Abstract
Introduction:
Amplification of airway inflammation and its destruction due to oxidative stress is a major step in the pathogenesis of chronic obstruction pulmonary disease (COPD). Exhaled carbon monoxide (eCO) may be quantified to evaluate the airway inflammation and oxidative stress in such patients.
Objectives:
To assess the disease severity of COPD and treatment response by measuring eCO as a biomarker.
Materials and Methods:
COPD patients diagnosed according to the global initiative for chronic obstructive lung disease guidelines and healthy individuals as controls were selected. One hundred and fifty patients with COPD and 125 controls were included in the study. Participants were further subdivided on the basis of their smoking habits. Clinical examinations and spirometry were done to diagnose COPD by following the standard protocol. eCO was measured using a piCO + Smokerlyzer (Breath CO Monitor, Bedfont Scientific Ltd., Kent, UK). It was a single-center cross-sectional study.
Results:
Mean (± standard error of mean) CO levels in ex-smokers with COPD were higher (5.21 ± 1.546 ppm; P < 0.05) than in nonsmoking controls (1.52 ± 0.571 ppm) but were lower than in current smokers with COPD (12.55 ± 4.514 ppm; P < 0.05). eCO levels were higher in current smokers with COPD (12.55 ± 4.514 ppm; P < 0.05) compared to healthy smokers (9.71 ± 5.649). There was a negative correlation between eCO and forced expiratory volume in 1 s (FEV1) in COPD (r = −0.28; P < 0.05). The mean eCO level was decreased (6.291–4.332; P < 0.001) with improvement in lung function (FEV1 38.75%–50.65%: P < 0.05) after treatment with inhaled steroid.
Conclusion:
Our study concludes that quantification of eCO level in COPD varies with different grades of airway obstruction and to measure the treatment response. Measuring the level of eCO can be used to assess the indirect assessment of airway inflammation, oxidative stress, and severity of airway obstruction in COPD patients.
KEY WORDS: Chronic obstructive pulmonary disease, exhaled carbon monoxide, forced expiratory volume in one second, parts per million
INTRODUCTION
Chronic obstruction pulmonary disease (COPD) is a disease of major public health importance. It is diagnosed only after spirometric evaluation according to the global initiative for chronic obstructive lung disease (GOLD) guidelines.[1] The forced expiratory spirogram is the most useful test of airflow dynamics. Postbronchodilator forced expiratory volume in 1 s (FEV1) is the mainstay of classification of severity of COPD, and it is strongly predictive of subsequent mortality from COPD.[2,3,4,5] There are limitations with the use of FEV1, since changes in it over time are small in relation to repeatability of the measurement.[6]
The use of exhaled biomarkers as a diagnostic tool for COPD was overviewed by van Beurden et al. in 2002, and they suggested that there was a need for “standardization of the measurements, for comparison of COPD patients with healthy individuals matched for age and smoking status, for data on reproducibility and variability, for correlation of exhaled markers with other parameters, and for intervention studies.”[7] Exhaled breath biomarkers of airway inflammation may aid in the early diagnosis of COPD. COPD could be diagnosed earlier in those smokers at risk of developing the disease before symptoms or changes in spirometry are present.[8] As they diagnosed so late in their disease, when available, therapeutic and preventive measures are limited.[8] This would lead to a significant achievement in COPD management. Hence, exhaled biomarkers which reflect airway inflammation and correlate with disease severity may therefore help improve monitoring and treatment of COPD.
