Abstract
Due to the involvement of SHP2 (SH2 domain‐containing protein‐tyrosine phosphatase) in human disease, including Noonan syndrome and cancer, several inhibitors targeting SHP2 have been developed. Here, we report that the commonly used SHP2 inhibitor NSC‐87877 does not exhibit robust inhibitory effects on growth factor‐dependent MAPK (mitogen‐activated protein kinase) pathway activation and that the recently developed active site‐targeting SHP2 inhibitors IIB‐08, 11a‐1, and GS‐493 show off‐target effects on ligand‐evoked activation/trans‐phosphorylation of the PDGFRβ (platelet‐derived growth factor receptor β). GS‐493 also inhibits purified human PDGFRβ and SRC in vitro, whereas PDGFRβ inhibition by IIB‐08 and 11a‐1 occurs only in the cellular context. Our results argue for extreme caution in inferring specific functions for SHP2 based on studies using these inhibitors.
Keywords: 11a‐1, GS‐493, IIB‐08, NSC‐87877, PDGFRβ, SHP2 inhibitor
Abbreviations
- 4‐OHT
4‐hydroxytamoxifen
- EGF
epidermal growth factor
- MAPK
mitogen‐activated protein kinase
- PDGF
platelet‐derived growth factor
- PDGFRβ
platelet‐derived growth factor receptor β
- PTP
protein‐tyrosine phosphatase
- pY
phosphotyrosyl
- RTK
receptor tyrosine kinase
- SHP2
SH2 domain‐containing protein‐tyrosine phosphatase
SHP2, encoded by PTPN11, is a classic protein‐tyrosine phosphatase (PTP), comprising two tandem SH2 domains (N‐SH2 and C‐SH2, respectively), followed by a catalytic (PTP) domain and a C‐terminal tail 1. In the absence of appropriate cellular stimuli, SHP2 resides in an auto‐inhibitory ‘closed’ structure, as a consequence of intramolecular interactions between the N‐SH2 and PTP domains 2. Upon stimulation of cells with appropriate growth factors, such as platelet‐derived growth factor (PDGF) or epidermal growth factor (EGF), the cognate receptor tyrosine kinase (RTK) is activated, resulting in receptor trans‐phosphorylation 3, 4, as well as phosphorylation of scaffolding proteins, such as GAB family members 5. SHP2 binds to phosphorylated RTKs and/or scaffold proteins via SH2 domain/phosphotyrosyl (pY) interactions, which are incompatible with intramolecular N‐SH2/PTP interaction and result in an enzymatically activated ‘open’ structure 1, 2.
Once activated, SHP2 functions as a key positive regulator of RTK‐evoked signal transduction, acting upstream of RAS in the ERK MAP kinase pathway 6, 7. Phosphatase activity is required for SHP2 action 8, although the key substrate(s) remain controversial. In addition, in response to most agonists, SHP2 itself undergoes tyrosyl phosphorylation on two C‐terminal tyrosines, which then function as GRB2 binding sites. This ‘adapter’ function for SHP2 enhances RAS/ERK signaling 9.
Mutations that affect residues at the N‐SH2/PTP interface of SHP2 cause aberrant enzymatic activation, due to inability to form the ‘closed’ structure. Such mutations are found frequently in, and are responsible for, several disorders, including Noonan syndrome 10, juvenile myelomonocytic leukemia (JMML) 1, 11, 12, and, less frequently, solid tumors such as neuroblastoma 13. In addition, SHP2, along with its binding partner GAB2, is required for BCR/ABL‐evoked transformation and leukemogenesis 1, 14, as well as some forms of breast 1, 15 and other cancers 1. Recent studies indicate that SHP2 deficiency blocks adaptive resistance to RAF inhibitors 16, and SHP2 is implicated as the major mediator of the programmed cell death 1 (PD‐1) 17, 18 and B‐ and T‐lymphocyte attenuator (BTLA) immune checkpoint pathways 19. For these reasons, SHP2 has emerged as an important drug target, and inhibitors targeting the active site, such as NSC‐87877 20, IIB‐08 21, 11a‐1 22, and GS‐493 23, and more recently, an allosteric inhibitor, SHP099 24, have been reported.
