Abstract
Accumulating evidence suggests that Propionibacterium acnes (P. acnes) is a novel pathogenic factor promoting intervertebral disc degeneration (IVDD). However, the underlying mechanisms by which P. acnes induces IVDD have been unclear. In this study, we quantified the severity of IVDD, as well as the expressions of inducible nitric oxide synthase (iNOS)/nitric oxide (NO) and cyclooxygenase (COX-2)/prostaglandin (PGE2) in human intervertebral discs (IVDs) infected with P. acnes. Compared with P. acnes-negative IVDs, P. acnes-positive IVDs showed increased iNOS/NO and COX-2/PGE2 activity concomitant with more severe IVDD. In order to detect the potential correlation between iNOS/NO expression, COX-2/PGE2 expression, and IVDD, we developed a P. acnes-induced IVDD rat model and found that the upregulation of iNOS/NO and COX-2/PGE2 was essential to the occurrence of P. acnes-induced IVDD. This finding was supported by the fact that the inhibition of iNOS/NO and COX-2/PGE2 activity ameliorated IVDD significantly, as evidenced by restored aggrecan and collagen II expression both in vivo and in vitro. Mechanistically, we found that P. acnes induced iNOS/NO and COX-2/PGE2 expressions via a reactive oxygen species- (ROS-) dependent NF-κB cascade. Furthermore, NADPH oxidase participated in P. acnes-induced ROS, iNOS/NO, and COX-2/PGE2 expressions. Overall, these findings further validated the involvement of P. acnes in the pathology of IVDD and provided evidence that P. acnes-induced iNOS/NO and COX-2/PGE2 activation via the ROS-dependent NF-κB pathway is likely responsible for the pathology of IVDD.
1. Introduction
Intervertebral disc degeneration (IVDD) is a prerequisite for a number of discogenic diseases and can produce many clinical symptoms including sciatica, low back pain, and physical dysfunction. All of these drastically affect patients' quality of life and productivity at work; they also significantly increase the burden of medical treatment [1]. Despite the common occurrence of IVDD, its pathogenesis is not fully understood.
Conventionally, excessive mechanical loading, nutritional disorder, traumatic injury, or genetic predisposition were considered the main etiologies for IVDD [1]. However, recent studies suggest that Propionibacterium acnes (P. acnes)—a low-virulence anaerobic bacterium found to reside latently within nonpyogenic intervertebral discs (IVDs)—can induce IVDD [2]. In patients with IVDD, the prevalence of P. acnes has ranged from 13% to 44% [3–7]. We also first identified the existence of the bacterium in IVDs via histologic observation [8]. Interestingly, our previous work suggested that patients with P. acnes-positive IVDs had more severe degeneration than those with P. acnes-negative IVDs [9, 10]. Furthermore, we found that the inoculation of P. acnes induced significant IVDD in animal models [11]. Therefore, P. acnes was considered to be one of the potential pathogenic factors of IVDD. However, until now, the mechanisms by which P. acnes causes IVDDs have not been fully understood. Our previous study revealed that P. acnes can induce IVDD by promoting apoptosis of the nucleus pulposus [10]. But other possible pathologic mechanisms remained to be explored.
Until now, the mechanisms by which P. acnes causes IVDDs have not been fully understood. Although our previous study revealed that P. acnes can induce IVDD by promoting apoptosis of the nucleus pulposus, inhibition of P. acnes-induced apoptosis of nucleus pulposus cells (NPCs) only partly relieves the IVDD [10], suggesting that there may be other pathophysiological mechanisms of P. acnes-induced IVDD. Therefore, whether P. acnes would cause the IVDD via other possible mechanism is an interesting problem worth investigating.
Nitric oxide (NO) and prostaglandin E2 (PGE2) are two well-known catabolic factors involved in the occurrence and development of IVDD [12, 13]. When stimulated, the activation of iNOS and COX-2 results in the production of NO and PGE2 in IVDs and causes harmful pathophysiologic effects such as inflammation, apoptosis, and degeneration [13–16]. Interestingly, P. acnes is able to stimulate various cells, such as macrophages and keratinocytes, to produce NO and PGE2 [17]. An important question, therefore, was whether P. acnes could promote the expression of NO and PGE2 in IVDs and thus induce IVDD.
We first sought to determine whether latent infection with P. acnes was responsible for IVDD by promoting the production of NO and PGE2. In addition, the underlying signaling pathway involving this process was further explored. To our knowledge, this is the first study to investigate the relationship between P. acnes infection and NO/PGE2, and our findings provide new insights for the prevention and treatment of degenerative disc diseases.
2. Materials and Methods
2.1. Patients, Bacterial Culture, and Match of Samples
A total of 46 patients were included in this study from September 2013 to May 2017. The patients underwent discectomy at the single-level lumbar spine due to intervertebral disc degeneration associated with low back pain and/or sciatica. All patients were assigned for surgery after failed attempts to improve their condition using conservative treatment for several months. Patients who received antibiotics within the month preceding surgery were not included. According to our previous protocol [8], all tissues were cultured in tryptone soy broth (TSB) for 14 days under anaerobic conditions, then the presence of bacteria in the culture was identified by amplifying the 16S rDNA gene by PCR.
According to previous study, the variables age, primary symptoms, duration of symptoms, and surgery level dramatically affected the severity of IVDD. Thus, to reveal the exact pathological effect of P. acnes and reduce heterogeneity, a case-controlled method was used for the quantitative analysis following a previous study [18]. After culture of the specimen and identification of the bacteria, the patients who had P. acnes only in IVDs were classified as the positive group (n = 23). Equal numbers of patients who were identified as completely bacteria-free in IVDs were selected to match each of the positive patients based on the following criteria: (1) same gender, (2) same surgery segment, (3) same symptoms of low back pain only, sciatica only, or both, (4) similar ages ± 5 years, and (5) similar duration of symptoms ± 3 months. These patients were named the negative group (n = 23).
2.2. Preparation of P. acnes Inoculum
A standard strain of P. acnes (ATCC: 6919, GIM: 1.243, Guangdong Microbiology Culture Center, Guangdong, China) was cultured on Gifu Anaerobic (GAM) broth (Nissui, Tokyo, Japan) for 3 d at 37°C under anaerobic conditions.