Oxidative stress is a major step in the pathogenesis of COPD and causes amplification of airway inflammation and its destruction.[9] The measurement of exhaled carbon monoxide (eCO) may represent a new method for the noninvasive monitoring of airway inflammation and oxidant stress in COPD patients. Carbon monoxide (CO) is produced ubiquitously in the body by heme oxygenase (HO) as a breakdown product of heme.[10,11,12,13]
CO in exhaled breath may be of endogenous or exogenous in origin. Major sources of endogenous CO in exhaled breath are enzymatic degradation of heme, nonheme-related release (lipid peroxidation, xenobiotic, and bacteria).[14] The most important source of CO (~85%) in the body is from the degradation of hemoglobin by the enzyme HO, and the rest arises from the degradation of myoglobin, catalase, nitric oxide (NO) synthases, guanylyl cyclase, and cytochromes.[15] Several bacteria also produce CO,[16] but this does not have any significance on the level of eCO. Approximately 85% of the CO is bound to hemoglobin in circulating erythrocytes and the remaining is bound to myoglobin and other compound, and <1% is unbound and dissolved in body fluid.[17] Approximately 80% of the CO formed from heme degradation is exhaled.[18]
The major exogenous sources producing CO are petroleum or diesel fuel during road transport and industrial processes using carbon compounds; these two are responsible for 80% of CO emitted to the atmosphere.[19] CO is also an indoor pollutant: as a result of the functioning of gas cookers and some heating systems.[20] Both active and passive smoking are the major cause for high levels of exhaled CO, although some exposure to CO may occur in normal day-to-day life because of environmental pollution.[14]
Exhaled CO is produced in healthy nonsmokers endogenously and increases in many inflammatory lung conditions.[21] Various factors that may influence eCO level are smoking,[22,23] airway pollution,[24,25] airway obstruction,[26] hyperbilirubinemia,[27] sex (cyclic variations in women),[28] race (increased in Japanese newborn),[29] and allergen challenge (early and late response).[30] It has been useful in monitoring various pulmonary inflammatory diseases such as asthma,[31,32,33,34,35,36] allergic rhinitis,[37] COPD (ex-smokers),[38] upper respiratory tract infections,[39] bronchiectasis,[40] lower respiratory tract infections,[41] interstitial lung disease,[42] cystic fibrosis,[43,44] and critically ill patients.[45]
CO causes bronchodilatation in vivo and this finding suggests a role for endogenous CO in inflammatory airway diseases.[46] eCO has also been used to quantify oxidative stress in stable asthma and bronchiectasis patients who have higher CO levels than healthy controls.[31,32] eCO has been found to be increase in stable cystic fibrosis patients but to a greater extent, during exacerbations.[44] Till now, most of the studies were performed in vitro that relate COPD with oxidative stress, using invasive techniques such as examination of bronchoalveolar lavage fluid or measurement of systemic rather than oxidant stress.[47]
Therefore, in this study, we have quantified lung oxidative stress in stable COPD patients by measuring eCO levels. This may contribute to the understanding of the pathophysiology of COPD and may suggest a potential new noninvasive method to monitor airway inflammation in this disease. In addition to it, spirometry has also been done, and correlation of level of airway obstruction (disease severity) with level of eCO has also been made. We have also tried to measure the Utility of eCO level in monitoring and treatment of COPD.
MATERIALS AND METHODS
Subjects
COPD patients diagnosed according to the GOLD guidelines and healthy individuals as controls were selected. One hundred and fifty patients with COPD and 125 controls were included in the study. Participants were further subdivided on the basis of their smoking habits. Clinical examinations and spirometry were done to diagnose COPD by following the standard protocol.
Patients with a history suggestive of asthma and other respiratory diseases were excluded. Patients with systemic diseases, vascular disease, thrombosis, alcoholism, renal disease, and hepatic disease were also excluded from the study.
Ex smoker are those patients who had stopped smoking for at least 6 months. Healthy smokers and current smokers with COPD refrained from smoking for at least 12 h before eCO measurements.
Study design
It was a single-center cross-sectional observational study.
Methods
Patients with typical symptoms of chronic cough with or without expectoration with shortness of breath on exertion were included in the study after confirming the diagnosis by FEV1/FVC <70% and postbronchodilator FEV1 <80% on spirometry as per the GOLD guidelines. Clinical examinations were made following the standard protocol/procedure. Chest X-ray, hematological and biochemical parameters, and ECG with echocardiography were made. After informed about the objectives and procedures related to the study, informed consent was taken from volunteers. After confirming the diagnosis, the patient has received inhaled corticosteroid (budesonide 400 mcg), long-acting beta-2 agonist with or without long-acting antimuscarinic agents for 6 months. The study was approved by the ethics committee.
