Abstract
We describe a 21-year-old male with a history of smoking and subacute onset of breathlessness with normal cardiorespiratory examination. The presence of “track marks” and digital infarcts prompted evaluation for infective endocarditis and confrontational history taking revealed anorexia, weight loss over 3 months along with intravenous drug abuse of reconstituted tablets of tapentadol. Echocardiography was normal and blood cultures were sterile; computed tomography showed bilateral, diffuse, small centrilobular nodules with “tree-in-bud” appearance. In this clinicopathologic conference, we discuss the clinical and radiological differential diagnosis of centrilobular nodules, lung biopsy findings, and management options for patients with such a presentation.
KEY WORDS: Excipient lung disease, intravascular talcosis, pulmonary foreign body angiogranulomatosis, talc granulomatosis
PRESENTATION OF THE CASE
A 21-year-old college student presented with breathlessness and left-sided chest pain for a week. Breathlessness was insidious in onset and persisted on walking at own speed at level. Chest pain was left-sided, intermittent, pricking and non-exertional. There was no history of fever, rhinitis, headache, hemoptysis, cough, or joint pains. He did not raise pets but admitted to smoking 7–8 cigarettes/day and consuming whisky (192 g of alcohol weekly). He denied any recent worsening of educational performance. He did not report any significant prior medical illness or pertinent illness in family or occupational dust exposure. He was initially seen in cardiology and evaluation showed normal cardiac and respiratory examination without any pallor, clubbing, or organomegaly. Body mass index was 18.6 kg/m2. The examination also showed extensive track marks over both arms with evidence of multiple peripheral digital infarcts [Figure 1], raising suspicion for infective endocarditis (IE). On confronting, he disclosed daily intravenous self-injections of three–four powdered and reconstituted 50 mg tablets of Tapentadol (Tydol, Sun Pharma) in sterile distilled water two–three times a day over the last 2 years. He also admitted to using inhaled cannabis twice weekly over the last 2 years. There was associated anorexia and unquantified weight loss over the last 3 months. Three sets of blood cultures were sterile. Electrocardiography showed T-wave inversions in leads V3–V6 and II, III, and aVF. Transthoracic echocardiography (TTE) showed normal valves without any evidence of vegetations or pulmonary hypertension and normal left ventricular function. Serum creatinine was 0.6 mg/dL; urine microscopy was normal without any albuminuria. Hemoglobin was 14.2 g/dL with normal leukocyte and platelet counts. He remained afebrile. Transesophageal echocardiography (TEE) was planned; in the meanwhile, review of his chest radiographs showed diffusely scattered fine nodules bilaterally [Figure 2, left]. Spirometry showed mild restriction with reduced diffusion capacity for carbon monoxide (forced expiratory volumes at 1 s 2.25 L [65%]%, forced vital capacity 2.87 L [71%], DLco 62%). There was desaturation to 92% while walking 6 minutes, with preserved walk distance (545 meters, 89% predicted). Human immunodeficiency virus (HIV), anti-nuclear antibodies, hepatitis B, and hepatitis C antibodies were negative by enzyme-linked immunosorbent assay. Immunoglobulin E levels were 9 IU/mL (normal <100 IU/mL). High-resolution computed tomography (HRCT) of the thorax showed bilateral profuse evenly distributed micronodules with “tree-in-bud” [Figure 2, right]. There was no sputum production on induction with 3% saline. A procedure was planned for diagnosis.
Figure 1.
