Abstract
Low-grade gliomas cause considerable morbidity and most will recur after initial therapy. At recurrence, low-grade gliomas can undergo transformation to high-grade gliomas (grade III or grade IV), which are associated with worse prognosis. Temozolomide (TMZ) provides survival benefit in patients with glioblastomas, but its value in patients with low-grade gliomas is less clear. A subset of TMZ-treated, isocitrate dehydrogenase‒mutant, low-grade astrocytomas recur as more malignant tumors with thousands of de novo, coding mutations bearing a signature of TMZ-induced hypermutation. Preliminary studies raise the hypothesis that TMZ-induced hypermutation may contribute to malignant transformation, although with highly variable latency. On the other hand, hypermutated gliomas have radically altered genomes that present new opportunities for therapeutic intervention. In light of these findings and the immunotherapy clinical trials they inspired, how do patients and providers approach the risks and benefits of TMZ therapy? This review discusses what is known about the mechanisms and consequences of TMZ-induced hypermutation and outstanding questions regarding its clinical significance.
Keywords: hypermutation, low-grade gliomas, temozolomide
Introduction
Disruption of DNA repair pathways increases mutagenesis and genomic instability, thereby promoting cancer progression and resistance to therapies.1,2 Isocitrate dehydrogenase (IDH)–mutant, low-grade astrocytomas treated with TMZ can recur as more malignant tumors with DNA mismatch repair (MMR) defects and a hypermutator phenotype,3–5 as observed in temozolomide (TMZ)-treated glioblastoma (GBM) patients.4–8 TMZ-induced hypermutation is defined by a dramatic increase in the mutation rate and a TMZ-associated mutational signature in posttreatment recurrences. In order to present the current knowledge of TMZ-induced hypermutation in gliomas, this review first provides relevant background on the new molecular classification of gliomas, TMZ clinical trial results, and mechanisms of TMZ action in relation to DNA repair and the genesis of hypermutated clones. The review focuses on TMZ-induced hypermutation in IDH-mutant gliomas. At the conclusion of the review, we discuss immune checkpoint inhibitors as a new experimental therapy for patients with hypermutated gliomas.
The 2016 World Health Organization Molecular Classification of Gliomas
Objective classification of gliomas is critical for understanding which gliomas may undergo TMZ-associated hypermutation, and how this event might influence therapeutic response and survival. In 2016, the guidelines for the World Health Organization (WHO) classification of gliomas were revised from the 2007 guidelines to incorporate molecular parameters.9 By molecular classification, grade II and grade III astrocytic tumors are further stratified into IDH-mutant (most of grade II and grade III) and IDH-wildtype groups. The majority of grade II and grade III astrocytic tumors falls into the IDH-mutant group and shows characteristic mutation of TP53 and loss of alpha thalassemia/mental retardation syndrome X-linked (ATRX) staining. Of note, current WHO grading poorly distinguishes prognoses for grades II and III IDH-mutant astrocytomas.10 Diagnostic criteria for grade II or III oligodendrogliomas includes both IDH mutation status and combined whole chromosome arm losses of 1p and 19q (1p/19q codeletion). The diagnoses of oligoastrocytoma and anaplastic oligoastrocytoma are now strongly discouraged.9
IDH mutation status and 1p/19q codeletion have prognostic value for gliomas. The Cancer Genome Atlas (TCGA) network performed genome-wide analyses of 293 grades II and III gliomas from adults.11 Three molecular subgroups of WHO grade II and grade III gliomas emerged which correlated with clinical outcomes, recurrence, and survival: (i) IDH-mutant gliomas with 1p/19q codeletion, (ii) IDH-mutant gliomas without 1p/19q codeletion, and (iii) IDH-wildtype gliomas without 1p/19q codeletion. The median survival for patients with mutant IDH plus 1p/19q codeletion, mutant IDH without 1p/19q codeletion, and IDH-wildtype gliomas without 1p/19q codeletion are 8, 6.3, and 1.7 years, respectively.11 Another study looking at 1087 grade II–IV gliomas demonstrated that nearly all tumors could be placed in one of 5 molecular subgroups on the basis of 3 markers: IDH mutation, 1p/19q codeletion, and telomerase reverse transcriptase (TERT) promoter mutations.12 Among patients with grade II or III gliomas, the molecular groups were independently associated with overall survival (OS).12 Given their longer OS, the mutagenic consequences of TMZ are particularly relevant in the low-grade glioma subgroups.
Grade IV GBMs are further stratified into IDH-wildtype (90%) or IDH-mutant (10%) molecular subtypes. IDH-wildtype GBMs are also known as primary GBMs that arise de novo. IDH-mutant GBMs, or secondary GBMs, may arise from a low-grade diffuse astrocytoma or anaplastic astrocytoma.13,14 IDH-mutant GBMs have high rates of ATRX mutations (~80%), which are less common in IDH-wildtype GBMs (7%); rather, up to 83% of primary GBMs have frequent TERT promoter mutations.15–17 Patients with IDH-wildtype GBMs have a median OS of 15 months, whereas patients with IDH-mutant GBMs have a median survival of 31 months.18 TMZ, given concurrently with radiation postoperatively and adjuvantly (the Stupp regimen), increases OS in GBMs and is therefore the standard of care.19
Malignant Transformation of Low-Grade Gliomas
As a group, low-grade gliomas are histologically and biologically heterogeneous and are associated with marked diversity in survival times. Although some patients may survive for more than a decade, most tumors recur. At recurrence, a tumor can undergo malignant transformation to a high-grade glioma (grade III or IV), which is associated with worse prognosis.20 On MRI, malignant transformation correlates with the development of focal contrast enhancement, which is often used as a radiographic surrogate of malignant transformation.21
The interval between initial presentation and malignant transformation is highly variable, with a reported incidence ranging from 17% to 73% and median interval after initial resection ranging from 2.1 to 10.1 years.20 Known risk factors for malignant transformation of an initial low-grade glioma include larger preoperative tumor volume, higher tumor growth rate, and decreased extent of surgical resection.22,23 Genetic alterations associated with malignant transformation in IDH-mutant gliomas include the acquisition of genetic alterations in the retinoblastoma (RB) and Akt‒mammalian target of rapamycin (mTOR) pathways, activation of the MYC and RTK-RAS‒phosphatidylinositol-3 kinase (PI3K) pathways, upregulation of the Forkhead box M1 (FOXM1)- and E2F2-mediated cell cycle transitions, and epigenetic silencing of developmental transcription factors.3,24 In a study of 204 patients with grade II gliomas treated prospectively on North Central Cancer Treatment Group clinical trials, malignant transformation occurred in 70% of low-grade astrocytomas compared with 45% in oligodendrogliomas.25 Of note, this study was performed prior to molecular subtyping of gliomas and thus the proportion of malignant transformation in the corresponding molecularly defined groups may differ.
