Short abstract
Background
Elderly patients are at high risk of complications after stroke, such as infections and fever. The occurrence of these complications has been associated with an increased risk of death or dependency.
Hypothesis: Prevention of aspiration, infections, or fever with metoclopramide, ceftriaxone, paracetamol, or any combination of these in the first four days after stroke onset will improve functional outcome at 90 days in elderly patients with acute stroke.
Design
International, 3 × 2-factorial, randomised-controlled, open-label clinical trial with blinded outcome assessment (PROBE) in 3800 patients aged 66 years or older with acute ischaemic stroke or intracerebral haemorrhage and an NIHSS score ≥ 6. Patients will be randomly allocated to any combination of oral, rectal, or intravenous metoclopramide (10 mg thrice daily); intravenous ceftriaxone (2000 mg once daily); oral, rectal, or intravenous paracetamol (1000 mg four times daily); or usual care, started within 24 h after symptom onset and continued for four days or until complete recovery or discharge from hospital, if earlier.
Outcome: The primary outcome measure is the score on the modified Rankin Scale at 90 days (± 14 days), as analysed with multiple regression.
Summary: This trial will provide evidence for a simple, safe and generally available treatment strategy that may reduce the burden of death or disability in patients with stroke at very low costs.
Planning: First patient included in May 2016; final follow-up of the last patient by April 2020.
Registration: ISRCTN, ISRCTN82217627, https://doi.org/10.1186/ISRCTN82217627
Keywords: Stroke, complications, elderly, ceftriaxone, metoclopramide, paracetamol
Introduction
In the first days after stroke, about half of all patients develop complications, including infections and fever. The risk of developing these events is greater in patients of higher age or with more severe stroke.1–3 Aspiration, infections and fever can impede functional recovery, prolong hospital admissions, and are independently associated with an increased risk of death or long-term dependency.1,2,4–9 In addition, systematic review of animal studies modelling ischaemic stroke has shown that hyperthermia during or shortly after the onset of ischaemia substantially increases infarct size, suggesting that the relation between fever and poor outcome observed in patients is at least in part causal.10
The risk of developing these complications can be reduced by very simple, safe and inexpensive measures, such as metoclopramide for the management of dysphagia, antibiotics for the prevention of infections and paracetamol for the prevention of fever, but it is uncertain whether these measures also improve functional outcome.11–14 In some, generally small, randomised trials, preventive treatment with these drugs not only convincingly reduced the risks of aspiration, infections, or fever by one third to one half, but was also associated with clear trends towards a lower risk of death or poor outcome.11–14 The cluster-randomised Quality in Acute Stroke Care (QASC) study demonstrated that implementation of nursing protocols for the management of fever, hyperglycaemia and swallowing dysfunction on a stroke unit resulted in better outcomes.15 However, in two recent large trials, preventive treatment with antibiotics did not improve functional outcomes.16,17
American guidelines for the treatment of patients with acute ischaemic stroke advocate screening for dysphagia; the use of antibiotics in patients with infections; and antipyretic drugs such as paracetamol in patients with subfebrile temperatures or fever.18 Guidelines of the European Stroke Organisation concluded that there is insufficient evidence from randomised trials to make strong recommendations on whether, when and to whom preventive antibiotic or antipyretic treatment should be given after ischaemic stroke or intracerebral haemorrhage.19,20 The authors called for randomised trials to allow for better-informed recommendations in the future.20
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial will assess whether a pharmacological strategy to prevent aspiration, infections, or fever with metoclopramide, ceftriaxone, paracetamol, or any combination of these in elderly patients with a moderately severe to severe acute stroke is more effective at reducing the risk of death and improving functional outcome than current clinical practice of waiting until these complications are manifest before initiating treatment.