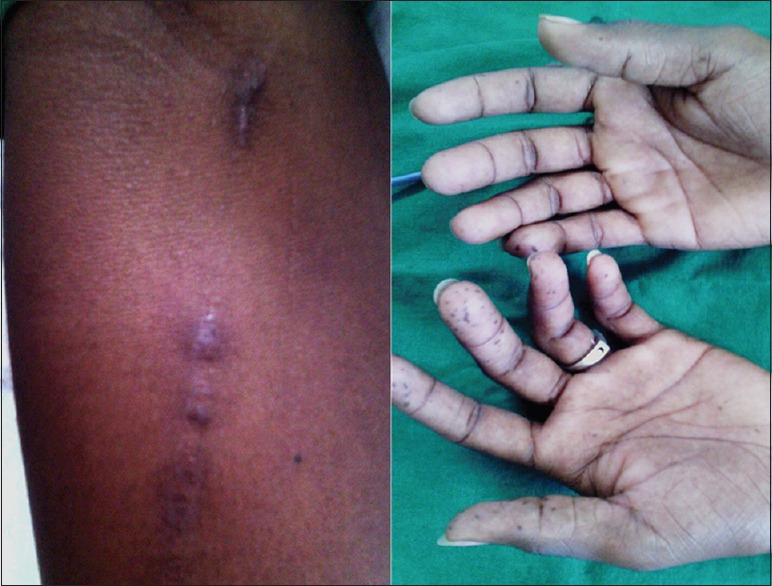
Composite image of the clinical photographs (left) of the right forearm showing “track marks,” indicating healed superficial septic thrombophlebitis and (right) both hands showing multiple bilateral distal digital pulp infarcts. Similar findings were also seen in both plantar surfaces (not shown)
Figure 2.
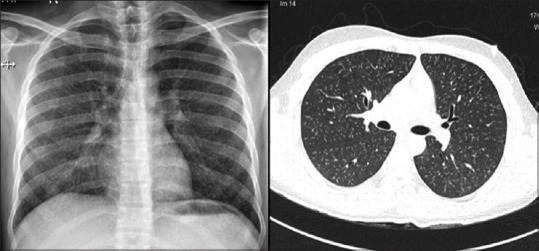
Composite image of the chest radiograph (left) showing small nodular opacities and high-resolution computed tomography of the thorax (right) showed bilateral profuse evenly distributed micronodules with tree-in-budding; pulmonary artery was normal in size, and there were no associated lymphadenopathy. Expiratory computed tomography did not show any air trapping (not shown)
DIFFERENTIAL DIAGNOSIS
Important features of this case
This HIV-negative young adult with significant smoking history, cannabis use, and intravenous drug abuse with reconstituted tablets over 2 years has breathlessness, anorexia, and weight loss with radiological findings of micronodules. There is evidence of distal embolic infarcts, but initial evaluation for endocarditis was negative. The reduced DLco, restriction on spirometry and unexpectedly low walk distance in a young individual with desaturation on walking implies extensive parenchymal and/or pulmonary vascular disease. The differential diagnosis of profuse bilateral micronodules is extensive [Table 1] but can be narrowed by careful history and analysis of HRCT findings in conjunction with the expected pulmonary complications of intravenous drug abuse [Table 2].[1] The first step is to characterize the distribution of the micronodules spatially within the lung and also the secondary pulmonary lobule. These nodules were uniform in size and were distributed evenly in a cephalocaudal direction, without any preferential lobar involvement. None of the nodules abut the pleural surface or the fissures [Figure 3]; there is clear evidence of extensive bilateral branching nodules in centrilobular distribution. There is no evidence of bronchiectasis; while no contrast was administered, in conjunction with echocardiography, there is no dilated pulmonary artery or lymphadenopathy. These findings limit the radiological differentials to causes of micronodules in a centrilobular distribution and extensive uniform “tree-in-bud” appearance. “Tree-in-bud” indicates the presence of dilated centrilobular bronchioles with lumen impacted by mucus, fluid, or pus and associated with peribronchiolar inflammation. “Tree-in-bud” indicates small airway disease and is associated with airway infection in the large majority but can also be rarely caused by arteriolar disease.[2] Its presence makes three common causes of centrilobular nodules unlikely: hypersensitivity pneumonitis, follicular bronchiolitis, and respiratory bronchiolitis.
Table 1.