2.3. Inoculation of P. acnes into Caudal IVDs of Rat
According to the previous study [10], the target vertebrae (Ca)6/7 to (Ca)8/9 (n = 3 per animal) of eight-week-old male Sprague-Dawley rats were identified and marked by palpation and X-ray before surgery. A volume of 2.5 μl P. acnes (OD600 = 3.0), P. acnes with NG-monomethyl-L-arginine, monoacetate salt (L-NMMA, iNOS inhibitor, 1 mM, no. S0011, Beyotime, Shanghai, China), P. acnes with diclofenac sodium (DS, COX-2 inhibitor, 200 μM, Selleck, Houston, USA), or saline was inoculated vertically into the nucleus pulposus using a microsyringe with a 28-gauge needle (Hamilton, Nevada, USA). All animal experiments were performed in accordance with the protocol approved by the Shanghai Jiao Tong University (SJTU) Animal Care and Use Committee [IACUC protocol number: SYXK (Shanghai)2011-0113] and in accordance with the Ministry of Science and Technology of the People's Republic of China Animal Care guidelines. All surgeries were performed under anesthesia, and all efforts were made to minimize suffering.
2.4. Cocultures of NPCs and P. acnes
Tissues of nucleus pulposus were harvested and cultured from 5 disc degenerated patients, including 3 males and 2 females, with a mean age of 36.5 years (28–50 years) following the above protocol. Cell samples from different patients were kept separate. All experiments were carried out in duplicate and were conducted with human NPCs from passages 2 to 3.
For coculture, the bacteria were harvested from the third day cultures in stationary phase and washed twice with phosphate-buffered saline (PBS). The bacterial density was adjusted to optical density (OD600 = 2). Then, P. acnes were added to the cell culture (5 × 105 cells/well) in a 6-well culture plate at a 100 : 1 multiplicity of infection (MOI) without antibiotics. After 12 and 24 hours, cocultured cells were washed three times with PBS and prepared for late-stage experiments.
2.5. Western Blot Analysis
For Western blot analysis, total proteins from the samples were separated by SDS-PAGE, transferred to nylon membranes, and incubated separately with the following primary antibodies: collagen II (dilution of 1 : 2000; cat. number ab34712, Abcam, Britain), aggrecan (dilution of 1 : 1000; cat. number ab36861, Abcam, Britain), iNOS (dilution of 1 : 500; cat. number ab3523, Abcam, Britain), COX-2 (dilution of 1 : 1000; cat. number 12282, CST Inc., MA, USA), and NF-κΒ/phospho-NF-κΒ (dilution of 1 : 1000; cat. number 8242S/3033S, CST Inc., MA, USA). β-Actin (dilution of 1 : 2000; cat. number CW0096, CW Bio, Beijing, China) was used as an internal control. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody, goat anti-rabbit IgG (dilution of 1 : 2000; cat. number CW0103s, CW Bio, Beijing, China), or goat anti-mouse IgG (dilution of 1 : 2000; cat. number CW0102s, CW Bio, Beijing, China) at room temperature for 2 h, and the bands were visualized using chemiluminescence (Pierce Biotechnology Inc., IL, USA). The images were analyzed using Fusion FX7 (Vilber Lourmat, Marne-la-Vallée, France).
2.6. Immunofluorescence
ROS level was quantified using dihydroethidium (DHE, 5 μM, S0063, Beyotime, Shanghai, China). NPCs were treated with DHE and incubated for 30 min at 37°C.
For iNOS and COX-2, P. acnes-induced cells were cultured on glass slides and then fixed for 30 min in 4% paraformaldehyde. The NPCs were incubated for 16 h at 4°C with iNOS (dilution of 1 : 500, cat. number Ab178945, Abcam, Britain) and COX-2 antibody (dilution of 1 : 1000, cat. number 12282, CST Inc., MA, USA). All images were observed using a fluorescence microscope (Axio, Carl Zeiss, Oberkochen, Germany).
2.7. Flow Cytometric Analysis
ROS level was determined using dichloro-dihydro-fluorescein diacetate (DCFH-DA, 10 μM, S0033, Beyotime, Shanghai, China) and directly treated with 2 μl of DCFH-DA (10 mM) dissolved in PBS (2 ml) at 37°C for 20 min. Fluorescence was analyzed using a FACScan (Becton Dickinson, Sunnyvale, CA) flow cytometer with excitation at 488 nm and emission at 530 nm.
The proportion of NPC apoptosis was detected using the Annexin V-FITC apoptosis detection kit (C1063, Beyotime, Shanghai, China) and calculated by the percentage of early apoptotic (Annexin V+/PI−) cells plus the percentage of late apoptotic (Annexin V+/PI+) cells using flow cytometry.
2.8. Measurement of PGE2 Production
Nucleus pulposus cells (NPCs) were subcultured in 6-well plates, cocultured with P. acnes for different times and incubated with indicated components, respectively. The supernatant (1 ml) of the culture medium was collected to determine PGE2 concentrations by an enzyme-linked immunosorbent assay (ELISA) (SKGE004B, R&D Systems Inc., Minneapolis, MN, USA).
2.9. Nitrite Assay
The nitrite concentration in the medium was measured as an indicator of NO production according to the Griess reaction [19]. A volume of 50 μl of each supernatant was mixed with the same volume of Griess reagent (S0021, Beyotime, Shanghai, China), and the absorbance of the mixture at 550 nm was determined with an ELISA plate reader (Dynatech MR-7000; Dynatech Laboratories).
2.10. Statistical Analysis
Data were collected from three or more independent experiments and expressed as the mean ± SD. A two-sided Student's t-test was used to analyze differences between two groups. One-way ANOVA was performed to show differences among multiple groups. P < 0.05 was considered significantly different.