Design
Overview and timelines
PRECIOUS is an international, multi-centre, 3 × 2-factorial, randomised, controlled, open-label clinical trial with blinded outcome assessment (PROBE) of the preventive use of metoclopramide, ceftriaxone, paracetamol, or any combination of these, for four days in elderly patients with acute ischaemic stroke or intracerebral haemorrhage. The primary outcome measure is the score on the modified Rankin Scale (mRS) at 90 days (±14 days).21 3800 patients will be recruited over a period of about four years in about 80 hospitals (both academic and regional) in 9 European countries (Figure 1). The first patient was included in May 2016 and the main results are anticipated to be available in 2020. The complete and most recent version of the study protocol is available at www.precious-trial.eu.
Figure 1:
Participating countries in PRECIOUS.
Study population
The study population consists of patients aged 66 years or older who are hospitalised with moderately severe to severe acute ischaemic stroke or intracerebral haemorrhage and can be treated within 24 h of stroke onset. In order to be eligible to participate, a patient must meet all inclusion criteria listed in Table 1 and none of the exclusion criteria listed in Table 2. Patients with an active infection are excluded.
Table 1.
Inclusion criteria.
CT: computed tomography; MRI: magnetic resonance imaging; NIHSS: National Institutes of Health Stroke Scale.
aA normal CT scan is considered compatible with ischaemic stroke.
bNIHSS is assessed at the time of inclusion.
cIn case of a stuttering stroke, treatment should start within 24 h of the moment the first symptoms occurred.
dInformed consent is given by the patient, legal representative or independent physician (depending on local and national regulations).
Table 2.
Exclusion criteria.
|
|
Criteria for censoring a treatment stratum:For the metoclopramide stratum:
|
For the ceftriaxone stratum:
|
For the paracetamol stratum:
|
mRS: modified Rankin Scale.
aAs judged by the treating clinical physician.
bScore 4 mRS: Moderately severe disability. Unable to attend to own body needs without assistance and unable to walk unassisted.
Patient enrolment
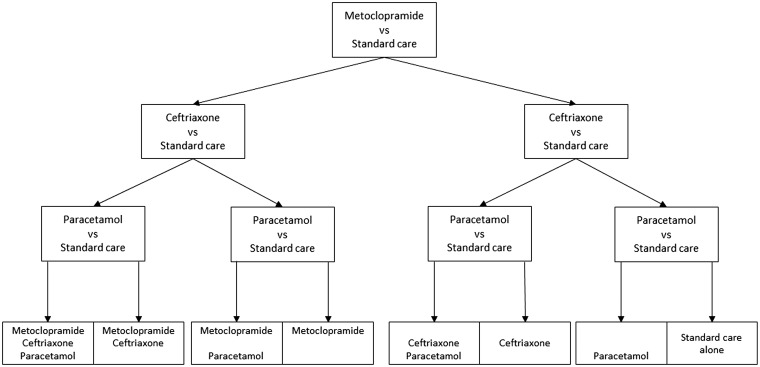
After written informed consent, patients are randomly allocated in a 3 × 2 factorial design to any combination of open-label oral, rectal, or intravenous metoclopramide (10 mg thrice daily); intravenous ceftriaxone (2000 mg once daily); oral, rectal, or intravenous paracetamol (1000 mg four times daily); or usual care, started within 24 h after symptom onset and continued for four days or until complete recovery or discharge from hospital, if earlier (Figure 2). The daily dose of metoclopramide is reduced to 3 times 5 mg in patients with moderate to severe renal impairment or with severe hepatic impairment, and to 3 times 2.5 mg in patients with end-stage renal disease.
Figure 2:
Treatment allocation will be based on proportional minimisation. Investigators will have the opportunity to censor a single randomisation stratum in a specific patient before randomisation. Each of the 8 subgroups is expected to consist of approximately 475 patients.
Allocation is based on proportional minimisation through a web-based allocation service. Treatment allocation is stratified by country and includes the following minimisation factors for balance in baseline characteristics: age (66–75 years vs. > 75 years); sex (male vs. female); stroke type (ischaemic stroke vs. intracerebral haemorrhage); stroke severity (NIHSS 6–12 vs. > 12); and diabetes mellitus (yes vs. no). Investigators have the opportunity to censor a single randomisation stratum in a specific patient before randomisation, for example in case of an allergy to one of the study medications (Table 2). Alongside the study treatment, all patients receive standard care as recommended by national or international guidelines or local protocols. This may include thrombolysis and endovascular treatment for acute ischaemic stroke, and treatment of hypertension for intracerebral haemorrhage.