Protein‐tyrosine phosphatase family members share a conserved PTP catalytic domain 25, so development of active site SHP2 inhibitors has focused largely on achieving specificity versus other PTPs. The above active site‐targeting inhibitors, except NSC‐87877, reportedly are highly specific for SHP2, compared with other PTPs, including SHP1, which has the highest similarity to SHP2 21, 22, 23. However, potential off‐target effects of these molecules against other enzyme families (e.g., kinases) have, in general, not been reported.
We tested the effects of the commonly used active site SHP2 inhibitors NSC‐87877, IIB‐08, 11a‐1, and GS‐493 on PDGF signaling in fibroblasts and found that they either failed to effectively inhibit SHP2 function in cells (NSC‐87877) or had off‐target effects on PTKs. These results argue strongly that such agents cannot be used alone to infer SHP2 functions in health or disease.
Materials and methods
Cell culture
Ptpn11 fl/fl MEFs expressing CRE‐ERTam 7, Swiss 3T3 (3T3 Swiss albino) fibroblasts, and HEK293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS). To induce deletion of Ptpn11, Ptpn11 fl/fl MEFs were treated with 1 μm 4‐hydroxytamoxifen (4‐OHT) for 4 days. All cells tested negative for mycoplasma with the Mycoplasma Plus detection kit (Agilent, Santa Clara, CA, USA).
Antibodies
Mouse monoclonal anti‐SHP2 (B‐1, sc‐7384), anti‐PDGFRβ‐pY716 (F‐10, sc‐365464), and anti‐ERK2 (D‐2, sc‐1647) antibodies, as well as rabbit polyclonal anti‐PDGFRβ (958, sc‐432) and anti‐PDGFRβpY579 (sc‐135671) antibodies, were purchased from Santa Cruz Biotechnology. Mouse monoclonal anti‐MEK1 (61B12, #2352), rabbit monoclonal anti‐PDGFRβpY857 (C43E9, #3170) and anti‐PDGFRβpY1009 (42F9, #3124) antibodies, as well as rabbit polyclonal anti‐pMEK S217/221 (#9121), anti‐pERK1/2 T202/Y204 (#9101), and anti‐EGFR (#2232) antibodies, were purchased from Cell Signaling. Mouse monoclonal anti‐phosphotyrosine antibody cocktail (4G10 platinum, 05‐1050) was purchased from Millipore. All antibodies were used at the concentrations recommended by their manufacturers.
Growth factors and chemical compounds
Recombinant human PDGF‐BB and EGF were purchased from Peprotech. 4‐OHT was purchased from Sigma. IIB‐08 and 11a‐1 were provided by Z.Y. Zhang (Purdue University). GS‐493 was provided by J. Rademann (Freie Universität Berlin) or was purchased from Bioduro. NSC‐87877 was purchased from Millipore. SHP099 was purchased from Alputon Inc.
Expression constructs, infection, and sorting
Retroviral expression vectors for wild‐type SHP2 (WT SHP2) and the mutant SHP2C459E were generated by subcloning human PTPN11 cDNA into pMSCV‐IRES‐EGFP (Clontech). Ptpn11 fl/fl MEFs expressing WT or mutant SHP2 were generated by retroviral infection, according to the manufacturer's protocol (pMSCV retrovirus system; Clontech), followed by fluorescence‐activated cell sorting (FACS) for EGFP‐positive cells.