3. Results
3.1. IVDs Infected with P. acnes Had Increased iNOS/NO and COX-2/PGE2 Expressions Concomitant with Severe Disc Degeneration in Patients
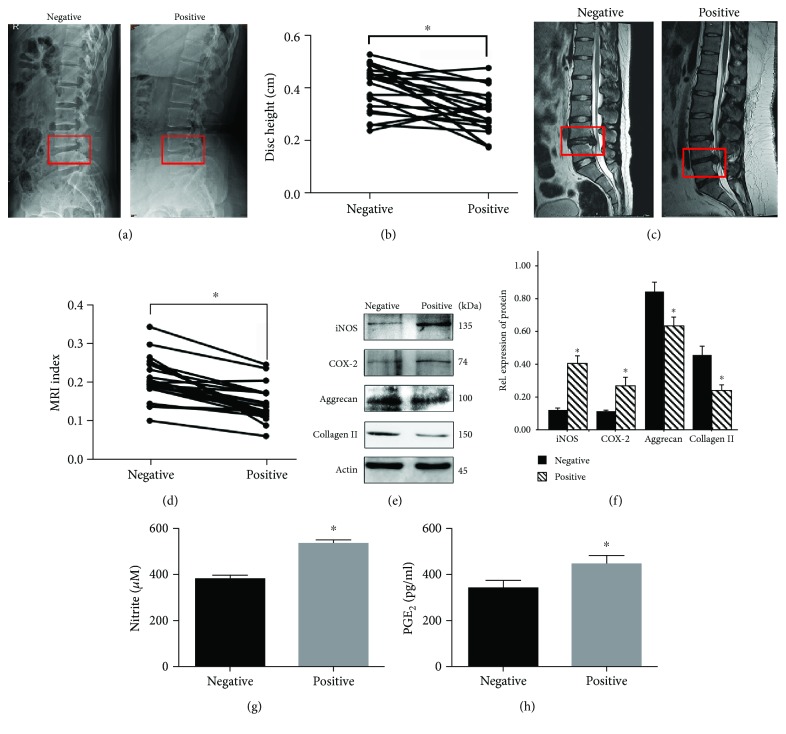
To explore the role of iNOS and COX-2 in P. acnes-induced IVDD, we first evaluated iNOS and COX-2 expressions in human IVDs infected with P. acnes. The radiographic analysis suggested that intervertebral height in the P. acnes-positive group was much lower than that in the P. acnes-negative group (Figures 1(a) and 1(b)). Moreover, magnetic resonance imaging (MRI) showed more hypointense signals in the midsagittal T2-weighted images of P. acnes-positive IVDs (Figures 1(c) and 1(d)) than in images of P. acnes-negative IVDs, thus further demonstrating that P. acnes infection is associated with more severe IVDD.
Figure 1.
The IVDs infected with P. acnes had increased iNOS/NO and COX-2/PGE2 expressions, concomitant with severe disc degeneration. (a–d) Representative images from lateral X-rays and magnetic resonance imaging point to severe degeneration in P. acnes-positive IVDs (n = 23 for each group). (e–f) Western blot analysis of aggrecan, collagen II, iNOS, and COX-2 in human IVDs. ∗The P. acnes-positive groups compared with the P. acnes-negative groups. (g, h) The concentration of NO and PGE2 were tested by the Griess reaction and ELISA, respectively. ∗The P. acnes-positive groups compared with the P. acnes-negative groups; P < 0.05. P values were analyzed by paired t-test, Student's t-test, and one-way ANOVA. Data are presented as the mean ± SD from three independent experiments.
In addition, quantitative analysis of protein suggested that the expression of iNOS and COX-2 increased significantly in the P. acnes-positive group compared with the P. acnes-negative group (Figures 1(e) and 1(f)). This was accompanied by a decrease in the expression of aggrecan and collagen II (Figures 1(e) and 1(f)) and an increase in the concentration of NO and PGE2 (Figures 1(g) and 1(h)). Taken together, these results show that P. acnes infection increases iNOS/NO and COX-2/PGE2 expressions in human IVDs, leading to the conclusion that P. acnes infection can promote the degeneration of IVDs by increasing iNOS/NO and COX-2/PGE2 activity.
3.2. P. acnes Infection Induced IVDD by Promoting the Expression of iNOS/NO and COX-2/PGE2
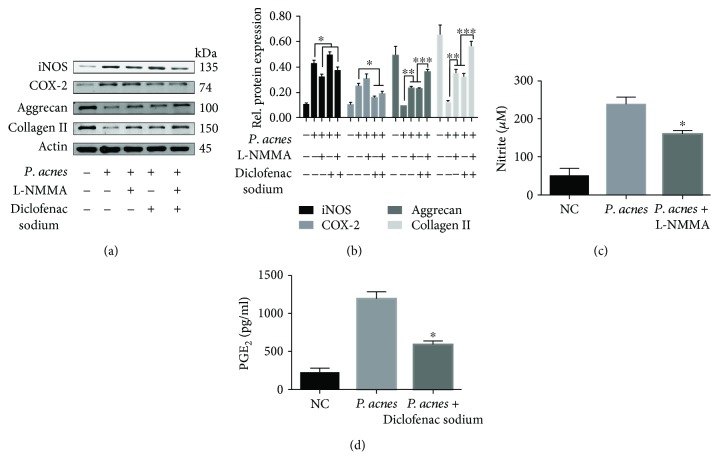
To further elucidate the relationship between iNOS/NO, COX-2/PGE2 activity, and IVDD caused by P. acnes infection, the bacteria were inoculated into the caudal IVDs of rats. After 72 hours, quantitative protein analysis showed that the expression of iNOS/NO and COX-2/PGE2 increased significantly in P. acnes-inoculated IVDs compared with control samples (Figures 2(a)–2(d)), whereas the expression of aggrecan and collagen II decreased significantly (Figures 2(a) and 2(b)). More importantly, when iNOS/NO and COX-2/PGE2 activation was inhibited by L-NMMA (inhibitor of iNOS) and diclofenac sodium (inhibitor of COX-2), respectively (Figures 2(a)–2(d)), P. acnes-induced IVDD was ameliorated, as evidenced by the partially restored expression of aggrecan and collagen II (Figures 2(a) and 2(b)). Interestingly, we found that iNOS and COX-2 exerted a synergistic effect in the promotion of IVDD, as the simultaneous application of L-NMMA and diclofenac sodium restored the expression of aggrecan and collagen II to a higher level than when L-NMMA or diclofenac sodium was used alone (Figures 2(a) and 2(b)). Collectively, these results show that iNOS/NO and COX-2/PGE2 activation is essential for P. acnes-decreased aggrecan and collagen II expression during IVDD in vivo.
Figure 2.
Caudal IVD inoculation with P. acnes-induced IVDD by promoting iNOS/NO and COX-2/PGE2 expression in rats. (a, b) Western blot analysis of aggrecan, collagen II, iNOS, and COX-2 expression in the IVDs of rats inoculated with P. acnes for 72 h, with or without L-NMMA (1 mM) and DS (200 μM) pretreatment. ∗/∗∗The infected group compared with the infection + L-NMMA and/or DS groups. ∗∗∗The infection + L-NMMA and DS group compared with the infection + L-NMMA or DS groups. (c, d) The concentration of NO and PGE2 as tested in different groups. ∗The infected group compared with the infection + L-NMMA or DS groups; P < 0.05. P values were analyzed by Student's t-test and one-way ANOVA. Data are presented as the mean ± SD from three independent experiments.
