Abstract
Breast cancer (BrCa) metabolism is geared toward biomass synthesis and maintenance of reductive capacity. Changes in glucose and glutamine metabolism in BrCa have been widely reported, yet the contribution of fatty acids (FAs) in BrCa biology remains to be determined. We recently reported that adipocyte coculture alters MCF‐7 and MDA‐MB‐231 cell metabolism and promotes proliferation and migration. Since adipocytes are FA‐rich, and these FAs are transferred to BrCa cells, we sought to elucidate the FA metabolism of BrCa cells and their response to FA‐rich environments. MCF‐7 and MDA‐MB‐231 cells incubated in serum‐containing media supplemented with FAs accumulate extracellular FAs as intracellular triacylglycerols (TAG) in a dose‐dependent manner, with MDA‐MB‐231 cells accumulating more TAG. The differences in TAG levels were a consequence of distinct differences in intracellular partitioning of FAs, and not due to differences in the rate of FA uptake. Specifically, MCF‐7 cells preferentially partition FAs into mitochondrial oxidation, whereas MDA‐MB‐231 cells partition FAs into TAG synthesis. These differences in intracellular FA handling underpin differences in the sensitivity to palmitate‐induced lipotoxicity, with MDA‐MB‐231 cells being highly sensitive, whereas MCF‐7 cells are partially protected. The attenuation of palmitate‐induced lipotoxicity in MCF‐7 cells was reversed by inhibition of FA oxidation. Pretreatment of MDA‐MB‐231 cells with FAs increased TAG synthesis and reduced palmitate‐induced apoptosis. Our results provide novel insight into the potential influences of obesity on BrCa biology, highlighting distinct differences in FA metabolism in MCF‐7 and MDA‐MB‐231 cells and how lipid‐rich environments modulate these effects.
Keywords: breast cancer, CPT1, DGAT, fatty acid oxidation, oleate, triacylglycerols
Abbreviations
- ATF4
activating transcription factor 4
- ATGL
adipose triglyceride lipase
- BrCa
breast cancer
- BSA
bovine serum albumin
- CPT1A
carnitine palmitoyltransferase 1A
- DGAT‐1
diacylglycerol O‐acyltransferase 1
- DMSO
dimethyl sulfoxide
- ER
endoplasmic reticulum
- FA
fatty acid
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3‐phosphate dehydrogenase
- PARP
poly (ADP ribose) polymerase
- TAG
triacylglycerols
- TLC
thin‐layer chromatography
1. Introduction
Cancer cells require adaptations across multiple metabolic processes to support increased rates of cell growth and division (DeBerardinis et al., 2008; Hanahan and Weinberg, 2011), highlighting potential new therapeutic strategies. One such adaptive alteration in cancer metabolism observed in most, but not all, cancer cells is termed the ‘Warburg effect’—the upregulation of glycolytic flux and lactate production even in the presence of adequate oxygen (Warburg, 1956). Similarly, demand for glutamine is greater than other nonessential amino acids in cancer cells (Coles and Johnstone, 1962). These alterations are characterized by redistribution of glucose carbons away from catabolism for ATP production toward macromolecule synthesis and maintenance of reductive capacity to sustain proliferation (Romero‐Garcia et al., 2011). In addition to enhanced glucose and glutamine metabolism, alterations in cancer cell fatty acid (FA) metabolism have been reported (Balaban et al., 2015). However, in general, these observations have predominantly been limited to the synthesis of new FAs from nonlipid carbon sources, that is, glucose and glutamine (Zhang and Du, 2012). One important observation is that FAs contributed more to total ATP turnover in breast cancer (BrCa) cells than that of glucose or glutamine (Guppy et al., 2002). However, the influence of readily available FAs in lipid‐rich environments (e.g., from stromal adipocytes or the circulation of obese patients) for utilization in BrCa cells remains to be fully elucidated.
FAs are important metabolic substrates for ATP, NADPH, and macromolecular synthesis and are major regulators in cellular signaling pathways (Glatz and Luiken, 2015). The intracellular‐free FA pool is supplied by de novo lipogenesis, intracellular triacylglycerols (TAG) contained in lipid droplets, and exogenous sources—including in the circulation or local microenvironment (Santos and Schulze, 2012). Interestingly, increased lipid droplet number is a feature of aggressive BrCa (Antalis et al., 2010; Chamras et al., 2002; Przybytkowski et al., 2007; Shiu and Paterson, 1984). We recently showed that the vast majority of carbons contributing to the intracellular lipid pool in BrCa cells arise from extracellular FAs and not from glucose or glutamine (Balaban et al., 2017). Cells take up FAs from the bloodstream or local microenvironment via various surface transport proteins (Glatz and Luiken, 2017). FAs are then condensed into and stored as TAG in lipid droplets (Farese and Walther, 2009) and other complex lipids including phospholipids (Louie et al., 2013), or enter the mitochondria for β‐oxidation (Bruce et al., 2009). Interestingly, exogenous FAs have anti‐/pro‐proliferative, promigratory, and antiapoptotic effects in BrCa cells depending on context, suggesting a link between extracellular FAs and BrCa cell behavior (Hardy et al., 2000, 2003; Li et al., 2014). However, the nature of this effect is dependent on the chemical structure of FAs, the saturation level. Unsaturated FAs, including oleate and linoleate, support cell growth, whereas saturated FAs such as palmitate are toxic to cells (Hardy et al., 2000; Listenberger et al., 2003). Recently, we showed that coculturing BrCa cells with FA‐rich adipocytes increased TAG levels and promoted BrCa cell proliferation and migration, which were enhanced in the presence of obese adipocytes (Balaban et al., 2017). Collectively, these observations suggest a link between intracellular FA metabolism and disease progression that is amplified in obese individuals. However, the mechanistic links between FA availability and metabolism to individual attributes of breast cancer cell behavior are yet to be resolved in detail.
The aim of this study was to characterize the FA metabolism of BrCa cells and their response to FA‐rich environments. Insights into these mechanisms will provide greater understanding of the role that elevated extracellular FA levels may play in linking obesity‐induced enhanced BrCa progression (Eheman et al., 2012).
2. Materials and methods
2.1. Cell culture
The estrogen receptor α‐positive MCF‐7 (ATCC HTB‐22), BT‐474 (ATCC HTB‐20), and MDA‐MB‐175 (ATCC HTB‐25) and the estrogen receptor α‐negative MDA‐MB‐231 (ATCC HTB‐26), BT‐549 (ATCC HTB‐122), and MDA‐MB‐468 (ATCC HTB‐132) human BrCa cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in high glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; HyClone, GE Healthcare Life Sciences, Pittsburgh, PA, USA) and 100 IU·mL−1 penicillin and 100 IU·mL−1 streptomycin (Life Technologies Australia Pty Ltd., Scoresby, Vic, Australia). Cell lines are validated periodically in house by Garvan Molecular Genetics using a test based on the Powerplex 18D Kit (DC1808, Promega, Madison, WI, USA) and tested for mycoplasma every 3 months (MycoAlert™ mycoplasma detection kit, Lonza, Basel, Switzerland).
To inhibit diacylglycerol O‐acyltransferase 1 (DGAT1) activity, cells were treated with AZD3988 (Tocris Bioscience, Invitrogen) (McCoull et al., 2012) or dimethyl sulfoxide (DMSO) control for 24 h in low‐glucose DMEM and no antibiotics. After treatment, cells were washed and sensitivity to palmitate‐induced apoptosis was assessed. To inhibit FA oxidation, cells were treated with etomoxir (Sigma‐Aldrich, Castle Hill, NSW, Australia) in low‐glucose DMEM and no antibiotics for times detailed in figure legends.
2.2. Lipid‐loading cells
Cells were incubated in medium supplemented with 0–450 μm of either oleate only or 1 : 2 : 1 palmitate/oleate/linoleate (FA mix) as indicated with 10% FBS (vol./vol.) in low‐glucose DMEM and no antibiotics for 24 h.
2.3. Substrate metabolism
Extracellular‐derived FA metabolism: Cells were incubated in assay medium containing 0.5 mmol·L−1 cold oleate or palmitate, [1‐14C]oleate or [1‐14C]palmitate (0.5 μCi·mL−1; PerkinElmer, Boston, MA), conjugated to 2% (wt/vol.) FA‐free bovine serum albumin (BSA), and 1 mm carnitine in low‐glucose DMEM for 4 h. Mitochondrial oxidation was determined from 14CO2 production as previously described (Meex et al., 2015). Cells were harvested in ice‐cold PBS to determine 14C‐oleate incorporation into intracellular lipid pools and protein content.
Cellular lipids were extracted by Folch method (Folch et al., 1957). Lipids were separated by thin‐layer chromatography (TLC) using heptane/isopropyl ether/acetic acid (60 : 40 : 3, v/v/v) as developing solvent for TAG or by a two‐step solvent system for ceramides where TLC plates were developed to one‐third of the total length of the plate in chloroform: methanol: 25% NH3 (20 : 4 : 0.2, v/v/v), dried, and then re‐chromotographed in heptane/isopropyl ether/acetic acid (60 : 40 : 3, v/v/v). 14C activity in the TAG and ceramide bands was determined by scintillation counting.
