Abstract
Scope
Micronutrients are in small amounts in foods, act in concert, and require variable amounts of time to see changes in health and risk for disease. These first principles are incorporated into an intervention study designed to develop new experimental strategies for setting target recommendations for food bioactives for populations and individuals.
Methods and results
A 6‐week multivitamin/mineral intervention is conducted in 9–13 year olds. Participants (136) are (i) their own control (n‐of‐1); (ii) monitored for compliance; (iii) measured for 36 circulating vitamin forms, 30 clinical, anthropometric, and food intake parameters at baseline, post intervention, and following a 6‐week washout; and (iv) had their ancestry accounted for as modifier of vitamin baseline or response. The same intervention is repeated the following year (135 participants). Most vitamins respond positively and many clinical parameters change in directions consistent with improved metabolic health to the intervention. Baseline levels of any metabolite predict its own response to the intervention. Elastic net penalized regression models are identified, and significantly predict response to intervention on the basis of multiple vitamin/clinical baseline measures.
Conclusions
The study design, computational methods, and results are a step toward developing recommendations for optimizing vitamin levels and health parameters for individuals.
Keywords: community‐based participatory research, metabolic health, micronutrients, targeted and systems nutrition
1. Introduction
Micronutrients are indispensable bioactive compounds for maintaining health and resiliency. However, the need for using vitamin and mineral supplements in most developed and developing countries remains controversial despite their essential role in biochemical reactions. The “Enough is Enough” editorial1 claiming that multiple micronutrient supplements were not needed in today's food environment sparked robust rebuttals (e.g., ref. [2, 3, 4]). Many of the controversies in this field stem from using chronic disease (e.g., ref. [5, 6, 7, 8]) as an outcome for randomized clinical trials (RCTs; reviewed9) and observational studies (reviewed in Ref. [10]) because of the (i) many factors that contribute to complex phenotypes, (ii) the (usually) long time necessary to develop disorders, (iii) differences between choice of micronutrients, their doses, and the length of observation, (iv) genetic and other differences between populations and between cases and controls, and (v) the inability to accurately measure exposure to environmental elements.
The debate on the value of multi‐micronutrient supplements may be simplified by returning to first principles: (micro)nutrients and most bioactive components (i) are present in food in small amounts (nutrients are not drugs), (ii) act in concert by interacting with each other and other nutrients, and (iii) may require short to long latency to affect biochemistry or physiology depending on the nutrient(s), individual genetic makeup, and the complexity of the outcome (e.g., intermediate risk factor, chronic disease, or death).11, 12, 13 Analyzing the effects of nutrients on human metabolism, that is, the design of human studies, should reflect these principles. With a few exceptions (e.g., ref. [14, 15]) the current research paradigm is (usually) to (i) add one or a few micronutrients to existing diets, (ii) collect and analyze physiological data without (usually) placing the individual in the context of their environment (e.g., ref. [16, 17, 18]), (iii) use chronic disease or even death as an outcome, (iv) mask interindividual variation in diet intakes, genetic makeup, and metabolic physiology by (v) using randomized control experiments that determine population attributable risk19 rather than individual risk or benefit.
We designed and conducted a multivitamin/mineral supplement intervention (Figure 1) in children and adolescents to address these limitations (Box 1). Our more comprehensive approach analyzed aggregated data from all participants but also allowed for analyzing individual responses, a variation of n‐of‐1 study designs20, 21, 22, 23, 24 (reviewed in ref. [25]).
Figure 1.
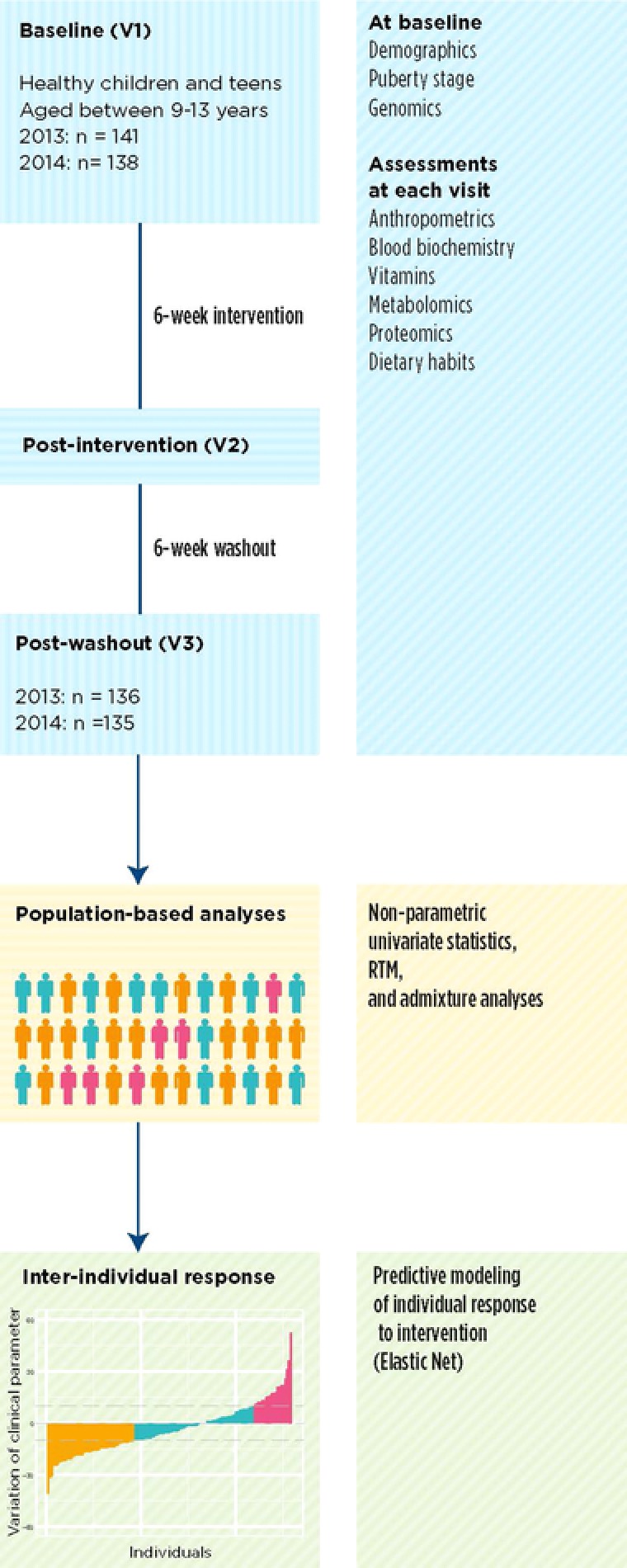
Intervention design and overview of statistical analysis.
Box 1: Features of the Experimental Design
Used a multivitamin/mineral supplement containing ≈100% daily recommended allowances and less than upper tolerable level of each of 12 vitamins and five minerals for 5 days a week for 6 weeks.
Monitored compliance to the intervention.
The same intervention was repeated the next year to test the replicability of the results.
Assessed effectiveness by comparing changes in clinical and omics variables at baseline, after the intervention, and following a 6‐week washout period. Clinical results were available at the end of intervention yielding assessment of effectiveness of the intervention.
Twenty‐four‐hour dietary recalls (24HR) and food frequency questionnaires (FFQ) were used to assess nutrient intakes at baseline, after the intervention, and following a washout.
An n‐of‐1 study with no control group but data analyzed by aggregation to determine population level responses as well as individual responses. Several statistical tests verified the effects of the intervention.
Clinical and other responses to the intervention were correlated with comprehensive analyses of plasma vitamins, clinical variables, and genetic profiles. Individuals with similar baseline or like responses can be grouped based on these correlations.
The diets of individuals aged 9–13 are often insufficient in micronutrients.135
The study was conducted using principles and methods for community based participatory research121, 122, 123 to accelerate the translation of research to knowledge for participants and their families.
2. Experimental Section
2.1. Population
Children and adolescents were recruited from three schools in the west side of Ribeirão Preto (Administrative district 2)—see Supporting Information 1 for more detailed description of the built environment. Volunteers were of 9–13 years, 11 months, and 29 days old at study initiation and clinically stable. Participants underwent assessment by a pediatrician (i) at baseline to assess pubertal stage according to Tanner criteria26 and (ii) at the three data collection visits to determine clinical status of the exclusion criteria. See Supporting Information 1 for exclusion criterion.
Enrolled participants signed the statement of informed assent and a parent of each participant signed informed consent. Participants received Institutional Review Board (Brazilian National Ethics Committee, CONEP 00969412.6.0000.5440) approved compensation as well as breakfast and lunch following blood draws on each assessment visit. The trial was registered on http://ClinTrials.gov (NCT01823744).
2.2. Study Design
The study design is described in Figure 1 and Box 1. The trial was conducted in the same period of the year (March to June) in two consecutive years (2013 and 2014). In order to achieve study objectives, the doses of micronutrients provided ≈100% daily recommended allowances and less than upper tolerable level for most nutrients. A preintervention test of the product determined that children under 12 years were more willing to accept two rather than three milk bars. Hence, participants who were aged between 9 and 11 years received two tablets of Nestrovit (Supporting Information Table S1) while those aged between 12 and 13 years received three tablets. These different doses also met a criterion of being <5% of estimated daily energy intake per age group27, 28 to avoid changes in appetite. Six of the authors individually monitored supplement intake at the beginning of each school period. The compliance rate was 98% over both years.
2.3. Assessments, Blood Collection, Sample Analysis
Assessments (Figure 1) were conducted on Saturdays and Sundays for all time points with 60–70 participants on each day. Trained researchers performed all physiological measurements and interviews which included 24HR and FFQ, anthropometric measurements, and impedance analysis (see Methods in Supporting Information 1). The questionnaire for socioeconomic rating was from Associação Brasileira de Empresas de Pesquisa.29
Fasted (12 h) blood samples (total of 21 mL) were taken immediately after check‐in procedures were completed. Blood was collected in EDTA tubes for metabolomics, proteomics, and RBC fatty acid profiling, in PAXgene for DNA analysis (additional 3 mL at baseline), and separately in ACD tubes for clinical biochemistry. Another blood sample was collected in heparin tubes (for NMR metabolomics) and frozen. All samples were coded at the time of collection, centrifuged, aliquoted, and frozen at –80 °C for further analyses. Clinical biochemistry analyses were done immediately after the blood draw in the USP Hospital Clinical Laboratory using standard procedures on a Weiner Lab CT 600i (Diamond Diagnostics). The variables analyzed (Supporting Information 1, Table 2) and methods of analyses of micronutrients in plasma, genotype analysis, and admixture determination are described in Supporting Information 1.
2.4. Assessment of Dietary Habits
Food frequency questionnaires (FFQs)30, 31, 32 and one 24HR32, 33 were used on the three assessment days to assess habitual dietary habits and day‐before‐blood‐draw nutrient intake, respectively. Both methods were used because habitual diets assessed through FFQs are considered more relevant for studying gene—nutrient interactions while 24‐h intakes may affect plasma metabolite levels (reviewed in ref. [34]). Energy intakes are often overestimated with FFQs30, 35 compared to 24HR (which was found in this study, manuscript in preparation). Since under and over reporting in food intake studies are well known,36, 37, 38, 39, 40, 41, 42 participants who reported consuming less than 0.79 or above 2.4 times their basal metabolic rate calculated using Schofield formula43 were therefore excluded from diet‐related analysis.44, 45 Eighty‐five participants in 2013 and 84 in 2014 were included to generate the aggregated FFQ food intake results.46, 47 DietWin Profissional software version 2011 (http://www.dietwin.com.br) was used for analyzing 24HR and FFQ dietary intake data (see Supporting Information 1 for more details). The nutritive value of the foods was analyzed for energy, minerals, and macro‐ and micronutrients. Values were adjusted for total energy intake prior to analysis. Revised Brazilian healthy eating index (HEI) scores were derived using the 24‐hr intake records48, 49, 50, 51 from the 3 assessment days.
2.5. Statistical Analyses
All statistical analyses (Figure 1) were performed with R statistical software version 3.0.1. Nonparametric Mann–Whitney and Kruskal–Wallis tests were used for between‐group and cohort comparisons, respectively. Chi‐square tests were used to compare distribution of categorical variables (i.e., sex and Tanner scores). Nominal association was set to 5% for unadjusted p‐values. When specified, p‐values were corrected for multiple testing calculating the false discovery rate (FDR) adjusted p‐values using the Benjamini–Hochberg procedure52 and the significance threshold set to 0.1, except where noted otherwise. All values are reported as median [1st quartile–3rd quartile] except where noted otherwise.