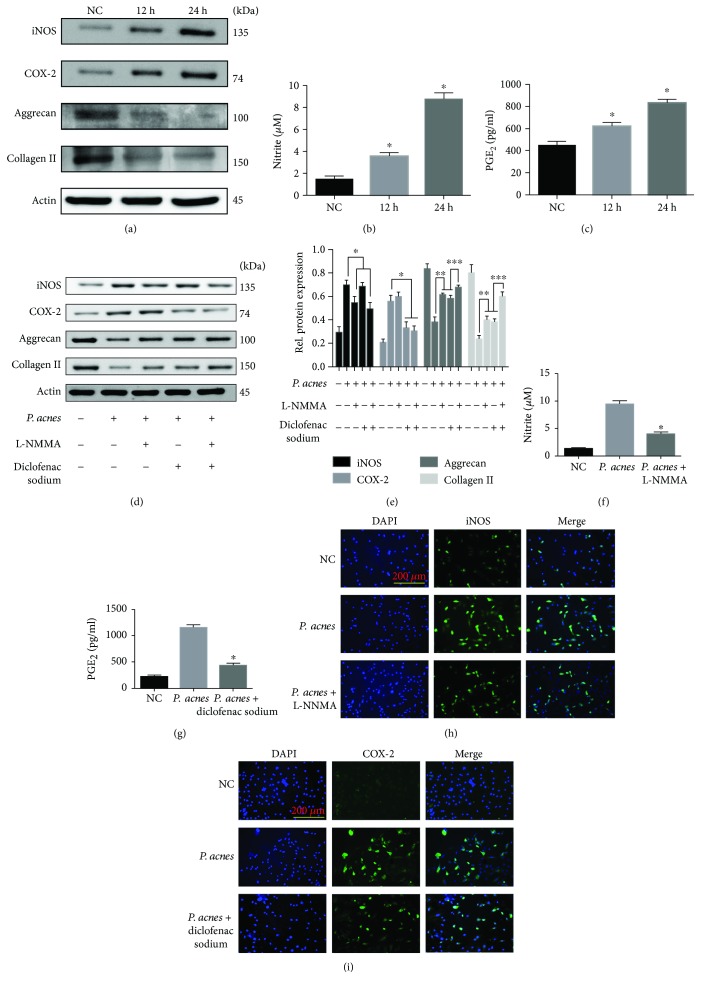
Next, we sought to investigate whether P. acnes could inhibit aggrecan and collagen II expression by promoting iNOS/NO and COX-2/PGE2 expressions in vitro. The results showed that P. acnes incubation (MOI = 100) increased iNOS/NO and COX-2/PGE2 expressions (Figures 3(a)–3(c)), whereas it decreased aggrecan and collagen II expressions in a time-dependent manner in nucleus pulposus cells (NPCs) (Figure 3(a)). However, these processes were significantly dampened in the presence of L-NMMA (100 μM) and diclofenac sodium (200 nM) (Figures 3(d)–3(i)). Taken together, these results show that P. acnes can induce the degeneration of NPCs by promoting iNOS/NO and COX-2/PGE2 activity in vitro.
Figure 3.
Inoculation with P. acnes induced iNOS/NO and COX-2/PGE2 expressions in NPCs. (a) Time curve expression of iNOS, COX-2, aggrecan, and collagen II in NPCs induced by P. acnes at a MOI = 100 : 1. (b, c) P. acnes inoculation increased NO and PGE2 production in a time-dependent manner. ∗The groups with different incubation times compared with the negative control group. (d, e) Western blot analysis of iNOS, COX-2, aggrecan, and collagen II expression in NPCs infected with P. acnes for 24 hours, pretreated with or without L-NMMA (100 μM) and DS (200 nM). ∗/∗∗The infected group compared with the infection + L-NMMA and/or DS groups. ∗∗∗The infection + L-NMMA and DS group compared with the infection + L-NMMA or DS groups. (f, g) The concentration of NO and PGE2 production induced by P. acnes infection for 24 hours, pretreated with or without L-NMMA (100 μM) and DS (200 nM). ∗The infection group compared with the infection + L-NMMA or DS groups. (h, i) Immunofluorescence analysis of iNOS and COX-2 expressions in NPCs induced by P. acnes infection for 24 hours, pretreated with or without L-NMMA (100 μM) and DS (200 nM); P < 0.05. P values were analyzed by one-way ANOVA. Data are presented as the mean ± SD from three independent experiments.
3.3. P. acnes Infection Induced iNOS/NO and COX-2/PGE2 Production via the Generation of ROS
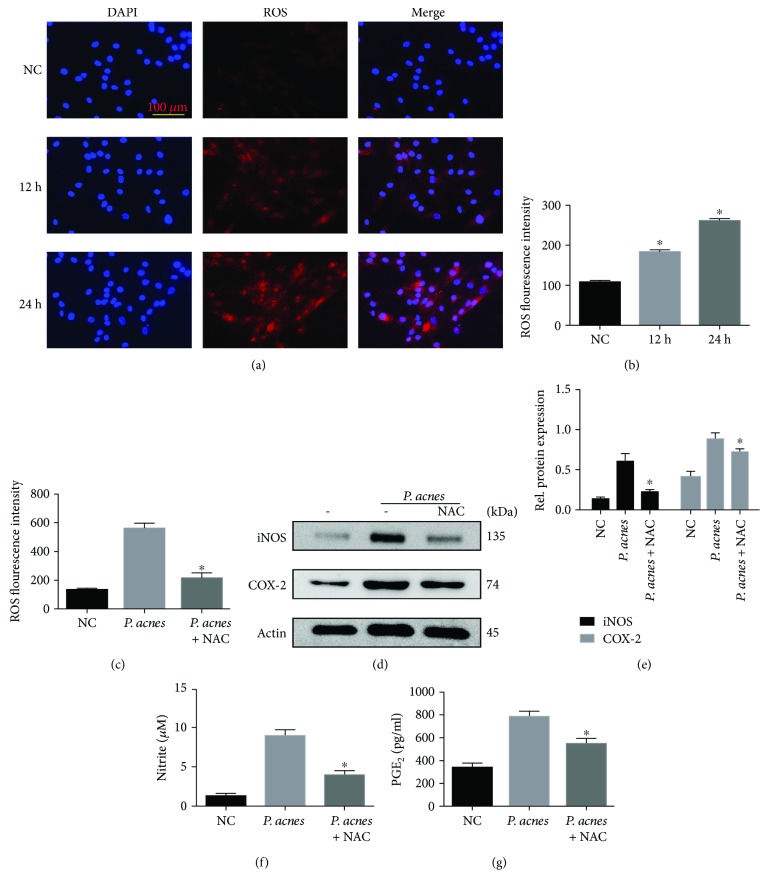
Having established the critical role of iNOS and PGE2 in P. acnes-induced IVDD, we next sought to explore the molecular mechanisms involved in the induction of iNOS and PGE2 by P. acnes in NPCs. Since ROS have been shown to regulate iNOS and COX-2 expressions in various cells [20], we hypothesized that ROS generation was required for iNOS and PGE2 expressions induced by P. acnes. To test this, we first detected the intracellular ROS levels in NPCs infected with P. acnes by DHE probe and DCFH-DA staining for 12 and 24 hours. The results showed that the incubation of P. acnes increased the intracellular ROS levels in a time-dependent manner (Figures 4(a) and 4(b)). To detect the causal role of ROS in iNOS and COX-2 expressions, the ROS scavenger N-acetyl-L-cysteine (NAC) was used. The results showed that pretreatment with NAC significantly reduced ROS levels induced by P. acnes (Figure 4(c)). Concomitantly, iNOS and COX-2 expressions, as well as NO and PGE2 production, were all abundantly dampened after NAC application (Figures 4(d)–4(g)). Taken together, these results indicate that an increase in ROS is essential for P. acnes-induced iNOS/NO and COX-2/PGE2 expressions in NPCs.