Data collection and follow-up
Baseline characteristics assessed are listed in Table 3. The presence of any treatment restriction, the method of food intake and the vital signs (including body temperature) are recorded at baseline and during the first seven days of hospitalisation. The recording and reporting period for all severe or serious adverse events begins after randomisation and ends on day 7, except for serious adverse reactions and suspected unexpected serious adverse reactions (SUSARs), which are recorded and reported up to 90 days. Death occurring before day 90 (± 14) is a study secondary outcome and is always documented and recorded.
Table 3.
Baseline characteristics.
|
mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale.
aAspirin in any formulation and in a daily dose of up to 300 mg is not considered an antipyretic drug.
bThe method of feeding on the day before the stroke and at noon of the relevant day will be recorded and classified as 1. normal food; 2. oral, soft or fluids only; 3. nasogastric tube; 4. percutaneous endoscopic gastrostomy (PEG); 5. intravenous only; 6. none.
cThe presence of any treatment restriction will be recorded at baseline and during the patients stay in the hospital, and will be classified as 1. Do not resuscitate; 2. Do not intubate and ventilate; 3. Withholding other treatments that may prolong life; 4. Withholding food; 5. Withholding fluids; and 6. Palliation with morphine or a benzodiazepine. Any combination of these strategies is possible.
dBlood pressure, pulse and body temperature will be assessed at baseline and at 12-h (± 3 h) intervals (where assessed as part of routine clinical practice). Both rectal and tympanic thermometry are allowed.
eIf assessed at baseline as part of routine clinical practice, the following laboratory tests will be collected: serum glucose; glomerular filtration rate; C-reactive protein (CRP); alkaline phosphatase (ALP); gamma-glutamyl transferase (GGT); alanine aminotransferase (ALT); and aspartate aminotransferase (AST); leucocyte count and differential.
At day 7 after admission to the hospital, or at discharge if earlier, the score on the mRS is assessed. During a follow-up visit at day 90 (± 14), the mRS is assessed by a trained, certified investigator in a standard fashion according to each centre’s normal practice, and the interview is recorded with a digital video camera. During this recording, no reference to the treatment allocation is made. The videos are uploaded and distributed for independent and blinded scoring by three certified expert raters from the same country as the patient. Additionally, the Barthel index (BI),22 Montreal Cognitive Assessment (MoCA)23 and EuroQol 5D-5L (EQ-5D-5L) are assessed at 90 days, as well as the patient’s location and number of nights spent at home over the first 90 days after stroke.
Outcome events
The primary outcome measure is the score on the mRS at 90 days (±14).24 The mRS is an ordinal hierarchical scale incorporating seven categories from 0 up to and including 6 and describes the range of disability encountered post stroke. ‘Death’ is assigned a score of 6. Secondary outcomes are outlined in Table 4.
Table 4.
Study outcomes.
Primary outcome
|
Secondary outcomesAt 7 days (± 1 day) or at discharge, if earlier:
|
| At 90 days (± 14 days): |
mRS: modified Rankin Scale; SAE: serious adverse event; ESBL: extended-spectrum beta-lactamase.
aAs assessed by three independent and blinded adjudicators based on a video recording of an mRS interview at the follow-up visit after 90 days.
bInfections will be categorised as diagnosed by the clinician, and as judged by an independent adjudication committee (masked to treatment allocation).
cDetected as part of routine clinical practice.
dConverted to units of defined daily doses according to the classification of the WHO Anatomical Therapeutic Chemical Classification System with Defined Daily Doses Index.
eAs detected by PCR in a rectal swab.
fDefined as mRS 3 to 6.
gAssessed with the Barthel index (BI).28
hAssessed with the Montreal Cognitive Assessment (MoCA).29
iAssessed with the EuroQol 5D-5L (EQ-5D-5L).
jThe number of nights among the first 90 since stroke onset that are spent in the patient’s own home or a relative’s home. Where final follow-up occurs earlier, the last known placement will be extrapolated to 90 days.
kHospital; rehabilitation service; chronic nursing facility; home.
