Abstract
Background:
Adequate dietary intake and nutritional status have important effects on brain functions and on brain health. Energy intake and specific nutrients excess or deficiency from diet differently affect cognitive processes, emotions, behaviour, neuroendocrine functions and synaptic plasticity with possible protective or detrimental effects on neuronal physiology. Lipids, in particular, play structural and functional roles in neurons. Here the importance of dietary fats and the need to understand the brain mechanisms activated by peripheral and central metabolic sensors. Thus, the manipulation of lifestyle factors such as dietary interventions may represent a successful therapeutic approach to maintain and preserve brain health along lifespan.
Methods:
This review aims at summarizing the impact of dietary fats on brain functions.
Results:
Starting from fat consumption, nutrient sensing and food-related reward, the impact of
gut-brain communications will be discussed in brain health and disease. A specific focus will be on the impact of fats on the molecular pathways within the hypothalamus involved in the control of reproduction via the expression and the release of Gonadotropin-Releasing Hormone. Lastly, the effects of specific lipid classes such as polyunsaturated fatty acids and of the “fattest” of all diets, commonly known as “ketogenic diets”, on brain functions will also be discussed.
Conclusion:
Despite the knowledge of the molecular mechanisms is still a work in progress, the clinical relevance of the manipulation of dietary fats is well acknowledged and such manipulations are in fact currently in use for the treatment of brain diseases.
Keywords: Fat, diet, PUFAs, endocannabinoids, neuroprotection, microbiota, nutrient sensing, hypothalamus, GnRH, reproduction, metabolic sensors, leptin, kisspeptin, ghrelin, neuroprotection, ketogenic diets, epilepsy
1. INTRODUCTION
The incidence of overweight and obesity in developed and developing countries is rising due to unbalanced diets, the large use of junk food and insufficient physical exercise. According to the World Health Organization (WHO) “Worldwide obesity has more than doubled since 1980. Most of the world's population live in countries where overweight and obesity kill more people than underweight. 41 million children under the age of 5 were overweight or obese in 2014…. Overall, about 13% of the world’s adult population (11% of men and 15% of women) were obese in 2014 [1].
The common perception of nutrition and food has usually been linked to the need of energy for cell methabolism homeostasis. In this respect, energy availability and the abundance or the depletion of specific nutrients from diet differently affect the functions of the whole body. In particular, the brain uses more energy than any other human organ and lipids represent about 50% of its dry weight. As a consequence, adequate dietary intake and nutritional status have a recognized impact on brain functions such as cognitive processes, emotions, behaviour, neuroendocrine functions and synaptic plasticity with consequences on health [2].
Food sensing, through the production of autocrine, paracrine and endocrine signals, represents the first step in the modulation of energy homeostasis and brain activity. Several nutrient-sensors released from peripheral tissues (i.e. gastrointestinal tract, adipose tissues and skeletal muscle) transmit information concerning the metabolic status and then enter the brain which in turn produces specific neuropeptides to orchestrate a wide range of biological functions and processes, comprising feeding behavior, energy expenditure and regulation of specific methabolic pathways, mainly through neuroendocrine mechanisms and autonomic terminals [3]. Here the importance and the need to understand the brain mechanisms activated by peripheral and central metabolic sensors.
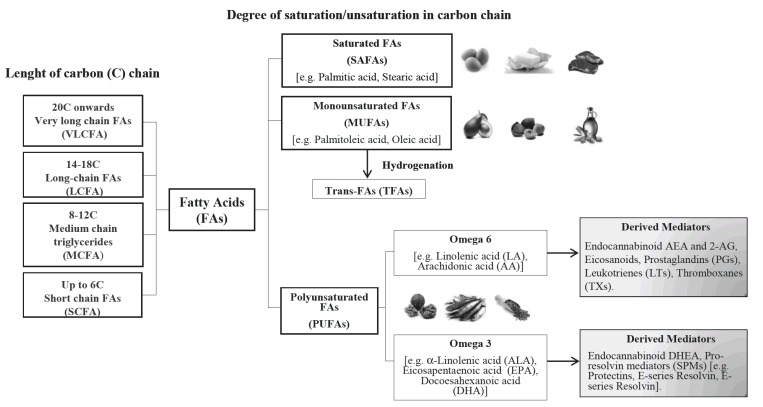
Foods contain mixture of different types of fatty acids (FAs), which are carboxylic acids with a long aliphatic chain that is either saturated or mono/polyunsaturated (SAFAs, MUFAs/PUFAs respectively). Their impact on health strongly depends on fat types (Fig. 1) and daily fat intake. Over the last year, dietary lipids have garnered recognition for their direct actions on the brain since they can affect multiple brain processes by regulating synaptic transmission, membrane fluidity and signal-transduction pathways. In this respect, omega-6 and omega-3 long chain (LC) PUFAs, also known as n-6 or n-3 PUFAs respectively, are nutrients widely present among dietary lipids with quite different proprieties as their inflammatory potential. In fact, the n-6 PUFA arachidonic acid (ARA) exerts pro-inflammatory and atherogenic actions via its conversion by the cyclooxygenase (COX) and lipooxygenase (LOX) enzymatic pathways to bioactive mediators known as eicosanoids (prostaglandins (PGs), thromboxanes (TXs) and leukotrienes (LTs). However, during the initial (acute) phase of an inflammatory response, the ARA metabolism can also switch to the production of lipoxins (LXs), another eicosanoid class with the capacity to limit the extent and duration of the inflammatory process and promote the resolution of inflammation [4]. LXs are considered the first identified members of the family of specialized pro-resolving mediators (SPMs) [4, 5]. The SPMs family also comprises n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)-derived pro-resolving mediators called resolvins (Rvs) and protectins (PDs) [6]. In the brain, the bioavailability of PUFAs and of their bioactive derivatives strongly depends on diet composition with an ideal ratio of 5 (n-6): 1 (n-3) [7]. Nevertheless, diets are often unbalanced and low n-3 PUFAs have been associated with neuropsychiatric and neurological disorders with inflammatory outcomes [8].
Fig. (1).
Schematic representation of fatty acids (FAs). FAs are classified depending on the degree of saturation/unsaturation in the carbon chain and on the lenght of carbon chain. The main source of saturated SAFAs is animal fat; MUFAs and PUFAs mainly derive from vegetables and (fish/vegetable) oils; trans-FAs (TFAs), are mainly formed by the industrial production of hydrogenated fats from vegetable oils.
Among PUFAs derivates, endocannabinoids (eCBs) deserve attention. These are lipid mediators that mainly act via type 1 and type 2 cannabinoid receptors (CB1 and CB2 respectively), which are Gi/o protein-coupled receptors. The eCB system is one of the most ancient signaling system of vertebrates [9, 10] with numerous pathways in the brain [11, 12]. The main eCBs are anandamide (AEA) and 2-arachidonoylglycerol (2-AG), which are the N-ethanolamide and the glyceryl ester of n-6 PUFA ARA respectively. Interestingly, eCBs link food lipids to synaptic activity, neuronal plasticity, and to neuroendocrine and reproductive functions [13-21]. This highlights, the importance of dietary lipids to preserve and maintain the specific molecular systems and mechanisms that regulate neuronal functions and the possibility to prevent or to treat brain diseases via diet manipulations. For instance, it has been recognized that dietary n-3 PUFAs have “antiaging” effects that support cognitive processes and maintain synaptic functions and plasticity [8, 22, 23]. In turn, diets that are high in saturated fats negatively impact brain functions and increase the risk of cardiovascular and neurological diseases [24].
Therefore, in this review we report the impact of dietary fats on brain functions. First we describe the gut-brain communications, with focuses on microbiota, dietary fats composition, lipid sensing, satiety and processing of hedonic food. Then we describe the functional interplay between diet and the hypothalamic control of reproduction, through the integrated activity of peripheral and centrally produced metabolic sensors that directly or indirectly convey metabolic cues on the hypothalamus-pituitary-gonad (HPG) axis. Then, we move to the description of the beneficial effect of dietary n-3 PUFA on the preservation of neuronal function and structure and lastly, we discuss the potential therapeutic effects of ketogenic diets, the “fattest” of all diets.
Taken together, we provide evidence that diet manipulation in fat content can preserve brain health and has a clinical relevance.
2. GUT-BRAIN COMMUNICATION THROUGH MICROBIOTA
There is probably no better expression to define the enteric nervous system (ENS) than the figure of speech of the “second brain” [25]. As far as our knowledge about the ENS extends, the more gaps in the gastrointestinal tract (GIT), neuroendocrine, immune and the central and autonomic nervous systems are reduced. Thus, the notion of “gut-brain axis” is now largely used to explain the close connection and the tight bidirectional communication between the gut and brain and the importance of their cross-talk for brain functions in health and disease [26-29]. Quite surprisingly, the integrity of gut-brain reciprocal signaling appears essential not only for the pathophysiology of GIT disorders such as the irritable bowel syndrome but also for several brain neurological and neuropsychiatric disorders including Parkinson’s disease, mood disorders (e.g., depression) and autism spectrum disorders (ASDs) [29-32]. The link between gut health and brain disorders can be, nonetheless, elusive as long as we do not consider the extraordinary number of commensal bacteria resident in the human gut, which is estimated to be around 100 trillion [33]. This giant population of symbiotic bacteria, known as microbiota, shapes GIT motility, epithelial barrier function, gut homeostasis and metabolism via nutrient processing and affects brain physiology and incidence of disease risk. Within this picture, the gut-brain axis constitutes the bidirectional route by which multiple signals (e.g., neural, neurohormonal and immunological) converging to the gut and in the gut re-encoded can access the brain. The microbiota is the living machinery composed of more than one thousand species of microorganisms (basically, Bacteroidetes and Firmicutes) whose genome outnumbered our genome about 150 times [33, 34].
2.1. Impact of Probiotics and Prebiotics on Brain Functions
The liability to mood disorders (e.g., depressive symptoms) is emblematic of the powerful impact that microbiota can exert on brain physiology and the pathogenetic consequences of microbiota dysfunction. Alterations in the microbial composition generate a state of dysbiosis that is a potential pathogenetic environment and a threat for the maintainance of host’s health. Dysbiosis of the microbiota is responsible for different pathogenetic events such as changes in the host immunity and intestinal permeability [35]. Dysfunctions of the intestinal barrier increase the risk of endotoxins access to systemic circulation and development of a chronic inflammatory state and higher susceptibility to brain disease [36]. Dysregulated eating produces a dramatic impact on the composition of gut microbial community. The importance of dietary factors may become even more critical than the different genotypic hosts. Indeed, the exposure of different inbred strains of mice to repeated shift between diets with a different nutritional profile demonstrates that gut microbiota can be drastically modeled by dietary factors regardless of the genotype [37]. Prebiotics, probiotics, synbiotics [38] and fecal transplantation are examples of different strategies to reshape the microbial community and counteract neurological and neuropsychiatric diseases. The term probiotic, as opposed to antibiotic, is not recent and can be tracked back in time up to 1965 [39]. According to Food and Agriculture Organization of the United Nations (FAO) and the WHO, we should describe a probiotic as a “live microorganism which when administered in adequate amounts, confers health benefit on the host” [40]. On the other hand, despite several revisions from the original meaning [41], the core concept of prebiotics as a “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon” has not radically changed [42]. Probiotics can confer (additional) cytoprotection against a variety of toxins and pathogens, thus reinforcing the epithelial cells of the intestinal wall (e.g., tight junction proteins) and the integrity and function of the intestinal mucosal barrier.
Derangement of gut microbiota has been associated with brain disorders and neurodegenerative diseases such as ASDs, depression and Alzheimer’s disease (AD). Abnormal increase of Clostridium type germs in the GIT [43] and atypical increase of fecal bacteria of Sutterella species in ASD children have been reported [44, 45]. A probiotic-based treatment (namely, Bacteroides fragilis) was showed to improve gut pearmeability and ASD-like features in mouse model of maternal immune activation [46] and probiotic treatment in ASD children is considered a potential therapeutic option [47].
Depression is known to be associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and a pivotal study has provided the intriguing evidence for an excessive corticosterone and adrenocorticotrophic hormone response in germ-free stressed (i.e., restrained) mice as well as the demonstration that exaggerated stress response can be reverted by recolonization with Bifidobacterium infantis [48]. The therapeutic potential of probiotic administration such as Lactobacillus rhamnosus has been demonstrated for the modulation of the expression of γ-aminobutyric acid (GABA) receptors in the central nervous system (CNS) and its ability to mitigate stress-induced corticosterone response as well as depression- and anxiety-like behaviors [49]. Similarly, there is evidence that chronic colitis can elicit anxiety-like behavior and that administration of Bifidobacterium longus can produce anxiolytic effects via the vagal connection between brain and the ENS [50]. Moreover, stress-associated alterations such as memory dysfunctions and altered neurotrophins expression in the hippocampus can be restored by treatment with probiotics [51], thus supporting the idea that changes of microbiota environment can be observed in neuropsychiatric disorders such as schizophrenia and ASDs [32, 52]. The bi-directional relationship throughout the brain-gut axis also implies that prolonged stressors affecting the CNS and the immune system can disrupt the microbiota homeostasis (i.e., dysbiosis) and elicit a chronic inflammatory state [53, 54] as for obesity. Interestingly, probiotics may produce similar anti-obesity and anti-inflammatory effects (i.e., macrophage infiltration into adipose tissue) through different underlying mechanisms as observed for the administration of Lactobacillus paracasei, Lactobacillus rhamnosus and Bifidobacterium animalis subsp. lactis [55].
Diet and nutrients are among the most relevant environmental stimuli for the enteric ecosystem but also the potential risk factors affecting the microbiota homeostasis. Indeed, dietary habits are critical determinants of microbiota microsystem and health. As a matter of fact, all the different routes by which visceral and interoceptive information can flow through the brain-gut axis can also be affected by dietary nutrients. The neural information generated in the GIT is conveyed in the brain via vagal and spinal afferent neurons and, at the same time, the endocrine and the immune information via gut hormones and cytokines, respectively. Diet and nutrients can determine microbiota composition and, via these signaling pathways, can even influence the metabolic phenotype and liability to develop metabolic dysfunction such as obesity [56-58]. The impact of macronutrients on microbiota composition has received attention with respect to carbohydrate ingestion, specifically dietary fiber. The ingestion of soluble dietary as fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) produces short chain fatty acids (SCFAs) and improves the microbiota environment by favoring bacteria known to be inversely associated with metabolic dysregulation and chronic inflammation (e.g., obesity). The ingestion of prebiotics as non-digestible carbohydrates (CHO) such as cellulose and inulin, fibers and polysaccharides is indeed a strategy known to improve the enteric ecology via the increase of beneficial gut bacteria population that may provide resilience against metabolic derangement. SCFAs are organic fatty acids with 2 up to 6 carbon atoms and are the expression of the major part of the metabolites produced by the fermentation of non-digestible CHO, with acetate (C2), propionate (C3) and butyrate (C4) as the main SCFAs present in different proportion in the cecum and colon population [59]. Thus, dietary fibers and derived SCFAs can regulate body weight, food ingestion, glucose homeostasis, and insulin sensitivity. There are several cases in which ingestion of prebiotics such as isolichenan (from the lichen Cetrariella islandica) and arabinoxylan from the yeast Triticumaestivum has been demonstrated to improve brain functions and elicit the recovery of ethanol-induced or vascular dementia-associated memory deficits in mice [60, 61]. Moreover, as observed for probiotics, also prebiotics can affect brain signaling pathways involved either in the prevention or in the pathophysiology of affective disorders. FOS and GOS can increase the hippocampal expression of brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartate (NMDAR) subunits that are key factors in memory function and synaptic plasticity [62]. In humans, GOS consumption has been observed to be associated with a reduced secretion of salivary cortisol at awakening, thus suggesting an antidepressant potential for GOS supplementation [63]. Considering that prebiotics supplementation favors the growth of beneficial bacteria (i.e., probiotics), there is therefore a virtue circle between probiotics and prebiotics ingestion, both increasing the production of SCFAs.
2.2. SCFAs, Microbiota and Neuroinflammation
Dietary-derived SCFAs are essential substrates for the defence of intestinal epithelial cells and the regulation of lipid metabolism. SCFAs can promote fatty acids oxidation and inhibit their synthesis thus contributing to the decrease of free fatty acids plasma levels and body weight [64, 65]. SCFAs provide beneficial effects for the whole-body energy homeostasis but SCFAs are also signaling molecules whose action is not yet totally understood. SCFAs are indeed ligands for G-protein-coupled receptors such as free fatty acid receptors 2 (FFAR2 or GPR43) and free fatty acid receptors 3 (FFAR3 or GPR41) that have been identified in hematopoietic tissues, in the large intestine, in pancreatic β-cells, in neurons (FFAR3), in leukocytes and in adipocytes (FFAR2) [66]. One key aspect of FFAR2-mediated signaling is the ability, upon their activation, to promote the synthesis of glucagon-like peptide 1 (GLP-1) in enteroendocrine L cells [67]. Remarkably, dietary butyrate supplementation enhances adaptive thermogenesis and fatty acid oxidation in mice [64] and both butyrate and propionate have been shown to inhibit high fat diet (HFD)-induced weight gain and food intake and stimulate glucose-dependent insulinotropic polypeptide (GIP) secretion in a manner independent of FFAR3 [65]. Dietary FOS has a drastic impact on microbiota ecosystem, increasing Bacteroidetes and decreasing Firmicutes and propionate and butyrate can improve glucose tolerance and insulin sensitivity via different but complementary mechanisms leading to the stimulation of intestinal gluconeogenesis (IGN) [68]. Butyrate-generating species are favored by the ingestion of dietary fibers but each macronutrient can modify the microbiota community in a diet-dependent manner [69]. Reminiscent of the alteration of microbiota described in obese individuals show that exposure to Western diet generates an unbalance between Bacteroidetes and Firmicutes favoring the increase of the latter phyla over the former or, in other words, an increase of the ratio of Firmicutes to Bacteroidetes [70]. By contrast, in subjects whose diet adheres to Mediterranean diet recommendations, there is an increase of fecal content of SCFA levels (an index of high CHO fermentation) and high prevalence of Bacteroidetes phylum [71, 72]. SCFAs have a major impact on enteroendocrine signaling, feeding homeostasis and appetite regulation. Multiple mechanisms can be involved, and appetite can be suppressed by SCFAs-dependent leptin secretion upon FFAR3 activation [73]. In addition to GLP-1 synthesis, SCFAs can influence the secretion of other satiety peptides such as cholecystokinin (CCK) and peptide YY (PYY) by the epithelial cells, specifically enteroendocrine L cells. The effects of prebiotics supplementation on the increase of plasma PYY and GLP-1 concentrations are paradigmatic of the host-gut microbiota interaction. Oligofructose (OFS) supplementation promotes GLP-1 secretion in proximal colon [74] and colonic infusion of propionate stimulates the release of GLP-1 and PYY [75].
With respect to the deleterious effects of obesity on microbiota ecosystem, the ingestion of dietary fats and in particular of fat-rich diet increases the circulating levels of lipopolysaccharide (LPS). LPS represents the most part of the outer layer of Gram-negative bacteria, an endotoxin originating from gut microbiota with a potent inflammatory action that is believed to underlie the obesity-associated intestinal permeability and increase of LPS plasma levels (i.e., metabolic endotoxaemia) [76]. Even acute high fat meal consumption can elicit a postprandial inflammatory response that is often observed as transitory increase of endotoxaemia [77]. Interestingly, consumption of fat meals enables the intestinal absorption of LPS from the gut and chylomicrons formation has been identified as one factor underlying LPS absorption and chronic inflammatory state [78]. However, different types of dietary fats can elicit different effects on lipaemia (i.e., excessive blood concentrations of lipids), chylomicrons accumulation and postprandial endotoxaemia. For instance, oil emulsification appears much more harmful for postprandial lipaemia than consumption of the same non-emulsified oil (sunflower oil) [79]. This study investigated some possible events linking systemic inflammation, lipid digestion and endotoxin absorption at the light of the large use of oil emulsification in several common industrial foodstuffs. The oral gavage in rats of emulsified sunflower oil produced higher endotoxaemia and hypertriglyceridemia than that caused by the ingestion of non-emulsified sunflower oil. Moreover, healthy humans consuming a mixed meal containing emulsified fat (e.g., margarine, butter) showed transient postprandial endotoxaemia (i.e., plasma endotoxin concentration) and increased plasma levels of the endotoxin soluble receptor sCD14 that is a marker of endotoxin exposure, and a peak of inflammatory cytokine IL-6 two hours after meal ingestion [79]. In contrast to the intake of a rapeseed oil-based diet, the intake of palm oil increases both plasma levels of pro-inflammatory IL-6 and ratio of LPS to the soluble form of binding-protein CD14 (sCD14), which tend to increase as a function of the intensification of the inflammatory response [80]. Thus, fatty acids profile can drastically impact the microbiota-gut-brain function increasing or decreasing chronic inflammation burden by the way of dietary lipid composition. In healthy adults, postprandial endotoxaemia was found maximum after intake of saturated fats (i.e., coconut oil) and reduced after n-3 PUFAs (i.e., DHA) ingestion [81].
As described in details in paragraph 4, PUFAs have a key role in inflammation. In fact, among ARA-derived mediators, LXs can participate to “resolve” inflammation as reported for their signaling activity addressing neutrophils to migrate towards the site of inflammation, accelerating neutrophils apoptosis and clearing, and delaying apoptosis of macrophages [82]. Among n-3 PUFAs, SPMs exert potent anti-inflammatory and protective actions via multiple mechanisms as the blockage of neutrophil and eosinophil infiltration, the activation of macrophage phagocytosis, scavenging of inflammatory chemokines and the stimulation of antimicrobial defense mechanism [83, 84]. Thus, n-3 PUFAs may regulate the immune response via the action of SPMs. Given the major impact of diet and nutrients on gut microbial composition, the immune function can be deregulated in case of dysbiosis (diet-induced metabolic endotoxaemia) and, on the contrary, protected by the generation of SCFAs. The fact that both SCFAs and n-3 PUFAs stimulate the immune function and regulate the inflammatory response is a challenge for our current vision of the interplay between nutrition, immune regulation and pathogenesis of inflammatory-dependent diseases.