Figure 4.
P. acnes induced iNOS/NO and COX-2/PGE2 production via increased ROS production in NPCs. (a) Immunofluorescence analysis of ROS in NPCs in response to P. acnes infection at a MOI = 100 : 1 for different time periods. (b) The ROS fluorescence intensity was tested by flow cytometric analysis. ∗The different incubation time groups compared with the negative control group. (c–g) The flow cytometric analysis of ROS, Western blot of iNOS and COX-2, and the production of NO and PGE2 in NPCs infected with P. acnes for 24 hours, with or without NAC (10 mM) preincubation. ∗The infection + NAC group compared with the infection group. P < 0.05; P values were analyzed by one-way ANOVA. Data are presented as the mean ± SD from three independent experiments.
3.4. P. acnes Induced iNOS/NO and COX-2/PGE2 Expressions via the ROS-Dependent NF-κB Cascade
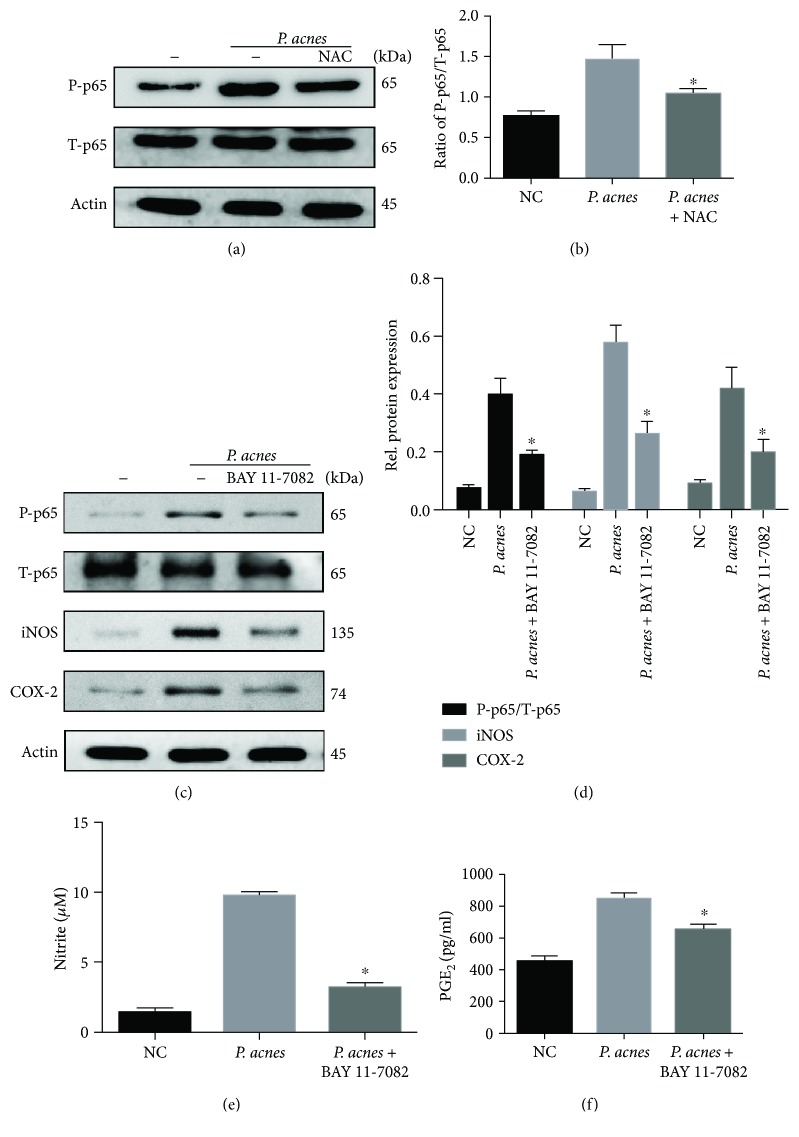
We further explored the ROS-mediated downstream signaling pathways of iNOS/NO and COX-2/PGE2 expressions. Since our previous report demonstrated that the NF-κB signaling pathway mediated P. acnes-induced IVDD by regulating IL-1β and TNF-α expressions [10], we speculated that NF-κB activation might be essential for ROS-induced iNOS/NO and COX-2/PGE2 expressions. The results show that ROS scavenger NAC significantly inhibited NF-κB activation induced by P. acnes (Figures 5(a) and 5(b)), demonstrating that the NF-κB pathway was downstream of ROS in NPCs.
Figure 5.
P. acnes induced iNOS/NO and COX-2/PGE2 expressions via a ROS-dependent NF-κB cascade. (a, b) Western blot analysis of NF-κB p65 activation in the presence of P. acnes for 1 hour, pretreated with or without NAC (10 mM) in NPCs. (c–f) The activation of NF-κB p65, expression of iNOS and COX-2 (c, d), and production of NO and PGE2 (e, f) were analyzed. The NPCs were incubated with P. acnes for 1 hour (for NF-κB p65 activation) and 24 hours (for iNOS, COX-2, NO, and PGE2 expression), pretreated with or without NF-κB-specific inhibitors (BAY 11-7082, 10 μM) for 1 hour. ∗The infection + BAY 11-7082/NCA group compared with the infection group; P < 0.05. P values were analyzed by one-way ANOVA. Data are presented as the mean ± SD from three independent experiments. P-p65: phosphorylated-p65; T-p65: total-p65.