Immunoblotting
Ptpn11 fl/fl MEFs expressing CRE‐ERTam, Swiss 3T3 fibroblasts, or HEK293T cells were seeded on 6‐well plates (MEFs and 3T3 cells, 2 × 105/well; HEK293T cells, 5 × 105/well), followed by serum starvation (MEFs and 3T3 cells, no FBS; HEK293T cells, no FBS or 0.1% FBS) for 16 h. Starved cells were then stimulated with PDGF‐BB (50 ng·mL−1, final concentration) or EGF (1 ng·mL−1 or 50 ng·mL−1, final concentration), as indicated. For inhibitor treatment, cells were pre‐incubated for 3 h (NSC‐87877) or 30 min (other SHP2 inhibitors) in media (1 mL/well) containing the indicated concentrations of each compound with 0.5% DMSO or 0.5% DMSO alone, followed by addition of 10 μL of medium containing 5 μg·mL−1 PDGF‐BB (50 ng·mL−1, final concentration). Cells were lysed in SDS lysis buffer (50 mm Tris/HCl pH 7.5, 100 mm NaCl, 1 mm EDTA, 1% SDS, 2 mm Na3VO4). Lysates were resolved by SDS/PAGE, followed by transfer to Immobilon‐FL PVDF membranes (Millipore). Membranes were blocked in 1% BSA/TBS containing 0.1% Tween‐20 for 30 min and treated with primary antibodies in blocking buffer for 1 h, followed by treatment with IRDye‐conjugated secondary antibodies (LI‐COR). Images were obtained using an ODYSSEY CLx quantitative IR fluorescent detection system (LI‐COR) with Image Studio software Ver. 5.2 (LI‐COR).
In vitro kinase assays
In vitro kinase assays were performed by the SelectScreen™ Biochemical Kinase Profiling Service with the Z’‐LYTE Kinase Assay (Thermo, Waltham, MA, USA). Purified recombinant human PDGFRβ or human SRC was incubated with Z’‐LYTE kinase substrate in the presence of serial dilutions of each inhibitor in kinase reaction buffer (50 mm HEPES, pH 7.5, 0.01% BRIJ‐35, 10 mm MgCl2, 2 mm MnCl2, 1 mm EGTA, 1 mm DTT) with 100 μm ATP (PDGFRβ) or 50 μm ATP (SRC) at room temperature for 1 h. Relative inhibition for each condition (average of technical duplicates) was calculated by setting the activity in the absence of inhibitor as 0%. IC50s were obtained by fitting the data to sigmoidal dose–response curves.
Results and Discussion
SHP2 catalytic activity is necessary for PDGF‐dependent MAPK activation but not for PDGFR phosphorylation
Exposure of PDGF‐BB to cells causes dimerization and activation of PDGFRβ on the plasma membrane, resulting in trans‐phosphorylation of multiple receptor tyrosine residues, including Y579, Y716, Y857, and Y1009 4. These pYs serve as binding sites for SH2 domain‐ or PTB domain‐containing signaling proteins, such as SHP2, which initiate downstream signal transduction 4. To evaluate the role of SHP2 catalytic activity in PDGF signaling, we employed immortalized mouse embryo fibroblasts (MEFs) from Ptpn11 fl/fl animals, which express Cre‐ERTam 7. MEFs were reconstituted with wild‐type (WT) SHP2 or a catalytically inactive SHP2 mutant (C459E) and then were treated with 4‐OHT (1 μm) for 4 days to delete endogenous Ptpn11. Upon PDGF‐BB‐treatment (50 ng·mL−1) after serum starvation, parental (undeleted) cells showed agonist‐evoked phosphorylation/activation of the ERK MAPK pathway components MEK1 and ERK1/2, whereas these were strongly suppressed by SHP2 depletion (Fig. 1). Suppressed ERK signaling in Ptpn11 knockout cells was rescued by re‐expression of WT SHP2. By contrast, SHP2C459E did not restore PDGF‐dependent ERK phosphorylation, despite expression at levels comparable to endogenous SHP2 in control cells (Fig. 1), confirming a requirement for SHP2 catalytic activity in PDGFR‐induced ERK activation in fibroblasts. Notably, overall PDGFR tyrosyl phosphorylation, as well as phosphorylation of multiple specific PDGFRβ tyrosyl residues (pY579, pY716, pY857, and pY1009 of PDGFRβ), was unaffected, or slightly increased, by the absence of catalytically active SHP2 in Ptpn11‐knockout cells (Fig. 1). These data indicate that SHP2 does not enhance (and might inhibit some) PDGFRβ tyrosyl phosphorylation events (at these time points) in this immortalized MEF line.
