Abstract
Case series
Patient: Male, 14 • Female, 11
Final Diagnosis: Improvement in bowel functions after bone marrow mononuclear cells transplantation
Symptoms: Impaired bowel functions • spina bifida
Medication: —
Clinical Procedure: Bone Marrow-Derived Mononuclear Cell Transplantation
Specialty: Pediatrics and Neonatology
Objective:
Congenital defects/diseases
Background:
Bowel dysfunction is observed in 42.2–71.2% of patients with spina bifida. Traditional treatments yield limited results. The objective of this paper is to report on improvement in bowel function in 2 children with spina bifida following bone marrow-derived mononuclear cells transplantation.
Case reports:
Two patients – 14 years old and 11 years old – with bowel dysfunction after myelomeningocele repair underwent 2 BMMNC transplantations without complications. Those patients had normal defecation, assessed through follow-ups of 21 months and 16 months, respectively.
Conclusions:
BMMNC transplantation can improve bowel function, as demonstrated in 2 patients with spina bifida.
MeSH Keywords: Adult Stem Cells, Bone Marrow Transplantation, Spina Bifida Occulta
Background
Spina bifida (SB) is the most common malformation due to neural tube defects and has a prevalence ranging from 3.5 to 42.8 per 10 000 births, with geographic variation. There are 2 types of SB – SB aperta and SB occulta – with different sub-types (myeloschisis, myelomeningocele, meningocele, lipomeningocele, and spinal dorsal dermal sinus tract) [1,2].
Although children with SB have been shown to have a low fatality rate, most of them suffer from long-term functional disorders such as urinary dysfunction, bowel dysfunction, paraplegia, distress, and other problems. These dysfunctions are sequelae of primary anatomical defects of the spinal cord and its injury during pregnancy and after delivery. During pregnancy, neural tissue destruction happened as a result of the spinal cord being exposed to amniotic fluid. Spinal nerves may be further damaged while awaiting surgery or during postnatal repair of SB [3–6].
Among many associated disabilities, bowel dysfunction (constipation, fecal incontinence, or both) is observed in 42.2—71.2% of patients with SB [7,8]. Quality of life of these patients is severely affected and they often experience embarrassment, anxiety, depression, and social isolation [9].
Multiple approaches have been conducted in an attempt to improve bowel function in patients with SB, including adjustment of diet and fluid intake, oral laxatives, antegrade continence enema, transanal irrigation, digital stimulation or extraction of stool, or nerve-stimulation implants, but these treatments sometimes fail to achieve fecal continence [10–12].
Recently, stem cell transplantation (SCT) has emerged as an alternative treatment option for patients with neurologic damage, and it has resulted in promising outcomes [13–19]. Especially in patients with spinal cord injury, improvements of bowel and bladder function were observed at different levels after STC transplantation [20].
SCT was also tried for SB in animal models and humans. In animals, injection of mesenchymal stem cells into amniotic fluids can induce partial or complete coverage of experimental SB [21–24]. In humans, Gupta et al. published the first paper on BMMNC transplantation for patients with meningomyelocele in 2007; the procedure was safe, without perioperative complications [25]. However, follow-up results have not been reported, and since then, to the best of our knowledge, no other studies using stem cells for SB have been published.
The aim of this report is to present results on the improvement of bowel function after BMMNC transplantation in 2 patients with SB.
Case Report
Patient 1
A boy was born in October 2001. No abnormality was detected during prenatal ultrasound. However, a myelomeningocele located in the lumbosacral area was detected after birth, which was repaired at 10 days of age, and ventriculoperito-neal shunting was carried out at 3 months of age due to hydrocephalus. After myelomeningocele repair, the patient could not defecate or void voluntarily. Daily enema was required to evacuate stool, and clean intermittent catheterization (CIC) was applied to empty the bladder. Leakage of urine occurred frequently. The patient had never had any urge sensation of defecation or urination.
On examination at Vinmec International Hospital in July 2015:
– Uremia: 5.3 mmol/l, Creatinemia: 96 µmol/l.
– Urinary tract sonography revealed a thickened bladder wall many diverticula.