Intracellular TAG‐derived FA metabolism: Cells were pulsed overnight for 18 h with [1‐14C]oleate (1 μCi·mL−1; PerkinElmer, Boston, MA, USA) and cold oleate (80 or 450 μm), to prelabel the endogenous TAG pool. Following the pulse, the specific activity of the TAG pool was determined in a cohort of cells by measuring the 14C activity in the TAG following lipid extraction and TLC as well as the biochemical assessment of the TAG pool (see Biochemical Measures). Lipolysis was determined in another cohort run in parallel where cells were chased for 4 h in low‐glucose DMEM containing 0.5% FA‐free BSA and 10 μm triacsin C to block FA recycling. TAG‐derived FA oxidation (endogenous FA oxidation) was measured by determination of 14CO2 production in the absence of triacsin C and in the presence of 1 mm carnitine.
2.4. Biochemical measures
Cell TAG were extracted using the method of Folch et al. (1957) and quantified using an enzymatic colorimetric method (GPO‐PAP reagent, Roche Diagnostics, North Ryde, NSW, Australia). Cell protein content was determined using Pierce Micro BCA protein assay (Life Technologies Australia Pty Ltd.).
2.5. Western blot analysis
Protein extraction was performed as described previously (Hoy et al., 2011). Cell lysates were subjected to SDS/PAGE, transferred to PVDF membranes (Merck Millipore, Bayswater, Vic, Australia) and then immunoblotted with antibodies for adipose triglyceride lipase (ATGL), poly (ADP ribose) polymerase (PARP, cleaved PARP), activating transcription factor 4 (ATF4), and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) obtained from Cell Signaling Technology (Danvers, MA, USA), and anti‐14‐3‐3 from Santa Cruz Biotechnology (ThermoFisher Scientific, Scoresby, Vic, Australia). Chemiluminescent visualization performed using Luminata Crescendo Western Horseradish Peroxidase Substrate (Merck Millipore) and imaged using the Bio‐Rad ChemiDoc MP Imaging System (Bio‐Rad laboratories, Hercules, CA, USA) using image lab software 4.1 (Bio‐Rad laboratories).
2.6. Palmitate treatment and cell viability
Cells were plated in triplicate in 96‐well plates (3 × 103 cells/well), and a group of cells were then lipid‐loaded for 24 h. The following day, the media were removed, cells were washed, and fresh low‐glucose DMEM with 10% FBS media ± 250 μm palmitate (Sigma‐Aldrich) added. MTT assays were performed as described previously (Roslan et al., 2014) at defined time points stated in figure legends. Viable cells were counted by trypan blue dye exclusion at indicated time points stated in figure legends. In a parallel cohort in 6‐well plates, cells were lysed for immunoblot analysis after 24 h of palmitate treatment.
2.7. Gene expression survival analysis
Analysis of DGAT1 gene expression, alteration frequencies, and patient outcomes (overall survival) in all cancers (n = 2051) from the METABRIC breast cancer cohort was performed using the cBioPortal for Cancer Genomics (Cerami et al., 2012; Gao et al., 2013). Hazard ratio was calculated using graphpad prism 7.03 (GraphPad Software, San Diego, CA, USA) from exported primary data from cBioPortal for Cancer Genomics.
2.8. Statistical analysis
Statistical analyses were performed with graphpad prism 7.03 (GraphPad Software). Differences among groups were assessed with appropriate statistical tests noted in figure legends. P ≤ 0.05 was considered significant. Data are reported as mean ± SEM.
3. Results
3.1. Increasing FA availability increases triacylglycerol content in MCF‐7 and MDA‐MB‐231 cells, leading to increased FA oxidation
We recently reported that adipocyte‐derived FA uptake promotes BrCa cell proliferation (Balaban et al., 2017), and others have described that the more aggressive MDA‐MB‐231 cells have increased neutral lipid stores in lipid droplets compared to the less aggressive MCF‐7 cells (Antalis et al., 2010; Chamras et al., 2002; Przybytkowski et al., 2007; Shiu and Paterson, 1984). Firstly, we determined whether the extracellular lipid environment could influence lipid droplet‐contained TAG levels in BrCa cells. MCF‐7 and MDA‐MB‐231 were exposed to a range of oleate concentrations or a FA mix consisting of palmitate, oleate, and linoleate in a 1 : 2 : 1 ratio that represents the mixture of FAs found in the plasma (Watt et al., 2012). Total intracellular TAG content of MCF‐7 and MDA‐MB‐231 increased in a dose‐dependent manner in response to increasing concentrations of either oleate alone or the FA mixture (Fig. 1A), showing that the accumulation of FAs in intracellular TAG is not FA species‐specific. Interestingly, MDA‐MB‐231 cells had a higher basal intracellular TAG content (MCF‐7: 8.2 ± 0.6 nmol·mg−1; MDA‐MB‐231: 12.7 ± 1.3 nmol·mg−1, n = 18; P = 0.004) and a higher capacity to incorporate exogenous FAs into storage compared to MCF‐7 cells (Fig. 1A).
Figure 1.
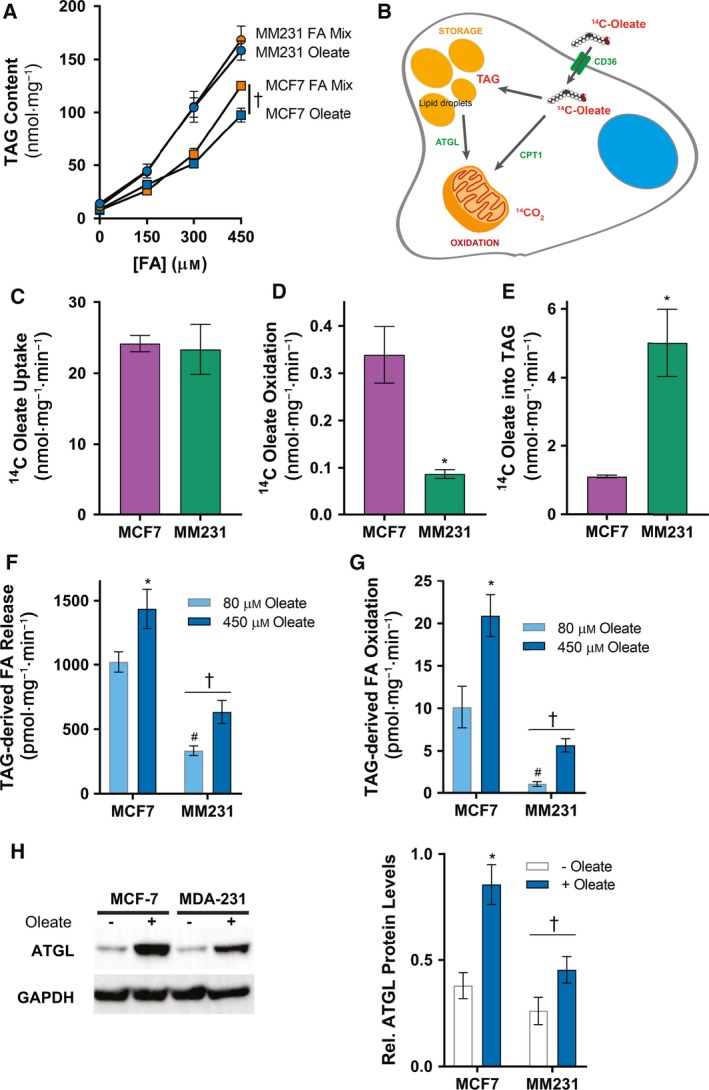
Effect of extracellular lipid availability on intracellular TAG content. (A) MCF‐7 and MDA‐MB‐231 (MM231) cells were treated with a concentration range (0–450 μm) of a lipid cocktail (1 : 2 : 1 palmitate/oleate/linoleate, P/O/L) or oleate alone for 24 h and then assayed for TAG content. (B) Schematic of the pathways mapped by radiotracing of oleate metabolism. 14C‐oleate (C) uptake, (D) oxidation, and (E) incorporation into triacylglycerol (TAG) in MCF‐7 and MDA‐MB‐231 cells. *P < 0.05 compared to MCF‐7 by unpaired Student's t‐test. (F) MCF‐7 and MDA‐MB‐231 cells were treated with 1 μCi/mL [1‐14C]‐oleate and 80 μm or 450 μm cold oleate for 24 h and were subsequently treated with 10 μm triacsin C for 4 h to measure the release of TAG‐derived FAs into lipid‐free media. (G) In another cohort, MCF‐7 and MDA‐MB‐231 cells were incubated with 100 mm carnitine for 4 h and 14 CO 2 production assessed. (H) Representative immunoblots and densitometric quantitation of ATGL in MCF‐7 and MDA‐MB‐231 cells with or without prior overnight incubation with oleate. Data show means ± SEM of three independent experiments performed in triplicate. (A) †P ≤ 0.05 main effect for cells by three‐way ANOVA. (C–E) *P < 0.05 compared to MCF‐7 by unpaired Student's t‐test. (F–H) †P ≤ 0.05 main effect for cells; *P ≤ 0.05 vs. – oleate; #P ≤ 0.05 vs. MCF‐7 cells – oleate by two‐way ANOVA followed by Tukey's multiple comparisons test.