2.3. Lipid Sensing, Satiety and Processing of Hedonic Food
An interesting point of convergence between epidemic obesity in Western countries and changes driven by dietary lipids in gut microbiota, dysbiosis and chronic systemic inflammation relies on the notions of “nutrient sensing” and metabolic-sensing neurons. Nutrients are indeed signaling molecules conveying information about whole-body energy status and microbiota changes to the brain and the immune system. In turn, the brain is the most powerful unit for the processing of nutrient-associated information, including food-associated reward. Neurons sensitive to metabolic and nutrient information are distributed in the ventromedial and arcuate nuclei (ARC) of the hypothalamus in which fuels, gut-derived signals and macronutrients can be detected [85, 86]. However, in extra-hypothalamic areas, metabolic sensing neurons have been identified in the nucleus of the solitary tract (NST) and in the midbrain ventral tegmental area. In particular, the NST emerges as an integrative structure for descending hypothalamic melanocortin inputs and ascending gut-derived satiety signals. Recently, the existence of aminoacid (L-leucine)-dependent satiety signaling detected by NST neurons [87] has been described, which can curb food intake unless animals are fed with HFD [88]. This desensitization of anorexiant signals transmitted through the gut-brain communication occurs as consequence of HFD intake, and is emblematic of the vulnerability of the homeostatic regulation of food-derived energy intake. For an efficient system of homeostatic control, we can predict that energy intake and time intervals between the meals generate molecular signals that are orchestrated with those generated by the energy reservoir (i.e., adipose tissue stores), as for instance by the leptin-dependent satiety signals. Interestingly, the satiety and satiation signaling system is organized redundantly and a plethora of signals are generated through the gut-brain axis. Without considering pancreatic and stomach hormones, in the proximal and distal intestine as well as in the duodenum, we can at least enumerate CCK, neurotensin, GLP-1, PYY, oxyntomodulin, serotonin, obestatin and nesfatin as satiation-triggering signals. In the last years, there has been an intense debate about the pressure exerted by our affluent environment and the prevailing model of food consumption where easily accessible energy-dense and high palatable food can hijack the homeostatic control of food ingestion. This obesogenic food environment is characterized by highly hedonic foods whose signals are processed by separate, though partly overlapping with the homeostatic system, neural circuitries. Hedonic eating involves cognitive, rewarding and emotional aspects (e.g., food seeking) that are processed by corticolimbic structures, including orbitofrontal cortex, ventral tegmental area (VTA), ventral striatum and amygdala [89]. As “canonical” satiety hormone leptin is a powerful example of the multiple homeostatic and non-homeostatic mechanisms underlying its anorexiant action. Indeed, leptin acts on hypothalamic ARC and dorsomedial nuclei to reduce food intake and increase thermogenesis but also on lateral hypothalamus to control mesolimbic dopaminergic (DA-ergic) transmission within VTA and projecting DA-ergic neurons to the ventral striatum. Fat consumption is highly attractive and pleasurable for humans as well as for rodents, and high palatability intensifies the consumption of larger meals and, consequently, the risk to develop brain insensitivity to leptin signaling. Nevertheless, there are dietary fats that can help in the fight against the obesogenic effects of hedonic eating and the potential derangement of leptin signaling system. On one side, eCBs are a special class of ARA derivatives that play a critical role in energy balance and feeding regulation. The best-known and described eCBs, AEA and 2-AG produce robust effects in terms of energy preservation, increasing energy intake and lipid storage, also contributing to the consumption of hedonic food via the increase of DA release in mesolimbic structures [90, 91]. Moreover, while the content of eCBs in the food is negligible to mimick eCB-mediated physiological effects [92], the eCBs levels can be modulated by dietary fatty acids intake, and in particular, by the ingestion of linoleic acid, the precursor of ARA [93]. The oral administration of the probiotic Lactobacillus acidophilus demonstrated to induce the expression of CB2 receptors in intestinal epithelial cells [94], providing a seminal evidence for the existence of a communication system between microbiota, probiotics and modulation of the eCB function.
On the other hand, the direct infusion of fat into the duodenum is a signal that can elicit satiety or reduce meal size (i.e., satiation), in both cases eliciting feeding inhibition and anorexigenic responses. The release of CCK from enteroendocrine cells is one of the mechanisms by which dietary fats such as PUFAs can produce inhibition of feeding responses [95]. There are lipid mediators such as oleoylethanolamide (OEA), palmitoylethanolamide (PEA) and linoleoylethanolamide (LEA) that are members of the family of fatty acids ethanolamide (namely N-acylethanolamines, NAEs) except they do not bind CB1 and CB2. For its nature of bioactive lipid mediator and the physiological role of satiety factor able to increase intervals between meals, OEA has been strongly investigated in the last years. OEA is synthesized in the upper intestine by duodenal and jejunal enterocytes that internalize dietary triacylglycerols-derived oleic acid as substrate and generate OEA [96]. OEA production in the jejunal and duodenal enterocytes upon intake of dietary fat-derived oleic acid is a well-characterized mechanism and site of OEA synthesis [96]. Nevertheless, depending on the nutritional status, different levels of OEA are found also in duodenum, stomach, colon, adipocytes, liver, kidney, lung, pancreas, heart and muscle [97-99]. The mechanisms underlying the action of OEA as satiety factor is paradigmatic of the role of post ingestive processes and fat sensing for the control of dietary fats intake. Several studies have contributed to reveal that OEA binds with high affinity the subtype alpha of the peroxisome proliferator-activated receptors (PPAR-alpha) producing satiety responses that are not observed in mice lacking PPAR-alpha [97]. Interestingly, it has been reported that OEA can also evoke the secretion of GLP-1 and produce anorexic effects by engaging the G protein-coupled receptor 119 (GPR119) [100]. The mode of action of OEA illustrates a case of lipid-derived sensor of dietary fats connecting food intake and fatty acids sensing in the gut to hypothalamic fatty acids sensing. Although controversial as for the subdiaphragmatic vagal deafferentation [101], OEA-induced hypophagia is abolished in case vagal sensory afferents are lost as for pharmacological treatment or surgical resection [97, 102]. Next, intraperitoneal OEA administration increases transcription of the early gene c-Fos in the NST [102], and protein expression in oxytocin-immuno-reactive neurons in hypothalamic paraventricular (PVN) and supraoptic nuclei (SON) and in the histaminergic tuberomammillary nucleus [103]. Thus, at least two neurotransmitter systems appear involved in the brain control of OEA-induced anorexigenic effects. First, PVN and SON activation increases oxytocin mRNA levels and neurosecretion whereas blockade of oxytocin receptors suppresses OEA-induced hypophagia [103, 104]. Secondly, the hypophagic effects of OEA are abolished in case brain histaminergic system is not fully functional and the integrity of histamine transmission is also required for the OEA-dependent increase of c-Fos expression in oxytocin neurons of the PVN [103]. This is the first demonstration that an intestinal lipid formed upon dietary fats ingestion generates a satiety signal that is decoded by the brain histaminergic system [103]. In spite of its selectivity, this mechanism may contribute to explain several physiological pathways linking intestinal fat sensing to brain circuits involved in energy intake but also in reward assignment to palatable food. Indeed, OEA activity can also help to disclose some mechanisms underlying the obesogenic effects triggered by the overconsumption of palatable energy-dense food, linking fat sensing within the gut-brain axis to homeostatic and hedonic processing of food intake. Prolonged intake of high fat food reduces the intestinal levels of NAEs [105] and also deregulates the nigrostriatal DA release evoked by intragastric lipid infusion [106]. Remarkably, intestinal OEA infusion can revert and normalize DA signaling, and exogenous OEA infusion also appears to reestablish the OEA-mediated anorexigenic effects but only in mice fed with a low-fat diet [106] that increased the intake of a lipid emulsion in parallel with the increase of striatal DA signaling of rewarding food. The link between the homeostatic control of energy intake and brain reward circuits is demonstrated by the increased intake of a non-palatable low fat emulsion following OEA administration in HFD-fed animals. Thus, prolonged intake of HFD may lead to overconsumption of dietary fats because of the lower intestinal OEA levels and reduced brain DA signaling. OEA links fatty acids sensing in the gut to central processing of hedonic hunger and its ability to affect brain DA signaling might contribute to reassign a reward value to foods normally considered as non-palatable or barely hedonic.
In this view, OEA is a member of a family of lipids that is worth investigating for a better understanding of the lipid-sensing gut-brain pathways and detection of effective drug targets for the treatment of obesity and eating disorders.
3. THE IMPACT OF DIET ON THE HYPO-THALAMIC CONTROL OF REPRODUCTION
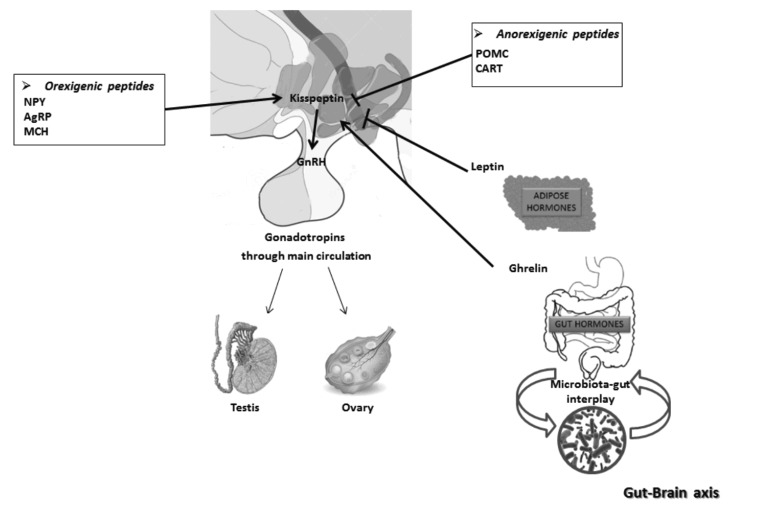
The survival and perpetuation of species are preserved through reproduction. This process requires adequate energy resources, likewise indispensable to support the biosynthesis of sex steroids, the production of high quality gametes, pregnancy and lactation in mammals. In conditions which cannot ensure the correct use of the metabolic resources, the body needs to define priorities to preserve physiological processes essential for survival. In this sense, puberty onset, fertility rate and successful pregnancy all depend on diet and energy homeostasis, as excellently reviewed elsewhere [107]. Nevertheless, it is interesting to note that the increase of metabolic disorders matches with increased rates of infertility in developed and developing countries, as a consequence of environmental factors such as over-nutrition and sedentary lifestyle [108]. Furthermore, nutritional status of parents may impact gamete epigenome with effects on metabolic and reproductive health of the offspring [109]. As described in the previous paragraph, several metabolic sensors from adipose tissues and gut sense the availability or the lack of nutritional resources and, via specific receptors located in peripheral tissues or in the brain, exert local activities on gonads or integrate the gut-brain communication to the HPG axis (Fig. 2) with outcomes depending on species, sex and life stages [110]. Therefore, in the next paragraphs, we provide a survey on the centrally mediated effects of diet on reproduction via the capturing peripheral signals in the brain (i.e. leptin, ghrelin) and in situ production of orexigenic and anorexigenic peptides that directly or indirectly convey dietary cues on HPG axis. Possible interplay with lipid mediators such as eCBs is lastly discussed.
Fig. (2).
A schematic view of the interplay between the HPG and gut-brain axis. Kisspeptin neurons convey to GnRH neurons a plethora of central and peripheral metabolic signals. Orexigenic and anorexigenic peptides influence kisspeptin neurons with stimulatory or inhibitory effects, respectively. In addition, peripheral hormones such as leptin and ghrelin, coming from adipose tissue and gut, respectively, via kisspeptin neurons, inform the HPG axis of metabolic reserves of the body. As final effect, in both sexes, the reproductive activity may be stimulated or inhibited.
3.1. Role of Orexigenic and Anorexigenic Peptides in Reproduction
A complicated network of neuronal circuits supervises reproductive functions, attempting to coordinate and converge towards the HPG axis environmental, hormonal and metabolic conditions (Table 1). A hierarchical actor in HPG axis is the decapeptide Gonadotropin-Releasing Hormone (GnRH), with its well-known role in the stimulation of pituitary gonadotropins and downstream of gonadal functions [140-144]. Due to their excellent position in the hypothalamus, GnRH neurons are the best candidate to relay metabolic information to the HPG axis, thus they start a dynamic interplay with the wide range of the signals involved in the metabolic control of reproduction [145, 146].
Table 1.
Peripherally and centrally produced metabolic sensors that link metabolism and reproduction.
| Main Site of Synthesis | Metabolic Effects | Reproductive Effects | Refs. | ||
|---|---|---|---|---|---|
| Peripheral hormones | Adiponectin | Adipose tissue | Anorexigenic | Acts on the late stages of folliculogenesis and on the development of a functional placenta |
[111] |
| CCK | Gastrointestinal tract | Anorexigenic | Regulates sperm capacitation and fertilization |
[112-114] | |
| Ghrelin | Gastrointestinal tract | Orexigenic | Downregulates kiss1 expression | [115-118] | |
| GLP-1 | L-cells in the small intestine and colon | Anorexigenic | Stimulates the HPG axis, in cell lines augments GnRH secretion |
[119, 120] | |
| Insulin | Pancreas | Anorexigenic | Controls pulsatile GnRH secretion | [116, 121] | |
| Irisin | Skeletal muscle cells | Regulator of body fat and muscle mass | Suggested role as trigger for the activation of the hypothalamic neuronal network monitoring the onset of puberty | [122] | |
| Leptin | White adipose tissue | Anorexigenic | Puberty onset | [116, 118, 123, 124] | |
| Obestatin | Gastrointestinal tract and testis | Anorexigenic | Modulates GnRH secretion and regulates testosterone secretion |
[125, 126] | |
| PYY | Entero-endocrine L-cells of the distal ileum and colon | Anorexigenic | Increases gonadotrophin secretion | [127] | |
| Neuropeptides | AgRP | ARC | Orexigenic | Mediates the orexigenic action of testosterone |
[116, 128, 129] |
| CART | ARC | Anorexigenic | Has a stimulatory effect on kisspeptin neurons |
[130, 131] | |
| GALP | ARC | Anorexigenic | Increases serum levels of LH and testosterone | [132] | |
| Kisspeptin | Hypothalamus | Anorexigenic | Regulates GnRH secretion | [124, 133] | |
| MCH | Hypothalamus | Orexigenic | Regulates LH release; stimulates hypothalamic GnRH |
[116, 134-136] | |
| NPY | ARC | Orexigenic | Regulates GnRH neurons influencing LH release |
[116, 128, 137] | |
| Orexin | Hypothalamus | Orexigenic | Has direct input to GnRH neurons in the human. | [138, 139] | |
| POMC | ARC | Anorexigenic | Contacts GnRH neurons | [116, 128, 133] | |
The relationship between metabolism and reproduction is centrally orchestrated by orexigenic and anorexigenic neuropeptides produced in the ARC [147] (Table 1). First class includes peptides able to stimulate the appetite, such as neuropeptide Y (NPY), agouti related protein (AgRP), melanin-concentrating hormone (MCH); the second one includes appetite inhibiting neuropeptides such as proopiomelanocortin (POMC) precursor and cocaine- and amphetamine-related transcript (CART) [116]. Strong evidence confers important roles in reproduction to these metabolic neuropeptides, through their ability to directly contact GnRH neurons [148-152], thus influencing gonadotropin discharge [108].
One of the major peripheral indicators of body metabolic reserves is leptin, a peptide hormone produced by white adipose tissue [123]. Leptin is an essential threshold factor in the control of puberty onset by food intake [118] and such an action has GnRH neurons as potential targets. Many data demonstrate that GnRH neurons are devoid of functional leptin receptors, thus intermediate neuronal pathways convey HPG nutritional cues [153]. In such a context, kisspeptins - kiss1 gene produced peptides [154] - have an important role through the activation of kisspeptin receptor GPR54 [155]. In fact, the kisspeptin system has a prominent role in the central and local regulation of reproduction as upstream modulators of GnRH [144, 156-160].
Kisspeptin system deeply responds to gonadal and metabolic factors [161-163], including leptin. In fact, ~40% of kisspeptin neurons located in the ARC express leptin receptor [124]. Under caloric restriction or fasting, leptin levels in blood dramatically decrease, causing a drop in hypothalamic kiss1 mRNA expression [164]; thereafter, leptin administration ameliorates kiss1 levels [124]. Interestingly, Ob/Ob mice, lacking leptin gene, show increased appetite and are affected by obesity and sterility. Reduced expression of kiss1 in the ARC, but not in the AVPV, causes hypogonadotropic hypogonadism in this animal model [124]. However, indirect action of leptin through other hypothalamic neurons in tight connection with kisspeptin neurons cannot be ruled-out [165, 166]. In fact, the selective ablation of leptin receptor from hypothalamic kisspeptin neurons does not alter puberty onset [167]. Consistently, functional crosstalk between kisspeptin neurons NPY and POMC has also been unraveled [133, 168].
Diet can affect reproduction through the inhibition of kisspeptin signaling in diabetes mellitus affected rats as well as diet-induced obese (DIO) male rats [169, 170]. Fasting also reduces kiss1 mRNA expression in the prepubertal rat [164] and adult mice [168] hypothalamus. Accordingly, kisspeptin administration reverses starvation-induced hypogonadotropism [164]. Similar responses in fasting conditions have been observed on GPR54 mRNA expression [124].
Lastly, under/overnutrition results in a time shift of puberty through kisspeptin system. In rats, maternal food restriction delays the onset of puberty in offspring of both sexes [171], whereas HFD during pregnancy advances puberty onset in female offspring [172]. Accordingly, HFD during lactation alters pubertal development in female offspring only [173]. All the above effects implicate the regulation of kisspeptin expression in the ARC depending on serum leptin levels [173].
Together with leptin, ghrelin also contributes to inform the hypothalamus of changes in peripheral energy balance. Ghrelin is a peptide secreted by the gastrointestinal tract, working as an orexigenic factor [115]; in volunteers, intravenous ghrelin infusion increases appetite and food intake by 28% [174]. Plasma levels of ghrelin rise almost 2-fold immediately due to fasting and fall after the ingestion of food [175]. However, the exact molecular mechanism by which this hormone regulates feeding is poorly understood. As far as the activity on the HPG axis is concerned, ghrelin has an inhibitory effect on both Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH) release. This effect is due to the inhibition of hypothalamic GnRH neurons and direct suppressive effects on gonads [176]. In animal models, ghrelin has the ability to delay puberty onset in response to insufficient energy states, via the modulation of kiss1 expression [115, 117, 177].
However, different sensitivity to ghrelin administration during pre-pubertal period has been reported between males and females [178].
Post-pubertally, circulating ghrelin levels drastically increase in case of body weight decrease induced by diet/exercise [179]; similar effects have been observed in young amenorrheic athletes which also show decreased LH and GnRH pulsatility [180]. Consistently, ghrelin administration disrupts fertility in adul female rats [117]. All these observations support the idea that abnormal eating behavior influences ghrelin levels, with possible suppression of the reproductive axis through kisspeptin regulation and GnRH/LH inhibition.
Therefore, a balance between leptin and ghrelin is maintained in the ARC, at the levels of NPY/AgRP and Kisspeptin populations. Leptin inhibits NPY/AgRP and stimulates Kisspeptin circuits, ghrelin does quite the opposite [181].
3.2. The Involvement of eCBs in the Central Pathways Integrating Metabolism and Reproduction
The functional interplay that links the main actors in food intake (i.e. peripherally and centrally produced orexigenic and anorexigenic peptides/hormones) to the main gatekeepers of reproduction (i.e. Kisspeptin and GnRH) finds in the n-6 PUFA ARA derived eCBs key signaling intermediates. [10, 13, 16, 17, 18, 141, 143].
Interestingly, during the 20th century, Western diet has been subjected to deep changes, with an enrichment in n-6, over n-3 PUFA assumption, thus contributing to the onset of overweight and obesity [182, 183]. Diet is an important source of ARA (further details in parapraph 4) and thus, in addition to enzymatic hydrolysis, it is realistic that the tone of eCBs might be modulated by the fatty acid composition of food [184], with different outcomes on health including the centrally orchestrated control of reproduction.
ECBs work as orexigenic factors, stimulating food intake and promoting the accumulation of body fat [13, 185-188]. Elevated circulating AEA and 2-AG levels and a parallel drop in the FAAH activity in obese subjects corroborate the role of the eCB system in human obesity [189]. Accordingly, the activation of CB1 increases food intake [190], while its genetic and pharmacological impairment implicates less food assumption [191, 192]. The reintroduction of n-3 PUFA in the Western diet, and so of EPA and DHA, may help to restore eCB tone [193, 194], since experimental data suggest that a dietary treatment with n-3 PUFA decreases the concentration of AEA and 2-AG in the visceral adipose tissue and AEA levels in the liver and heart [195].
Therefore, CB1 seems to cooperate with key hypothalamic peptides involved in the regulation of energy homeostasis, like POMC/CART neurons into ARC [131]. In this regard, the orexigenic effect of AEA may be centrally mediated by inactivation of CART immunoreactive fibers [196]. This crosstalk seems to be well demonstrated in male mice chronically exposed to Bisphenol A (BPA) via drinking water [197]. In these animals, BPA works as a powerful anorexigenic signal, depressing cannabinoid signalling and upregulating the hypothalamic expression of CART [197].
However, some evidence support the hypothesis that the regulation of food intake by eCBs within the hypothalamus might be more complex with a bimodal orexigenic and anorexigenic action, depending on whether CB1 is activated in glutamatergic or GABAergic terminals, respectively [198].
A strong interaction has also been suggested between eCB system and leptin, the latter producing its anorexigenic effects through the inhibition of hypothalamic eCB levels [199]; accordingly, in Ob/Ob mice, there is an overactivation of the hypothalamic eCB signalling [199]. Similarly, a functional synergic overlap in promoting food intake has been demonstrated between ghrelin and eCBs, at both central and peripheral levels [200]. Ghrelin positively regulates the tone of eCBs and hypothalamic 2-AG levels increase after food deprivation and decrease after food consumption in parallel to ghrelin [201]. Thus, both leptin and ghrelin require a functional CB1 signalling to exert their respective anorexigenic and orexigenic effects, since in the hypothalamus of CB1 knockout mice, these effects are abolished [202].
An eCB system has been detected in GnRH secreting neurons, in hypothalamic cell lines, both in in vitro and in in vivo models and either in mammalian and non-mammalian vertebrates [203-205]. AEA can modulate GnRH biosynthesis, release or signaling via CB1 both centrally and peripherally [14, 17, 18, 204, 206-210]. Outcomes on steroid biosynthesis, gametogenesis, gamete quality and fertility rate have been reported [16-18, 142, 211, 212, 213]. Consistently, the characterization of the reproductive phenotype in CB1 knockout mice revealed impairment of HPG axis at multiple levels [214, 215]. At molecular levels, several mechanisms have been reported. Classically, eCBs act as retrograde signals to reduce GABAergic synaptic transmission to GnRH secreting neurons, but involvement of glial cells and the direct activity on subsets of GnRH secreting neurons that express CB1 have been reported [17, 18].
