Abstract
Background
Parkinson’s disease (PD) is a common age-related neurodegenerative disorder, but effective therapeutic agents for PD remain largely limited.
Material/Methods
In the present study, we evaluated the beneficial effects and underlying mechanisms of punicalagin (PN) in human neuroblastoma SH-SY5Y cells treated with 6-hydroxydopamine (6-OHDA) to mimic PD in vitro. Cell viability was monitored by MTT assay and LDH release assay. Cell apoptosis was assayed by Annexin V-FITC/PI double-staining. Intracellular ROS production was assessed by DCFH-DA staining. The expression levels of protein and mRNA were determined by Western blotting and qRT-PCR analysis, respectively.
Results
The results showed that pretreatment of SH-SY5Y cells with PN (50, 100, and 200 μM) prior to exposure to 200 μM 6-OHDA for 2 h resulted in increased cell viability and decreased cell apoptosis. PN also inhibited excessive oxidative stress in 6-OHDA-treated SH-SY5Y cells. Moreover, PN treatment effectively restored mitochondrial function and enhanced phosphorylation of AMPK. Furthermore, PN blocked 6-OHDA-induced NF-κB activation and IL-1β expression.
Conclusions
Our study shows that PN exhibited neuroprotective effects on the 6-OHDA-treated SH-SY5Y cells, thus providing a potential theoretical insight for the clinical application of PN against PD.
MeSH Keywords: AMP-Activated Protein Kinases, NF-kappa B, Oxidative Stress, Parkinson Disease
Background
Parkinson’s disease (PD), a neurodegenerative, progressive disorder first reported by James Parkinson in 1817 [1], affects about 1% of the population over the age of 50 years [2]. AD is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta, along with the abnormal aggregation of α-synuclein in Lewy bodies [3]. PD severely affects a patient’s physical and mental condition and imposes a substantial economic burden on society. Therefore, novel effective drugs with limited adverse effects and other forms of therapeutic approaches for PD are required.
Drug development for PD remains a major challenge. In recent years, considerable attention has been paid to the beneficial effects of natural products in neurodegenerative diseases, including PD [4]. Punica granatum (pomegranate) is an antioxidant-rich fruit. Punicalagin (2,3-hexahydroxydiphenoyl-gallagyl-D-glucose; PN; Figure 1) is the major antioxidant polyphenol ingredient in pomegranate, and it is beneficial for overall good health. It is well established that PN possesses many physiological activities, including strong anti-oxidative and anti-inflammatory properties. For example, PN has been reported to exert inhibitory effects on lipopolysaccharide-induced neuroinflammation and oxidative stress in cultured astrocytes and microglial BV-2 cells [5]. However, there are limited reports on the protective activities of PN in PD.
Figure 1.
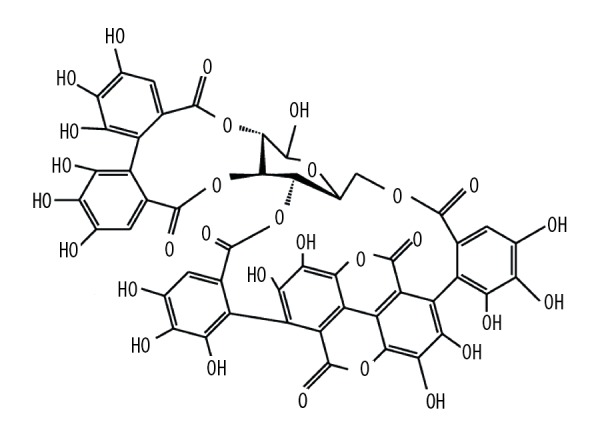
The chemical structure of punicalagin (PN).
6-hydroxydopamine (6-OHDA) is a synthetic organic compound widely used to generate an experimental cellular model of PD in vitro [6]. In the present study, we used 6-OHDA to induce cytotoxicity in human neuroblastoma SH-SY5Y cells to investigate the potential protective role of PN in PD, as well as the related underlying mechanisms.