Figure 1.
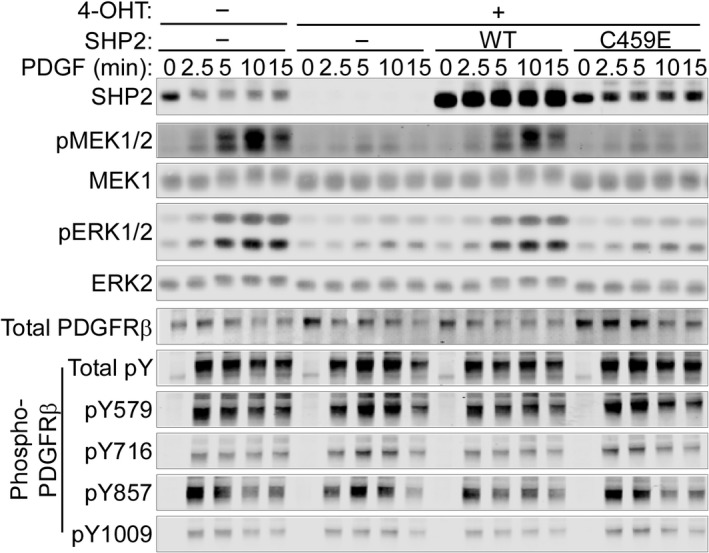
SHP2 catalytic activity is required for PDGF‐evoked ERK MAPK signaling, but not for PDGFRβ phosphorylation. Ptpn11 fl/fl MEFs expressing CRE‐ERT am with or without retroviral expression of WT or C459E mutant SHP2 were treated with 4‐OHT (1 μm) for 4 days or left untreated. Cells were then serum‐starved, followed by treatment with PDGF‐BB (50 ng·mL−1) for the indicated times. Lysates were subjected to immunoblotting with the indicated antibodies. Representative immunoblots are shown from one of three experiments.
Active site‐targeting SHP2 inhibitors suppress PDGF‐evoked ERK activation and PDGFRβ phosphorylation
We then tested the effects of the active site‐targeting SHP2 inhibitors, NSC‐87877, IIB‐08, 11a‐1, and GS‐493, at first using Swiss 3T3 fibroblasts. After serum starvation, cells were pretreated with the reported inhibitory concentrations of NSC‐87877 (20 or 50 μm, 3 h) 20, IIB‐08 (10 μm, 30 min) 21, 11a‐1 (5 μm, 30 min) 22, or GS‐493 (5 μm, 30 min) 23 and then stimulated with PDGF‐BB (50 ng·mL−1, final concentration). NSC‐87877 had no detectable effect on ERK MAPK signaling (Fig. 2A). We also failed to reproduce reported inhibitory effects of this compound on EGF (1 ng·mL−1 or 50 ng·mL−1)‐stimulated ERK activation in HEK293T cells 20 (Fig. 2B). By contrast, the allosteric SHP2 inhibitor SHP099 effectively suppressed PDGF‐ or EGF‐stimulated ERK MAPK pathway in Swiss 3T3 cells or HEK293T cells, respectively (Fig. 2C).
Figure 2.