Lastly, updates in the modulation of GnRH via eCBs recently come from a non-mammalian vertebrate model, the anuran amphibian Pelophylax esculentus and address kisspeptin secreting neurons as a new target for eCBs in the modulation of HPG axis [207, 210]. Interestingly, in male rats exposed to immobilization stress, kiss1 mRNA decreases in the medial preoptic area rather than in the ARC and serum LH concentrations reduce as a consequence of the HPG axis suppression [216]. Thus, despite the fact that the involvement of eCBs in the functional interplay between kisspeptin and GnRH-secreting neurons in physiological conditions has never been investigated so far in mammals, data in amphians deserve attention since most master regulatory systems are evolutionarily conserved [143].
4. NEUROPROTECTIVE EFFECTS OF N-3 PUFAs
The main n-6 and n-3 LC PUFAs found in the brain are the ARA (20:4 n-6) and DHA (22:6 n-3) respectively. Both these LC PUFAs have pivotal roles in brain physiology as they regulate fundamental neurobiological processes, in particular those involved in cognition [8, 217]. Neuroprotective activities of LC n-3 PUFAs DHA and EPA are believed to be potent for protection and/or treatment of cognitive decline and dementia [218-223]. We refer to recent seminal reviews reporting on the mechanisms potentially involved in the neuroprotective activities of LC n-3 PUFA [8, 223-227]. Here, we briefly discuss recent findings on LC n-3 PUFAs role in neuroinflammatory pathways and eCB system regulation.
4.1. Dietary PUFAs and Brain PUFAs Metabolism
In the brain, LC-PUFAs are largely esterified to the phospholipid cell membrane of neurons and glial cells (astrocytes, microglia and oligodendrocytes). ARA represents 5 to 11% of total brain phospholipids while DHA represents 13 to 22% [228]. Brain phospholipids enriched with DHA are especially present in grey matter and synapses. Due to the limited capacity of the brain to synthesize LC PUFAs [229, 230], they have to be provided through the diet either as precursors [n-6 linoleic acid (LA) and n-3 αlinolenic acid (ALA) found in vegetable oils and grain products] or as preformed ARA and DHA (found in meat products and fish, respectively) [231, 232]. Hence, increased consumption of n-3 PUFAs rich products results in a partial replacement of ARA by DHA in brain cell membrane [228]. Conversely, a lower n-3 PUFAs intake leads to lower brain levels of DHA with increased ARA levels. LA and ALA are metabolized into ARA and DHA through a series of elongation and desaturation steps. The fatty acid desaturase (ΔD, gene FADS) Δ5D and Δ6D are rate-limiting enzymes. In humans, carriers of specific genetic variants of FADS display higher biological status of LA and ALA (PUFAs precursors) and lower status of LC-PUFA products, ARA and DHA [233].
Of note, observational studies in humans revealed a gender difference in PUFAs levels, with higher ARA and DHA reported in women as compared to men [233, 234]. These differences could be linked to sex hormones as they influence PUFAs metabolism with estrogen stimulating, and testosterone inhibiting the conversion of both n-3 and n-6 precursors into their respective long chain metabolites [235]. However, whether these differences in PUFAs have a role in specific brain diseases with a gender component has been poorly questioned and requires further investigations.
As the endogenous synthesis of DHA from ALA is extremely inefficient, the consumption of preformed DHA from fish sources is recommended. DHA is likely to enter in the brain from the blood, but the mechanisms involved are still a matter of debate [8, 236]. While numerous data show that non-esterified DHA freely enters the brain [8], an orphan receptor, the major facilitator superfamily domain-containing protein 2a (Mfsd2a) has been described as important to transport DHA in the form of lysophosphatidylcholine (LPC) through the blood brain barrier (BBB) [237]. In retinal cells, adiponectin receptor 1 has been recently identified as a key factor for DHA uptake and retention [238].
4.2. Role of PUFAs in the Regulation of Neuroinflammatory Processes
DHA, ARA and their mediators regulate several processes within the brain, such as neurotransmission and neuroinflammation, which are key processes in cognition [8, 216, 239]. Upon activation of phospholipase A2 (PLA2), unesterified ARA and DHA are released from cell membrane and exert their effects directly or upon conversion to a series of bioactive mediators [240]. The major enzymes involved in the synthesis of ARA and DHA bioactive mediators are COX, LOX and cytochrome P450 [241].
Neuroinflammatory pathways are involved in cognitive impairment and neurodegenerative diseases such as Alzheimer’s disease. Neuroinflammation involves the brain innate immune system, mainly microglial cells [242, 243]. It is now well accepted that in the healthy brain, these cells modulate synaptic functions, in particular through the phagocytosis of unnecessary synapses [244] and protect neurons from infection or damage [245, 246]. Indeed, microglia deregulation due to aging or neurodegenerative processes participates to synaptic loss observed not only in Alzheimer’s disease [247] but also in chronic stress conditions [248] or dietary lipid unbalance [249, 250]. In addition, aging and neurodegenerative diseases are accompanied by increased proinflammatory factors such as cytokines and reactive oxygen species (ROS) production, which participate to neuronal death, neuropathological processes and promote cognitive deficit [23, 251].
COX and LOX enzymes facilitate the conversion of ARA into several prostanoids including PG, LT, TX and LX. These enzymes play a crucial role in the progression of inflammation, either within the brain [8]. COX2 expression is particularly high in neurons. Among ARA/COX2 derived prostanoids, PGE2 is a potent signaling molecule, which regulates neurotransmitter release, neuroinflammation and cerebral blood flow. COX2 expression is upregulated during inflammatory processes in neurons and microglia and its role, together with PGE2, has been consistently reported in inflammation-associated memory impairment [252, 253]. Interestingly, several studies report that blocking COX1/2 with non-steroidal anti-inflammatory drugs (NSAIDs) protects against cognitive impairments in animal model of Alzheimer’s disease suggesting a role for PGE2 and COX2 in memory impairment [254]. This is consistent with the observation that in normal population, NSAIDs assumption prevents Alzheimer’s disease [255]. Such a protective effect of COX inhibition relies on the prevention of neuroinflammatory processes and the promotion of adequate microglia activity. Indeed, in animal models of Alzheimer’s disease, PGE2/EP2 blockade in microglia potently reverses the Aβ42 driven pathogenicity and memory loss [256]. However, no beneficial effects of NSAIDs treatment in Alzheimer’s disease patients have been reported, suggesting that preventing COX activation is efficient before the establishment of the disease. LOX-derived ARA prostanoids are also incriminated in neuroinflammatory processes and memory loss. 5-LOX expression is increased in the brain of patients with degenerative diseases, including Alzheimer’s disease [257] and the role of ARA-derived LOX bioactive mediators in neuroinflammatory processes is well established [258]. Indeed, a dual COX/5-LOX inhibition to reduce production of PG and LT with proinflammatory activities is proposed as a new therapeutic approach for neurodegenerative diseases [259]. In this context, it would be of high interest to consider n-6 and n-3 PUFAs status, as both fatty acids are metabolized through the COX/LOX pathways into derivatives with opposite properties in inflammation [8, 23]. While it has long been recognized that bioactive metabolites of ARA are responsible for many of the actions of ARA, bioactive metabolites of DHA have been identified and characterized more recently [241]. In the brain, several DHA-derived mediators are detected, like, in particular, the LOX-derived SPMs neuroprotectin D1 (NPD1), resolvin D5 (RvD5) and maresin 1 (MaR1) [241, 260]. Both DHA and derived SPMs display potent anti-inflammatory activities in vivo and target microglia [252, 261-264]. DHA and SPMs are impaired at the periphery and within the brain of Alzheimer’s disease patients [265, 266] and in accelerated aging mice model [267]. Interestingly, decreased DHA distribution in Alzheimer’s disease patient’s brains correlates with synaptic loss than Aβ42 deposition [268]. In addition, DHA or SPMs promote phagocytosis of Aβ42 by microglia [269] and modulate the number and activation of microglial cells in vivo [270]. Whether SPMs play a role in the protective activity of long chain n-3 PUFAs on neurological disorders remains to be established.
4.3. Role of PUFAs in the Regulation of Brain eCB System
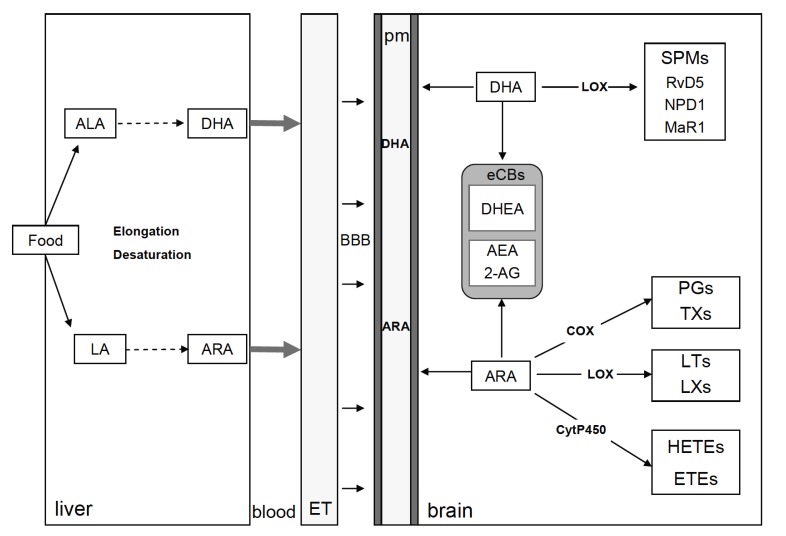
As previously mentioned, eCBs are important PUFAs-derived lipid mediators within the brain and are produced by both neurons and glial cells [271]. The main brain ARA-derived eCBs are AEA and 2-AG, while docosahexaenoylethanolamide (DHEA or synaptamide) is an eCB-like molecule derived from DHA [194]. ECBs’ half-life within the brain is regulated by specific catabolizing enzymes like fatty acid amide hydrolase (FAAH) for AEA and DHEA and monoacylglycerol lipase (MAGL) for 2-AG. Importantly, COX and LOX oxidize ARA-derived eCBs into bioactive PG, which promotes inflammation [272]. DHEA has a lower binding affinity for CB1 and CB2 as compared to AEA and 2-AG and binds GPR receptors, in particular GPR110 within the brain [273, 274]. Dietary n-3/n-6 PUFAs ratio directly influences the proportion of ethanolamides derived from ARA and DHA [193]. Importantly, these changes in eCBs are accompanied by impairment of CB1 activity in the brain, leading to synaptic activity impairment in several brain structures [20, 21, 275]. 2-AG and AEA regulate synaptic functions suppressing excitatory and inhibitory synapse neurotransmitter release by acting as retrograde messengers at presynaptic CB1 [276]. Recent studies show that ARA-derived eCBs also modulate synaptic transmission through post-synaptic receptor TRPV1 or through CB receptors expressed on astrocytes [271, 277]. The importance of brain eCB signalling in the understanding of how altered dietary intake of PUFAs correlates with a range of neurological disorders is of high interest. However, further studies are necessary to demonstrate these links [278]. A schematic representation of PUFAs synthesis from food, PUFAs entry across the BBB and production of PUFAs bioactive derivates in neuronal cells are provided in Fig. (3).
Fig. (3).
Schematic representation of PUFAs synthesis from food, PUFAs entry across the BBB and production of PUFAs bioactive derivates in neuronal cells. ET, endothelial cell; pm, plasma membrane of brain cells, HETE, hydroxyeicosatetraenoic acid; ETE, ecosatetraenoic acid.
4.4. Clinical Trials with Dietary n-3 PUFAs
Several correlative epidemiological studies highlight that the consumption of n-3 PUFAs-rich food has potential neuroprotective effects through their anti-inflammatoy activities within the brain [239]. In particular, these studies revealed the positive association between n-3 PUFAs and cognitive performances in elderly and/or patients with neurodegenerative diseases such as Alzheimer’s disease [8, 279]. These observations led to a number of clinical trials aimed at assessing whether dietary supplementation with LC n-3 PUFAs, mainly EPA and DHA, can restore cognitive function in elderly, patients suffering of Alzheimer’s disease or at risks of cognitive decline, psychosis or mood disorders [217, 280, 281]. Some of the clinical trials studying the impact of LC n-3 PUFAs on cognition in healthy or unhealthy subjects are presented in Table 2. Overall, the results are mitigated. In particular, several trials reveal no improvement of cognitive decline in Alzheimer’s disease patients (Table 2). Of note, some clinical trials report an improvement in cognition in subjects with mild cognitive decline (MCI). Interestingly, a randomized control trial (Multidomain Alzheimer Preventive Trial, MAPT) reported that elderly with the lowest quartile of DHA in erythrocytes get benefit from the LC n-3 PUFAs supplementation with regard to cognitive improvement [292]. This promising study further highlights the importance of assessing dietary intake of n-3 PUFAs and/or biological index of these FA to supplement subjects at risk of cognitive decline.
Table 2.
Clinical trials assessing the effect of long chain n-3 PUFAs dietary supplementation on cognition in healthy and unhealthy adult and aged subjects.
| Subjects | Treatment/day and Duration | Primary Outcome | Results | Refs. |
|---|---|---|---|---|
| Healthy adult subjects | ||||
| Healthy adults 18–35 years |
1 g EPA+DHA 12 weeks |
Cognition and mood | No improvement of memory or mood Improvement of cognitive fatigue |
[282] |
| Young adult females | 400 mg of DHA 50 days |
Memory, mood, reaction times, vigilance or visual acuity | Improvement of memory | [283] |
| Healthy aged subjects with cognitive complaint or deficit | ||||
| Subjects at risk for developing late age-related macular degeneration | 1 g LC PUFAs and/or 10 mg lutein/2 mg zeaxanthin 5 years |
Cognitive function | No effect | [284] |
| Healthy subjects 50-75 years |
2.2 g/d of n-3 PUFA | Executive function MRI |
Improvement of executive function and gray matter volume | [285] |
| Subjects with subjective memory impairment aged 62-80 years | Fish oil (EPA+DHA: 2.4 g/d) 24 weeks | Erythrocyte membrane EPA+DHA fMRI posterior cingulate cortex working memory task |
Positive effect on all parameters | [286] |
| Healthy adults 50-70 years | 240 mg EPA + 240 mg DHA or 480 mg EPA + 480 mg DHA with a multivitamin or 480 mg EPA + 480 mg DHA 6 weeks and 16 weeks |
Cognitive and cardiovascular function Erythrocyte n-3 PUFAs |
Increased n-3 PUFAs No effect on primary cognitive outcome Improvement in spatial memory |
[287] |
| Postmenopausal women 60-84 years |
1g DHA, 160 mg EPA, 240 mg Ginkgo biloba, 60 mg phosphatidylserine, 20mg d-α tocopherol, 1mg folic acid and 20 µg vitamin B12 6 months | Mobility Cognition |
Partial improvement | [288] |
| Cognitively healthy individuals aged 50-75 years |
n-3 LC-PUFAs 2,200 mg/day 26 weeks |
Object location memory | Improvement of recall of object locations | [289] |
| Healthy older adults aged 50-70 years with subjective memory deficits | DHA or DHA plus Gingko biloba, phosphatidylserine and vitamins B6 and B12 6 months |
Cerebral hemodynamics and cognitive functions | No improvement | [290] |
| Healthy middle aged to elderly subjects | 3g Fish oil n-3 PUFA 5 weeks |
Working memory | Improvement | [291] |
| Healthy elderly >55 years with subjective memory complaint |
900 mg of algal DHA 24 weeks |
Paired Associate Learning | Improvement | [292] |
| Non demented patients with cognitive complaints 70 years |
800 mg DHA and 225 mg EPA and/or cognitive training and physical activity 36 months | Cognition | No improvement In a subset of patients with high EPA+DHA in erythrocytes, less cognitive decline |
[293] |
| Unhealthy subjects with cognitive impairment | ||||
| Elderly people (average 83 yr) with moderately severe dementia from thrombotic cerebrovascular disorder | 4.32 g/day of DHA 3, 6, and 12 months |
Cognition (MMSE) Erythrocyte n-3 PUFAs |
Improvement of the dementia score Increase in DHA erythrocyte content |
[294] |
| Subjects | Treatment/day and Duration | Primary Outcome | Results | Refs. |
| Unhealthy subjects with cognitive impairment | ||||
| Patients with coronary artery disease | 1.9 g/d n-3 PUFA 12 weeks |
Vascular cognitive impairment | No effect on cognitive performance Verbal memory impairment in a subgroup of nondepressed patients |
[295] |
| Individuals with cognitive impairment no dementia or Alzheimer’s disease | 600 mg EPA and 625 mg DHA 4 months |
Cognitive functions Mood |
No improvement | [296] |
| Subjects with diagnosis of probable Alzheimer’s disease > 55 years |
675 mg DHA and 975 mg EPA/day or 675 mg DHA and 975 mg EPA plus 600 mg lipoic acid (LA)/day 12 months |
Urine lipid oxidation (F2-isoprostane) Cognition Functional impairment |
No significant change of F2-is prostane Improvement of the cognitive decline in the DHA+EPA+LA group Improvement of functional impairment in all groups |
[297] |
| Elderly patients with MCI | 1.74 g/d of EPA/DHA 12 months |
Memory, psychomotor speed, executive function and attention, and visual-constructive skills | Improvement in memory | [298] |
| Alzheimer’s disease patients | ||||
| Drug-naıve patients with mild Alzheimer’s disease | DHA, EPA, phospholipids, choline, UMP, vitamin B12, B6, and folate, vitamins C and E, and selenium 24 weeks |
Memory performance EEG |
Improvement | [299] |
| Individuals with mild to moderate Alzheimer’s disease | 2 g of algal DHA/d 18 months |
Alzheimer’s disease assessment and dementia | No improvement of cognitive and functional decline | [280] |
| Alzheimer’s disease patients | 2.3 g n-3 PUFAs 6 months |
Cognitive performance | Positive correlation between n-3 PUFA rize and cognitive performance | [300] |
| Alzheimer’s disease patients, Vascular dementia, Dementia with Lewis Bodies, Parkinson disease dementia, Frontotemporal dementia |
n-3 LC-PUFAs 6, 12 and 18 months |
Mental health Dementia |
No positive effects Moderate effect on instrumental activity |
[301] |
| Alzheimer’s disease patients | 1.7 g DHA and 0.6 g of EPA 6 months |
Cognition (MMSE) | No improvement of cognition Positive effect in a small group of patients with very mild Alzheimer’s disease |
[302] |
5. THE THERAPEUTIC “FAT DIETS”: THE KETOGENIC DIETS
After so many observations about the detrimental effects of the excess of dietary fats on health, it could seem a paradox that a diet composed of fat by more than 90% is currently used as a therapeutic resource. These kinds of therapeutic diets are nearly free of sugar, contain an adequate amount of proteins, vitamins and minerals, and are calorie restricted by roughly 10-25%. They are commonly named “ketogenic diets” (KDs). In fact, there is a lack of glucose from the diet and from neoglucogenesis, given the poor amount of aminoacids assumed in the diet, thus the liver produces ketone bodies: acetoacetate, beta-hydroxybutyrate and acetone [303].
KDs have an anti-epileptic effect and are currently used to treat drug-resistant epilepsies in children [304] even in less restrictive and more palatable forms such as the low-glycemic index diet and the modified Atkins diet [305]. The use of KDs in adults is limited due to poor compliance to the treatment, but recent studies suggest that KDs can be effective in adults as well [306, 307]. The use of KDs as a treatment for epilepsy originated from the very ancient observations about the beneficial effects of fasting, since the times of Hyppocrates. After understanding metabolic pathways in modern era, the attention was focused on the ketogenic effects of fasting, so a ketogenic diet was proposed for the treatment of epilepsy since 1921 [308, 309].
The mechanism for such anti-epileptic effect is actually unknown. Because one of the most evident metabolic effects of KDs is the elevation in blood levels of ketone bodies, many studies have searched for possible anti-epileptic effects of ketone bodies [303], but the results of these studies fail to ultimately confirm that this is the main mechanism of action of KDs.
Simple clinical observations, for example, give somewhat strange data. In fact, while the elevation of blood ketones can be simply obtained by fasting for 12-24 hours, an effective anti-epileptic effect is only observed several weeks
after the initiation of a KD. This observation suggests that ketone bodies should not have a direct, clinically relevant, anti-epileptic effect. On the other hand, the acute assumption of a small amount of sugar is able to abruptly abolish the anti-epileptic effect of KDs, with even possible immediate induction of seizures [310]. This would suggest an important role for low glicaemia or high blood ketones.
In rat models, some studies have shown that ketone bodies can have acute anti-seizure effects [311, 312] and that an acute administration of a synthetic ketone ester can have a small anti-seizure effect [313]. In vitro studies have also suggested that acetoacetate and beta-hydroxybutyrate can decrease the activity of neurons within the substantia nigra, which is an important center for the propagation of seizures [314] and that acetoacetate inhibits vesicular glutamate transporters [315]. However, it has not been proven that a prolonged administration of a synthetic ketone precursor produces a significant anti-epileptic effect. Actually, calorie restriction is believed to have an important role for the effects of KDs [316, 317]. In fact, even fasting is known to have anti-epileptic effects since the beginning of the 20th century [309]. It has been argued that the energy metabolism could be involved in the anticonvulsant mechanism of KDs [318] and that some of the biochemical effects induced and maintained by KDs could have anti-seizure properties. The biochemical pathways evaluated until now include ketone bodies production, GABA and glutamate synthesis and recycling, adenosine production, mitochondrial oxidative metabolism, tricarboxylic acid cycle refueling and fatty acids oxidation [319]. It is supposed that such metabolic effects act on brain neurotransmitter production or on ion channel functioning [320] and that the substantia nigra could be particularly involved in these effects [314, 321].
Surprisingly, despite KD is a high-fat diet, few studies have assessed the effects of KD on lipid levels or proportion within the central nervous system. Two recent studies reported an increase in the content of unsaturated fatty acids within the hippocampus [322, 323], but this topic certainly deserves to be further investigated.
Taken together the above data indicate that blood levels of ketone bodies have not a direct, clinically relevant, anti-epileptic effect, but they should be considered an index of the compliance of patients to the diet regimen.
Interestingly, KDs have been proposed to treat many other neurological diseases, like brain trauma, Alzheimer’s disease, Parkinson’s disease, Amyotrophic Lateral Sclerosis, mitochondriopathies, alternating hemiplegia of childhood, brain tumors, migraine and autism spectrum disorders [324, 325]. Again, the mechanism for the beneficial effects of KDs in these pathologies is unknown, but it is supposed to involve an amelioration of the metabolic pathways for energy utilization in the cells of the nervous system.