Material and Methods
Cell culture and drug treatment
Human neuroblastoma cell line SH-SY5Y cells, obtained from American Type Culture Collection (ATCC; Manassas, VA, USA), were plated onto culture plates coated with poly-d-lysine and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin-streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. SH-SY5Y cells were treated with 200 μM 6-OHDA (dissolved in ascorbic acid; Sigma, St. Louis, MO, USA) for 24 h to establish the PD model in vitro. At 2 h prior to 6-OHDA treatment, SH-SY5Y cells were pretreated with PN (50, 100, and 200 μM; Tauto Biotech, Shanghai, China) or vehicle (0.1% v/v DMSO; Sigma). In some cases, cells were pretreated with the AMPK inhibitor compound c (5 μM; Calbiochem, San Diego, CA, USA).
Cell viability analysis
Cell viability was monitored by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After the aforementioned treatments, SH-SY5Y cells were treated with 20 μl of MTT solution (5 mg/ml; Sigma) at 37°C. After another 4 h, the supernatants were replaced with DMSO to dissolve the formazan crystals, and the absorbance was measured with a microplate reader (Dynex, Chantilly, VA, USA) at a wavelength of 570 nm.
Lactate dehydrogenase (LDH) release assay
Cell injury was determined by measuring the level of lactate dehydrogenase (LDH) released into the medium when cell membranes are damaged. Total and released LDH levels were determined by colorimetric method using an LDH assay kit (Jiancheng, Nanjing, China). Absorbance of all samples was measured at 490 nm using a microplate reader. Released LDH was normalized to total LDH.
Cell apoptosis analysis
Cell apoptosis was assayed by an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit (MultiSciences Biotech, Co., Ltd., Suzhou, China) [7]. Briefly, after treatments, SH-SY5Y cells were collected, washed, and resuspended in the binding buffer. Then, the cells were incubated with 5 μl Annexin V-FITC and stained with 5 μl PI at room temperature for 15 min in the dark. The apoptosis rates were detected by FACScan flow cytometer (BD Biosciences, Franklin lakes, NJ, USA) equipped with Cell Quest software.
Measurement of intracellular ROS accumulation
Intracellular ROS production was assessed with 2′, 7′-dichlorofluorescein diacetate (DCFH-DA) fluorescent probe (Sigma). In brief, after various treatments, the cells were incubated with DCFH-DA (10 μM) in the dark at 37°C for 30 min. Then, the cells were washed 3 times with PBS and the fluorescence was monitored by flow cytometry.
Measurement of superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity was measured using a commercial SOD assay kit (Jiancheng). After treatments, the cells were homogenized and lysed on ice. Then, lysates were centrifuged and the supernatant was collected to analyze the SOD activity. The luminescence was measured using a microplate reader.
Measurement of intracellular ATP level
The ATP levels in the cells were detected using a commercial ATP assay kit (Beyotime). In brief, after treatments, the cells were incubated with the assay buffer with shaking for 15 min at room temperature. Then, the chemiluminescence of the samples was detected using a microplate reader.
Measurement of mitochondrial membrane potential (MMP)
The MMP was measured using the dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1; Cayman Chemical Co., Ann Arbor, MI, USA). At high membrane potentials, JC-1 enters the mitochondria and forms aggregates that have a red fluorescence, whereas JC-1 shows as a green fluorescence at a low potential. Briefly, after the aforementioned treatments, the cells were washed with PBS and incubated with 0.5 ml JC-1-fluorescent dye for 30 min at 37°C in the dark. The fluorescence of green monomer and red aggregates were quantified by flow cytometry. Results are expressed as the ratio of the red/green fluorescence.
Western blotting
Cellular proteins were prepared using RIPA lysis buffer (Beyotime). For detection of cytochrome c, the mitochondrial and cytosolic fractions were prepared using the Mitochondria/Cytosol Fractionation kit (Abcam, Cambridge, MA, USA). For detection of NF-κB p65, the nuclear and cytosolic fractions were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Rockford, IL, USA). The protein concentrations were assessed using a BCA protein assay kit (Beyotime) [8]. Lysates containing equivalent amounts of protein were loaded on SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Then, the membranes were incubated with primary antibodies overnight at 4°C, followed by incubation with HRP-conjugated secondary antibodies for 2 h at room temperature. Protein bands were visualized using the enhanced chemiluminescence detection system (Amersham, Little Chalfont, UK). GAPDH, COX IV, or Lamin B was considered as a loading control.
