we consider two individuals with the same extent of memory-impairing neuropathology, one may exhibit clinical signs of dementia, while the other remains cognitively intact [114, 115]. The difference is believed to stem from their individual levels of the cerebral reserves, not the severity of the underlying pathology in their brains. Cerebral reserve includes 1) brain reserve [114, 116, 117], related to differences in brain size and structure, such as neuronal and synaptic density; 2) cognitive reserve [118-120], about the differences in the ability to make flexible and efficient use of existing brain resources when engaged in a cognitive task; and 3) network maintenance [121], referring to the differential capacity to initiate synaptic repair, synaptogenesis, and neurogenesis. The three components appear working together and supporting the cognitive leisure built through life’s experiences. Evidence has been provided that lifestyle and environmental factors, especially of those cognitively challenging, play a strong role in shaping the expression of cerebral reserve. Higher IQ, education in early life, and bilingualism, for instance, contribute to higher cerebral reserve. Bilingualism has been shown to delay the AD onset by 5.1 years on average [122], in comparison to monolingualism. If the age of dementia onset in all cases could be delayed by just 5 years, the number of dementia cases could be drastically reduced [123]. As we know more and more of the cognitive mechanisms, facilitating cerebral reserve through pharmacological means would certainly become a reality in the future. There are pharmacological agents that can mimic the cognitive leisure and enhance cognitive capacities [124] (Fig. 1). These agents could be developed to be enhancers of cerebral reserve, in order to delay and prevent (when delayed for an enough long period) VaD and other memory disorders. Caution, however, need to be taken, since the concept of cerebral reserve implies both the chance to mitigate VCID by increasing the cerebral reserve and the danger of underestimating severity of the cerebrovascular damage when just evaluating the VCID [114].
Fig. (1).
The cerebral reserve hypothesis and pharmacology. Cerebral reserve represents the memory function, which is impaired by damaging neuropathology in memory disorders or reduced in aging. Memory therapy 1 is the ‘conventional’ therapeutic strategy, which antagonizes the neuropathology of memory disorders and underlying cerebrovascular pathology as triggered by vascular incidence/disease and associated with aging. Memory pharmacology 2 enhances cerebral reserve, so that cognitive function is maintained to a much greater amount of pathology before the function starts to drop (Memory therapy 2) at a much later time (2). The clinical diagnosis of dementia would also occur later (2), at greater neuropathology, but at greater rate of progression.
CONCLUSION
Although VCID represents a great challenge in modern clinical practice, therapeutic options remain rather limited, because molecular and cellular mechanisms that underlie cerebrovascular disease are still poorly understood. In addition, the co-existence of VaD and AD pathology would certainly complicate the treatment [125]. Although AD and vascular dementia have been largely targeted as entirely separate entities in pathogenic and therapeutic studies, a growing body of epidemiological, neuroimaging, pathologic, and clinical evidence indicates that vascular pathology and amyloid pathology commonly occur together in clinically diagnosed patients suffering from dementia. So far, scientific investigation of dementia has mainly focused on either amyloid-based pathology or vascular pathology. The amyloid-based pathology of AD and the vascular pathology of VaD are viewed and clinically treated as separate disease entities. Despite intensive investigation worldwide, this approach, however, has not led to a cure of either disorder. A recent study indicates that in patients with AD and VCID, the anti-AD treatment alone would not lead to cognitive benefits unless the vascular pathology is also co-treated [125]. One approach for a successful treatment would be through a combined pharmacological treatment of both the cerebrovascular disease and synaptic deficits [51].
VCI and VaD after stroke and/or cardiovascular episodes are extremely common [126, 127], even after successful clinical recovery from the cardiovascular disorders. VCID is one of the dementias in which prevention is possible, especially for those with modifiable cardiovascular risk factors. Managing cardiovascular risk factors, such as hypertension, diabetes, dyslipidemia, heart function, obesity, smoking, and physical and mental inactivity, would greatly reduce the chances of developing VCID in later life. As we understand more of the cognitive mechanisms and their vulnerability to a variety of injuries, effective anti-dementia drugs are expected to be developed in the near future. In addition, building cerebral reserve pharmacologically through mimicking cognitive leisure may represent an important new strategy in fighting the war with dementia. The therapeutic approach could stop or even reverse VCI’s progression to VaD, and eventually delay and prevent the VCID and other dementias, including AD.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Nagata K., Yamazaki T., Takano D., Maeda T., Fujimaki Y., Nakase T., Sato Y. Cerebral circulation in aging. Ageing Res. Rev. 2016;30:49–60. doi: 10.1016/j.arr.2016.06.001. [http://dx.doi.org/10.1016/j.arr.2016.06.001]. [PMID: 27484894]. [DOI] [PubMed] [Google Scholar]
- 2.Yu K.H., Cho S.J., Oh M.S., Jung S., Lee J.H., Shin J.H., Koh I.S., Cha J.K., Park J.M., Bae H.J., Kang Y., Lee B.C. Cognitive impairment evaluated with vascular cognitive impairment harmonization standards in a multicenter prospective stroke cohort in Korea. Stroke. 2013;44(3):786–788. doi: 10.1161/STROKEAHA.112.668343. [http://dx.doi.org/10.1161/ STROKEAHA.112.668343]. [PMID: 23271507]. [DOI] [PubMed] [Google Scholar]
- 3.Sachdev P.S., Chen X., Brodaty H., Thompson C., Altendorf A., Wen W. The determinants and longitudinal course of post-stroke mild cognitive impairment. J. Int. Neuropsychol. Soc. 2009;15(6):915–923. doi: 10.1017/S1355617709990579. [http://dx.doi.org/10.1017/S1355617709990579]. [PMID: 19891821]. [DOI] [PubMed] [Google Scholar]
- 4.Kjörk E., Blomstrand C., Carlsson G., Lundgren-Nilsson Å., Gustafsson C. Daily life consequences, cognitive impairment, and fatigue after transient ischemic attack. Acta Neurol. Scand. 2015;133:103–110. doi: 10.1111/ane.12435. [http://dx.doi.org/10.1111/ane.12435]. [PMID: 25955112]. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C., Launer L.J., Laurent S., Lopez O.L., Nyenhuis D., Petersen R.C., Schneider J.A., Tzourio C., Arnett D.K., Bennett D.A., Chui H.C., Higashida R.T., Lindquist R., Nilsson P.M., Roman G.C., Sellke F.W., Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [http://dx.doi.org/10.1161/STR.0b013e3182299496]. [PMID: 21778438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He W., Sengupta M., Velkoff V.A., DeBarros K.A.U.S. U.S. 2005.