The Role of Chemotherapy in the Management of Gliomas
The role of TMZ treatment in patients with high-grade gliomas is well established. In general, the treatment paradigm is maximal safe surgical resection followed by adjuvant chemoradiation. The landmark European Organisation for Research and Treatment of Cancer–National Cancer Institute of Canada (EORTC-NCIC) study established that postoperative radiotherapy with concurrent and adjuvant TMZ improved survival in patients with GBM compared with adjuvant radiotherapy alone, and a subsequent study demonstrated improved survival with adjuvant TMZ even for elderly patients receiving adjuvant hypofractionated radiotherapy.26,27 Studies in anaplastic oligodendrogliomas have demonstrated a benefit with the adjuvant procarbazine, lomustine (CCNU), and vincristine (PCV) chemotherapy regimen.28,29 Recently, it has been shown that adjuvant TMZ is associated with a survival benefit in patients with 1p/19q intact anaplastic tumors (the Concurrent and/or Adjuvant TMZ for 1p/19q Nondeleted Tumors [CATNON] trial).30 The Radiation Therapy Oncology Group (RTOG) 9813 phase III study of radiotherapy with TMZ versus nitrosourea in adults with anaplastic astrocytoma, showed no significant difference in survival between the 2 treatment arms, but TMZ was better tolerated; 1p/19q status was not reported, but IDH1 mutation was found to be prognostic for OS.31 An ongoing study (CODEL) is directly comparing radiation plus concurrent and adjuvant TMZ versus radiation plus adjuvant PCV in patients with 1p/19q codeleted, high-risk, grade II and III gliomas.32 Thus, TMZ in combination with adjuvant radiotherapy constitutes standard of care for all WHO grade IV and 1p/19q intact WHO grade III gliomas.
The clinical management of low-grade gliomas remains controversial but is evolving rapidly. After resection, treatment options include observation, adjuvant radiation alone, chemotherapy alone, or radiation plus chemotherapy. Patients with low-grade glioma are typically young and have longer expected survival compared with patients with high-grade gliomas. Thus, treatment-related toxicities and quality of life are important clinical considerations. Early studies demonstrated that adjuvant radiotherapy improved progression-free survival (PFS) compared with observation for patients with low-grade gliomas, but that salvage radiotherapy at the time of progression yielded equivalent OS.33 These results led many to advocate for initial observation after surgical resection in an effort to delay adjuvant radiotherapy and associated risk of long-term neurocognitive decline. More recent work has focused on tailoring adjuvant treatment based on individual risk factors, with the RTOG 9802 study demonstrating improved PFS and OS with the addition of adjuvant PCV to radiotherapy versus radiotherapy alone for patients with high-risk low-grade gliomas.34 Although IDH mutation status was assessed post-hoc in a subset of patients, 1p/19q status was not reported, so the differences between IDH-mutated subtypes are unknown. Whether TMZ can be substituted for PCV chemotherapy remains unproven. Furthermore, it is not clear how TMZ and PCV compare in the induction of hypermutation. In addition to RTOG 9813, RTOG 0424, a phase II study of radiation with concurrent and adjuvant TMZ, demonstrated promising results; many practitioners have extrapolated positive results with TMZ in malignant gliomas to the low-grade setting, given the favorable toxicity profile of TMZ.31,35 The results of the CODEL study should determine whether TMZ is an appropriate substitute for PCV.
While RTOG 9802 clearly established a role for adjuvant chemotherapy in patients with low-grade gliomas, the study did not address the underlying controversy regarding the timing of radiation and chemotherapy. Appropriately selected patients could be considered for adjuvant chemotherapy alone, with combination chemoradiotherapy reserved for the time of progression, but it remains to be seen if this strategy yields survival results comparable to those observed in RTOG 9802. A recently published phase II study examined the utility of adjuvant TMZ alone in patients with high-risk low-grade gliomas, and found that TMZ yielded a high rate of radiographic stability and meaningfully delayed the receipt of radiotherapy, with most patients on the study not having received radiation with a median follow-up of almost 6 years.36 Long-term survival was comparable to that of patients enrolled on RTOG 9802 receiving adjuvant radiation alone, but patients on the study were not salvaged with combined chemoradiotherapy, as would currently be considered standard of care. In an unplanned subgroup analysis, the study demonstrated particularly favorable results for patients with 1p/19q codeletion and limited residual disease after surgical resection, suggesting that a selected subgroup of patients may be appropriate candidates for adjuvant TMZ alone. It is also important to note that malignant transformation was observed in 33 of 55 patients on the study who underwent surgical resection for progression after therapy, similar to previously observed rates of malignant transformation.22,33,37 Thus, the appropriate role for adjuvant therapies in the management of low-grade gliomas remains controversial. Future adjuvant treatment decisions for low-grade gliomas are likely to be based on an individual patient’s risk profile, incorporating clinical, molecular, and radiographic features, and assuming biomarkers can be identified, an assessment of the risk of hypermutation.