– Lumbar spine MRI: The spinal cord terminated at S2 level (Figure 1).
– Cystography showed the bladder with an abnormal shape, mildly deviated to the right side with many diverticula (Figure 2A). Rectomanometry revealed no rectal sensation (Table 1A).
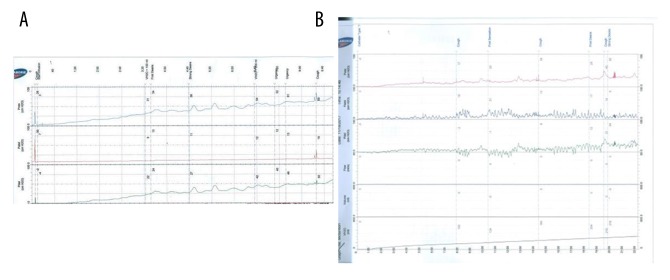
– The urodynamic investigation revealed very poor detrusor function during filling phase (Table 1B, Figure 3A).
Figure 1.
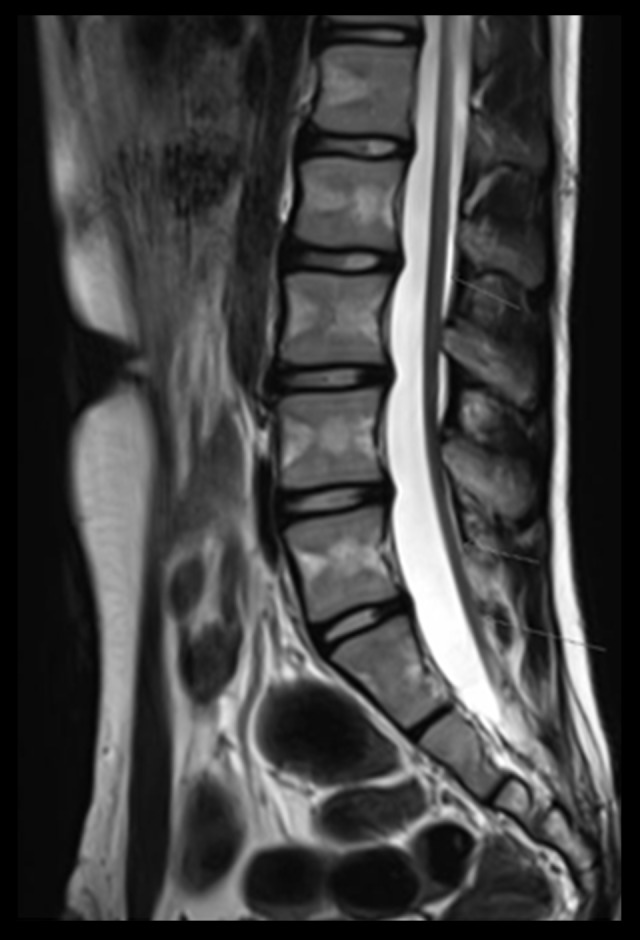
MRI shows a low termination of the spinal cord.
Figure 2.
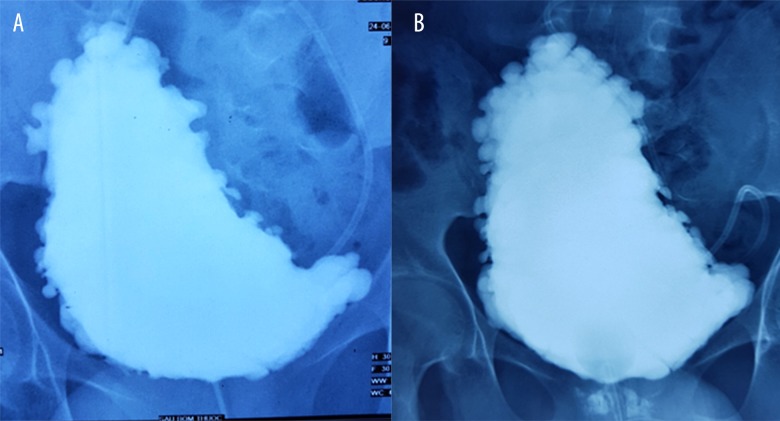
(A) Pretransplantation cystography of the first patient demonstrates many diverticula. (B) Post-transplantation cystography of the first patient still shows many diverticula.
