Abstract
Scope
Urinary biomarkers are used to estimate the nutritional intake of humans. The aim of this study was to distinguish between low, medium, and high apple consumption by quantifying possible intake biomarkers in urine samples after apple consumption by HPLC‐MS/MS. Apples were chosen as they are the most consumed fruits in Germany.
Methods and Results
Thirty subjects took part in 7‐day study. They abstained from apples and apple products except for one weighed apple portion resembling one, two, or four apples. Before apple consumption and during the following days spot urine samples were collected. These urine samples were incubated with β‐glucuronidase, diluted, and directly measured by HPLC‐MS/MS. Phloretin, epicatechin, procyanidin B2, and quercetin were detected in urine using Scheduled MRMTM mode. Phloretin was confirmed as a urinary biomarker of apple intake and had the ability to discriminate between low or medium (one or two apples) and high apple consumption (four apples). The groups also differ in the excretion of epicatechin and procyanidin B2.
Conclusion
Apple consumption can be monitored by urinary biomarkers for a period of at least 12 h after consumption. Furthermore the amount of apples consumed can be estimated by the concentration of certain biomarkers.
Keywords: Apple, Food consumption marker, Intake biomarker, Phloretin, Urine
Abbreviation
- ACN
acetonitrile
- BMI
body mass index
- DIN
deutsche Industrienorm
- f
female
- FA
formic acid
- FFQ
food frequency questionnaire
- HPLC‐MS/MS
high‐performance liquid chromatography coupled to tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- m
male
- MRM
multiple reaction monitoring
- RA
apparent recovery
1. Introduction
Intake biomarkers are constituents of food or metabolites thereof, which can be detected in urine or serum samples and are indicative for nutritional intake. They allow to draw conclusions concerning nutritional intake and health aspects. Up to now mostly food frequency questionnaires and/or nutrition diaries are used to obtain dietary data. However it is well known, that these methods have limitations as they are subjective and therefore are prone to error. Urinary intake biomarkers are objective and are preferable to those in serum 1, 2, 3. Urine sampling is not invasive and the metabolite profile after nutritional intake is more striking than in serum, as the body tries to keep the conditions in blood stable. In contrast urine is a filtrate of blood and there is no need to keep the concentrations of different substances stable 4.
In the last years different possible biomarkers for various food groups were described. Intake biomarkers for fruit and vegetable consumption are of special interest for the assessment of healthy nutritional intake. When selecting intake biomarkers it is important to pay attention to the specific relation between the intake biomarker and the corresponding food item. For example flavonoids are discussed as possible biomarkers for fruit and vegetable, but some major flavonoid sources, as red wine, tea, or coffee have to be considered as well. For this reason, it is important not to choose flavonoids or other possible substances, as intake biomarkers whose main source are nonfruit foods 5. Despite the specific character of intake biomarkers it is important that there is a robust analytical method to identify and quantify the biomarker. Furthermore in an ideal case the change in concentration of an intake biomarker in urine is directly linked to a lower or higher dietary exposure 6. It is possible to use global intake biomarkers for a food group such as fruit and vegetables, but individual intake biomarkers allow to get detailed information about the individual nutrition status 7.
Mennen et al. found 13 dietary flavonoids and phenolic acids as possible biomarkers for polyphenol intake. In their study, where they analyzed 24 h urine and spot urine samples from a cohort of 154 participants, possible biomarkers for consumption of citrus fruits, apples, onions, chocolate, coffee, and tea have been found 8, 9. The correlations between consumption and excretion could be shown for 24 h urine as well as spot urine samples 9. In most cases intake biomarkers were detected in 24 h urine, which has the drawback that no kinetics of excretion can be monitored. Mennen et al. showed that it is possible to use morning spot urine samples to detect intake biomarkers 9. During the study presented in this paper spot urine samples, morning urine samples as well as samples during the day were collected and analyzed in order to get also kinetic data.
Despite the quantitative correlation between an intake biomarker after consumption of a given food it is also of interest to correlate intake biomarkers with the size of the portion of a certain food item. There are some studies covering this aspect, but the size of the portions was not always realistic. Krogholm et al. compared low and high fruit consumption by serving a mixed fruit dish (300 or 600 g). The size of the apple portion was with 40/80 g very low corresponding to one fourth or half an apple. It was not possible to distinguish between low and high apple consumption, but it was discussed that urinary phloretin might be sensitive enough to reflect more realistic, higher apple intakes 10. Brevik et al. found similar results in a study using low or high mixed fruit consumption (apple content 20 or 50 g). No correlation between higher apple consumption and an increasing phloretin excretion was detectable 11, but again, a rather low apple portion had been consumed.
Since apples are the most prominent fruit in Germany with an average consumption of 23.5 kg/year [http://de.statista.com/statistik/daten/studie/247425/umfrage/die-beliebtesten-obstsorten-der-deutschen/ (Accessed 19 July, 2016)], they were chosen for this study. Aim of the study was to investigate the correlation between low, medium, and high apple consumption using realistic portions and concentrations of possible intake biomarkers in urine. Therefore a targeted approach was chosen and measurements were done by HPLC‐MS/MS.
Phloretin might be a specific biomarker for apples as phloretin glycosides such as phloridzin (phloretin‐2′‐O‐glucoside) and phloretin‐2′‐O‐(2′′‐O‐xylosyl)glucoside as well as phloretin itself are nearly unique in apples 12. Phloridzin was also found in plum 13 and pomegranate 14, but both fruits are not consumed in considerable amounts. Besides phloretin some other possible biomarkers that have been described in literature are quercetin, epicatechin, and different phenolic acids 8, 9, 15, 16, 17. Procyanidins B1, B2, B5, and B7 can be found in apples as well and different apple cultivars nearly have the same procyanidin distribution 18. Furthermore Bittner et al. and Rzeppa et al. studied the systemic absorption and metabolism of procyanidins in pigs and showed that procyanidin dimers and trimers are excreted in urine 19, 20. Therefore some B‐type procyanidins were included in this study to investigate their suitability as urinary intake biomarkers.