We next examined differences in the capacity of MCF‐7 and MDA‐MB‐231 cells to store extracellular FAs as intracellular TAG (Fig. 1A,B). Interestingly, there was no difference in FA uptake rate (Fig. 1C) but striking differences in intracellular handling of FAs, with MDA‐MB‐231 cells having lower FA oxidation (Fig. 1D) and increased TAG synthesis (Fig. 1E) compared to MCF‐7 cells. The differences in FA oxidation align with our previously reported observations of elevated carnitine palmitoyltransferase 1A (CPT1A) protein levels in MCF‐7 cells compared to MDA‐MB‐231 cells, with MCF‐7 cells have greater amounts of CPT1A compared to MDA‐MB‐231 cells (Balaban et al., 2017).
Lipid droplet‐contained TAG is a temporary storage destination for FAs and acts as an easily mobilized supply of intracellular FAs, so we investigated whether increased intracellular lipid pools altered FA metabolism in these cells. Overnight exposure to media containing an additional 450 μm oleate and 0.2 μCi·mL−1 14C‐oleate increased TAG levels in both MCF‐7 and MDA‐MB‐231 cells compared to cells treated with 80 μm oleate and 0.2 μCi·mL−1 14C‐oleate (data not shown, similar to Fig. 1A). The release of FA into the media (i.e., lipolysis) was significantly greater from MCF‐7 cells compared to MDA‐MB‐231 cells (Fig. 1F). Further, overnight lipid‐loading increased lipolysis in both cell lines (Fig. 1F). Similar patterns were observed in the oxidation of intracellular TAG‐derived FAs (Fig. 1G), which was consistent with differences in the protein levels of the TAG lipase ATGL (Fig. 1H). Therefore, these experiments demonstrate that 1) MDA‐MB‐231 cells accumulate more FAs as TAG due to low oxidative and TAG hydrolysis rates and increased flux of FAs into TAG and 2) MCF‐7 cells have high oxidative and TAG hydrolysis rates and low rate of FA incorporation into TAG.
3.2. MCF‐7 cells have mild sensitivity to palmitate‐induced apoptosis
Both MDA‐MB‐231 and MCF‐7 cells have the capacity to accumulate TAG following incubation in FA‐rich media (Fig. 1). Although FA accumulation in lipid droplets of BrCa cells is well‐described (Antalis et al., 2010; Chamras et al., 2002; Przybytkowski et al., 2007; Shiu and Paterson, 1984), the role of this lipid‐rich, energy‐dense metabolic substrate in cellular behavior is not well defined. While exogenous FAs have both anti‐ and pro‐proliferative effects as well as promigratory and antiapoptotic effects in BrCa cells (Hardy et al., 2000, 2003; Louie et al., 2013), little is known about the responsiveness of lipid‐loaded BrCa cells to apoptotic stimuli. To assess this, we challenged MCF‐7 cells to high levels of palmitate in serum‐containing media, which can induce apoptosis (Hardy et al., 2000, 2003; Kamili et al., 2015; Listenberger et al., 2003). MCF‐7 cells incubated in palmitate‐containing media displayed a striking attenuation in cell viability, as judged by MTT signal (Fig. 2A,B) and cell number (Fig. 2C)—suggesting either inhibition of cell proliferation and/or induction of apoptosis—compared to cells grown in FBS only media. We failed to detect evidence that apoptotic signaling was activated by the addition of palmitate to serum‐containing media (data not shown). Lipid‐loaded MCF‐7 cells with either oleate alone (Fig. 2A) or FA mix (Fig. 2B) were protected from this palmitate‐induced reduction in MTT and cell number (Fig. 2C). These results suggest that extracellular FA availability, and the associated increase in intracellular lipid stores, is associated with protection of MCF‐7 cells from palmitate‐induced apoptosis.
Figure 2.
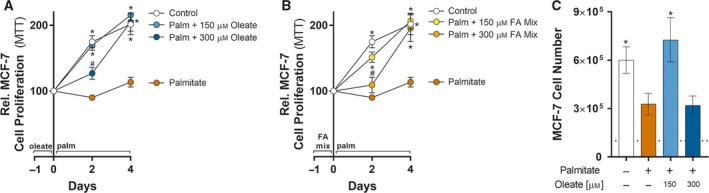
The MCF‐7 cells are not sensitive to palmitate‐induced apoptosis. MTT assays of MCF‐7 cells treated with 250 μm palmitate for 4 days with or without prior overnight incubation with (A) oleate or (B) 1 : 2 : 1 mixture of palmitate/oleate/linoleate (FA mix). MTT results are presented as percentages of MTT absorbance at indicated time points relative to that at Day 0 for each group (five independent experiments performed in quadruplicate). (C) Cell number of MCF‐7 cells treated with 250 μm palmitate for 4 days with or without prior overnight incubation with oleate. The dashed line represents the number of cells present at Day 0 (three independent experiments performed in triplicate). Data are presented as mean ± SEM. *P ≤ 0.05 vs. palmitate; #P ≤ 0.05 vs. control by two‐way ANOVA (A and B) or one‐way ANOVA (D) followed by Tukey's multiple comparisons test.
3.3. Increasing intracellular TAG levels protects MDA‐MB‐231 cells from palmitate‐induced apoptosis and serum starvation
Palmitate treatment reduced cell proliferation in MCF‐7 cells, and exposing these cells to additional extracellular FAs protected cells from palmitate‐induced apoptosis. The same approach was used in MDA‐MB‐231 cells in order to determine whether BrCa cell lines of different hormone receptor status show varied response to palmitate.
The addition of 250 μm palmitate to FBS media strikingly reduced MTT absorbance within 2 days, reducing even further by 4 days compared to cells cultured in FBS (Fig. 3A,B). This reduced reductive metabolic capacity (measured via MTT assay) was associated with activation of PARP and ATF4 signaling after 1 day of palmitate treatment (Fig. 3C), which are markers of apoptosis (Elmore, 2007; Sano and Reed, 2013). Further, the early activation of apoptotic signaling corresponded with reduced cell number (Fig. 3D) and cellular protein amount (Fig. 3E,F) following 4 days of palmitate treatment. Similar to MCF‐7 cells, MDA‐MB‐231 cells lipid‐loaded with either oleate alone (Fig. 3A) or FA mixture (Fig. 3B) were partly protected from the effects of palmitate‐induced reduction in MTT activity. Further, the effect of palmitate on PARP and ATF4 signaling (Fig. 3B), cell number (Fig. 3D), and cellular protein amount (Fig. 3E,F) was reduced by prior incubation of either oleate alone or FA mixture. In the absence of serum, lipid‐loaded MDA‐MB‐231 cells were similarly, but less strikingly, protected from serum starvation‐induced apoptosis (Fig. 3G). Collectively, these data demonstrate that MDA‐MB‐231 cells are highly sensitive to palmitate‐induced apoptosis, whereas MCF‐7 have attenuated cell proliferation when cultured in palmitate‐rich media, and that intracellular lipid stores influence MCF‐7 and MDA‐MB‐231 sensitivity to palmitate‐induced apoptosis.
Figure 3.

The MDA‐MB‐231 cells are sensitive to palmitate‐induced apoptosis and lipid‐loading protects MDA‐MB‐231 cells from palmitate‐induced apoptosis. MTT assays of MDA‐MB‐231 cells treated with 250 μm palmitate for 4 days with or without prior overnight incubation with (A) oleate or (B) 1 : 2 : 1 mixture of palmitate/oleate/linoleate (FA mix). MTT results are presented as percentages of MTT absorbance at indicated time points relative to that at Day 0 for each group. (five independent experiments performed in quadruplicate). (C) Representative immunoblots of cPARP and ATF4 levels of MDA‐MB‐231 cells treated with 250 μm palmitate for 1 day with or without prior overnight incubation with oleate or 1 : 2 : 1 mixture of palmitate/oleate/linoleate (FA mix; representative of three independent experiments performed in triplicate). (D) Cell number of MDA‐MB‐231 cells treated with 250 μm palmitate for 4 days with or without prior overnight incubation with oleate. The dashed line represents the number of cells present at Day 0 (three independent experiments performed in duplicate). Cell protein content of MDA‐MB‐231 cells treated with 250 μm palmitate for 4 days with or without prior overnight incubation with (E) oleate or (F) 1 : 2 : 1 mixture of palmitate/oleate/linoleate (FA mix) (three independent experiments performed in duplicate). (G) MTT assays of MDA‐MB‐231 cells serum‐starved for 4 days with or without prior overnight incubation with oleate. (H) Palmitate sensitivity of MCF‐7, BT‐474, MDA‐MB‐175, MDA‐MB‐231, BT‐549, and MDA‐MB‐468 cells expressed as the difference in MTT signal between Day 0 (D0) and Day 4 (D4) for cells cultured in serum‐containing media supplemented with 250 μm. Data are presented as mean ± SEM. *P ≤ 0.05 vs. palmitate; #P ≤ 0.05 vs. control by two‐way ANOVA (A and B) or one‐way ANOVA (D–F) followed by Tukey's multiple comparisons test. *P ≤ 0.05 vs. Day 0 MTT signal by Student's t‐test (H).