- 7.Khan A., Kalaria R.N., Corbett A., Ballard C. Update on vascular dementia. J. Geriatr. Psychiatry Neurol. 2016;29(5):281–301. doi: 10.1177/0891988716654987. [http://dx.doi.org/10.1177/0891988716654987]. [PMID: 27502303]. [DOI] [PubMed] [Google Scholar]
- 8.Ihle-Hansen H., Thommessen B., Wyller T.B., Engedal K., Øksengård A.R., Stenset V., Løken K., Aaberg M., Fure B. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement. Geriatr. Cogn. Disord. 2011;32(6):401–407. doi: 10.1159/000335361. [http://dx.doi. org/10.1159/000335361]. [PMID: 22311341]. [DOI] [PubMed] [Google Scholar]
- 9.Douiri A., Rudd A.G., Wolfe C.D. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995-2010. Stroke. 2013;44(1):138–145. doi: 10.1161/STROKEAHA.112.670844. [http://dx.doi.org/10.1161/STROKEAHA.112. 670844]. [PMID: 23150656]. [DOI] [PubMed] [Google Scholar]
- 10.Arboix A., Martí-Vilalta J.L. A study of lacunar infarcts based on analysis of the main anatomopathological series in the literature. Rev. Neurol. 1998;26(151):365–367. [PMID: 9585943]. [PubMed] [Google Scholar]
- 11.Schneider J.A., Arvanitakis Z., Bang W., Bennett D.A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [http://dx.doi.org/10.1212/01.wnl.0000271090.28148.24]. [PMID: 17568013]. [DOI] [PubMed] [Google Scholar]
- 12.Yoshitake T., Kiyohara Y., Kato I., Ohmura T., Iwamoto H., Nakayama K., Ohmori S., Nomiyama K., Kawano H., Ueda K. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45(6):1161–1168. doi: 10.1212/wnl.45.6.1161. [http://dx.doi.org/10. 1212/WNL.45.6.1161]. [PMID: 7783883]. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Zhang X., Yang D., Luo G., Chen S., Le W. Hypoxia increases Abeta generation by altering β- and γ-cleavage of APP. Neurobiol. Aging. 2009;30(7):1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [http://dx.doi.org/10. 1016/j.neurobiolaging.2007.10.011]. [PMID: 18063223]. [DOI] [PubMed] [Google Scholar]
- 14.Zhiyou C., Yong Y., Shanquan S., Jun Z., Liangguo H., Ling Y., Jieying L. Upregulation of BACE1 and β-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochem. Res. 2009;34(7):1226–1235. doi: 10.1007/s11064-008-9899-y. [http://dx.doi.org/10.1007/s11064-008-9899-y]. [PMID: 19123057]. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T., Hasegawa Y., Uekawa K., Senju S., Nakagata N., Matsui K., Kim-Mitsuyama S. Transient mild cerebral ischemia significantly deteriorated cognitive impairment in a mouse model of alzheimer’s disease via angiotensin AT1 receptor. Am. J. Hypertens. 2017;30(2):141–150. doi: 10.1093/ajh/hpw099. [http://dx.doi.org/10.1093/ajh/ hpw099]. [PMID: 27572961]. [DOI] [PubMed] [Google Scholar]
- 16.Prasad K., Wiryasaputra L., Ng A., Kandiah N. White matter disease independently predicts progression from mild cognitive impairment to Alzheimer’s disease in a clinic cohort. Dement. Geriatr. Cogn. Disord. 2011;31(6):431–434. doi: 10.1159/000330019. [http://dx.doi.org/10. 1159/000330019]. [PMID: 21757908]. [DOI] [PubMed] [Google Scholar]
- 17.Bowler J.V., Munoz D.G., Merskey H., Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 1998;64(1):18–24. doi: 10.1136/jnnp.64.1.18. [http://dx.doi.org/10.1136/jnnp.64.1.18]. [PMID: 9436722]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zekry D., Hauw J.J., Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J. Am. Geriatr. Soc. 2002;50(8):1431–1438. doi: 10.1046/j.1532-5415.2002.50367.x. [http://dx.doi.org/10.1046/j.1532-5415.2002.50367.x]. [PMID: 12165002]. [DOI] [PubMed] [Google Scholar]
- 19.Langa K.M., Foster N.L., Larson E.B. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292(23):2901–2908. doi: 10.1001/jama.292.23.2901. [http://dx.doi.org/10.1001/jama.292.23.2901]. [PMID: 15598922]. [DOI] [PubMed] [Google Scholar]
- 20.Niwa K., Porter V.A., Kazama K., Cornfield D., Carlson G.A., Iadecola C. A β-peptides enhance vasoconstriction in cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2001;281(6):H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [http://dx.doi.org/10.1152/ajpheart.2001.281.6.H2417]. [PMID: 11709407]. [DOI] [PubMed] [Google Scholar]
- 21.Palmer J.C., Tayler H.M., Love S. Endothelin-converting enzyme-1 activity, endothelin-1 production, and free radical-dependent vasoconstriction in Alzheimer’s disease. J. Alzheimers Dis. 2013;36(3):577–587. doi: 10.3233/JAD-130383. [PMID: 23629587]. [DOI] [PubMed] [Google Scholar]