Table 1A.
Rectal manometry before and after BMMNCs transplantations of the first patient.
| Indicator | Pre-transplantation | Post-transplantation | Normal value [26] |
|---|---|---|---|
| Rectal pressure: Resting/squeeze/strain | 9.8/9.6/24 | 10.5/14.8/23.7 | No information |
| Anal resting pressure | 16 | 44.4 | 96 mmHg |
| Anal squeeze pressure | 18 | 76.8 | 229 mmHg |
| First sensation | None | 20 ml | 22.1 ml |
| Urge | No defecation urge sensation | 60 ml | 55 ml |
| Rectoanal inhibitory reflex (RAIR) | 40 ml | 20 ml | 18.6 ml |
Table 1B.
Urodynamic investigation before and after transplantations of the first patient.
| Indicator | Pre-transplantation | Post-transplantation | Normal value [27,28] |
|---|---|---|---|
| Vesical capacity | 273 ml | 333 ml | 480–510 ml |
| First sensation of voiding | 200ml | 185 ml | 211 ml (200–330 ml) |
| Strong desire to void | >350 ml | ||
| P det max | 5 cm H2O | 15 cm H2O | 6–15 cm H2O |
| Detrusor function during filling phase | Poor detrusor contraction | Improved | Stable |
| Leakage | The leak was not noticed during filling phase |
Figure 3.
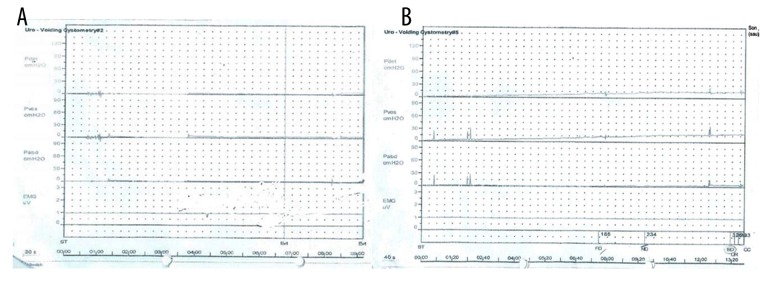
(A) Pre-transplantation urodynamic investigation of the first patient reveals a very underactive detrusor function. (B) Post-transplantation urodynamic investigation of the first patient shows increased detrusor function.
Diagnosis of bowel and bladder dysfunction was confirmed, and BMMNC transplantation was indicated after receiving approval of the hospital board and written informed consent from the parent. BMMNC transplantation performed twice with an interval of 6 months.
The first BMMNC transplantation was performed on August 10th, 2015
Bone marrow aspiration was performed through the bilateral anterior iliac crests under general anaesthesia in the operating room. We obtained 250 ml of bone marrow. The density gradient centrifugation method using Ficoll was conducted to separate and collect BMMNCs.
The total blood components and the number of BMMNCs were analyzed and calculated before and after the Ficoll separating process using a Beckman Coulter LH780. To identify the number of hematopoietic progenitor cells (CD34+) from the patient’s bone marrow after the Ficoll process, the samples were freshly prepared and incubated with Stem Kit™ Reagent (Beckman Coulter) according to the manufacturer’s protocol, followed by running through a flow cytometer (Navios Beckman Coulter, Navios software V2.0). Before transfusion, the BMMNC solutions were examined for Mycoplasma and Endotoxin using the MycoAlert™ PLUS Mycoplasma detection kit (Lonza) and Endosafe-PTS kit (Charles River), respectively.