Causes of diffuse micronodular disease on computed tomography

Table 2.
Pulmonary problems in intravenous drug abusers

Figure 3.
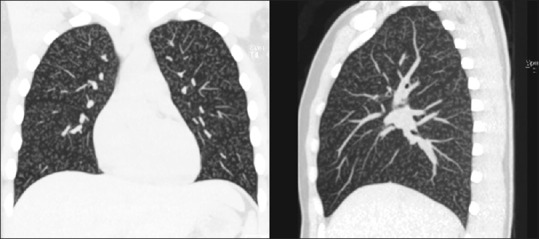
Coronal and sagittal reconstruction of the maximum intensity projection formatting of the high-resolution computed tomography images of the thorax showing diffuse, extensive, bilateral centrilobular nodules with branching and without any lobar predominance
Endobronchial tuberculosis
Mycobacterium tuberculosis is endemic in India, with more than 2.2 million new cases each year. Delayed recognition and extensive disease are related to associated substance abuse, poverty, and malnutrition, all of which were present in this patient. Extensive endobronchial spread of tuberculosis can cause extensive tree-in-bud opacities although asymmetry is usually seen.[3] Tuberculosis is also the most common cause of micronodules with a random pattern (miliary). Even though the “pretest probability” of tuberculosis is high in this patient, the marked symmetry and the absence of any cavitation or consolidation is odd; digital infarcts are unusual but could be caused by an associated immune complex phenomenon that can occur with tuberculosis. Mycobacterium avium-intracellulare infection can also cause centrilobular nodules with “tree-in-bud,” bronchiectasis, and consolidation. While it can be seen in immunocompetent patients, the classic phenotype is a middle-aged woman with slender build and without any constitutional symptoms.
Bronchopneumonia and viral bronchiolitis
Infectious bronchiolitis due to viruses, Mycoplasma, and chlamydia is a common cause of profuse and symmetric centrilobular nodules with “tree-in-bud;”[3] however, the prolonged illness and associated embolic phenomenon are unusual.
Allergic bronchopulmonary aspergillosis
Centrilobular nodules with “tree-in-bud” or miliary nodules can be caused by allergic bronchopulmonary aspergillosis but the absence of asthma, central bronchiectasis, and normal IgE levels rule out this diagnosis.[2,4]
Diffuse panbronchiolitis
Diffuse panbronchiolitis (DPB) is a rare disease of unknown cause presenting in males between the second and fifth decade with productive cough, diffuse bilateral centrilobular nodules with “tree-in-bud” and sinusitis. While reported in Indians,[5] it is extremely rare in our population but can explain all the clinical and radiological features.[6] Cold agglutinins are frequent in DPB and pulp infarcts may be due to an associated connective tissue disease. The absence of productive sputum, obstruction on spirometry, sinusitis, and air trapping on expiratory CT are unusual in DPB and necessitate further evaluation with surgical lung biopsy to establish the diagnosis.[6]
Excipient lung disease
Centrilobular nodules are usually caused by bronchiolar disease but can rarely be caused by arteriolar disease. Arteriolar causes include pulmonary edema and hemorrhage, excipient lung disease (ELD), granulomatosis with polyangiitis (GPA), plexogenic arteriopathy (PH), veno-occlusive disease (VOD), pulmonary capillary hemangiomatosis (PCH), and metastatic calcification.[2] There was no evidence of heart failure, renal failure or anemia and hemoptysis to suggest alveolar hemorrhage. The absence of pulmonary hypertension makes centrilobular nodules due to PH, VOD, and PCH very unlikely. Nodules due to GPA are larger, fewer, and associated with cavitation. Excipients are insoluble inert particulate filler materials that bind and protect the active drug during production as well as shape and lubricate tablets for easy swallowing. Excipients include talc (hydrated magnesium silicate), microcrystalline cellulose, crospovidone, and starch. Typically, excipients constitute 5% of a tablet's mass. On intravenous injection, excipient particles lodge in pulmonary arterioles and trigger an angiogranulomatous reaction; progressive disease is associated with dyspnea and pulmonary hypertension.[7] However, the presence of finger pulp infarcts, “tree-in-bud” appearance and absence of pulmonary hypertension are unusual in ELD.