- 22.Frances A., Sandra O., Lucy U. Vascular cognitive impairment, a cardiovascular complication. World J. Psychiatry. 2016;6(2):199–207. doi: 10.5498/wjp.v6.i2.199. [http://dx.doi.org/10.5498/wjp.v6.i2.199]. [PMID: 27354961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiesmann M., Kiliaan A.J., Claassen J.A. Vascular aspects of cognitive impairment and dementia. J. Cereb. Blood Flow Metab. 2013;33(11):1696–1706. doi: 10.1038/jcbfm.2013.159. [http://dx.doi.org/10.1038/jcbfm.2013. 159]. [PMID: 24022624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corriveau R.A., Bosetti F., Emr M., Gladman J.T., Koenig J.I., Moy C.S., Pahigiannis K., Waddy S.P., Koroshetz W. The science of vascular contributions to cognitive impairment and dementia (VCID): A framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell. Mol. Neurobiol. 2016;36(2):281–288. doi: 10.1007/s10571-016-0334-7. [http://dx.doi.org/10.1007/s10571-016-0334-7]. [PMID: 27095366]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalaria R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016;131(5):659–685. doi: 10.1007/s00401-016-1571-z. [http://dx.doi.org/10.1007/s00401-016-1571-z]. [PMID: 27062261]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker K.A., Power M.C., Gottesman R.F. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr. Hypertens. Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3. [http://dx.doi.org/10. 1007/s11906-017-0724-3]. [PMID: 28299725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachdev P. Vascular cognitive disorder. Int. J. Geriatr. Psychiatry. 1999;14(5):402–403. [http://dx.doi.org/10.1002/(SICI)1099-1166 (199905)14:5<402:AID-GPS958>3.0.CO;2-H]. [PMID: 10389048]. [PubMed] [Google Scholar]
- 28.O’Brien J.T., Erkinjuntti T., Reisberg B., Roman G., Sawada T., Pantoni L., Bowler J.V., Ballard C., DeCarli C., Gorelick P.B., Rockwood K., Burns A., Gauthier S., DeKosky S.T. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [http://dx. doi.org/10.1016/S1474-4422(03)00305-3]. [PMID: 12849265]. [DOI] [PubMed] [Google Scholar]
- 29.Ritter A., Pillai J.A. Treatment of vascular cognitive impairment. Curr. Treat. Options Neurol. 2015;17(8):367. doi: 10.1007/s11940-015-0367-0. [http://dx.doi.org/ 10.1007/s11940-015-0367-0]. [PMID: 26094078]. [DOI] [PubMed] [Google Scholar]
- 30.Helman A.M., Murphy M.P. Vascular cognitive impairment: Modeling a critical neurologic disease in vitro and in vivo. Biochim. Biophys. Acta. 2016;1862(5):975–982. doi: 10.1016/j.bbadis.2015.12.009. [http://dx.doi.org/10.1016/ j.bbadis.2015.12.009]. [PMID: 26704178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pendlebury S.T., Cuthbertson F.C., Welch S.J., Mehta Z., Rothwell P.M. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41(6):1290–1293. doi: 10.1161/STROKEAHA.110.579888. [http://dx.doi. org/10.1161/STROKEAHA.110.579888]. [PMID: 20378863]. [DOI] [PubMed] [Google Scholar]
- 32.Godefroy O., Fickl A., Roussel M., Auribault C., Bugnicourt J.M., Lamy C., Canaple S., Petitnicolas G. Is the montreal cognitive assessment superior to the mini-mental state examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42(6):1712–1716. doi: 10.1161/STROKEAHA.110.606277. [http:// dx.doi.org/10.1161/STROKEAHA.110.606277]. [PMID: 21474808]. [DOI] [PubMed] [Google Scholar]
- 33.Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E., Powers W.J., DeCarli C., Merino J.G., Kalaria R.N., Vinters H.V., Holtzman D.M., Rosenberg G.A., Wallin A., Dichgans M., Marler J.R., Leblanc G.G. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [http://dx.doi.org/10.1161/ 01.STR.0000237236.88823.47]. [PMID: 16917086]. [DOI] [PubMed] [Google Scholar]
- 34.Roman G.C., Tatemichi T.K., Erkinjuntti T. Vascular dementia: diagnostic criteria for research studies. Report NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 35.Rabe-Jablonska J., Bienkiewicz W. 1994. [PubMed] [Google Scholar]
- 36.Chui H.C., Victoroff J.I., Margolin D., Jagust W., Shankle R., Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42(3 Pt 1):473–480. doi: 10.1212/wnl.42.3.473. [http://dx.doi.org/10.1212/WNL.42.3.473]. [PMID: 1549205]. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg G.A., Wallin A., Wardlaw J.M., Markus H.S., Montaner J., Wolfson L., Iadecola C., Zlokovic B.V., Joutel A., Dichgans M., Duering M., Schmidt R., Korczyn A.D., Grinberg L.T., Chui H.C., Hachinski V. Consensus statement for diagnosis of subcortical small vessel disease. J. Cereb. Blood Flow Metab. 2016;36(1):6–25. doi: 10.1038/jcbfm.2015.172. [http://dx.doi.org/10.1038/jcbfm.2015.172]. [PMID: 26198175]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada M.K. A link between vascular damage and cognitive deficits after whole-brain radiation therapy for cancer: A clue to other types of dementia? Drug Discov. Ther. 2016;10(2):79–81. doi: 10.5582/ddt.2016.01004. [http://dx.doi.org/10.5582/ddt.2016.01004]. [PMID: 27087553]. [DOI] [PubMed] [Google Scholar]
- 39.Ikram M.A., Bersano A., Manso-Calderón R., Jia J.P., Schmidt H., Middleton L., Nacmias B., Siddiqi S., Adams H.H.H. Genetics of vascular dementia - review from the ICVD working group. BMC Med. 2017;15(1):48. doi: 10.1186/s12916-017-0813-9. [http://dx.doi.org/10.1186/s12916-017-0813-9]. [PMID: 28260527]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skrobot O.A., McKnight A.J., Passmore P.A., Seripa D., Mecocci P., Panza F., Kalaria R., Wilcock G., Munafò M., Erkinjuntti T., Karhunen P., Pessi T., Martiskainen M., Love S., Kehoe P.G. A validation study of vascular cognitive impairment genetics meta-analysis findings in an independent collaborative cohort. J. Alzheimers Dis. 2016;53(3):981–989. doi: 10.3233/JAD-150862. [http://dx.doi.org/ 10.3233/JAD-150862]. [PMID: 27314523]. [DOI] [PubMed] [Google Scholar]