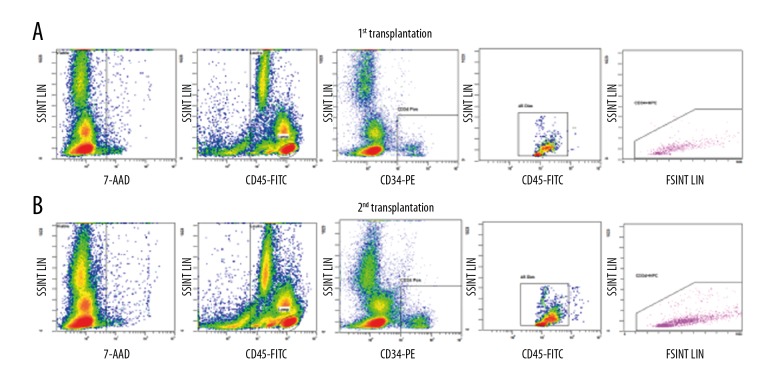
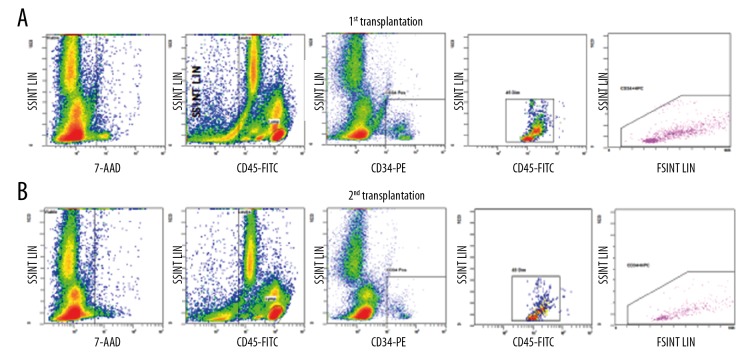
A transplant solution with a volume of 10 ml was obtained after processing, containing a total of 2.22×108 MNCs and 2.89×107 CD34 cells (Figure 4A), equal to 4.93×106 MNC/kg and 6.43×105 CD34 cells/kg, respectively, and were infused intrathecally between the 4th and 5th lumbar space. There were no adverse events during or after the procedure. The patient was discharged 2 days after the transplantation.
Figure 4.
Flow cytometry of CD34+ analysis of the first patient. Calculation of CD34+ population from the BMMNCs at the 1st transplantation (A) and 2nd transplantation (B).
Evolution after the first transplantation:
– Bowel function: At 5 months after the transplantation, the patient could feel the urge to defecate and was able to defecate once every 2–3 days.
– Bladder function: He could feel the urge to urinate and was able to urinate about 10 ml at a time.
The second stem cell transplantation was carried out on July 6th, 2017
We collected and processed 250 ml of bone marrow, as above. After processing, 10 ml of solution containing a total of 3×108 MNC and 1.69×107 CD34 cells (Figure 4B), which was approximately 6.67×106 MNC/kg and 3.76×105 CD34 cells/kg, respectively, were infused intrathecally through the 4th and 5th lumbar space without perioperative adverse events. The patient was discharged 2 days after the transplantation.
Follow-up at 21 months after the first transplantation
– Bowel function: The patient was able to defecate once every 1D2 days without soiling or fecal incontinence. Rectal sensation was observed on rectomanometry (Table 1A).
– Bladder function: Increased detrusor contractility was observed on urodynamic investigation (Table 1B, Figure 3B).
He could feel the urge to urination and was able to urinate voluntarily about 40 ml at a time; however, his bladder did not entirely empty, so CIC was still required. Many diverticula still appeared on cystography (Figure 2B).
Patient 2
A girl was born in January 2005. A small mass located in the lumbar coccygeal area was detected after birth. However, no investigation was carried out. At 9 months old, she could not defecate spontaneously. Urinary infections occurred repeatedly.
MRI revealed SB with a small myelomeningocele located at L5 and S1. Daily colon enemas were required to evacuate stool. The operation to repair the myelomeningocele was performed at 28 months old. After the operation, she was still unable to defecate spontaneously and dysuria occurred. A daily colon enema and CIC were required. The child never experienced an urge to defecate or to urinate.
On examination at Vinmec International Hospital in May 2016:
– Uremia: 3.07 mmol/l, Creatininemia: 60 µmol/L.
– Abdominal ultrasound showed a left dilated ureter and irregular bladder wall with many diverticula.
– Vertebral MRI: The spinal cord terminated at S3–S4 level (Figure 5).