KDs also have other interesting therapeutic effects. In fact, it has been acknowledged that KDs produce weight loss and ameliorate glycaemic control and blood lipid profile in metabolic syndrome, insulin resistance and type 2 diabetes [324]. These effects are probably due to the low carbohydrate intake which obviously ameliorates the glycaemic control and promotes lipid catabolism.
There are also some studies reporting possible beneficial effects of KDs on tumor progression [326, 327]. It can be argued that such effects could be ascribed to the restricted availability of nutrients from the diet, particularly aminoacids, which are obviously a limiting factor for cellular growth and, thus, for tumor growth. Another possibility is that KDs could enhance the oxidative stress in cancer cells resulting in a greater vulnerability of these cells to radiation therapies [328].
KDs can have transient adverse effects, like gastrointestinal distress, acidosis, hypoglycaemia, dehydration, hyperlipidaemia, and lethargy. Long treatment with KDs is a risk for vitamin and mineral deficiency which can produce easy bruising, impairment of growth and bone fractures. Hyperuricaemia and kidney stones have also been described [329, 330]. KDs are generally considered safe treatments and most of the side effects can be prevented or treated. One of the greatest problems with KDs is the poor palatability and the difficulty for patients to follow severe dietary restrictions. This is why some less restrictive kinds of KDs are currently proposed to patients [305]. However, the greatest challenge for present research is to understand the true mechanisms of KDs and to find a pharmacological alternative, thus realizing a sort of “KD in a pill”, with the same or greater efficacy of a KD but without its dietary limitations [331]; to this aim, the anti-seizure efficacy of a synthetic ketone was tested in animal models and some acute effects have been described [313, 317, 332], but much work is still needed to find an effective drug substitute for KDs.
CONCLUSION
Due to the above observation, we can conclude that dietary fats are both friends and foes for brain functions. Lipids have a structural role in brain and are key factors in neuron activity and health, as for n-3 PUFAs and their bioactive derivates. By contrast, disorders in energy homeostasis have been linked to several neurological and neuropsychiatric diseases pointing out the importance of the microbiota-gut-brain communication for brain health. As a consequence, the manipulation of lifestyle factors, such as dietary interventions is a recognized approach to maintain and preserve brain health along lifespan. The identification of all the molecular mechanisms is far away to be completed, of course, and further work is necessary in the future. Many efforts are required to assess the optimal conditions for therapeutic use in humans. However, dietary manipulation for the treatment of brain disorders is not just a promise for the future, but yet a reality. In fact, the clinical relevance of the manipulation of dietary lipids, as for KDs, is well-known and a currently in use for the treatment of brain diseases.
ACKNOWLEDGEMENTS
Rosaria Meccariello, Rosanna Chianese, Roberto Coccurello, Andrea Viggiano and Riccardo Pierantoni designed the study; Rosanna Chianese, Roberto Coccurello, Andrea Viggiano, Marika Scafuro, Marco Fiore, Giangennaro Coppola, Francesca Felicia Operto, Sophie Layé, and Rosaria Meccariello draft the manuscript; Rosanna Chianese and Rosaria Meccariello designed and prepared figures; Silvia Fasano, Riccardo Pierantoni, Andrea Viggiano and Rosaria Meccariello designed and prepared the graphical abstract; Silvia Fasano, Riccardo Pierantoni and Rosaria Meccariello critically revised the manuscript; all the authors approved the final version of the manuscript.
LIST OF ABBREVIATIONS
- AEA
Anandamide
- 2-AG
Arachidonoylglycerol
- AgRP
Agouti related protein
- ALA
n-3 α-linolenic acid
- ARA
Arachidonic acid
- ARC
Arcuate nucleus
- ASDs
Autism spectrum disorders
- BBB
Blood brain barrier
- BDNF
Brain-derived neurotrophic factor
- BPA
Bisphenol A
- CART
Cocaine- and amphetamine-related transcript
- CB1
Type 1 cannabinoid receptor
- CB2
Type 2 cannabinoid receptor
- CCK
Cholecystokinin
- CHO
Carbohydrates
- COX
Cyclooxygenase
- CNS
Central nervous system
- ΔD
Fatty acid desaturase
- DA-ergic
Dopaminergic
- DHA
Docosahexaenoic acid
- DHEA
Docosahexaenoylethanolamide or synaptamide
- DIO
Diet-induced obese
- eCBs
Endocannabinoids
- ENS
Enteric nervous system
- EPA
Eicosapentaenoic acid
- FAAH
Fatty acid amide hydrolase
- FAs
Fatty acids
- FSH
Follicle stimulating hormone
- FFAR
Free fatty acid receptors
- FOS
Fructo-oligosaccharides
- GABA
γ-aminobutyric acid
- GALP
Galanin like peptide;
- GIP
Glucose-dependent insulinotropic polypeptide
- GIT
Gastrointestinal tract
- GLP-1
Glucagon-like peptide 1
- GnRH
Gonadotropin-releasing hormone
- GOS
Galacto-oligosaccharides
- GPR
G protein-coupled receptor
- HFD
High fat diet
- HPA
Hypothalamic-pituitary-adrenal
- HPG
Hypothalamus-pityitary-godad
- IGN
Intestinal gluconeogenesis
- KDs
Ketogenic diets
- LA
n-6 linoleic acid
- LC-PUFAs
Long chain polyunsaturated fatty acids
- LEA
Linoleoylethanolamide
- LH
Luteinizing hormone
- LOX
Lipoxygenases
- LPC
Lysophosphatidylcholine
- LPS
Lipopolysaccharide
- LTs
Leukotrienes
- LXs
Lipoxins
- MAGL
Monoacylglycerol lipase
- MaR1
Maresin 1
- MCH
Melanin-concentrating hormone
- Mfsd2a
Major facilitator superfamily domain-containing protein 2a
- MUFAs
Monounsaturated FAs
- NAEs
N-acylethanolamines
- NMDAR
N-methyl-d-aspartate receptor
- NPD1
Neuroprotectin D1
- NPY
Neuropeptide Y
- NSAIDs
Non-steroidal anti-inflammatory drugs
- NST
Nucleus of the solitary tract
- OEA
Oleoylethanolamide
- OFS
Oligofructose
- PEA
Palmitoylethanolamide
- PGs
Prostaglandins
- POMC
Proopiomelanocortin precursor
- PLA2
Phospholipase A2
- PPAR-alpha
Peroxisome proliferator-activated receptors alpha
- PVN
Paraventricular nucleus
- PYY
Peptide YY
- ROS
Oxygen species
- RvD5
Resolvin D5
- sCD14
Soluble form of binding-protein CD14
- SCFAs
Short chain fatty acids
- SAFAs
Saturated FAs
- SON
Supraoptic nucleus
- SPMs
Specialized pro-resolving mediators
- TFAs
Trans-FAs
- TX
Thromboxanes
- VTA
Ventral tegmental area
- WHO
World Health Organization
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.2016 http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9(7):568–578. doi: 10.1038/nrn2421. [http://dx.doi.org/10.1038/nrn2421]. [PMID: 18568016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi C.X., Tschöp M.H. Brain-gut-adipose-tissue communication pathways at a glance. Dis. Model. Mech. 2012;5(5):583–587. doi: 10.1242/dmm.009902. [http://dx.doi.org/10.1242/dmm.009902]. [PMID: 22915019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73(3-4):141–162. doi: 10.1016/j.plefa.2005.05.002. [http://dx.doi.org/10.1016/j.plefa.2005.05.002]. [PMID: 16005201]. [DOI] [PubMed] [Google Scholar]
- 5.Serhan C.N. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta. 1994;1212(1):1–25. doi: 10.1016/0005-2760(94)90185-6. [http://dx.doi.org/10.1016/0005-2760(94)90185-6]. [PMID: 8155718]. [DOI] [PubMed] [Google Scholar]
- 6.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011;111(10):5922–5943. doi: 10.1021/cr100396c. [http://dx. doi.org/10.1021/cr100396c]. [PMID: 21766791]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simopoulos A.P. Essential fatty acids in health and chronic diseases. Forum Nutr. 2003;56:67–70. [PMID: 15806801]. [PubMed] [Google Scholar]
- 8.Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15(12):771–785. doi: 10.1038/nrn3820. [http://dx.doi.org/10.1038/nrn3820]. [PMID: 25387473]. [DOI] [PubMed] [Google Scholar]
- 9.Elphick M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3201–3215. doi: 10.1098/rstb.2011.0394. [http://dx.doi.org/10.1098/rstb. 2011.0394]. [PMID: 23108540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano S., Meccariello R., Cobellis G., Chianese R., Cacciola G., Chioccarelli T., Pierantoni R. The endocannabinoid system: an ancient signaling involved in the control of male fertility. Ann. N. Y. Acad. Sci. 2009;1163:112–124. doi: 10.1111/j.1749-6632.2009.04437.x. [http://dx.doi.org/10.1111/ j.1749-6632.2009.04437.x]. [PMID: 19456333]. [DOI] [PubMed] [Google Scholar]
- 11.Marsicano G., Lutz B. Neuromodulatory functions of the endocannabinoid system. J. Endocrinol. Invest. 2006;29(3) Suppl.:27–46. [PMID: 16751707]. [PubMed] [Google Scholar]
- 12.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–1030. doi: 10.1002/glia.20983. [http://dx.doi.org/10.1002/glia.20983]. [PMID: 20468046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006;27(1):73–100. doi: 10.1210/er.2005-0009. [http://dx.doi.org/10.1210/er.2005-0009]. [PMID: 16306385]. [DOI] [PubMed] [Google Scholar]
- 14.Chianese R., Cobellis G., Pierantoni R., Fasano S., Meccariello R. Non-mammalian vertebrate models and the endocannabinoid system: relationships with gonadotropin-releasing hormone. Mol. Cell. Endocrinol. 2008;286(1-2) Suppl. 1:S46–S51. doi: 10.1016/j.mce.2008.01.009. [http://dx. doi.org/10.1016/j.mce.2008.01.009]. [PMID: 18325658]. [DOI] [PubMed] [Google Scholar]
- 15.Cacciola G., Chianese R., Chioccarelli T., Ciaramella V., Fasano S., Pierantoni R., Meccariello R., Cobellis G. Cannabinoids and reproduction: A lasting and intriguing history. Pharmac. 2010;3:3275–3323. [http://dx.doi.org/10.3390/ph3103275]. [Google Scholar]
- 16.Battista N., Meccariello R., Cobellis G., Fasano S., Di Tommaso M., Pirazzi V., Konje J.C., Pierantoni R., Maccarrone M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 2012;355(1):1–14. doi: 10.1016/j.mce.2012.01.014. [http://dx.doi.org/10.1016/j.mce.2012.01.014]. [PMID: 22305972]. [DOI] [PubMed] [Google Scholar]
- 17.Bovolin P., Cottone E., Pomatto V., Fasano S., Pierantoni R., Cobellis G., Meccariello R. Endocannabinoids are involved in male vertebrate reproduction: regulatory mechanisms at central and gonadal level. Front. Endocrinol. 2014;5:54. doi: 10.3389/fendo.2014.00054. [http://dx.doi.org/10.3389/fendo.2014.00054]. [PMID: 24782832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cobellis G., Meccariello R., Chianese R., Chioccarelli T., Fasano S., Pierantoni R. Effects of neuroendocrine CB1 activity on adult Leydig cells. Front. Endocrinol. (Lausanne) 2016;7:47. doi: 10.3389/fendo.2016.00047. [http://dx.doi.org/10.3389/fendo.2016.00047]. [PMID: 27375550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meccariello R., Battista N., Bradshaw H.B., Wang H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014;2014:412354. doi: 10.1155/2014/412354. [http://dx.doi.org/10.1155/2014/ 412354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafourcade M., Larrieu T., Mato S., Duffaud A., Sepers M., Matias I., De Smedt-Peyrusse V., Labrousse V.F., Bretillon L., Matute C., Rodríguez-Puertas R., Layé S., Manzoni O. J. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011;14(3):345–350. doi: 10.1038/nn.2736. [http://dx.doi.org/10.1038/nn.2736]. [PMID: 21278728]. [DOI] [PubMed] [Google Scholar]
- 21.Thomazeau A., Bosch-Bouju C., Manzoni O., Layé S. Nutritional n-3 PUFA deficiency abolishes endocannabinoid gating of hippocampal long-term potentiation. Cereb. Cortex. 2016;27(4):2571–2579. doi: 10.1093/cercor/bhw052. [http://dx.doi.org/10.1093/cercor/bhw052]. [PMID: 26946127]. [DOI] [PubMed] [Google Scholar]
- 22.Cederholm T., Salem N., Jr, Palmblad J. ω-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. 2013;4(6):672–676. doi: 10.3945/an.113.004556. [http://dx.doi.org/10.3945/an.113.004556]. [PMID: 24228198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layé S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82(4-6):295–303. doi: 10.1016/j.plefa.2010.02.006. [http://dx.doi.org/10.1016/j.plefa.2010.02.006]. [PMID: 20227866]. [DOI] [PubMed] [Google Scholar]
- 24.Barnard N.D., Bunner A.E., Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol. Aging. 2014;35(Suppl. 2):S65–S73. doi: 10.1016/j.neurobiolaging.2014.02.030. [http://dx.doi.org/10.1016/j.neurobiolaging. 2014.02.030]. [PMID: 24916582]. [DOI] [PubMed] [Google Scholar]
- 25.Gershon M.D. 1998. [Google Scholar]
- 26.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [http://dx.doi.org/10.1038/nrgastro.2009.35]. [PMID: 19404271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [http://dx. doi.org/10.1038/nrn3071]. [PMID: 21750565]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [http://dx.doi.org/10.1038/nrmicro2876]. [PMID: 23000955]. [DOI] [PubMed] [Google Scholar]
- 29.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [http://dx.doi.org/10.1038/nrn3346]. [PMID: 22968153]. [DOI] [PubMed] [Google Scholar]
- 30.Evrensel A., Ceylan M.E. The gut-brain axis: The missing link in depression. Clin. Psychopharmacol. Neurosci. 2015;13(3):239–244. doi: 10.9758/cpn.2015.13.3.239. [http://dx.doi.org/10.9758/cpn.2015.13.3.239]. [PMID: 26598580]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klingelhoefer L., Reichmann H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015;11(11):625–636. doi: 10.1038/nrneurol.2015.197. [http://dx.doi.org/10.1038/ nrneurol.2015.197]. [PMID: 26503923]. [DOI] [PubMed] [Google Scholar]
- 32.Vuong H.E., Hsiao E. Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. 2016. [DOI] [PMC free article] [PubMed]
- 33.Gill S.R., Pop M., Deboy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., Gordon J.I., Relman D.A., Fraser-Liggett C.M., Nelson K.E. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [http://dx.doi.org/10. 1126/science.1124234]. [PMID: 16741115]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D.R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J.M., Hansen T., Le Paslier D., Linneberg A., Nielsen H.B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Doré J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., Bork P., Ehrlich S.D., Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [http://dx.doi.org/10.1038/nature08821]. [PMID: 20203603]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noble E.E., Hsu T.M., Kanoski S.E. Gut to brain dysbiosis: Mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front. Behav. Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009. [http://dx.doi.org/10.3389/fnbeh.2017.00009]. [PMID: 28194099]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [http://dx.doi.org/10.3389/fncel. 2015.00392]. [PMID: 26528128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmody R.N., Gerber G.K., Luevano J.M., Jr, Gatti D.M., Somes L., Svenson K.L., Turnbaugh P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [http://dx.doi.org/10.1016/j.chom.2014.11.010]. [PMID: 25532804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [http://dx.doi.org/10.1007/10_2008_097]. [PMID: 18461293]. [DOI] [PubMed] [Google Scholar]
- 39.Lilly D.M., Stillwell R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science. 1965;147(3659):747–748. doi: 10.1126/science.147.3659.747. [http://dx.doi.org/10.1126/science.147.3659.747]. [PMID: 14242024]. [DOI] [PubMed] [Google Scholar]
- 40.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [http://dx.doi.org/10.1038/nrgastro.2014.66]. [PMID: 24912386]. [DOI] [PubMed] [Google Scholar]
- 41.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [PMID: 7782892]. [DOI] [PubMed] [Google Scholar]
- 42.Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A., Garau M., Murphy E.F., Saulnier D., Loh G., Macfarlane S., Delzenne N., Ringel Y., Kozianowski G., Dickmann R., Lenoir-Wijnkook I., Walker C., Buddington R. Dietary prebiotics: current status and new definition. Food Sci. Technol. Bull: Funct. Foods. 2010;7:1–19. [Google Scholar]
- 43.Finegold S.M., Molitoris D., Song Y., Liu C., Vaisanen M.L., Bolte E., McTeague M., Sandler R., Wexler H., Marlowe E.M., Collins M.D., Lawson P.A., Summanen P., Baysallar M., Tomzynski T.J., Read E., Johnson E., Rolfe R., Nasir P., Shah H., Haake D.A., Manning P., Kaul A. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002;35(Suppl. 1):S6–S16. doi: 10.1086/341914. [http://dx.doi.org/10.1086/341914]. [PMID: 12173102]. [DOI] [PubMed] [Google Scholar]
- 44.Williams B.L., Hornig M., Parekh T., Lipkin W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3(1):1–11. doi: 10.1128/mBio.00261-11. [http://dx.doi.org/10.1128/mBio.00261-11]. [PMID: 22233678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism. 2013;4(1):42. doi: 10.1186/2040-2392-4-42. [http://dx.doi.org/10.1186/ 2040-2392-4-42]. [PMID: 24188502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., Patterson P.H., Mazmanian S.K. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [http://dx.doi.org/10. 1016/j.cell.2013.11.024]. [PMID: 24315484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro F., Liu Y., Rhoads J.M. Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 2016;22(46):10093–10102. doi: 10.3748/wjg.v22.i46.10093. [http://dx.doi.org/10.3748/wjg.v22.i46.10093]. [PMID: 28028357]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X-N., Kubo C., Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [http://dx.doi.org/10.1113/ jphysiol.2004.063388]. [PMID: 15133062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse in vivothe vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [http://dx.doi.org/10.1073/pnas.1102999108]. [PMID: 21876150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bercik P., Park A.J., Sinclair D., Khoshdel A., Lu J., Huang X., Deng Y., Blennerhassett P.A., Fahnestock M., Moine D., Berger B., Huizinga J.D., Kunze W., McLean P.G., Bergonzelli G.E., Collins S.M., Verdu E.F. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [http://dx.doi.org/10.1111/j.1365-2982.2011.01796.x]. [PMID: 21988661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., Macqueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [http://dx.doi.org/10.1136/gut.2009.202515]. [PMID: 20966022]. [DOI] [PubMed] [Google Scholar]
- 52.Nemani K., Hosseini Ghomi R., McCormick B., Fan X. Schizophrenia and the gut-brain axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;56:155–160. doi: 10.1016/j.pnpbp.2014.08.018. [http://dx.doi.org/10.1016/ j.pnpbp.2014.08.018]. [PMID: 25240858]. [DOI] [PubMed] [Google Scholar]
- 53.Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [http://dx.doi.org/10.1016/j.bbi.2010.10.023]. [PMID: 21040780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galley J.D., Nelson M.C., Yu Z., Dowd S.E., Walter J., Kumar P.S., Lyte M., Bailey M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14:189. doi: 10.1186/1471-2180-14-189. [http://dx.doi.org/10.1186/ 1471-2180-14-189]. [PMID: 25028050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Tang H., Zhang C., Zhao Y., Derrien M., Rocher E., van-Hylckama V.J.E., Strissel K., Zhao L., Obin M., Shen J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. doi: 10.1038/ismej.2014.99. [http://dx.doi.org/10.1038/ismej.2014.99]. [PMID: 24936764]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serino M., Luche E., Gres S., Baylac A., Bergé M., Cenac C., Waget A., Klopp P., Iacovoni J., Klopp C., Mariette J., Bouchez O., Lluch J., Ouarné F., Monsan P., Valet P., Roques C., Amar J., Bouloumié A., Théodorou V., Burcelin R. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61(4):543–553. doi: 10.1136/gutjnl-2011-301012. [http://dx.doi.org/10.1136/gutjnl-2011-301012]. [PMID: 22110050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cano P.G., Santacruz A., Trejo F.M., Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) 2013;21(11):2310–2321. doi: 10.1002/oby.20330. [http://dx.doi.org/10.1002/ oby.20330]. [PMID: 23418126]. [DOI] [PubMed] [Google Scholar]
- 58.Verdam F.J., Fuentes S., de Jonge C., Zoetendal E.G., Erbil R., Greve J.W., Buurman W.A., de Vos W.M., Rensen S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring) 2013;21(12):E607–E615. doi: 10.1002/oby.20466. [http://dx.doi.org/10.1002/oby.20466]. [PMID: 23526699]. [DOI] [PubMed] [Google Scholar]
- 59.Wong J.M., de Souza R., Kendall C.W., Emam A., Jenkins D.J. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [http://dx.doi.org/10.1097/ 00004836-200603000-00015]. [PMID: 16633129]. [DOI] [PubMed] [Google Scholar]