Infections will be categorised as diagnosed by the clinician, and as judged by an independent adjudication committee (masked to treatment allocation) according to modified Centres for Disease Control and Prevention criteria.25 The scoring algorithms for infections that will be used by this committee have been described previously and are in line with recommendations of the Pneumonia in Stroke Consensus Group.26 Clostridium difficile infection will be defined as diarrhoea in combination with a positive Clostridium difficile toxin test.
Substudy
To detect selection of bacteria with third generation cephalosporin resistance caused by increased antibiotic pressure, a nested case-control substudy will be performed in 1000 patients in 30 centres in different participating countries. The presence of extended spectrum beta-lactamase (ESBL) producing bacteria will be assessed with polymerase chain reaction (PCR). For this purpose, two rectal swabs will be collected in each patient, after specific informed consent, on admission and at day 7 (± 1 day, or at discharge, if earlier).
Sample size calculation and statistical analysis plan
The primary effect estimate will be the difference between groups in the mRS scores obtained through centralised adjudications and assessed using multiple regression, and will be expressed as a mean difference with 95% confidence interval. PRECIOUS is powered to detect a statistically significant shift in the difference in the proportion of patients with mRS 0 to 2 at 90 days, assuming an effect that leads to a 5% absolute increase (from 36 to 41%)26 in the cumulative proportion of patients with mRS 0 to 2 in any intervention group, compared with controls. The effect size of 5% is based on previous smaller studies and/or meta-analyses thereof, performed in more general stroke populations.12–14,16 The statistical analyses will be performed according to the intention-to-treat principle and adjusted for the minimisation factors mentioned, other relevant baseline characteristics, and treatment allocation for the other two strata of the trial. Three separate primary analyses will be performed, looking at the main effects of each of the three interventions compared with their respective controls. Although the study is not powered to detect interactions between the three interventions, such interactions will be investigated in secondary analyses. Two sensitivity analyses will be performed in which all patients who are lost to follow-up will be classified as having the worst possible outcome (death) or the best possible outcome (mRS = 0), respectively.
Secondary efficacy outcomes will be analysed using similar methods as for the primary efficacy analysis, with binary logistic regression used for binary outcomes, including death, unfavourable outcome and SAEs. Ordinal logistic regression will be used for ordered categorical data and multiple regression for continuous outcomes. Wilcoxon rank sum test will be used for continuous outcome measures which are not normally distributed. Several subgroup analyses will be performed based on age, sex, stroke type and severity, diabetes mellitus, presence of atrial fibrillation, pre-stroke mRS score, treatment with alteplase or other recanalisation method, treatment allocation for the other two trial strata and time to treatment. A full statistical analysis plan will be completed before the final follow-up of the last patient.
Discussion
Because several complications in the first days after stroke have consistently been associated with a higher risk of death or poor functional outcome, prevention of these complications appears a logical, simple and safe approach to improve outcome after stroke. In the past two decades, several trials aimed at prevention of complications have been performed, but – besides organised care in a designated stroke unit – no treatment to prevent complications has convincingly shown to improve the functional outcome in patient with stroke.13,14,16 However, most of these trials were underpowered, started treatment too late after stroke onset, or aimed at only one specific complication after stroke. Strengths of PRECIOUS are the assessment in an elderly population with moderately severe to severe stroke (with an increased risk of complications and poor outcome), the start of treatment within 24 h after stroke onset, and its multifactorial design. The trial will provide high-quality evidence that could be broadly generalisable. Because of its pragmatic design and the use of safe, inexpensive, and generally available drugs, the results of PRECIOUS could be implemented rapidly throughout Europe and the rest of the world.