2. Materials and methods
2.1. Chemicals and reagents
Solvents used for sample dilution and chromatography were purchased from Carl Roth (Karlsruhe, Germany), Sigma‐Aldrich (Steinheim, Germany), and VWR (Darmstadt, Germany) in gradient or analytical grade. Formic acid (FA) was obtained from Grüssing (Filsum, Germany). Water for dilution and HPLC was purified using a Milli‐Q Gradient A10 system (Millipore, Schwalbach, Germany). β‐Glucuronidase from Helix pomatia was provided from Sigma‐Aldrich.
Phloretin and quercetin were obtained from Sigma‐Aldrich and (−)‐EC was obtained from Carl Roth. Procyanidins B1, B2, B5, B7 as references for quantification have been previously isolated from plant material or synthesized according to Rzeppa et al. 18. Taxifolin was obtained from “Leblang” Foxtank GmbH Berlin.
All analytes were dissolved in ACN or ACN/H2O and the multianalyte stock solutions were prepared at a concentration of 10 μg/mL in ACN/H2O and stored at −80°C. In addition working solutions containing 1.0, 0.1, and 0.01 μg/mL in ACN/H2O (10/90) were used for matrix‐matched calibration.
2.2. Study and study design
A short‐term intervention study was accomplished with 30 subjects (14 females and 16 males, aged 26.7 ± 2.9 years). Prior to the start of the study all subjects were informed about the aim and scope of this study and gave written consent about their participation. To ensure the pseudonymization of subjects six‐digit random numbers were allocated to each participant to tag their samples and personal data. The study was approved by the research ethical committee of the University Hospital Münster, Germany (File reference: 2014‐632‐f‐S).
2.2.1. Subjects
The subjects were divided into three group (low, medium, and high consumption), with ten subjects in each group. The low consumption group consisted out of four female and six male subjects, with a mean BMI of 21.35 ± 2.98 kg/m2 and a mean age of 25.6 ± 1.8 years. The medium consumption group consisted out of seven female and three male subjects, with a mean BMI of 24.18 ± 4.19 kg/m2 and a mean age of 26.3 ± 3.2 years. The high consumption group consisted out of three female and seven male subjects, with a mean BMI of 23.98 ± 2.79 kg/m2 and a mean age of 28.4 ± 3.2 years. All subjects were healthy and had no signs of diseases related to the gastrointestinal tract or any form of liver disease as well as no allergy to apple or apple products. All subjects signed an informed consent form.
2.2.2. Apple samples
The apples used in this study were polyphenol‐rich Boskoop apples from the “Alte Land”‐region, Germany. They were purchased in a local supermarket.
2.2.3. Study design
Study participants were not allowed to consume any apples or apple products on all seven study days with the exception of the single portion at day 3 (see Fig. 1A). The additional diet before and during the study could be freely chosen by the subjects. Subjects recorded their dietary intake via a food diary, with special attention to fruit and vegetables as well as polyphenol rich foods and beverages.
Figure 1.
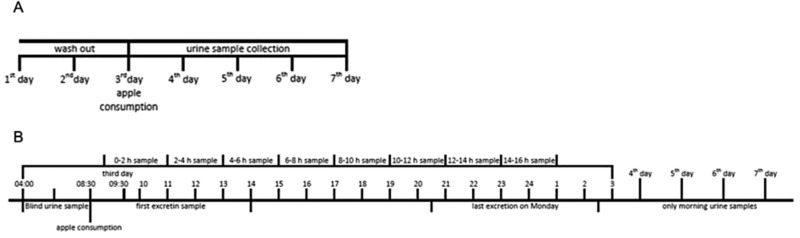
(A) Seven‐day study design, (B) timeline of urine sample collection.
In the morning of day 3 (Monday) the subjects collected one blank spot urine sample after the two wash‐out days. After this all subjects were split up into three groups, low, medium, and high consumption, and were provided with a portion of Boskoop apples. The low consumption group got 200 ± 10 g, medium consumption group 400 ± 10 g, and high consumption group 790 ± 10 g of apple quavers, corresponding to one, two, or four apples, respectively. The subjects got their portion in quavers of whole apples without the apple cores but with the apple skin.
The subjects consumed their apple portion within 1 h. Subsequently, they collected a spot urine sample from every time they urinated on day 3. From day 4–7 they just collected a spot urine sample of morning urine (see Fig. 1B). Urine samples were prefrozen at −20 °C and then stored at −65 °C.
2.3. Analysis
2.3.1. Analysis of urine samples
On the day of sample analysis the urine samples were thawed to room temperature and vortexed. An aliquot of 100 μL was mixed with 10 μL 1.5 M sodium acetate solution (pH 4.8) and 25 μL of the injection standard taxifolin (1 μg/mL). Then 10 μL of β‐glucuronidase solution (500 U in 150 mM sodium acetate solution pH 4.8) was added and the samples were incubated at 37°C for 3 h. Afterwards, 100 μL ACN (+0.1% FA) was added to stop the incubation. The solution was vortexed and then 755 μL water (+0.1% FA) was added, so the urine was diluted 1/10 (v/v) over all steps. After centrifugation at 15 000 × g for 5 min to remove solid residues the supernatant was directly used for HPLC‐MS/MS analysis. All measurements were performed in duplicate.
For each sample the urinary creatinine levels were determined to normalize biomarker concentrations to nanogram per milligram creatinine. Therefore, the samples were measured by the central laboratory of the University Hospital Münster on an ADVIA 1800 clinical chemistry analyzer using the Jaffé method (Siemens Healthcare Diagnostics, Eschborn, Germany).
2.3.2. Statistical analysis and data presentation
To test for significant differences in the amount of excreted intake biomarkers between the three groups a statistical analysis was carried out. For statistical analysis excreted concentrations for day 3 were summed up for each subject. A student nonparametric t‐test was used for significance testing between groups and a paired t‐test for testing significant differences between the blank urine from the morning of day 3 and the following samples. Significant changes were accepted at a p‐value of 0.05. Statistical analysis was realized with GraphPad Prism 5.02. Besides the discussion of most significant changes in Section 3 below, a detailed summary on the significance of compared groups and time points, provided as p‐values for all Student t‐tests can be found in Supporting information Tables 1–4.