– Cystography showed an irregular thickness of the bladder wall, with multiple pseudodiverticula, especially in the bladder fundus region (Figure 6A).
– Rectomanometry: The first rectal sensation was perceived at 40 ml and constant sensation at 100 ml (Table 2A).
– The urodynamic investigation showed a high vesical pressure and P det (Table 2B, Figure 7A).
Figure 5.
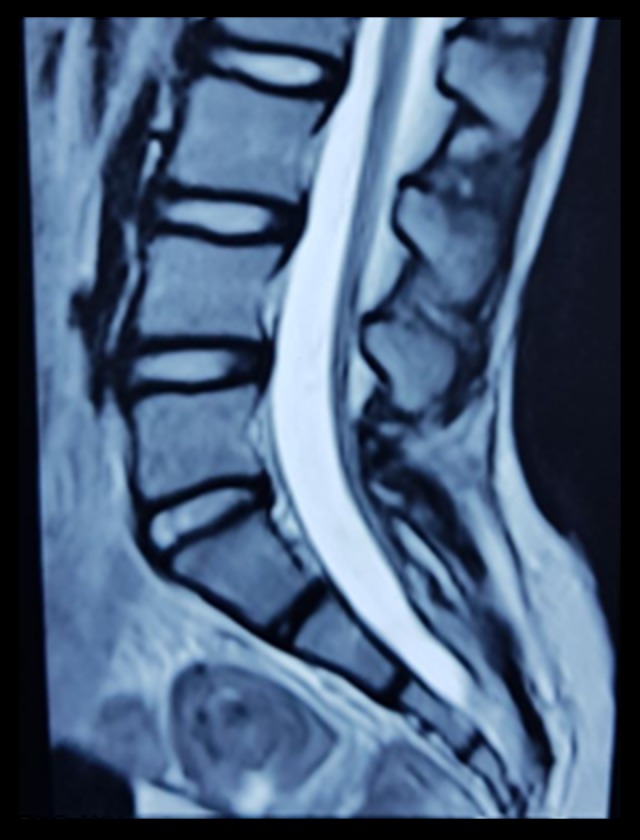
Spinal MRI of the second patient demonstrates a low termination of the spinal cord.
Figure 6.
(A) Pre-transplantation cystography of the second patient shows multiple diverticula. (B) Post-transplantation cystography of the second patients still shows multiple diverticula.
Table 2A.
Rectal manometry before and after BMMNCs transplantations of the second patient.
| Indicator | Pre-transplantation | Post-transplantation | Normal value [26] |
|---|---|---|---|
| Rectal pressure: Resting/squeeze/strain | 5/6/15 mmHg | 3/12/23 mmHg | No information |
| Anal resting pressure | 18 | 47 | 94 mmHg |
| Anal squeeze pressure | 26 | 67 | 206 mmHg |
| First sensation | 40 ml | 20 ml | 14.7 ml |
| Urge | No defecation urge sensation | 80 ml | 36.3 ml |
| Rectoanal inhibitory reflex (RAIR) | 20 ml | 10 ml | 14.7 ml |
Table 2B.
Urodynamic investigation before and after transplantations of the second patient.
| Indicator | Pre-transplantation | Post-transplantation | Normal value [27,28] |
|---|---|---|---|
| Vesical capacity | 230 ml | 246 ml | 360–400 ml |
| First sensation of voiding | 105 ml | 124 ml | No information |
| Strong desire to void | 140 ml | 219 ml | No information |
| P det max | 50 cm H2O | 40 cm H2O | <20 cm H2O |
| Detrusor function during filling phase | Overactive | Improved | Stable |
Figure 7.
(A) Pre-transplantation urodynamic investigation of the second patient shows hyperactive detrusor functions. (B) Post-transplantation urodynamic investigation of the second patient reveals reduced hyperactive detrusor function.
The diagnosis of bowel and bladder dysfunction was confirmed. The first BMMNC transplantation was performed on July 29th, 2016 with the approval of the Hospital’s Board of Directors and written informed consent from her parent.