Vitamin and clinical variables were excluded if there were more than 25% missing values at any time point. Outliers were identified using principal component analysis for clinical and vitamin parameters separately and for each time point. Outliers were defined as samples falling outside of the 99% confidence ellipse at any time point. Six individuals were identified as statistical outliers for clinical variables in 2013 and three individuals in 2014. Six individuals were statistical outliers for circulating vitamin levels in 2013 and seven individuals in 2014. These subjects were excluded from further analyses.
2.6. Regression to the Mean
The algorithm of Ostermann et al.53 assesses the significance of intervention after accounting for regression to the mean (RTM), which may occur in situations of repeated measurements when extreme values have a tendency to regress to the population mean. This method required specification of the “true” population mean for each analyzed variable. Due to the lack of population‐level assessments on the many of the measurements described here, averages of values obtained at study initiation (time point 1) and following washout (time point 3) were used as true population mean for each response variable.
2.7. Prediction Model of Response to Intervention
We also performed an analysis to identify predictive models that explain the variability in response to intervention. The 2013 data were used as the training set and therefore z‐score normalized and then tested on 2014 results by normalizing that data using 2013 scaling values (mean and SD). We performed elastic net regression to explore whether multiple variables (including baseline clinical biochemistry, blood vitamin levels, and dietary intakes) could explain variation in response to intervention in each of clinical endpoint using the glmnet 54 R package. We used tenfold cross‐validation for selection of lambda (and thus severity of penalty) that minimized mean squared error. We used these results to build models to predict response of each clinical variable and vitamin (with response defined as change between visit 1 (V1) and 2 (V2)—i.e., V2–V1) from baseline clinical biochemistry and vitamin levels.
For each response variable, elastic net coefficients were then estimated with 1000 bootstrapped samples of 2013 data using the approach proposed by Bach55 that included ten clinical response variables, age, sex, BMI, plasma levels of 14 vitamins, four subclasses of vitamins, and three minerals since these variables are associated with physiological effects. Each bootstrapped model was then tested for accuracy of prediction of 2014 response to intervention to determine model performance. Accuracy was defined as Pearson correlation coefficient and p‐value from Pearson correlation test between predicted and observed 2014 response to intervention. All p‐values were adjusted for multiple testing using the Benjamini & Hochberg procedure56 and a significance threshold was set at p = 0.1. A simple model was also tested that included only a variable's own baseline as predictor (e.g., glucose response–glucose baseline), to compare with the more complex models. We assessed (i) prediction of blood vitamin response to intervention from baseline clinical and vitamin levels, and (ii) prediction of clinical response to intervention from vitamin response to intervention.
3. Results
3.1. Baseline Population Characteristics
Of the 141 and 139 children and adolescents enrolled in 2013 and 2014, respectively, 136 and 135 participants completed all assessments and were included in subsequent analyses (Figure 1 and Supporting Information Table S3). After removing outliers and siblings, 120 and 133 participants were analyzed for 2013 and 2014 (Table 1). The percentages of this cohort who were severely thin, underweight, eutrophic, overweight, and obese, was similar between years and 2 year averages were 2.5, 9.3, 44.8, 21.4, and 21.8%, respectively. Almost 16% of all participants were classified as having dyslipidemias (Supporting Information Table S3), defined for Brazilian children in ref. [57]. Waist circumference and fat mass were similar between years as were fasting glucose and lipid profiles (Table 1). Baseline values of a subset of clinical parameters (Table 1, marked by*) and plasma levels of vitamins and minerals (Table 2, marked by letters) were significantly different between years. These differences could not be explained by age, gender, pubertal status, or corrected HEI dietary intake scores differences (see 3.2) and may reflect variations in unmeasured environmental factors.
Table 1.
Baseline demographics, anthropometric, and clinical characteristics of participants in 2013 (n = 120) and 2014 (n = 133)
| Median v1 2013 | Q1–Q3 2013 | Median v1 2014 | Q1–Q3 2014 | Mann–Whitney p‐Value 2013 versus 2014 | |
|---|---|---|---|---|---|
| n (outliers removed) | 120 | – | 133 | – | – |
| Sex (% female) | 57.1 | – | 54.9 | – | 6.8 × 10–01 |
| Tanner Score (for stages 1/2/3/4/5)a | 12/52/40/12/3 | – | 7/33/60/30/3 | – | 1.7 × 10–03 |
| Age (years)a | 12.1 | 11.1–12.9 | 12.52 | 11.7–13.5 | 1.5 × 10–04 |
| Weight (kg) | 44.6 | 36.5–57.8 | 45.5 | 38.5–59.1 | 3.3 × 10–01 |
| Height (cm)a | 152.6 | 146.7–158.9 | 154.8 | 150.1–159.1 | 2.4 × 10–02 |
| BMI (kg m–2) | 19.2 | 16.3–22.5 | 19.0 | 16.6–23.4 | 8.9 × 10–01 |
| Waist circumference (cm) | 71.2 | 62.3–80.7 | 66.5 | 60.7–83.1 | 1.9 × 10–01 |
| Socio economic status (A1/A2/B1/B2/C1/C2/D) | 0/6/19/42/33/14/4 | – | 0/4/17/45/37/18/9 | – | 2.3 × 10–01 |
| Fat free mass (% of body weight) | 75.8 | 69.6–81.0 | 76.8 | 72.0–78.5 | 2.1 × 10–01 |
| Fat mass (% of body weight) | 24.1 | 19.8–30.0 | 23.2 | 18.8–30.0 | 2.4 × 10–01 |
| Glucose (mg dL–1) | 91.5 | 89.0 | 94 | 90.0 | 9.4 × 10–02 |
| Total cholesterol (mg dL–1) | 167 | 144.8–179.5 | 161 | 141–175.5 | 2.5 × 10–01 |
| LDL‐cholesterol (mg dL–1) | 106.5 | 86.8–116.5 | 100 | 83–106.8 | 2.1 × 10–01 |
| HDL‐cholesterol (mg dL–1) | 45.5 | 38.8–53.0 | 45 | 39.0–52.0 | 8.5 × 10–01 |
| Triglycerides (mg dL–1) | 68 | 48.0–82.3 | 60 | 46.0–90.8 | 1.4 × 10–01 |
| Mean corpuscular volume (fl)a | 85.35 | 82.4–87.7 | 82.65 | 81.0–84.7 | < 1 × 10–02 |
| Mean corpuscular hemoglobin (pg)a | 28.25 | 27.2–29 | 30.2 | 29.4–31.0 | < 1 × 10–02 |
| Basophils (% of total white blood cells)a | 1.11 | 0.9–1.5 | 1.3 | 1.1–1.6 | < 1 × 10–02 |
| Platelets (number of cells x 103/μL)a | 277 | 243–323 | 250 | 222–292 | < 1 × 10–02 |
| Albumin (g dL–1) | 4.5 | 4.4–4.7 | 4.5 | 4.4–4.7 | 9.8 × 10–01 |
| Calcium (mmol L–1)a | 10.4 | 10.2 –10.5 | 10.8 | 10.3–10.5 | 1.7 × 10–16 |
| Iron (mg dL–1)a | 93 | 73.0 –110.2 | 82 | 64.0–108.8 | 4.1 × 10–03 |
| Phosphate (mg dL–1)a | 4.9 | 4.6–5.2 | 4.7 | 4.4–5.3 | 2.5 × 10–02 |
Significant difference between year marked by.
Table 2.
Baseline vitamin levels of participants in 2013 and 2014 and normal population rangesa
| Median 2013b | Q1–Q3 | Median 2014b | Q1–Q3 | Mean or Median Refe | 95% CI Ref | Age Year | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Retinol (μg mL–1) (Vit A) | 0.3 (n = 106) | 0.3–0.4 | 0.4c (n = 96) | 0.3–0.5 | 0.36 | 0.36–0.37 | 6–11 | 58 |
| 0.47 | 0.45–0.48 | 12–19 | ||||||
| β–Carotene (μg mL–1) (Vit A precursor)* | 0.2 (n = 103) | 0.1–0.3 | 0.2 (n = 94) | 0.1–0.3 | 0.13 | 0.12–0.14 | 6–11 | 58 |
| 0.09 | 0.09–0.10 | 12–19 | ||||||
| Thiamine (Vit B1)* | 3.3 (n = 73) | 2.5–4.3 | 2.7 (n = 74) | 2.4–3.4 | 6.8 | 4.5–7.0 | 5–12 | 129 |
| Thiamine monophosphate (VitB1) | 8.5 (n = 105) | 6.0–11.4 | 10.0c (n = 93) | 7.6–14.1 | 6.8 | 5.8–9.0 | 5–12 | 129 |
| Thiamine triphosphate (VitB1)* | 4.6 (n = 4) | 4.07–5.28 | 3.3 (n = 80) | 2.8–3.8 | 9.0 | 8.4–10.7 | 5–12 | 129 |
| Riboflavin (VitB2)* | 10.8 (n = 98) | 8.0–15 | 12.1 (n = 92) | 8.2–16.5 | 20.1 ± 3.0 | 12.5–44.6 | 10–18 | 130 |
| Flavin adenine dinucleotide (VitB2) | 44.8 (n = 100) | 36.0–52.0 | 31.9c (n = 95) | 28.1–36.9 | 55.0 | 30.0–120.0 | 10–18 | 130 |
| Flavin mononucleotide (VitB2) | 10.6 (n = 98) | 8.2–13.7 | 8.0c (n = 79) | 6.7–10.2 | 13.0 ± 0.7 | 10.2–18.4 | 10–18 | 130 |
| Nicotinamide (VitB3) | 384.0 (n = 106) | 310.0–454.0 | 446.5c (n = 97) | 369–521.2 | 261.0 ± 217 | 20–34 | 131 | |
| Nudifloramide (VitB3) | 870.0 (n = 106) | 656.0–1305.0 | 943.5 (n = 97) | 691.0 –1397.0 | no ref | – | – | – |
| Pantothenic Acid (Vit B5) | 212.0 (n = 106) | 180.0–260.0 | 197.0d (n = 96) | 168.5–234.5 | no ref | – | – | – |
| Pyridoxic Acid (Vit B6)f | 16.8 (n = 106) | 12.3–23.0 | 22.4 (n = 68) | 17.3–26.8 | 23.5 | 21.8–25.5 | 6–11 | 58 |
| 20.9 | 19.9–22.0 | 12–19 | ||||||
| Pyridoxal (Vit B6) | 7.3 (n = 104) | 5.9–9.4 | 7.9 (n = 97) | 6.2–9.5 | 21.1 | Range 8.8–58.7 | 1–18 | 132 |
| Pyridoxal 5′–phosphate (Vit B6)f | 32.9 (n = 76) | 24.4–44.4 | 32.7 (n = 88) | 24.8–45.7 | 33.9 | Range 20.5–151 | 1–18 | 132 |
| Folate (ng mL–1) (Vit B9) | 4.9 (104) | 3.8–6.5 | 4.17c (95) | 3.2–5.3 | 16.1 | 15.6–16.6 | 6–11 | 58 |
| 11.2 | 11.0–11.5 | 12–19 | ||||||
| 5–Methyl–tetrahydrofolic acid (Vit B9) | 21.1 (n = 104) | 12.4–29.6 | 20.8 (n = 95) | 10.0–31.1 | 91.0 | Low–High 26.4–219.7 | 11–15.9 | 133 |
| Para– aminobenzoylglutamic acid (Vit B9)f | 3.6 (n = 51) | 2.7–4.7 | 7.3 (n = 97) | 4.7–9.8 | 11.9 ± 7.6 | – | 40 ± 1 | 134 |
| Cobalamin (pg mL–1) (Vit B12) | 371 (n = 105) | 290.8–464.5 | 410c (n = 96) | 319–550 | 728 | 713–743 | 6–11 | 58 |
| 510 | 499–521 | 12–19 | ||||||
| 25–hydroxycholecalciferol (25‐OH‐VitD3) | 64 (n = 106) | 54.7–82.4 | 70.2 (n = 96) | 56.8–81.2 | 63.8 | 61.6–66.1 | 6–11 | 58 |
| 55.1 | 52.4–58.0 | 12–19 | ||||||
| α‐tocopherol (μg mL–1) (Vit E) | 5.8 (n = 104) | 5.1–6.8 | 6.2 (n = 97) | 5.4–7.0 | 8.2 | 8.0–8.4 | 6–11 | 58 |
| 7.6–7.8 | 12–19 | |||||||
| γ‐tocopherol (μg mL–1) (Vit E) | 0.8 (n = 106) | 0.5–1.1 | 0.8 (n = 97) | 0.7–1.1 | 1.82 | 1.7–1.9 | 6–11 | 58 |
| 1.79 | 1.7–1.9 | 12–19 |
Although 26 circulating forms were analyzed, 22 had detectable levels or greater than 25% missing values at any time point.