No statistical analysis of covariance was carried out for other possible parameters such as biological sex or BMI, because of the already small number of participants in each group. Furthermore a slight correlation between biological sex and BMI was visible within the participants with males tending to higher BMI values, so the use of covariances might lead to false‐positive results.
Most of the data are presented as box whiskers plots after Tukey. In these the data between the first and the third quartile are in the box, as well as the median (marked as a band inside the box). The lowest and highest data points are shown by the whiskers, when they are still in the 1.5 interquartiles range, when they are outside of these range, they will be considered as outliners and shown as dots. In Figs. 2, 5 and 6 the bars representing the first excretion and the last excretion on day 3 are marked with # as these bars contain data that are also part of the 0–2 h bar or the 2–4 h bar, respectively, of the 10–12 h bar or 12–14 h bar or 14–16 h bar, depending on when the single subjects gave their first and last urine sample on day 3.
Figure 2.
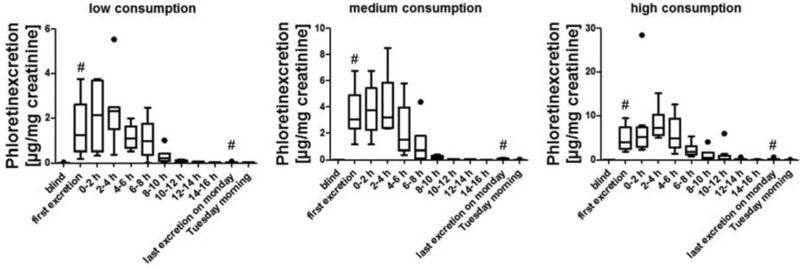
Phloretin excretion kinetics for low, medium, and high apple consumption group (•: outliners, #: data presented in these bars are also part of other bars depending on the time of the first and last urine excretion of each individual, see Section 2.3.2 for details).
Figure 5.
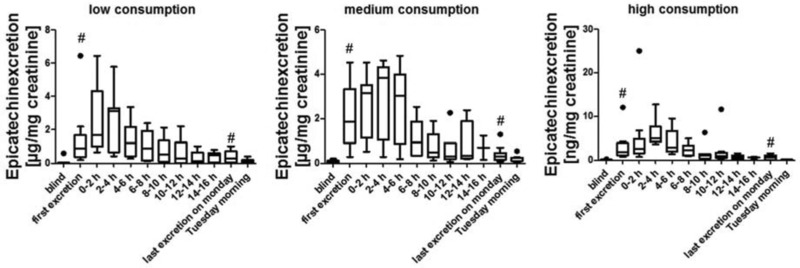
Epicatechin excretion kinetics for low, medium, and high consumption group (•: outliners, #: data presented in these bars are also part of other bars depending on the time of the first and last urine excretion of each individual, see Section 2.3.2 for details).
Figure 6.
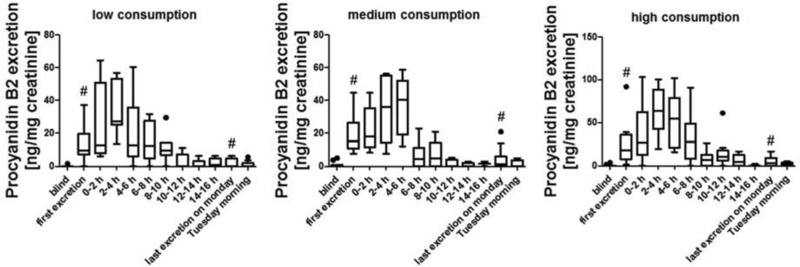
Procyanidin B2 excretion kinetics for low, medium, and high consumption group (•: outliners, #: data presented in these bars are also part of other bars depending on the time of the first and last urine excretion of each individual, see Section 2.3.2 for details).
2.4. HPLC‐MS/MS conditions
The HPLC‐MS/MS analysis was carried out using a NexeraTM system (Shimadzu, Duisburg, Germany) with a LC‐20ADXR solvent delivery module, a SIL‐20AXR autosampler, a DGU‐20A5R degasser, a CBM‐20A communications bus module, and a CT0‐10ASVP column oven coupled to an SCIEX QTRAP® 5500 mass spectrometer.
2.4.1. HPLC setup
Chromatographic separation was carried out on a Dr. Maisch Reprospher 100 phenyl‐hexyl column (3 μm, 150 × 2 mm, Dr. Maisch GmbH, Ammerbuch‐Entringen, Germany) equipped with a Gemini C6‐Phenyl guard column (3 μm, 2 × 4 mm, Phenomenex Inc., Aschaffenburg, Germany). Column oven temperature was set to 50 °C. A binary gradient consisting of ACN (A) and H2O (B), both with 0.1% FA with a flow rate of 300 μL/min was used. The gradient started at 10% A for 1 min, then solvent A was increased to 25% (1.5 min), further to 45% (5 min), and finally to 100% A (9 min) this was held for 2 min, then the column was reequilibrated to starting conditions for 6 min. Injection volume was 30 μL.
2.4.2. MS/MS setup
Detection of analytes was carried out on a SCIEX QTRAP® 5500 mass spectrometer with ESI ionization in Scheduled MRMTM detection mode using Analyst 1.6.2 as software. The ESI source parameters were as follows: source temperature: 450 °C, curtain gas: 35 psi, gas 1: 35 psi, gas 2: 45 psi, entrance potential: 10 V. Ion spray voltage was set to −4500 V in negative ionization mode. Two characteristic multiple reaction monitoring transitions per analyte were monitored to ensure accurate identification. For each analyte, the MS/MS parameters were optimized by direct infusion of pure standard solutions. The target scan time was set to 0.25 s. MS parameters for all analytes can be found in Table 1.