Spirometry
Spirometry is a method of assessing lung function by measuring the volume of air; the patient can exhale out from the lungs after maximal inspiration. Spirometry was measured and the best value from the three maneuvers was expressed as an absolute value (in liters) and as a percentage of the predicted value. Necessary instructions were given to patients before test. Reversibility testing was also performed. Postbronchodilator FEV1 was recorded in all cases to assess severity of airway obstruction and to categorize into mild (≥80% predicted), moderate (50% ≤ FEV1 <80% predicted), severe (30% ≤ FEV1 <50% predicted), and very severe (<30% predicted) according to the GOLD guidelines.
Exhaled carbon monoxide
Breath CO monitoring was performed using a portable piCO + Smokerlyzer (Breath CO monitor, Bedfont Scientific Ltd., Kent, UK). The participants were asked to exhale completely, inhale fully, and then hold their breath for as long as possible. Following breath holding, the participants were asked to exhale slowly into the Smokerlyzer and were encouraged to exhale fully to sample the alveolar air. This procedure was repeated and two successive recordings were made, and the mean values were used in all calculations.
Statistical analysis
Data were analyzed using IBM SPSS 20 Statistics and Microsoft Office Excel 2013 software. Independent t-test was used to compare the mean eCO levels of two groups. One-way analysis of variance (ANOVA) and Tukey's honestly significant difference (HSD) post hoc test were performed to compare the mean values in four stages of airway obstructions (mild, moderate, severe, and very severe) among the COPD cases. Spearman's rho bivariate correlation coefficient was used to quantify the extent of correlation between FEV1 with the eCO levels among COPD cases. Paired t-test was used to compare the mean percentage predicted FEV1 and mean eCO before and after treatment. The results are mentioned in mean ± standard deviation. For all statistical analysis, P < 0.05 was considered statistically significant.
RESULTS
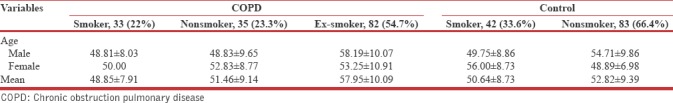
There were 150 COPD patients, aged from 23 to 80 years (mean 54.43 ± 10.18) and 125 healthy controls, age from 35 to 85 years (mean 52.09 ± 9.2). Among COPD patients and healthy controls, there were 122 (81.3%) males and 28 (18.7%) females and 92 (73.6%) males and 33 (26.4%) females, respectively. COPD patients were divided into three groups: smokers (33), ex-smokers (82), and nonsmokers (35), while healthy controls were divided into smokers (42) and nonsmokers (83). Of 75 smokers, 68 were male while 7 were female. In 118 nonsmokers, 68 were male and 50 females. Out of 82 ex-smokers, 78 were male while 4 were female.
Among COPD males, 12 were nonsmokers, 78 ex-smokers, and 32 smokers. In COPD females, 23 were nonsmokers, 4 ex-smokers, and 1 smoker. Among healthy controls males, 36 were smoker and 56 nonsmoker. Among female healthy controls, 6 were smoker and 27 nonsmoker [Table 1].
Table 1.
The demographic pattern of study population
Comparisons of exhaled carbon monoxide in different groups of participants
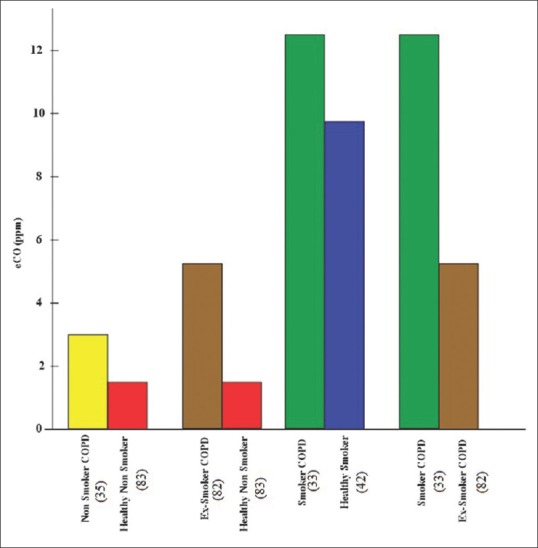
Exhaled CO levels were higher in nonsmokers with COPD (2.94 ± 0.873 ppm; P < 0.001), compared to healthy nonsmokers (1.52 ± 0.571 ppm) [Figures 1 and 2].