To complement our novel observations of heterogeneity in the response of MCF‐7 and MDA‐MB‐231 cells to palmitate supplementation, we assessed the sensitivity of other BrCa cells to palmitate and whether this effect was altered by prior lipid‐loading. Unlike MCF‐7 cells, BT‐474 and MDA‐MB‐175 cells incubated in palmitate‐containing media displayed a striking reduction in MTT signal (Fig. S1A–D) and cells lipid‐loaded with either oleate alone (Fig. S1A and C) or FA mixture (Fig. S1B and D) were partly protected from the effects of palmitate. Interestingly, BT‐549 cells cultured in palmitate‐containing media displayed attenuated cell growth (Fig. S1E and F), whereas MDA‐MB‐468 cells more closely phenocopied MDA‐MB‐231 cells in that palmitate supplementation of serum‐containing media induced apoptosis which was partly prevented by prior oleate (Fig. S1G) or FA mixture (Fig. S1H) treatment. Overall, we observed no consistent response to palmitate supplementation across the three estrogen receptor α‐positive (MCF‐7, BT‐474, and MDA‐MB‐175), and three estrogen receptor α‐negative (MDA‐MB‐231, BT‐549, and MDA‐MB‐468) human BrCa cells (Fig. 3H).
3.4. Differences in palmitate handling explain the differential response to palmitate‐induced apoptosis in MCF‐7 and MDA‐MB‐231 cells
The striking differences in palmitate sensitivity between MDA‐MB‐231 and MCF‐7 cells, both in the basal state and after lipid‐loading, may be due to altered intracellular palmitate metabolism, similar to the patterns of oleate metabolism (Fig. 1). To test this, the fate of extracellularly‐derived radiolabeled FAs was assessed in MDA‐MB‐231 and MCF‐7 cells incubated in 14C‐palmitate, in either the basal state or following overnight exposure to oleate. Total palmitate uptake was similar in MDA‐MB 231 and MCF‐7 cells, and we observed no effect of overnight oleate exposure on palmitate uptake (Fig. 4A). Interestingly, MDA‐MB‐231 cells had dramatically lower palmitate oxidation (Fig. 4B) and greater incorporation of palmitate into TAG (Fig. 4C) compared to MCF‐7 cells, consistent with our previous observations of oleate metabolism (Balaban et al., 2017). Pretreatment with oleate increased palmitate oxidation in MCF‐7 but not MDA‐MB‐231 cells (Fig. 4B), whereas MDA‐MB‐231 TAG synthesis was increased (Fig. 4C). Hence, the overall effect is a greater partitioning of palmitate into storage relative to oxidation in MDA‐MB‐231 cells compared to MCF‐7 cells, and this intracellular partitioning in MDA‐MB‐231 cells is increased by pretreatment with oleate (Fig. 4D).
Figure 4.
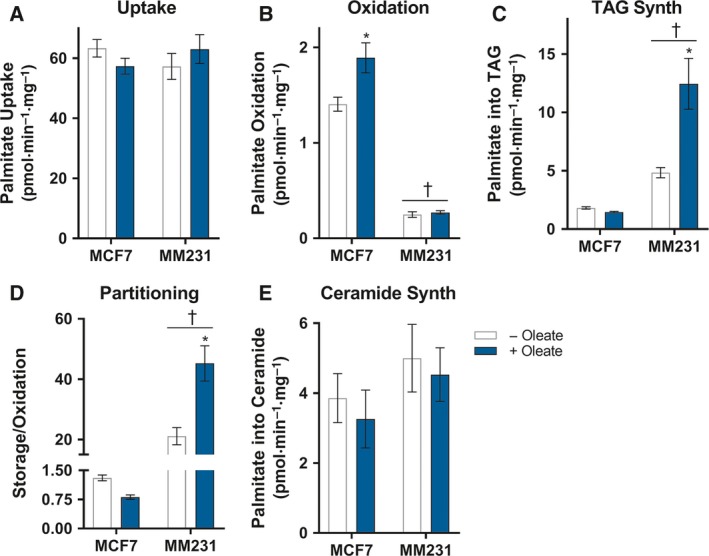
The MCF‐7 and MDA‐MB‐231 cells metabolize palmitate differently and this is selectively altered by pretreatment with oleate. 14C‐palmitate (A) uptake, (B) oxidation, (C) incorporation into triacylglycerol (TAG), (D) intracellular partitioning of 14C‐palmitate expressed as the ratio of 14C‐palmitate incorporation into triacylglycerol (storage) vs. 14C‐palmitate oxidation, 14C‐palmitate incorporation into (E) ceramide in MCF‐7 and MDA‐MB‐231 cells with or without prior overnight incubation with 150 μm oleate. Data are presented as mean ± SEM of three independent experiments performed in triplicate. †P ≤ 0.05 main effect for cells; *P ≤ 0.05 vs. – oleate; #P ≤ 0.05 vs. MCF‐7 cells – oleate by two‐way ANOVA followed by Tukey's multiple comparisons test.
Palmitate is a critical precursor of de novo ceramide synthesis (Kitatani et al., 2008), which can activate apoptosis (Tohyama et al., 1999). As such, one hypothesis to explain the enhanced sensitivity to palmitate in MDA‐MB‐231 cells compared to MCF‐7 cells was enhanced ceramide synthesis in MDA‐MB‐231 cells. However, there was no difference in the rate of palmitate incorporation into ceramide in MCF‐7 and MDA‐MB‐231 cells, and this was not altered by oleate pretreatment (Fig. 4E), thereby excluding this mechanism. Collectively, these experiments demonstrate that MCF‐7 and MDA‐MB‐231 cells incorporate exogenous palmitate at similar rates, but they metabolize this saturated FA differently. Specifically, MCF‐7 cells have higher rates of palmitate oxidation compared to MDA‐MB‐231 cells, whereas MDA‐MB‐231 cells have a higher rate of storing palmitate as TAG and this is enhanced by pretreatment with oleate. As such, the differences in palmitate handling may explain the differential sensitivity to palmitate‐induced apoptosis.
3.5. Inhibition of mitochondrial FA oxidation sensitizes MCF‐7 cells to palmitate‐induced apoptosis
MCF‐7 cells are protected from palmitate‐induced apoptosis compared to MDA‐MB‐231 cells, which may be due to higher palmitate oxidation (Fig. 4B) related to CPT1A protein levels (Balaban et al., 2017). Therefore, we tested whether inhibiting palmitate oxidation sensitized MCF‐7 cells to palmitate‐induced apoptosis. Treating MCF‐7 cells with the CPT1 inhibitor etomoxir lowered basal palmitate oxidation (Fig. 5A). The addition of 250 μm palmitate to growth media slowed cell growth but the combination of palmitate and etomoxir further reduced the MTT signal (Fig. 5B). This reduction in MTT signal was associated with reduced MCF‐7 cell number (Fig. 5C) and cellular protein amount (Fig. 5D) after 4 days of treatment, as well as activation of PARP signaling after 1 day of treatment (Fig. 5E). Inhibition of FA oxidation sensitizes MCF‐7 cells to palmitate‐induced apoptosis, indicating that FAO is an important part of apoptosis resistance in these cells. There were some discrepancies in the measured effect of palmitate and etomoxir alone between individual readouts (i.e., MTT, cell number, and cellular protein), which likely reflect differential effects on the cellular characteristic being measured. For example, MTT is a redox/cell viability measure which may not necessarily correlate with cell number and cellular protein levels in all instances.
Figure 5.