The Cytotoxic Mechanism of TMZ
The mechanism of TMZ-induced cytotoxicity and mutagenicity is well studied. TMZ is a lipophilic, monofunctional alkylating agent that is administered orally and is well tolerated, with main toxicities of mild nausea, vomiting, and dose limiting myelosuppression.38 TMZ is a prodrug that is absorbed intact at acidic pH, allowing oral administration, but rapidly decomposes at pH >7 to form monomethyl triazene 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC). MTIC further reacts with water to form 5-aminoimidazole-4-carboxamide and the methyldiazonium cation. The methyldiazonium cation then preferentially adds methyl groups to DNA at N7 positions of guanine in guanine-rich regions (N7-MeG), N3 adenine (N3-MeA), and O6 guanine residues (O6-meG).39,40
Although N7-MeG is the major DNA adduct induced by TMZ, the cytotoxicity and mutagenicity is primarily attributed to the O6-meG lesion.40 O6-meG lesions are directly repaired in cells by the suicide repair protein, O6-methylguanine DNA methyltransferase (MGMT), which removes the methyl adduct in a one-step alkyl transfer reaction that transfers the alkyl group from the oxygen in the DNA to the sulfur of a cysteine residue in the catalytic pocket of MGMT, thereby restoring guanine and inactivating MGMT. A single MGMT molecule can repair only one alkyl adduct, therefore the repair of O6-meG adducts is dependent on the number of MGMT molecules per cell and on the rate of MGMT regeneration.41
Unrepaired O6-meG DNA adducts are cytotoxic. The methylation of the O6 position of guanine changes the normal hydrogen bonding of guanine with cytosine resulting in mispairing of O6-meG with thymine during DNA replication.40 The DNA MMR machinery recognizes the mispairing of O6-meG with thymine and repairs the daughter strand but leaves behind the O6-meG in the template strand. This leads to repeated attempts by the MMR pathway to repair the same mismatched base in a process called futile cycling. Futile cycling occurs in the context of DNA replication and results in replication-associated DNA double-strand breaks, which are repaired by homologous recombination; unrepaired DNA double-strand breaks result in cell death.42 Thus, cytotoxicity from TMZ is dependent on an intact MMR pathway and low levels of MGMT.
Indeed, in GBM patients, epigenetic silencing of the MGMT gene by promoter methylation is associated with longer OS when patients were treated with alkylating chemotherapy (either carmustine or TMZ) and radiation.43,44 A comprehensive characterization by a TCGA consortium of more than 500 GBMs showed that the MGMT promoter methylation status distinguished TMZ responders from nonresponders in the classical subtype of GBM.45 Methylation of MGMT is associated with longer survival in patients with GBM who receive TMZ.46 In agreement with these results, high MGMT protein staining in GBM patient samples is associated with TMZ resistance.47 In the phase III EORTC-NCIC trial, the addition of concomitant and adjuvant TMZ to adjuvant radiotherapy improved survival in GBM patients compared with adjuvant radiotherapy alone; MGMT promoter methylation was the strongest predictor for outcome and benefit from TMZ.26 Yet, even for gliomas with unmethylated MGMT promoters, for which TMZ is less effective, clinical data showed a slight trend toward survival benefit with adjuvant TMZ.46 As a result, TMZ is often also given to patients with high-grade glioma with unmethylated MGMT.46
Mechanism of TMZ-Induced Hypermutation
Alkylating agents, such as TMZ and procarbazine, are also mutagenic, due to the O6-meG adducts they induce.40,48 TMZ, like other alkylating agents, is a known carcinogen, with case reports linking TMZ use to the development of acute myelogenous leukemia and acute lymphoblastic leukemia.49,50 The stoichiometric limitation of MGMT-mediated repair of O6-meG adducts renders MMR pathway status a critical determinant of whether tumor cells die or exhibit resistance in response to alkylating agents. In the context of cellular MGMT deficiency, futile cycling by intact MMR causes cell death, whereas loss of MMR function can lead to the mispairing of guanine with thymine.47 Resistance to TMZ can arise in cells deficient in MMR, as unrepaired DNA damage can no longer lead to cell death in this context.51,52
In a radiographic study of untreated low-grade gliomas before and after TMZ, tumor growth was slower among untreated 1p/19q codeleted tumors compared with untreated non-codeleted tumors (3.4 vs 5.9 mm/y). Tumor growth was also slower among untreated tumors that do not overexpress p53, measured by immunohistochemistry, compared with untreated tumors overexpressing p53 (4.2 vs 6.3 mm/y).53 After the administration of TMZ, the growth of both astrocytoma and oligodendroglioma subtypes were slowed. However, the duration of the effect was shorter in patients whose tumors overexpressed p53 (an immunohistochemical result that often but not always reflects TP53 mutation)54 and did not harbor 1p/19q codeletion, suggesting a more accelerated acquisition of TMZ resistance in astrocytomas versus oligodendrogliomas and a potential relationship between TP53 mutation and glioma cell response to TMZ.53
In the absence of MGMT-mediated repair and intact MMR, cells may incur a large number of G:C>A:T transitions throughout the genome upon DNA replication, leading to TMZ resistance and a “hypermutator phenotype” in recurrent tumors.6,7 Spontaneous deamination of 5-methylcytosine to uracil can also cause G:C>A:T transitions, though at much lower rates and mainly at cytosine-phosphate-guanine (CpG) dinucleotides.55 Therefore, hypermutation is defined not only by the abundance of mutations, but also by a strong signature of G:C>A:T transitions at CpG and non-CpG sites.