Next, we sought to determine whether NF-κB activation was critical for ROS-induced iNOS/NO and COX-2/PGE2. The results show that in line with the dampened p65 phosphorylation, iNOS and COX-2 expressions (Figures 5(c) and 5(d)) as well as the concentration of NO and PGE2 were significantly decreased by NF-κB inhibitor-BAY11 pretreatment (Figures 5(e) and 5(f)). Taken together, the data demonstrate that ROS-dependent NF-κB activation contributes to P. acnes-induced iNOS/NO and COX-2/PGE2 activation in NPCs.
3.5. NADPH Oxidase Participates in P. acnes-Induced ROS, iNOS/NO, and OX-2/PGE2 Expressions
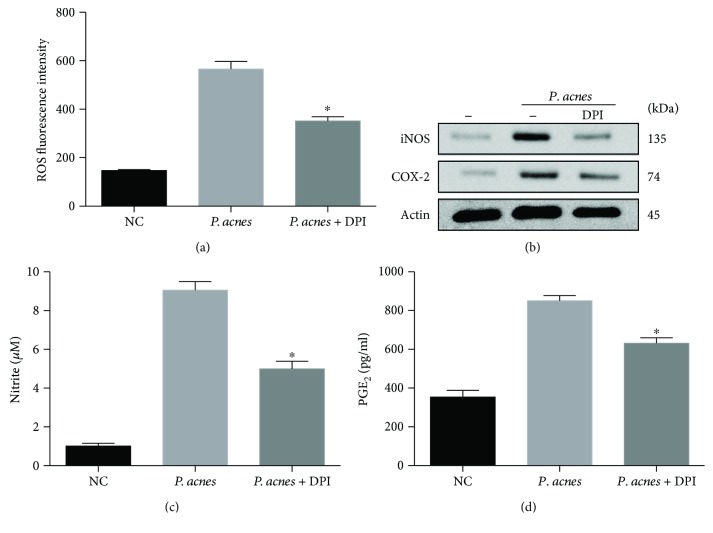
Since NADPH oxidase is an enzymatic source for the production of ROS under various pathologic conditions [21], we next wanted to establish the role of NADPH oxidase in P. acnes-induced ROS, iNOS, and COX-2 expressions. The quantitative results of DCFH-DA-positive cells via flow cytometric analysis showed that treatment with the NADPH oxidase inhibitor diphenyliodonium chloride (DPI) significantly decreased the ROS levels of NPCs induced by P. acnes (Figure 6(a)). In line with the change of intracellular ROS levels, iNOS and COX-2 expressions, as well as the increased concentrations of NO and PGE2 induced by P. acnes, were significantly dampened by the application of DPI (inhibitor of NADPH oxidase) (Figures 6(b)–6(d)). Taken together, these data indicate that activation of NADPH oxidase contributed to P. acnes-induced ROS production as well as iNOS/NO and COX-2/PGE2 expressions in NPCs.
Figure 6.
Participation of NADPH oxidase in P. acnes-induced ROS, iNOS/NO, and COX-2/PGE2 expressions. (a) Flow cytometric analysis of ROS fluorescence intensity in NPCs infected with P. acnes for 24 hours, pretreated with or without DPI (10 μM). ∗The infection + DPI group compared with the infection group. (b–d) DPI inhibited P. acnes-induced iNOS/NO and COX-2/PGE2 production in NPCs. ∗The infection + DPI group compared with the infection group; P < 0.05. P values were analyzed by one-way ANOVA. Data are presented as the mean ± SD from three independent experiments.
4. Discussion
Latent infection with P. acnes has been thought to be crucial to the pathogenesis of IVDD, and the relationship between P. acnes and IVDD has gradually become a hot spot for research [8–11]. More importantly, we found that the pathologic mechanism of P. acnes-induced IVDD was the activation of iNOS/NO and the COX-2/PGE2 system in NPCs. Finally, the signaling pathway involved in the activation of the iNOS/NO and COX-2/PGE2 system by P. acnes has proved to be the ROS-dependent NF-κB pathway.
iNOS and COX-2 are genes that produce NO and PGE2 in cells; they are not only cellular signaling factors but also well-known pathogenic factors for IVDD. Both NO and PGE2 are able to inhibit the synthesis of aggrecan in NPCs, which then leads to the destruction of extracellular matrix in IVDs [15, 22]. In addition, increased PGE2 affects the catabolic and anabolic balance in IVDs—for example, by promoting the expression of matrix metalloproteinase (MMP) and inhibiting the expression of insulin-like growth factor-1 (IGF-1) as well as accelerating the degeneration of IVDs [16]. Also, NO is a proapoptotic factor for NPCs, and the apoptosis of NPCs has been proved to be the key cause of IVDD [14]. However, whether NO and PGE2 are involved in P. acnes-induced IVDD is unknown. We found that P. acnes induced IVDD as well as the overexpression of iNOS/NO and COX-2/PGE2 in vivo and in vitro, whereas elimination of iNOS and COX-2 activity significantly decreased NO and PGE2 production while also ameliorating IVDD. More interestingly, inhibition of both NO and PGE2 generation simultaneously was more effective than inhibiting either one alone, suggesting that the two factors have a synergetic effect in promoting P. acnes-induced IVDD. Therefore, it is reasonable to conclude that P. acnes-induced iNOS/NO and P. acnes-induced COX-2/PGE2 are direct factors leading to the development of IVDD. Since our precious report has demonstrated that P. acnes infection increases NPC degeneration via inducing the apoptosis of NPCs [10], we next detected whether the inhibition of iNOS and COX-2 activity had an effect on the survival of NPCs. The results (Supplemental Figure 1) showed that L-NMMA and diclofenac sodium treatment significantly decreased P. acnes-induced apoptosis of NPCs. In addition, we found that L-NMMA and diclofenac sodium exerted a synergistic effect on the inhibition of NPC apoptosis induced by P. acnes, as the simultaneous application of L-NMMA and diclofenac sodium reduced the apoptosis percentage to a lower level than that in the groups where L-NMMA or diclofenac sodium was used alone. Taken together, these results demonstrated that iNOS- and COX-2-mediated NPC degeneration might also decrease the apoptosis of NPCs induced by P. acnes.
