Abstract
Objective
To clarify the relevance of measuring interleukin‐6 (IL‐6) and C‐reactive protein (CRP) levels in order to predict clinical response to tocilizumab (TCZ) in rheumatoid arthritis patients.
Methods
In a pooled, post hoc analysis of 5 pivotal trials of TCZ, we examined the distributions of baseline serum concentrations of IL‐6 and CRP, stratified by randomized treatment group, and week 24 Disease Activity Score in 28 joints (DAS28) status (DAS28 <2.6 versus DAS28 ≥2.6). Relationships between early biomarker changes and later changes in DAS28 scores were evaluated using Spearman's correlations and scatterplots. Finally, percentage changes from baseline in IL‐6 and CRP levels were evaluated.
Results
Distributions of baseline IL‐6 and CRP levels were similar for patients who achieved DAS28 scores <2.6 within 6 months of TCZ initiation and those who did not. Correlations between early changes in these 2 biomarkers and change in DAS28 scores were low (rho < 0.3 for all). Mean percentage increases from baseline in IL‐6 concentrations were observed in all treatment groups (highest in the 8 mg/kg dose group); mean percentage decreases in CRP concentrations were greater at week 2 and at all visits for the 8 mg/kg dose group.
Conclusion
Baseline serum concentrations of IL‐6 and CRP may not be predictive of clinical outcomes after TCZ treatment. Data demonstrate the efficacy of TCZ in patients across a broad range of baseline serum IL‐6 and CRP concentrations. Similarly, changes in these biomarkers after TCZ dosing are expected and may or may not correspond to changes in other clinical signs and symptoms. These results complement previous reports describing the complex interactions among biomarker changes, other therapeutic mechanisms of action, and clinical outcomes.
Introduction
In the past 15 years, therapeutic options for patients with rheumatoid arthritis (RA) have grown extensively; 9 biologic therapies and 1 novel oral medication have been approved by the US Food and Drug Administration, allowing physicians and patients more choices for disease control. However, because of the heterogeneity of RA and differences in the targeted mechanisms of action of the drugs, not every patient will respond initially or maintain response to a given therapy. The American College of Rheumatology and the European League Against Rheumatism recommend treat‐to‐target as a standard of care 1, 2, 3. Implicit in this approach is the maintenance of tight disease control, influenced by regular assessments of disease activity, which may require switching therapies. At the same time, interest has been growing in identifying biomarkers to help clinicians stratify patients and match them to personalized, efficacious treatment options 4.
Box 1. Significance & Innovations.
The interaction between the mechanism of action and biomarkers is often assumed to be correlated with clinical effects, but may not be.
Baseline values of C‐reactive protein (CRP) and interleukin‐6 (IL‐6) are not predictive of clinical outcomes after tocilizumab (TCZ) treatment.
Changes in CRP and IL‐6 levels after TCZ treatment may or may not correspond to changes in other clinical signs and symptoms.
Given the targeted nature of modern medicines, the rheumatologist is tempted to attribute prognostic relevance to serum markers thought to be associated with disease. However, given the complexity of the immunologic networks that drive RA, limited progress has been made in identifying generalized or treatment‐specific biomarkers. Progress has also been restricted by the intrinsic impact of a treatment's specific mechanism of action. Wang et al 5 attempted to address these constraints by investigating the molecular pathway of the target of tocilizumab (TCZ) therapy but found that the variation in pathway activity, as measured in blood, may not be a strong predictor of treatment response in RA.
TCZ is a recombinant, humanized, anti–human monoclonal antibody directed against the soluble interleukin‐6 receptor (sIL‐6R) and the membrane‐bound IL‐6 receptor (mIL‐6R). IL‐6 is a proinflammatory, multifunctional cytokine produced by a variety of cell types and involved in diverse physiologic and immunologic processes. Moreover, elevated tissue and serum levels of IL‐6 have been implicated in the pathology of several inflammatory and autoimmune disorders, including RA. TCZ has been shown to inhibit sIL‐6R– and mIL‐6R–mediated signaling 6. Inhibition of this signaling is associated with the rapid reduction of acute‐phase reactants, such as C‐reactive protein (CRP) levels, and the elevation of serum IL‐6 levels after TCZ dosing 7.