All values are in nmol L–1. Values in parentheses represent the number of individuals included in analysis.
Mann–Whitney FDR adjusted p‐value < 0.05 versus 2013.
Mann–Whitney FDR adjusted p‐value < 0.1 versus 2013.
Values from58 are geometric means.
Variables were not considered in other analyses since they had more than 25% missing values at any time point.
3.2. Dietary Habits and HEI Quality
Variation of energy, carbohydrate, protein, total fat, vitamins, and minerals intakes between time points were also analyzed (data not shown); dietary habits did not change during the course of the intervention and washout periods in 2013 while only intakes of saturated fat and calcium increased (p < 0.1) during washout period in 2014 after removing over‐ and under‐reporters and statistical outliers. Usual dietary habits, estimated by averaging all time points per year, were similar in reported total energy and macronutrient intake between years. Average intakes of polyunsaturated fat, β‐carotene and retinol (vitamin A), vitamin C, and heme iron were higher while total fiber was lower in 2013 (p < 0.05; Supporting Information Table S4). Total scores for the revised Brazilian HEI were 53.7 [46.2–60.7] and 54.5 [47.5–63.8] for 2013 and 2014, respectively (p > 0.05), corresponding to sub‐optimal diets with a lack of vegetables, fruits, and whole cereals (detailed in a separate, submitted manuscript).
3.3. Variability of Baseline Vitamin Levels
The circulating levels of most vitamins were below normal ranges for a pediatric and adolescent population based on best available reference values (Table 2) as per the Center for Disease Control and Prevention cutoff references values,58 which ranged from 2.1% for vitamin B12 to 23.1% for α‐tocopherol (Table 3). These data suggest that the study cohort had hidden hunger,59, 60 defined as sufficient energy intake but with insufficient consumption of micronutrients. None of the baseline variables correlated with age, anthropometric measurements, diet, and only vitamin D showed an association with sex: 25‐hydroxy‐vitamin D3 was significantly lower in females (median = 61 and 68 for 2013 and 2014, respectively) than males (median = 74 and 71 for 2013 and 2014, respectively). Certain circulating metabolites were statistically correlated with other metabolites (nutrient–metabolite or nutrient–nutrient interactions; Supporting Information Figures S4–S9) in one or both years, but none of these correlations were significant after multiple testing.
Table 3.
Prevalence of deficiencies based on CDC cutoffsa
| Median 2013 | Q1–Q3 | Median 2014 | Q1–Q3 | Cutoff | Deficiencies 2013 (%) | Deficiencies 2014 (%) | |
|---|---|---|---|---|---|---|---|
| Folate (ng mL–1) | 4.9 | 3.8–6.5 | 4.17b | 3.2–5.3 | < 2 | 4.8 (n = 104) | 7.4 (n = 95) |
| Retinol (μg mL–1) | 0.3 | 0.3–0.4 | 0.4b | 0.3–0.5 | < 0.2 | 3.8 (n = 106) | 3.1 (n = 96) |
| α‐Tocopherol (μg mL–1) | 5.8 | 5.1–6.8 | 6.2 | 5.4–7.0 | < 5.0 | 23.1 (n = 104) | 15.5 (n = 97) |
| Vit B12 (pg mL–1) | 371 | 290.8–464.5 | 410b | 319–550 | < 200 | 7.6 (n = 105) | 2.1 (n = 94) |
| 25 OH VitD3 (nmol L–1) | 64 | 54.7–82.4 | 70.2 | 56.8–81.2 | < 50 | 10.4 (n = 106) | 13.5 (n = 96) |
From Appendix C of. [58] Note that CDC references are from serum rather than plasma used in this study.
Mann Whitney FDR adjusted p‐value < 0.05 vs. 2013.
We tested the relationship between an individual's genetic ancestry and micronutrient levels because the Brazilian population is highly admixed. Linear regressions between ancestral components (Q) model (Figure 2A) and baseline vitamin levels showed higher thiamine monophosphate (TMP) levels (Figure 2B) with higher European ancestry (component Q = 1). Plasma vitamin B12 (Figure 2C) was negatively associated with increasing Native American ancestry (Q = 5). Finally, Native American ancestry was associated with lower baseline folate levels (Q = 5, Figure 2D) and greater response to the intervention (Q = 5, Figure 2E).
Figure 2.
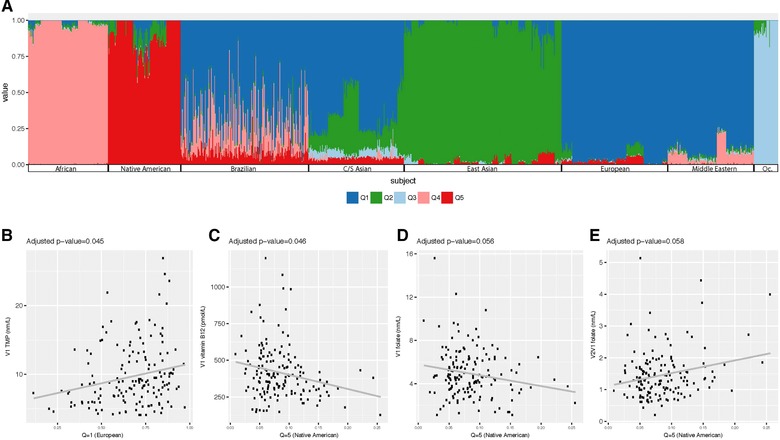
Admixture analysis. Influence of genetic ancestry on baseline vitamin levels. Ancestry markers from the Human Genome Diversity Project (HGDP) reference populations were used A) to identify admixture in data from unrelated participants from both years as per methods. To test whether linear regression between the ancestral components and baseline vitamin levels existed, a k = 5 model was used to the following covariates: trial year, sex, age, fat mass, and tanner score. Adjusted p‐value of 0.05 was used as significance threshold. B) Baseline TMP and Q1 (Europe) with estimate of regression coefficient (ERC) 4.57, C) baseline vitamin B12 and Q5 (Native American) ECR = 186.53, D) baseline folate and Q5 (Native American) ECR = 2.13, and E) folate response as ratio of V2/V1 and k5 (Native American) with ECR = 0.77.
3.4. Effect of Intervention on Clinical Parameters, Vitamin Levels, Population Level Dyslipidemia, and Interindividual Response
A published algorithm53 tested the response to the intervention for RTM. LDL–cholesterol (LDL‐c), mean corpuscular volume (MCV), albumin, glucose, and the levels of nine circulating vitamin forms were significantly influenced by the intervention in both years (Table 5) after accounting for RTM. Other vitamins passed the RTM in 1 year but not both (data not shown). The fasting levels of LDL‐c, glucose, and albumin decreased, while MCV increased during the intervention in both years (Table 5). Flavine mononucleotide (FMN), nudifloramide (N‐methyl‐2‐pyridoxone‐5‐carboxamide), pantothenic acid, pyridoxal, α‐tocopherol, total folate, and vitamin B12 increased in both years during the intervention and, in most cases, returned toward baseline values after the washout (Table 5). The levels of γ‐tocopherol and calcium decreased during the intervention. The concentrations of α‐ and γ‐tocopherols are known to be inversely correlated.61 Permutation testing was also conducted to confirm the effect of the intervention and showed that variations in LDL‐c (Supporting Information Figure S1), albumin, total cholesterol, and MCV (data not shown) exceeded random effect.
Table 5.
Effect of intervention above regression to the mean for participants in 2013 and 2014
| Variable | Intervention 2013 | Washout 2013 | Intervention 2014 | Washout 2014 | ||||
|---|---|---|---|---|---|---|---|---|
| Effect | p‐Value | Effect | p‐Value | Effect | p‐Value | Effect | p‐Value | |
| Albumin (g dL–1) | –0.04 | 9.98 × 10–04 | –0.02 | 1.69 × 10–02 | –0.06 | 3.67 × 10–05 | –0.006 | 6.59 × 10–01 |
| Basophils (103 mm–3) | –0.08 | 2.48 × 10–02 | 0.06 | 1.16 × 10–01 | –0.08 | 1.37 × 10–02 | 0.006 | 8.57 × 10–01 |
| Calcium (mmol L–1) | –0.12 | 1.91 × 10–05 | 0.06 | 1.66 × 10–02 | –0.51 | 8.51 × 10–27 | –0.397 | 2.38 × 10–25 |
| Glucose (mg dL–1) | –2.06 | 4.78 × 10–04 | –0.46 | 2.83 × 10–01 | –1.37 | 8.74 × 10–03 | 0.306 | 5.61 × 10–01 |
| LDL‐Cholesterol (mg dL–1) | –8.08 | 8.30 × 10–10 | 0.11 | 9.27 × 10–01 | –4.17 | 3.70 × 10–04 | –4.823 | 7.64 × 10–05 |
| Mean corpuscular volume (fl) | 1.39 | 1.13 × 10–22 | –2.83 | 6.08 × 10–47 | 0.59 | 6.11 × 10–13 | –0.583 | 8.18 × 10–08 |
| FMN (nmol L–1) | 2.66 | 6.45 × 10–05 | –1.50 | 2.13 × 10–04 | 2.66 | 5.12 × 10–05 | –2.293 | 1.04 × 10–06 |
| Nudifloramide (nmol L–1) | 351.6 | 1.82 × 10–10 | –159.36 | 2.75 × 10–03 | 384.7 | 1.32 × 10–08 | –151.174 | 4.18 × 10–03 |
| Pantothenic Acid (nmol L–1) | 146.3 | 5.59 × 10–46 | –52.12 | 1.03 × 10–09 | 80.44 | 1.26 × 10–15 | –15.148 | 2.62 × 10–01 |
| Pyridoxal (nmol L–1) | 5.71 | 2.28 × 10–30 | –0.75 | 8.85 × 10–02 | 3.52 | 4.96 × 10–11 | –2.344 | 7.33 × 10–22 |
| α–Tocopherol (μg mL–1) | 0.47 | 2.54 × 10–04 | –0.94 | 7.86 × 10–16 | 0.59 | 2.90 × 10–04 | 0.006 | 9.67 × 10–01 |
| γ–Tocopherol (μg mL–1) | –0.20 | 1.31 × 10–09 | –0.01 | 5.38 × 10–01 | –0.12 | 9.58 × 10–05 | 0.095 | 7.23 × 10–03 |
| 5–methyltetrahydrofolic acid | 6.38 | 1.61 × 10–10 | –4.32 | 2.99 × 10–09 | 4.30 | 4.83 × 10–03 | 0.053 | 9.64 × 10–01 |
| Folate (ng mL–1) | 1.87 | 1.56 × 10–12 | –0.34 | 1.67 × 10–01 | 0.79 | 1.17 × 10–04 | –0.373 | 5.88 × 10–02 |
| Vitamin B12 (pg mL–1) | 69.87 | 1.49 × 10–04 | –45.18 | 2.39 × 10–04 | 69.07 | 1.45 × 10–07 | –21.144 | 1.83 × 10–01 |
All variables are in nmol L–1 except where noted otherwise.
The micronutrient supplement improved markers of dyslipidemia in a high percentage of individuals between baseline and postintervention (Table 4) although a few individuals had increased dyslipidemia markers (Table 4). These analyses did not exclude statistical outliers and siblings since the clinical definition of dyslipidemia is based on cutoff values for individuals.57
Table 4.