Table 1.
Detailed MS/MS parameters
| substance | Q1 (m/z) | Q3 (m/z) | Declustering potential (V) | Collision energy (V) | Cell exit potential (V) |
|---|---|---|---|---|---|
| Phloretin | 273 | 167/123 | −20 | −25 | −6 |
| Phloretin glucuronide | 449 | 273/123 | −152 | −20 | −7 |
| Procyanidin B1 | 577 | 289/407 | −35 | −18 | −12 |
| Procyanidin B2 | 577 | 407/289 | −35 | −33 | −12 |
| Procyanidin B5 | 577 | 425/289 | −35 | −18 | −12 |
| Procyanidin B7 | 577 | 425/289 | −35 | −18 | −12 |
| (Epi)catechin | 289 | 245/109 | −45 | −21 | −7 |
| Quercetin | 301 | 179/273 | −30 | −25 | −8 |
Multiple reaction monitoring transitions were tuned on an AB Sciex Qtrap 5500® mass spectrometer with direct infusion of neat standard solutions (or diluted urine samples for phloretin glucuronide). The declustering potential, entrance potential, collision energy, and collision cell exit potential were optimized for each multiple reaction monitoring transition. The two most intense multiple reaction monitoring transitions were used as quantifier/qualifier to build the final method.
2.5. Validation of the analytical method
The analytical method for the analytes phloretin, epicatechin, the procyanidins B1, B2, and B5 and quercetin was subject to an in‐house validation with focus on linearity, apparent recovery (RA), and determination of LOD and LOQ (the validation data are summarized in Table 2). Procyanidin B7 was not validated because it was not detectable in any urine sample. Analytes were quantified by using an external matrix‐matched calibration with spiked blank urine samples, which were prepared along with the samples (Section 2.3.1). The blank urine was produced by a volunteer who ate a polyphenol‐free diet for 24 h and then gave one spot urine sample. The calibration curves were adjusted for each analyte (see Table 2). For some analytes the calibration curve was split into two for better linear correlations.
Table 2.
Validation parameters obtained during in‐house validation
| substance | Retention time (min) | Linear range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) | RA (%) |
|---|---|---|---|---|---|
| Phloretin | 8.4 | 0.075‐10 | 0.075 | 0.25 | 49.6 ± 1.0 |
| 10–75 | |||||
| Epicatechin | 5.5 | 2.5–50 | 0.86 | 3.14 | 43.6 ± 2.5 |
| Procyanidin B1 | 4.2 | 1–50 | 0.19 | 0.75 | 40.8 ± 0.3 |
| Procyanidin B2 | 5.6 | 0.5–5 | 0.1 | 0.37 | 44.2 ± 3.1 |
| Procyanidin B5 | 6.5 | 1–50 | 0.44 | 1.61 | 42.3 ± 1.2 |
| Quercetin | 8.0 | 2.5–50 | 4.1 | 13.3 | 35.5 ± 5.1 |
Blank urine samples were spiked with analyte solutions and prepared as described in Section 2.1.1, resulting in a 1/10 dilution of the urine sample and analyzed by HPLC‐MS/MS. The data given in nanogram per milliliter are the concentration of the analytes in the diluted sample. LOD; LOQ; and
RA, apparent recovery were determined, all three were calculated according to the calibration method of the German Standard DIN 32635, as described before by Kleigrewe et al. 21.
The LOD and LOQ were calculated according to the calibration method of the German Standard DIN 32635, as described before by Kleigrewe et al. 21 .
For matrix‐matched calibration 100 μL urine was spiked with multianalyte standard solution, then sodium acetate solution and β‐glucuronidase were added and the samples were prepared as described above. The spiking levels correlate to concentration levels from 0.05, 0.075, 0.1, 0.25, 0.5, 0.75, 1, 2.5, 5, 7.5, 10, 25, 50, 100, and 250 ng/mL of each analyte in samples measured by HPLC‐MS/MS and tenfold higher concentrations in pure urine. Each calibration point was prepared in triplicate, and each sample was measured thrice. The calibration curves were calculated by linear regression. The recovery was determined from the slope of the linear calibration curves of the matrix‐matched calibration and neat standard solution.
3. Results
3.1. Method validation
In order to analyze the urine samples from the human study an in‐house validation of the HPLC‐MS/MS method was performed. For matrix‐matched calibration the validation parameters RA, LOD, and LOQ were determined. Blank urine samples were spiked with phloretin, epicatechin, procyanidins B1, B2, and B5 and quercetin. The method allowed the quantitative analysis of concentrations from 0.25 ng/mL for phloretin, 3.14 ng/mL for epicatechin, 0.37 ng/mL for procyanidin B2, and 13.3 ng/mL for quercetin. The recovery rates varied from 35.6 ± 5.1% for quercetin to 49.6 ± 1.0% for phloretin. A summary of the validation parameters can be found in Table 2.
3.2. Analysis of urine samples
The excretion concentrations of phloretin, epicatechin, and procyanidin B2 significantly increases following apple consumption in comparison to their concentration in the blind urine samples. The p‐values for the comparison of the blind urine sample with the first excreted sample (see Figs. 2, 5, 6) ranged between <0.001 and 0.047. The significant distinction between the morning urine sample of day 4 (day after apple consumption; fourth day morning in Figs. 2, 5, 6) and the blind urine sample is no longer possible. As the individual urine samples were collected at different time points the samples were grouped into 2‐h time slots for better comparison as shown in Fig. 1B.