Figure 1.
Bar chart showing comparison of exhaled carbon monoxide level in different groups of chronic obstruction pulmonary disease with controls
Figure 2.
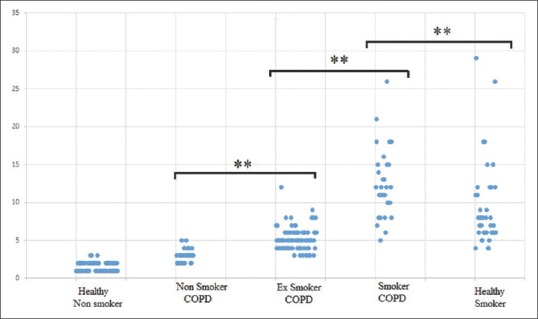
Exhaled carbon monoxide level in different groups of individuals. **Shows significant difference among these groups (P < 0.05)
Exhaled CO levels were higher in ex-smokers with COPD (5.21 ± 1.546 ppm; P < 0.001), compared to healthy nonsmokers (1.52 ± 0.571 ppm) [Figures 1 and 2].
Exhaled CO levels were higher in current smokers with COPD (12.55 ± 4.514 ppm; P < 0.05), compared to healthy smokers (9.71 ± 5.649 ppm) [Figures 1 and 2].
Exhaled CO levels were higher in current smokers with COPD (12.55 ± 4.514 ppm; P < 0.05), compared to ex-smokers (5.21 ± 1.546 ppm) [Figures 1 and 2].
On applying one-way ANOVA, difference in the mean eCO level was found to be significant (F (4, 270) = 120.25 P < 0.001). On applying Tukey's HSD post hoc test, the significant difference in mean is found in different study groups.
Comparison of exhaled carbon monoxide and forced expiratory volume in one second in different stages of chronic obstruction pulmonary disease
On applying one-way ANOVA, difference in the mean eCO level was found to be significant (F (2, 147) = 6.648, P = 0.01). On applying Tukey's HSD post hoc test, the difference in mean is found to be significant between Stage II (4.75 ± 2.92) and Stage IV (7.90 ± 4.86) and Stage III (5.75 ± 3.82) and Stage IV (7.90 ± 4.86) (P < 0.05) as shown in Figure 3.
Figure 3.
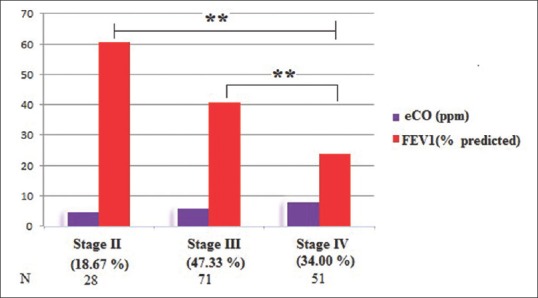
Comparison of exhaled carbon monoxide and forced expiratory volume in 1 s in different stages of chronic obstruction pulmonary disease. **Shows significant difference (P < 0.05)
Correlations between exhaled carbon monoxide and forced expiratory volume in one second in chronic obstruction pulmonary disease
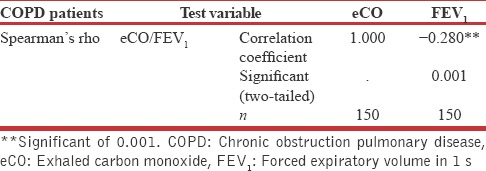
The correlation between FEV1 and exhaled CO was found to be negative (P < 0.01). The level of eCO increases with increase in severity of disease [Figure 4 and Table 2].
Figure 4.