The first report of hypermutation induced by alkylating agents in human GBMs was a targeted sequencing study of 518 protein kinases across 9 patients.5 Consistent with a lack of MGMT-mediated repair, 2 patients with TMZ-treated, recurrent tumors with inactivating mutations in the MMR pathway gene MSH6 had an increase in G:C>A:T transitions at non-CpG dinucleotides. The TCGA consortium also found 7 hypermutated recurrences from patients treated with TMZ or CCNU, which chloroethylates DNA. All hypermutated recurrences showed MGMT promoter methylation and higher proportions of non-CpG site G:C>A:T transitions.4 MMR mutations in patients with MGMT promoter methylation were exclusively G:C>A:T transitions at non-CpG sites, suggesting that MMR inactivation and hypermutation were results of MGMT promoter methylation and chemotherapy-induced mutagenesis rather than spontaneous deamination. In pairs of primary and recurrent GBMs from 80 patients who were treated with radiation and TMZ, reduced expression of MMR proteins, but not changes in MGMT promoter methylation, was characteristic of GBMs recurring after standard of care treatment.56 Hypermutation was not assessed in this study, however. A recent comprehensive analysis of hypermutation across >81000 adult and pediatric tumors describes a mutation signature based on mutations in a specific nucleotide context that identifies hypermutated brain tumors with alkylating-associated mutations.57
TMZ-Induced Hypermutation and Malignant Transformation of Low-Grade Astrocytomas
Johnson et al performed a comparison of the mutational profile of 23 IDH-mutant, low-grade astrocytomas at initial diagnosis versus at tumor recurrence to determine the extent to which mutations in the initial tumors differ from their subsequent recurrent tumors and how treatment with TMZ affects the mutational profile.3 Among the 10 TMZ-treated low-grade astrocytomas, 6 harbored TMZ-induced hypermutation, composed of thousands of coding mutations which were not seen in the initial tumor prior to TMZ therapy. The vast majority of the new mutations were G:C>A:T transitions, the signature of TMZ-induced mutagenesis.3,5 All 6 hypermutated tumors underwent malignant transformation to GBM and had acquired new somatic mutations in MMR genes as well as increased methylation of the MGMT promoter region.3,58 Furthermore, TMZ-induced mutations consistently affected genes in the RB and Akt-mTOR signaling pathways, which are frequently deregulated in GBMs.3,4 This preliminary study and others24 led to the hypothesis that TMZ-induced hypermutation could drive malignant transformation in low-grade astrocytomas.
These studies proposed an evolutionary path for the TMZ-resistant tumor clones that repopulate hypermutated recurrences (Fig. 1).3,58 A TMZ-associated mutation (G:C>A:T transition unique to the post-TMZ recurrence) in at least one MMR gene was observed in 5 of 6 hypermutated recurrences.3 In 2 cases, preexisting heterozygous deletions encompassing MGMT, or an MMR gene, were followed by TMZ-associated mutations in one of the genes of interest. Hypermutated recurrences showed more than 1000 TMZ-related, clonal mutations in coding regions and had significantly higher average MGMT promoter methylation levels compared with TMZ-treated non-hypermutated recurrences, or compared with the untreated initial tumors. These studies suggest that during TMZ treatment, a selective advantage may exist for a minority of cells with higher MGMT promoter methylation once MMR activity is abrogated by TMZ-induced mutation. In this theoretical context, each resistant tumor cell has a mostly unique repertoire of mutations, in some cases conferring a distinct, selective advantage, depending on which genes and pathways are altered. Indeed, alterations of PI3K signaling, the p53 and RB pathways, and cell cycle gene expression serve as one explanation for the dominant clones in hypermutated recurrences.3,59 The increased mutational load from TMZ includes new driver mutations that are linked to malignant transformation of IDH-mutant astrocytomas, though additional cases are needed to verify these trends.3
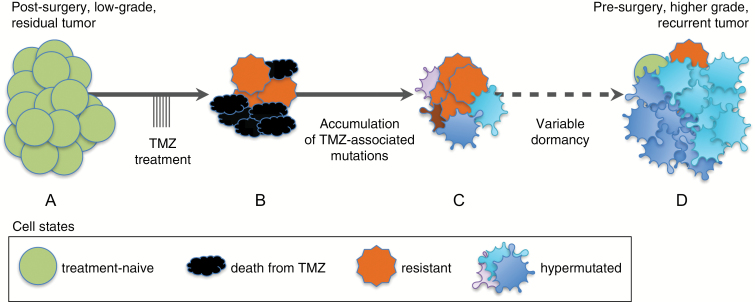
Fig. 1.
Model of TMZ-associated malignant transformation. An initial low-grade glioma (A) is resected and residual disease is treated with TMZ, causing tumor cell death (B). If DNA repair capacity is low and TMZ-associated mutations occur within key amino acids of MMR genes, the loss of MMR function may render cells resistant to TMZ. Resistant cells can acquire high numbers of de novo TMZ-associated mutations, including thousands in coding regions, resulting in hypermutation (C). After widely varying periods of dormancy, clonal expansions of hypermutated cells drive formation of higher-grade tumor recurrences. Multiple unique hypermutated tumor clones may expand concurrently, as depicted by the different colored groups of hypermutated cells (D).
Outstanding Questions on Hypermutated Gliomas
These studies raise several clinically important questions. First, what is the true risk of TMZ-induced malignant transformation among IDH-mutant, low-grade gliomas treated with TMZ? Knowing the risk of TMZ-induced hypermutation is essential for patients and clinicians to accurately assess the risks and benefits of TMZ treatment. This may be difficult to quantify, however, as reoperations are reserved for selected patients, and thus hypermutation status cannot be determined directly in an unbiased cohort. Detection of tumor-associated mutations in cell-free DNA from blood or cerebral spinal fluid may represent a minimally invasive technique that can address this bias.60
Are there patient or tumor factors that are predictive for risk of hypermutation? It is important to determine why hypermutation occurred in some, but not all, astrocytomas treated with TMZ. Knowledge of markers of susceptibility to TMZ-induced hypermutation could be key to strategies to prevent or delay malignant transformation in low-grade glioma patients treated with TMZ.
Are there a threshold dose and/or duration of TMZ exposure that increases the risk of TMZ-associated hypermutation? The time between the initiation of TMZ treatment and radiographic progression of a hypermutated tumor ranged from 12 to 90 months, and the duration of TMZ treatment also varied.3 Clinical or biological factors underlying this wide range of latencies, from creation of the hypermutated cells during treatment to a clinically significant clonal expansion, have not been defined. It is important to identify a TMZ regimen that maximizes treatment benefit while minimizing the risk of TMZ-induced hypermutation and hematologic toxicities.