3.2.1. Excretion of phloretin after apple consumption
For the analysis of each consumption, group box plot diagrams are used and presented in Fig. 2. Each box consists of all samples collected up to that moment (first excretion after consumption, last excretion at consumption day and group samples every 2 h (0–2 h, 2–4 h up to 14–16 h after the apple consumption). As can be seen from Fig. 2, phloretin is quickly excreted and levels in urine rise directly after apple consumption. In the first urine sample collected after apple consumption the phloretin concentration is significantly higher than in the blind urine sample (p‐value between <0.001 and 0.004). In each consumption group the maximum concentration of phloretin is reached after 3 h (± 60 min). In the following urine samples, the phloretin concentration is decreasing, but still higher than in the blind urine sample. For all samples given on the day of apple consumption it is possible to distinguish between blind urine samples and spot urine samples, which can be seen as the phloretin concentration in the samples is higher as in the blind urine samples. In Fig. 3, the excretion values of phloretin for all three consumption groups (low, medium, and high) are summed up. Significant differences (t‐test, p‐value < 0.05) between the high consumption group and medium consumption group as well as to the low consumption group are clearly visible. Differences between low and medium consumption group are less distinct resulting in a p‐value of 0.075. All boxes and whiskers in all boxplot diagrams in Figs. 2 and 3 are quite wide indicating the high interindividual variations in the groups. The excretion kinetics and the excretion during the day differ between the ten subjects in each group due to interindividual variations. In Fig. 4, the excretion curves for three probands from each group (low, medium, or high consumption; 1, 2, or 4 apple) for the first 24 h after apple consumption are presented. To show the interindividual differences one curve for low, medium, and high phloretin excretion is presented for each consumption group. For example subject f (medium consumption group) shows a higher excretion rate than subjects h and g from the high consumption group. Excretion curves for every individual can be found in Supporting Information Fig. 1.
Figure 3.
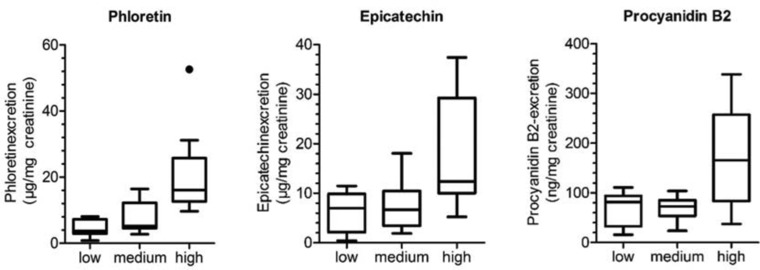
Summed up phloretin/epicatechin/procyanidin B2 excretion from the first excretion after apple consumption to the last excretion on the day of consumption for all three consumption groups (•: outliners, see Section 2.3.2 for details).
Figure 4.
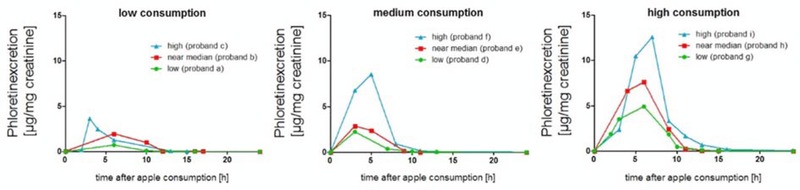
Phloretin excretion kinetics (μg/mg creatinine) for three individuals from the low, medium, and high consumption group.
3.2.2. Epicatechin excretion after apple consumption
The epicatechin excretion is similar to the phloretin excretion and the results for each consumption group are summarized in Fig. 5. The epicatechin levels in urine rise directly after consumption and the highest concentration can be detected after 3 h (± 60 min) in each group. It is possible to distinguish clearly between the blind urine samples and all samples excreted on consumption day, although epicatechin is already detectable in low background concentrations in the blind urine samples. Similar to the phloretin excretion there are high interindividual variations. The single excretion curves for each individual can be found in Supporting Information Fig. 2. Summed up epicatechin levels for the comparison of all three consumption groups are shown in Fig. 3. Alike to phloretin the distinction between low or medium and high consumption can be clearly seen, while distinction between low and medium consumption is less decided.
3.2.3. Procyanidin excretion after apple consumption
Furthermore, the excretion of the dimeric procyanidins B1, B2, B5, and B7 were analyzed. Procyanidins B1 and B7 were not detectable in any urine sample. Procyanidin B5 was found in some samples but mostly below the LOQ. Procyanidin B2 was quantifiable and a clear difference between the three groups could be seen (high consumption group 70% positive samples, medium consumption group 52% positive samples, and low consumption group 50% positive samples). The highest concentration of procyanidin B2 was detected after 3–6 h in each consumption group (Fig. 6). As for phloretin and epicatechin the high interindividual variations are responsible for the wide boxes and whiskers, but again for the samples of the consumption day it is possible to distinguish between the blind urine samples, collected before the apple consumption and up to 10 h after the consumption. Also, similar to phloretin and epicatechin, it is not possible to distinguish between urine sample from the morning after the consumption day (fourth day morning) and the blind urine sample indicating a fast excretion rate. Excretion curves for every individual can be found in Supporting Information Fig. 3. For the summed up procyanidin B2 excretion, it is possible to distinguish significantly between the low or medium consumption and the high consumption (p‐value 0.019), but not between the low and the medium consumption (p‐value 0.796), as shown in Fig. 3.
3.2.4. Quercetin excretion after apple consumption
In terms of quercetin excretion only slight effects can be seen and these were generally in relation to the high consumption group. Concentrations measured were mainly lower than the LOQ, thus no quantitation or statistical analysis was carried out.
4. Discussion
The interest in monitoring human nutrition is high due to the fact that nutrition and health are closely related and several chronic diseases are directly linked to the human diet. Up to date nutritional intake is analyzed by using mostly self‐reported data from different questionnaires or diaries, but it is well known, that these methods have some limitations as they are subjective. Therefore, there is an interest to find alternative methods to evaluate nutritional intake and the use of intake biomarkers is a promising alternative approach 1, 2, 3. The current study aimed for detection of apple consumption by analyzing intake biomarkers in urine and to distinguish between low, medium, and high apple consumption by quantifying these biomarkers.
After apple consumption a fast elevation of the concentrations of phloretin, epicatechin, and procyanidin B2 in urine could be observed in this study. We showed that phloretin, epicatechin, and procyanidin B2 can be used as short‐term intake biomarkers for apple consumption. Besides being qualitative biomarkers for apple intake, they allow for quantitative distinction between low and high intake of apples (see Fig. 3).