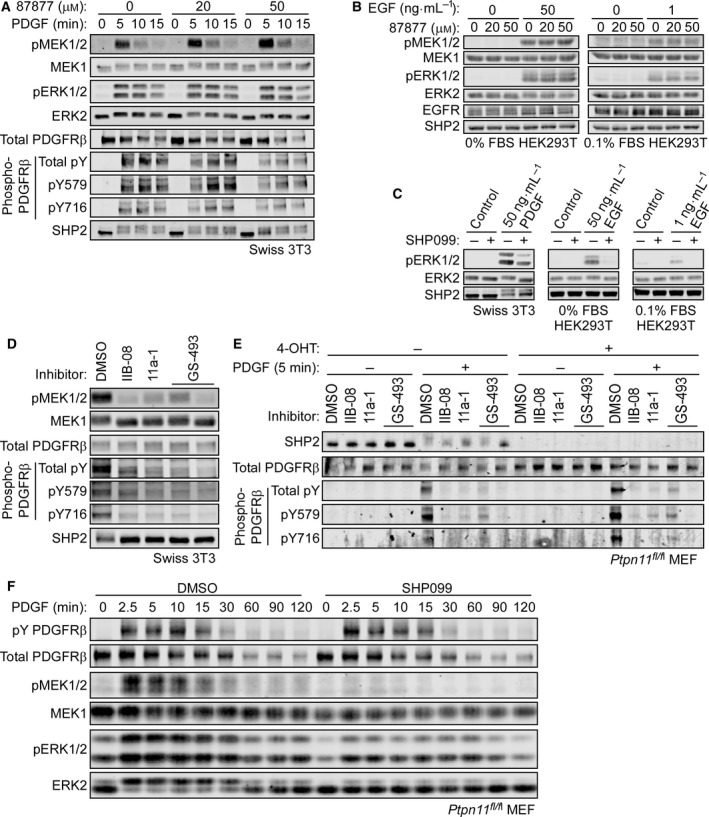
SHP2‐independent suppression of PDGFRβ phosphorylation by active site‐targeting SHP2 inhibitors. (A) Serum‐starved Swiss 3T3 cells were pretreated with NSC‐87877 (20 or 50 μm) for 3 h, followed by addition of PDGF‐BB (50 ng·mL−1, final concentration) for 5 min. (B) HEK293T cells were serum‐starved without FBS (left) or with 0.1% FBS (right, according to ref. 20) for 16 h and pretreated with NSC‐87877 (20 or 50 μm) for 3 h, followed by addition of EGF (50 ng·mL−1 or 1 ng·mL−1, final concentration, respectively) for 5 min. (C) Serum‐starved Swiss 3T3 cells or HEK293T cells were pretreated with SHP099 (10 μm) for 30 min, followed by addition of PDGF‐BB (50 ng·mL−1) or EGF (50 ng·mL−1 or 1 ng·mL−1) for 5 min as indicated. Serum‐starved Swiss 3T3 cells (D) or Ptpn11 fl/fl MEFs expressing CRE‐ERT am treated with or without 4‐OHT (E) were pretreated with IIB‐08 (10 μm), 11a‐1 (5 μm), GS‐493 (5 μm), or DMSO for 30 min, followed by addition of PDGF‐BB (50 ng·mL−1, final concentration) for 5 min. Lysates were then subjected to immunoblotting with the indicated antibodies. Representative immunoblots are shown from one of two experiments, respectively. (F) Ptpn11 fl/fl MEFs without Ptpn11 deletion were serum‐starved and pretreated with SHP099 (10 μm) for 30 min. Cells were then stimulated with PDGF‐BB (50 ng·mL−1) for the indicated times. Lysates were subjected to immunoblotting with the indicated antibodies. Representative immunoblots are shown from one of three experiments.
The other active site inhibitors tested efficiently abolished PDGF‐evoked MEK1 phosphorylation, but also strongly suppressed ligand‐dependent tyrosine phosphorylation of PDGFRβ (Fig. 2D). As Ptpn11 knockout (Fig. 1) did not attenuate PDGFR phosphorylation, we tested these inhibitors in MEFs. In contrast to the effects of SHP2 depletion or replacement of SHP2 with a catalytically inactive SHP2 mutant, IIB‐08, 11a‐1, and GS‐493 suppressed ligand‐dependent PDGFRβ phosphorylation in wild‐type MEFs. Even more importantly, these inhibitors also blocked PDGFRβ phosphorylation in cells lacking SHP2 (Fig. 2E). By contrast, treatment of MEFs with SHP099 suppressed PDGF‐dependent MEK/ERK activation without suppressing PDGFRβ phosphorylation (Fig. 2F). These observations show unambiguously that suppression of PDGFRβ phosphorylation by these active site inhibitors at their recommended concentrations is independent of SHP2, and indicate that they have off‐target effects, at least in fibroblasts.