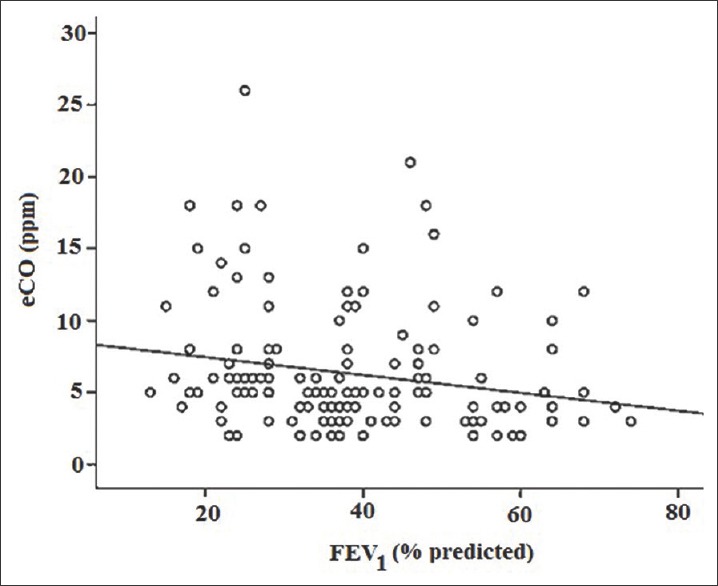
Scattered dot showing correlations between exhaled carbon monoxide and forced expiratory volume in 1 s in chronic obstruction pulmonary disease (n = 150) (r = -0.280; P < 0.01)
Table 2.
Correlations between exhaled carbon monoxide and forced expiratory volume in 1 s using Spearman's rho bivariate
Treatment response with inhaled steroids
The mean exhaled CO level was decreased (6.29 ± 0.344 to 4.33 ± 0.237) with improvement in disease (FEV1 38.75 ± 1.164 to 50.65 ± 1.137) after treatment with inhaled corticosteroid. It was statistically significant (P < 0.001).
DISCUSSION
Recently, there has been lots of interest in the analysis of exhaled breath constituents for the monitoring of inflammation and oxidative stress in the lungs. Most available studies have concentrated on exhaled NO; several other volatile gases (CO, ethane, and pentane) have also been used recently. The assessment of noninvasive biomarkers in COPD is an area of intensive investigation. Oxidative stress is a major component of airway inflammation in patients with COPD.[47] Hence, eCO is a simple method for detecting and monitoring airway inflammation and oxidative stress.
In view of this, we have studied levels of CO in exhaled air of COPD patients. We have found a >3-fold increase in the level of exhaled CO in ex-smokers with COPD compared to healthy nonsmokers, as was previously reported. Since ex-smokers with COPD had stopped smoking for at least the last 6 months, increased CO levels in these patients are likely to be the result of enhanced oxidant stress in their lungs. An increase in eCO was observed in current smokers with COPD compared to healthy smokers matched for age and smoking habits, similar to the study of Montuschi et al.[48] This may indicate higher oxidative stress in the former group. We have found higher eCO levels in current smokers with COPD than in ex-smokers with COPD, similar to the earlier study,[38,48,49] but comparisons between the two groups are not possible because of the influence of cigarette smoke on exhaled CO levels. In current smokers with COPD, it is difficult to discriminate between the amount of increased exhaled CO due to lung oxidative stress and that due to CO contained in cigarette smoke.[50] There was negative correlation between eCO levels in COPD patients and lung function (FEV1 values) and it was statistically significant (P < 0.01), similar to previous study.[6] However, our results differ from a study that showed no negative correlation between CO levels and lung function.[48]
We have large sample size with adequate number of participants in each group as compared to the previous studies.[6,38,48,49] To the best of our knowledge, only one study from India by Sivagnaname had been published till now.[6] Hence, our study is second from India.
Most of the COPD patients in our study were treated with steroids. In patients with moderate asthma (i.e., FEV1 67%–63% of predicted), an open study[31] has shown that CO is sensitive to inhaled steroid treatment. We had similar results in our study in COPD patients. In previous study,[51] exhaled CO and NO levels were similar in COPD patients treated and not treated with inhaled and/or oral steroids. However, in our study, there was a significant difference in eCO levels after treatment with inhaled steroids.