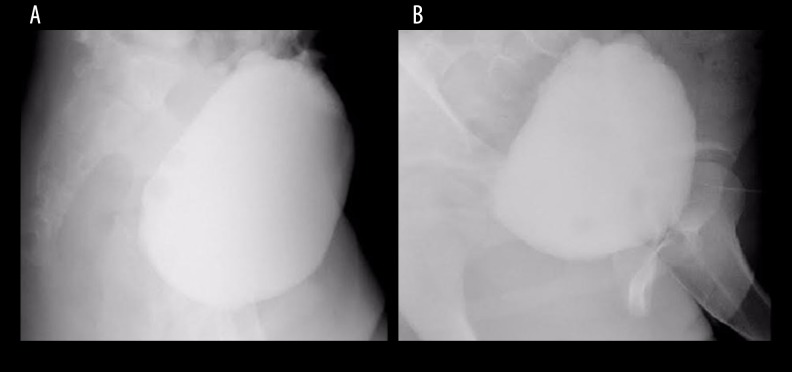
Similar BMMNC volume, preparation, and quality control were applied as described in the first patient. A 10-ml solution containing 5.2×108 MNCs and 3.65×107 CD34 cells in total (1.68×107 MNC/kg and 1.18×105 CD34 cells/kg, respectively) were infused intrathecally through the vertebral space between the 4th and 5th space (Figure 8A).
Figure 8.
Flow cytometry of CD34+ analysis of the second patient. Calculation of the CD34+ population from the BMMNCs at the 1st transplantation (A) and 2nd transplantation (B).
Evolution after the first transplantation
– Bowel function: At 4 months after the transplantation, the patient experienced urge sensation of defecation and was able to defecate voluntarily 1–2 times per day without laxatives.
– Bladder function: The patient developed urinary urge and was able to urinate spontaneously at a small volume; however, her bladder could not be emptied completely, so CIC was still needed.
The 2nd stem cell transplantation was carried out on March 24th, 2017
Similar BMMNCs volume, preparation and quality control were applied as described previously. A 10-ml solution containing 6.4×108 MNCs and 1.12×107 CD34 cells (approximately 1.78×107 MNC/kg and 3.1×105 CD34 cells/kg, respectively) were infused intrathecally through the vertebral space between the 4th and 5th space without adverse events (Figure 8B). The patient was discharged 2 days after the transplantation.
Follow-up 16 months after the first transplantation:
– Bowel function: The patient was able to defecate spontaneously 1–2 times per day without soiling or fecal incontinence. Rectal sensation was improved. The first sensation was perceived at 20 ml and constant sensation at 60 ml (Table 2A).
– Bladder function: The patient had urinary urge and was able to urinate a small amount, but CIC was still required to empty her bladder completely. Urodynamic analysis showed decreased vesical pressure and P det (Table 2B, Figure 7B). Multiple diverticula were still observed on cystography (Figure 6B).
Discussion
To the best of our knowledge, this is the first report showing improvement of bowel function in patients with SB after BMMNC transplantation. Our results reveal that intrathecal stem cell transplantation was safe and effective for 2 patients with SB. There were no intraoperative or postoperative complications. Bowel function was remarkably improved after BMMNC transplantation. These 2 patients could feel the urge to defecation and pass stool voluntarily at 5 months and, 4 months, respectively, after the first transplantation. At follow-up 21 months and 16 months, respectively, after the first transplantation, the patients achieved normal defecation. Oral laxatives or other supportive measures were not required to evacuate stools.
Improvement of bladder function was not significant compared to that of bowel function in our patients after BMMNC transplantations. Although urodynamic investigation showed some improvements in the 2 patients and they could feel the urge to urinate and could spontaneously void a small amount of urine, their bladders could not be emptied completely.
The failure to achieve urinary continence may have been due to performing BMMNC transplantation at the late stage, when the bladder already has suffered from severe fibrosis and has poor function. To determine whether better bladder function could be achieved through earlier STC transplantation, additional studies with larger patient numbers and longer follow-up need to be performed and evaluated.
The mechanism by which stem cells improve bowel and bladder function in SB patients is still poorly understood. However, there are similarities between spinal cord injury in patients with SB and in patients with spinal cord injury due to trauma. The improvement of bowel and bladder function in our 2 patients could be explained based on knowledge of STC transplantation in patients with spinal cord injury.