CLINICAL DIAGNOSIS
In summary, the differential diagnosis of extensive centrilobular nodules with “tree-in-bud” without bronchiectasis or pulmonary hypertension in this young adult with smoking, cannabis use, and intravenous drug abuse of reconstituted tablets can be narrowed down to extensive endobronchial tuberculosis, diffuse panbronchiolitis, and ELD.
Dr. RM. PL. Ramanthan: With these clinicoradiological possibilities in mind, this patient underwent fundus examination and bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsy, with a plan for surgical biopsy if evaluation was inconclusive. Can we now have the findings?
Dr. K. Divya: Dilated fundus examination confirmed refractile particles scattered along both the retinal arteries and retinal veins [Figure 4], confirming systemic embolization of small (<5 μm) talc particles, consistent with intravenous talcosis. Inhalational talc exposure does not cause such marked retinal deposition as the inhaled particles are larger and are restricted by bronchioles and pulmonary circulation.[8,9]
Figure 4.
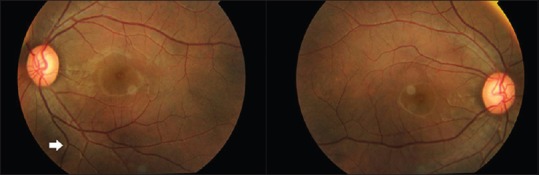
Fundus photograph of both eyes showing refractile particles scattered along both the retinal arteries and retinal veins, confirming the diagnosis of intravenous talcosis
Dr. Nidhya Ganesan: BAL was negative for acid-fast bacilli and for M. tuberculosis by Xpert MTB/RIF; occasional needle-shaped crystals were seen on cytology. The transbronchial lung biopsy specimen confirmed perivascular granulomatous reaction to talc particles [Figure 5, left] that appear as birefringent needle-shaped crystals within the granulomas under polarized light [Figure 5, right]. Review of the composition of Tydol confirmed the presence of talc as excipient. Microcrystalline cellulose appears rod like while starch has a maltese cross pattern and crospovidone is nonbirefringent on polarization.[7]
Figure 5.
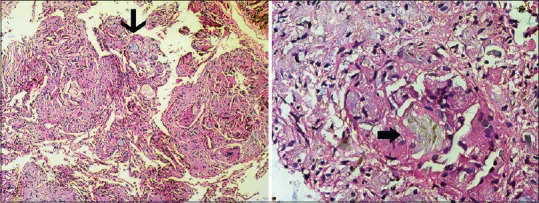
Photomicrographs of the transbronchial lung tissue biopsy specimen (left) demonstrating perivascular foreign body granulomatous reaction to the talc particles (thin arrow, H and E, ×100). Under polarized light, birefringent 20–95 μm needle-shaped crystals were seen in the granuloma, consistent with talc (block arrow, H and E, ×400)
Dr. Srinivas Rajagopala: A diagnosis of ELD (talc granulomatosis) was made. Psychiatry services were involved, and the patient underwent counseling, arrangement for family supervision, and daily-observed methadone replacement. He has successfully discontinued intravenous drug abuse and remains stable at 3 months of follow-up. Serial spirometry, DLco, and radiographs have been advised at 6 monthly intervals. Three unusual findings need an explantation; 1) The presence of “tree-in-bud” in a process that is hematogenous in origin 2)The absence of pulmonary hypertension in ELD and 3)The cause of the bilateral digital infarcts observed.