CONCLUSION
Our study concludes that quantification of exhaled CO levels in COPD cases varies with different grades of airway obstruction. We concluded that measuring the level of eCO in COPD cases along with spirometry forms a new approach for better understanding of pathophysiology of COPD cases, with indirect assessment of airway inflammation, oxidative stress, and severity of airway obstruction and to assess the response to drug treatment. Further clinical studies may refine clinical applications for eCO, as a biomarker of disease severity in COPD.
Limitation of study
In tertiary care centers, most of the patients presented in their late stage of disease. Most of our patients are smokers or ex-smokers that could be confounding the eCO level.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Salameh P, Khayat G, Waked M. Validation of the respiratory toxics exposure score (RTES) for chronic obstructive pulmonary disease screening. Int J Occup Med Environ Health. 2011;24:339–47. doi: 10.2478/s13382-011-0043-x. [DOI] [PubMed] [Google Scholar]
- 2.Carter R, Peavler M, Zinkgraf S, Williams J, Fields S. Predicting maximal exercise ventilation in patients with chronic obstructive pulmonary disease. Chest. 1987;92:253–9. doi: 10.1378/chest.92.2.253. [DOI] [PubMed] [Google Scholar]
- 3.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–80. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins C, Rodríguez-Roisin R. Quality of life, stage severity and COPD. Eur Respir J. 2009;33:953–5. doi: 10.1183/09031936.00019009. [DOI] [PubMed] [Google Scholar]
- 5.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291:755–8. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- 6.Sivagnaname Y. Utility of measuring exhaled carbon monoxide (ECO) level in addition to pulmonary function test (Spirometry) in the monitoring of chronic obstructive pulmonary disease (COPD) [Last accessed on 2018 May 04];Int J Med Sci Public Health. 2014 3:289–94. Available from: http://www.ijmsph.com/?mno=48517 . [Google Scholar]
- 7.van Beurden WJ, Dekhuijzen PN, Smeenk FW. Exhaled biomarkers in COPD: Their potential role in diagnosis, treatment and prognosis. Monaldi Arch Chest Dis. 2002;57:258–67. [PubMed] [Google Scholar]
- 8.O'Reilly P, Bailey W. Clinical use of exhaled biomarkers in COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:403–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–48. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 10.Cantoni L, Rossi C, Rizzardini M, Gadina M, Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J. 1991;279(Pt 3):891–4. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YM, Bergonia HA, Müller C, Pitt BR, Watkins WD, Lancaster JR, Jr, et al. Loss and degradation of enzyme-bound heme induced by cellular nitric oxide synthesis. J Biol Chem. 1995;270:5710–3. doi: 10.1074/jbc.270.11.5710. [DOI] [PubMed] [Google Scholar]
- 12.Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 13.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 15.Berk PD, Rodkey FL, Blaschke TF, Collison HA, Waggoner JG. Comparison of plasma bilirubin turnover and carbon monoxide production in man. J Lab Clin Med. 1974;83:29–37. [PubMed] [Google Scholar]
- 16.Levine AS, Bond JH, Prentiss RA, Levitt MD. Metabolism of carbon monoxide by the colonic flora of humans. Gastroenterology. 1982;83:633–7. [PubMed] [Google Scholar]
- 17.Coburn RF. Endogenous carbon monoxide production. N Engl J Med. 1970;282:207–9. doi: 10.1056/NEJM197001222820407. [DOI] [PubMed] [Google Scholar]
- 18.Vreman HJ, Baxter LM, Stone RT, Stevenson DK. Evaluation of a fully automated end-tidal carbon monoxide instrument for breath analysis. Clin Chem. 1996;42:50–6. [PubMed] [Google Scholar]