Although identifying predictive biomarkers is a desirable endeavor, equally important is the consideration of how an individual drug can be differentiated according to its mechanism of action 8. One might argue that recognizing the nonpredictive nature of otherwise “obvious” targets is imperative in order to avoid unnecessary expense and lost time (time during which a patient's disease could progress). The objective of this brief report is to describe the relevance of measuring IL‐6 and CRP levels in the context of patient selection and clinical response prediction for TCZ therapy.
Patients and methods
Detailed descriptions of the pivotal trial study designs, patient populations (n = 4,186 randomized), and results that demonstrated the efficacy and safety of TCZ, leading to its initial approval for the treatment of patients with RA, have been published previously 9, 10, 11, 12, 13. In brief, clinical signs and symptoms were measured at baseline and every 4 weeks thereafter; CRP levels were measured at baseline, at weeks 2 and 4, and then every 4 weeks thereafter; and IL‐6 was measured in all patients at baseline, at week 12, and at week 24. All serum samples except those at week 2 were drawn just before dosing. In this post hoc analysis, distributions of baseline serum concentrations of IL‐6 and CRP, stratified by randomized treatment group, and week 24 Disease Activity Score in 28 joints (DAS28) status (did or did not achieve DAS28 <2.6) were examined using box plots. Spearman's correlations and scatterplots were used to evaluate relationships between early changes in these biomarkers versus later changes in DAS28 status. Finally, means and 95% confidence intervals for percentage changes from baseline in IL‐6 and CRP levels were estimated by visit and treatment group. Patients were analyzed according to their randomized treatment groups. DAS28 status was assessed using nonresponder imputation: patients with missing data were counted as having DAS28 ≥2.6. DAS28 change from baseline was analyzed as observed, except in the event of patient escape, when scores were set to missing. CRP and IL‐6 biomarkers were analyzed as observed, with no imputation for missing data, and IL‐6 values below the limit of quantitation were set at the lower limit (3.13 pg/ml).
Results
Mean ± SD baseline CRP levels were 2.6 ± 3.2 mg/dl (elevated as per the inclusion criterion), and mean ± SD baseline IL‐6 levels were 40.1 ± 59.50 pg/ml. Neither baseline nor change from baseline level of CRP or IL‐6 was associated with clinical response to TCZ in the 4 mg/kg or 8 mg/kg doses. Distributions of baseline CRP and IL‐6 values were similar for patients who did and did not achieve DAS28 <2.6 at week 24 (Figures 1A and 2A), and no meaningful correlations or other obvious associations were observed between early changes in these 2 biomarkers and change in DAS28 to week 24 in any treatment group (all rho < 0.30) (Figures 1B and 2B). The same analyses conducted using the Clinical Disease Activity Index (CDAI) outcome in lieu of the DAS28 also did not indicate associations between biomarkers at baseline and changes from baseline with clinical response (data not shown). Mean percentage increases in IL‐6 concentrations from baseline were observed in all treatment groups and were highest in the 8 mg/kg dose group (Figure 1C). Mean percentage decreases in CRP concentrations were observed after the first dose of study medication in a dose‐dependent manner, with the largest decreases occurring in the 8 mg/kg group (Figure 2C).
Figure 1.
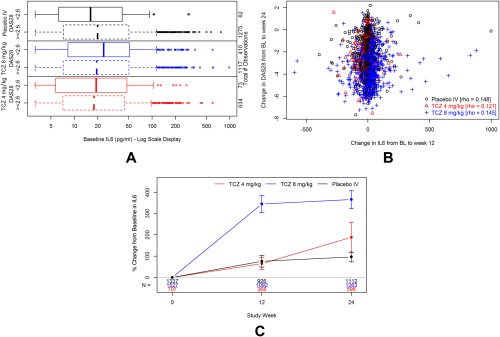
Interleukin‐6 (IL6) serum concentrations during study treatment with tocilizumab (TCZ) 8 mg/kg with or without a concomitant disease‐modifying antirheumatic drug (DMARD), TCZ 4 mg/kg plus DMARD, or placebo (intravenous [IV]) plus DMARD. A, Box plot showing similar distributions of baseline (BL) IL‐6 levels by treatment arm and week 24 Disease Activity Score in 28 joints (DAS28) status. B, Scatterplot with Spearman's rank correlation for change from BL to week 12 in IL6 levels versus change from baseline to week 24 in DAS28 status. C, IL6 mean percentage change from baseline with 95% confidence interval by visit.