Quantitative reverse transcription-PCR (qRT-PCR)
The mRNA levels of IL-1β were measured by quantitative reverse transcription-PCR (qRT-PCR). After the aforementioned treatments, total RNA was isolated from the cells using the Trizol reagent (Invitrogen). First-strand cDNA was synthesized using the reverse transcription kit (Takara, Tokyo, Japan). qPCR was performed on the LightCycler 480 System II (Roche Diagnostics, Rotkreuz, Switzerland) using the fluorescent dye SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). The data were calculated using the 2−ΔΔCt method with GAPDH serving as an internal control [9]. The sequences of primers used here were shown as follows: IL-1β, forward primer: 5′-GAGCTCGCCAGTGAAATGATG-3′, reverse primer: 5′-AGTGGTGGTCGGAGATTCGT-3′; GAPDH, forward primer: 5′-ACAGTCAGCCGCATCTTCTT-3′, reverse primer: 5′-GACAAGCTTCCCGTTCTCAG-3′.
Statistical analysis
All results are expressed by the mean ± standard deviation (SD) and the data were from at least 3 independent experiments performed in triplicate. One-way ANOVA followed by Tukey’s multiple comparison were performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The difference was considered as significant when the P-value was less than 0.05.
Results
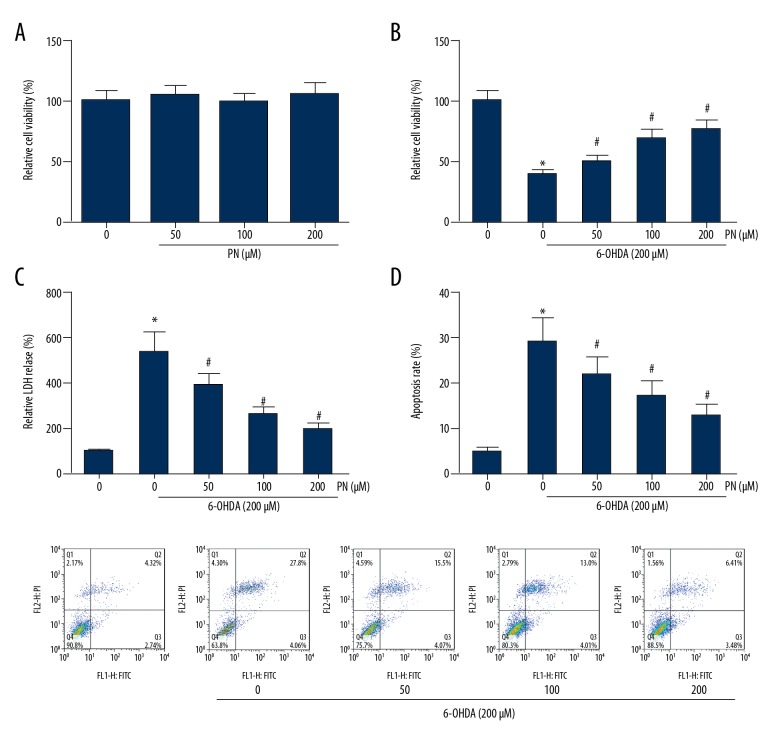
PN attenuates 6-OHDA-induced cytotoxicity in SH-SY5Y cells
First, as indicated by MTT assay, PN alone had no obvious effect on SH-SY5Y cell viability (Figure 2A). Then, the effects of PN on the 6-OHDA-treated SH-SY5Y cells were determined, and we found that the average viability of the cells treated with 6-OHDA alone was reduced to approximately 40%, and PN pretreatment exerted protective effects against 6-OHDA-induced injury (Figure 2B). The protective role of PN in 6-OHDA-injured SH-SY5Y cells was further confirmed by LDH release assay. As expected, the LDH release was significantly eliminated by PN pretreatment (Figure 2C). SH-SY5Y cells were further stained with apoptosis marker Annexin V/PI and analyzed with flow cytometry, and the results demonstrated that PN dose-dependently rescued SH-SY5Y cells from 6-OHDA-induced apoptosis (Figure 2D).