- 19.Téllez J, Rodríguez A, Fajardo A. Carbon monoxide contamination: An environmental health problem. Rev Salud Publica (Bogota) 2006;8:108–17. doi: 10.1590/s0124-00642006000100010. [DOI] [PubMed] [Google Scholar]
- 20.Harrison RM, Thornton CA, Lawrence RG, Mark D, Kinnersley RP, Ayres JG, et al. Personal exposure monitoring of particulate matter, nitrogen dioxide, and carbon monoxide, including susceptible groups. Occup Environ Med. 2002;59:671–9. doi: 10.1136/oem.59.10.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horváth I, MacNee W, Kelly FJ, Dekhuijzen PN, Phillips M, Döring G, et al. Haemoxygenase-1 induction and exhaled markers of oxidative stress in lung diseases. summary of the ERS research seminar in Budapest, Hungary, September, 1999. Eur Respir J. 2001;18:420–30. doi: 10.1183/09031936.01.00231201. [DOI] [PubMed] [Google Scholar]
- 22.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117:758–63. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis MJ, Russell MA, Saloojee Y. Expired air carbon monoxide: A simple breath test of tobacco smoke intake. Br Med J. 1980;281:484–5. doi: 10.1136/bmj.281.6238.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewat VN, Foster EV, O'Brien GD, Town GI. Ambient and exhaled carbon monoxide levels in a high traffic density area in christchurch. N Z Med J. 1998;111:343–4. [PubMed] [Google Scholar]
- 25.Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, et al. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med. 2000;162:161–6. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- 26.Togores B, Bosch M, Agustí AG. The measurement of exhaled carbon monoxide is influenced by airflow obstruction. Eur Respir J. 2000;15:177–80. doi: 10.1183/09031936.00.15117700. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson DK, Vreman HJ. Carbon monoxide and bilirubin production in neonates. Pediatrics. 1997;100:252–4. doi: 10.1542/peds.100.2.252. [DOI] [PubMed] [Google Scholar]
- 28.Delivoria-Papadopoulos M, Coburn RF, Forster RE. Cyclic variation of rate of carbon monoxide production in normal women. J Appl Physiol. 1974;36:49–51. doi: 10.1152/jappl.1974.36.1.49. [DOI] [PubMed] [Google Scholar]
- 29.Fischer AF, Nakamura H, Uetani Y, Vreman HJ, Stevenson DK. Comparison of bilirubin production in Japanese and Caucasian infants. J Pediatr Gastroenterol Nutr. 1988;7:27–9. doi: 10.1097/00005176-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Paredi P, Leckie MJ, Horvath I, Allegra L, Kharitonov SA, Barnes PJ, et al. Changes in exhaled carbon monoxide and nitric oxide levels following allergen challenge in patients with asthma. Eur Respir J. 1999;13:48–52. doi: 10.1183/09031936.99.13104899. [DOI] [PubMed] [Google Scholar]
- 31.Zayasu K, Sekizawa K, Okinaga S, Yamaya M, Ohrui T, Sasaki H, et al. Increased carbon monoxide in exhaled air of asthmatic patients. Am J Respir Crit Care Med. 1997;156:1140–3. doi: 10.1164/ajrccm.156.4.96-08056. [DOI] [PubMed] [Google Scholar]
- 32.Horváth I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ, et al. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: A new marker of oxidative stress. Thorax. 1998;53:668–72. doi: 10.1136/thx.53.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaya M, Sekizawa K, Ishizuka S, Monma M, Sasaki H. Exhaled carbon monoxide levels during treatment of acute asthma. Eur Respir J. 1999;13:757–60. doi: 10.1034/j.1399-3003.1999.13d10.x. [DOI] [PubMed] [Google Scholar]
- 34.Stirling RG, Lim S, Kharitonov SA, Chung FK, Barnes PJ. Exhaled breath carbon monoxide is minimally elevated in severe but not mild atopic asthma. Am J Respir Crit Care Med. 2000;161:A922. [Google Scholar]