DuPont et al. showed that phloretin can be detected in 24 h urine after consumption of 1.1 L apple cider 22. But due to the fact that they used 24 h urine, no kinetics could be detected. In our present study, it was shown that the phloretin concentration rises to a maximum after 3 h (± 60 min) and decreases afterwards within the first 12 h after apple consumption. Mennen et al. showed that phloretin might be a potential biomarker for apple consumption, when they measured spot urine samples and 24 h urine samples from 53 subjects from the SU.VI.MAX study and found a correlation between phloretin in spot urine samples and 24 h urine samples and apple consumption 8. Since phloretin is reported to be nearly unique for apples and apple products 12, it may be used as a specific biomarker for apple consumption. Similar to the results of our study Marks et al. showed that the phloretin glucuronide concentration rises and decreases within 8 h in urine and serum from healthy subjects. The maximum in serum is reached 30 min after apple cider consumption, and in urine about 2 h after apple consumption 12. Kahle et al. also reported a fast absorption and excretion of phloretin. Their assumption is based on the fact that phloretin glucuronides were detected in ileostoma effluent from ileostoma patients so they suggest that the metabolism of phloretin and its glycosides is fast and the absorption of the aglycon is probably taking place in the small intestine 16. This fits well to our results where a maximum phloretin excretion after glucuronidase cleavage can be found already about 3 h after apple consumption and in total the phloretin is excreted within 12 h. With this result, it was proven that phloretin is a short‐term intake biomarker for apple consumption.
Kristensen et al. described epicatechin as an exposure marker of apple consumption, a marker that cannot be found in blank urine but in urine samples of rats after apple consumption 23. Kahle et al. found that after apple juice consumption besides epicatechin no other monomeric or dimeric flavanol were detectable in urine and serum 24. Previous studies from our group showed that procyanidins B1, B2, B5, and B7 were found in apples 18 and that dimeric procyanidins could be detected in pig urine after feeding a monomer‐reduced grape seed extract 20 or pure procyanidin B4 to pigs 19. We showed in the present study that besides epicatechin also procyanidins B5 and B2 were detected in human urine after apple consumption and procyanidin B2 could be quantified and might be also suitable as a supporting short‐term intake biomarker for apple consumption. It is excreted within the first 12 h after consumption, depending on the size of the apple portion. Epicatechin and procyanidin B2 are suited as biomarkers to distinguish between a low or medium and high apple consumption, but they are not specific for apples and especially epicatechin can be found in many other sources 5. In this study, we could not observe any difference between individuals drinking tea or coffee and other individuals, but epicatechin is nearly ubiquitously found in many different food samples leading to background concentrations in urine. So other than Kristensen et al. 23 in the present study epicatechin was present in low background concentrations in blind urine and rising in sample urine after apple consumption. Reason for this might be the standardized feed material used in the rat study 23 compared to the freely chosen additional diet within our study. This effect was not seen for procyanidin B2. Although it is also not unique for apples and is found in legumes as well as many different fruits 18, 25, we did not observe a higher background concentration in the blind urine. However, in our study it was possible to distinguish between a low or medium and a high apple consumption as well as between urine samples collected before and after apple consumption by quantifying the biomarkers epicatechin and procyanidin B2 in urine.
For the three biomarkers, the p‐values are lower than 0.019 for the differentiation between low and high or medium and high consumption. The differentiation between the low and medium consumption is possible by using phloretin as a biomarker (p‐value 0.075), but due to the high interindividual variations the differences between the two groups are not that clear. Using epicatechin and procyanidin B2, it was not possible to distinguish between low and medium consumption due to high variations. But for procyanidin B2, it has to be taken into account, that for low and medium consumption after 9 and 11 h, respectively, excreted concentrations are below the LOQ.
Regarding the excretion kinetics of the biomarkers it was possible to distinguish between blind urine samples and urine samples obtained after apple consumption on the day of consumption (third day), but for the most individuals it is no longer possible to distinguish between the blind urine sample and the urine sample from the next morning, 24 h after apple consumption (fourth day morning). This clearly shows that phloretin, epicatechin, and procyanidin B2 are feasible as short‐term biomarkers only.
In earlier studies Krogholm et al. (40 g against 80 g of apple) 10 and Brevik et al. (20 g against 50 g of apple) 11 did not detect a correlation between higher apple consumption and an increase in phloretin excretion. Krogholm et al. reported that high interindividual variations were observed, even though a standardized diet was used 10. While high interindividual variations have been reported quite often and were also detected in our study (see Fig. 4), all biomarkers identified in this study were suitable to monitor apple consumption despite the freely chosen additional diet of the participants. Reasons for these interindividual variations might be age, weight, smoking, and other factors that influence the absorption, metabolism, and distribution of biomarkers in the human body 26. But as discussed despite these variations it is possible to detect apple consumption by analyzing urine samples.
Quercetin is reported as a potential biomarker 17 for apples but we could not detect quercetin in concentrations over the LOQ. Since onions are a major source of quercetin and absorption of quercetin from onions is much higher than from apples, the suitability of quercetin as a biomarker for apple consumption is highly questionable 27. Hollman et al. reported that the percentage of quercetin absorbed from apples was only one third of that from onions 28
The major advantages from the study design used here are on the one hand the relatively realistic amounts of apples consumed, which in addition are high enough to show a correlation between apple intake and excreted biomarkers. On the other hand all intake biomarkers identified in this study are not influenced by the normal diet of the subjects. Furthermore, it is possible to follow the kinetics since spot urine samples were analyzed and not 24 h urine samples. The main limitation of the study is that all identified biomarkers are only short‐term biomarkers and a retrospective analysis of diet is only possible for about 1 day. However, this can be overcome by collecting several urine samples from the same individual over a longer period of time.