Do TMZ-induced hypermutated GBMs arising from initially low-grade, IDH-mutant gliomas have different prognoses compared with their non-hypermutated GBM counterparts? Hypermutation has been associated with improved prognosis in other cancer sites. Patients with MMR-deficient hypermutated sporadic colorectal cancers have improved outcomes over patients with non-hypermutated cancers of the same stage, although hypermutation in these cases are not treatment related.61 Patients with hypermutated uterine serous carcinomas with somatic mutations in MMR genes and the polymerase epsilon gene (POLE) exhibit significantly better prognoses compared with patients with non-hypermutated uterine serous carcinomas, although the reasons are unclear.62 Wang et al examined longitudinal genomic and transcriptomic data from 114 GBM patients and showed that 17 out of 100 GBM patients treated with TMZ recurred with hypermutated tumors.63 The median survival time of patients with hypermutated primary GBM with wildtype IDH1 was 24 months, versus 18 months in other patients with GBM having wildtype IDH1, although it is unclear whether this difference was statistically significant and whether expanded studies of IDH-mutant gliomas will yield similar results.63 Kim et al identified 5 hypermutated GBMs from a cohort of tumor samples from 21 patients with pairs of primary and first recurrent GBMs.8 Three of the patients with hypermutation were treated with TMZ and radiation, all 5 had MGMT promoter methylation and mutations in MMR genes, and 1 had a secondary GBM with mutated IDH1; it is not clear if the 3 patients treated with TMZ had TMZ-induced mutations. The 5 patients with hypermutated GBMs survived for 35, 64, 107, 191, and 245 days after their second surgeries at recurrence compared with a previously reported median survival of 7.8 months after surgery upon first recurrence.8,64 Although these data suggest that hypermutated GBMs may have worse clinical outcomes, conclusions cannot be drawn from the small sample size.
Are TMZ-induced hypermutated gliomas more sensitive to radiation or other chemotherapies? A study of germline ultra-hypermutated cancers with biallelic MMR deficiency and with loss of polymerase proofreading function suggested that there is an upper limit of 10000 to 20000 exonic mutations, above which further mutational burden may be incompatible with cell survival.65 With the onslaught of additional DNA damage in the setting of a high mutational burden, hypermutated cancers may have increased sensitivity to DNA-damaging agents.66 In addition, treatment of MGMT-deficient GBMs with TMZ introduces a strong selective pressure to lose MMR function. Radiation can induce a variety of DNA damage lesions, including DNA double-strand breaks, single strand breaks, and oxidative base damage. However, the relationship between MMR deficiency and radiosensitivity is not straightforward; MMR proficiency has been associated with radiosensitivity following low-dose-rate ionizing radiation (IR), whereas loss of MMR was associated with radiosensitivity following acute high-dose-rate IR.67
In addition to their canonical role in the repair of base-base mismatches and insertion and deletion loops during replication, MMR proteins have noncanonical functions in the repair of oxidatively damaged DNA lesions and in modulating homologous recombination repair of DNA double-strand breaks which may be therapeutically exploited.68,69 Hewish et al screened a pair of isogenic mutL homolog 1 (MLH1)-deficient and MLH1-proficient cancer cell lines with a library of clinically used drugs and found that cytarabine was selectively toxic to MLH1- and mutS homolog 2 (MSH2)-deficient tumor cells due to increased levels of cellular oxidative stress, suggesting that MMR-deficient cancers may be more sensitive to cytarabine-based chemotherapy regimens.70 Martin et al showed that inhibition of the base excision repair protein DNA polymerase beta (POLB) was synthetically lethal with MSH2 deficiency, and inhibition of mitochondrial DNA polymerase gamma (POLG) was synthetically lethal with MLH1 deficiency due to accumulation of the toxic and mutagenic oxidative DNA lesions 8-oxoG, providing a rationale for the development of POLB and POLG inhibitors.71 In a small molecule screen to identify drugs that are selectively lethal to cells lacking functional MSH2, methotrexate was identified as being highly selective for cells with MSH2 deficiency, which was attributed to the persistence of 8-oxoG lesions in the MSH2-deficient cells.72 Dietlan et al performed a functional screen which identified a druggable synthetic lethal interaction between MSH3 deficiency and the DNA-dependent protein kinase catalytic subunit, an essential component of the machinery for nonhomologous end-joining repair of DNA double-strand breaks.73 Aquilina et al showed that methylation-tolerant cell lines that were generated after multiple exposures to the alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine were more sensitive to CCNU compared with the parental cell line.74 In addition, methylation-tolerant cell lines generated after multiple exposures to the alkylating agent N-methyl-N-nitrosourea (MNU) that were also MMR deficient were more sensitive to CCNU than isogenic parental cell lines with functional MMR.74 Sensitivity to CCNU and tolerance to MNU (via loss of MMR) were both detectable only when the MGMT repair protein was inactivated by the MGMT inhibitor, O6-benzylguanine. The study did not identify the lesion induced by CCNU that is the substrate for MMR, but it may involve an interstrand DNA cross-link, which is the cytotoxic lesion formed by CCNU.75 A more recent preclinical study showed similar findings in GBM cells; TMZ resistance mediated by MMR deficiency in MGMT-methylated GBM cells was accompanied by increased sensitivity to CCNU and to combined CCNU and TMZ.76 Since MMR proficiency is inversely related to CCNU toxicity, hypermutated glioma cells may be sensitive to CCNU, although this remains to be determined. Interestingly, the combination of radiotherapy, CCNU, and TMZ has shown promising long-term survival data in patients with newly diagnosed glioblastoma, especially in the patients with MGMT methylated tumors.77 Overall, exploiting the loss of the noncanonical functions of MMR may yield new therapeutic strategies for patients with hypermutated cancers.