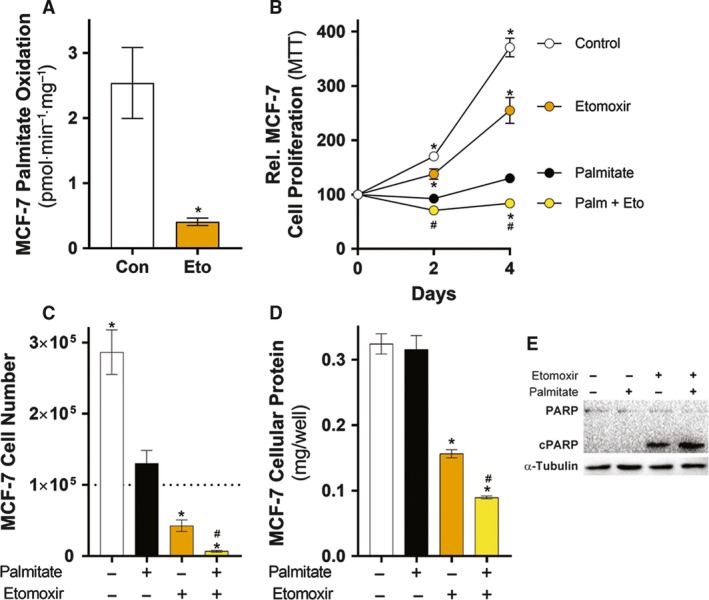
Inhibition of fatty acid oxidation in MCF‐7 cells sensitizes cells to palmitate‐induced apoptosis. (A) 14C‐palmitate oxidation in MCF‐7 cells that were treated with or without 100 μM etomoxir (Eto) (five independent experiments performed in triplicate). (B) MTT assays of MCF‐7 cells treated with 250 μm palmitate (Palm), 100 μm etomoxir (Eto), or a combination for 4 days. MTT results are presented as percentages of MTT absorbance at indicated time points relative to that at Day 0 for each group (MTT: six independent experiments performed in quadruplicate). (C) Cell number and (D) protein amount of MCF‐7 cells treated with 250 μm palmitate (Palm), 100 μm etomoxir (Eto), or a combination for 4 days. The dashed line represents the number of cells present at Day 0 (three independent experiments performed in triplicate). (E) Representative immunoblots of cPARP of MCF‐7 cells treated with 250 μm palmitate, 100 μm etomoxir, or a combination for 1 day (three independent experiments performed in triplicate). Data are presented as mean ± SEM. (A) *P ≤ 0.05 vs. control by unpaired Student's t‐test. (B) *P ≤ 0.05 vs. palmitate; #P ≤ 0.05 vs. etomoxir by two‐way ANOVA followed by Tukey's multiple comparisons test. (C and D) *P ≤ 0.05 vs. palmitate; #P ≤ 0.05 vs. etomoxir by one‐way ANOVA followed by Tukey's multiple comparisons test.
3.6. Inhibition of oleate‐stimulated TAG synthesis restores sensitivity to palmitate‐induced apoptosis in lipid‐loaded MDA‐MB‐231 cells
Pretreating MDA‐MB‐231 cells with either oleate alone or the FA mixture protected from apoptosis induced by either palmitate or serum starvation (Fig. 3). Radiometric analysis of palmitate metabolism points to an increase in TAG synthesis (Fig. 4C) as a potential mechanism by which FA pretreatment protects from palmitate apoptosis by shunting palmitate into lipid droplet‐contained TAG. We directly tested this by inhibiting TAG synthesis through the addition of an inhibitor of DGAT1, which catalyzes the final reaction in TAG synthesis (Cases et al., 1998, 2001), only during the preincubation of oleate prior to palmitate treatment. As expected, DGAT1 inhibition attenuated the increase in MDA‐MB‐231 TAG content in response to oleate administration (Fig. 6A) as a consequence of a reduction in the rate of incorporation of radiolabeled oleate into TAG (Fig. 6B). As previously observed, palmitate treatment induced apoptosis and preincubation with oleate blunted this effect (Fig. 6C); however, lowering intracellular TAG levels by DGAT1 inhibition in the presence of oleate pretreatment restored MDA‐MB‐231 cell sensitivity to palmitate at Day 2 (Fig. 6C). After 4 days of palmitate treatment, the MTT signal in MDA‐MB‐231 cells pretreated with the DGAT1 inhibitor and oleate did increase but it was still lower than MDA‐MB‐231 cells that were pretreated with oleate alone (Fig. 6C). The reduction in MTT signal was associated with reactivation of PARP signaling (Fig. 6D) and reduced cellular protein amount (Fig. 6E). Therefore, TAG synthesis is required for the protective effects of pretreating MDA‐MB‐231 cells with FAs to palmitate‐induced apoptosis. Analysis of the METABRIC breast cancer cohort (Clark, 1989; Curtis et al., 2012) showed DGAT1 amplification in 21% (434/2051) of breast cancer samples. Analysis of overall survival in this cohort showed a significant decrease in overall survival in patients with DGAT1 amplification (143 months vs 158.6 months, HR: 0.844 (95% CI: 0.727–0.980), P = 0.0193; Fig. 6F).
Figure 6.
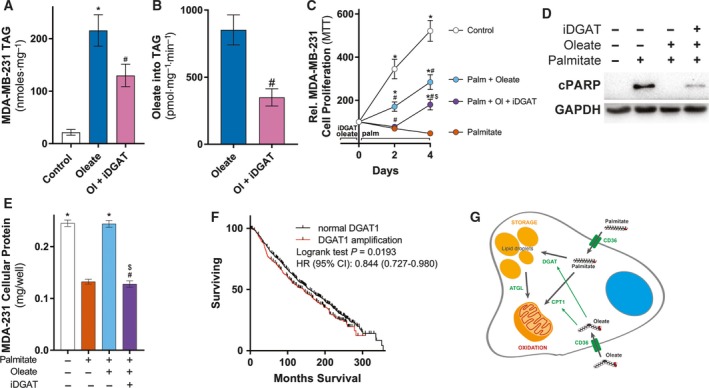
Inhibition of TAG synthesis in MDA‐MB‐231 cells blunts the protective effects of prior oleate treatment to palmitate‐induced apoptosis. (A) MDA‐MB‐231 cell triacylglycerol (TAG) levels in cells treated with 300 μm oleate with or without 0.6 nm DGAT inhibitor AZD3988 (iDGAT) for 24 h (four independent experiments performed in duplicate). (B) 14C‐oleate incorporation into TAG in MDA‐MB‐231 cells that were treated with or without DGAT inhibitor (iDGAT; three independent experiments performed in duplicate). (C) MTT assays of MDA‐MB‐231 cells treated with 250 μm palmitate (Palm) for 4 days following prior incubation with 300 μM oleate (Ol) with or without 0.6 nm DGAT inhibitor AZD3988 (iDGAT). MTT results are presented as percentages of MTT absorbance at indicated time points relative to that at Day 0 for each group (four independent experiments performed in quadruplicate). (D) Representative immunoblots of cPARP of MDA‐MB‐231 cells treated with 250 μm palmitate for 1 days following prior incubation with oleate with or without DGAT1 inhibitor (three independent experiments performed in triplicate). (E) Protein amount of MDA‐MB‐231 cells treated with 250 μm palmitate for 4 days following prior incubation with 300 μM oleate with or without DGAT inhibitor (iDGAT; three independent experiments performed in triplicate). (F) Differential overall survival among breast cancer cases parsed by DGAT1 amplification. (G) Schematic of the mechanisms by which oleate pretreatment prevents palmitate‐induced apoptosis. Data are presented as mean ± SEM. (A) *P ≤ 0.05 vs. control; #P ≤ 0.05 vs. oleate by one‐way ANOVA followed by Tukey's multiple comparisons test. (B) #P ≤ 0.05 vs. oleate by unpaired Student's t‐test. (C) *P ≤ 0.05 vs. palmitate; #P ≤ 0.05 vs. control; $P ≤ 0.05 vs. palm + oleate by two‐way ANOVA followed by Tukey's multiple comparisons test. (E) *P ≤ 0.05 vs. palmitate; #P ≤ 0.05 vs. control; $P ≤ 0.05 vs. palm + oleate by one‐way ANOVA followed by Tukey's multiple comparisons test.
4. Discussion
Cancer cells require adaptations across multiple metabolic processes to support increased rate of cell growth and division (DeBerardinis et al., 2008). The majority of interest in tumor metabolism to date has focused on glucose metabolism (e.g., Warburg effect) and glutamine addiction in cancer cells (Ward and Thompson, 2012). FAs have received far less attention, even though the contribution of FAs to total ATP turnover in BrCa cells is greater than that of glucose or glutamine (Guppy et al., 2002). In this study, we demonstrate that MCF‐7 and MDA‐MB‐231 cells differ in their intracellular handling of FAs and in response to palmitate‐induced apoptosis. These differences in palmitate‐induced apoptosis were reflected across a range of other BrCa cell lines, independent of estrogen receptor α‐status, highlighting the striking heterogeneity in palmitate sensitivity. Specifically, MCF‐7 cells have increased FA oxidative capacity that underpins their resistance to palmitate‐induced apoptosis. MDA‐MB‐231 cells have increased TAG synthesis capacity and are highly sensitive to palmitate‐induced apoptosis which was inhibited by prior lipid‐loading to stimulate TAG synthesis.