- 60.Smriga M., Chen J., Zhang J.T., Narui T., Shibata S., Hirano E., Saito H. Isolichenan, an alpha-glucan isolated from lichen cetrariella islandica, repairs impaired learning behaviors and facilitates hippocampal synaptic plasticity. Proc. Jpn. Acad. B. Phys. Biol. Sci. 1999;75:219–223. [Google Scholar]
- 61.Han H.S., Jang J-H., Jang J.H., Choi J.S., Kim Y.J., Lee C., Lim S.H., Lee H-K., Lee J. Water extract of Triticum aestivum L. and its components demonstrate protective effect in a model of vascular dementia. J. Med. Food. 2010;13(3):572–578. doi: 10.1089/jmf.2009.1242. [http://dx.doi.org/10.1089/jmf.2009.1242]. [PMID: 20521983]. [DOI] [PubMed] [Google Scholar]
- 62.Savignac H.M., Corona G., Mills H., Chen L., Spencer J.P., Tzortzis G., Burnet P.W. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem. Int. 2013;63(8):756–764. doi: 10.1016/j.neuint.2013.10.006. [http://dx. doi.org/10.1016/j.neuint.2013.10.006]. [PMID: 24140431]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt K., Cowen P.J., Harmer C.J., Tzortzis G., Errington S., Burnet P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl.) 2015;232(10):1793–1801. doi: 10.1007/s00213-014-3810-0. [http://dx.doi.org/10.1007/s00213-014-3810-0]. [PMID: 25449699]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M., Cefalu W.T., Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [http://dx.doi.org/10.2337/db08-1637]. [PMID: 19366864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin H.V., Frassetto A., Kowalik E.J., Jr, Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., Marsh D.J. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones in vivo free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [http://dx.doi.org/10.1371/journal.pone.0035240]. [PMID: 22506074]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of short chain fatty acid receptors. Mol. Pharmacol. 2016;89:388–398. doi: 10.1124/mol.115.102301. [http://dx.doi.org/10.1124/mol. 115.102301]. [PMID: 26719580]. [DOI] [PubMed] [Google Scholar]
- 67.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion in vivothe G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [http://dx.doi.org/10.2337/db11-1019]. [PMID: 22190648]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits in vivogut-brain neural circuits. Cell. 2014;156(1-2):84–96. doi: 10.1016/j.cell.2013.12.016. [http://dx.doi.org/10.1016/j.cell.2013.12.016]. [PMID: 24412651]. [DOI] [PubMed] [Google Scholar]
- 69.Graf D., Di Cagno R., Fåk F., Flint H.J., Nyman M., Saarela M., Watzl B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015;26:26164. doi: 10.3402/mehd.v26.26164. [PMID: 25656825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [http://dx.doi.org/10.1038/4441022a]. [PMID: 17183309]. [DOI] [PubMed] [Google Scholar]
- 71.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., Serrazanetti D.I., Di Cagno R., Ferrocino I., Lazzi C., Turroni S., Cocolin L., Brigidi P., Neviani E., Gobbetti M., O’Toole P.W., Ercolini D. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi: 10.1136/gutjnl-2015-309957. [PMID: 26416813]. [DOI] [PubMed] [Google Scholar]
- 72.Gutiérrez-Díaz I., Fernández-Navarro T., Sánchez B., Margolles A., González S. Mediterranean diet and faecal microbiota: a transversal study. Food Funct. 2016;7(5):2347–2356. doi: 10.1039/c6fo00105j. [http://dx. doi.org/10.1039/C6FO00105J]. [PMID: 27137178]. [DOI] [PubMed] [Google Scholar]
- 73.Xiong Y., Miyamoto N., Shibata K., Valasek M.A., Motoike T., Kedzierski R.M., Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA. 2004;101(4):1045–1050. doi: 10.1073/pnas.2637002100. [http://dx.doi.org/10.1073/pnas.2637002100]. [PMID: 14722361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cani P.D., Neyrinck A.M., Maton N., Delzenne N.M. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like Peptide-1. Obes. Res. 2005;13(6):1000–1007. doi: 10.1038/oby.2005.117. [http://dx.doi.org/10.1038/oby.2005.117]. [PMID: 15976142]. [DOI] [PubMed] [Google Scholar]
- 75.Psichas A., Sleeth M.L., Murphy K.G., Brooks L., Bewick G.A., Hanyaloglu A.C., Ghatei M.A., Bloom S.R., Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion in vivofree fatty acid receptor 2 in rodents. Int. J. Obes. 2015;39(3):424–429. doi: 10.1038/ijo.2014.153. [http://dx.doi.org/10.1038/ijo.2014.153]. [PMID: 25109781]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmée E., Cousin B., Sulpice T., Chamontin B., Ferrières J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [http://dx.doi.org/10.2337/db06-1491]. [PMID: 17456850]. [DOI] [PubMed] [Google Scholar]
- 77.Erridge C., Attina T., Spickett C.M., Webb D.J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [PMID: 17991637]. [DOI] [PubMed] [Google Scholar]
- 78.Ghoshal S., Witta J., Zhong J., de Villiers W., Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. [http://dx.doi.org/10.1194/ jlr.M800156-JLR200]. [PMID: 18815435]. [DOI] [PubMed] [Google Scholar]
- 79.Laugerette F., Vors C., Géloën A., Chauvin M.A., Soulage C., Lambert-Porcheron S., Peretti N., Alligier M., Burcelin R., Laville M., Vidal H., Michalski M.C. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011;22(1):53–59. doi: 10.1016/j.jnutbio.2009.11.011. [http://dx. doi.org/10.1016/j.jnutbio.2009.11.011]. [PMID: 20303729]. [DOI] [PubMed] [Google Scholar]
- 80.Laugerette F., Furet J.P., Debard C., Daira P., Loizon E., Géloën A., Soulage C.O., Simonet C., Lefils-Lacourtablaise J., Bernoud-Hubac N., Bodennec J., Peretti N., Vidal H., Michalski M.C. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 2012;302(3):E374–E386. doi: 10.1152/ajpendo.00314.2011. [http://dx.doi.org/10.1152/ajpendo.00314.2011]. [PMID: 22094473]. [DOI] [PubMed] [Google Scholar]
- 81.Lyte J.M., Gabler N.K., Hollis J.H. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis. 2016;15(1):186. doi: 10.1186/s12944-016-0357-6. [http://dx.doi.org/10.1186/s12944-016-0357-6]. [PMID: 27816052]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chandrasekharan J.A., Sharma-Walia N. Lipoxins: nature’s way to resolve inflammation. J. Inflamm. Res. 2015;8:181–192. doi: 10.2147/JIR.S90380. [PMID: 26457057]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [http://dx.doi.org/10.1084/jem. 20020760]. [PMID: 12391014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serhan C.N., Chiang N., Van Dyke T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [http://dx.doi.org/10.1038/ nri2294]. [PMID: 18437155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jo Y.H., Su Y., Gutierrez-Juarez R., Chua S., Jr Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J. Neurophysiol. 2009;101(5):2305–2316. doi: 10.1152/jn.91294.2008. [http://dx.doi.org/10. 1152/jn.91294.2008]. [PMID: 19261705]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levin B.E., Magnan C., Dunn-Meynell A., Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152(7):2552–2557. doi: 10.1210/en.2011-0194. [http://dx.doi.org/10.1210/en.2011-0194]. [PMID: 21521751]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blouet C., Schwartz G.J. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 2012;16(5):579–587. doi: 10.1016/j.cmet.2012.10.003. [http://dx.doi.org/10.1016/j.cmet.2012.10.003]. [PMID: 23123165]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cavanaugh A.R., Schwartz G.J., Blouet C. High-fat feeding impairs nutrient sensing and gut brain integration in the caudomedial nucleus of the solitary tract in mice. PLoS One. 2015;10(3):e0118888. doi: 10.1371/journal.pone.0118888. [http://dx.doi.org/10.1371/journal.pone.0118888]. [PMID: 25774780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng H., Lenard N.R., Shin A.C., Berthoud H.R. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int. J. Obes. 2009;33(Suppl. 2):S8–S13. doi: 10.1038/ijo.2009.65. [http://dx.doi.org/10.1038/ijo.2009.65]. [PMID: 19528982]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahler S.V., Smith K.S., Berridge K.C. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32(11):2267–2278. doi: 10.1038/sj.npp.1301376. [http://dx.doi.org/10.1038/ sj.npp.1301376]. [PMID: 17406653]. [DOI] [PubMed] [Google Scholar]
- 91.Melis T., Succu S., Sanna F., Boi A., Argiolas A., Melis M.R. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci. Lett. 2007;419(3):231–235. doi: 10.1016/j.neulet.2007.04.012. [http://dx.doi.org/10.1016/j.neulet.2007. 04.012]. [PMID: 17462824]. [DOI] [PubMed] [Google Scholar]
- 92.Di Marzo V., Sepe N., De Petrocellis L., Berger A., Crozier G., Fride E., Mechoulam R. Trick or treat from food endocannabinoids? Nature. 1998;396(6712):636–637. doi: 10.1038/25267. [http://dx.doi.org/10. 1038/25267]. [PMID: 9872309]. [DOI] [PubMed] [Google Scholar]
- 93.Alvheim A.R., Malde M.K., Osei-Hyiaman D., Lin Y.H., Pawlosky R.J., Madsen L., Kristiansen K., Frøyland L., Hibbeln J.R. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012;20(10):1984–1994. doi: 10.1038/oby.2012.38. [http://dx.doi.org/10.1038/oby.2012.38]. [PMID: 22334255]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rousseaux C., Thuru X., Gelot A., Barnich N., Neut C., Dubuquoy L., Dubuquoy C., Merour E., Geboes K., Chamaillard M., Ouwehand A., Leyer G., Carcano D., Colombel J.F., Ardid D., Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007;13(1):35–37. doi: 10.1038/nm1521. [http://dx.doi.org/10.1038/nm1521]. [PMID: 17159985]. [DOI] [PubMed] [Google Scholar]
- 95.Motter A.L., Ahern G.P. TRPA1 is a polyunsaturated fatty acid sensor in mammals. PLoS One. 2012;7(6):e38439. doi: 10.1371/journal.pone.0038439. [http://dx. doi.org/10.1371/journal.pone.0038439]. [PMID: 22723860]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piomelli D. A fatty gut feeling. Trends Endocrinol. Metab. 2013;24(7):332–341. doi: 10.1016/j.tem.2013.03.001. [http://dx.doi.org/10.1016/j.tem.2013.03.001]. [PMID: 23567058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu J., Gaetani S., Oveisi F., Lo Verme J., Serrano A., Rodríguez De Fonseca F., Rosengarth A., Luecke H., Di Giacomo B., Tarzia G., Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425(6953):90–93. doi: 10.1038/nature01921. [http://dx.doi.org/10.1038/ nature01921]. [PMID: 12955147]. [DOI] [PubMed] [Google Scholar]
- 98.Izzo A.A., Piscitelli F., Capasso R., Marini P., Cristino L., Petrosino S., Di Marzo V. Basal and fasting/refeeding-regulated tissue levels of endogenous PPAR-alpha ligands in Zucker rats. Obesity (Silver Spring) 2010;18(1):55–62. doi: 10.1038/oby.2009.186. [http://dx.doi.org/10.1038/oby.2009.186]. [PMID: 19521349]. [DOI] [PubMed] [Google Scholar]
- 99.Gonthier M.P., Hoareau L., Festy F., Matias I., Valenti M., Bès-Houtmann S., Rouch C., Robert-Da Silva C., Chesne S., Lefebvre d’Hellencourt C., Césari M., Di Marzo V., Roche R. Identification of endocannabinoids and related compounds in human fat cells. Obesity (Silver Spring) 2007;15(4):837–845. doi: 10.1038/oby.2007.581. [http://dx.doi.org/10.1038/oby.2007.581]. [PMID: 17426318]. [DOI] [PubMed] [Google Scholar]
- 100.Hansen K.B., Rosenkilde M.M., Knop F.K., Wellner N., Diep T.A., Rehfeld J.F., Andersen U.B., Holst J.J., Hansen H.S. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 2011;96(9):E1409–E1417. doi: 10.1210/jc.2011-0647. [http://dx.doi.org/10.1210/jc.2011-0647]. [PMID: 21778222]. [DOI] [PubMed] [Google Scholar]
- 101.Azari E.K., Ramachandran D., Weibel S., Arnold M., Romano A., Gaetani S., Langhans W., Mansouri A. Vagal afferents are not necessary for the satiety effect of the gut lipid messenger oleoylethanolamide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307(2):R167–R178. doi: 10.1152/ajpregu.00067.2014. [http://dx.doi.org/10.1152/ajpregu. 00067.2014]. [PMID: 24829501]. [DOI] [PubMed] [Google Scholar]
- 102.Rodríguez de Fonseca F., Navarro M., Gómez R., Escuredo L., Nava F., Fu J., Murillo-Rodríguez E., Giuffrida A., LoVerme J., Gaetani S., Kathuria S., Gall C., Piomelli D. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–212. doi: 10.1038/35102582. [http://dx.doi.org/10.1038/35102582]. [PMID: 11700558]. [DOI] [PubMed] [Google Scholar]
- 103.Provensi G., Coccurello R., Umehara H., Munari L., Giacovazzo G., Galeotti N., Nosi D., Gaetani S., Romano A., Moles A., Blandina P., Passani M.B. Satiety factor oleoylethanolamide recruits the brain histaminergic system to inhibit food intake. Proc. Natl. Acad. Sci. USA. 2014;111(31):11527–11532. doi: 10.1073/pnas.1322016111. [http://dx.doi.org/10.1073/pnas.1322016111]. [PMID: 25049422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gaetani S., Fu J., Cassano T., Dipasquale P., Romano A., Righetti L., Cianci S., Laconca L., Giannini E., Scaccianoce S., Mairesse J., Cuomo V., Piomelli D. The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J. Neurosci. 2010;30(24):8096–8101. doi: 10.1523/JNEUROSCI.0036-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.0036-10.2010]. [PMID: 20554860]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Diep T.A., Madsen A.N., Holst B., Kristiansen M.M., Wellner N., Hansen S.H., Hansen H.S. Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J. 2011;25(2):765–774. doi: 10.1096/fj.10-166595. [http://dx.doi.org/10.1096/fj.10-166595]. [PMID: 20959516]. [DOI] [PubMed] [Google Scholar]
- 106.Tellez L.A., Medina S., Han W., Ferreira J.G., Licona-Limón P., Ren X., Lam T.T., Schwartz G.J., de Araujo I.E. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341(6147):800–802. doi: 10.1126/science.1239275. [http://dx.doi.org/10.1126/science. 1239275]. [PMID: 23950538]. [DOI] [PubMed] [Google Scholar]
- 107.Cheng G., Buyken A.E., Shi L., Karaolis-Danckert N., Kroke A., Wudy S.A., Degen G.H., Remer T. Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr. Rev. 2012;70(3):133–152. doi: 10.1111/j.1753-4887.2011.00461.x. [http://dx.doi.org/10.1111/ j.1753-4887.2011.00461.x]. [PMID: 22364156]. [DOI] [PubMed] [Google Scholar]
- 108.Wade G.N., Jones J.E. Neuroendocrinology of nutritional infertility. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(6):R1277–R1296. doi: 10.1152/ajpregu.00475.2004. [http://dx.doi.org/10.1152/ajpregu.00475.2004]. [PMID: 15528398]. [DOI] [PubMed] [Google Scholar]
- 109.Yan M., Zhai Q. Sperm tsRNAs and acquired metabolic disorders. J. Endocrinol. 2016;230(3):F13–F18. doi: 10.1530/JOE-16-0185. [http://dx.doi.org/10.1530/ JOE-16-0185]. [PMID: 27340033]. [DOI] [PubMed] [Google Scholar]
- 110.Comninos A.N., Jayasena C.N., Dhillo W.S. The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update. 2014;20(2):153–174. doi: 10.1093/humupd/dmt033. [http://dx.doi.org/10.1093/humupd/dmt033]. [PMID: 24173881]. [DOI] [PubMed] [Google Scholar]
- 111.Dos Santos E., Pecquery R., de Mazancourt P., Dieudonné M.N. Adiponectin and reproduction. Vitam. Horm. 2012;90:187–209. doi: 10.1016/B978-0-12-398313-8.00008-7. [http://dx.doi.org/10.1016/B978-0-12-398313-8.00008-7]. [PMID: 23017717]. [DOI] [PubMed] [Google Scholar]
- 112.Stanley S., Wynne K., McGowan B., Bloom S. Hormonal regulation of food intake. Physiol. Rev. 2005;85(4):1131–1158. doi: 10.1152/physrev.00015.2004. [http://dx.doi.org/10.1152/physrev.00015.2004]. [PMID: 16183909]. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Y., Ru Y., Shi H., Wang Y., Wu B., Upur H., Zhang Y. Cholecystokinin receptors regulate sperm protein tyrosine phosphorylation in vivouptake of HCO3-. Reproduction. 2015;150(4):257–268. doi: 10.1530/REP-15-0138. [http://dx.doi.org/10.1530/REP-15-0138]. [PMID: 26175429]. [DOI] [PubMed] [Google Scholar]
- 114.Zelezná B., Maixnerová J., Matysková R., Haugvicová R., Blokesová D., Maletínská L. Anorexigenic effect of cholecystokinin is lost but that of CART (Cocaine and Amphetamine Regulated Transcript) peptide is preserved in monosodium glutamate obese mice. Physiol. Res. 2009;58(5):717–723. doi: 10.33549/physiolres.931511. [PMID: 19093718]. [DOI] [PubMed] [Google Scholar]
- 115.Roa J., García-Galiano D., Castellano J.M., Gaytan F., Pinilla L., Tena-Sempere M. Metabolic control of puberty onset: new players, new mechanisms. Mol. Cell. Endocrinol. 2010;324(1-2):87–94. doi: 10.1016/j.mce.2009.12.018. [http://dx.doi.org/10.1016/j.mce.2009.12.018]. [PMID: 20026241]. [DOI] [PubMed] [Google Scholar]
- 116.Wilson J.L., Enriori P.J. A talk between fat tissue, gut, pancreas and brain to control body weight. Mol. Cell. Endocrinol. 2015;418(Pt 2):108–119. doi: 10.1016/j.mce.2015.08.022. [http://dx.doi.org/10.1016/j.mce.2015.08.022]. [PMID: 26316427]. [DOI] [PubMed] [Google Scholar]
- 117.Forbes S., Li X.F., Kinsey-Jones J., O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci. Lett. 2009;460(2):143–147. doi: 10.1016/j.neulet.2009.05.060. [http://dx.doi.org/10.1016/j.neulet.2009.05.060]. [PMID: 19477231]. [DOI] [PubMed] [Google Scholar]
- 118.Tena-Sempere M. Roles of ghrelin and leptin in the control of reproductive function. Neuroendocrinology. 2007;86(3):229–241. doi: 10.1159/000108410. [http://dx.doi.org/10.1159/000108410]. [PMID: 17851226]. [DOI] [PubMed] [Google Scholar]
- 119.Tang-Christensen M., Larsen P.J., Göke R., Fink-Jensen A., Jessop D.S., Møller M., Sheikh S.P. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am. J. Physiol. 1996;271(4 Pt 2):R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [PMID: 8897973]. [DOI] [PubMed] [Google Scholar]
- 120.Beak S.A., Heath M.M., Small C.J., Morgan D.G., Ghatei M.A., Taylor A.D., Buckingham J.C., Bloom S.R., Smith D.M. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J. Clin. Invest. 1998;101(6):1334–1341. doi: 10.1172/JCI610. [http://dx.doi.org/10.1172/ JCI610]. [PMID: 9502775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sliwowska J.H., Fergani C., Gawałek M., Skowronska B., Fichna P., Lehman M.N. Insulin: its role in the central control of reproduction. Physiol. Behav. 2014;133:197–206. doi: 10.1016/j.physbeh.2014.05.021. [http://dx.doi.org/10.1016/j.physbeh.2014.05.021]. [PMID: 24874777]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wahab F., Shahab M., Behr R. Hypothesis: Irisin is a metabolic trigger for the activation of the neurohormonal axis governing puberty onset. Med. Hypotheses. 2016;95:1–4. doi: 10.1016/j.mehy.2016.08.003. [http://dx.doi.org/10.1016/j.mehy.2016.08.003]. [PMID: 27692156]. [DOI] [PubMed] [Google Scholar]
- 123.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., Lallone R.L., Burley S.K., Friedman J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [http://dx.doi.org/10.1126/science.7624777]. [PMID: 7624777]. [DOI] [PubMed] [Google Scholar]
- 124.Smith J.T., Acohido B.V., Clifton D.K., Steiner R.A. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006;18(4):298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [http://dx.doi.org/10.1111/ j.1365-2826.2006.01417.x]. [PMID: 16503925]. [DOI] [PubMed] [Google Scholar]
- 125.Sarwat J., Shakeel A., Nazifa T. Hizbullah. Possible modulation of testosterone secretion by obestatin in pubertal male rats. Pak. J. Zool. 2011;43:799–803. [Google Scholar]
- 126.Zhang J.V., Ren P.G., Avsian-Kretchmer O., Luo C.W., Rauch R., Klein C., Hsueh A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310(5750):996–999. doi: 10.1126/science.1117255. [http://dx.doi.org/10.1126/science. 1117255]. [PMID: 16284174]. [DOI] [PubMed] [Google Scholar]
- 127.Pinilla L., Fernández-Fernández R., Roa J., Castellano J.M., Tena-Sempere M., Aguilar E. Selective role of neuropeptide Y receptor subtype Y2 in the control of gonadotropin secretion in the rat. Am. J. Physiol. Endocrinol. Metab. 2007;293(5):E1385–E1392. doi: 10.1152/ajpendo.00274.2007. [http://dx.doi.org/10.1152/ajpendo.00274.2007]. [PMID: 17785504]. [DOI] [PubMed] [Google Scholar]
- 128.Broberger C., Johansen J., Johansson C., Schalling M., Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA. 1998;95(25):15043–15048. doi: 10.1073/pnas.95.25.15043. [http://dx.doi.org/10.1073/pnas.95.25.15043]. [PMID: 9844012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nestor C.C., Qiu J., Padilla S.L., Zhang C., Bosch M.A., Fan W., Aicher S.A., Palmiter R.D., Rønnekleiv O.K., Kelly M.J. Optogenetic Stimulation of arcuate nucleus kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol. Endocrinol. 2016;30(6):630–644. doi: 10.1210/me.2016-1026. [http://dx. doi.org/10.1210/me.2016-1026]. [PMID: 27093227]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.True C., Verma S., Grove K.L., Smith M.S. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology. 2013;154(8):2821–2832. doi: 10.1210/en.2013-1156. [http://dx.doi.org/10.1210/en.2013-1156]. [PMID: 23736294]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lindberg D., Chen P., Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 2013;521(14):3167–3190. doi: 10.1002/cne.23338. [http://dx.doi.org/10.1002/cne.23338]. [PMID: 23696474]. [DOI] [PubMed] [Google Scholar]