Immune Checkpoint Inhibitors for Hypermutated Tumors
Immune checkpoint inhibition is an attractive therapeutic avenue that exploits the mutational burden and clonal mutational architecture of hypermutated tumors. There has been considerable enthusiasm regarding the success of immune checkpoint inhibitors in treating recurrences from a variety of cancer types. The most pronounced responses have been among tumors known to have high mutational burdens,78,79 such as subsets of non–small cell lung cancers,80–84 malignant melanomas,85,86 renal cell carcinomas,87 and MMR-deficient tumors.88 Observations from a phase II clinical trial of immune checkpoint inhibitors among colorectal cancers and MMR-deficient extracranial malignancies support these hypotheses. Response rates of 40% were observed for MMR-deficient colorectal cancers, compared with 0% for MMR-intact colorectal cancers. Interestingly, all patients with somatic MMR deficits had objective responses to checkpoint inhibition, compared with 27% of patients with germline MMR deficits.88 On May 23, 2017, the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab was approved for the treatment of adult and pediatric patients with unresectable or metastatic, microsatellite instability–high, or MMR-deficient solid tumors regardless of tumor site or histology.89
Even among purportedly immunogenic tumors, not all cases have durable responses to checkpoint inhibition, and mixed responses are often observed. Biomarkers of response to immune checkpoint inhibitors have included programmed death ligand 1 (PD-L1) staining and lymphocyte infiltration. However, the predictive power of these markers are variable across histologies and are complicated by inconsistencies in immunohistochemistry technique.90,91 More recently, neoantigen burden92 and tumor clonal architecture93 have been proposed as novel genomic biomarkers for efficacious checkpoint inhibition. These findings suggest that checkpoint inhibition may stochastically amplify T-cell clones with specificity for a small number of tumor neoantigens. For tumors with highly branched evolution, T-cell specificity against some but not all tumor cells may result in partial responses to treatment. Thus, the extent of intratumoral heterogeneity of hypermutated glioma could be a critical factor in response prediction.93
Several immune-based therapies, including immune checkpoint inhibitors, peptide vaccines, dendritic cell vaccines, and engineered T-cell therapy are under active investigation for gliomas. Secondary high-grade gliomas with alkylator-induced hypermutation may elicit a robust immunogenic response. Given the high mutational burden and the clonal mutational architecture of TMZ-induced hypermutated GBMs, immune checkpoint therapies may be promising for GBMs with TMZ-associated hypermutation. Furthermore, these tumors are highly enriched for mutations in the Akt-mTOR pathway,3 which results in posttranscriptional increase in PD-L1 membrane presentation, an immune escape mechanism in these tumors94; perhaps there is a rationale for the use of PI3K or mTOR kinase inhibitors in conjunction with immune checkpoint inhibitors.
The potential for immune checkpoint inhibitors in hypermutated GBMs is highlighted by 2 case reports. A case report of 2 siblings with hypermutated GBMs arising from germline biallelic MMR deficits demonstrated clinically significant responses to the PD-1 inhibitor nivolumab.95 This was the first report demonstrating durable responses of recurrent GBM to immune checkpoint inhibition. Another case report showed clinical response to the PD-1 inhibitor pembrolizumab (MK-3475) in a patient with a germline POLE mutation and GBM with primitive neuroectodermal tumor features who developed metastatic disease to the spine after receiving chemoradiation and maintenance TMZ.96 Genomic analyses on the tissue from the pretreatment frontotemporal GBM and 2 spinal metastases showed that all samples were hypermutated and between 2040 and 3254 neoantigenic mutations were identified per sample. Immunohistochemical analyses performed on the resected spinal metastases before and 3 weeks after treatment with pembrolizumab showed cytolytic lymphocyte infiltration into the tumors post-pembrolizumab.96 These reports provide preliminary support for the utility of immune checkpoint inhibitors in hypermutated gliomas. However, response to immune checkpoint inhibitors in gliomas with TMZ-induced hypermutation may differ compared with hypermutated gliomas arising from germline or somatic mutations in MMR genes or in POLE or the polymerase delta gene.
Immune checkpoint inhibitors are now being evaluated for newly diagnosed GBM (CheckMate-498, NCT02617589, and CheckMate-548, NCT02667587) and for recurrent GBM (CheckMate-143, NCT02017717). Thus far, results have been negative. In a preliminary report of CheckMate-143, a phase II/III randomized clinical trial comparing nivolumab versus bevacizumab for patients with recurrent GBM, nivolumab did not improve PFS (median 1.5 vs 3.5 mo) or OS (9.8 vs 10.0 mo). Although fewer objective responses were observed in the nivolumab arm (8% vs 23%), responses were more durable than those observed with bevacizumab (11.1 vs 5.3 mo). These results are disappointing, but they indicate that a small proportion of patients do have durable responses to checkpoint blockade.97 Based on Kim et al, a low risk for TMZ-induced hypermutation in IDH1-wildtype primary GBMs under the Stupp regimen might be expected, though the requirement for a second surgery results in a biased cohort.98 A recent study looked at tumor mutational burden, PD-1/PD-L1 expression, and DNA MMR defects in 327 glioma patients and showed that biomarkers of response to immune checkpoint inhibitors occur infrequently in gliomas; a high tumor mutation load was associated with loss of MLH1, MSH2, MSH6, and postmeiotic segregation increased 2 (PMS2) proteins, but was found in only 3.5% of patients with newly diagnosed GBM.99 These results underscore the importance of patient selection in clinical trials of immune checkpoint inhibitors in gliomas. Currently, pembrolizumab is being investigated in patients with recurrent malignant gliomas with a hypermutator phenotype (NCT02658279). “Hypermutator phenotype” in this study is defined as tumors with at least 30 mutations detected by the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets oncopanel (or comparable next-generation sequencing) or if tumors have a mutation in an MMR gene or in genes known to be associated with hypermutator phenotypes or microsatellite instability. This trial will help address the question of whether hypermutated gliomas (grades II–IV) are vulnerable to immune checkpoint inhibitor therapies.
Concluding Remarks
Randomized controlled trials have shown the clear benefit of TMZ in the management of gliomas. However, preliminary studies show that some low-grade gliomas develop TMZ-induced hypermutation and malignant transformation. A true estimate of risk of TMZ-induced hypermutation, why it occurs in some low-grade gliomas but not others, and its prognostic significance are not well understood; thus, currently there is insufficient evidence to change clinical practice in the use of TMZ in low-grade gliomas. Future studies are needed to better understand the risk and mechanisms of TMZ-induced hypermutation in low-grade gliomas and, once hypermutated, whether they are more vulnerable to certain therapies.
Funding
This effort was generously supported by a gift from the Dabbiere family. Additional support was by the National Institutes of Health R01CA169316 (J.F.C.), P01CA118816-06 (J.F.C.), P50CA097257 (S.M.C. and J.F.C.), and 5T32CA151022-07 (M.R.G.); and ABR Holman Research Pathway (S.C.).
Conflict of interest statement
There are no conflicts of interest to report.