All cells and organisms store lipids to provide a buffer for energy fluctuations and promote survival. This lipid is stored in cytosolic lipid droplets, which are often in close proximity to other cellular organelles, notably mitochondria and endoplasmic reticulum (ER). Lipid droplets are highly conserved in yeast through to mammals (Le Lay and Dugail, 2009), but the size and number of lipid droplets varies between cell types. Lipid droplets store neutral lipids including TAG and cholesteryl esters as temporary complex lipid storage forms of FAs and cholesterol. A number of studies have reported that the number of lipid droplets in estrogen receptor α‐positive MCF‐7 cells is lower than in the more aggressive ERα‐negative MDA‐MB‐231 cells (Antalis et al., 2010; Chamras et al., 2002; Przybytkowski et al., 2007; Shiu and Paterson, 1984). Consistent with these observations, we demonstrated that both MCF‐7 and MDA‐MB‐231 cells can respond to changing levels of extracellular FAs and accumulate these as TAG in a dose‐dependent manner, independent of FA species. Interestingly, MDA‐MB‐231 cells accumulated more FAs in TAG compared to MCF‐7 cells, which is supported by a previous report that investigated the addition of oleate only to culture media (Przybytkowski et al., 2007). This increased ability of MDA‐MB‐231 cells to accumulate more TAG is a consequence of increased partitioning of FAs into TAG, and lower oxidative and TAG lipolytic capacity compared to MCF‐7 cells. The lower rates of FA oxidation and lipolysis correlate with protein levels of the rate limiting enzymes in these pathways, CPT1 (Balaban et al., 2017) and ATGL, respectively. These observations suggest that MDA‐MB‐231 cells are more sensitive to extracellular FA availability and have higher capacity to store FAs as TAG than MCF‐7 cells. However, the role of estrogen receptor α‐mediated signaling in driving the differences in FA metabolism between these two cell lines remains to be determined.
Exposure to elevated levels of the saturated FA palmitate induces apoptosis in a range of cells including 3T3 fibroblasts (Kamili et al., 2015), peripheral blood mononuclear cells (RostamiRad et al., 2018), human cardiac progenitor cells (Leonardini et al., 2017), pancreatic β cells (Boslem et al., 2011; Luo et al., 2017), macrophages (Kim et al., 2017), and hepatocytes (Penke et al., 2017). Palmitate also impairs MDA‐MB‐231 cell proliferation and activates apoptosis (Baumann et al., 2016; Hardy et al., 2000, 2003; Kourtidis et al., 2009; Wu et al., 2017) via a number of mechanisms, including ER stress (Baumann et al., 2016; Boslem et al., 2011), impaired autophagy (RostamiRad et al., 2018; Wu et al., 2017), altered NAD metabolism (Penke et al., 2017), and ceramide synthesis (Luo et al., 2017). Importantly, many of the deleterious effects of palmitate on cellular function are mitigated by cotreatment with other FAs, in particular the monounsaturated FA oleate (Colvin et al., 2017; Kim et al., 2017; Penke et al., 2017; Sargsyan et al., 2016), which itself is pro‐proliferative and activates phosphoinositide 3‐kinase signaling (Hardy et al., 2000).
We provide new insight into both the cell‐specific effects of palmitate as well as the mechanism by which palmitate alters BrCa cell biology. Firstly, MCF‐7 cells are less sensitive to the apoptotic effects of palmitate treatment in the presence of serum compared to MDA‐MB‐231 cells, which is similar in response to serum starvation (Przybytkowski et al., 2007), and palmitate treatment in serum‐free conditions (Hardy et al., 2000). The differential response was not due to differences in palmitate uptake but associated with postuptake intracellular handling of palmitate. Specifically, MDA‐MB‐231 cells partition palmitate toward storage as lipid droplet confined TAG, likely explaining the differences in lipid droplet amount between MDA‐MB‐231 and MCF‐7 cells (Antalis et al., 2010; Chamras et al., 2002; Przybytkowski et al., 2007; Shiu and Paterson, 1984). MCF‐7 cells preferentially partition palmitate toward mitochondrial oxidation, which is associated with increased CPT1A protein levels in these cells (Balaban et al., 2017). Mitochondrial FA oxidation is the primary bioenergetic pathway in many nontumor tissues (Bonnefont et al., 2004) and is a greater contributor to total ATP turnover than glucose or glutamine in MCF‐7 cells (Guppy et al., 2002). Pharmacological inhibition of this metabolic pathway impairs cell growth and viability in a range of cancer cells (Rodriguez‐Enriquez et al., 2015), including MYC‐driven triple‐negative BrCa (Camarda et al., 2016). We and others have reported that MCF‐7 cells have high rates of mitochondrial oxidation of exogenous FAs compared to MDA‐MB‐231 cells (Przybytkowski et al., 2007), and we now report that this is also the case for the oxidation of intracellular‐derived FAs. This elevated FA oxidative capacity in MCF‐7 cells was associated with higher levels of CPT1A (Balaban et al., 2017) and ATGL protein levels, and was enhanced by overnight oleate treatment. We have recently reported that CPT1A and oxidative phosphorylation protein levels and FA oxidation rate are also increased following coculture with adipocytes (Balaban et al., 2017), which was also observed with ZR‐75‐1 BrCa cells (Wang et al., 2017). In fact, inhibition of FA oxidation reduced adipocyte‐stimulated ZR‐75‐1 BrCa cell invasion (Wang et al., 2017) and we report here that inhibition of FA oxidation sensitized MCF‐7 cells to palmitate‐induced apoptosis, as determined by a combination of MTT, cell number, cellular protein amount, and PARP signaling. Our results shed new light on the contribution of FA oxidation in MCF‐7 cells where pharmacological inhibition of FA oxidation sensitized MCF‐7 cells to palmitate‐induced apoptosis and suggests that targeting FA oxidation is an attractive therapeutic strategy in BrCa. The addition or presence of oleate ameliorates the cytotoxic effects of palmitate (Capel et al., 2016; Colvin et al., 2017; Kim et al., 2017; Kwon and Querfurth, 2015; Penke et al., 2017; Sargsyan et al., 2016). Proposed mechanisms for these observations include attenuating palmitate‐induced ER stress (Colvin et al., 2017), preventing activation of the unfolded protein response (Sommerweiss et al., 2013), activating prosurvival pathways of ER stress (Sargsyan et al., 2016), restoring insulin stimulated protein kinase B (Akt) signaling (Capel et al., 2016), and activating AMP‐activated protein kinase and mechanistic target of rapamycin signaling (Kwon and Querfurth, 2015). Oleate treatment in a range of cells also modulates palmitate metabolism including stimulating TAG synthesis, preventing diacylglycerol accumulation (Capel et al., 2016; Kwon et al., 2014), and preventing mitochondrial reactive oxygen species production and dysfunction (Kwon et al., 2014). These observations were predominantly made by cotreatment with oleate and palmitate. In the current study, we also observed that pretreatment with either oleate or a FA mixture to increase intracellular TAG levels prevented palmitate‐induced apoptosis. This alteration in the response to palmitate treatment was observed in both MCF‐7 and MDA‐MB‐231 cells and was associated with increased palmitate oxidation in MCF‐7 cells and increased TAG synthesis in MDA‐MB‐231 cells. There was no change in palmitate uptake or palmitate incorporation into ceramide following overnight oleate treatment. This is counter to the recent report that oleate cotreatment of RAW264.7 macrophage cells prevented the palmitate‐induced increase in the mRNA level of the FA transporter CD36 (Kim et al., 2017), hinting at cell type‐specific mechanisms. Collectively, oleate and FA treatment influences MCF‐7 and MDA‐MB‐231 cell palmitate metabolism and thereby avoid the deleterious effects of lipotoxicity.
Several studies have demonstrated that the ability to synthesize TAG plays a critical role in the protection from palmitate‐induced lipotoxicity (Kamili et al., 2015; Listenberger et al., 2003; Przybytkowski et al., 2007). For example, oleate supplementation promotes TAG synthesis in CHO cells and also prevents palmitate‐induced apoptosis in mouse embryonic fibroblasts but this protection was not seen in DGAT1−/− mouse embryonic fibroblasts (Listenberger et al., 2003). We demonstrate that pretreatment of MDA‐MB‐231 cells with oleate or FA mix protects from both palmitate‐induced and serum starvation‐induced apoptosis. The ability of oleate pretreatment to prevent serum starvation‐induced apoptosis is consistent with previous observations in other models (Przybytkowski et al., 2007). The pro‐apoptotic effects of palmitate in MDA‐MB‐231 cells has been shown to require autophagy protein 5 (Wu et al., 2017), whereas we show that the protection from palmitate‐induced apoptosis by prior lipid‐loading required the upregulation of TAG synthesis via DGAT1. Further, DGAT1 amplification in a large breast cancer cohort was associated with a significant decrease in overall survival. This latter observation was not seen in H4IIEC3 rat hepatoma cells when inhibiting TAG synthesis through siRNA silencing of DGAT1 and DGAT2 (Leamy et al., 2016). Although MDA‐MB‐231 cells have a high incorporation rate of FAs into intracellular TAG pools compared to MCF‐7 cells, this rate appears to be inadequate to prevent palmitate‐induced apoptosis and only with cosupplementation with oleate or pretreatment with oleate or the FA mix is the synthesis of TAG sufficient to partition lipotoxic palmitate into safe storage in intracellular lipid droplet‐contained TAG.