5. Conclusion
Phloretin, epicatechin, and procyanidin B2 can be used as short‐term biomarkers for apple consumption. They are not just biomarkers for apple intake but also to distinguish between low and high apple consumption. All three biomarkers are suitable to monitor apple consumption and are not influenced by a varied daily diet. To overcome the problem that the identified compounds are only short‐term biomarkers and are in most cases no longer detectable 24 h after apple consumption, the collection of spot urine samples over a period of 1–3 month is recommended to evaluate the individual apple consumption. Although biomarkers are not able to replace traditional methods to analyze nutrition, the identification of robust biomarkers will strengthen and validate food frequency questionnaires and other traditional methods and may in the future provide an alternative to those.
The authors have declared no conflict of interest.
Supporting information
Supporting Information Figure S1. Single probands kinetics for phloretin excretion of all 30 individuals taking part in the study, presented in groups of low, medium and high consumption (timepoint 0 h = moment of apple consumption; every six‐digit number represents one individual).
Supporting Information Figure S2. Single probands kinetics for epicatechin excretion of all 30 individuals taking part in the study, presented in groups of low, medium and high consumption (timepoint 0 h = moment of apple consumption; every six‐digit number represents one individual).
Supporting Information Figure S3. Single probands kinetics for procyanidin B2 excretion of all 30 individuals taking part in the study, presented in groups of low, medium and high consumption (timepoint 0 h = moment of apple consumption; every six‐digit number represents one individual).
Supporting Information Table S1. p‐values determined by paired t test, comparing phloretin excretion in blind urine sample with first excretion samples, samples after 0‐2 h, 2‐4 h, etc., last excretion on third day and fourth day morning sample; n is the number of samples in this group, numbers lower than 10 resulting because not every individual gave urine samples in every time slot.
Supporting Information Table S2. p‐values determined by paired t test, comparing epicatechin excretion in blind urine sample with first excretion samples, samples after 0‐2 h, 2‐4 h, etc., last excretion on third day and fourth day morning sample; n is the number of samples in this group, numbers lower than 10 resulting because not every individual gave urine samples in every time slot.
Supporting Information Table S3. p‐values determined by paired t test, comparing procyanidin B2 excretion in blind urine sample with first excretion samples, samples after 0‐2 h, 2‐4 h, etc., last excretion on third day and fourth day morning sample; n is the number of samples in this group, numbers lower than 10 resulting because not every individual gave urine samples in every time slot.
Supporting Information Table S4. P‐values determined by Mann Whitney test, comparing summed up excretions of phloretin, epicatechin and procyanidin B2 for the low, medium and high consumption groups; n is 10 for each group.
Acknowledgments
We thank Sciex for providing us with a QTRAP 5500 mass spectrometer. We are grateful to S. Lürwer for the assistance in the analysis of the urine samples.
T.S., F.H., and H.‐U.H. were involved in the concept and design of the study. T.S. and F.H. participated in the method development, sample analysis, and interpretation of data. T.S., F.H., and H.‐U.H. involved in the preparation of the manuscript. H.‐U.H. was involved in study supervision. The copyright line for this article was changed on 17 August 2018 after original online publication.
Saenger T., Hübner F., Humpf H.‐U., Mol. Nutr. Food Res. 2017, 1600629.
6 References
- 1. Shim, J.‐S. , Oh, K. , Kim, H. C. , Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Potischman, N. , Biologic and methodologic issues for nutritional biomarkers. J. Nutr. 2003, 133 (Suppl 3), 875S–880S. [DOI] [PubMed] [Google Scholar]
- 3. Zamora‐Ros, R. , Urpí‐Sardà, M. , Lamuela‐Raventós, R. M. , Estruch, R. et al., Resveratrol metabolites in urine as a biomarker of wine intake in free‐living subjects: The PREDIMED Study. Free Radic. Biol. Med. 2009, 46, 1562–1566. [DOI] [PubMed] [Google Scholar]
- 4. Gao, Y. , Urine‐an untapped goldmine for biomarker discovery? Sci. China. Life Sci. 2013, 56, 1145–1146. [DOI] [PubMed] [Google Scholar]
- 5. Krogholm, K. S. , Flavonoids as Fruit and Vegetable Intake Biomakers: Developement, Validation and Application of Flavonoid Biomarkers in Nutritional Research. Ph.D. thesis, University of Copenhagen, Copenhagen: 2011. [Google Scholar]
- 6. Spencer, J. P. E. , El Mohsen, A. , Manal M, Minihane, A.‐M. et al., Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [DOI] [PubMed] [Google Scholar]
- 7. Nielsen, S. E. , Freese, R. , Kleemola, P. , Mutanen M., Flavonoids in human urine as biomarkers for intake of fruits and vegetables. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 459–466. [PubMed] [Google Scholar]
- 8. Mennen, L. I. , Sapinho, D. , Ito, H. , Bertrais, S . et al., Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol‐rich foods. Br. J. Nutr. 2006, 96, 191–198. [DOI] [PubMed] [Google Scholar]
- 9. Mennen, L. I. , Sapinho, D. , Ito, H. , Galan, P. et al., Urinary excretion of 13 dietary flavonoids and phenolic acids in free‐living healthy subjects—variability and possible use as biomarkers of polyphenol intake. Eur. J. Clin. Nutr. 2008, 62, 519–525. [DOI] [PubMed] [Google Scholar]
- 10. Krogholm, K. S. , Haraldsdottier, J. , Knuthsen, P. , Rassmussen, S. E. Urinary total flavonoid excretion but not 4‐pyridoxic acid or potassium can be used as a biomarker for the intake of fruits and vegetables. J. Nutr. 2004, 134, 445–451. [DOI] [PubMed] [Google Scholar]