GS‐493, but not IIB‐08 and 11a‐1, inhibits PDGFRβ and SRC kinase activities in vitro
To investigate how IIB‐08, 11a‐1, and GS‐493 block PDGFRβ tyrosine phosphorylation, we first asked whether they directly inhibit PDGFRβ kinase activity in vitro. GS‐493 inhibited the purified PDGFRβ kinase domain (IC50 = 1.6 μm) and also inhibited recombinant SRC (IC50 = 746 nm). The reported in vitro IC50s of GS‐493 for SHP2, SHP1, and PTP1B are 71 nm, 2.08 μm, and 3.17 μm, respectively 23. By contrast, IIB‐08 or 11a‐1 did not significantly inhibit either isolated kinase domain in vitro (Fig. 3).
Figure 3.
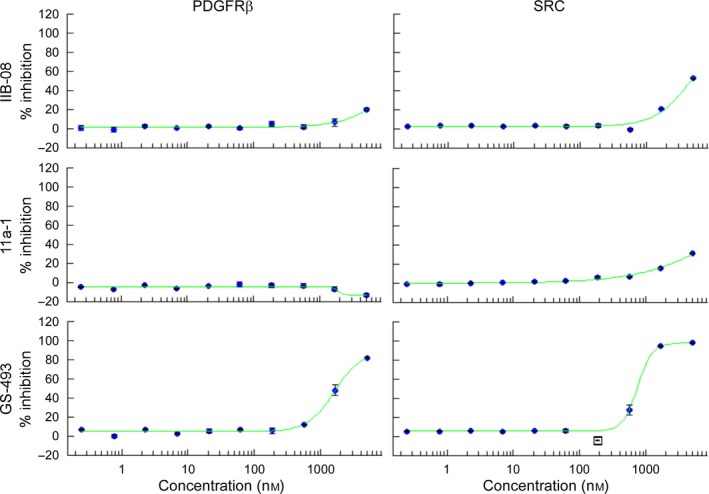
Inhibition of PDGFRβ and SRC in vitro by GS‐493, but not IIB‐08 and 11a‐1. Kinase activities of purified human PDGFRβ or human SRC in vitro were measured in the presence of serial dilutions of IIB‐08, 11a‐1, or GS‐493. Graphs show relative inhibition of kinase activity in the presence of indicated concentration of each inhibitor, setting the activity without SHP2 inhibitor as 0%. The bars indicate the range of technical duplicates, and the blue dots indicate the average of the two values obtained (n = 1). Data were fit to sigmoidal dose–response curves (green lines). No statistical analysis was performed. IC 50s for GS‐493 are found in the main text.
Taken together, our data indicate that NSC‐87877—or at least versions of this compound available commercially—lacks clear inhibitory effects on the ERK MAPK pathway, at least in the context of PDGF or EGF stimulation of fibroblasts and HEK293T cells. By contrast, GS‐493, IIB‐08, and 11a‐1 suppress PDGFRβ phosphorylation independently of SHP2 and thereby block downstream signaling in PDGF‐stimulated cells. GS‐493 suppresses ligand‐evoked trans‐phosphorylation of PDGFRβ, likely by directly inhibiting the PDGFRβ kinase domain. GS‐493 also inhibits SRC in vitro, and given the conservation of kinase domains, might well inhibits other PTKs as well. How IIB‐08 and 11a‐1 impair PDGFRβ activation in cells, while not inhibiting the PDGFRβ kinase domain in vitro, remains unclear. Potential mechanisms include (a) modulation of receptor affinity for PDGF, (b) inhibition of receptor dimerization or allosteric inhibition via another region of PDGFRβ not included in the recombinant kinase domain, (c) activation of PTP(s) that dephosphorylates PDGFRβ, or (d) PDGFRβ inhibition via metabolite(s) generated within cells. As with GS‐493, additional potential off‐target effects of IIB‐08 and 11a‐1 on other PTKs cannot, and should not, be excluded.