This study has focused on the contribution of FA metabolism in mediating palmitate‐induced apoptosis. Extensive literature exists describing the effects of FAs on BrCa cell proliferation and migration/invasion (see review (Kinlaw et al., 2016)). For example, oleate increases proliferation, migration and invasion in MDA‐MB‐231 cells (Hardy et al., 2005; Wu et al., 2017) and MCF‐7 cells (Soto‐Guzman et al., 2008), but this effect on MCF‐7 cells is not always observed (Hardy et al., 2005). Other literature has described the effects of other FA species such as the omega‐3 or omega‐6 FAs on BrCa cell biology (Mansara et al., 2015; Tiwari et al., 1991; Zhang et al., 2012). However, the breadth of FA diversity, driven by differences in chain length and saturation/desaturation status, and combinations of different FA species continue to challenge the broader understanding of the influence of FAs has on BrCa cell proliferation, migration, and invasion.
5. Conclusion
In conclusion, we revealed that extracellular FAs are used as both fuel and substrates for complex lipid synthesis such as TAG and ceramides in MCF‐7 and MDA‐MB‐231 cells. In addition, high extracellular lipid availability further enhanced FA flux in these cells, supported by an increase in ATGL protein levels. Furthermore, palmitate is preferentially incorporated into TAG for storage in the more aggressive MDA‐MB‐231 cells, whereas less aggressive cell line MCF‐7 shunts palmitate into FA oxidation, highlighting for the first time striking differences in FA metabolism between 2 commonly used BrCa cells. Indeed, inhibiting CPT1A activity blocked palmitate oxidation and increased palmitate toxicity in MCF‐7 cells, thus suggesting a potential therapeutic target for estrogen receptor α‐positive BrCa types which possess higher levels of CPT1A (Balaban et al., 2017). The increased complex lipid synthesis phenotype in MDA‐MB‐231 cells is enhanced in a lipid‐rich environment and prevents palmitate‐induced apoptosis, and therefore, this pathway represents a potential therapeutic target in these cells (Fig. 6G). The outcomes from these experiments may inform on the potential role that obesity‐associated dyslipidemia may play in influencing BrCa progression (De Angel et al., 2013; Hall et al., 2013; Liu et al., 2012), and therefore, these metabolic traits might be the basis for novel therapeutic targeting in BrCa, particularly in obese patients.
Author contributions
SB, LSL, BV, AA, QG, and RFS performed experiments and analyzed data. DNS and TG provided intellectual input and edited the manuscript. AJH conceived the general ideas for this study, designed and performed experiments, analyzed data, and wrote the manuscript. All authors read and approved the final version of the manuscript.
Supporting information
Fig. S1. Breast cancer cell response to palmitate supplementation.
Acknowledgements
AJH is supported by Helen and Robert Ellis Postdoctoral Research Fellowship and funding from the University of Sydney, Sydney Medical School Foundation, and Movember Revolutionary Team Award. SB was a recipient of a University of Sydney Australian Postgraduate Award. DNS is supported by the National Health and Medical Research Council (Project Grant GNT1052963). TG is supported by the University of Sydney (U7007, U7042, U7113, RY253), Sydney, Australia.
Thanks to the Bosch Institute Molecular Biology Facility for technical support.
References
- Antalis CJ, Arnold T, Rasool T, Lee B, Buhman KK and Siddiqui RA (2010) High ACAT1 expression in estrogen receptor negative basal‐like breast cancer cells is associated with LDL‐induced proliferation. Breast Cancer Res Treat 122, 661–670. [DOI] [PubMed] [Google Scholar]
- Balaban S, Lee LS, Schreuder M and Hoy AJ (2015) Obesity and cancer progression: is there a role of fatty acid metabolism? Biomed Res Int 2015, 274585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T et al (2017) Adipocyte lipolysis links obesity to breast cancer growth: adipocyte‐derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann J, Wong J, Sun Y and Conklin DS (2016) Palmitate‐induced ER stress increases trastuzumab sensitivity in HER2/neu‐positive breast cancer cells. BMC Cancer 16, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont JP, Djouadi F, Prip‐Buus C, Gobin S, Munnich A and Bastin J (2004) Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med 25, 495–520. [DOI] [PubMed] [Google Scholar]
- Boslem E, MacIntosh G, Preston AM, Bartley C, Busch AK, Fuller M, Laybutt DR, Meikle PJ and Biden TJ (2011) A lipidomic screen of palmitate‐treated MIN6 beta‐cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J 435, 267–276. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Hoy AJ, Turner N, Watt MJ, Allen TL, Carpenter K, Cooney GJ, Febbraio MA and Kraegen EW (2009) Overexpression of carnitine palmitoyltransferase‐1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high‐fat diet‐induced insulin resistance. Diabetes 58, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, Eyob H, Kajimura S, Tward A, Krings G et al (2016) Inhibition of fatty acid oxidation as a therapy for MYC‐overexpressing triple‐negative breast cancer. Nat Med 22, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel F, Cheraiti N, Acquaviva C, Henique C, Bertrand‐Michel J, Vianey‐Saban C, Prip‐Buus C and Morio B (2016) Oleate dose‐dependently regulates palmitate metabolism and insulin signaling in C2C12 myotubes. Biochim Biophys Acta 1861, 2000–2010. [DOI] [PubMed] [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ et al (1998) Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 95, 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T and Farese RV Jr (2001) Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 276, 38870–38876. [DOI] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamras H, Ardashian A, Heber D and Glaspy JA (2002) Fatty acid modulation of MCF‐7 human breast cancer cell proliferation, apoptosis and differentiation. J Nutr Biochem 13, 711–716. [DOI] [PubMed] [Google Scholar]
- Clark RJ (1989) The treatment of chemical dependence. JAMA 261, 3239. [PubMed] [Google Scholar]
- Coles NW and Johnstone RM (1962) Glutamine metabolism in Ehrlich ascites‐carcinoma cells. Biochem J 83, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin BN, Longtine MS, Chen B, Costa ML and Nelson DM (2017) Oleate attenuates palmitate‐induced endoplasmic reticulum stress and apoptosis in placental trophoblasts. Reproduction 153, 369–380. [DOI] [PubMed] [Google Scholar]
- Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y et al (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angel RE, Blando JM, Hogan MG, Sandoval MA, Lansakara PD, Dunlap SM, Hursting SD and Cui Z (2013) Stearoyl gemcitabine nanoparticles overcome obesity‐induced cancer cell resistance to gemcitabine in a mouse postmenopausal breast cancer model. Cancer Biol Ther 14, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G and Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7, 11–20. [DOI] [PubMed] [Google Scholar]
- Eheman C, Henley SJ, Ballard‐Barbash R, Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE, Kohler BA et al (2012) Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 118, 2338–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV Jr and Walther TC (2009) Lipid droplets finally get a little R‐E‐S‐P‐E‐C‐T. Cell 139, 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M and Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226, 497–509. [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz JF and Luiken JJ (2015) Fatty acids in cell signaling: historical perspective and future outlook. Prostaglandins Leukot Essent Fatty Acids 92, 57–62. [DOI] [PubMed] [Google Scholar]
- Glatz JF and Luiken JJ (2017) From fat to FAT (CD36/SR‐B2): understanding the regulation of cellular fatty acid uptake. Biochimie 136, 21–26. [DOI] [PubMed] [Google Scholar]
- Guppy M, Leedman P, Zu X and Russell V (2002) Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF‐7 breast cancer cells. Biochem J 364, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RG 2nd, Jean GW, Sigler M and Shah S (2013) Dosing considerations for obese patients receiving cancer chemotherapeutic agents. Annals Pharmacother 47, 1666–1674. [DOI] [PubMed] [Google Scholar]
- Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hardy S, El‐Assaad W, Przybytkowski E, Joly E, Prentki M and Langelier Y (2003) Saturated fatty acid‐induced apoptosis in MDA‐MB‐231 breast cancer cells. A role for cardiolipin. J Biol Chem 278, 31861–31870. [DOI] [PubMed] [Google Scholar]
- Hardy S, Langelier Y and Prentki M (2000) Oleate activates phosphatidylinositol 3‐kinase and promotes proliferation and reduces apoptosis of MDA‐MB‐231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res 60, 6353–6358. [PubMed] [Google Scholar]
- Hardy S, St‐Onge GG, Joly E, Langelier Y and Prentki M (2005) Oleate promotes the proliferation of breast cancer cells via the G protein‐coupled receptor GPR40. J Biol Chem 280, 13285–13291. [DOI] [PubMed] [Google Scholar]