References
- 1. Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017;58(5):235–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12(9):587–598. [DOI] [PubMed] [Google Scholar]
- 3. Johnson BE, Mazor T, Hong C et al. . Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLendon R, Friedman A, Bigner D et al. . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunter C, Smith R, Cahill DP et al. . A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 2006;66(8):3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yip S, Miao J, Cahill DP et al. . MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15(14):4622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cahill DP, Levine KK, Betensky RA et al. . Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13(7):2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim H, Zheng S, Amini SS et al. . Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louis DN, Perry A, Reifenberger G et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 10. Reuss DE, Mamatjan Y, Schrimpf D et al. . IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brat DJ, Verhaak RGW, Aldape KD et al. . Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eckel-Passow JE, Lachance DH, Molinaro AM et al. . Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. [DOI] [PubMed] [Google Scholar]
- 14. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu XY, Gerges N, Korshunov A et al. . Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124(5):615–625. [DOI] [PubMed] [Google Scholar]
- 16. Arita H, Narita Y, Fukushima S et al. . Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267–276. [DOI] [PubMed] [Google Scholar]
- 17. Killela PJ, Reitman ZJ, Jiao Y et al. . TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan H, Parsons DW, Jin G et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 20. Sanai N, Chang S, Berger MS. Low-grade gliomas in adults: a review. J Neurosurg. 2011;115(5):948–965. [DOI] [PubMed] [Google Scholar]
- 21. Upadhyay N, Waldman AD. Conventional MRI evaluation of gliomas. Br J Radiol. 2011;84(Spec No 2):S107–S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rees J, Watt H, Jäger HR et al. . Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72(1):54–64. [DOI] [PubMed] [Google Scholar]
- 23. Smith JS, Chang EF, Lamborn KR et al. . Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 24. Bai H, Harmancı AS, Erson-Omay EZ et al. . Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet. 2016;48(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaeckle KA, Decker PA, Ballman KV et al. . Transformation of low grade glioma and correlation with outcome: an NCCTG database analysis. J Neurooncol. 2011;104(1):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 27. Perry JR, Laperriere N, O’Callaghan CJ et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 28. van den Bent MJ, Brandes AA, Taphoorn MJ et al. . Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 29. Cairncross JG, Wang M, Jenkins RB et al. . Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Bent MJ, Baumert B, Erridge SC et al. . Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang S, Zhang P, Cairncross JG et al. . Phase III randomized study of radiation and temozolomide versus radiation and nitrosourea therapy for anaplastic astrocytoma: results of NRG Oncology RTOG 9813. Neuro Oncol. 2017;19(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaeckle K, Vogelbaum M, Ballman K, Giannini C. ATCT-16. CODEL (ALLIANCE-N0577; EORTC-26081/2208; NRG-1071; NCIC-CEC-2): phase III randomized study of RT vs. RT + TMZ vs. TMZ for newly diagnosed 1p/19q- codeleted anaplastic glioma analysis of patients treated on the original protocol design. Neuro Oncol. 2015;91(3):497–504. [Google Scholar]
- 33. van den Bent MJ, Afra D, de Witte O et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 34. Buckner JC, Shaw EG, Pugh SL et al. . Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fisher BJ, Hu C, Macdonald DR et al. . Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys. 2015;91(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wahl M, Phillips JJ, Molinaro AM et al. . Chemotherapy for adult low-grade gliomas: clinical outcomes by molecular subtype in a phase II study of adjuvant temozolomide. Neuro Oncol. 2017;19(2):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leu S, von Felten S, Frank S, Boulay JL, Mariani L. IDH mutation is associated with higher risk of malignant transformation in low-grade glioma. J Neurooncol. 2016;127(2):363–372. [DOI] [PubMed] [Google Scholar]
- 38. Newlands ES, Blackledge GR, Slack JA et al. . Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer. 1992;65(2):287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Denny BJ, Wheelhouse RT, Stevens MF, Tsang LL, Slack JA. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33(31):9045–9051. [DOI] [PubMed] [Google Scholar]
- 40. Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969;223(5202):206–207. [DOI] [PubMed] [Google Scholar]
- 41. Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst). 2007;6(8):1079–1099. [DOI] [PubMed] [Google Scholar]
- 42. Roos WP, Batista LF, Naumann SC et al. . Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26(2):186–197. [DOI] [PubMed] [Google Scholar]
- 43. Esteller M, Garcia-Foncillas J, Andion E et al. . Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 44. Hegi ME, Diserens AC, Godard S et al. . Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 45. Brennan CW, Verhaak RG, McKenna A et al. ; TCGA Research Network The somatic genomic landscape of glioblastoma. Cell. 2013; 155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hegi ME, Diserens AC, Gorlia T et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 47. Friedman HS, McLendon RE, Kerby T et al. . DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol. 1998;16(12):3851–3857. [DOI] [PubMed] [Google Scholar]
- 48. Bodell WJ, Gaikwad NW, Miller D, Berger MS. Formation of DNA adducts and induction of lacI mutations in Big Blue Rat-2 cells treated with temozolomide: implications for the treatment of low-grade adult and pediatric brain tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(6):545–551. [PubMed] [Google Scholar]
- 49. Noronha V, Berliner N, Ballen KK et al. . Treatment-related myelodysplasia/AML in a patient with a history of breast cancer and an oligodendroglioma treated with temozolomide: case study and review of the literature. Neuro Oncol. 2006;8(3):280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Vita S, De Matteis S, Laurenti L et al. . Secondary Ph+ acute lymphoblastic leukemia after temozolomide. Ann Hematol. 2005;84(11):760–762. [DOI] [PubMed] [Google Scholar]
- 51. Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4(1):1–6. [PubMed] [Google Scholar]
- 52. Marra G, D’Atri S, Corti C et al. . Tolerance of human MSH2+/- lymphoblastoid cells to the methylating agent temozolomide. Proc Natl Acad Sci U S A. 2001;98(13):7164–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ricard D, Kaloshi G, Amiel-Benouaich A et al. . Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61(5):484–490. [DOI] [PubMed] [Google Scholar]