Changes in Dyslipidemia from baseline to after interventiona
| Lipid | Age | Dyslipidemia Cutoff | Above Cutoff @ Baseline | Below Cutoff After Intervention | % Decrease |
|---|---|---|---|---|---|
| Total cholesterol | 9–13 | >200 mg dL–1 | 34 (280) | 19 (275) | 55.9 |
| LDL | 9–13 | >130 mg dL–1 | 42 (280) | 21 (275) | 50.0 |
| Triglyceride | 9 | >100 mg dL–1 | 1 (8) | 1 (7) | 100.0 |
| Triglyceride | 10–13 | >130 mg dL–1 | 21 (272) | 10 (268) | 47.8 |
| Lipid | Age | Cutoff | Below Cutoff @ Baseline | Above Cutoff After Intervention | % Increase |
|---|---|---|---|---|---|
| Total cholesterol | 9–13 | >200 mg dL–1 | 246 (280) | 5 (275) | 2.0 |
| LDL | 9–13 | >130 mg dL–1 | 230 (280) | 3 (275) | 1.1 |
| Triglyceride | 9 | >100 mg dL–1 | 7 (8) | 0 (7) | 0 |
| Triglyceride | 10–13 | >130 mg dL–1 | 251 (272) | 17 (268) | 6.3 |
Cutoffs from [57]. Number in parenthesis = sample number, no data for five samples after intervention. Percent decrease was calculated as number of individuals below cutoff after intervention divided by number of individuals above cutoff @ baseline. Percent increase was calculated from number of individuals above cutoff after intervention by number of individuals below cutoff at base line.
Interindividual variability occurred in every parameter tested (data not shown). Assessing the biological relevance of the response of each metabolite is challenging since in most cases, the day‐to‐day measurement variation of these variables are often unknown especially in healthy, age‐matched subjects. An exception is LDL, which typically varies by less than 10%.62 Using this stringent range of variation, three LDL response groups were identified (Figure 3): (i) individuals whose LDL decreased by more than 10% (responders), (ii) those in whom LDL increased by more than 10% (opposite responders), and (iii) those with LDL variations in between those limits (nonresponders). The small number of opposite responders precluded statistical analysis to determine correlations with anthropometric or other biochemical measurements. Nevertheless, we identified a plausible mechanism for variation in LDL levels by (i) using a published cofactor protein network63 and analysis of 515 cofactor connected proteins involved using DAVID annotation (https://david.ncifcrf.gov/ and ref. [64]) and MetaCore tools (http://www.portal.gene.com). A network was identified for sterol metabolism (highest Benjamini & Hochberg corrected p = 9.7 × 10–20) that linked control of LDL catabolism through the liver X receptor65 with six replicated plasma vitamins analyzed in this study (Supporting Information Figures S2 and S3). We chose not to estimate expected day‐to‐day variation for other metabolic markers since their ranges in comparable cohorts are not available (i.e., healthy and age‐matched subjects).
Figure 3.
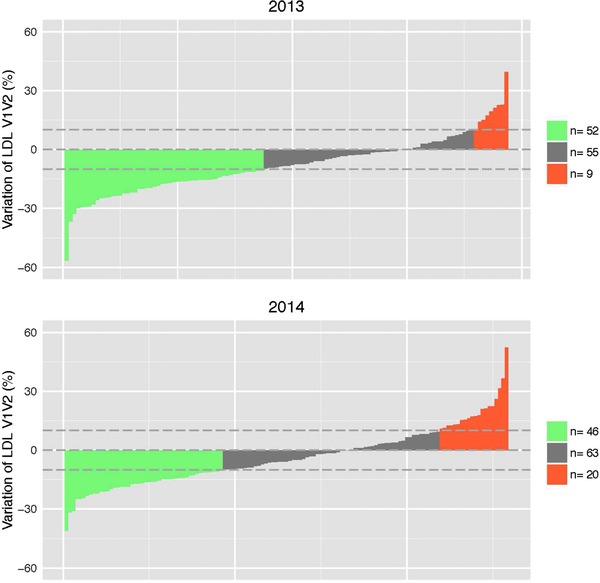
Interindividual variability in response to intervention for LDL. We have identified individuals whose response exceeded normal within individual day‐to‐day variation (reported to be 10%62). Green: opposite‐responders, gray: non‐responders, and orange: responders to intervention.
3.5. Modeling the Variability of the Response to Intervention
To explain individual responses and identify variables that might contribute to responses of measured metabolites, bootstrapped elastic net regressions were performed (Methods). Simple models that consisted of only a variable's own baseline as predictor performed best in terms of Pearson correlation and its significance between predicted and observed 2014 response to intervention (Figure 4A, B). The coefficients of these single metabolite predictors were always negative, indicating that a lower baseline level predicted more positive response of a given metabolite to the supplement, and a higher baseline predicted a less positive response (Figure 4A, B).
Figure 4.
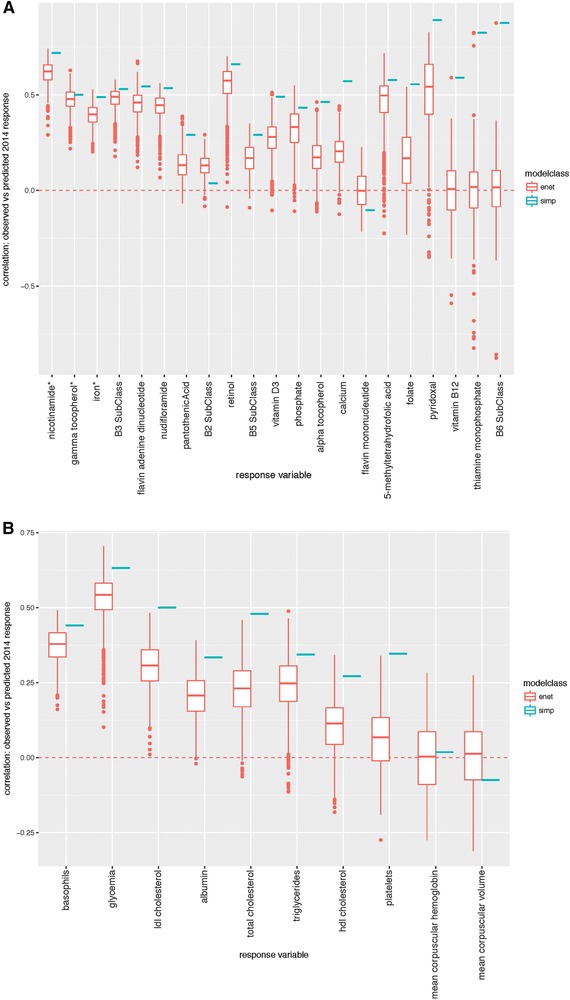
Comparison of elastic net and simple model performance across bootstrapped analyses. A) Vitamin and B) clinical variable response. Model performance is shown for each response variable and modeling approach. Performance is measured as a correlation between predicted and observed 2014 response to intervention.
While the simple models consistently had the strongest p‐values for prediction of 2014 response, elastic net (enet) performed well for some response variables (e.g., Figure 4A, B and Supporting Information Figures S10–S13) based on mean p‐values across bootstrapped analyses. We consider enet models to be successful when 100% of bootstrapped models significantly predict 2014 response (at adjusted p < 0.1). Enet‐selected predictors of a given metabolite level may provide insights about the molecular physiological state that contributes to response to the intervention. Note that prediction of response to intervention from baseline dietary habit parameters was also evaluated but did not produce strong prediction of clinical response. Detailed results from the elastic net regressions can be found in Supporting Information 2.
3.6. Prediction of Vitamin Response to Intervention
Enet predictions of vitamin response (Figure 4A) performed well, producing consistently significant predictive models for nicotinamide (Figure 5A, B), γ‐tocopherol (Supporting Information Figure S10), and iron (Supporting Information Figure S11). The best performing nicotinamide response enet model (score 0.742; adjusted p = 1.78 × 10–11) had a negative coefficient for baseline nicotinamide, vitamin D3, and age (retained in 100.0, 98.9, and 90.1% of bootstraps, respectively). Predictors with positive coefficients for predicting nicotinamide response included platelets, calcium, and iron (retained in 99.7, 95.1, 89.6 of bootstraps, respectively; Figure 5A).
Figure 5.
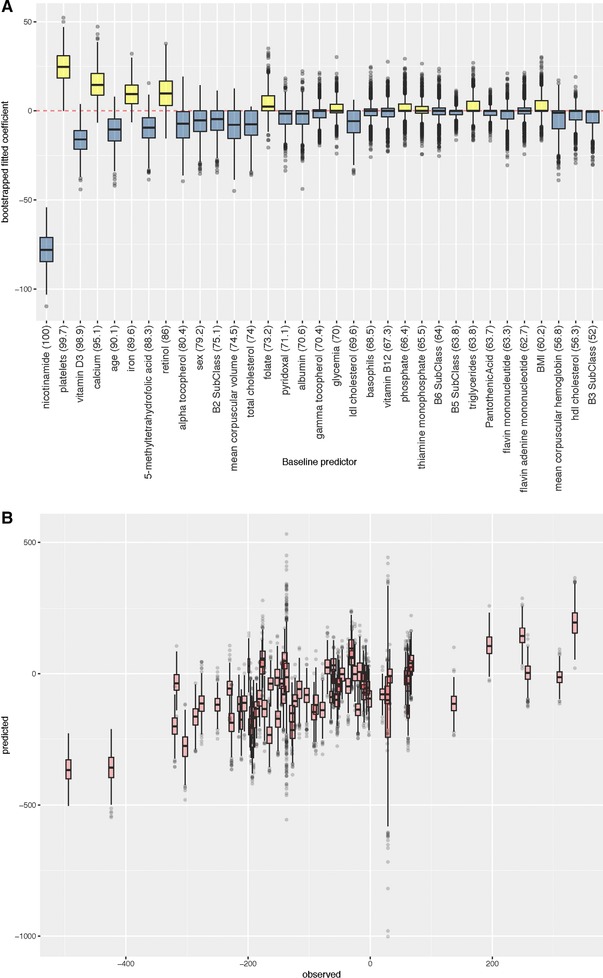
Modeling for nicotinamide response. A) Fitted coefficients and frequency (in parentheses) of predictor variables across 1000 bootstrapped analyses. B) Observed versus predicted response to intervention across bootstrapped analyses. Each boxplot corresponds to an individual participant, and thus shows variation in predicted response for each individual across the bootstrapped analyses.
3.7. Prediction of Clinical Response to Intervention
Although no predictive model of clinical responses met the significance threshold for all bootstraps, the model for glucose response significantly predicted 2014 response in 95.5% of cases. The best performing elastic net model for glucose response (Figure 4B) scored 0.706 (p = 1.11 × 10–09) with every bootstrapped model including baseline glucose as predictor (Supporting Information Figure S12) with a negative coefficient, indicating that lower baseline glucose is predictive of a greater positive glycemia change. γ‐Tocopherol and HDL‐cholesterol were retained in 83.6 and 73.2% of the bootstrapped models, respectively, with negative coefficients in most cases. The models retained mean corpuscular hemoglobin (MCH), vitamin D3, and triglycerides (82.2, 75.5, and 75.4%, respectively), all with positive coefficients.
Two models of basophil response (0.2% of bootstraps) failed to meet our threshold for prediction of 2014 response (Figure 4B). The best performing model scored 0.49 (p = 5.58 × 10–04) (Supporting Information Figure S13). Baseline basophil levels were retained in all bootstraps with a negative coefficient, while positive coefficients were typically estimated for FMN (86.9% of bootstraps), TMP (76.4%), and glycemia (72.8%). Triglycerides (78.6%) typically had negative fitted coefficients.
4. Discussion
The study described here was devised to assess physiological changes in response to adding ≈100% DRIs of 12 vitamins and five minerals for 6 weeks for individuals consuming a free‐living diet. Others have also analyzed the effect of different 24‐ingredient unsupervised nutrient interventions lasting 24 weeks15 or 1 year14 on intermediate risk factors of disease in adults. In contrast, many studies use one to several micronutrients and either intermediate risk factors or reduction in chronic disease as outcomes (reviewed in refs. [5, 6, 7, 8, 9, 10, 64, 66]). We highlight key results of our study focusing on organic vitamin results. Given word limitations, we provide additional discussion in Supporting Information 1.