It may be questioned whether the effects of prophylactic antibiotics in patients with stroke should still be assessed after the neutral results in two recent phase III clinical trials. Ceftriaxone is an off-patent, broad-spectrum antibiotic with proven efficacy against bacteria most frequently causing infection in patients with acute stroke.12,27 In the PASS trial, 2550 patients with stroke were randomly assigned to standard care or intravenous ceftriaxone, started within 24 h of stroke onset, continued for four days. Preventive ceftriaxone reduced the incidence of infections in general (from 7% to 3%; p < 0.0001), but did not have an effect on the occurrence of pneumonia or the risk of a poor outcome at 90 days.16 However, the median score on the NIHSS of patients in PASS-was 5, which could explain the relatively low incidence of infections. In the cluster-randomised STROKE-INF trial, which included 1217 stroke patients with dysphagia, prophylactic antibiotics did not change the incidence of post-stroke pneumonia or poor functional outcome.17 However, antibiotic treatment may have started too late (up to 48 h after stroke onset) to prevent early infections. In addition, a considerable proportion of patients in the treatment group received only a limited number of antibiotic doses, while 34% of the patients in the control group received an antibiotic at least once during the first seven days. Finally, individual centres included only a small number of patients over an extended period of time; in a cluster-randomised study, this may induce selection bias decreasing the discriminative power. Because of the limitations of these two trials, and the strong association between infections and a poor functional outcome,4,28 additional evidence on the effect of preventive antibiotics is still strongly needed.
The PRECIOUS trial will also be able to assess whether antibiotics work in isolation, or whether the effect is dependent on the combination of drugs that are used in the trial. The results of PASS and STROKE-INF support the concept that post-stroke pneumonia might be a respiratory syndrome resulting from complex bacterial, chemical and immunological causes that might not be prevented by antibiotics alone. The combination of treatments in PRECIOUS, especially the combination of metoclopramide and ceftriaxone, targets different pathways in the development of post-stroke pneumonia, potentially resulting in a larger reduction in the risk of complications than with the individual treatments alone.
The prevention of complications with the treatments proposed in PRECIOUS was safe in previous trials and not associated with an increased risk of SAEs.13,14,16 In addition, the risk of developing Clostridium difficile overgrowth was smaller than 1% in previous studies with ceftriaxone, and there was no association with an increase in antimicrobial resistance.16,17
PRECIOUS uses paracetamol for the prevention of increases in body temperature because this was safe in doses up to 6 g per day in randomised clinical trials in patients with acute stroke, reduced the risk of subfebrile temperatures or fever at 24 h by 50% and was associated with a trend towards an improvement in functional outcome in the PAIS trial. This trial was underpowered to detect a benefit on functional outcome because this was terminated prematurely due to lack of funding after inclusion of 1400 patients, against a target of 2500 patients.10 For PRECIOUS, we have selected a maximum daily dose of 4 g to comply with the drug’s summary of product characteristics.
Given the potential benefit of the prevention of complications to the patients included in PRECIOUS, future stroke patients, their caregivers, and society, the risk-benefit balance is strongly in favour of conducting this clinical trial.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Philip Bath is Stroke Association Professor of Stroke Medicine and is an NIHR Senior Investigator; Janika Kõrv has received fees as a consultant or lecture fees from Bayer, Pfizer, Boehringer Ingelheim, ReNeuron; Götz Thomalla has received fees as a consultant or lecture fees from Acandis, Bayer Vital, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Daichii Sankyo, GlaxoSmithKline, and Stryker and received a research grant from Bayer. Bart van der Worp has received speaker’s fees from Boehringer Ingelheim and Bayer; the other authors report no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PRECIOUS has received funding from the European Union’s Horizon, 2020 research and innovation programme under grant agreement No 634809.
Ethical approval
The trial has been approved by national or local research ethics boards for all active clinical sites.
Informed consent
Not applicable
Guarantor
HBW.