- 54. Newcomb EW, Madonia WJ, Pisharody S, Lang FF, Koslow M, Miller DC. A correlative study of p53 protein alteration and p53 gene mutation in glioblastoma multiforme. Brain Pathol. 1993;3(3):229–235. [DOI] [PubMed] [Google Scholar]
- 55. Bello MJ, Alonso ME, Amiñoso C et al. . Hypermethylation of the DNA repair gene MGMT: association with TP53 G:C to A:T transitions in a series of 469 nervous system tumors. Mutat Res. 2004;554(1-2):23–32. [DOI] [PubMed] [Google Scholar]
- 56. Felsberg J, Thon N, Eigenbrod S et al. ; German Glioma Network Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129(3):659–670. [DOI] [PubMed] [Google Scholar]
- 57. Campbell BB, Light N, Fabrizio D et al. . Comprehensive analysis of hypermutation in human cancer. Cell. 2017;171(5):1042–1056.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Thuijl HF, Mazor T, Johnson BE et al. . Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol. 2015;129(4):597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mazor T, Pankov A, Johnson BE et al. . DNA methylation and somatic mutations converge on the cell cycle and define similar evolutionary histories in brain tumors. Cancer Cell. 2015;28(3):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pentsova EI, Shah RH, Tang J et al. . Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carethers JM, Jung BH. Genetics and genetic biomarkers in sporadic colorectal cancer. Gastroenterology. 2015;149(5):1177–1190.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Santin AD, Bellone S, Centritto F, Schlessinger J, Lifton R. Improved survival of patients with hypermutation in uterine serous carcinoma. Gynecol Oncol Rep. 2015;12:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Cazzato E, Ladewig E et al. . Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sughrue ME, Sheean T, Bonney PA, Maurer AJ, Teo C. Aggressive repeat surgery for focally recurrent primary glioblastoma: outcomes and theoretical framework. Neurosurg Focus. 2015;38(3):E11. [DOI] [PubMed] [Google Scholar]
- 65. Shlien A, Campbell BB, de Borja R et al. ; Biallelic Mismatch Repair Deficiency Consortium Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet. 2015;47(3):257–262. [DOI] [PubMed] [Google Scholar]
- 66. Schlesner M, Eils R. Hypermutation takes the driver’s seat. Genome Med. 2015;7(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martin LM, Marples B, Coffey M et al. . DNA mismatch repair and the DNA damage response to ionizing radiation: making sense of apparently conflicting data. Cancer Treat Rev. 2010;36(7):518–527. [DOI] [PubMed] [Google Scholar]
- 68. Macpherson P, Barone F, Maga G, Mazzei F, Karran P, Bignami M. 8-Oxoguanine incorporation into DNA repeats in vitro and mismatch recognition by MutSalpha. Nucleic Acids Res. 2005;33(16): 5094–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spies M, Fishel R. Mismatch repair during homologous and homeologous recombination. Cold Spring Harb Perspect Biol. 2015;7(3):a022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hewish M, Martin SA, Elliott R, Cunningham D, Lord CJ, Ashworth A. Cytosine-based nucleoside analogs are selectively lethal to DNA mismatch repair-deficient tumour cells by enhancing levels of intracellular oxidative stress. Br J Cancer. 2013;108(4):983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martin SA, McCabe N, Mullarkey M et al. . DNA polymerases as potential therapeutic targets for cancers deficient in the DNA mismatch repair proteins MSH2 or MLH1. Cancer Cell. 2010;17(3):235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martin SA, McCarthy A, Barber LJ et al. . Methotrexate induces oxidative DNA damage and is selectively lethal to tumour cells with defects in the DNA mismatch repair gene MSH2. EMBO Mol Med. 2009;1(6-7):323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dietlein F, Thelen L, Jokic M et al. . A functional cancer genomics screen identifies a druggable synthetic lethal interaction between MSH3 and PRKDC. Cancer Discov. 2014;4(5):592–605. [DOI] [PubMed] [Google Scholar]
- 74. Aquilina G, Ceccotti S, Martinelli S, Hampson R, Bignami M. N-(2-chloroethyl)-N’-cyclohexyl-N-nitrosourea sensitivity in mismatch repair-defective human cells. Cancer Res. 1998;58(1):135–141. [PubMed] [Google Scholar]
- 75. Tong WP, Kirk MC, Ludlum DB. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N’-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1982;42(8): 3102–3105. [PubMed] [Google Scholar]
- 76. Stritzelberger J, Distel L, Buslei R, Fietkau R, Putz F. Acquired temozolomide resistance in human glioblastoma cell line U251 is caused by mismatch repair deficiency and can be overcome by lomustine. Clin Transl Oncol. 2017; doi: 10.1007/s12094-017-1743-x. [DOI] [PubMed] [Google Scholar]
- 77. Glas M, Happold C, Rieger J et al. . Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27(8):1257–1261. [DOI] [PubMed] [Google Scholar]
- 78. Snyder A, Makarov V, Merghoub T et al. . Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGranahan N, Furness AJ, Rosenthal R et al. . Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brahmer J, Reckamp KL, Baas P et al. . Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Borghaei H, Paz-Ares L, Horn L et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gettinger SN, Horn L, Gandhi L et al. . Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Garon EB, Rizvi NA, Hui R et al. ; KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 84. Herbst RS, Baas P, Kim DW et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 85. Hodi FS, O’Day SJ, McDermott DF et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Larkin J, Chiarion-Sileni V, Gonzalez R et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Motzer RJ, Escudier B, McDermott DF et al. ; CheckMate 025 Investigators Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Le DT, Uram JN, Wang H et al. . PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site—when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–1412. [DOI] [PubMed] [Google Scholar]
- 90. Herbst RS, Soria JC, Kowanetz M et al. . Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. [DOI] [PubMed] [Google Scholar]
- 92. Snyder A, Makarov V, Merghoub T et al. . Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McGranahan N, Furness AJ, Rosenthal R et al. . Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Parsa AT, Waldron JS, Panner A et al. . Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. [DOI] [PubMed] [Google Scholar]
- 95. Bouffet E, Larouche V, Campbell BB et al. . Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 96. Reardon DA, Omuro A, Brandes AA et al. . OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: checkmate 143. Neuro Oncol. 2017;19(suppl 3):iii21. [Google Scholar]
- 97. Johanns TM, Miller CA, Dorward IG et al. . Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim J, Lee IH, Cho HJ et al. . Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015;28(3):318–328. [DOI] [PubMed] [Google Scholar]
- 99. Hodges TR, Ott M, Xiu J et al. . Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19(8): 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]