Figure 2.
PN attenuates 6-OHDA-induced cytotoxicity in SH-SY5Y cells. (A) MTT analysis of SH-SY5Y cells treated with different doses of PN alone for 24 h. (B) MTT analysis of 6-OHDA-treaed SH-SY5Y cells pretreated with different doses of PN. (C) LDH release was assessed using a commercial kit. (D) Cell apoptosis was determined through Annexin V-FITC/PI double-staining. * P<0.05 vs. 6-OHDA-untreated cells; # P<0.05 vs. PN-untreated cells.
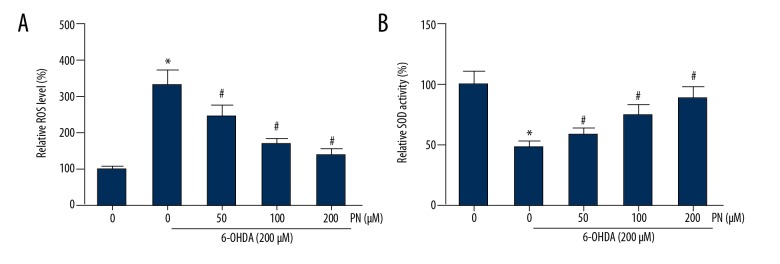
PN reduces 6-OHDA-induced oxidative stress in SH-SY5Y cells
ROS accumulation and the induced oxidative stress play a critical role in cell apoptosis [10]. The level of intracellular ROS was examined by DCFH-DA staining. The results showed that SH-SY5Y cells treated with 6-OHDA showed a significant increase of intracellular ROS, and this increase was significantly restored by PN pretreatment (Figure 3A). Administration of PN also dose-dependently enhanced the SOD activity in 6-OHDA-treated SH-SY5Y cells (Figure 3B).
Figure 3.
PN reduces 6-OHDA-induced oxidative stress in SH-SY5Y cells. (A) Intracellular ROS accumulation was detected through DCFH-DA staining. (B) Intracellular SOD activity was estimated using a commercial kit. *P<0.05 vs. 6-OHDA-untreated cells; # P<0.05 vs. PN-untreated cells.
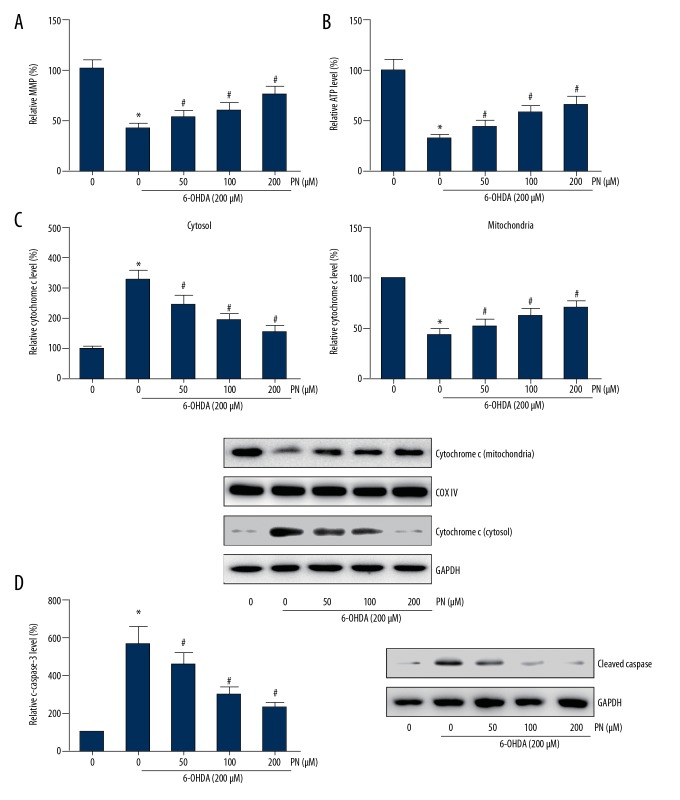
PN attenuates 6-OHDA-induced mitochondrial dysfunction in SH-SY5Y cells
To further assess the effects of PN on mitochondrial injury induced by 6-OHDA, we analyzed the MMP of treated cells by JC-1 staining. The results showed that PN remarkably restored the 6-OHDA-induced disruption of MMP (Figure 4A). Consistently, we also observed that PN pretreatment increased the intracellular ATP levels in the 6-OHDA-treated cells (Figure 4B). Furthermore, as shown in Figure 4C, 6-OHDA treatment resulted in an increase of cytosolic cytochrome c, whereas PN significantly reduced the cytochrome c release in a dose-dependent manner. The mitochondria-mediated apoptosis involves the activation of caspase cascade. Here, we also observed that PN dose-dependently attenuated the increased expression levels of cleaved caspase-3 induced by 6-OHDA exposure (Figure 4D).