- 35.Horváth I, Barnes PJ. Exhaled monoxides in asymptomatic atopic subjects. Clin Exp Allergy. 1999;29:1276–80. doi: 10.1046/j.1365-2222.1999.00661.x. [DOI] [PubMed] [Google Scholar]
- 36.Uasuf CG, Jatakanon A, James A, Kharitonov SA, Wilson NM, Barnes PJ, et al. Exhaled carbon monoxide in childhood asthma. J Pediatr. 1999;135:569–74. doi: 10.1016/s0022-3476(99)70054-5. [DOI] [PubMed] [Google Scholar]
- 37.Monma M, Yamaya M, Sekizawa K, Ikeda K, Suzuki N, Kikuchi T, et al. Increased carbon monoxide in exhaled air of patients with seasonal allergic rhinitis. Clin Exp Allergy. 1999;29:1537–41. doi: 10.1046/j.1365-2222.1999.00684.x. [DOI] [PubMed] [Google Scholar]
- 38.Culpitt SV, Paredi P, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide is increased in COPD patients regardless of their smoking habit. Am J Respir Crit Care Med. 1998;157:A787. [Google Scholar]
- 39.Yamaya M, Sekizawa K, Ishizuka S, Monma M, Mizuta K, Sasaki H, et al. Increased carbon monoxide in exhaled air of subjects with upper respiratory tract infections. Am J Respir Crit Care Med. 1998;158:311–4. doi: 10.1164/ajrccm.158.1.9711066. [DOI] [PubMed] [Google Scholar]
- 40.Horvath I, Loukides S, Wodehouse T, Kharitonov SA, Cole PJ, Barnes PJ, et al. Increased levels of exhaled carbon monoxide in bronchiectasis: A new marker of oxidative stress. Thorax. 1998;53:867–70. doi: 10.1136/thx.53.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biernacki WA, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide in patients with lower respiratory tract infection. Respir Med. 2001;95:1003–5. doi: 10.1053/rmed.2001.1196. [DOI] [PubMed] [Google Scholar]
- 42.Antuni JD, Du Bois AB, Ward S, Cramer DS, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide may be a marker of deterioration of lung function in cryptogenic fibrosing alveolitis and scleroderma. Am J Respir Crit Care Med. 1999;159:A51. [Google Scholar]
- 43.Paredi P, Shah PL, Montuschi P, Sullivan P, Hodson ME, Kharitonov SA, et al. Increased carbon monoxide in exhaled air of patients with cystic fibrosis. Thorax. 1999;54:917–20. doi: 10.1136/thx.54.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antuni JD, Kharitonov SA, Hughes D, Hodson ME, Barnes PJ. Increase in exhaled carbon monoxide during exacerbations of cystic fibrosis. Thorax. 2000;55:138–42. doi: 10.1136/thorax.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scharte M, Bone HG, Van Aken H, Meyer J. Increased carbon monoxide in exhaled air of critically ill patients. Biochem Biophys Res Commun. 2000;267:423–6. doi: 10.1006/bbrc.1999.1936. [DOI] [PubMed] [Google Scholar]
- 46.Cardell LO, Ueki IF, Stjärne P, Agusti C, Takeyama K, Lindén A, et al. Bronchodilatation in vivo by carbon monoxide, a cyclic GMP related messenger. Br J Pharmacol. 1998;124:1065–8. doi: 10.1038/sj.bjp.0701878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med. 1997;156:341–57. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 48.Montuschi P, Kharitonov SA, Barnes PJ. Exhaled carbon monoxide and nitric oxide in COPD. Chest. 2001;120:496–501. doi: 10.1378/chest.120.2.496. [DOI] [PubMed] [Google Scholar]
- 49.Hanta I, Kocabas A, Olgunus O, Satar S, Seydaoglu G. Does the expired-air carbon monoxide level reflect the severity of inflammation in COPD? Bratisl Lek Listy. 2007;108:255–8. [PubMed] [Google Scholar]
- 50.Chatkin G, Chatkin JM, Aued G, Petersen GO, Jeremias ET, Thiesen FV, et al. Evaluation of the exhaled carbon monoxide levels in smokers with COPD. J Bras Pneumol. 2010;36:332–8. doi: 10.1590/s1806-37132010000300011. [DOI] [PubMed] [Google Scholar]
- 51.Paredi P, Kharitonov SA, Leak D, Ward S, Cramer D, Barnes PJ, et al. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:369–73. doi: 10.1164/ajrccm.162.2.9909025. [DOI] [PubMed] [Google Scholar]