Figure 2.

C‐reactive protein (CRP) serum concentrations during study treatment with tocilizumab (TCZ) 8 mg/kg with or without a concomitant disease‐modifying antirheumatic drug (DMARD), TCZ 4 mg/kg plus DMARD, or placebo (intravenous [IV]) plus DMARD. A, Box plot showing similar distributions of baseline (BL) CRP by treatment arm and week 24 DAS28 status. B, Scatterplot with Spearman's rank correlation for change from BL to week 4 in CRP levels versus change from BL to week 24 in DAS28 status. C, CRP mean percentage change from BL with 95% confidence interval by visit.
Discussion
These results hold important implications for clinical practice. Although it might seem intuitive that IL‐6 or CRP levels would have predictive value in identifying patients more likely to respond to the unique mechanism of action of TCZ, here we show that the baseline values of these biomarkers are not predictive of clinical outcomes after TCZ treatment. Data demonstrate that TCZ can be efficacious in patients across a broad range of serum IL‐6 and CRP concentrations at baseline. Similarly, dramatic changes in these biomarkers after dosing are expected for many patients and may or may not correspond to changes in other clinical signs and symptoms. These results are similar to a previous study with TCZ. CRP levels were normalized during treatment; however, remission rates were comparable when disease activity was analyzed using an index that included CRP (Simplified Disease Activity Index) or did not include CRP (CDAI) 14, 15. This study is only 1 example of the complex interactions between key inflammatory mediators in RA that can be further complicated by the diversity of therapeutic mechanisms of action, making identification of predictive biomarkers challenging. The lack of effective biomarkers at this stage to predict clinical response to any treatment has been highlighted by others 8. In the meantime, the rheumatologist is left to rely on evaluating therapeutic response through comprehensive clinical assessments of how the disease is progressing and how the patient feels and functions.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Devenport had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Wang, Devenport, Low, Yu, Hitraya.
Acquisition of data
Wang, Devenport, Low, Yu, Hitraya.
Analysis and interpretation of data
Wang, Devenport, Low, Yu, Hitraya.
ROLE OF THE STUDY SPONSOR
Hoffmann‐La Roche and Genentech sponsored this post hoc analysis using existing data from earlier pivotal studies conducted by Roche. Publication of this article was not contingent upon approval by Hoffmann‐La Roche and Genentech.
Supported by Hoffmann‐La Roche and Genentech.
The copyright line for this article was changed on 14 August 2018 after original online publication.
REFERENCES
- 1. Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux‐Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson WH, Lindstrom TM, Cheung RK, Sokolove J. Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat Rev Rheumatol 2013;9:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Platt A, Upmanyu R, Germer S, Lei G, Rabe C, et al. IL‐6 pathway‐driven investigation of response to IL‐6 receptor inhibition in rheumatoid arthritis. BMJ Open 2013;3:e003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishimoto N, Kishimoto T. Humanized antihuman IL‐6 receptor antibody, tocilizumab. Handb Exp Pharmacol 2008;181:151–60. [DOI] [PubMed] [Google Scholar]
- 7. Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, et al. Tocilizumab inhibits signal transduction mediated by both mIL‐6R and sIL‐6R, but not by the receptors of other members of IL‐6 cytokine family. Int Immunopharmacol 2005;5:1731–40. [DOI] [PubMed] [Google Scholar]
- 8. Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol 2013;9:154–63. [DOI] [PubMed] [Google Scholar]
- 9. Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, et al. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 10. Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin‐6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease‐modifying antirheumatic drugs: the Tocilizumab in Combination With Traditional Disease‐Modifying Antirheumatic Drug Therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 11. Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL‐6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti‐tumour necrosis factor biologicals: results from a 24‐week multicentre randomised placebo‐controlled trial. Ann Rheum Dis 2008;67:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez‐Reino JJ, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremer JM, Blanco R, Brzosko M, Burgos‐Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double‐blind treatment phase of a randomized placebo‐controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609–21. [DOI] [PubMed] [Google Scholar]
- 14. Smolen JS, Aletaha D. Interleukin‐6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute‐phase reactants. Arthritis Rheum 2011;63:43–52. [DOI] [PubMed] [Google Scholar]
- 15. Kay J, Morgacheva O, Messing SP, Kremer JM, Greenberg J, Reed GW, et al. Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res Ther 2014;16:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]