Figure 4.
PN attenuates 6-OHDA-induced mitochondrial dysfunction in SH-SY5Y cells. (A) The disruption of MMP was determined through JC-1 staining. (B) Intracellular ATP level was estimated using a commercial kit. (C) Western blot analysis was performed to detect the protein levels of cytochrome c in the cytosolic and mitochondrial fractions. (D) Western blot analysis was performed to detect the protein levels of cleaved caspase-3. * P<0.05 vs. 6-OHDA-untreated cells; # P<0.05 vs. PN-untreated cells.
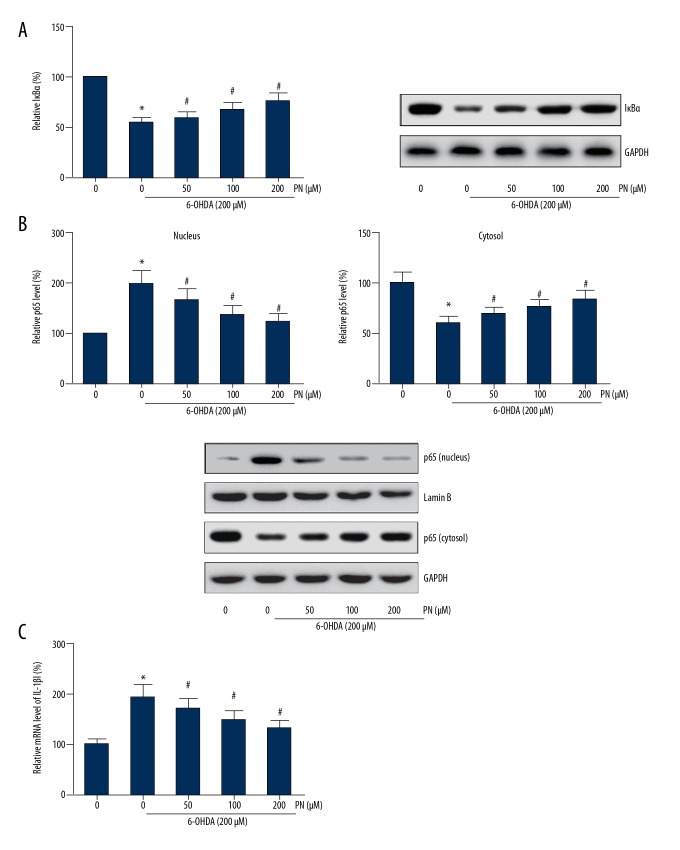
Figure 6.
PN suppresses NF-κB activation and inhibits IL-1β expression in 6-OHDA-treated SH-SY5Y cells. (A) Western blot analysis was performed to detect the protein levels of IκBα. (B) Western blot analysis was performed to detect the protein levels of p65 in the cytosolic and nuclear fractions. (C) qRT-PCR was performed to detect the mRNA levels of IL-1β. * P<0.05 vs. 6-OHDA-untreated cells; # P<0.05 vs. PN-untreated cells.