Inhibiting either SHP2 or the PDGFR can inhibit RAS/ERK/MAPK signaling. Hence, the biological effects of the above inhibitors are difficult, if not impossible, to attribute to SHP2 inhibition. Nevertheless, these inhibitors have been used extensively to probe SHP2 action (reviewed in refs. 26, 27), often as major perturbants of SHP2 function. By contrast, the allosteric inhibitor SHP099 does not have off‐target effects on SRC or other tyrosine kinases in vitro 24 or in the cellular contexts described here. Even so, comparing the effects of SHP099 and SHP2 depletion (via chemical degradation) indicates that SHP099 also can have off‐target effects in at least some settings 28.
In summary, the above study and our results indicate that reports using SHP2 inhibitors as the major means of inferring SHP2 function must be re‐evaluated and potentially re‐interpreted. For SHP099‐like compounds, defined drug‐resistant mutants can be used in rescue experiments to demonstrate specificity 24. Current allosteric SHP2 inhibitors act as ‘molecular glue’ stabilizing the closed, inactive form of the enzyme, and thereby act like ‘chemical nulls’ that potentially block multiple, if not all, aspects of SHP2 function 29. For this reason, it would certainly be useful to have validated ‘on‐target’ catalytic inhibitors. The off‐target effects revealed here argue that additional efforts are necessary to achieve this goal, and emphasize the need for careful control experiments and multiple lines of evidence to confidently assign functions to SHP2 using inhibitor approaches.
Author contributions
RT and BGN conceived and designed the experiments and wrote the manuscript. RT and HR performed the experiments. BGN supervised the research.
Acknowledgements
We thank Z. Y. Zhang (Purdue University) and J. Rademann (Freie Universität Berlin) for providing materials. We also thank Ms. X. Wang (Neel Lab) for synthesizing plasmids. This work was supported by NIH R37CA49132 to BGN.
References
- 1. Chan G and Neel BG (2016) Role of PTPN11 (SHP2) in Cancer. Protein Tyrosine Phosphatases in Cancer In Protein Tyrosine Phosphatases in Cancer (Neel BG. and Tonks NK, eds), pp. 115–143. Springer, New York. [Google Scholar]
- 2. Hof P, Pluskey S, Dhe‐Paganon S, Eck MJ and Shoelson SE (1998) Crystal structure of the tyrosine phosphatase SHP‐2. Cell 92, 441–450. [DOI] [PubMed] [Google Scholar]
- 3. Lemmon MA and Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heldin CH and Lennartsson J (2013) Structural and functional properties of platelet‐derived growth factor and stem cell factor receptors. Cold Spring Harb Perspect Biol 5, a009100 10.1101/cshperspect.a009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103, 211–225. [DOI] [PubMed] [Google Scholar]
- 6. Bennett AM, Tang TL, Sugimoto S, Walsh CT and Neel BG (1994) Protein‐tyrosine‐phosphatase SHPTP2 couples platelet‐derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA 91, 7335–7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang W, Klaman LD, Chen B, Araki T, Harada H, Thomas SM, George EL and Neel BG (2006) An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev Cell 10, 317–327. [DOI] [PubMed] [Google Scholar]
- 8. Zhao R and Zhao ZJ (1999) Tyrosine phosphatase SHP‐2 dephosphorylates the platelet‐derived growth factor receptor but enhances its downstream signaling. Biochem J 338, 35–39. [PMC free article] [PubMed] [Google Scholar]
- 9. Li W, Nishimura R, Kashishian A, Batzer AG, Kim WJ, Cooper JA and Schlessinger J (1994) A new function for a phosphotyrosine phosphatase: linking GRB2‐Sos to a receptor tyrosine kinase. Mol Cell Biol 14, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts AE, Allanson JE, Tartaglia M and Gelb BD (2013) Noonan syndrome. Lancet 381, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hählen K, Hasle H, Licht JD and Gelb BD (2003) Somatic mutations in PTPN11 in juvenile myelomonocytic leucemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet 34, 148–150. [DOI] [PubMed] [Google Scholar]