- 41.Pendlebury S.T., Rothwell P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [http://dx.doi.org/10.1016/S1474-4422(09)70236-4]. [PMID: 19782001]. [DOI] [PubMed] [Google Scholar]
- 42.Solfrizzi V., Scafato E., Frisardi V., Seripa D., Logroscino G., Maggi S., Imbimbo B.P., Galluzzo L., Baldereschi M., Gandin C., Di Carlo A., Inzitari D., Crepaldi G., Pilotto A., Panza F. Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimers Dement. 2013;9(2):113–122. doi: 10.1016/j.jalz.2011.09.223. [http://dx.doi.org/10.1016/j.jalz.2011.09.223]. [PMID: 23245560]. [DOI] [PubMed] [Google Scholar]
- 43.Wardlaw J.M., Smith C., Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12(5):483–497. doi: 10.1016/S1474-4422(13)70060-7. [http://dx.doi.org/10.1016/S1474-4422(13)70060-7]. [PMID: 23602162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akoudad S., Wolters F.J., Viswanathan A., de Bruijn R.F., van der Lugt A., Hofman A., Koudstaal P.J., Ikram M.A., Vernooij M.W. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934–943. doi: 10.1001/jamaneurol.2016.1017. [http://dx.doi. org/10.1001/jamaneurol.2016.1017]. [PMID: 27271785]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [http://dx.doi.org/10.1016/S1474-4422(10) 70104-6]. [PMID: 20610345]. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence A.J., Patel B., Morris R.G., MacKinnon A.D., Rich P.M., Barrick T.R., Markus H.S. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St. George’s cognition and neuroimaging in stroke (SCANS) study. PLoS One. 2013;8(4):e61014. doi: 10.1371/journal.pone.0061014. [http://dx.doi.org/ 10.1371/journal.pone.0061014]. [PMID: 23613774]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenberg S.M., Vernooij M.W., Cordonnier C., Viswanathan A., Al-Shahi Salman R., Warach S., Launer L.J., Van Buchem M.A., Breteler M.M. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [http://dx. doi.org/10.1016/S1474-4422(09)70013-4]. [PMID: 19161908]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valenti R., Del Bene A., Poggesi A., Ginestroni A., Salvadori E., Pracucci G., Ciolli L., Marini S., Nannucci S., Pasi M., Pescini F., Diciotti S., Orlandi G., Cosottini M., Chiti A., Mascalchi M., Bonuccelli U., Inzitari D., Pantoni L. Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: The Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J. Neurol. Sci. 2016;368:195–202. doi: 10.1016/j.jns.2016.07.018. [http://dx.doi. org/10.1016/j.jns.2016.07.018]. [PMID: 27538632]. [DOI] [PubMed] [Google Scholar]
- 49.Yzeiraj E., Tam D.M., Gorodeski E.Z. Management of cognitive impairment in heart failure. Curr. Treat. Options Cardiovasc. Med. 2016;18(1):4. doi: 10.1007/s11936-015-0425-7. [http://dx.doi.org/10.1007/s11936-015-0425-7]. [PMID: 26747626]. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese V., Giordano J., Signorile A., Laura Ontario M., Castorina S., De Pasquale C., Eckert G., Calabrese E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016;94(12):1588–1603. doi: 10.1002/jnr.23925. [http://dx.doi.org/10.1002/jnr.23925]. [PMID: 27662637]. [DOI] [PubMed] [Google Scholar]
- 51.Sun M-K., Nelson T.J., Alkon D.L. Towards universal therapeutics for memory disorders. Trends Pharmacol. Sci. 2015;36(6):384–394. doi: 10.1016/j.tips.2015.04.004. [http://dx.doi.org/10.1016/j.tips.2015.04.004]. [PMID: 25959522]. [DOI] [PubMed] [Google Scholar]
- 52.Zarow C., Vinters H.V., Ellis W.G., Weiner M.W., Mungas D., White L., Chui H.C. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann. Neurol. 2005;57(6):896–903. doi: 10.1002/ana.20503. [http://dx.doi.org/10.1002/ana.20503]. [PMID: 15929035]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ihara M., Tomimoto H. Lessons from a mouse model characterizing features of vascular cognitive impairment with white matter changes. J. Aging Res. 2011;2011:978761. doi: 10.4061/2011/978761. [http://dx.doi.org/10. 4061/2011/978761]. [PMID: 22132331]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saggu R., Schumacher T., Gerich F., Rakers C., Tai K., Delekate A., Petzold G.C. Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol. Commun. 2016;4(1):76. doi: 10.1186/s40478-016-0350-3. [http:// dx.doi.org/10.1186/s40478-016-0350-3]. [PMID: 27487766]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yassi N., Desmond P.M., Masters C.L. Magnetic resonance imaging of vascular contributions to cognitive impairment and dementia. J. Mol. Neurosci. 2016;60(3):349–353. doi: 10.1007/s12031-016-0799-3. [http://dx.doi.org/ 10.1007/s12031-016-0799-3]. [PMID: 27437942]. [DOI] [PubMed] [Google Scholar]
- 56.Reijmer Y.D., Fotiadis P., Piantoni G., Boulouis G., Kelly K.E., Gurol M.E., Leemans A., O’Sullivan M.J., Greenberg S.M., Viswanathan A. Small vessel disease and cognitive impairment: The relevance of central network connections. Hum. Brain Mapp. 2016;37(7):2446–2454. doi: 10.1002/hbm.23186. [http://dx.doi.org/10.1002/hbm.23186]. [PMID: 27004840]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Folsom A.R., Rasmussen M.L., Chambless L.E., Howard G., Cooper L.S., Schmidt M.I., Heiss G. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. Diabetes Care. 1999;22(7):1077–1083. doi: 10.2337/diacare.22.7.1077. [http://dx. doi.org/10.2337/diacare.22.7.1077]. [PMID: 10388971]. [DOI] [PubMed] [Google Scholar]