Contributorship
All co-authors have contributed to the trial design, protocol development, and the writing of this manuscript.
References
- 1.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol 2010; 9: 105–118. [DOI] [PubMed] [Google Scholar]
- 2.Hesse K, Fulton RL, Abdul-Rahim AH, et al. VISTA Collaborators. Characteristic adverse events and their incidence among patients participating in acute ischemic stroke trials. Stroke 2014; 45: 2677–2682. [DOI] [PubMed] [Google Scholar]
- 3.Westendorp W, Vermeij J-D, Hilkens NA, et al. Development and internal validation of a prediction rule for post-stroke infection and post-stroke pneumonia in acute stroke patients. Eur Stroke J 2018; 3: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westendorp WF, Nederkoorn PJ, Vermeij J-D, et al. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 2011; 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Hertog H, van der Worp B, van Gemert M, et al. Therapeutic hypothermia in acute ischemic stroke. Expert Rev Neurother 2007; 7: 155–164. [DOI] [PubMed] [Google Scholar]
- 6.Greer DM, Funk SE, Reaven NL, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke 2008; 39: 3029–3035. [DOI] [PubMed] [Google Scholar]
- 7.Saini M, Saqqur M, Kamruzzaman A, et al. ; VISTA Investigators. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke 2009; 40: 3051–3059. [DOI] [PubMed] [Google Scholar]
- 8.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005; 36: 2756–2763. [DOI] [PubMed] [Google Scholar]
- 9.Geurts M, Scheijmans FEV, van Seeters T, et al. Temporal profile of body temperature in acute ischemic stroke: relation to infarct size and outcome. BMC Neurol 2016; 16: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jonge JC, Wallet J and van der Worp HB. Fever worsens outcomes in animal models of acute ischaemic stroke: a systematic review and meta-analysis. European Stroke Journal 2018; DOI: 10.1177/2396987318776421. [DOI] [PMC free article] [PubMed]
- 11.den Hertog HM, van der Worp HB, van Gemert HMA, et al. An early rise in body temperature is related to unfavorable outcome after stroke: data from the PAIS study. J Neurol 2011; 258: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westendorp WF, Vermeij J-D, Vermeij F, et al. Antibiotic therapy for preventing infections in patients with acute stroke. Cochrane Database Syst Rev 2012; 1: CD008530. [DOI] [PubMed] [Google Scholar]
- 13.den Hertog HM, van der Worp HB, van Gemert HMA, et al. The paracetamol (Acetaminophen) in stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol 2009; 8: 434–440. [DOI] [PubMed] [Google Scholar]
- 14.Warusevitane A, Karunatilake D, Sim J, et al. Safety and effect of metoclopramide to prevent pneumonia in patients with stroke fed via nasogastric tubes trial. Stroke 2015; 46: 454–460. [DOI] [PubMed] [Google Scholar]
- 15.Middleton S, McElduff P, Ward J, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet 2011; 378: 1699–1706. [DOI] [PubMed] [Google Scholar]
- 16.Westendorp WF, Vermeij J-D, Zock E, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015; 385: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 17.Kalra L, Irshad S, Hodsoll J, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015; 386: 1835–1844. [DOI] [PubMed] [Google Scholar]
- 18.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 19.Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–855. [DOI] [PubMed] [Google Scholar]
- 20.Ntaios G, Dziedzic T, Michel P, et al. European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke 2015; 10: 941–949. [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 23.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 24.Lees KR, Bath PMW, Schellinger PD, et al. Contemporary outcome measures in acute stroke research. Stroke 2012; 43: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 25.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 26.Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke 2015; 46: 2335–2340. [DOI] [PubMed] [Google Scholar]
- 27.van de Beek D, Wijdicks EFM, Vermeij FH, et al. Preventive antibiotics for infections in acute stroke. Arch Neurol 2009; 66: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 28.Vermeij FH, Scholte Op Reimer WJM, de Man P, et al. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis 2009; 27: 465–471. [DOI] [PubMed] [Google Scholar]