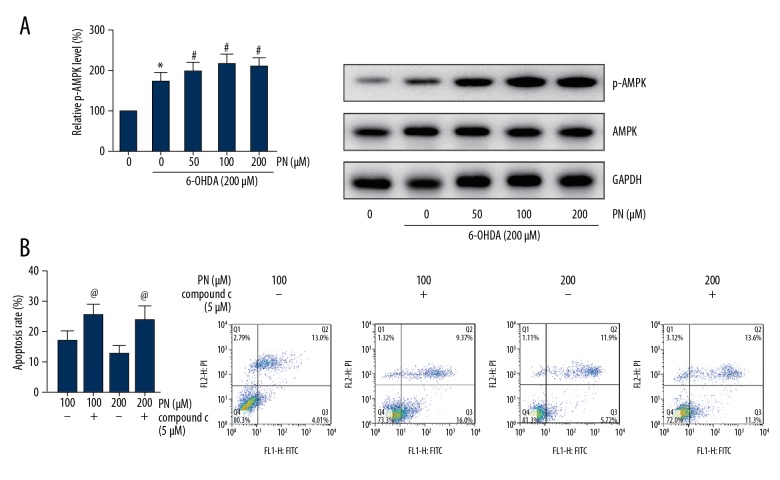
PN rescued 6-OHDA-induced phosphorylation of AMPK in SH-SY5Y cells
As demonstrated in Figure 5A, we found that 6-OHDA exposure could promote phosphorylation of AMPK. However, intriguingly, pretreatment of PN enhanced the phosphorylation levels of AMPK. We then chose compound c as the AMPK inhibitor to analyze whether AMPK inhibition could block the protective role of PN in 6-OHDA-induced cell apoptosis. The p-AMPK level was significantly reduced due to the application of compound c (data not shown), and 5 μM of compound c remarkably increased the apoptotic rate of SH-SY5Y cells pretreated with PN before 6-OHDA exposure (Figure 5B).
Figure 5.
PN rescued 6-OHDA-induced phosphorylation of AMPK in SH-SY5Y cells. (A) Western blot analysis was performed to detect the protein levels of p-AMPK. (B) Cell apoptosis was determined through Annexin V-FITC/PI double-staining. * P<0.05 vs. 6-OHDA-untreated cells; # P<0.05 vs. PN-untreated cells; @ P<0.05 vs. compound c-untreated cells.
PN suppresses NF-κB activation and inhibits IL-1β expression in 6-OHDA-treated SH-SY5Y cells
We found that SH-SY5Y cells treated with 6-OHDA had significant promotion of IκBα degradation and p65 nuclear translocation, which were dose-dependently inhibited by PN pretreatment (Figure 6A, 6B). We also found that 6-OHDA exposure increased the mRNA levels of IL-1β in SH-SY5Y cells, and pretreatment with PN remarkably abrogated the 6-OHDA-induced IL-1β expression (Figure 6C).
Discussion
Many studies have demonstrated that natural products derived from fruits and vegetables have neuroprotective effects. In the present study, we established a sensitive PD model using 6-OHDA on SH-SY5Y cell line due to its neuronal background and neuron-like properties [11], and observed that 6-OHDA exposure led to impaired viability and increased apoptosis of SH-SY5Y cells. These effects were significantly counteracted by pretreatment of PN. These experimental results clearly illustrate the neuroprotective effects of PN on 6-OHDA-injured SH-SY5Y cells.
ROS increases with age, and oxidative stress caused by excessive ROS is closely related to the pathogenesis of PD [12]. The mitochondrion is a critical organelle for cellular metabolism, and the impairment of mitochondrial function is also linked to aging and neurodegeneration [13]. 6-OHDA can produce oxidative stress, thereby inducing a ROS-related collapse in MMP [14,15]. Here, we found that PN eliminated ROS production, stabilized MMP, and therefore inhibited cytochrome c release in 6-OHDA-treated SH-SY5Y cells. Cytochrome c release further induces the activation of the caspase cascade, thus eventually leading to apoptosis [16]. In the present study, the cleavage of caspase-3 was also significantly suppressed by PN pretreatment.