Dr. B. Devanand: Diseases involving pulmonary arterioles can cause centrilobular nodules as arterioles run in the peribronchovascular interstitium adjacent to bronchioles. Talcosis associated with intravenous abuse is most commonly associated with diffuse fine centrilobular nodules, ground-glass abnormalities or a combination of nodules and lower-lobe emphysema (Ritalin lung with methylphenidate).[9,10] The “tree-in-bud” pattern is unusual for arteriolar disease; it can be caused by intra-arteriolar accumulation of talc with associated adjacent perivascular granulomatous reaction giving the appearance of branching centrilobular nodules and has been described in a few cases of ELD earlier.[11]
Dr. Srinivas Rajagopala: What is the course of ELD? Can the disease progress despite cessation of abuse?
Dr. Kancherla Roopa: Lung fibrosis and pulmonary hypertension are related to the extent of vascular obstruction and granulomatous inflammation and are usually irreversible with talc, cellulose, and crospovidone as these cannot be mobilized; starch can be removed by metabolism and pulmonary hypertension can be reversible to a large degree. Disease progression can occur despite cessation due to ongoing granulomatous foreign-body reaction to the deposited, unmobilizable talc. The initial series of patients described all had progressive symptoms leading to death over 9 months to 5 years.[10,12] The index patient has a large load of perivascular disease and is at risk for pulmonary hypertension and disease progression despite cessation of further abuse.
Dr. Srinivas Rajagopala: How common is systemic embolization of talc given that the lung acts as an effective filter?
Dr. K. Divya: The talc that is trapped in the pulmonary arterioles is typically around 10 μm, but smaller particles can escape to the systemic circulation and be detected in the retina, spleen, and virtually every organ on autopsy.[12] Finding excipient crystals in fundoscopy is a rapid noninvasive method to confirm intravenous talcosis.
Dr. Srinivas Rajagopala: Given the presence of digital infarcts, is TEE needed to exclude IE in this patient?
Dr. G. Rajendiran: The first consideration in this patient with possible unsafe needle practices, no murmurs, weight loss, breathlessness, and digital embolic manifestations was subacute native valve IE. TTE has low sensitivity compared to TEE (46% vs. 93%) although both are highly specific (96%).[13] Embolic manifestations are seen in about half of patients with IE.[14] He was not administered any antibiotics, three sets of blood cultures were sterile, and TTE showed all valves clearly without any vegetations. TEE is restricted in our practice to high probability for IE or in whom TTE is rendered less sensitive due to obesity, lung hyperinflation, or prosthetic valves. The index patient met only 2 minor Modified Duke's criteria and was considered low risk for IE after the initial evaluation and TEE deferred.[15] Furthermore, a clear alternative explanation for the observed distal infarcts in the form of talc deposition in the distal microcirculation with thrombosis is available and corroborated by fundoscopy. While this physical finding has not been described in ELD previously, it is consistent with the distal digital embolization and subsequent microthrombosis caused by talc.
Dr. Srinivas Rajagopala: What are the options for those with progressive breathlessness despite cessation of intravenous drug abuse?
Dr. RM. PL. Ramanthan: There are previous small series of lung allografts after a careful selection process in ELD with excellent outcomes.[16] Cadaveric bilateral sequential lung transplantation is a reality in our country today, and he can be listed for the same at our center if he meets criteria and remains free of substance abuse for at least 6 months with adequate psychiatry counseling.
SUMMARY
Clinical examination findings continue to have relevance in this era of investigation-driven medicine. Excipient lung disease can result from intravenous injection of reconstituted tablets and can present with ground glass opacities, small nodules, and emphysema. Pulmonary arteriolar diseases must be considered in the differential diagnosis of centrilobular nodules on CT.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that names, identifying marks, and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Jagadeshwari, Research coordinator, and Nissy, Nurse, PSG Institute of Pulmonology, for their help in completing the paperwork for Ethics approval and collating the images for the manuscript.