- 58.Saedi E., Gheini M.R., Faiz F., Arami M.A. Diabetes mellitus and cognitive impairments. World J. Diabetes. 2016;7(17):412–422. doi: 10.4239/wjd.v7.i17.412. [http://dx.doi.org/10.4239/wjd.v7.i17.412]. [PMID: 27660698]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de la Monte S.M. Relationships between diabetes and cognitive impairment. Endocrinol. Metab. Clin. North Am. 2014;43(1):245–267. doi: 10.1016/j.ecl.2013.09.006. [http://dx.doi.org/10.1016/j.ecl.2013.09.006]. [PMID: 24582101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCrimmon R.J., Ryan C.M., Frier B.M. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [http://dx.doi. org/10.1016/S0140-6736(12)60360-2]. [PMID: 22683129]. [DOI] [PubMed] [Google Scholar]
- 61.Dejgaard A., Gade A., Larsson H., Balle V., Parving A., Parving H.H. Evidence for diabetic encephalopathy. Diabet. Med. 1991;8(2):162–167. doi: 10.1111/j.1464-5491.1991.tb01564.x. [http://dx.doi.org/10.1111/j.1464-5491.1991. tb01564.x]. [PMID: 1827403]. [DOI] [PubMed] [Google Scholar]
- 62.Wallin K., Boström G., Kivipelto M., Gustafson Y. Risk factors for incident dementia in the very old. Int. Psychogeriatr. 2013;25(7):1135–1143. doi: 10.1017/S1041610213000409. [http://dx.doi.org/10.1017/S1041610213000409]. [PMID: 23574921]. [DOI] [PubMed] [Google Scholar]
- 63.Sonnen J.A., Larson E.B., Crane P.K., Haneuse S., Li G., Schellenberg G.D., Craft S., Leverenz J.B., Montine T.J. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann. Neurol. 2007;62(4):406–413. doi: 10.1002/ana.21208. [http://dx.doi.org/10. 1002/ana.21208]. [PMID: 17879383]. [DOI] [PubMed] [Google Scholar]
- 64.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., Harrington M.G., Chui H.C., Law M., Zlokovic B.V. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [http://dx.doi.org/10.1016/j.neuron. 2014.12.032]. [PMID: 25611508]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esiri M.M., Wilcock G.K., Morris J.H. Neuropathological assessment of the lesions of significance in vascular dementia. J. Neurol. Neurosurg. Psychiatry. 1997;63(6):749–753. doi: 10.1136/jnnp.63.6.749. [http://dx. doi.org/10.1136/jnnp.63.6.749]. [PMID: 9416809]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X., Wu B., Nie K., Jia Y., Yu J. Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem. Int. 2014;65:23–29. doi: 10.1016/j.neuint.2013.12.004. [http://dx.doi.org/10.1016/ j.neuint.2013.12.004]. [PMID: 24361538]. [DOI] [PubMed] [Google Scholar]
- 67.Venkat P., Chopp M., Chen J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [http://dx.doi.org/ 10.1016/j.expneurol.2015.05.006]. [PMID: 25987538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ueno M., Chiba Y., Matsumoto K., Murakami R., Fujihara R., Kawauchi M., Miyanaka H., Nakagawa T. Blood-brain barrier damage in vascular dementia. Neuropathology. 2016;36(2):115–124. doi: 10.1111/neup.12262. [http://dx.doi.org/10.1111/neup.12262]. [PMID: 26607405]. [DOI] [PubMed] [Google Scholar]
- 69.Wardlaw J.M., Sandercock P.A.G., Dennis M.S., Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34(3):806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [http://dx.doi.org/10.1161/01.STR.0000058480.77236.B3]. [PMID: 12624314]. [DOI] [PubMed] [Google Scholar]
- 70.Srinivasan V., Braidy N., Chan E.K., Xu Y.H., Chan D.K.Y. Genetic and environmental factors in vascular dementia: an update of blood brain barrier dysfunction. Clin. Exp. Pharmacol. Physiol. 2016;43(5):515–521. doi: 10.1111/1440-1681.12558. [http://dx.doi.org/10.1111/1440-1681.12558]. [PMID: 26859837]. [DOI] [PubMed] [Google Scholar]
- 71.Buckley J.S., Salpeter S.R. A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging. 2015;32(6):453–467. doi: 10.1007/s40266-015-0266-9. [http://dx.doi.org/10.1007/s40266-015-0266-9]. [PMID: 25941104]. [DOI] [PubMed] [Google Scholar]
- 72.Russ T.C., Morling J.R. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst. Rev. 2012;9(9):CD009132. doi: 10.1002/14651858.CD009132.pub2. [PMID: 22972133]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birks J., Craig D. Galantamine for vascular cognitive impairment. Cochrane Database Syst. Rev. 2006;2013(4):CD004746. doi: 10.1002/14651858.CD004746.pub2. [PMID: 23862185]. [DOI] [PubMed] [Google Scholar]
- 74.Witter D., McCord M., Suryadevara U. Delirium associated with memantine use in a patient with vascular dementia. J. Clin. Psychopharmacol. 2015;35(6):736–737. doi: 10.1097/JCP.0000000000000420. [http://dx.doi.org/10.1097/ JCP.0000000000000420]. [PMID: 26448402]. [DOI] [PubMed] [Google Scholar]