- Hoy AJ, Bruce CR, Turpin SM, Morris AJ, Febbraio MA and Watt MJ (2011) Adipose triglyceride lipase‐null mice are resistant to high‐fat diet‐induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology 152, 48–58. [DOI] [PubMed] [Google Scholar]
- Kamili A, Roslan N, Frost S, Cantrill LC, Wang D, Della‐Franca A, Bright RK, Groblewski GE, Straub BK, Hoy AJ et al (2015) TPD52 expression increases neutral lipid storage within cultured cells. J Cell Sci 128, 3223–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Cho YM, Lee KH, Jeong SW and Kwon OJ (2017) Oleate protects macrophages from palmitate‐induced apoptosis through the downregulation of CD36 expression. Biochem Biophys Res Commun 488, 477–482. [DOI] [PubMed] [Google Scholar]
- Kinlaw WB, Baures PW, Lupien LE, Davis WL and Kuemmerle NB (2016) Fatty acids and breast cancer: make them on site or have them delivered. J Cell Physiol 231, 2128–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitatani K, Idkowiak‐Baldys J and Hannun YA (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20, 1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Srinivasaiah R, Carkner RD, Brosnan MJ and Conklin DS (2009) Peroxisome proliferator‐activated receptor‐gamma protects ERBB2‐positive breast cancer cells from palmitate toxicity. Breast Cancer Res 11, R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B, Lee HK and Querfurth HW (2014) Oleate prevents palmitate‐induced mitochondrial dysfunction, insulin resistance and inflammatory signaling in neuronal cells. Biochim Biophys Acta 1843, 1402–1413. [DOI] [PubMed] [Google Scholar]
- Kwon B and Querfurth HW (2015) Palmitate activates mTOR/p70S6K through AMPK inhibition and hypophosphorylation of raptor in skeletal muscle cells: reversal by oleate is similar to metformin. Biochimie 118, 141–150. [DOI] [PubMed] [Google Scholar]
- Le Lay S and Dugail I (2009) Connecting lipid droplet biology and the metabolic syndrome. Prog Lipid Res 48, 191–195. [DOI] [PubMed] [Google Scholar]
- Leamy AK, Hasenour CM, Egnatchik RA, Trenary IA, Yao CH, Patti GJ, Shiota M and Young JD (2016) Knockdown of triglyceride synthesis does not enhance palmitate lipotoxicity or prevent oleate‐mediated rescue in rat hepatocytes. Biochim Biophys Acta 1861, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardini A, D'Oria R, Incalza MA, Caccioppoli C, Andrulli Buccheri V, Cignarelli A, Paparella D, Margari V, Natalicchio A, Perrini S et al (2017) GLP‐1 receptor activation inhibits palmitate‐induced apoptosis via ceramide in human cardiac progenitor cells. J Clin Endocrinol Metab 102, 4136–4147. [DOI] [PubMed] [Google Scholar]
- Li S, Zhou T, Li C, Dai Z, Che D, Yao Y, Li L, Ma J, Yang X and Gao G (2014) High metastaticgastric and breast cancer cells consume oleic acid in an AMPK dependent manner. PLoS ONE 9, e97330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS and Schaffer JE (2003) Triglyceride accumulation protects against fatty acid‐induced lipotoxicity. Proc Natl Acad Sci USA 100, 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Scholz C, Zang C, Schefe JH, Habbel P, Regierer AC, Schulz CO, Possinger K and Eucker J (2012) Metformin and the mTOR inhibitor everolimus (RAD001) sensitize breast cancer cells to the cytotoxic effect of chemotherapeutic drugs in vitro. Anticancer Res 32, 1627–1637. [PubMed] [Google Scholar]
- Louie SM, Roberts LS, Mulvihill MM, Luo K and Nomura DK (2013) Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta 1831, 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Feng Y, Ma H, Liu C, Chen G, Wei X, Mao X, Li X, Xu Y, Tang S et al (2017) Neutral ceramidase activity inhibition is involved in palmitate‐induced apoptosis in INS‐1 cells. Endocr J 64, 767–776. [DOI] [PubMed] [Google Scholar]
- Mansara PP, Deshpande RA, Vaidya MM and Kaul‐Ghanekar R (2015) Differential ratios of omega fatty acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA‐MB‐231. PLoS ONE 10, e0136542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoull W, Addie MS, Birch AM, Birtles S, Buckett LK, Butlin RJ, Bowker SS, Boyd S, Chapman S, Davies RD et al (2012) Identification, optimisation and in vivo evaluation of oxadiazole DGAT‐1 inhibitors for the treatment of obesity and diabetes. Bioorg Med Chem Lett 22, 3873–3878. [DOI] [PubMed] [Google Scholar]
- Meex RC, Hoy AJ, Mason RR, Martin SD, McGee SL, Bruce CR and Watt MJ (2015) ATGL‐mediated triglyceride turnover and the regulation of mitochondrial capacity in skeletal muscle. Am J Physiol Endocrinol Metab 308, E960–E970. [DOI] [PubMed] [Google Scholar]
- Penke M, Schuster S, Gorski T, Gebhardt R, Kiess W and Garten A (2017) Oleate ameliorates palmitate‐induced reduction of NAMPT activity and NAD levels in primary human hepatocytes and hepatocarcinoma cells. Lipids Health Dis 16, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybytkowski E, Joly E, Nolan CJ, Hardy S, Francoeur AM, Langelier Y and Prentki M (2007) Upregulation of cellular triacylglycerol – free fatty acid cycling by oleate is associated with long‐term serum‐free survival of human breast cancer cells. Biochem Cell Biol 85, 301–310. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Enriquez S, Hernandez‐Esquivel L, Marin‐Hernandez A, El Hafidi M, Gallardo‐Perez JC, Hernandez‐Resendiz I, Rodriguez‐Zavala JS, Pacheco‐Velazquez SC and Moreno‐Sanchez R (2015) Mitochondrial free fatty acid beta‐oxidation supports oxidative phosphorylation and proliferation in cancer cells. Int J Biochem Cell Biol 65, 209–221. [DOI] [PubMed] [Google Scholar]
- Romero‐Garcia S, Lopez‐Gonzalez JS, Baez‐Viveros JL, Aguilar‐Cazares D and Prado‐Garcia H (2011) Tumor cell metabolism: an integral view. Cancer Biol Ther 12, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslan N, Bieche I, Bright RK, Lidereau R, Chen Y and Byrne JA (2014) TPD52 represents a survival factor in ERBB2‐amplified breast cancer cells. Mol Carcinog 53, 807–819. [DOI] [PubMed] [Google Scholar]
- RostamiRad A, Ebrahimi SSS, Sadeghi A, Taghikhani M, Meshkani R (2018) Palmitate‐induced impairment of autophagy turnover leads to increased apoptosis and inflammation in peripheral blood mononuclear cells. Immunobiology 223, 269–278. [DOI] [PubMed] [Google Scholar]
- Sano R and Reed JC (2013) ER stress‐induced cell death mechanisms. Biochem Biophys Acta 1833, 3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CR and Schulze A (2012) Lipid metabolism in cancer. FEBS J 279, 2610–2623. [DOI] [PubMed] [Google Scholar]
- Sargsyan E, Artemenko K, Manukyan L, Bergquist J and Bergsten P (2016) Oleate protects beta‐cells from the toxic effect of palmitate by activating pro‐survival pathways of the ER stress response. Biochim Biophys Acta 1861, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Shiu RP and Paterson JA (1984) Alteration of cell shape, adhesion, and lipid accumulation in human breast cancer cells (T‐47D) by human prolactin and growth hormone. Cancer Res 44, 1178–1186. [PubMed] [Google Scholar]
- Sommerweiss D, Gorski T, Richter S, Garten A and Kiess W (2013) Oleate rescues INS‐1E beta‐cells from palmitate‐induced apoptosis by preventing activation of the unfolded protein response. Biochem Biophys Res Commun 441, 770–776. [DOI] [PubMed] [Google Scholar]
- Soto‐Guzman A, Robledo T, Lopez‐Perez M and Salazar EP (2008) Oleic acid induces ERK1/2 activation and AP‐1 DNA binding activity through a mechanism involving Src kinase and EGFR transactivation in breast cancer cells. Mol Cell Endocrinol 294, 81–91. [DOI] [PubMed] [Google Scholar]
- Tiwari RK, Mukhopadhyay B, Telang NT and Osborne MP (1991) Modulation of gene expression by selected fatty acids in human breast cancer cells. Anticancer Res 11, 1383–1388. [PubMed] [Google Scholar]
- Tohyama J, Oya Y, Ezoe T, Vanier MT, Nakayasu H, Fujita N and Suzuki K (1999) Ceramide accumulation is associated with increased apoptotic cell death in cultured fibroblasts of sphingolipid activator protein‐deficient mouse but not in fibroblasts of patients with Farber disease. J Inherit Metab Dis 22, 649–662. [DOI] [PubMed] [Google Scholar]
- Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V et al (2017) Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O (1956) On the origin of cancer cells. Science 123, 309–314. [DOI] [PubMed] [Google Scholar]
- Ward PS and Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF and Hoy AJ (2012) Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia 55, 2741–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wu Q, Li JJ, Chen C, Sun S, Wang CH and Sun SR (2017) Autophagy mediates free fatty acid effects on MDA‐MB‐231 cell proliferation, migration and invasion. Oncol Lett 14, 4715–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F and Du G (2012) Dysregulated lipid metabolism in cancer. World J Biol Chem 3, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhou L, Shi W, Song N, Yu K and Gu Y (2012) A mechanism underlying the effects of polyunsaturated fatty acids on breast cancer. Int J Mol Med 30, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Breast cancer cell response to palmitate supplementation.