With further investigation, we found that the activation of P. acnes-induced iNOS/NO and COX-2/PGE2 was mediated by ROS. ROS are a family of unstable and highly reactive molecules with or without free radicals, mainly including superoxide anion (O2 −), hydrogen peroxide (H2O2). ROS are inevitably produced via the oxygen-using metabolic process [23]. Previous studies have demonstrated that keratinocytes and macrophages generate many ROS with P. acnes stimulation [17]. When we cocultured NPCs with P. acnes, the concentration of ROS significantly increased, along with an increase of iNOS/NO and COX-2/PGE2, whereas excessive iNOS/NO and COX-2/PGE2 expressions were significantly decreased when ROS were neutralized by NAC (Figures 4(d)–4(f)), suggesting that ROS are the key molecules involved in P. acnes-facilitated iNOS/NO and COX-2/PGE2 activation.
There are two main sources of ROS in cells: the mitochondrial and nonmitochondrial pathways [21, 24]. In the nonmitochondrial pathway, the ROS are mainly generated from the cytosolic enzymes of NADPH oxidase. In this study, the concentration of ROS significantly decreased when the activity of NADPH oxidase was inhibited (Figure 6(a)), suggesting that the P. acnes-induced ROS stem primarily from the nonmitochondrial pathway. However, our previous study proved that P. acnes could activate the TLR-2/JNK/mitochondrial pathway during the apoptosis of NPCs [10], and dysfunction of the mitochondrion was shown to be another source of ROS in many cells [25, 26]. Therefore, we speculated that some ROS may arise from the mitochondrial dysfunction induced by P. acnes, which corresponds with the finding that the NADPH oxidase inhibitor DPI cannot completely scavenge the ROS production induced by P. acnes.
Accumulated ROS appear to regulate cellular function via downstream signaling pathways, such as NF-κB [27, 28]. NF-κB is an important transcriptional factor that plays pivotal roles in inflammatory responses by regulating genes that encode proinflammatory proteins such as iNOS and COX-2 [29]. However, whether activation of the NF-κB pathway is critical for iNOS and COX-2 activation induced by ROS in the presence of P. acnes is less clear. We found that accumulated ROS increased the phosphorylation of NF-κB p65 and inhibited NF-κB p65 with BAY 11-7082 pretreatment, thus alleviating the overexpression of iNOS/NO and COX-2/PGE2. These findings suggest that ROS mediate iNOS/NO and COX-2/PGE2 via NF-κB p65 in P. acnes-stimulated NPCs.
In conclusion, this study demonstrates that P. acnes induces IVDD by promoting iNOS/NO and COX-2/PGE2 activation via a ROS-dependent NF-κB pathway (Figure 7). The confirmation of P. acnes as a pathogenic factor for IVDD and elucidation of the underlying mechanisms provide new insights into IVDD and may ultimately lead to the development of new treatment regimens for IVDD.
Figure 7.
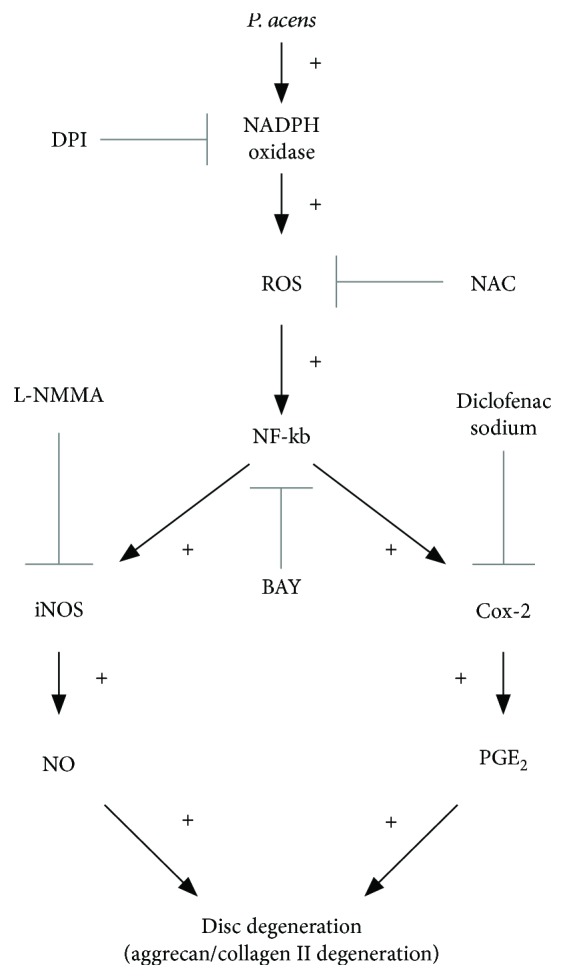
Schematic illustration of P. acnes-induced IVDD by promoting iNOS/NO and COX-2/PGE2.
Acknowledgments
This work was supported by grants from the Science and Technology Commission of Shanghai Municipality, Shanghai, China (no.15DZ1942604), the Ruijin Hospital North, Shanghai Jiao Tong University School of Medicine, Shanghai, China (no.2015FY03), the Shanghai Sailing Program, Shanghai, China (no. 16YF1410100), the National Natural Science Fund, China (NSFC no.81702188), the Natural Science Fund of Jiangsu Province, China (no. BK20161274), and the Doctor Creative Fund of Shanghai Jiao Tong University School of Medicine, China (BXJ201818).
Abbreviations
- P. acnes:
Propionibacterium acnes
- IVD:
Intervertebral disc
- IVDD:
Intervertebral disc degeneration
- NPCs:
Nucleus pulposus cells
- MOI:
Multiplicity of infection
- DS:
Diclofenac sodium
- DCFH-DA:
Dichloro-dihydro-fluorescein diacetate
- L-NMMA:
NG-monomethyl-L-arginine, monoacetate salt
- DPI:
Diphenyliodonium chloride
- MMP:
Matrix metalloproteinase
- IGF-1:
Insulin-like growth factor-1.
Contributor Information
Changwei Li, Email: changwei393331@163.com.
Zhe Chen, Email: drchenzhe@live.com.
Peng Cao, Email: dr_caopeng8@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Yazhou Lin and Guoqing Tang contributed equally to this work.
Supplementary Materials
Supplemental Figure 1: the inhibition of iNOS and/or COX-2 prevents apoptotic cell death of NPCs. Flow cytometric analysis of apoptosis in NPCs infected with P. acnes for 24 hours, pretreated with or without L-NMMA (100 μM) and DS (200 nM). DS: diclofenac sodium.