- 75.Sun M-K., Hongpaisan J., Nelson T.J., Alkon D.L. Poststroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc. Natl. Acad. Sci. USA. 2008;105(36):13620–13625. doi: 10.1073/pnas.0805952105. [http://dx.doi.org/10.1073/pnas.0805952105]. [PMID: 18768786]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun M-K., Hongpaisan J., Alkon D.L. Postischemic PKC activation rescues retrograde and anterograde long-term memory. Proc. Natl. Acad. Sci. USA. 2009;106(34):14676–14680. doi: 10.1073/pnas.0907842106. [http://dx.doi. org/10.1073/pnas.0907842106]. [PMID: 19667190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wetsel W.C., Khan W.A., Merchenthaler I., Rivera H., Halpern A.E., Phung H.M., Negro-Vilar A., Hannun Y.A. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J. Cell Biol. 1992;117(1):121–133. doi: 10.1083/jcb.117.1.121. [http://dx.doi.org/10. 1083/jcb.117.1.121]. [PMID: 1556149]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chou W.H., Messing R.O. Protein kinase C isozymes in stroke. Trends Cardiovasc. Med. 2005;15(2):47–51. doi: 10.1016/j.tcm.2005.01.003. [http://dx.doi.org/10. 1016/j.tcm.2005.01.003]. [PMID: 15885569]. [DOI] [PubMed] [Google Scholar]
- 79.Shimohata T., Zhao H., Steinberg G.K. ε PKC may contribute to the protective effect of hypothermia in a rat focal cerebral ischemia model. Stroke. 2007;38(2):375–380. doi: 10.1161/01.STR.0000254616.78387.ee. [http://dx.doi.org/10.1161/ 01.STR.0000254616.78387.ee]. [PMID: 17204679]. [DOI] [PubMed] [Google Scholar]
- 80.Masi G., Brovedani P. The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression. CNS Drugs. 2011;25(11):913–931. doi: 10.2165/11595900-000000000-00000. [http://dx.doi.org/10. 2165/11595900-000000000-00000]. [PMID: 22054117]. [DOI] [PubMed] [Google Scholar]
- 81.Hund T.J., Lerner D.L., Yamada K.A., Schuessler R.B., Saffitz J.E. Protein kinase Cepsilon mediates salutary effects on electrical coupling induced by ischemic preconditioning. Heart Rhythm. 2007;4(9):1183–1193. doi: 10.1016/j.hrthm.2007.05.030. [http://dx.doi.org/10.1016/j.hrthm.2007.05. 030]. [PMID: 17765619]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Della-Morte D., Raval A.P., Dave K.R., Lin H.W., Perez-Pinzon M.A. Post-ischemic activation of protein kinase C ε protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci. Lett. 2011;487(2):158–162. doi: 10.1016/j.neulet.2010.10.013. [http://dx.doi.org/10.1016/j.neulet.2010.10.013]. [PMID: 20951185]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dave K.R., DeFazio R.A., Raval A.P., Torraco A., Saul I., Barrientos A., Perez-Pinzon M.A. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J. Neurosci. 2008;28(16):4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. [http://dx.doi.org/ 10.1523/JNEUROSCI.5471-07.2008]. [PMID: 18417696]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raval A.P., Dave K.R., DeFazio R.A., Perez-Pinzon M.A. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184(1184):345–353. doi: 10.1016/j.brainres.2007.09.073. [http://dx.doi.org/10.1016/ j.brainres.2007.09.073]. [PMID: 17988655]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhuang S., Li J., Wang X., Wang H.F., Zhang W.J., Wang H.Y., Xing C.M. Renin-angiotensin system-targeting antihypertensive drugs and risk of vascular cognitive impairment: A meta-analysis. Neurosci. Lett. 2016;615:1–8. doi: 10.1016/j.neulet.2016.01.011. [http://dx.doi.org/10.1016/ j.neulet.2016.01.011]. [PMID: 26797651]. [DOI] [PubMed] [Google Scholar]
- 86.Gareri P., Castagna A., Cotroneo A.M., Putignano S., De Sarro G., Bruni A.C. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin. Interv. Aging. 2015;10:1421–1429. doi: 10.2147/CIA.S87886. [http://dx.doi.org/10.2147/CIA.S87886]. [PMID: 26366063]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cotroneo A.M., Castagna A., Putignano S., Lacava R., Fantò F., Monteleone F., Rocca F., Malara A., Gareri P. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin. Interv. Aging. 2013;8:131–137. doi: 10.2147/CIA.S38420. [PMID: 23403474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alvarez-Sabín J., Ortega G., Jacas C., Santamarina E., Maisterra O., Ribo M., Molina C., Quintana M., Román G.C. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc. Dis. 2013;35(2):146–154. doi: 10.1159/000346602. [http://dx.doi.org/10.1159/000346602]. [PMID: 23406981]. [DOI] [PubMed] [Google Scholar]
- 89.Grieb P. Neuroprotective properties of citicoline: facts, doubts and unresolved issues. CNS Drugs. 2014;28(3):185–193. doi: 10.1007/s40263-014-0144-8. [http://dx. doi.org/10.1007/s40263-014-0144-8]. [PMID: 24504829]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L., Zhang J., Sun H., Zhu H., Liu H., Yang Y. An enriched environment elevates corticosteroid receptor levels in the hippocampus and restores cognitive function in a rat model of chronic cerebral hypoperfusion. Pharmacol. Biochem. Behav. 2013;103(4):693–700. doi: 10.1016/j.pbb.2012.12.023. [http://dx.doi.org/10.1016/j.pbb.2012.12. 023]. [PMID: 23290935]. [DOI] [PubMed] [Google Scholar]
- 91.Bayat M., Sharifi M.D., Haghani M., Shabani M. Enriched environment improves synaptic plasticity and cognitive deficiency in chronic cerebral hypoperfused rats. 2015. [DOI] [PubMed]
- 92.Wang F., Chang G.M., Yu Q., Geng X. The neuroprotection of repetitive transcranial magnetic stimulation pre-treatment in vascular dementia rats. J. Mol. Neurosci. 2015;56(1):198–204. doi: 10.1007/s12031-014-0480-7. [a]. [DOI] [PubMed] [Google Scholar]