AMPK is an important cellular energy sensor in most cells [17]. Owing to mitochondrial damage, the ATP/AMP ratio may be reduced, thus leading to AMPK activation. A recent study reported that PD toxins induced phosphorylation of AMPK in PC12 cells and primary neurons [18]. AMPK activation appears to affect neuronal energy homeostasis, help preserve cellular energy, and then exert neuroprotective effects [19]. Our data show that compound c application reduced the p-AMPK level, and the apoptotic cells were then increased due to AMPK inactivation, indicating that AMPK activation is also involved in the beneficial effects of PN on 6-OHDA-treated cells.
Increasing evidence has emerged to indicate that neuroinflammation is implicated in the pathogenesis and development of PD [20,21]. The nuclear factor-κB (NF-κB) signaling pathway plays a critical function in regulating inflammatory responses. Nuclear translocation of NF-κB is increased in dopaminergic neurons of PD patients [22], and NF-κB activation is also found in 6-OHDA induced dopaminergic neuronal cell death [23]. Many studies have reported the anti-neuroinflammatory ability of PN [5,24], and here we also found that PN led to the inhibition of NF-κB activation and IL-1β expression in 6-OHDA-treated SH-SY5Y cells, indicating the potential anti-inflammatory role of PN in PD.
Conclusions
Our findings provided direct experimental evidence that PN exerts its neuroprotective effects against 6-OHDA-induced cytotoxicity in SH-SY5Y cells through attenuating mitochondrial dysfunction and inflammatory responses. Although the in vivo effects require further exploration, the present study provides support for the potential role of PN in preventing neuronal cell death in the process of PD.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Lees AJ. Unresolved issues relating to the shaking palsy on the celebration of James Parkinson’s 250th birthday. Mov Disord. 2007;22(Suppl 17):S327–34. doi: 10.1002/mds.21684. [DOI] [PubMed] [Google Scholar]
- 2.Miller DB, O’Callaghan JP. Biomarkers of Parkinson’s disease: Present and future. Metabolism. 2015;64(3 Suppl 1):S40–46. doi: 10.1016/j.metabol.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasser T. Molecular pathogenesis of Parkinson disease: Insights from genetic studies. Exp Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Bai L, He J, et al. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur J Med Chem. 2017;141:257–72. doi: 10.1016/j.ejmech.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 5.Kim YE, Hwang CJ, Lee HP, et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology. 2017;117:21–32. doi: 10.1016/j.neuropharm.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/845618. 845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 2011;(50) doi: 10.3791/2597. pii: 2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 10.Clutton S. The importance of oxidative stress in apoptosis. Br Med Bull. 1997;53(3):662–68. doi: 10.1093/oxfordjournals.bmb.a011637. [DOI] [PubMed] [Google Scholar]
- 11.Xicoy H, Wieringa B, Martens GJ. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol Neurodegener. 2017;12(1):10. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345(1–2):91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 13.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta. 2010;1802(1):29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Lotharius J, Dugan LL, O’Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci. 1999;19(4):1284–93. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Lazaro M, Galindo MF, Concannon CG, et al. 6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA. J Neurochem. 2008;104(6):1599–612. doi: 10.1111/j.1471-4159.2007.05115.x. [DOI] [PubMed] [Google Scholar]
- 16.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Liu C, Chen S, et al. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal. 2014;26(8):1680–89. doi: 10.1016/j.cellsig.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104(17):7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Dis. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8(4):382–97. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 22.Hunot S, Brugg B, Ricard D, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci USA. 1997;94(14):7531–36. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SH, Choi WS, Yoon SY, et al. Activation of NF-kappaB is involved in 6-hydroxydopamine-but not MPP+ -induced dopaminergic neuronal cell death: Its potential role as a survival determinant. Biochem Biophys Res Commun. 2004;322(3):727–33. doi: 10.1016/j.bbrc.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 24.Olajide OA, Kumar A, Velagapudi R, et al. Punicalagin inhibits neuroinflammation in LPS-activated rat primary microglia. Mol Nutr Food Res. 2014;58(9):1843–51. doi: 10.1002/mnfr.201400163. [DOI] [PubMed] [Google Scholar]