- 132.Krasnow S.M., Fraley G.S., Schuh S.M., Baumgartner J.W., Clifton D.K., Steiner R.A. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144(3):813–822. doi: 10.1210/en.2002-220982. [http://dx.doi.org/10.1210/en.2002-220982]. [PMID: 12586757]. [DOI] [PubMed] [Google Scholar]
- 133.Fu L.Y., Van den Pol A.N. Kisspeptin directly excites anorexigenic POMC neurons, but inhibits orexigenic NPY cells by indirect synaptic mechanism. J. Neurosci. 2010;30:10205–10219. doi: 10.1523/JNEUROSCI.2098-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.2098-10.2010]. [PMID: 20668204]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Skofitsch G., Jacobowitz D.M., Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res. Bull. 1985;15(6):635–649. doi: 10.1016/0361-9230(85)90213-8. [http://dx.doi.org/10.1016/0361-9230(85)90213-8]. [PMID: 4084816]. [DOI] [PubMed] [Google Scholar]
- 135.Santollo J., Eckel L.A. The orexigenic effect of melanin-concentrating hormone (MCH) is influenced by sex and stage of the estrous cycle. Physiol. Behav. 2008;93(4-5):842–850. doi: 10.1016/j.physbeh.2007.11.050. [http://dx.doi.org/10.1016/j.physbeh.2007.11.050]. [PMID: 18191424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Naufahu J., Cunliffe A.D., Murray J.F. The roles of melanin-concentrating hormone in energy balance and reproductive function: Are they connected? Reproduction. 2013;146(5):R141–R150. doi: 10.1530/REP-12-0385. [http://dx.doi.org/10.1530/REP-12-0385]. [PMID: 23884861]. [DOI] [PubMed] [Google Scholar]
- 137.Li C., Chen P., Smith M.S. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology. 1999;140(11):5382–5390. doi: 10.1210/endo.140.11.7093. [http://dx.doi.org/10.1210/endo.140.11.7093]. [PMID: 10537170]. [DOI] [PubMed] [Google Scholar]
- 138.Skrapits K., Kanti V., Savanyú Z., Maurnyi C., Szenci O., Horváth A., Borsay B.Á., Herczeg L., Liposits Z., Hrabovszky E. Lateral hypothalamic orexin and melanin-concentrating hormone neurons provide direct input to gonadotropin-releasing hormone neurons in the human. Front. Cell. Neurosci. 2015;9:348. doi: 10.3389/fncel.2015.00348. [http://dx.doi.org/10.3389/fncel.2015.00348]. [PMID: 26388735]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goforth P.B., Myers M.G. Roles for orexin/hypocretin in the control of energy balance and metabolism. Curr. Top. Behav. Neurosci. 2017;33:137–156. doi: 10.1007/7854_2016_51. [PMID: 27909992]. [DOI] [PubMed] [Google Scholar]
- 140.Pierantoni R., Cobellis G., Meccariello R., Fasano S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002;218:69–141. doi: 10.1016/s0074-7696(02)18012-0. [http://dx.doi.org/10.1016/S0074-7696(02)18012-0]. [PMID: 12199520]. [DOI] [PubMed] [Google Scholar]
- 141.Pierantoni R., Cobellis G., Meccariello R., Cacciola G., Chianese R., Chioccarelli T., Fasano S. CB1 activity in male reproduction: mammalian and nonmammalian animal models. Vitam. Horm. 2009;81:367–387. doi: 10.1016/S0083-6729(09)81014-5. [http://dx.doi.org/10.1016/S0083-6729(09)81014-5]. [PMID: 19647119]. [DOI] [PubMed] [Google Scholar]
- 142.Pierantoni R., Cobellis G., Meccariello R., Cacciola G., Chianese R., Chioccarelli T., Fasano S. Testicular gonadotropin-releasing hormone activity, progression of spermatogenesis, and sperm transport in vertebrates. Ann. N. Y. Acad. Sci. 2009;1163:279–291. doi: 10.1111/j.1749-6632.2008.03617.x. [http://dx.doi.org/10.1111/j.1749-6632.2008.03617.x]. [PMID: 19456349]. [DOI] [PubMed] [Google Scholar]
- 143.Chianese R., Chioccarelli T., Cacciola G., Ciaramella V., Fasano S., Pierantoni R., Meccariello R., Cobellis G. The contribution of lower vertebrate animal models in human reproduction research. Gen. Comp. Endocrinol. 2011;171(1):17–27. doi: 10.1016/j.ygcen.2010.12.011. [http://dx.doi.org/10.1016/j.ygcen.2010.12.011]. [PMID: 21192939]. [DOI] [PubMed] [Google Scholar]
- 144.Meccariello R., Chianese R., Chioccarelli T., Ciaramella V., Fasano S., Pierantoni R., Cobellis G. Intra-testicular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front. Endocrinol. (Lausanne) 2014;5:69. doi: 10.3389/fendo.2014.00069. [http://dx.doi.org/10.3389/fendo.2014.00069]. [PMID: 24847312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cornejo M.P., Hentges S.T., Maliqueo M., Coirini H., Becu-Villalobos D., Elias C.F. Neuroendocrine Regulation of Metabolism. J. Neuroendocrinol. 2016;28(7):12395. doi: 10.1111/jne.12395. [http://dx.doi.org/10.1111/jne.12395]. [PMID: 27114114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hill J.W., Elmquist J.K., Elias C.F. Hypothalamic pathways linking energy balance and reproduction. Am. J. Physiol. Endocrinol. Metab. 2008;294(5):E827–E832. doi: 10.1152/ajpendo.00670.2007. [http://dx.doi.org/10. 1152/ajpendo.00670.2007]. [PMID: 18285524]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wynne K., Stanley S., McGowan B., Bloom S. Appetite control. J. Endocrinol. 2005;184(2):291–318. doi: 10.1677/joe.1.05866. [http://dx.doi.org/10.1677/ joe.1.05866]. [PMID: 15684339]. [DOI] [PubMed] [Google Scholar]
- 148.Qian S., Chen H., Weingarth D., Trumbauer M.E., Novi D.E., Guan X., Yu H., Shen Z., Feng Y., Frazier E., Chen A., Camacho R.E., Shearman L.P., Gopal-Truter S., MacNeil D.J., Van der Ploeg L.H., Marsh D.J. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002;22(14):5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [http://dx.doi.org/10.1128/MCB.22.14.5027-5035.2002]. [PMID: 12077332]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T., Plum L., Balthasar N., Hampel B., Waisman A., Barsh G.S., Horvath T.L., Brüning J.C. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8(10):1289–1291. doi: 10.1038/nn1548. [http://dx.doi.org/10.1038/nn1548]. [PMID: 16158063]. [DOI] [PubMed] [Google Scholar]
- 150.Dicken M.S., Hughes A.R., Hentges S.T. Gad1 mRNA as a reliable indicator of altered GABA release from orexigenic neurons in the hypothalamus. Eur. J. Neurosci. 2015;42(9):2644–2653. doi: 10.1111/ejn.13076. [http://dx.doi.org/10.1111/ejn.13076]. [PMID: 26370162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elias C.F., Saper C.B., Maratos-Flier E., Tritos N.A., Lee C., Kelly J., Tatro J.B., Hoffman G.E., Ollmann M.M., Barsh G.S., Sakurai T., Yanagisawa M., Elmquist J.K. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 1998;402(4):442–459. [http://dx.doi.org/10.1002/(SICI)1096-9861(19981228)402:4<442: AID-CNE2>3.0.CO;2-R]. [PMID: 9862320]. [PubMed] [Google Scholar]
- 152.Jarvie B.C., Hentges S.T. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J. Comp. Neurol. 2012;520(17):3863–3876. doi: 10.1002/cne.23127. [http://dx. doi.org/10.1002/cne.23127]. [PMID: 22522889]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hausman G.J., Barb C.R., Lents C.A. Leptin and reproductive function. Biochimie. 2012;94(10):2075–2081. doi: 10.1016/j.biochi.2012.02.022. [http://dx.doi.org/10.1016/j.biochi.2012.02.022]. [PMID: 22980196]. [DOI] [PubMed] [Google Scholar]
- 154.Kotani M., Detheux M., Vandenbogaerde A., Communi D., Vanderwinden J.M., Le Poul E., Brézillon S., Tyldesley R., Suarez-Huerta N., Vandeput F., Blanpain C., Schiffmann S.N., Vassart G., Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001;276(37):34631–34636. doi: 10.1074/jbc.M104847200. [http://dx.doi.org/10.1074/jbc.M104847200]. [PMID: 11457843]. [DOI] [PubMed] [Google Scholar]
- 155.Thompson E.L., Patterson M., Murphy K.G., Smith K.L., Dhillo W.S., Todd J.F., Ghatei M.A., Bloom S.R. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J. Neuroendocrinol. 2004;16(10):850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [http://dx.doi.org/10.1111/j.1365-2826.2004.01240.x]. [PMID: 15500545]. [DOI] [PubMed] [Google Scholar]
- 156.Messager S., Chatzidaki E.E., Ma D., Hendrick A.G., Zahn D., Dixon J., Thresher R.R., Malinge I., Lomet D., Carlton M.B., Colledge W.H., Caraty A., Aparicio S.A. Kisspeptin directly stimulates gonadotropin-releasing hormone release in vivoG protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [http://dx.doi.org/10.1073/pnas.0409330102]. [PMID: 15665093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clarkson J., Herbison A.E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [http://dx.doi.org/10.1210/en.2006-0787]. [PMID: 16959837]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chianese R., Cobellis G., Chioccarelli T., Ciaramella V., Migliaccio M., Fasano S., Pierantoni R., Meccariello R. Kisspeptins, estrogens and male fertility. Curr. Med. Chem. 2016;23(36):4070–4091. doi: 10.2174/0929867323666160902155434. [http://dx.doi.org/10.2174/0929867323666160902155434]. [PMID: 27593959]. [DOI] [PubMed] [Google Scholar]
- 159.Chianese R., Ciaramella V., Fasano S., Pierantoni R., Meccariello R. Kisspeptin receptor, GPR54, as a candidate for the regulation of testicular activity in the frog Rana esculenta. Biol. Reprod. 2013;88(3):73. doi: 10.1095/biolreprod.112.103515. [http://dx.doi.org/10.1095/biolreprod.112.103515]. [PMID: 23365413]. [DOI] [PubMed] [Google Scholar]
- 160.Chianese R., Ciaramella V., Fasano S., Pierantoni R., Meccariello R. Kisspeptin drives germ cell progression in the anuran amphibian Pelophylax esculentus: a study carried out in ex vivo testes. Gen. Comp. Endocrinol. 2015;211:81–91. doi: 10.1016/j.ygcen.2014.11.008. [http://dx.doi.org/10.1016/j.ygcen.2014.11.008]. [PMID: 25452028]. [DOI] [PubMed] [Google Scholar]
- 161.Revel F.G., Ansel L., Klosen P., Saboureau M., Pévet P., Mikkelsen J.D., Simonneaux V. Kisspeptin: a key link to seasonal breeding. Rev. Endocr. Metab. Disord. 2007;8(1):57–65. doi: 10.1007/s11154-007-9031-7. [http://dx.doi.org/10.1007/s11154-007-9031-7]. [PMID: 17380397]. [DOI] [PubMed] [Google Scholar]
- 162.Fernandez-Fernandez R., Martini A.C., Navarro V.M., Castellano J.M., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. Novel signals for the integration of energy balance and reproduction. Mol. Cell. Endocrinol. 2006;254-255:127–132. doi: 10.1016/j.mce.2006.04.026. [http://dx. doi.org/10.1016/j.mce.2006.04.026]. [PMID: 16759792]. [DOI] [PubMed] [Google Scholar]
- 163.Franceschini I., Lomet D., Cateau M., Delsol G., Tillet Y., Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci. Lett. 2006;401(3):225–230. doi: 10.1016/j.neulet.2006.03.039. [http://dx.doi.org/10.1016/ j.neulet.2006.03.039]. [PMID: 16621281]. [DOI] [PubMed] [Google Scholar]
- 164.Castellano J.M., Navarro V.M., Fernández-Fernández R., Nogueiras R., Tovar S., Roa J., Vazquez M.J., Vigo E., Casanueva F.F., Aguilar E., Pinilla L., Dieguez C., Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146(9):3917–3925. doi: 10.1210/en.2005-0337. [http://dx. doi.org/10.1210/en.2005-0337]. [PMID: 15932928]. [DOI] [PubMed] [Google Scholar]
- 165.Finn P.D., Cunningham M.J., Pau K.Y., Spies H.G., Clifton D.K., Steiner R.A. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139(11):4652–4662. doi: 10.1210/endo.139.11.6297. [http://dx.doi.org/10.1210/endo.139.11.6297]. [PMID: 9794477]. [DOI] [PubMed] [Google Scholar]
- 166.Wahab F., Shahab M., Behr R. The involvement of gonadotropin inhibitory hormone and kisspeptin in the metabolic regulation of reproduction. J. Endocrinol. 2015;225(2):R49–R66. doi: 10.1530/JOE-14-0688. [http://dx. doi.org/10.1530/JOE-14-0688]. [PMID: 25957191]. [DOI] [PubMed] [Google Scholar]
- 167.Donato J., Jr, Cravo R.M., Frazão R., Gautron L., Scott M.M., Lachey J., Castro I.A., Margatho L.O., Lee S., Lee C., Richardson J.A., Friedman J., Chua S., Jr, Coppari R., Zigman J.M., Elmquist J.K., Elias C.F. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Invest. 2011;121(1):355–368. doi: 10.1172/JCI45106. [http://dx.doi.org/10.1172/JCI45106]. [PMID: 21183787]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luque R.M., Kineman R.D., Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148(10):4601–4611. doi: 10.1210/en.2007-0500. [http://dx.doi.org/10.1210/en.2007-0500]. [PMID: 17595226]. [DOI] [PubMed] [Google Scholar]
- 169.Castellano J.M., Navarro V.M., Roa J., Pineda R., Sánchez-Garrido M.A., García-Galiano D., Vigo E., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. Alterations in hypothalamic KiSS-1 system in experimental diabetes: early changes and functional consequences. Endocrinology. 2009;150(2):784–794. doi: 10.1210/en.2008-0849. [http://dx.doi.org/10.1210/en.2008-0849]. [PMID: 18845637]. [DOI] [PubMed] [Google Scholar]
- 170.Dudek M., Kołodziejski P.A., Pruszyńska-Oszmałek E., Sassek M., Ziarniak K., Nowak K.W., Sliwowska J.H. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides. 2016;56:41–49. doi: 10.1016/j.npep.2016.01.005. [http://dx.doi.org/10.1016/j.npep.2016.01.005]. [PMID: 26853724]. [DOI] [PubMed] [Google Scholar]
- 171.Léonhardt M., Lesage J., Croix D., Dutriez-Casteloot I., Beauvillain J.C., Dupouy J.P. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol. Reprod. 2003;68(2):390–400. doi: 10.1095/biolreprod.102.003269. [http://dx.doi.org/10.1095/biolreprod.102.003269]. [PMID: 12533401]. [DOI] [PubMed] [Google Scholar]
- 172.Connor K.L., Vickers M.H., Beltrand J., Meaney M.J., Sloboda D.M. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J. Physiol. 2012;590(9):2167–2180. doi: 10.1113/jphysiol.2011.223305. [http://dx.doi.org/10.1113/ jphysiol.2011.223305]. [PMID: 22411006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Takumi K., Shimada K., Iijima N., Ozawa H. Maternal high-fat diet during lactation increases Kiss1 mRNA expression in the arcuate nucleus at weaning and advances puberty onset in female rats. Neurosci. Res. 2015;100:21–28. doi: 10.1016/j.neures.2015.06.004. [http://dx.doi.org/10.1016/ j.neures.2015.06.004]. [PMID: 26094983]. [DOI] [PubMed] [Google Scholar]
- 174.Wren A.M., Seal L.J., Cohen M.A., Brynes A.E., Frost G.S., Murphy K.G., Dhillo W.S., Ghatei M.A., Bloom S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001;86(12):5992. doi: 10.1210/jcem.86.12.8111. [http://dx.doi.org/10.1210/ jcem.86.12.8111]. [PMID: 11739476]. [DOI] [PubMed] [Google Scholar]
- 175.Cummings D.E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol. Behav. 2006;89(1):71–84. doi: 10.1016/j.physbeh.2006.05.022. [http://dx.doi.org/10.1016/j.physbeh.2006.05.022]. [PMID: 16859720]. [DOI] [PubMed] [Google Scholar]
- 176.Tena-Sempere M. Ghrelin: novel regulator of gonadal function. J. Endocrinol. Invest. 2005;28(5) Suppl.:26–29. [PMID: 16114272]. [PubMed] [Google Scholar]
- 177.Martini A.C., Fernández-Fernández R., Tovar S., Navarro V.M., Vigo E., Vazquez M.J., Davies J.S., Thompson N.M., Aguilar E., Pinilla L., Wells T., Dieguez C., Tena-Sempere M. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology. 2006;147(5):2374–2382. doi: 10.1210/en.2005-1422. [http://dx.doi.org/10.1210/en.2005-1422]. [PMID: 16455774]. [DOI] [PubMed] [Google Scholar]
- 178.Fernández-Fernández R., Tena-Sempere M., Aguilar E., Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci. Lett. 2004;362(2):103–107. doi: 10.1016/j.neulet.2004.03.003. [http://dx.doi.org/10.1016/j.neulet.2004.03.003]. [PMID: 15193764]. [DOI] [PubMed] [Google Scholar]
- 179.Leidy H.J., Gardner J.K., Frye B.R., Snook M.L., Schuchert M.K., Richard E.L., Williams N.I. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J. Clin. Endocrinol. Metab. 2004;89(6):2659–2664. doi: 10.1210/jc.2003-031471. [http://dx.doi.org/10.1210/jc.2003-031471]. [PMID: 15181038]. [DOI] [PubMed] [Google Scholar]
- 180.Ackerman K.E., Slusarz K., Guereca G., Pierce L., Slattery M., Mendes N., Herzog D.B., Misra M. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am. J. Physiol. Endocrinol. Metab. 2012;302(7):E800–E806. doi: 10.1152/ajpendo.00598.2011. [http://dx.doi.org/10.1152/ajpendo.00598.2011]. [PMID: 22252944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fuqua J.S., Rogol A.D. Neuroendocrine alterations in the exercising human: implications for energy homeostasis. Metabolism. 2013;62(7):911–921. doi: 10.1016/j.metabol.2013.01.016. [http://dx.doi.org/10.1016/j.metabol.2013. 01.016]. [PMID: 23415825]. [DOI] [PubMed] [Google Scholar]
- 182.Blasbalg T.L., Hibbeln J.R., Ramsden C.E., Majchrzak S.F., Rawlings R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [http://dx.doi.org/10.3945/ajcn.110. 006643]. [PMID: 21367944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cascio M.G. PUFA-derived endocannabinoids: an overview. Proc. Nutr. Soc. 2013;72(4):451–459. doi: 10.1017/S0029665113003418. [http://dx.doi.org/10.1017/ S0029665113003418]. [PMID: 24020830]. [DOI] [PubMed] [Google Scholar]
- 184.Bisogno T., Maccarrone M. Endocannabinoid signaling and its regulation by nutrients. Biofactors. 2014;40(4):373–380. doi: 10.1002/biof.1167. [http://dx.doi.org/10.1002/biof.1167]. [PMID: 24753395]. [DOI] [PubMed] [Google Scholar]
- 185.Matias I., Gonthier M.P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., Roche R., Maj M., Pagotto U., Monteleone P., Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 2006;91(8):3171–3180. doi: 10.1210/jc.2005-2679. [http://dx.doi.org/10.1210/jc.2005-2679]. [PMID: 16684820]. [DOI] [PubMed] [Google Scholar]
- 186.Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J.P., Di Marzo V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007;31(4):692–699. doi: 10.1038/sj.ijo.0803539. [http://dx.doi.org/10.1038/sj.ijo.0803539]. [PMID: 17224929]. [DOI] [PubMed] [Google Scholar]
- 187.Williams C.M., Kirkham T.C. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl.) 1999;143(3):315–317. doi: 10.1007/s002130050953. [http://dx.doi.org/10.1007/ s002130050953]. [PMID: 10353436]. [DOI] [PubMed] [Google Scholar]
- 188.Cristino L., Becker T., Di Marzo V. Endocannabinoids and energy homeostasis: an update. Biofactors. 2014;40(4):389–397. doi: 10.1002/biof.1168. [http://dx.doi.org/10.1002/biof.1168]. [PMID: 24752980]. [DOI] [PubMed] [Google Scholar]
- 189.Di Marzo V., Côté M., Matias I., Lemieux I., Arsenault B.J., Cartier A., Piscitelli F., Petrosino S., Alméras N., Després J.P. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52(2):213–217. doi: 10.1007/s00125-008-1178-6. [http://dx.doi.org/10. 1007/s00125-008-1178-6]. [PMID: 18972095]. [DOI] [PubMed] [Google Scholar]
- 190.Quarta C., Bellocchio L., Mancini G., Mazza R., Cervino C., Braulke L.J., Fekete C., Latorre R., Nanni C., Bucci M., Clemens L.E., Heldmaier G., Watanabe M., Leste-Lassere T., Maitre M., Tedesco L., Fanelli F., Reuss S., Klaus S., Srivastava R.K., Monory K., Valerio A., Grandis A., De Giorgio R., Pasquali R., Nisoli E., Cota D., Lutz B., Marsicano G., Pagotto U. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11(4):273–285. doi: 10.1016/j.cmet.2010.02.015. [http://dx.doi.org/10.1016/j.cmet. 2010.02.015]. [PMID: 20374960]. [DOI] [PubMed] [Google Scholar]
- 191.Ravinet Trillou C., Delgorge C., Menet C., Arnone M., Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. 2004;28(4):640–648. doi: 10.1038/sj.ijo.0802583. [http://dx.doi.org/10.1038/sj.ijo.0802583]. [PMID: 14770190]. [DOI] [PubMed] [Google Scholar]
- 192.Després J.P., Golay A., Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 2005;353(20):2121–2134. doi: 10.1056/NEJMoa044537. [http://dx.doi.org/10.1056/NEJMoa044537]. [PMID: 16291982]. [DOI] [PubMed] [Google Scholar]
- 193.Kim J., Li Y., Watkins B.A. Fat to treat fat: emerging relationship between dietary PUFA, endocannabinoids, and obesity. Prostaglandins Other Lipid Mediat. 2013;104-105:32–41. doi: 10.1016/j.prostaglandins.2012.11.005. [http://dx.doi.org/10.1016/j.prostaglandins.2012.11.005]. [PMID: 23466458]. [DOI] [PubMed] [Google Scholar]
- 194.Kim H.Y., Spector A.A. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88(1):121–125. doi: 10.1016/j.plefa.2012.08.002. [http://dx.doi.org/10.1016/j.plefa.2012.08.002]. [PMID: 22959887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Batetta B., Griinari M., Carta G., Murru E., Ligresti A., Cordeddu L., Giordano E., Sanna F., Bisogno T., Uda S., Collu M., Bruheim I., Di Marzo V., Banni S. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J. Nutr. 2009;139(8):1495–1501. doi: 10.3945/jn.109.104844. [http://dx.doi.org/10.3945/jn.109.104844]. [PMID: 19549757]. [DOI] [PubMed] [Google Scholar]