- 93.Choi D.H., Lee K.H., Lee J. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol. Med. Rep. 2016;13(4):2981–2990. doi: 10.3892/mmr.2016.4891. [http://dx.doi.org/ 10.3892/mmr.2016.4891]. [PMID: 26934837]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin Y., Lu X., Dong J., He X., Yan T., Liang H., Sui M., Zheng X., Liu H., Zhao J., Lu X. Involuntary, forced and voluntary exercises equally attenuate neurocognitive deficits in vascular dementia by the BDNF-pCREB mediated pathway. Neurochem. Res. 2015;40(9):1839–1848. doi: 10.1007/s11064-015-1673-3. [http://dx.doi.org/10.1007/s11064-015-1673-3]. [PMID: 26240057]. [DOI] [PubMed] [Google Scholar]
- 95.Gomez-Pinilla F., Zhuang Y., Feng J., Ying Z., Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [http://dx.doi.org/10.1111/j.1460-9568.2010.07508. x]. [PMID: 21198979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang H.Y., Crupi D., Liu J., Stucky A., Cruciata G., Di Rocco A., Friedman E., Quartarone A., Ghilardi M.F. Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J. Neurosci. 2011;31(30):11044–11054. doi: 10.1523/JNEUROSCI.2125-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.2125-11.2011]. [PMID: 21795553]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang N., Xing M., Wang Y., Tao H., Cheng Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience. 2015;311:284–291. doi: 10.1016/j.neuroscience.2015.10.038. [http://dx.doi.org/10.1016/j.neuroscience.2015.10.038]. [PMID: 26518460]. [DOI] [PubMed] [Google Scholar]
- 98.Langdon K.D., Granter-Button S., Harley C.W., Moody-Corbett F., Peeling J., Corbett D. A cognitive rehabilitation paradigm effective in male rats lacks efficacy in female rats. J. Cereb. Blood Flow Metab. 2014;34(10):1673–1680. doi: 10.1038/jcbfm.2014.132. [http://dx.doi.org/10.1038/ jcbfm.2014.132]. [PMID: 25052554]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.André S., Heinrich S., Kayser F., Menzler K., Kesselring J., Khader P.H., Lefaucheur J-P., Mylius V. At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. J. Neurol. Sci. 2016;369:185–190. doi: 10.1016/j.jns.2016.07.065. [http://dx.doi.org/10.1016/j.jns.2016.07.065]. [PMID: 27653887]. [DOI] [PubMed] [Google Scholar]
- 100.Howlett O.A., Lannin N.A., Ada L., McKinstry C. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 2015;96(5):934–943. doi: 10.1016/j.apmr.2015.01.013. [http://dx.doi.org/10.1016/j.apmr.2015.01.013]. [PMID: 25634620]. [DOI] [PubMed] [Google Scholar]
- 101.Gillespie D.C., Bowen A., Chung C.S., Cockburn J., Knapp P., Pollock A. Rehabilitation for post-stroke cognitive impairment: an overview of recommendations arising from systematic reviews of current evidence. Clin. Rehabil. 2015;29(2):120–128. doi: 10.1177/0269215514538982. [http://dx.doi.org/10.1177/0269215514538982]. [PMID: 24942480]. [DOI] [PubMed] [Google Scholar]
- 102.Khan M.B., Hoda M.N., Vaibhav K., Giri S., Wang P., Waller J.L., Ergul A., Dhandapani K.M., Fagan S.C., Hess D.C. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl. Stroke Res. 2015;6(1):69–77. doi: 10.1007/s12975-014-0374-6. [http://dx.doi.org/10.1007/s12975-014-0374-6]. [PMID: 25351177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L., Zhang G., Khan A.A., Guo X., Gu Y. Clinical efficacy and meta-analysis of stem cell therapies for patients with brain ischemia. Stem Cells Int. 2016;2016:6129579. doi: 10.1155/2016/6129579. [http://dx.doi.org/ 10.1155/2016/6129579]. [PMID: 27656217]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ravaglia G., Forti P., Maioli F., Martelli M., Servadei L., Brunetti N., Pantieri G., Mariani E. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement. Geriatr. Cogn. Disord. 2006;21(1):51–58. doi: 10.1159/000089515. [http://dx.doi.org/10.1159/000089515]. [PMID: 16276110]. [DOI] [PubMed] [Google Scholar]
- 105.Zhu C.Y., Wang Y., Zeng Q.X., Qian Y., Li H., Yang Z.X., Yang Y.M., Zhang Q., Li F.F., Liu S.L. Combined effects of age and polymorphisms in Notch3 in the pathogenesis of cerebral infarction disease. Metab. Brain Dis. 2016;31(5):1157–1164. doi: 10.1007/s11011-016-9868-0. [http://dx.doi.org/10.1007/s11011-016-9868-0]. [PMID: 27370894]. [DOI] [PubMed] [Google Scholar]
- 106.Rouch L., Cestac P., Hanon O., Cool C., Helmer C., Bouhanick B., Chamontin B., Dartigues J.F., Vellas B., Andrieu S. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 2015;29(2):113–130. doi: 10.1007/s40263-015-0230-6. [http://dx.doi.org/10.1007/ s40263-015-0230-6]. [PMID: 25700645]. [DOI] [PubMed] [Google Scholar]
- 107.Wharton W., Goldstein F.C., Zhao L., Steenland K., Levey A.I., Hajjar I. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 2015;63(9):1749–1756. doi: 10.1111/jgs.13627. [http://dx.doi.org/10. 1111/jgs.13627]. [PMID: 26389987]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharp S.I., Aarsland D., Day S., Sønnesyn H., Ballard C. Hypertension is a potential risk factor for vascular dementia: systematic review. Int. J. Geriatr. Psychiatry. 2011;26(7):661–669. doi: 10.1002/gps.2572. [http://dx.doi.org/10.1002/gps.2572]. [PMID: 21495075]. [DOI] [PubMed] [Google Scholar]