In animals with spinal cord injury, transplantation of bone marrow-derived cells or bone marrow mesenchymal stem cells can produce myelin repair, improvement of degeneration of axons, an increase of axonal regrowth, promotion of neurite outgrowth of spinal neurons, and secretion of neurotrophic factors [29]. Evidence of improved bladder function has been reported in 2 studies, which illustrated the potential mechanism of SCT in spinal cord injury via inhibition of unmediated C-fiber sprouting from bladder afferents. These effects contributed to the decreases of C-fiber formation in bladders, directly controlled by bladder-to-bladder spinal micturition reflex [30,31]. In patients with spinal cord injury, Jiang et al. showed improved bowel function at different levels in 9 of 12 cases (60%), and improved urinary bladder function in 8 of 10 patients (80%) after stem cell transplantation [20].
Conclusions
In conclusion, BMMNC transplantation significantly improved defecation function in 2 patients with SB. A study with a more patients and longer follow-up is required to confirm the efficacy of this treatment.
Footnotes
Statement
All laboratory equipment and stem cell preparations were provided by Vinmec Research Institute of Stem Cell and Gene Technology. Clinical standard operations and drugs were performed and provided by medical doctors and staff at Vinmec International Hospital.
Conflict of Interests
The authors have declared that no competing interests exist.
References:
- 1.Zaganjor I, Sekkarie A, Tsang BL, et al. Describing the prevalence of neural tube defects worldwide: A systematic literature review. PLoS One. 2016;11:e151586. doi: 10.1371/journal.pone.0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 3.Meuli M, Meuli-Simmen C, Yingling CD, et al. Creation of myelomeningocele in utero: A model of functional damage from spinal cord exposure in fetal sheep. J Pediatr Surg. 1995;30:1028–33. doi: 10.1016/0022-3468(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 4.Müslüman AM, Karşıdağ S, DÖ Sucu, et al. Clinical outcomes of myelomeningocele defect closure over 10 years. J Clin Neurosci. 2012;9(7):984–90. doi: 10.1016/j.jocn.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Perry VL, Albright AL, Adelson PD. Operative nuances of myelomeningocele closure. Neurosurgery. 2002;51(3):719–23. [PubMed] [Google Scholar]
- 6.Mattogno PP, Massimi L, Tamburrini G, et al. Myelomeningocele repair: Surgical management based on a 30-year experience. Acta Neurochir Suppl. 2017;124:143–48. doi: 10.1007/978-3-319-39546-3_22. [DOI] [PubMed] [Google Scholar]
- 7.Choi EK, Im YJ, Han SW. Bowel management and quality of life in children with spina bifida in South Korea. Gastroenterol Nurs. 2017;40(3):208–15. doi: 10.1097/SGA.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli A, Lacobelli BD, Silveri M, et al. Colorectal dysfunction and faecal incontinence in children with spina bifida. Br J Urol. 1988;81(Suppl. 3):117–19. doi: 10.1046/j.1464-410x.1998.00026.x. [DOI] [PubMed] [Google Scholar]
- 9.Lemelle JL, Guillemin F, Aubert D, et al. Quality of life and continence in patients with spina bifida. Qual Life Res. 2006;15(9):1481–92. doi: 10.1007/s11136-006-0032-x. [DOI] [PubMed] [Google Scholar]
- 10.Choi EK, Im YJ, Han SW, et al. Long-term outcome of transanal irrigation for children with spina bifida. Spinal Cord. 2015;53:216–20. doi: 10.1038/sc.2014.234. [DOI] [PubMed] [Google Scholar]
- 11.Vande Velde S, Van Biervliet S, Van Renterghem K, et al. Achieving fecal continence in patients with spina bifida: A descriptive cohort study. J Urol. 2007;178:2640–44. doi: 10.1016/j.juro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Lansen-Koch SM, Govaert B, Oerlemans D, et al. Sacral nerve modulation for defaecation and micturition disorders in patients with spina bifida. Colorectal Dis. 2012;14(4):508–14. doi: 10.1111/j.1463-1318.2011.02678.x. [DOI] [PubMed] [Google Scholar]