References
- 1.Modic M. T., Ross J. S. Lumbar degenerative disk disease. Radiology. 2007;245(1):43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z., Cao P., Zhou Z., Yuan Y., Jiao Y., Zheng Y. Overview: the role of Propionibacterium acnes in nonpyogenic intervertebral discs. International Orthopaedics. 2016;40(6):1291–1298. doi: 10.1007/s00264-016-3115-5. [DOI] [PubMed] [Google Scholar]
- 3.Stirling A., Worthington T., Rafiq M., Lambert P. A., Elliott T. S. J. Association between sciatica and Propionibacterium acnes . Lancet. 2001;357(9273):2024–2025. doi: 10.1016/S0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- 4.Albert H. B., Lambert P., Rollason J., et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? European Spine Journal. 2013;22(4):690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coscia M. F., Denys G. A., Wack M. F. Propionibacterium acnes, coagulase-negative Staphylococcus, and the “biofilm-like” intervertebral disc. Spine. 2016;41(24):1860–1865. doi: 10.1097/BRS.0000000000001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao P. J., Phan K., Reddy R., Scherman D. B., Taylor P., Mobbs R. J. DISC (degenerate-disc infection study with contaminant control): pilot study of Australian cohort of patients without the contaminant control. Spine. 2016;41(11):935–939. doi: 10.1097/BRS.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 7.Capoor M. N., Ruzicka F., Schmitz J. E., et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS One. 2017;12(4, article e0174518) doi: 10.1371/journal.pone.0174518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan Y., Zhou Z., Jiao Y., et al. Histological identification of Propionibacterium acnes in nonpyogenic degenerated intervertebral discs. BioMed Research International. 2017;2017:7. doi: 10.1155/2017/6192935.6192935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z., Chen Z., Zheng Y., et al. Relationship between annular tear and presence of Propionibacterium acnes in lumbar intervertebral disc. European Spine Journal. 2015;24(11):2496–2502. doi: 10.1007/s00586-015-4180-y. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y., Jiao Y., Yuan Y., et al. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerging Microbes & Infections. 2018;7(1):p. 1. doi: 10.1038/s41426-017-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Zheng Y., Yuan Y., et al. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: a pilot study. BioMed Research International. 2016;2016:7. doi: 10.1155/2016/9612437.9612437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang J. D., Georgescu H. I., McIntyre-Larkin L., Stefanovic-Racic M., Donaldson W. F., III, Evans C. H. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21(3):271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kang J. D., Stefanovic-Racic M., McIntyre L. A., Georgescu H. I., Evans C. H. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22(10):1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kohyama K., Saura R., Doita M., Mizuno K. Intervertebral disc cell apoptosis by nitric oxide: biological understanding of intervertebral disc degeneration. The Kobe Journal of Medical Sciences. 2000;46(6):283–295. [PubMed] [Google Scholar]
- 15.Liu G. Z., Ishihara H., Osada R., Kimura T., Tsuji H. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine. 2001;26(2):134–141. doi: 10.1097/00007632-200101150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Vo N. V., Sowa G. A., Kang J. D., Seidel C., Studer R. K. Prostaglandin E2 and prostaglandin F2α differentially modulate matrix metabolism of human nucleus pulposus cells. Journal of Orthopaedic Research. 2010;28(10):1259–1266. doi: 10.1002/jor.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai H. H., Lee W. R., Wang P. H., Cheng K. T., Chen Y. C., Shen S. C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. Journal of Dermatological Science. 2013;69(2):122–131. doi: 10.1016/j.jdermsci.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell M. J., Chin S. L., Rangarajan S., et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. The Lancet. 2016;388(10046):761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 19.Granger D. L., Taintor R. R., Boockvar K. S., Hibbs J. B., Jr. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods in Enzymology. 1996;268:142–151. doi: 10.1016/S0076-6879(96)68016-1. [DOI] [PubMed] [Google Scholar]
- 20.Jayasooriya R. G. P. T., Lee K. T., Choi Y. H., Moon S. K., Kim W. J., Kim G. Y. Antagonistic effects of acetylshikonin on LPS-induced NO and PGE2 production in BV2 microglial cells via inhibition of ROS/PI3K/Akt-mediated NF-κB signaling and activation of Nrf2-dependent HO-1. In Vitro Cellular & Developmental Biology - Animal. 2015;51(9):975–986. doi: 10.1007/s11626-015-9922-y. [DOI] [PubMed] [Google Scholar]
- 21.Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4(3):181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 22.Lowe G. N., Fu Y. H., McDougall S., et al. Effects of prostaglandins on deoxyribonucleic acid and aggrecan synthesis in the RCJ 3.1C5.18 chondrocyte cell line: role of second messengers. Endocrinology. 1996;137(6):2208–2216. doi: 10.1210/endo.137.6.8641167. [DOI] [PubMed] [Google Scholar]
- 23.Benhar M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radical Biology & Medicine. 2018 doi: 10.1016/j.freeradbiomed.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Turrens J. F. Mitochondrial formation of reactive oxygen species. The Journal of Physiology. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding F., Shao Z. W., Yang S. H., Wu Q., Gao F., Xiong L. M. Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis. 2012;17(6):579–590. doi: 10.1007/s10495-012-0708-3. [DOI] [PubMed] [Google Scholar]
- 26.Gruber H. E., Watts J. A., Riley F. E., Fulkerson M. B., Norton H. J., Hanley E. N., Jr. Mitochondrial bioenergetics, mass, and morphology are altered in cells of the degenerating human annulus. Journal of Orthopaedic Research. 2013;31(8):1270–1275. doi: 10.1002/jor.22361. [DOI] [PubMed] [Google Scholar]
- 27.Jeong K. Y., Suh G. J., Kwon W. Y., Kim K. S., Jung Y. S., Kye Y. C. The therapeutic effect and mechanism of niacin on acute lung injury in a rat model of hemorrhagic shock: down-regulation of the reactive oxygen species-dependent nuclear factor κB pathway. Journal of Trauma and Acute Care Surgery. 2015;79(2):247–255. doi: 10.1097/TA.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 28.Louradour I., Sharma A., Morin-Poulard I., et al. Reactive oxygen species-dependent Toll/NF-κB activation in the Drosophila hematopoietic niche confers resistance to wasp parasitism. eLife. 2017;6 doi: 10.7554/eLife.25496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nature Reviews Immunology. 2018;18(5):309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: the inhibition of iNOS and/or COX-2 prevents apoptotic cell death of NPCs. Flow cytometric analysis of apoptosis in NPCs infected with P. acnes for 24 hours, pretreated with or without L-NMMA (100 μM) and DS (200 nM). DS: diclofenac sodium.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.