4.1. Population Anthropometric and Dietary Data
The 9–13 year old participants in this study had a high prevalence67 of overweight and obesity based on waist circumference and body fat mass above normal.68 These conditions are consistent with the increased incidence of the metabolic syndrome in adolescents in developed and in developing countries.69, 70, 71, 72 Total energy, carbohydrate, protein, and fat consumption in the studied population approximated the consumption for children and adolescents reported in NHANES.71 The average HEI (54.4 ± 7.5 averaged over both years) revealed a poor quality of diet among the participants similar to those reported in other nutritional studies in Brazil and other countries.72, 73, 74, 75 The majority of participants in this study are in the middle segment of the socioeconomic spectrum with access to affordable fresh and whole foods (manuscript in preparation). Poor diets may contribute to dyslipidemia found in this and other populations of similar ages.76
4.2. Population Vitamin Status
The ‘normal’ range in levels of many of the 36 circulating vitamins have not been well characterized in this76, 77 or other age groups. Environment (ref. [78] and references therein) and physiological state (e.g., obesity)79, 80, 81, 82 may alter the plasma levels of micronutrients. Given these caveats, high percentages of this cohort had micronutrient insufficiencies or deficiencies compared to other populations based on the best available reference data. The National Demographic Survey of Child and Women's Health in Brazil showed that 17.4% of children had inadequate levels of vitamin A83 compared to 3.5% (2‐year average) found in this study. The prevalence of vitamin D insufficiency, defined here as below 57 nmol L–1,84 was 30.2 and 28.1% in the first and second years of intervention, respectively. The similarity in vitamin D measures was expected since the intervention was conducted in the same season in the same physical environment in both years. As noted by others,85 vitamin D was affected by sex and might reflect differences in time spent in the sun, fat mass differences, or metabolic needs of females at these ages.86 Anemia prevalence in this cohort was low (2‐year average of 2.1% based on hemoglobin status; Supporting Information 1, Table 3) although the mean estimated prevalence of anemia for children in other Brazilian studies ranged between 10.4 and 68.8%.87, 88
4.3. Population Level Responses to the Intervention
The combination of multi‐micronutrients used for this intervention reproducibly increased or decreased the levels of circulating forms of nine organic vitamin metabolites and three clinical variables. Many of the other vitamins and minerals also showed increased plasma levels in both years, but did not pass the stringent regression above the mean tests53 suggesting that differences in genetic makeup, environment, or their interactions differed between years.
Total cholesterol, LDL‐c, and fasting glucose decreased in response to the intervention and remained stable during the 6‐week washout. Decreases in average levels of blood lipids and glucose suggest that one or a combination of vitamins and minerals in the supplement influenced metabolism. Lipid profiles and glucose are associated with either vitamin baseline levels (e.g., refs. [89, 90, 91, 92]) and change in response to single (e.g., Refs. [93, 94, 95, 96, 97, 98, 99]) or multiple micronutrient supplementation.14 Levels of these clinical variables are often associated with risk of metabolic syndrome and chronic disease.100 Data mining and pathway analyses (MetaCor and DAVID) indicated that folate, niacin, pantothenic acid, tocopherols, and pyridoxal forms are cofactors for proteins involved in the sterol responsive nuclear liver X receptor transcription factor network.69 Changes in the circulating levels or bioavailability of these micronutrients following intervention may have interacted with cholesterol pathways and networks, and led to the observed changes in plasma levels of total cholesterol and LDL‐c (Supporting Information Figures S2 and S3). These findings require and deserve further investigation.
4.4. Interindividual Variability
Targeted nutrition and precision medicine depend on the ability to predict individual responses to dietary recommendations and drug treatments which typically are not the goals of randomized control trials or epidemiological studies. Bootstrapped elastic net penalized regression was used in this study to build predictive models fitted to baseline and response data from the 2013 cohort and able to predict responses of individuals in the 2014 cohort (Figures 4 and 5, and Supporting Information Figures S10–S13). The best statistical models for all variables were simple and relied only on a given variable's own baseline levels to predict its response. That is, lower baseline plasma levels of clinical measures or vitamins tended to increase while higher baseline levels tended to decrease showing a system‐wide adaptation to the intervention.
Although the simple models were best at predicting clinical and vitamin responses to the micronutrient intervention, models produced by elastic net were statistically significant in terms of the correlation between predicted and observed response in the test set (see Figures 4 and 5 and Supporting Information Figures S10–S13) and thus may be useful in identifying variables contributing to the system‐level responses.
4.5. Prediction of Vitamin Response from Baseline Vitamin and Clinical Parameters
The bootstrapped model for predicting nicotinamide response produced a set of predictors wherein higher baseline levels of platelets, calcium, iron, and retinol and lower levels of nicotinamide, 25‐OH vitamin D3, and 5‐methyltetrahydrofolic acid predicted a more positive response to intervention. Nicotinamide is a metabolic product of the NADP present in Nestrovit suggesting that active niacin metabolism contributed to the change in nicotinamide responsiveness. The level of nudifloramide, an end product of niacin metabolism, was also increased in response to the intervention. Overall, 99.7% of models for nicotinamide response included platelets, nucleate cells that function in thrombosis, hemostasis, and immune surveillance in health states and, when dysregulated, in disease pathologies.101 Although the molecular and cellular mechanisms remain poorly characterized, this finding is reinforced by reports showing that nicotinamide enhances in vitro megakaryocytic cell maturation into platelets.102 Vitamin D and calcium also were selected in 98.9 and 95.1% of bootstrapped models for nicotinamide response consistent with well‐studied interactions between these two micronutrients.103 The mechanism involved in vitamin D, calcium, and nicotinamide response are unclear. Similarly, interactions between retinol and nicotinamide have not been described previously. However folic acid (of which the active metabolite is 5‐methyltetrahydrofolic acid) supplementation has been found to increase NADPH oxidase activity in rats.104 The γ‐tocopherol response is discussed further in Supporting Information 1.
4.6. Prediction of Clinical Response from Baseline Vitamins and Clinical Parameters: Basophils and Glucose Levels
The bootstrapped predictive models for basophils included baseline basophil levels and triglycerides with negative coefficients, and baseline FMN, TMP, and glucose with positive coefficients (Supporting Information Figure S13). FMN is a metabolic product of vitamin B2 (Flavin adenine dinucleotide; FAD) present in Nestrovit indicating that flux through riboflavin pathways may be as important as homeostatic levels of FMN. Although basophils represent ≈1% of total white blood cells, they are thought to play a role in allergic reactions (along with neutrophils and macrophages) when activated by cytokines, antibodies, and proteases.105 An association between FMN with basophils has not been noted before and the role of basophils in maintenance of the healthy state in the absence of allergens or pathogens is unknown.
Elastic net regression of the response of glucose to the intervention retained baseline glucose levels in 100% of the bootstrapped models, as expected from simple model results. Baseline γ‐tocopherol, MCH, vitamin D3, and triglycerides occurred in >75% of the models with low baseline levels of tocopherol and high baseline MCH, triglycerides, and vitamin D predicting increased glucose response. α‐ and γ‐Tocopherol may stimulate glucose uptake by skeletal muscle cells, at least in vitro.106 Vitamin E intake may reduce glucose levels in type 2 diabetics and in subjects with baseline serum vitamin E deficiencies.107 High doses of vitamin E (>400 IU d–1) supplemented for longer periods (>12 weeks) significantly reduced HbA1c levels and fasting insulinemia.108
25‐OH vitamin D3 has also been implicated in glucose control because of an inverse correlation between plasma levels and impaired glucose tolerance (rev in refs. [109, 110]). However, supplementing diets with vitamin D with or without calcium produces mixed effects on glucose regulation in healthy individuals or diabetics.111, 112, 113 Mean corpuscular hemoglobin concentration (MCHC) is weakly and inversely correlated with HbA1c, suggesting an interaction that may need to be accounted for in diagnosing and monitoring diabetes.114 However, a mechanism by which MCHC influences or alters fasting glycemia is not obvious. LDL‐c levels may indirectly alter glucose levels by altering bile acid metabolism and by their roles as transcriptional regulators of cholesterol catabolism.115
4.7. Prediction of Vitamin or Clinical Responses from Dietary Intake Baseline Measures
The clinical and vitamin responses to the intervention could not be predicted from baseline dietary intake measures. These results may be due to combinations of dietary recall bias, differences in bioavailability of nutrients due to interindividual variation in transport (e.g., ref. [116]) or metabolism of micronutrients (e.g., ref. [117]), differences in microbiomes of individuals (e.g., ref. [118]), or other unmeasured factors. In addition, reported nutrient content in food databases may differ with the actual levels of food components consumed by the participants (see http://www.fao.org/docrep/008/y4705e/y4705e06.htm).
4.8. Ancestral Background Affects Baseline Vitamin Levels and Responses to Intervention
Linear regressions between ancestral components and baseline vitamin levels showed lower baseline folate and an increased folate response associated with Native American ancestry. The folate results are consistent with the known allele frequencies of genes involved in folate metabolism. For example, frequencies of methylenetetrahydrofolate reductase C677T (TT) and methylenetetrahydroflate dehydrogenease (MTHFD1) G1958A (AA) risk alleles are higher in Amerindians (57 and 58%, respectively), while being lower in African populations (0 and 4 %, respectively).119
Genome wide association studies120 and candidate gene studies (reviewed in ref. [121]) have identified single nucleotide polymorphisms associated with homeostatic levels of micronutrients. However, the majority of such associations have small effect sizes and the sum of all variants identified for a trait explain only a small proportion of the phenotype.122 The distinction between previous studies and this study is that micronutrient levels were associated with larger regions of ancestral DNA. Differences in ancestral background and their effect on plasma metabolite levels support the importance of including genomic data in determining micronutrient requirements for different populations. In addition, ethnic groups can exhibit substantial differences in disease incidence, severity, progression, and response to treatment (e.g., ref. [123, 124]). We121 and others (e.g., ref. [10, 125]) previously proposed that differences in fasting and response to micronutrients may contribute to differences in the incidence of some common diseases.
4.9. Conclusions
All micronutrients and minerals should ideally be consumed as part of a healthy diet. However, food access and availability in developed and developing economies vary across geography, built environment, climate, and socioeconomic status.8, 10, 12 Supplemental micronutrients are warranted in populations without access to nutritious food or those with poor diets. However, determining optimal doses remains not only an experimental but also translational challenge.
Our study was designed to obtain both public health results (e.g., percent insufficiencies in cohort) as well as how healthy Brazilian children and teens responded to multi‐micronutrients (interindividuality). The study followed “first principles” that recognized that all individuals are genetically, culturally, and environmentally unique. The limitations and strengths are described in Box 2. n‐of‐1 Study designs are increasingly used for precision medicine (e.g., ref. [24]) and personalized nutrition25 because of the understanding that population‐based treatment guidelines and recommendations may not apply to every individual. We used a simple intervention, washout approach, because the number of individuals analyzed was larger than most n‐of‐1 intervention studies and the participant burden (6‐week intervention and three assessments of 4–5 h each) for children and families was high for community based participatory research. Our results indicated that total cholesterol, LDL‐c, glycemia, MCV, and the circulating levels of nine vitamin metabolites responded to the 6‐week intervention in two consecutive years demonstrating that multi‐micronutrients mediated the physiology of systems associated with metabolic health. Predictive modeling of response variables was motivated by the need to develop micronutrient intake recommendations that account for the interindividuality in metabolic requirements for optimizing personal health.
Box 2: Strength and Limitations
Limitations
The following are the limitations of the study:
Small sample size.
Unique genetic admixture.
Context specificity due to urban, middle class culture in Brazil makes the results difficult to generalize to other populations.
Lack of control group and repeated measures between start and finish of the intervention limit traditional statistical approaches.
Strengths
The following are the strengths of the study:
n‐of‐1 Study design with each participant having his/her own control accounting for interindividual variability.
Replication of results in two interventions.
Adherence to intervention was monitored.
Comprehensive analysis of 36 circulating levels of vitamins.
Genomic analysis (5 million single nucleotide polymorphisms) and whole exome sequence (in preparation) which will eventually allow for associating individual variants to metabolic responses.
Aggregated data can be used to determine population level responses.
Effect of intervention was verified using stringent statistical tests.
The study followed principles and methods for community based participatory research126, 127, 128 to accelerate the translation of research to knowledge for participants and families.