- 196.Osei-Hyiaman D., Depetrillo M., Harvey-White J., Bannon A.W., Cravatt B.F., Kuhar M.J., Mackie K., Palkovits M., Kunos G. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81(4):273–282. doi: 10.1159/000087925. [http://dx.doi.org/10.1159/000087925]. [PMID: 16131814]. [DOI] [PubMed] [Google Scholar]
- 197.Suglia A., Chianese R., Migliaccio M., Ambrosino C., Fasano S., Pierantoni R., Cobellis G., Chioccarelli T. Bisphenol A induces hypothalamic down-regulation of the the cannabinoid receptor 1 and anorexigenic effects in male mice. 2016. [DOI] [PubMed]
- 198.Bellocchio L., Lafenêtre P., Cannich A., Cota D., Puente N., Grandes P., Chaouloff F., Piazza P.V., Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010;13(3):281–283. doi: 10.1038/nn.2494. [http://dx.doi.org/10.1038/ nn.2494]. [PMID: 20139974]. [DOI] [PubMed] [Google Scholar]
- 199.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G.I., Palmiter R.D., Sugiura T., Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [http://dx.doi.org/10.1038/35071088]. [PMID: 11298451]. [DOI] [PubMed] [Google Scholar]
- 200.Cani P.D., Montoya M.L., Neyrinck A.M., Delzenne N.M., Lambert D.M. Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br. J. Nutr. 2004;92(5):757–761. doi: 10.1079/bjn20041256. [http://dx.doi.org/10.1079/BJN20041256]. [PMID: 15533263]. [DOI] [PubMed] [Google Scholar]
- 201.Kirkham T.C., Williams C.M., Fezza F., Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002;136(4):550–557. doi: 10.1038/sj.bjp.0704767. [http://dx.doi.org/10.1038/sj.bjp.0704767]. [PMID: 12055133]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A.B., Fekete C., Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3(3):e1797. doi: 10.1371/journal.pone.0001797. [http://dx.doi.org/10.1371/ journal.pone.0001797]. [PMID: 18335063]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gammon C.M., Freeman G.M., Jr, Xie W., Petersen S.L., Wetsel W.C. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology. 2005;146(10):4491–4499. doi: 10.1210/en.2004-1672. [http:// dx.doi.org/10.1210/en.2004-1672]. [PMID: 16020480]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Scorticati C., Fernández-Solari J., De Laurentiis A., Mohn C., Prestifilippo J.P., Lasaga M., Seìlicovich A., Billi S., Franchi A., McCann S.M., Rettori V. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc. Natl. Acad. Sci. USA. 2004;101(32):11891–11896. doi: 10.1073/pnas.0404366101. [http://dx.doi.org/10.1073/pnas.0404366101]. [PMID: 15280536]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Meccariello R., Chianese R., Cacciola G., Cobellis G., Pierantoni R., Fasano S. Type-1 cannabinoid receptor expression in the frog, Rana esculenta, tissues: a possible involvement in the regulation of testicular activity. Mol. Reprod. Dev. 2006;73(5):551–558. doi: 10.1002/mrd.20434. [http://dx.doi.org/10.1002/mrd.20434]. [PMID: 16485273]. [DOI] [PubMed] [Google Scholar]
- 206.Meccariello R., Franzoni M.F., Chianese R., Cottone E., Scarpa D., Donna D., Cobellis G., Guastalla A., Pierantoni R., Fasano S. Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology. 2008;149(5):2149–2158. doi: 10.1210/en.2007-1357. [http://dx.doi.org/10.1210/en.2007-1357]. [PMID: 18218699]. [DOI] [PubMed] [Google Scholar]
- 207.Chianese R., Ciaramella V., Fasano S., Pierantoni R., Meccariello R. Anandamide modulates the expression of GnRH-II and GnRHRs in frog, Rana esculenta, diencephalon. Gen. Comp. Endocrinol. 2011;173(3):389–395. doi: 10.1016/j.ygcen.2011.07.001. [http://dx.doi.org/10.1016/j.ygcen. 2011.07.001]. [PMID: 21802420]. [DOI] [PubMed] [Google Scholar]
- 208.Chianese R., Ciaramella V., Scarpa D., Fasano S., Pierantoni R., Meccariello R. Anandamide regulates the expression of GnRH1, GnRH2, and GnRH-Rs in frog testis. Am. J. Physiol. Endocrinol. Metab. 2012;303(4):E475–E487. doi: 10.1152/ajpendo.00086.2012. [http://dx.doi.org/10. 1152/ajpendo.00086.2012]. [PMID: 22669247]. [DOI] [PubMed] [Google Scholar]
- 209.Chianese R., Ciaramella V., Scarpa D., Fasano S., Pierantoni R., Meccariello R. Endocannabinoids and endovanilloids: a possible balance in the regulation of the testicular GnRH signalling. Int. J. Endocrinol. 2013;2013:904748. doi: 10.1155/2013/904748. [http://dx.doi.org/10.1155/2013/904748]. [PMID: 24072997]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ciaramella V., Meccariello R., Chioccarelli T., Sirleto M., Fasano S., Pierantoni R., Chianese R. Anandamide acts in vivokisspeptin in the regulation of testicular activity of the frog, Pelophylax esculentus. Mol. Cell. Endocrinol. 2016;420:75–84. doi: 10.1016/j.mce.2015.11.011. [http://dx.doi.org/10.1016/j.mce.2015.11.011]. [PMID: 26586207]. [DOI] [PubMed] [Google Scholar]
- 211.Wenger T., Ledent C., Csernus V., Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem. Biophys. Res. Commun. 2001;284(2):363–368. doi: 10.1006/bbrc.2001.4977. [http://dx.doi.org/10.1006/bbrc.2001.4977]. [PMID: 11394887]. [DOI] [PubMed] [Google Scholar]
- 212.Chianese R., Ciaramella V., Fasano S., Pierantoni R., Meccariello R. Hypothalamus-pituitary axis: an obligatory target for endocannabinoids to inhibit steroidogenesis in frog testis. Gen. Comp. Endocrinol. 2014;205:88–93. doi: 10.1016/j.ygcen.2014.02.010. [http://dx.doi.org/10.1016/j.ygcen. 2014.02.010]. [PMID: 24566122]. [DOI] [PubMed] [Google Scholar]
- 213.Meccariello R., Battista N., Bradshawa H.B., Wang H. Endocannabinoids and reproduction. Int. J. Endocrinol. 2014;2014:378069. doi: 10.1155/2014/378069. [http://dx.doi.org/10.1155/2014/378069]. [PMID: 24575130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cacciola G., Chioccarelli T., Fasano S., Pierantoni R., Cobellis G. Estrogens and spermiogenesis: new insights from type 1 cannabinoid receptor knockout mice. Int. J. Endocrinol. 2013;2013:501350. doi: 10.1155/2013/501350. [http://dx.doi.org/10.1155/2013/501350]. [PMID: 24324492]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cacciola G., Chioccarelli T., Altucci L., Ledent C., Mason J.I., Fasano S., Pierantoni R., Cobellis G. Low 17beta-estradiol levels in CNR1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biol. Reprod. 2013;88(6):152. doi: 10.1095/biolreprod.112.105726. [http://dx.doi.org/10.1095/biolreprod.112.105726]. [PMID: 23677985]. [DOI] [PubMed] [Google Scholar]
- 216.Karamikheirabad M., Behzadi G., Faghihi M., Raoofian R., Ejtemaei Mehr S., Zuure W.A., Sadeghipour H.R. A role for endocannabinoids in acute stress-induced suppression of the hypothalamic-pituitary-gonadal axis in male rats. Clin. Exp. Reprod. Med. 2013;40(4):155–162. doi: 10.5653/cerm.2013.40.4.155. [http://dx.doi.org/10.5653/cerm.2013.40.4.155]. [PMID: 24505561]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Joffre C., Nadjar A., Lebbadi M., Calon F., Layé S. n-3 LCPUFA improves cognition: the young, the old and the sick. Prostaglandins Leukot. Essent. Fatty Acids. 2014;91(1-2):1–20. doi: 10.1016/j.plefa.2014.05.001. [http://dx.doi.org/10.1016/j.plefa.2014.05.001]. [PMID: 24908517]. [DOI] [PubMed] [Google Scholar]
- 218.Bazan N.G. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263–271. doi: 10.1016/j.tins.2006.03.005. [http://dx.doi.org/10.1016/j.tins.2006.03.005]. [PMID: 16580739]. [DOI] [PubMed] [Google Scholar]
- 219.Calon F., Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77(5-6):287–293. doi: 10.1016/j.plefa.2007.10.019. [http://dx.doi.org/10.1016/j.plefa.2007.10.019]. [PMID: 18037281]. [DOI] [PubMed] [Google Scholar]
- 220.Dyall S.C., Michael-Titus A.T. Neurological benefits of omega-3 fatty acids. Neuromol. Med. 2008;10(4):219–235. doi: 10.1007/s12017-008-8036-z. [http://dx.doi.org/10.1007/s12017-008-8036-z]. [PMID: 18543124]. [DOI] [PubMed] [Google Scholar]
- 221.Carrié I., Abellan Van Kan G., Rolland Y., Gillette-Guyonnet S., Vellas B. PUFA for prevention and treatment of dementia? Curr. Pharm. Des. 2009;15(36):4173–4185. doi: 10.2174/138161209789909764. [http://dx.doi.org/10.2174/ 138161209789909764]. [PMID: 20041819]. [DOI] [PubMed] [Google Scholar]
- 222.Cunnane S.C., Chouinard-Watkins R., Castellano C.A., Barberger-Gateau P. Docosahexaenoic acid homeostasis, brain aging and Alzheimer’s disease: Can we reconcile the evidence? Prostaglandins Leukot. Essent. Fatty Acids. 2013;88(1):61–70. doi: 10.1016/j.plefa.2012.04.006. [http://dx. doi.org/10.1016/j.plefa.2012.04.006]. [PMID: 22575581]. [DOI] [PubMed] [Google Scholar]
- 223.Salem N., Jr, Vandal M., Calon F. The benefit of docosahexaenoic acid for the adult brain in aging and dementia. Prostaglandins Leukot. Essent. Fatty Acids. 2015;92:15–22. doi: 10.1016/j.plefa.2014.10.003. [http://dx.doi.org/10.1016/j.plefa.2014.10.003]. [PMID: 25457546]. [DOI] [PubMed] [Google Scholar]
- 224.Dyall S.C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [http://dx.doi.org/10.3389/ fnagi.2015.00052]. [PMID: 25954194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Denis I., Potier B., Vancassel S., Heberden C., Lavialle M. Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms. Ageing Res. Rev. 2013;12(2):579–594. doi: 10.1016/j.arr.2013.01.007. [http://dx.doi.org/10.1016/j.arr.2013.01.007]. [PMID: 23395782]. [DOI] [PubMed] [Google Scholar]
- 226.Palacios-Pelaez R., Lukiw W.J., Bazan N.G. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol. Neurobiol. 2010;41(2-3):367–374. doi: 10.1007/s12035-010-8139-z. [http://dx. doi.org/10.1007/s12035-010-8139-z]. [PMID: 20467837]. [DOI] [PubMed] [Google Scholar]
- 227.Cole G.M., Frautschy S.A. DHA may prevent age-related dementia. J. Nutr. 2010;140(4):869–874. doi: 10.3945/jn.109.113910. [http://dx.doi.org/10.3945/ jn.109.113910]. [PMID: 20181786]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Joffre C., Grégoire S., De Smedt V., Acar N., Bretillon L., Nadjar A., Layé S. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot. Essent. Fatty Acids. 2016;114:1–10. doi: 10.1016/j.plefa.2016.09.003. [http://dx.doi.org/10.1016/j.plefa.2016.09. 003]. [PMID: 27926457]. [DOI] [PubMed] [Google Scholar]
- 229.Plourde M., Cunnane S.C. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007;32(4):619–634. doi: 10.1139/H07-034. [http://dx.doi.org/10.1139/H07-034]. [PMID: 17622276]. [DOI] [PubMed] [Google Scholar]
- 230.DeMar J.C., Jr, Lee H.J., Ma K., Chang L., Bell J.M., Rapoport S.I., Bazinet R.P. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim. Biophys. Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [PMID: 16920015]. [DOI] [PubMed] [Google Scholar]
- 231.Alashmali S.M., Hopperton K.E., Bazinet R.P. Lowering dietary n-6 polyunsaturated fatty acids: interaction with brain arachidonic and docosahexaenoic acids. Curr. Opin. Lipidol. 2016;27(1):54–66. doi: 10.1097/MOL.0000000000000255. [http://dx.doi.org/10.1097/MOL.0000000000000255]. [PMID: 26709472]. [DOI] [PubMed] [Google Scholar]
- 232.Domenichiello A.F., Kitson A.P., Chen C.T., Trépanier M.O., Stavro P.M., Bazinet R.P. The effect of linoleic acid on the whole body synthesis rates of polyunsaturated fatty acids from α-linolenic acid and linoleic acid in free-living rats. J. Nutr. Biochem. 2016;30:167–176. doi: 10.1016/j.jnutbio.2015.11.016. [http://dx.doi.org/10.1016/j.jnutbio.2015.11.016]. [PMID: 27012633]. [DOI] [PubMed] [Google Scholar]
- 233.Minihane A.M. Impact of genotype on EPA and DHA status and responsiveness to increased intakes. Nutrients. 2016;8(3):123. doi: 10.3390/nu8030123. [http://dx.doi.org/10.3390/nu8030123]. [PMID: 26950146]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kitson A.P., Stroud C.K., Stark K.D. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45(3):209–224. doi: 10.1007/s11745-010-3391-6. [http://dx.doi.org/10.1007/s11745-010-3391-6]. [PMID: 20151220]. [DOI] [PubMed] [Google Scholar]
- 235.Decsi T., Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011;94(6) Suppl.:1914S–1919S. doi: 10.3945/ajcn.110.000893. [http://dx.doi.org/10.3945/ajcn.110.000893]. [PMID: 22089435]. [DOI] [PubMed] [Google Scholar]
- 236.Song B.J., Elbert A., Rahman T., Orr S.K., Chen C.T., Febbraio M., Bazinet R.P. Genetic ablation of CD36 does not alter mouse brain polyunsaturated fatty acid concentrations. Lipids. 2010;45(4):291–299. doi: 10.1007/s11745-010-3398-z. [http://dx.doi.org/10.1007/s11745-010-3398-z]. [PMID: 20306148]. [DOI] [PubMed] [Google Scholar]
- 237.Nguyen L.N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., Wenk M.R., Goh E.L., Silver D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–506. doi: 10.1038/nature13241. [http://dx.doi.org/10.1038/ nature13241]. [PMID: 24828044]. [DOI] [PubMed] [Google Scholar]
- 238.Rice D.S., Calandria J.M., Gordon W.C., Jun B., Zhou Y., Gelfman C.M., Li S., Jin M., Knott E.J., Chang B., Abuin A., Issa T., Potter D., Platt K.A., Bazan N.G. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat. Commun. 2015;6:6228. doi: 10.1038/ncomms7228. [http://dx.doi.org/10.1038/ ncomms7228]. [PMID: 25736573]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vauzour D., Martinsen A., Layé S. Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem. Int. 2015;89:63–74. doi: 10.1016/j.neuint.2015.08.004. [http://dx.doi.org/10.1016/j. neuint.2015.08.004]. [PMID: 26260547]. [DOI] [PubMed] [Google Scholar]
- 240.Burke J.E., Dennis E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009;50(Suppl.):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [http://dx.doi.org/10.1194/jlr.R800033-JLR200]. [PMID: 19011112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [http://dx.doi.org/10.1038/nature13479]. [PMID: 24899309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015;16(3):229–236. doi: 10.1038/ni.3102. [http://dx.doi.org/10.1038/ni.3102]. [PMID: 25689443]. [DOI] [PubMed] [Google Scholar]
- 243.Perry V.H., Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10(4):217–224. doi: 10.1038/nrneurol.2014.38. [http://dx.doi.org/10.1038/nrneurol.2014.38]. [PMID: 24638131]. [DOI] [PubMed] [Google Scholar]
- 244.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [http://dx.doi.org/10.1038/nn1997]. [PMID: 17965659]. [DOI] [PubMed] [Google Scholar]
- 245.Ransohoff R.M., Perry V.H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [http://dx.doi.org/10.1146/annurev.immunol.021908.132528]. [PMID: 19302036]. [DOI] [PubMed] [Google Scholar]
- 246.Tremblay M.È., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. The role of microglia in the healthy brain. J. Neurosci. 2011;31(45):16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [http://dx.doi.org/10.1523/ JNEUROSCI.4158-11.2011]. [PMID: 22072657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hong S., Dissing-Olesen L., Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [http://dx.doi.org/10.1016/j.conb. 2015.12.004]. [PMID: 26745839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Delpech J.C., Madore C., Nadjar A., Joffre C., Wohleb E.S., Layé S. 2015. [DOI] [PubMed]
- 249.Nadjar A., Leyrolle Q., Joffre C., Layé S. Bioactive lipids as new class of microglial modulators: When nutrition meets neuroimunology. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;104-105:32–41. doi: 10.1016/j.pnpbp.2016.07.004. [PMID: 27392882]. [DOI] [PubMed] [Google Scholar]
- 250.Madore C., Leyrolle Q., Lacabanne C., Benmammar-Badel A., Joffre C., Nadjar A., Layé S. Neuroinflammation in autism: Plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural Plast. 2016;2016:3597209. doi: 10.1155/2016/3597209. [http://dx.doi.org/10. 1155/2016/3597209]. [PMID: 27840741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [http://dx.doi.org/10.1126/science.aag2590]. [PMID: 27540165]. [DOI] [PubMed] [Google Scholar]
- 252.Lukiw W.J., Bazan N.G. Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem. Res. 2000;25(9-10):1173–1184. doi: 10.1023/a:1007627725251. [http://dx.doi.org/10.1023/A:1007627725251]. [PMID: 11059791]. [DOI] [PubMed] [Google Scholar]
- 253.Sil S., Ghosh T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer’s Disease. J. Neuroimmunol. 2016;291:115–124. doi: 10.1016/j.jneuroim.2015.12.003. [http://dx.doi.org/10.1016/j.jneuroim.2015.12.003]. [PMID: 26857505]. [DOI] [PubMed] [Google Scholar]
- 254.Cunningham C., Skelly D.T. Non-steroidal anti-inflammatory drugs and cognitive function: are prostaglandins at the heart of cognitive impairment in dementia and delirium? J. Neuroimmune Pharmacol. 2012;7(1):60–73. doi: 10.1007/s11481-011-9312-5. [http://dx.doi.org/10.1007/s11481-011-9312-5]. [PMID: 21932048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Etminan M., Gill S., Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327(7407):128. doi: 10.1136/bmj.327.7407.128. [http://dx.doi.org/10.1136/bmj.327.7407.128]. [PMID: 12869452]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Johansson J.U., Woodling N.S., Wang Q., Panchal M., Liang X., Trueba-Saiz A., Brown H.D., Mhatre S.D., Loui T., Andreasson K.I. Prostaglandin signaling suppresses beneficial micro- glial function in Alzheimer’s disease models. J. Clin. Invest. 2015;125(1):350–364. doi: 10.1172/JCI77487. [http://dx.doi.org/10.1172/JCI77487]. [PMID: 25485684]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Manev H., Chen H., Dzitoyeva S., Manev R. Cyclooxygenases and 5-lipoxygenase in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(2):315–319. doi: 10.1016/j.pnpbp.2010.07.032. [http://dx.doi.org/10.1016/j.pnpbp.2010.07.032]. [PMID: 20691748]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Czapski G.A., Czubowicz K., Strosznajder J.B., Strosznajder R.P. The Lipoxygenases: Their Regulation and Implication in Alzheimer’s Disease. Neurochem. Res. 2016;41(1-2):243–257. doi: 10.1007/s11064-015-1776-x. [http://dx.doi.org/10.1007/s11064-015-1776-x]. [PMID: 26677076]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kong W., Hooper K.M., Ganea D. The natural dual cyclooxygenase and 5-lipoxygenase inhibitor flavocoxid is protective in EAE through effects on Th1/Th17 differentiation and macrophage/microglia activation. Brain Behav. Immun. 2016;53:59–71. doi: 10.1016/j.bbi.2015.11.002. [http://dx.doi.org/10.1016/j.bbi.2015.11.002]. [PMID: 26541818]. [DOI] [PubMed] [Google Scholar]
- 260.Bazan N.G. The docosanoid neuroprotectin D1 induces homeostatic regulation of neuroinflammation and cell survival. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88(1):127–129. doi: 10.1016/j.plefa.2012.08.008. [http://dx.doi.org/10.1016/j.plefa.2012.08.008]. [PMID: 23022417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.De Smedt-Peyrusse V., Sargueil F., Moranis A., Harizi H., Mongrand S., Layé S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008;105(2):296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.05129.x]. [PMID: 18021297]. [DOI] [PubMed] [Google Scholar]
- 262.Madore C., Nadjar A., Delpech J.C., Sere A., Aubert A., Portal C., Joffre C., Layé S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav. Immun. 2014;41:22–31. doi: 10.1016/j.bbi.2014.03.021. [http://dx.doi.org/10.1016/j.bbi.2014.03.021]. [PMID: 24735929]. [DOI] [PubMed] [Google Scholar]
- 263.Rey C., Nadjar A., Buaud B., Vaysse C., Aubert A., Pallet V., Layé S., Joffre C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 2016;55:249–259. doi: 10.1016/j.bbi.2015.12.013. [http://dx.doi.org/10.1016/j.bbi.2015.12.013]. [PMID: 26718448]. [DOI] [PubMed] [Google Scholar]
- 264.Orr S.K., Palumbo S., Bosetti F., Mount H.T., Kang J.X., Greenwood C.E., Ma D.W., Serhan C.N., Bazinet R.P. Unesterified docosahexaenoic acid is protective in neuroinflammation. J. Neurochem. 2013;127(3):378–393. doi: 10.1111/jnc.12392. [http://dx.doi.org/10.1111/ jnc.12392]. [PMID: 23919613]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhu M., Wang X., Hjorth E., Colas R.A., Schroeder L., Granholm A.C., Serhan C.N., Schultzberg M. Pro-resolving lipid mediators improve neuronal survival and increase Aβ42 phagocytosis. Mol. Neurobiol. 2016;53(4):2733–2749. doi: 10.1007/s12035-015-9544-0. [http://dx.doi.org/10.1007/s12035-015-9544-0]. [PMID: 26650044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Freund-Levi Y., Hjorth E., Lindberg C., Cederholm T., Faxen-Irving G., Vedin I., Palmblad J., Wahlund L.O., Schultzberg M., Basun H., Eriksdotter Jönhagen M. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer’s disease: the OmegAD study. Dement. Geriatr. Cogn. Disord. 2009;27(5):481–490. doi: 10.1159/000218081. [http://dx.doi.org/10.1159/000218081]. [PMID: 19439966]. [DOI] [PubMed] [Google Scholar]