- 13.Liem NT, Anh TN, Chinh VD, et al. Outcomes of autologous bone marrow mononuclear cells for cerebral palsy: An open-label uncontrolled clinical trial. BMC Pediatr. 2017;17:104. doi: 10.1186/s12887-017-0859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abi Chahine NH, Wehbe TW, Hilal RA, et al. Treatment of cerebral palsy with stem cells: A report of 17 cases. Int J Stem Cells. 2016;9(1):90–95. doi: 10.15283/ijsc.2016.9.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 16.Park HS, Park HC, Shim YS, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913–22. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 17.Callera F, do Nascimento RX. Delivery of autologous bone marrow precursor cells into the spinal cord via lumbar puncture technique in patients with spinal cord injury: A preliminary safety study. Exp Hematol. 2006;34:130–31. doi: 10.1016/j.exphem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Sykova’ E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675–87. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 19.Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical train. Stem Cells. 2007;25:2066–73. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- 20.Jiang PC, Xiong WP, Wang G, et al. A clinical report of autologous bone marrow-derived mesenchymal stem cell transplantation in patients with spinal cord injury. Exp Ther Med. 2013;6:140–46. doi: 10.3892/etm.2013.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Gao F, Ma L, et al. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat foetuses with spina bifida aperta. J Cell Mol Med. 2012;16(7):1606–17. doi: 10.1111/j.1582-4934.2011.01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dionigi B, Ahmed A, Brazzo J, 3rd, et al. Partial or complete coverage of experimental spina bifida by simple intra-amniotic injection of concentrated amniotic mesenchymal stem cells. J Pediatr Surg. 2015;50(1):69–73. doi: 10.1016/j.jpedsurg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Yuan Z, Wei X, et al. Application potential of bone marrow mesenchymal stem cell (BMSCs) based tissue-engineering for spinal cord defect repair in rat fetuses with spina bifida aperta. J Mater Sci Mater Med. 2016;27(4):77. doi: 10.1007/s10856-016-5684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng C, Graham CD, Connors JP, et al. A comparison between placental and amniotic mesenchymal stem cells for transamniotic stem cell therapy (TRASCET) in experimental spina bifida. J Pediatr Surg. 2016;51(6):1010–13. doi: 10.1016/j.jpedsurg.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 25.Gupta DK, Sharma S, Venugopal P, et al. Stem cells as a therapeutic modality in pediatric malformations. Transplant Proc. 2007;39(3):700–2. doi: 10.1016/j.transproceed.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 26.Banasiuk M, Banaszkiewicz A, Dziekiewicz M, et al. Values from three-dimensional high-resolution anorectal manometry analysis of children without lower gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2016;14(7):993–1000. doi: 10.1016/j.cgh.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Wahl EF, Churchill BM. Detrusor pressure rise in a normal bladder. BJU Int. 2004;94:901–2. doi: 10.1111/j.1464-410X.2004.05056.x. [DOI] [PubMed] [Google Scholar]
- 28.Mahfouz W, Al Afraa T, Campeau L, et al. Normal urodynamic parameters in women: Part II – invasive urodynamics. Int Urogynecol J. 2012;23:267–77. doi: 10.1007/s00192-011-1585-y. [DOI] [PubMed] [Google Scholar]
- 29.Gu W, Zhang F, Xue Q, et al. Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology. 2010;30:205–17. doi: 10.1111/j.1440-1789.2009.01063.x. [DOI] [PubMed] [Google Scholar]
- 30.Park WB, Kim SY, Lee SH, et al. The effect of mesenchymal stem cell transplantation on the recovery of bladder and hindlimb function after spinal cord contusion in rats. BMC Neurosci. 2010;11:119–30. doi: 10.1186/1471-2202-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsui T, Kakizaki H, Tanaka H, et al. Immortalized neural stem cells transplanted into the injured spinal cord promote recovery of voiding function in the rat. J Urol. 2013;170:1421–25. doi: 10.1097/01.ju.0000075501.05758.33. [DOI] [PubMed] [Google Scholar]