Abbreviations
- 24hrDR
24 hr dietary recalls
- 25‐OH‐VitD3
25‐hydroxy Vitamin D3
- 3‐epi‐25OH VitD3
C3‐epimer of 25‐hydroxy vitamin D3
- 5‐Me THF
5‐methyl tetrahydrofolic acid
- DAVID
DAVID bioinformatic resources
- ERC
estimate of regression coefficient
- FA
folic Acid
- FAD
flavin adenine dinucleotide
- FFQ
food frequency questionnaire
- FMN
flavin mononucleotide
- HEI
healthy eating index
- HGDP
human genome diversity project
- IRB
Institutional Review Board
- LDL‐c
LDL‐cholesterol
- MCH
mean corpuscular hemoglobin
- MCV
mean corpuscular volume
- MHDI
municipal human development index
- NA
nicotinic Acid
- NM
nicotinamide
- NUA
nicotinuric acid
- Nudi
nudifloramide
- PA
4‐pyridoxic Acid
- p‐ABGA
para‐aminobenzoylglutamic acid
- PCA
principal component analysis
- PL
pyridoxal
- PLP
pyridoxal 5’‐Phosphate
- PM
pyridoxamine
- PMP
pyridoxamine 5’‐Phosphate
- PN
pyridoxine
- RCTs
randomized control trials
- RTM
regression to the mean
- THF
tetrahydrofolate
- TMP
thiamine monophosphate
- TPP
thiamine pyrophosphate
- V1
visit 1
- V2
visit 2
- Vit A
retinol
Conflict of Interest
J.K., A.G., E.B., A.L., J.‐M.O., C.G., J.C., M.K., P.D., S.M., C.F.D., N.C., and S.C.M. worked for the Nestle Institute of Health Science or Nestle at the time this study was conducted. J.K. currently works for Vydiant Inc.
Supporting information
Supporting Information Figure S1. Permutation analysis of LDL response
Supporting Information Figure S2. Functional Annotation of Cofactor Interacting Proteins.
Supporting Information Figure S3. Sterol metabolic network analyzed in MetaCore.
Supporting Information Figure S4. Correlation between fat soluble vitamins and clinical cofactors.
Supporting Information Figure S5. Correlation between water soluble vitamins and clinical cofactors.
Supporting Information Figure S6. Correlation between water and fat soluble vitamins.
Supporting Information Figure S7. Correlation between fat soluble vitamins and clinical variables after intervention.
Supporting Information Figure S8. Correlation between water soluble vitamins and clinical variables after intervention.
Supporting Information Figure S9. Correlation between water and fat soluble vitamins after intervention.
Supporting Information Figure S10. Enet selected predictors of γ‐tocopherol response from vitamin and clinical baseline.
Supporting Information Figure S11. Frequency (parenthesis) and fitted coefficients of predictor variables across bootstrapped analyses of iron response.
Fig. S12. Frequency and fitted coefficients of predictor variables across bootstrapped analyses of glycemia response.
Supporting Information Figure S13. Frequency and fitted coefficients of predictor variables across bootstrapped analyses of basophil response.
Supporting Information File 1
Detailed Description of Environment
Additional Methods
Additional Results
Extended Discussion
Supporting Information Table S1: Micronutrient Genomics Project Variables
Supporting Information Table S2: Demographic, nutritional status, pubertal, economic status, anemia, and dyslipidemia prevalence of all children and adolescents at baseline in Brazil Micronutrient Project (outliers and sibs included)
Supporting Information File 2. ElasticNet coefficients
Acknowledgments
M.G.M., C.D.A.C., S.L., M.M., and M.‐P.S.‐B. contributed equally to this work. This research was funded by the Nestle Institute of Health Sciences (Lausanne, Switzerland). Funding for nine graduate students at the University of São Paulo Ribeirao Preto was obtained from by the Fundação de Apoio a Pesquisa do Estado de São Paulo and Comissão de Aperfeiçoamento de Pessoal de Nível Superiors. The authors thank the children, teens, and parents who participated in this study as well as the principals, teachers, and school district officials who made the schools available for activities related to the project.
Experimental design: J.P.M. and J.K. Assessments: M.G.M., C.A.C.L., R.G.S., R.B.D.T., M.O.R.V.A., J.M.C., E.H., T.T.B., J.S.C.J., G.Z.C., S.K.B.M., M.M.M., R.Q.S., T.F.L., I.R.R., R.R., J.R.J., M.L.F., M.C.R., P.V.S.S., L.L.F., T.H.A.C., T.M.M.D., T.H.T., G.C.A.S., M.M.O., V.N.P., M.T.M., J.K., and J.P.M. Laboratory analysis: L.C.H., V.P.V., E.C.G., K.M., A.G., E.B., A.L., J.M.O., C.G., M.K., P.D., and S.M. Data analysis: J.C., M.P.S.B., S.L., M.L.M., J.P.M., and C.P. Primary authors: J.K., J.P.M., M.‐P.S.B., S.L., M.J.M., M.G.M., C.A.C.L., and R.G.S.
Mathias M. G., de A. Coelho‐Landell C., Scott‐Boyer M., Lacroix S., Morine M. J., Salomão R. G., Toffano R. B. D., do V. Almada M. O. R., Camarneiro J. M., Hillesheim E., de Barros T. T., Camelo‐Junior J. S., Campos Giménez E., Redeuil K., Goyon A., Bertschy E., Lévêques A., Oberson J., Giménez C., Carayol J., Kussmann M., Descombes P., Métairon S., Draper C. F., Conus N., Mottaz S. C., Corsini G. Z., Myoshi S. K. B., Muniz M. M., Hernandes L. C., Venâncio V. P., Antunes L. M. G., da Silva R. Q., Laurito T. F., Rossi I. R., Ricci R., Jorge J. R., Fagá M. L., Quinhoneiro D. C. G., Reche M. C., Silva P. V. S., Falquetti L. L., da Cunha T. H. A., Deminice T. M. M., Tambellini T. H., de Souza G. C. A., de Oliveira M. M., Nogueira‐Pileggi V., Matsumoto M. T., Priami C., Kaput J., Monteiro J. P., Mol. Nutr. Food Res. 2018, 62, 1700613 10.1002/mnfr.201700613
Present address: Marie‐Pier Scott‐Boyer, University of Laval, Quebec Canada
Present address: Sébastien Lacroix, Institut sur la Nutrition et les Aliments Fonctionnels (INAF), Université Laval, Québec, Canada
Present address: Martin Kussmann, Liggins Institute, University of Auckland, New Zealand
Present address: Nelly Conus, Bayer Consumer Care AG, Basel, Switzerland
Present address: Vinícius Paula Venâncio, Department of Nutrition and Food Science, Texas A&M. College Station Texas
Present address: Corrado Priami. Department of Computer Science, University of Pisa, Italy
Present address: Jim Kaput, Vydiant, Gold River, CA 95670
References
- 1. Guallar E., Stranges S., Mulrow C., Appel L. J., E. R. Miller, III , Ann. Intern. Med. 2013, 159, 850. [DOI] [PubMed] [Google Scholar]
- 2. Frei B., Ames B. N., Blumberg J. B., Willet W. C., Ann. Intern. Med. 2013, 160, 807. [DOI] [PubMed] [Google Scholar]
- 3. Friberg T. R., Ann. Intern. Med. 2013, 160, 808. [DOI] [PubMed] [Google Scholar]
- 4. Friberg T. R., Frei B., Ames B. N., Blumberg J. B., Willet W. C., Fargnoli J., Greenleaf J., Mafner M., Frei B., Ames B. N., Blumberg J. B., Willet W. C., Stepp D., Friberg T. R., Mason I., Guallar E., Stranges S., Mulrow C., Appel L. J., E. R. Miller, III , Ann. Intern. Med. 2013, 160, 808. [Google Scholar]
- 5. Grodstein F., Brien J. O., Kang J. H., Dushkes R., Cook N. R., Okereke O., Manson J. E., Glynn R. J., Buring J. E., Gaziano J. M., Sesso H. D., Colton T., Henderson I. C., Lacroix A., Prentice R., Wenger N., Cotch M. F., Cutler J., Ferris F., Fleg J., Greenwald P., Kurinij N., Parnes H., Perloff M., Schron E., Zonderman A. Ann. Int. 2013, 159, 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hercberg S., Galan P., Preziosi P., Bertrais S., Mennen L., Malvy D., Roussel A.‐M., Favier A., Briancon S., Arch. Intern. 2004, 164, 2335. [DOI] [PubMed] [Google Scholar]
- 7. Bryc K., Durand E. Y., Macpherson J. M., Reich D., Mountain J. L., Am. J. Hum. Genet. 2015, 96, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macpherson H., Pipingas A., Pase M. P., Am. J. Clin. Nutr. 2013, 97, 437. [DOI] [PubMed] [Google Scholar]
- 9. Rautiainen S., Manson J. E., Lichtenstein A. H., Sesso H. D., Nat. Rev. Endocrinol. 2016, 12, 407. [DOI] [PubMed] [Google Scholar]
- 10. Angelo G., Drake V. J., Frei B., Crit. Rev. Food Sci. Nutr. 2015, 55, 1968. [DOI] [PubMed] [Google Scholar]
- 11. Heaney R. P., J. Nutr. 2008, 138, 1591. [DOI] [PubMed] [Google Scholar]
- 12. Blumberg J., Heaney R. P., Huncharek M., Scholl T., Stampfer M., Vieth R., Weaver C. M., Zeisel S. H., Nutr. Rev. 2010, 68, 478. [DOI] [PubMed] [Google Scholar]
- 13. Ames B. N., McCann J. C., Stampfer M. J., Willet W. C., Am. J. Clin. Nutr. 2007, 86, 522. [DOI] [PubMed] [Google Scholar]
- 14. Earnest C., Cooper K. H., Marks A., Mitchell T. L., Nutrition 2002, 18, 738. [DOI] [PubMed] [Google Scholar]
- 15. Mikirova N., Hunninghake R., Casciari J., Guilliams V., Vitam. Miner. 2014, 3, 1. [Google Scholar]
- 16. Zimmermann M. B., Kohrle J., Thyroid 2002, 12, 867. [DOI] [PubMed] [Google Scholar]
- 17. Moretti D., Zimmermann M. B., Wegmuller R., Walczyk T., Zeder C., Hurrell R. F., Am. J. Clin. Nutr. 2006, 83, 632. [DOI] [PubMed] [Google Scholar]
- 18. Gordon J. I., Dewey K. G., Mills D. A., Medzhitov R. M., Sci. Transl. Med. 2012, 4, 137ps12. [DOI] [PubMed] [Google Scholar]
- 19. Rockhill B., Newman B., Weinberg C., Am. J. Public Heal. 1998, 88, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nikles C. J., McKinlay, L. Mitchell G. K., Carmont S.‐A. S., Senior H. E., Waugh M.‐C. A., Epps A., Schluter P. J., Lloyd O. T., Trials 2014, 15, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X., Chen P., PLoS One 2014, 9, e87752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lillie E. O., Patay B., Diamant J., Issell B., Topol E. J., Schork N. J., Health S., Jolla L., Per. Med. 2012, 8, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyatt G. H., Keller J. L., Jaeschke R., Rosenbloom D., Adachi J. D., Newhouse M. T., Ann. Intern. Med. 1990, 112, 293. [DOI] [PubMed] [Google Scholar]
- 24. Schork N. J., Nature 2015, 520, 609. [DOI] [PubMed] [Google Scholar]
- 25. Schork N. J., Goetz L. H., Annu. Rev. Nutr. 2017, 37, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanner J. M., Growth at Adolescence, 2nd ed., Blackwell Scientific Publishing, Oxford: 1962. [Google Scholar]
- 27. CNPP , Center for Nutrition Policy and Promotion , Estimated amounts of calories. 2002. https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/EstimatedCalorieNeedsPerDayTable.pdf
- 28. Institute of Medicine , Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids, The National Academies Press, Washington, DC: 2002. [DOI] [PubMed] [Google Scholar]
- 29. Associação Brasileira de Empresas de Pesquisa , Critério de classificação econômica Brasil. Associação Brasileira de Empresas de Pesquisa, Sao Paulo: 2015, 1. [Google Scholar]
- 30. Fumagalli F., Pontes Monteiro J., Sartorelli D. S., Vieira M. N. C. M., de Lourdes Pires Bianchi M., Nutrition 2008, 24, 427. [DOI] [PubMed] [Google Scholar]