- 12. Emanuel PD (2008) Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia 22, 1335–1342. [DOI] [PubMed] [Google Scholar]
- 13. Bentires‐Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, Sugarbaker DJ et al (2004) Activating mutations of the noonan syndrome‐associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res 64, 8816–8820. [DOI] [PubMed] [Google Scholar]
- 14. Sattler M, Mohi MG, Pride YB, Quinnan LR, Malouf NA, Podar K, Gesbert F, Iwasaki H, Li S, Van Etten RA et al (2002) Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 1, 479–492. [DOI] [PubMed] [Google Scholar]
- 15. Bentires‐Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N, Harris LN, Richardson A, Neel BG and Gu H (2006) A role for the scaffolding adapter GAB2 in breast cancer. Nat Med 12, 114–121. [DOI] [PubMed] [Google Scholar]
- 16. Prahallad A, Heynen GJ, Germano G, Willems SM, Evers B, Vecchione L, Gambino V, Lieftink C, Beijersbergen RL, Di Nicolantonio F et al (2015) PTPN11 is a central node in intrinsic and acquired resistance to targeted cancer drugs. Cell Rep 12, 1978–1985. [DOI] [PubMed] [Google Scholar]
- 17. Okazaki T, Chikuma S, Iwai Y, Fagarasan S and Honjo T (2013) A rheostat for immune responses: the unique properties of PD‐1 and their advantages for clinical application. Nat Immunol 14, 1212–1218. [DOI] [PubMed] [Google Scholar]
- 18. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I et al (2017) T cell costimulatory receptor CD28 is a primary target for PD‐1‐mediated inhibition. Science 355, 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gavrieli M, Watanabe N, Loftin S, Murphy TL and Murphy KM (2003) Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP‐1 and SHP‐2. Biochem Biophys Res Commun 312, 1236–1243. [DOI] [PubMed] [Google Scholar]
- 20. Chen L, Sung S, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ and Wu J (2006) Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol 70, 562–570. [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, He Y, Liu S, Yu Z, Jiang ZX, Yang Z, Dong Y, Nabinger SC, Wu L, Gunawan AM et al (2010) Salicylic acid based small molecule inhibitor for the oncogenic Src homology‐2 domain containing protein tyrosine phosphatase‐2 (SHP2). J Med Chem 53, 2482–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng LF, Zhang RY, Yu ZH, Li S, Wu L, Gunawan AM, Lane BS, Mali RS, Li X, Chan RJ et al (2014) Therapeutic potential of targeting the oncogenic SHP2 phosphatase. J Med Chem 57, 6594–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grosskopf S, Eckert C, Arkona C, Radetzki S, Böhm K, Heinemann U, Wolber G, von Kries JP, Birchmeier W and Rademann J (2015) Selective inhibitors of the protein tyrosine phosphatase SHP2 block cellular motility and growth of cancer cells in vitro and in vivo. ChemMedChem 10, 815–826. [DOI] [PubMed] [Google Scholar]
- 24. Chen YN, LaMarche MJ, Chan HM, Fekkes P, Garcia‐Fortanet J, Acker MG, Antonakos B, Chen CH, Chen Z, Cooke VG et al (2016) Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 535, 148–152. [DOI] [PubMed] [Google Scholar]
- 25. Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7, 833–846. [DOI] [PubMed] [Google Scholar]
- 26. Butterworth S, Overduin M and Barr AJ (2014) Targeting protein tyrosine phosphatase SHP2 for therapeutic intervention. Future Med Chem 6, 1423–1437. [DOI] [PubMed] [Google Scholar]
- 27. Stanford SM and Bottini N (2017) Targeting tyrosine phosphatases: time to end the stigma. Trends Pharmacol Sci 38, 524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fulcher LJ, Hutchinson LD, Macartney TJ, Turnbull C and Sapkota GP (2017) Targeting endogenous proteins for degradation through the affinity‐directed protein missile system. Open Biol 7 pii: 170066, 10.1098/rsob.170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hao R, Tsutsumi R, Araki T and Neel BG (2016) Sticking it to cancer with molecular glue for SHP2. Cancer Cell 30, 194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]