- 267.Wang X., Puerta E., Cedazo-Minguez A., Hjorth E., Schultzberg M. Insufficient resolution response in the hippocampus of a senescence-accelerated mouse model--SAMP8. J. Mol. Neurosci. 2015;55(2):396–405. doi: 10.1007/s12031-014-0346-z. [http://dx.doi.org/10.1007/s12031-014-0346-z]. [PMID: 24913689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yuki D., Sugiura Y., Zaima N., Akatsu H., Takei S., Yao I., Maesako M., Kinoshita A., Yamamoto T., Kon R., Sugiyama K., Setou M. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer’s disease. Sci. Rep. 2014;4:7130. doi: 10.1038/srep07130. [http://dx.doi.org/10.1038/ srep07130]. [PMID: 25410733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hjorth E., Zhu M., Toro V.C., Vedin I., Palmblad J., Cederholm T., Freund-Levi Y., Faxen-Irving G., Wahlund L.O., Basun H., Eriksdotter M., Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimers Dis. 2013;35(4):697–713. doi: 10.3233/JAD-130131. [PMID: 23481688]. [DOI] [PubMed] [Google Scholar]
- 270.Hopperton K.E., Trépanier M.O., Giuliano V., Bazinet R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-β 1-40 in mice. J. Neuroinflammation. 2016;13(1):257. doi: 10.1186/s12974-016-0721-5. [http://dx.doi.org/10.1186/s12974-016-0721-5]. [PMID: 27688126]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Di Marzo V., Stella N., Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015;16(1):30–42. doi: 10.1038/nrn3876. [http://dx.doi.org/10.1038/nrn3876]. [PMID: 25524120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Alhouayek M., Muccioli G.G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 2014;35(6):284–292. doi: 10.1016/j.tips.2014.03.001. [http://dx.doi.org/10.1016/j.tips.2014. 03.001]. [PMID: 24684963]. [DOI] [PubMed] [Google Scholar]
- 273.Lee J.W., Huang B.X., Kwon H., Rashid M.A., Kharebava G., Desai A., Patnaik S., Marugan J., Kim H.Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 2016;7:13123. doi: 10.1038/ncomms13123. [http://dx.doi.org/10.1038/ncomms13123]. [PMID: 27759003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., Sharkey K.A., Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36(5):277–296. doi: 10.1016/j.tips.2015.02.008. [http://dx.doi.org/10.1016/j.tips.2015.02.008]. [PMID: 25796370]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Larrieu T., Madore C., Joffre C., Layé S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J. Physiol. Biochem. 2012;68(4):671–681. doi: 10.1007/s13105-012-0179-6. [http://dx.doi.org/10.1007/s13105-012-0179-6]. [PMID: 22707188]. [DOI] [PubMed] [Google Scholar]
- 276.Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76(1):70–81. doi: 10.1016/j.neuron.2012.09.020. [http://dx.doi.org/10.1016/j.neuron.2012.09.020]. [PMID: 23040807]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Han J., Kesner P., Metna-Laurent M., Duan T., Xu L., Georges F., Koehl M., Abrous D.N., Mendizabal-Zubiaga J., Grandes P., Liu Q., Bai G., Wang W., Xiong L., Ren W., Marsicano G., Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148(5):1039–1050. doi: 10.1016/j.cell.2012.01.037. [http://dx.doi.org/10.1016/j.cell.2012.01.037]. [PMID: 22385967]. [DOI] [PubMed] [Google Scholar]
- 278.Bosch-Bouju C., Layé S. Dietary omega-6/omega-3 and endocannabinoids: Implications for brain health and diseases. Cannabinoids in Health and Disease; 2016. pp. 111–142. [http://dx.doi.org/10.5772/ 62498] [Google Scholar]
- 279.Yassine H.N., Braskie M.N., Mack W.J., Castor K.J., Fonteh A.N., Schneider L.S., Harrington M.G., Chui H.C. Association of docosahexaenoic acid supplementation with alzheimer disease stage in apolipoprotein E ε4 carriers: A review. JAMA Neurol. 2017;74(3):339–347. doi: 10.1001/jamaneurol.2016.4899. [http://dx.doi.org/10.1001/jamaneurol.2016. 4899]. [PMID: 28114437]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Quinn J.F., Raman R., Thomas R.G., Yurko-Mauro K., Nelson E.B., Van Dyck C., Galvin J.E., Emond J., Jack C.R., Jr, Weiner M., Shinto L., Aisen P.S. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [http://dx.doi.org/10.1001/jama.2010.1510]. [PMID: 21045096]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yurko-Mauro K., Alexander D.D., Van Elswyk M.E. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120391. doi: 10.1371/journal.pone.0120391. [http://dx.doi.org/10.1371/journal.pone.0120391]. [PMID: 25786262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jackson P.A., Deary M.E., Reay J.L., Scholey A.B., Kennedy D.O. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18-35 years. Br. J. Nutr. 2012;107(8):1232–1243. doi: 10.1017/S000711451100403X. [http://dx.doi.org/10.1017/S000711451100403X]. [PMID: 21864417]. [DOI] [PubMed] [Google Scholar]
- 283.Benton D., Donohoe R.T., Clayton D.E., Long S.J. Supplementation with DHA and the psychological functioning of young adults. Br. J. Nutr. 2013;109(1):155–161. doi: 10.1017/S0007114512000566. [http://dx.doi.org/10. 1017/S0007114512000566]. [PMID: 22715808]. [DOI] [PubMed] [Google Scholar]
- 284.Chew E.Y., Clemons T.E., Agrón E., Launer L.J., Grodstein F., Bernstein P.S. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: The AREDS2 randomized clinical trial. JAMA. 2015;314(8):791–801. doi: 10.1001/jama.2015.9677. [http://dx. doi.org/10.1001/jama.2015.9677]. [PMID: 26305649]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Witte A.V., Kerti L., Hermannstädter H.M., Fiebach J.B., Schreiber S.J., Schuchardt J.P., Hahn A., Flöel A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex. 2014;24(11):3059–3068. doi: 10.1093/cercor/bht163. [http://dx.doi.org/10.1093/cercor/bht163]. [PMID: 23796946]. [DOI] [PubMed] [Google Scholar]
- 286.Boespflug E.L., McNamara R.K., Eliassen J.C., Schidler M.D., Krikorian R. Fish oil supplementation increases event-related posterior cingulate activation in older adults with subjective memory impairment. J. Nutr. Health Aging. 2016;20(2):161–169. doi: 10.1007/s12603-015-0609-6. [http://dx.doi.org/10.1007/s12603-015-0609-6]. [PMID: 26812512]. [DOI] [PubMed] [Google Scholar]
- 287.Pase M.P., Grima N., Cockerell R., Stough C., Scholey A. ; Sali A., Pipingas A. The effects of long-chain omega-3 fish oils and multivitamins on cognitive and cardiovascular function: a randomized, controlled clinical trial. J. Am. Coll. Nutr. 2015;34(1):21–31. doi: 10.1080/07315724.2014.880660. [http://dx.doi.org/10.1080/07315724.2014.880660]. [PMID: 25565485]. [DOI] [PubMed] [Google Scholar]
- 288.Strike S.C., Carlisle A., Gibson E.L., Dyall S.C. A high omega-3 fatty acid multinutrient supplement benefits cognition and mobility in older women: A randomized, double-blind, placebo-controlled pilot study. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(2):236–242. doi: 10.1093/gerona/glv109. [http://dx.doi.org/10.1093/gerona/glv109]. [PMID: 26265727]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Külzow N., Witte A.V., Kerti L., Grittner U., Schuchardt J.P., Hahn A., Flöel A. Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J. Alzheimers Dis. 2016;51(3):713–725. doi: 10.3233/JAD-150886. [http://dx.doi.org/10.3233/JAD-150886]. [PMID: 26890759]. [DOI] [PubMed] [Google Scholar]
- 290.Jackson P.A., Forster J.S., Bell J.G., Dick J.R., Younger I., Kennedy D.O. DHA supplementation alone or in combination with other nutrients does not modulate cerebral hemodynamics or cognitive function in healthy older adults. Nutrients. 2016;8(2):86. doi: 10.3390/nu8020086. [http://dx.doi.org/10.3390/nu8020086]. [PMID: 26867200]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nilsson A., Radeborg K., Salo I., Björck I. Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: a randomized controlled cross-over study. Nutr. J. 2012;11:99. doi: 10.1186/1475-2891-11-99. [http://dx.doi.org/10.1186/1475-2891-11-99]. [PMID: 23173831]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A., Salem N., Jr, Stedman M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6(6):456–464. doi: 10.1016/j.jalz.2010.01.013. [http://dx.doi.org/10.1016/j.jalz.2010.01.013]. [PMID: 20434961]. [DOI] [PubMed] [Google Scholar]
- 293.Andrieu S., Guyonnet S., Coley N., Cantet C., Bonnefoy M., Bordes S., Bories L., Cufi M.N., Dantoine T., Dartigues J.F., Desclaux F., Gabelle A., Gasnier Y., Pesce A., Sudres K., Touchon J., Robert P., Rouaud O., Legrand P., Payoux P., Caubere J.P., Weiner M., Carrié I., Ousset P.J., Vellas B. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389. doi: 10.1016/S1474-4422(17)30040-6. [http://dx.doi.org/10.1016/S1474-4422(17)30040-6]. [PMID: 28359749]. [DOI] [PubMed] [Google Scholar]
- 294.Terano T., Fujishiro S., Ban T., Yamamoto K., Tanaka T., Noguchi Y., Tamura Y., Yazawa K., Hirayama T. Docosahexaenoic acid supplementation improves the moderately severe dementia from thrombotic cerebrovascular diseases. Lipids. 1999;34(Suppl.):S345–S346. doi: 10.1007/BF02562338. [http://dx.doi.org/10.1007/BF02562338]. [PMID: 10419198]. [DOI] [PubMed] [Google Scholar]
- 295.Mazereeuw G., Herrmann N., Oh P.I., Ma D.W., Wang C.T., Kiss A., Lanctôt K.L. Omega-3 fatty acids, depressive symptoms, and cognitive performance in patients with coronary artery disease: Analyses from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2016;36(5):436–444. doi: 10.1097/JCP.0000000000000565. [http://dx.doi.org/10.1097/JCP.0000000000000565]. [PMID: 27529771]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Phillips M.A., Childs C.E., Calder P.C., Rogers P.J. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable alzheimer’s disease: A randomised controlled trial. Int. J. Mol. Sci. 2015;16(10):24600–24613. doi: 10.3390/ijms161024600. [http://dx.doi.org/10.3390/ijms161024600]. [PMID: 26501267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shinto L., Quinn J., Montine T., Dodge H.H., Woodward W., Baldauf-Wagner S., Waichunas D., Bumgarner L., Bourdette D., Silbert L., Kaye J. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 2014;38(1):111–120. doi: 10.3233/JAD-130722. [PMID: 24077434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lee L.K., Shahar S., Rajab N., Yusoff N.A., Jamal R.A., Then S.M. The role of long chain omega-3 polyunsaturated fatty acids in reducing lipid peroxidation among elderly patients with mild cognitive impairment: a case-control study. J. Nutr. Biochem. 2013;24(5):803–808. doi: 10.1016/j.jnutbio.2012.04.014. [http://dx.doi.org/10.1016/j.jnutbio.2012.04.014]. [PMID: 22898566]. [DOI] [PubMed] [Google Scholar]
- 299.Scheltens P., Twisk J.W., Blesa R., Scarpini E., von Arnim C.A., Bongers A., Harrison J., Swinkels S.H., Stam C.J., de Waal H., Wurtman R.J., Wieggers R.L., Vellas B., Kamphuis P.J. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J. Alzheimers Dis. 2012;31(1):225–236. doi: 10.3233/JAD-2012-121189. [PMID: 22766770]. [DOI] [PubMed] [Google Scholar]
- 300.Eriksdotter M., Vedin I., Falahati F., Freund-Levi Y., Hjorth E., Faxen-Irving G., Wahlund L.O., Schultzberg M., Basun H., Cederholm T., Palmblad J. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s Disease patients during oral omega-3 fatty acid supplementation: The OmegAD study. J. Alzheimers Dis. 2015;48(3):805–812. doi: 10.3233/JAD-150102. [http://dx.doi.org/10.3233/ JAD-150102]. [PMID: 26402079]. [DOI] [PubMed] [Google Scholar]
- 301.Burckhardt M., Herke M., Wustmann T., Watzke S., Langer G., Fink A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev. 2016;4:CD009002. doi: 10.1002/14651858.CD009002.pub3. [PMID: 27063583]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Freund-Levi Y., Eriksdotter-Jönhagen M., Cederholm T., Basun H., Faxén-Irving G., Garlind A., Vedin I., Vessby B., Wahlund L.O., Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [http://dx.doi.org/10.1001/archneur.63.10.1402]. [PMID: 17030655]. [DOI] [PubMed] [Google Scholar]
- 303.Masino S.A., Rho J.M. Mechanisms of Ketogenic Diet Action. 2012. [PubMed]
- 304.Coppola G., Veggiotti P., Cusmai R., Bertoli S., Cardinali S., Dionisi-Vici C., Elia M., Lispi M.L., Sarnelli C., Tagliabue A., Toraldo C., Pascotto A. The ketogenic diet in children, adolescents and young adults with refractory epilepsy: an Italian multicentric experience. Epilepsy Res. 2002;48(3):221–227. doi: 10.1016/s0920-1211(01)00315-1. [http://dx.doi.org/10.1016/S0920-1211(01)00315-1]. [PMID: 11904241]. [DOI] [PubMed] [Google Scholar]
- 305.Coppola G., D’Aniello A., Messana T., Di Pasquale F., della Corte R., Pascotto A., Verrotti A. Low glycemic index diet in children and young adults with refractory epilepsy: first Italian experience. Seizure. 2011;20(7):526–528. doi: 10.1016/j.seizure.2011.03.008. [http://dx.doi.org/10.1016/ j.seizure.2011.03.008]. [PMID: 21489826]. [DOI] [PubMed] [Google Scholar]
- 306.Kverneland M., Selmer K.K., Nakken K.O., Iversen P.O., Taubøll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197–201. doi: 10.1016/j.yebeh.2015.10.021. [http://dx.doi.org/10.1016/j.yebeh.2015.10.021]. [PMID: 26588588]. [DOI] [PubMed] [Google Scholar]
- 307.Cervenka M.C., Henry B.J., Felton E.A., Patton K., Kossoff E.H. Establishing an adult epilepsy diet center: Experience, efficacy and challenges. Epilepsy Behav. 2016;58:61–68. doi: 10.1016/j.yebeh.2016.02.038. [http://dx. doi.org/10.1016/j.yebeh.2016.02.038]. [PMID: 27060389]. [DOI] [PubMed] [Google Scholar]
- 308.Wilder R.M. The effect on ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921;2:307. [Google Scholar]
- 309.Wheless J.W. History of the ketogenic diet. Epilepsia. 2008;49(Suppl. 8):3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [http://dx.doi.org/10.1111/j.1528-1167.2008.01821.x]. [PMID: 19049574]. [DOI] [PubMed] [Google Scholar]
- 310.Huttenlocher P.R. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976;10(5):536–540. doi: 10.1203/00006450-197605000-00006. [http://dx.doi.org/10.1203/00006450-197605000-00006]. [PMID: 934725]. [DOI] [PubMed] [Google Scholar]
- 311.Rho J.M., Anderson G.D., Donevan S.D., White H.S. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 2002;43(4):358–361. doi: 10.1046/j.1528-1157.2002.47901.x. [http://dx.doi.org/10.1046/j.1528-1157.2002.47901.x]. [PMID: 11952765]. [DOI] [PubMed] [Google Scholar]
- 312.Likhodii S.S., Serbanescu I., Cortez M.A., Murphy P., Snead O.C., III, Burnham W.M. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann. Neurol. 2003;54(2):219–226. doi: 10.1002/ana.10634. [http://dx.doi.org/10.1002/ana.10634]. [PMID: 12891674]. [DOI] [PubMed] [Google Scholar]
- 313.Viggiano A., Pilla R., Arnold P., Monda M., D’Agostino D., Coppola G. Anticonvulsant properties of an oral ketone ester in a pentylenetetrazole-model of seizure. Brain Res. 2015;1618:50–54. doi: 10.1016/j.brainres.2015.05.023. [http://dx.doi.org/10.1016/j.brainres.2015.05.023]. [PMID: 26026798]. [DOI] [PubMed] [Google Scholar]
- 314.Ma W., Berg J., Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J. Neurosci. 2007;27(14):3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [http://dx.doi.org/10.1523/ JNEUROSCI.0132-07.2007]. [PMID: 17409226]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Juge N., Gray J.A., Omote H., Miyaji T., Inoue T., Hara C., Uneyama H., Edwards R.H., Nicoll R.A., Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99–112. doi: 10.1016/j.neuron.2010.09.002. [http://dx.doi.org/10.1016/j.neuron.2010.09. 002]. [PMID: 20920794]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bough K.J., Valiyil R., Han F.T., Eagles D.A. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35(1):21–28. doi: 10.1016/s0920-1211(98)00125-9. [http://dx.doi.org/10.1016/ S0920-1211(98)00125-9]. [PMID: 10232791]. [DOI] [PubMed] [Google Scholar]
- 317.Viggiano A., Pilla R., Arnold P., Monda M., D’Agostino D., Zeppa P., Coppola G. Different calorie restriction treatments have similar anti-seizure efficacy. Seizure. 2016;35:45–49. doi: 10.1016/j.seizure.2016.01.003. [http://dx. doi.org/10.1016/j.seizure.2016.01.003]. [PMID: 26794009]. [DOI] [PubMed] [Google Scholar]
- 318.Bough K. Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl. 8):91–93. doi: 10.1111/j.1528-1167.2008.01846.x. [http://dx.doi.org/10.1111/j.1528-1167.2008.01846.x]. [PMID: 19049599]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rogawski M.A., Löscher W., Rho J.M. Mechanisms of action of antiseizure drugs and the ketogenic diet. Cold Spring Harb. Perspect. Med. 2016;6(5):a022780. doi: 10.1101/cshperspect.a022780. [http://dx.doi.org/10.1101/ cshperspect.a022780]. [PMID: 26801895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rho J.M. How does the ketogenic diet induce anti-seizure effects? Neurosci. Lett. 2017;637:4–10. doi: 10.1016/j.neulet.2015.07.034. [http://dx.doi.org/10.1016/j.neulet. 2015.07.034]. [PMID: 26222258]. [DOI] [PubMed] [Google Scholar]
- 321.Viggiano A., Stoddard M., Pisano S., Operto F.F., Iovane V., Monda M., Coppola G. Ketogenic diet prevents neuronal firing increase within the substantia nigra during pentylenetetrazole-induced seizure in rats. Brain Res. Bull. 2016;125:168–172. doi: 10.1016/j.brainresbull.2016.07.001. [http://dx.doi.org/10.1016/j.brainresbull.2016.07.001]. [PMID: 27381979]. [DOI] [PubMed] [Google Scholar]
- 322.Chwiej J., Skoczen A., Matusiak K., Janeczko K., Patulska A., Sandt C., Simon R., Ciarach M., Setkowicz Z. The influence of the ketogenic diet on the elemental and biochemical compositions of the hippocampal formation. Epilepsy Behav. 2015;49:40–46. doi: 10.1016/j.yebeh.2015.04.042. [http://dx.doi.org/10.1016/j.yebeh.2015.04.042]. [PMID: 25986320]. [DOI] [PubMed] [Google Scholar]
- 323.Chwiej J., Skoczen A., Janeczko K., Kutorasinska J., Matusiak K., Figiel H., Dumas P., Sandt C., Setkowicz Z. The biochemical changes in hippocampal formation occurring in normal and seizure experiencing rats as a result of a ketogenic diet. Analyst (Lond.) 2015;140(7):2190–2204. doi: 10.1039/c4an01857e. [http://dx.doi.org/10.1039/ C4AN01857E]. [PMID: 25705743]. [DOI] [PubMed] [Google Scholar]
- 324.Paoli A., Rubini A., Volek J.S., Grimaldi K.A. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013;67(8):789–796. doi: 10.1038/ejcn.2013.116. [http://dx.doi.org/10.1038/ejcn.2013.116]. [PMID: 23801097]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Verrotti A., Iapadre G., Pisano S., Coppola G. Ketogenic diet and childhood neurological disorders other than epilepsy: an overview. Expert Rev. Neurother. 2016;21:1–13. doi: 10.1080/14737175.2017.1260004. [PMID: 27841033]. [DOI] [PubMed] [Google Scholar]
- 326.Bozzetti F., Zupec-Kania B. Toward a cancer-specific diet. Clin. Nutr. 2016;35(5):1188–1195. doi: 10.1016/j.clnu.2015.01.013. [http://dx.doi.org/10.1016/j.clnu. 2015.01.013]. [PMID: 25707910]. [DOI] [PubMed] [Google Scholar]
- 327.Branco A.F., Ferreira A., Simões R.F., Magalhães-Novais S., Zehowski C., Cope E., Silva A.M., Pereira D., Sardão V.A., Cunha-Oliveira T. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur. J. Clin. Invest. 2016;46(3):285–298. doi: 10.1111/eci.12591. [http://dx.doi.org/10.1111/eci.12591]. [PMID: 26782788]. [DOI] [PubMed] [Google Scholar]
- 328.Allen B.G., Bhatia S.K., Anderson C.M., Eichenberger-Gilmore J.M., Sibenaller Z.A., Mapuskar K.A., Schoenfeld J.D., Buatti J.M., Spitz D.R., Fath M.A. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [http://dx.doi.org/10.1016/j.redox.2014.08.002]. [PMID: 25460731]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lee P.R., Kossoff E.H. Dietary treatments for epilepsy: management guidelines for the general practitioner. Epilepsy Behav. 2011;21(2):115–121. doi: 10.1016/j.yebeh.2011.03.008. [http://dx.doi.org/10.1016/j.yebeh.2011.03.008]. [PMID: 21514240]. [DOI] [PubMed] [Google Scholar]
- 330.Ułamek-Kozioł M., Pluta R., Bogucka-Kocka A., Czuczwar S.J. To treat or not to treat drug-refractory epilepsy by the ketogenic diet? That is the question. Ann. Agric. Environ. Med. 2016;23(4):533–536. doi: 10.5604/12321966.1226841. [http://dx.doi.org/10.5604/12321966.1226841]. [PMID: 28030918]. [DOI] [PubMed] [Google Scholar]
- 331.Rho J.M., Sankar R. The ketogenic diet in a pill: is this possible? Epilepsia. 2008;49(Suppl. 8):127–133. doi: 10.1111/j.1528-1167.2008.01857.x. [http://dx.doi.org/10.1111/ j.1528-1167.2008.01857.x]. [PMID: 19049610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.D’Agostino D.P., Pilla R., Held H.E., Landon C.S., Puchowicz M., Brunengraber H., Ari C., Arnold P., Dean J.B. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(10):R829–R836. doi: 10.1152/ajpregu.00506.2012. [http://dx.doi.org/10.1152/ajpregu. 00506.2012]. [PMID: 23552496]. [DOI] [PubMed] [Google Scholar]