- 31. Slater B., Philippi S. T., Fisberg R. M., Latorre M. R., Eur J Clin Nutr 2003, 57, 629. [DOI] [PubMed] [Google Scholar]
- 32. Monteiro J. P., Pfrimer K., Tremeschin M. H., de Camargo Molina M., Chiarello P., Consumo Alimentar ‐ Visualizando porções, 1st ed., Guanabara, Rio de Janeiro: 2007. [Google Scholar]
- 33. Thompson F. E., Byers T., J. Nutr. 1994, 124, 2245S. [DOI] [PubMed] [Google Scholar]
- 34. Tucker K. L., Smith C. E., Lai C.‐Q., Ordovas J. M., Annu. Rev. Nutr. 2013, 33, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hernández‐Avila M., Romieu I., Parra S., Hernández‐Avila J., Madrigal H., Willett W., Salud Publica Mex. 1999, 40, 133. [DOI] [PubMed] [Google Scholar]
- 36. d. Santos L. C., Pascoal M. N., Fisberg M., Cintra I. P., Martini L. A., J. Pediatr. (Rio. J). 2010, 86, 400. [DOI] [PubMed] [Google Scholar]
- 37. Savage J. S., Mitchell D. C., Smiciklas‐Wright H., Symons Downs D., Birch L. L., J. Am. Diet. Assoc. 2008, 108, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scagliusi F. B., Lancha Júnior A. H., Rev. Nutr. 2003, 16, 471. [Google Scholar]
- 39. Börnhorst C., Huybrechts I., Hebestreit A., Krogh V., De Decker A., Barba G., Moreno L. A., Lissner L., Tornaritis M., Loit H. M., Molnár D., Pigeot I., Int. J. Obes. (Lond). 2014, 38, S115. [DOI] [PubMed] [Google Scholar]
- 40. Rennie K. L., Coward A., Jebb S. A., Br. J. Nutr. 2007, 97, 1169. [DOI] [PubMed] [Google Scholar]
- 41. Lanctot J. Q., Klesges R. C., Stockton M. B., Klesges L. M., Obesity (Silver Spring) 2008, 16, 1407. [DOI] [PubMed] [Google Scholar]
- 42. Singh R., Martin B. R., Hickey Y., Teegarden D., Campbell W. W., Craig B. A., Schoeller D. A., Kerr D. A., Weaver C. M., Am. J. Clin. Nutr. 2009, 89, 1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schofield W. N., Hum. Nutr. Clin. Nutr. 1985, 39, 5.4044297 [Google Scholar]
- 44. Goldberg G. R., Black A. E., Jebb S. A., Cole T. J., Murgatroyd P. R., Coward W. A., Prentice A. M., Eur. J. Clin. Nutr. 1991, 45, 569. [PubMed] [Google Scholar]
- 45. Black A. E., Bingham S. A., Johansson G., Coward W. A., Eur. J. Clin. Nutr. 1997, 51, 405. [DOI] [PubMed] [Google Scholar]
- 46. Núcleo de estudos e pesquisas em alimentação ‐ NEPA , Tabela Brasileira de Composicao de Alimentos ‐ TACO 4 Edicao Ampliada e Revisada, 4th ed., Núcleo de estudos e pesquisas em alimentação ‐ NEPA, Campinas: 2011. [Google Scholar]
- 47. Food Composition | Food and Nutrition Information Center. https://ndb.nal.usda.gov/ndb/
- 48. Previdelli A. N., Andrade S. C. D., Pires M. M., Ferreira S. R. G., Fisberg R. M., Marchioni D. M., Rev. Saude Publica 2011, 45, 794. [DOI] [PubMed] [Google Scholar]
- 49. Guenther P. M., Reedy J., Krebs‐Smith S. M., J. Am. Diet. Assoc. 2008, 108, 1896. [DOI] [PubMed] [Google Scholar]
- 50. Previdelli A. N., Andrade S. C., Pires M. M., Ferreira S. R. G., Fisberg R. M., Marchioni D. M., Rev. Saúde Pública 2011, 45, 794. [DOI] [PubMed] [Google Scholar]
- 51. Bowman S. A., Lino M., Gerrior S. A., Basiotis P. P., The Healthy Eating Index 1994‐96, Center for Nutrition Policy and Promotion, U.S. Dept. of Agriculture, Washington, D.C. 1998. [Google Scholar]
- 52. Benjamini Y., Hochberg Y., J. R. Stat. Soc. Ser. B Stat. Method 1995, 57, 289. [Google Scholar]
- 53. Ostermann T., Willich S. N., Lüdtke R., BMC Med. Res. Methodol. 2008, 8, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Friedman J., Hastie T., Tibshirani R., J. Stat. Softw. 2010, 33, 1. [PMC free article] [PubMed] [Google Scholar]
- 55. Bach F. R., ICML ’08 Proc. 25th Int. Conf. Mach. Learn. 2008, 33 https://hal.archives-ouvertes.fr/hal-00271289
- 56. Hochberg Y., Benjamini Y., Stat. Med. 1990, 9, 811. [DOI] [PubMed] [Google Scholar]
- 57. Simão A. F., Precoma D. B., Andrade J. P., Correa Filho H., Saraiva J. F. K., Oliveira G. M. M., Murro A. L. B., Campos A., Alessi A., Avezum Junior A., Miguel A. C. M. G., Sousa A. C. S., Lotemberg A. M. P., Lins A. P., Falud A. A., Brandão A. A., Sanjuliani A. F., Sbissa A. S., Santos Filho A. C., Herdy A. H., Polanczyk C. A., Lantieri C. J., Machado C. A., Scherr C., Stoll C., Amodeo C., Araújo C. G. S., Saraiva D., Moriguchi E. H., Mesquita E. T., Cesena F. H. Y., Fonseca F. A. H., Campos G. P., Soares G. P., Feitosa G. S., Xavier H. T., Castro I., Giuliano I. C. B., Rivera I. V., Guimaraes I. C. B., Issa J. S., Souza J. R. M., Faria Neto J. R., Cunha L. B. N., Pellanda L. C., Bortolotto L. A., Bertolami M. C., Miname M. H., Gomes M. A. M., Tambascia M., Malachias M. V. B., Silva M. A. M., Iza M. C. O., Magalhães M. E. C., Bacellar M. S. C., Milani M., Wajngarten M., Ghorayeb N., Coelho O. R., Villela P. B., Jardim P. C. B. V., Santos Filho R.D., Stein R., Cassani R. S. L., D'Avila R. L., Ferreira R. M., Barbosa R. B., Povoa R. M. S., Kaiser S. E., Ismael S. C., Carvalho T., Giraldez V. Z. R., Coutinho W., Souza W. K. S. B., Arq. Bras. Cardiol. 2013, 101, 1. [Google Scholar]
- 58. CDC , Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population, 2012, Createspace Independent, North Charleston, SC: 2014. [Google Scholar]
- 59. Muthayya S., Rah J. H., Sugimoto J. D., Roos F. F., Kraemer K., Black R. E., PLoS One 2013, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Semba R. D., J. Nutr. 2012, 142, 143S. [DOI] [PubMed] [Google Scholar]
- 61. Huang H.‐Y., Appel L. J., J. Nutr. 2003, 133, 3137. [DOI] [PubMed] [Google Scholar]
- 62. O'Hanesian M. A., Rosner B., Bishop L. M., Sacks F. M., Am. J. Clin. Nutr. 1996, 64, 53. [DOI] [PubMed] [Google Scholar]
- 63. Scott‐Boyer M. P., Lacroix S., Scotti M., Morine M. J., Kaput J., Priami C., Troesch B., Hoeft B., McBurney M., Eggersdorfer M., Weber P., Tzioumis E., Adair L. S., Winichagoon P., Bastos Carrera P., Fontes‐Villalba M., O'Keefe J. H., Lindeberg S., Cordain L., Ames B. N., Fenech M. F., Ommen B. V., Kaput J., Berti C., Lowe W. L., Karban J., Ramakrishnan U., Gonzalez‐Cossio T., Neufeld L. M., Rivera J., Martorell R., Ames B. N., Soares M. J., Pathak K., Calton E. K., Verkerk R. H., Manios Y., Moschonis G., Mavrogianni C., Bos R., Singh‐Povel C., Jenab M., Slimani N., Bictash M., Ferrari P., Bingham S. A., Comerford K. B., Lopes da Silva S., Mayne S. T., Ferrucci L. M., Cartmel B., Goh K. I., Leoni G., Rosato A., Perozzi G., Murgia C., Fischer J. D., Holliday G. L., Thornton J. M., UniProt C., Bairoch A., Andreini C., Bertini I., Cavallaro G., Holliday G. L., Thornton J. M., Kohl M., Wiese S., Warscheid B., Keshava Prasad T. S., Bader G. D., Hogue C. W., Brown K. R., Jurisica I., Barabasi A. L., Oltvai Z. N., Uhlen M., Liu C. C., Gray K. A., Yates B., Seal R. L., Wright M. W., Bruford E. A., Almeida‐Neto M., Ulrich W., Rodríguez‐Gironés M. A., Santamaría L., Dormann C. F., Fruend J., Bluethgen N., Gruber B., Csardi G., Nepusz T., Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I., Fenech M. F., Fuss J. O., Tsai C. L., Ishida J. P., Tainer J. A., Monteiro J. P., Fabian E., Bogner M., Kickinger A., Wagner K. H., Elmadfa I., Ferrier I. N., Ames B. N., Elson‐Schwab I., Silver E. A., McCann J. C., Yeger‐Lotem E., Sharan R., Ames B. N., Sci. Rep. 2016, 6, 1.28442746 [Google Scholar]
- 64. Huang D. W., Sherman B. T., Tan Q., Collins J. R., Alvord W. G., Roayaei J., Stephens R., Baseler M. W., Lane H. C., Lempicki R. A., Genome Biol. 2007, 8, R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moschetta A., Atheroscler. Suppl. 2015, 17, 9. [DOI] [PubMed] [Google Scholar]
- 66. Biesalski H. K., Tinz J., Nutrition 2017, 36, 60. [DOI] [PubMed] [Google Scholar]
- 67. WHO The WHO Child Growth Standards. WHO, Geneva: 2016. [Google Scholar]
- 68. McCarthy H. D., Jarrett K. V., Crawley H. F., Eur. J. Clin. Nutr. 2001, 55, 902. [DOI] [PubMed] [Google Scholar]
- 69. Niehues J. R., Gonzales A. I., Lemos R. R., Bezerra P. P., Haas P., Int. J. Pediatr. 2014, 2014, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Y.‐X., Zhao J.‐S., Chu Z.‐H., Tan H.‐L., Ann. Nutr. Metab. 2014, 64, 137. [DOI] [PubMed] [Google Scholar]
- 71. Cole N., Fox M. K., Diet Quality of American School‐Age Children by School Lunch Participation Status: Data from the National Health and Nutrition Examination Survey, 1999–2004, U.S. Department of Agriculture, Food and Nutrition Service, Office of Research, Nutrition and Analysis, Alexandria, VA: 2008. [Google Scholar]
- 72. Fungwe T., Guenther P. M., Juan W. Y., Hiza H., Lino M., Nutr. Insight 2009, 43. [Google Scholar]
- 73. Tek N. A., Yildiran H., Gamze A., Bilici S., Koksal E., Makbule G. K., Nevin S., Nutr. Res. Pract. 2011, 5, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Torres R., Santos E., Orraca L., Elias A., Palacios C., J. Acad. Nutr. Diet. 2014, 114, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tanaka L. F., Dias de Oliveira Latorre M. d. R., Medeiros da Silva A., Roma de Oliveira Konstantyner T. C., Mendes E. C., Sousa Marques H. H., J. Pediatr. (Rio. J). 2015, 91, 152. [DOI] [PubMed] [Google Scholar]
- 76. Greaves R. F., Clin. Biochem. Rev. 2012, 33, 123. [PMC free article] [PubMed] [Google Scholar]
- 77. Holler U., Bakker S. J. L., Dusterloh A., Frei B., Kohrle J., Konz T. F., Lietz G., McCann A., Michels A. J., Molloy A. M., Murakami H. R., Dietrich S., Wim H. M., Schmidt K., Shimbo K., Schumacher S., Vermeer C., Kaput J., Weber P., Eggersdorfer M., Rezzi S., Trends in Analytical Sciences, in press. [Google Scholar]
- 78. Kaput J., van Ommen B., Kremer B., Priami C., Monteiro J. P., Pontes J., Morine M., Pepping F., Diaz Z., Fenech M., He Y., Albers R., Drevon C. A., Evelo C. T., Hancock R. E., Ijsselmuiden C., Lumey L. H., Minihane A. M., Muller M., Murgia C., Radonjic M., Sobral B., K. P. West, Jr. , Genes Nutr. 2014, 9, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. García O. P., Long K. Z., Rosado J. L., Nutr. Rev. 2009, 67, 559. [DOI] [PubMed] [Google Scholar]
- 80. Astrup A., Bügel S., Int. J. Obes. (Lond). 2010, 34, 947. [DOI] [PubMed] [Google Scholar]
- 81. Tussing‐Humphreys L. M., Nemeth E., Fantuzzi G., Freels S., Guzman G., Holterman A.‐X. L., Braunschweig C., Obesity 2010, 18, 1449. [DOI] [PubMed] [Google Scholar]
- 82. Cheng H. L., Bryant C., Cook R., O'Connor H., Rooney K., Steinbeck K., Obes. Rev. 2012, 13, 150. [DOI] [PubMed] [Google Scholar]
- 83. BRAZIL., Ministério da Saúde , PNDS: Pesquisa nacional de demografia e saúde da criança e da mulher 2006: resultados sobre anemia e hipovitaminose A no Brasil [Folder]. 2009http://bvsms.saude.gov.br/bvs/folder/folder_micronutrientes.pdf.