REFERENCES
- 1.Marchiori E, Souza AS, Jr, Franquet T, Müller NL. Diffuse high-attenuation pulmonary abnormalities: A pattern-oriented diagnostic approach on high-resolution CT. AJR Am J Roentgenol. 2005;184:273–82. doi: 10.2214/ajr.184.1.01840273. [DOI] [PubMed] [Google Scholar]
- 2.Webb R, Muller NL, Naidich DP. High-Resolution CT of the Lung. 5th ed. Philadelphia: LWW Wolters Kluwer; 2014. HRCT Findings: Multiple nodules and nodular opacities; pp. 125–9. [Google Scholar]
- 3.Walker CM, Abbott GF, Greene RE, Shepard JA, Vummidi D, Digumarthy SR, et al. Imaging pulmonary infection: Classic signs and patterns. AJR Am J Roentgenol. 2014;202:479–92. doi: 10.2214/AJR.13.11463. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R, et al. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43:850–73. doi: 10.1111/cea.12141. [DOI] [PubMed] [Google Scholar]
- 5.Nath A, Aggarwal AN, Gupta R. Diffuse panbronchiolitis: Report of a rare disease from India. Indian J Chest Dis Allied Sci. 2010;52:43–5. [PubMed] [Google Scholar]
- 6.Poletti V, Casoni G, Chilosi M, Zompatori M. Diffuse panbronchiolitis. Eur Respir J. 2006;28:862–71. doi: 10.1183/09031936.06.00131805. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen VT, Chan ES, Chou SH, Godwin JD, Fligner CL, Schmidt RA, et al. Pulmonary effects of i.v. Injection of crushed oral tablets: “Excipient lung disease”. AJR Am J Roentgenol. 2014;203:W506–15. doi: 10.2214/AJR.14.12582. [DOI] [PubMed] [Google Scholar]
- 8.Akira M, Kozuka T, Yamamoto S, Sakatani M, Morinaga K. Inhalational talc pneumoconiosis: Radiographic and CT findings in 14 patients. AJR Am J Roentgenol. 2007;188:326–33. doi: 10.2214/AJR.05.0865. [DOI] [PubMed] [Google Scholar]
- 9.Ward S, Heyneman LE, Reittner P, Kazerooni EA, Godwin JD, Müller NL, et al. Talcosis associated with IV abuse of oral medications: CT findings. AJR Am J Roentgenol. 2000;174:789–93. doi: 10.2214/ajr.174.3.1740789. [DOI] [PubMed] [Google Scholar]
- 10.Feigin DS. Talc: Understanding its manifestations in the chest. AJR Am J Roentgenol. 1986;146:295–301. doi: 10.2214/ajr.146.2.295. [DOI] [PubMed] [Google Scholar]
- 11.Bendeck SE, Leung AN, Berry GJ, Daniel D, Ruoss SJ. Cellulose granulomatosis presenting as centrilobular nodules: CT and histologic findings. AJR Am J Roentgenol. 2001;177:1151–3. doi: 10.2214/ajr.177.5.1771151. [DOI] [PubMed] [Google Scholar]
- 12.Sieniewicz DJ, Nidecker AC. Conglomerate pulmonary disease: A form of talcosis in intravenous methadone abusers. AJR Am J Roentgenol. 1980;135:697–702. doi: 10.2214/ajr.135.4.697. [DOI] [PubMed] [Google Scholar]
- 13.Bashore TM, Cabell C, Fowler V., Jr Update on infective endocarditis. Curr Probl Cardiol. 2006;31:274–352. doi: 10.1016/j.cpcardiol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 14.McDonald JR. Acute infective endocarditis. Infect Dis Clin North Am. 2009;23:643–64. doi: 10.1016/j.idc.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 16.Weinkauf JG, Puttagunta L, Nador R, Jackson K, LaBranche K, Kapasi A, et al. Long-term outcome of lung transplantation in previous intravenous drug users with talc lung granulomatosis. Transplant Proc. 2013;45:2375–7. doi: 10.1016/j.transproceed.2012.11.004. [DOI] [PubMed] [Google Scholar]