- 11. Brevik, A. , Rasmussen, S. E. , Drevon, C. A. , Frost Andersen, L. , Urinary excretion of flavonoids reflects even small changes in the dietary intake of fruits and vegetables. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 843–849. [PubMed] [Google Scholar]
- 12. Marks, S. C. , Mullen, W. , Borges, G. , Crozier, A. , Absorption, metabolism, and excretion of cider dihydrochalcones in healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2009, 57, 2009–2015. [DOI] [PubMed] [Google Scholar]
- 13. van Gorsel, H. , Li, C. , Kerbel, E. L. , Smits, M. , Kader, A. A. , Compositional characterization of prune juice. J. Agric. Food Chem. 1992, 40, 784–789. [Google Scholar]
- 14. Poyrazoğlu, E. , Gökmen, V. , Artιk, N. , Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Comp. Anal. 2002, 15, 567–575. [Google Scholar]
- 15. Young, J. F. , Nielsen, S. E. , Haraldsdottier, J. , Daneshvar, B. et al., Effect of fruit juice intake on urinary quercetin excretion and biomarkers of antioxidative status. Am. J. Clin. Nutr. 1999, 69, 87–94. [DOI] [PubMed] [Google Scholar]
- 16. Kahle, K. , Huemmer, W. , Kempf, M. , Scheppach, W. et al., Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [DOI] [PubMed] [Google Scholar]
- 17. Kahle, K. , Kempf, M. , Schreier, P. , Scheppach, W. et al., Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [DOI] [PubMed] [Google Scholar]
- 18. Rzeppa, S. , Bargen, C. , von Bittner, K. , Humpf, H.‐U. , Analysis of flavan‐3‐ols and procyanidins in food samples by reversed phase high‐performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry (RP‐HPLC‐ESI‐MS/MS). J. Agric. Food Chem. 2011, 59, 10594–10603. [DOI] [PubMed] [Google Scholar]
- 19. Bittner, K. , Kemme, T. , Peters, K. , Kersten, S. et al., Systemic absorption and metabolism of dietary procyanidin B4 in pigs. Mol. Nutr. Food Res. 2014, 58, 2261–2273. [DOI] [PubMed] [Google Scholar]
- 20. Rzeppa, S. , Bittner, K. , Döll, S. , Dänicke, S. , Humpf, H.‐U. , Urinary excretion and metabolism of procyanidins in pigs. Mol. Nutr. Food Res. 2012, 56, 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleigrewe, K. , Sohnel, A.‐C. , Humpf, H.‐U. , A new high‐performance liquid chromatography‐tandem mass spectrometry method based on dispersive solid phase extraction for the determination of the mycotoxin fusarin C in corn ears and processed corn samples. J. Agric. Food Chem. 2011, 59, 10470–10476. [DOI] [PubMed] [Google Scholar]
- 22. DuPont, M. S. , Bennett, R. N. , Mellon, F. A. , Williamson, G. , Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans. Human Nutr. Metab. 2001, 172–176. [DOI] [PubMed] [Google Scholar]
- 23. Kristensen, M. , Engelsen, S. B. , Dragsted, L. O. , LC–MS metabolomics top‐down approach reveals new exposure and effect biomarkers of apple and apple‐pectin intake. Metabolomics 2012, 8, 64–73. [Google Scholar]
- 24. Kahle Kathrin, Polyphenole aus Apfelsaft: Studien zur Verfügbarkeit im Humanstoffwechsel. Ph.D. thesis, Julius‐Maximilians‐Universität, Würzburg: 2008. [Google Scholar]
- 25. Bittner, K. , Rzeppa, S. , Humpf, H.‐U. , Distribution and quantification of flavan‐3‐ols and procyanidins with low degree of polymerization in nuts, cereals, and legumes. J. Agric. Food Chem. 2013, 61, 9148–9154. [DOI] [PubMed] [Google Scholar]
- 26. Krogholm, K. S. , Bysted, A. , Brantsæter, A. L. , Jakobsen, J. et al., Evaluation of flavonoids and enterolactone in overnight urine as intake biomarkers of fruits, vegetables and beverages in the Inter99 cohort study using the method of triads. Br. J. Nutr. 2012, 108, 1904–1912. [DOI] [PubMed] [Google Scholar]
- 27. Manach, C. , Williamson, G. , Morand, C. , Scalbert, A. , Rémésy, C. , Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [DOI] [PubMed] [Google Scholar]
- 28. Hollman, P. C. , van Trijp, J M , Buysman, M. N. , van der Gaag, M S et al., Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1. Single probands kinetics for phloretin excretion of all 30 individuals taking part in the study, presented in groups of low, medium and high consumption (timepoint 0 h = moment of apple consumption; every six‐digit number represents one individual).
Supporting Information Figure S2. Single probands kinetics for epicatechin excretion of all 30 individuals taking part in the study, presented in groups of low, medium and high consumption (timepoint 0 h = moment of apple consumption; every six‐digit number represents one individual).
Supporting Information Figure S3. Single probands kinetics for procyanidin B2 excretion of all 30 individuals taking part in the study, presented in groups of low, medium and high consumption (timepoint 0 h = moment of apple consumption; every six‐digit number represents one individual).
Supporting Information Table S1. p‐values determined by paired t test, comparing phloretin excretion in blind urine sample with first excretion samples, samples after 0‐2 h, 2‐4 h, etc., last excretion on third day and fourth day morning sample; n is the number of samples in this group, numbers lower than 10 resulting because not every individual gave urine samples in every time slot.
Supporting Information Table S2. p‐values determined by paired t test, comparing epicatechin excretion in blind urine sample with first excretion samples, samples after 0‐2 h, 2‐4 h, etc., last excretion on third day and fourth day morning sample; n is the number of samples in this group, numbers lower than 10 resulting because not every individual gave urine samples in every time slot.
Supporting Information Table S3. p‐values determined by paired t test, comparing procyanidin B2 excretion in blind urine sample with first excretion samples, samples after 0‐2 h, 2‐4 h, etc., last excretion on third day and fourth day morning sample; n is the number of samples in this group, numbers lower than 10 resulting because not every individual gave urine samples in every time slot.
Supporting Information Table S4. P‐values determined by Mann Whitney test, comparing summed up excretions of phloretin, epicatechin and procyanidin B2 for the low, medium and high consumption groups; n is 10 for each group.