- 84. Looker A. C., Johnson C. L., Lacher D. A., Pfeiffer C. M., Schleicher R. L., Sempos C. T., NCHS Data Brief. 2011, 59, 1. [PubMed] [Google Scholar]
- 85. Yu H.‐J., Kwon M.‐J., Woo H.‐Y., Park H., J. Clin. Lab. Anal. 2016, 30, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Razzaghy‐Azar M., Shakiba M., Ann. Hum. Biol. 2010, 37, 692. [DOI] [PubMed] [Google Scholar]
- 87. Saraiva B. C. A., Soares M. C. C., Santos L. C. d., Pereira S. C. L., Horta P. M., J. Pediatr. (Rio. J). 2014, 90, 593. [DOI] [PubMed] [Google Scholar]
- 88. Pedraza D. F., Rocha A. C. D., Cien. Saude Colet. 2016, 21, 1525. [DOI] [PubMed] [Google Scholar]
- 89. Ashraf A. P., Alvarez J. A., Gower B. A., Saenz K. H., McCormick K. L., Obesity (Silver Spring). 2011, 19, 2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kelishadi R., Farajzadegan Z., Bahreynian M., Int. J. Food Sci. Nutr. 2014, 65, 404. [DOI] [PubMed] [Google Scholar]
- 91. Partida‐Hernández G., Arreola F., Fenton B., Cabeza M., Román‐Ramos R., Revilla‐Monsalve M. C., Biomed. Pharmacother. 2006, 60, 161. [DOI] [PubMed] [Google Scholar]
- 92. Barbagallo M., Dominguez L. J., Galioto A., Ferlisi A., Cani C., Malfa L., Pineo A., Busardo’ A., Paolisso G., Mol. Aspects Med. 2003, 24, 39. [DOI] [PubMed] [Google Scholar]
- 93. Heng E. C., Karsani S. A., Abdul Rahman M., Abdul Hamid N. A., Hamid Z., Wan Ngah W. Z., Eur. J. Nutr. 2013, 52, 1811. [DOI] [PubMed] [Google Scholar]
- 94. Burdeos G. C., Nakagawa K., Kimura F., Miyazawa T., Lipids 2012, 47, 471. [DOI] [PubMed] [Google Scholar]
- 95. Qureshi A. A., Sami S. A., Salser W. A., Khan F. A., J. Nutr. Biochem. 2001, 12, 318. [DOI] [PubMed] [Google Scholar]
- 96. Wu S.‐J., Liu P.‐L., Ng L.‐T., Mol. Nutr. Food Res. 2008, 52, 921. [DOI] [PubMed] [Google Scholar]
- 97. Nasser A.‐A., Omar A.‐A., Nasser A.‐D., Majed A., Sherif A.‐A., Benjamin V., Shaun S., Clin. Med. Insights Endocrinol. Diabetes 2014, 7, 1.24550684 [Google Scholar]
- 98. Doshi S. N., McDowell I. F. W., Moat S. J., Payne N., Durrant H. J., Lewis M. J., Goodfellow J., Circulation 2002, 105, 22. [DOI] [PubMed] [Google Scholar]
- 99. Birjmohun R. S., Hutten B. A., Kastelein J. J. P., Stroes E. S. G., J. Am. Coll. Cardiol. 2005, 45, 185. [DOI] [PubMed] [Google Scholar]
- 100. Kaur J., Cardiol. Res. Pract. 2014, 2014, 21. [Google Scholar]
- 101. Xu X. R., Zhang D., Oswald B. E., Carrim N., Wang X., Hou Y., Zhang Q., Lavalle C., Keown Mc.T., Marshall A. H., Ni H., Crit. Rev. Clin. Lab. Sci. 2016, 8363, 1. [DOI] [PubMed] [Google Scholar]
- 102. Giammona L. M., Fuhrken P. G., Papoutsakis E. T., Miller W. M., Br. J. Haematol. 2006, 135, 554. [DOI] [PubMed] [Google Scholar]
- 103. Lips P., Scand. J. Clin. Lab. Invest. Suppl. 2012, 72, 60. [DOI] [PubMed] [Google Scholar]
- 104. Hwang S.‐Y., Siow Y. L., Au‐Yeung K. K.W., House J. O. K., Am. J. Physiol. Renal Physiol. 2011, 300, F189. [DOI] [PubMed] [Google Scholar]
- 105. Siracusa M. C., Kim B. S., Spergel J. M., Artis D., J. Allergy Clin. Immunol. 2013, 132, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Singh I., Carey A. L., Watson N., Febbraio M. A., Hawley J. A., Eur. J. Nutr. 2008, 47, 387. [DOI] [PubMed] [Google Scholar]
- 107. Suksomboon N., Poolsup N., Sinprasert S., J. Clin. Pharm. Ther. 2011, 36, 53. [DOI] [PubMed] [Google Scholar]
- 108. Xu R., Zhang S., Tao A., Chen G., Zhang M., PLoS One 2014, 9, e95008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Heer M., Egert S., Diabetes. Metab. Res. Rev. 2015, 31, 14. [DOI] [PubMed] [Google Scholar]
- 110. Seida J. C., Mitri J., Colmers I. N., Majumdar S. R., Davidson M. B., Edwards A. L., Hanley D. A., Pittas A. G., Tjosvold L., Johnson J. A., J. Clin. Endocrinol. Metab. 2014, 99, 3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pittas A. G., Lau J., Hu F. B., Dawson‐Hughes B., J. Clin. Endocrinol. Metab. 2007, 92, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. George P. S., Pearson E. R., Witham M. D., Diabet. Med. 2012, 29, e142. [DOI] [PubMed] [Google Scholar]
- 113. Krul‐Poel Y. H. M., Westra S., ten Boekel E., ter Wee M. M., van Schoor N. M., van Wijland H., Stam F., Lips P. T. A. M., Simsek S., Diabetes Care 2015, 38, 1420. [DOI] [PubMed] [Google Scholar]
- 114. Rodriguez‐Segade S., Rodriguez J., García‐López J. M., Gude F., Casanueva F. F., RS‐Alonso S., Camiña F., Clin. Chem. 2016, 62, 1578. [DOI] [PubMed] [Google Scholar]
- 115. Staels B., Postgrad. Med. 2009, 121, 25. [DOI] [PubMed] [Google Scholar]
- 116. Shaghaghi M. A., Kloss O., Eck P., Adv. Nutr. 2016, 7, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Young V. R., Scrimshaw N. S., Am. J. Clin. Nutr. 1979, 32, 486. [DOI] [PubMed] [Google Scholar]
- 118. Magnúsdóttir S., Ravcheev D., De Crécy‐Lagard V., Thiele I., Front. Genet. 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Binia A., Contreras A. V., Canizales‐Quinteros S., Alonzo V.‐A., Tejero M. E., Silva‐Zolezzi I., Genes Nutr. 2014, 9, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tanaka T., Scheet P., Giusti B., Bandinelli S., Piras M. G., Usala G., Lai S., Mulas A., Corsi A. M., Vestrini A., Sofi F., Gori A. M., Abbate R., Guralnik J., Singleton A., Abecasis G. R., Schlessinger D., Uda M., Ferrucci L., Am. J. Hum. Genet. 2009, 84, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Monteiro J. P., Kussmann M., Kaput J., Genes Nutr. 2015, 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Witte J. S., Visscher P. M., Wray N. R., Nat. Rev. Genet. 2014, 15, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wells J. C. K., Obes. Rev. 2012, 13, 14. [DOI] [PubMed] [Google Scholar]
- 124. Burchard E. G., Ziv E., Coyle N., Gomez S. L., Tang H., Karter A. J., Mountain J. L., Pérez‐Stable E. J., Sheppard D., Risch N., N. Engl. J. Med. 2003, 348, 1170. [DOI] [PubMed] [Google Scholar]
- 125. van Ommen B., Fairweather‐Tait S., Freidig A., Kardinaal A., Scalbert A., Wopereis S., Br. J. Nutr. 2008, 99, S72. [DOI] [PubMed] [Google Scholar]
- 126. International Food Policy Research Institute , Global Nutrition Report 2016: From Promise to Impact: Ending Malnutrition by 2030, International Food Policy Research Institute, Washington DC: 2016. [Google Scholar]
- 127. Bogart L. M., Uyeda K., Health Psychol. 2009, 28, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. McCabe‐Sellers B., Lovera D., Nuss H., Wise C., Ning B., Teitel C., Clark B. S., Toennessen T., Green B., Bogle M. L., Kaput J., OMICS 2008, 12, 263. [DOI] [PubMed] [Google Scholar]
- 129. Anwar A., Marini M., Abruzzo P. M., Bolotta A., Ghezzo A., Visconti P., Thornalley P. J., Rabbani N., Free Radic. Res. 2016, 0, 1. [DOI] [PubMed] [Google Scholar]
- 130. Capo‐chichi C. D., Feillet F., Guéant J.‐L., Amouzou K. S., Zonon N., Sanni A., Am. J. Clin. Nutr. 2000, 71, 978. [DOI] [PubMed] [Google Scholar]
- 131. Rios‐Avila L., Coats B., Chi Y.‐Y., Midttun Ø., Ueland P. M., Stacpoole P. W., Gregory J. F., J. Nutr. 2015, 145, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Albersen M., Bosma M., Jans J. J. M., Hofstede F. C., Van Hasselt, P.M. De Sain‐van Der Velden M. G. M., Visser G., Verhoeven‐Duif N. M., PLoS One 2015, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Opladen T., Ramaekers V. T., Heimann G., Blau N., Mol. Genet. Metab. 2006, 87, 61. [DOI] [PubMed] [Google Scholar]
- 134. Sokoro A. A. H., Etter M. L., Lepage J., Weist B., Eichhorst J., Lehotay D. C., J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 832, 9. [DOI] [PubMed] [Google Scholar]
- 135. Scientific Report of the 2015 Dietary Guidelines Advisory Committee . https://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1. Permutation analysis of LDL response
Supporting Information Figure S2. Functional Annotation of Cofactor Interacting Proteins.
Supporting Information Figure S3. Sterol metabolic network analyzed in MetaCore.
Supporting Information Figure S4. Correlation between fat soluble vitamins and clinical cofactors.
Supporting Information Figure S5. Correlation between water soluble vitamins and clinical cofactors.
Supporting Information Figure S6. Correlation between water and fat soluble vitamins.
Supporting Information Figure S7. Correlation between fat soluble vitamins and clinical variables after intervention.
Supporting Information Figure S8. Correlation between water soluble vitamins and clinical variables after intervention.
Supporting Information Figure S9. Correlation between water and fat soluble vitamins after intervention.
Supporting Information Figure S10. Enet selected predictors of γ‐tocopherol response from vitamin and clinical baseline.
Supporting Information Figure S11. Frequency (parenthesis) and fitted coefficients of predictor variables across bootstrapped analyses of iron response.
Fig. S12. Frequency and fitted coefficients of predictor variables across bootstrapped analyses of glycemia response.
Supporting Information Figure S13. Frequency and fitted coefficients of predictor variables across bootstrapped analyses of basophil response.
Supporting Information File 1
Detailed Description of Environment
Additional Methods
Additional Results
Extended Discussion
Supporting Information Table S1: Micronutrient Genomics Project Variables
Supporting Information Table S2: Demographic, nutritional status, pubertal, economic status, anemia, and dyslipidemia prevalence of all children and adolescents at baseline in Brazil Micronutrient Project (outliers and sibs included)
Supporting Information File 2. ElasticNet coefficients