- 109.Reis J.P., Loria C.M., Launer L.J., Sidney S., Liu K., Jacobs D.R., Jr, Zhu N., Lloyd-Jones D.M., He K., Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann. Neurol. 2013;73(2):170–179. doi: 10.1002/ana.23836. [http://dx.doi.org/10. 1002/ana.23836]. [PMID: 23443990]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peila R., White L.R., Masaki K., Petrovitch H., Launer L.J. Reducing the risk of dementia: efficacy of long-term treatment of hypertension. Stroke. 2006;37(5):1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [http://dx.doi.org/10. 1161/01.STR.0000217653.01615.93]. [PMID: 16601212]. [DOI] [PubMed] [Google Scholar]
- 111.Haag M.D., Hofman A., Koudstaal P.J., Breteler M.M., Stricker B.H. Duration of antihypertensive drug use and risk of dementia: A prospective cohort study. Neurology. 2009;72(20):1727–1734. doi: 10.1212/01.wnl.0000345062.86148.3f. [http://dx.doi.org/10.1212/01.wnl.0000345062.86148.3f]. [PMID: 19228584]. [DOI] [PubMed] [Google Scholar]
- 112.Richard E., Moll van Charante E.P., van Gool W.A. Vascular risk factors as treatment target to prevent cognitive decline. J. Alzheimers Dis. 2012;32(3):733–740. doi: 10.3233/JAD-2012-120772. [http://dx.doi.org/10.3233/JAD-2012-120772]. [PMID: 22886011]. [DOI] [PubMed] [Google Scholar]
- 113.McGuinness B., Craig D., Bullock R., Passmore P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2016;(1):CD003160. doi: 10.1002/14651858.CD003160.pub3. [PMID: 26727124]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinter D., Enzinger C., Fazekas F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J. Neurol. 2015;262(11):2411–2419. doi: 10.1007/s00415-015-7776-6. [http://dx.doi.org/10.1007/s00415-015-7776-6]. [PMID: 25976029]. [DOI] [PubMed] [Google Scholar]
- 115.Murray A.D., Staff R.T., McNeil C.J., Salarirad S., Ahearn T.S., Mustafa N., Whalley L.J. The balance between cognitive reserve and brain imaging biomarkers of cerebrovascular and Alzheimer’s diseases. Brain. 2011;134(Pt 12):3687–3696. doi: 10.1093/brain/awr259. [http://dx.doi.org/10.1093/brain/awr259]. [PMID: 22102649]. [DOI] [PubMed] [Google Scholar]
- 116.Katzman R., Terry R., DeTeresa R., Brown T., Davies P., Fuld P., Renbing X., Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [http://dx.doi.org/10.1002/ana.410230206]. [PMID: 2897823]. [DOI] [PubMed] [Google Scholar]
- 117.Valenzuela M.J. Brain reserve and the prevention of dementia. Curr. Opin. Psychiatry. 2008;21(3):296–302. doi: 10.1097/YCO.0b013e3282f97b1f. [http://dx.doi.org/10. 1097/YCO.0b013e3282f97b1f]. [PMID: 18382231]. [DOI] [PubMed] [Google Scholar]
- 118.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [http://dx.doi.org/10. 1016/S1474-4422(12)70191-6]. [PMID: 23079557]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barulli D., Stern Y. 2013. [Google Scholar]
- 120.Whalley L.J., Staff R.T., Fox H.C., Murray A.D. Cerebral correlates of cognitive reserve. Psychiatry Res. Neuroimaging. 2016;247:65–70. doi: 10.1016/j.pscychresns.2015.10.012. [http://dx.doi.org/10.1016/j.pscychresns.2015.10.012]. [PMID: 26774854]. [DOI] [PubMed] [Google Scholar]
- 121.Nyberg L., Lövdén M., Riklund K., Lindenberger U., Bäckman L. 2012. [Google Scholar]
- 122.Craik F.I., Bialystok E., Freedman M. Delaying the onset of Alzheimer disease: bilingualism as a form of cognitive reserve. Neurology. 2010;75(19):1726–1729. doi: 10.1212/WNL.0b013e3181fc2a1c. [http://dx.doi.org/10.1212/ WNL.0b013e3181fc2a1c]. [PMID: 21060095]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seshadri S., Wolf P.A. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6(12):1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [http://dx.doi.org/10.1016/ S1474-4422(07)70291-0]. [PMID: 18031707]. [DOI] [PubMed] [Google Scholar]
- 124.Sun M-K., Alkon D.L. Pharmacological enhancement of synaptic efficacy, spatial learning, and memory. In: Seel N.M., editor. Encyclopedia of the Sciences of Learning. Berlin, Germany: Springer; 2012. pp. 2605–2608. [Google Scholar]
- 125.Weekman E.M., Sudduth T.L., Caverly C.N., Kopper T.J., Phillips O.W., Powell D.K., Wilcock D.M. Reduced efficacy of anti-Aβ immunotherapy in a mouse model of amyloid deposition and vascular cognitive impairment comorbidity. J. Neurosci. 2016;36(38):9896–9907. doi: 10.1523/JNEUROSCI.1762-16.2016. [http://dx.doi.org/10.1523/JNEUROSCI.1762-16.2016]. [PMID: 27656027]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tatemichi T.K., Paik M., Bagiella E., Desmond D.W., Stern Y., Sano M., Hauser W.A., Mayeux R. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology. 1994;44(10):1885–1891. doi: 10.1212/wnl.44.10.1885. [http://dx.doi.org/10.1212/WNL.44.10.1885]. [PMID: 7936242]. [DOI] [PubMed] [Google Scholar]
- 127.Leys D., Hénon H., Mackowiak-Cordoliani M.A., Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4(11):752–759. doi: 10.1016/S1474-4422(05)70221-0. [http:// dx.doi.org/10.1016/S1474-4422(05)70221-0]. [PMID: 16239182]. [DOI] [PubMed] [Google Scholar]