Abstract
Background
The current standard of care for glioblastoma (GBM) constitutes maximal safe surgical resection, followed by fractionated radiation and temozolomide. This treatment regimen is logistically burdensome, and in a health care system in which access to care is variable, there may be patients with worsened outcomes due to inadequate access to optimal treatment.
Methods
The National Cancer Database was queried for patients with diagnoses of GBM in 2006–2014. Patients were grouped according to insurance status: private insurance, Medicare, Medicaid, or uninsured. Treatments provided (surgery, radiation, and chemotherapy) were compared between groups in univariate and multivariable logistic regression analysis.
Results
A total of 61614 patients were analyzed. Compared with private insurance, the odds of surgery for Medicaid and uninsured patients were 0.72 (95% CI: 0.66–0.79) and 0.77 (95% CI: 0.69–0.87), respectively (P < 0.001). The multivariable odds of receiving radiotherapy were 0.91 (95% CI: 0.86–0.96), 0.62 (95% CI: 0.57–0.68), and 0.47 (95% CI: 0.43–0.52) for Medicare, Medicaid, and uninsured patients, respectively (all P < 0.001). In addition, the odds of receiving chemotherapy were 0.94 (95% CI: 0.89–0.99), 0.53 (95% CI: 0.49–0.57), and 0.41 (95% CI: 0.38–0.46) for Medicare, Medicaid, and uninsured patients, respectively (all P < 0.001).
Conclusion
Insurance status and type of insurance coverage appear to impact treatments rendered for GBM, independently of other variables. Furthermore, we find that such differential access to care significantly impacts survival. Ensuring adequate access to care for all patients with diagnoses of glioblastoma is critical to optimize survival, especially as therapies continue to advance.
Keywords: glioblastoma, health care access, insurance, Medicare, Medicaid
Importance of the Study
Several studies have suggested a deleterious impact of lower socioeconomic status (SES) on outcome for a number of non–central nervous system malignancies. However, literature evaluating the role of SES in GBM is less clear. In the present study, we utilized data from the largest cancer registry in the United States, the National Cancer Database, in order to decipher the effect of insurance status on access to care and survival for patients with diagnoses of glioblastoma. We found that uninsured and Medicaid patients had significantly lower odds of receiving the current standard of care (ie, surgery) followed by fractionated radiation and temozolomide, after adjusting for available demographics, comorbidities, and hospital characteristics. The findings of this investigation highlight for the first time the disparities in care for patients with GBM and underline that ensuring adequate access to care for these patients is critical to optimize survival, especially as therapies continue to advance.
Grade IV astrocytoma or glioblastoma (GBM) is the most common and most aggressive primary CNS neoplasm. The current standard of care for GBM is maximal safe surgical resection followed by the Stupp protocol with fractionated radiation and daily temozolomide, followed by a subsequent 6 cycles of adjuvant temozolomide.1 The addition of radiation and chemotherapy increased the 2-year survival rate from 10.4 to 26.5% and the median survival from 12.1 to 14.6 months.
Several studies have suggested a deleterious impact of lower socioeconomic status (SES) on outcome for a number of non-CNS malignancies. Studies evaluating the role of SES on GBM have been less clear.2–5 At issue with several of these studies is that one or more proxies for SES such as zip code tabulation areas, census tracts, income, occupation, and the Index of Relative Socioeconomic Disadvantage are used, which may not adequately capture actual access to care. In addition, the majority of previously published work on GBM using population-based data in the United States has utilized the Surveillance, Epidemiology, and End Results (SEER) program, which is severely limited by the absence of chemotherapy- and hospital-related variables.6–8 In this study, we compared patients with GBM based on insurance status using a national, surgeon-endorsed cancer registry with the hypothesis that the latter is a sensitive indicator of actual access to accepted standards of care with ramifications for outcome.
Materials and Methods
Data Source and Patient Cohort
The National Cancer Database (NCDB) was queried for patients with diagnoses of GBM between 2006 and 2014. The NCDB, established in 1989, is one of the largest cancer registries in the United States, capturing 70% of all newly diagnosed malignancies in the US annually and currently containing almost 34 million cases from over 1500 hospitals.9 Data are collected from selected health registries accredited by the American Cancer Society and the Commission on Cancer of the American College of Surgeons.10 It was developed mainly for surveillance and quality improvement in cancer care and captures a large number of cancer cases with de-identified data. It can be used to identify high risk groups, to study cancer care over time, patterns of care, and related patient outcomes.9
Cases were identified using the International Classification of Disease for Oncology, third edition (ICD-O-3) pathology codes (9440, 9441, and 9442). We stratified patients by insurance type at the time of diagnosis as private, Medicare, Medicaid, or uninsured. Patients were excluded based on the following criteria: age <18 years; systemic metastases at the time of diagnosis; any tumor occurring prior to their GBM diagnosis; or failure to undergo surgery, radiation, or chemotherapy due to medical comorbidities. The NCDB Participant User File data are de-identified and therefore exempt from institutional review board approval. The American College of Surgeons has executed a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals.
Outcomes of Interest
The primary outcome of interest was receipt of surgery, radiation, and/or chemotherapy. Secondary outcome was overall survival from the time of diagnosis until death or censoring due to loss to follow-up or administrative limitations.
Covariates
We recorded additional variables in 3 categories as follows: (i) patient demographics: age, sex, race, Charlson-Deyo comorbidity score, zip code household income, and distance between residence and the treatment facility; (ii) hospital characteristics: treatment facility type (community cancer programs, comprehensive community cancer programs, academic/research facilities, and integrative network cancer care programs [definitions provided in Supplementary Table S1]) and US census region of reporting facility; (iii) treatment parameters: days from diagnosis to surgical treatment, surgery, adjuvant radiotherapy, and adjuvant chemotherapy. Surgery was categorized based on the universal Collaborative Stage Data Collection System into biopsy, subtotal, gross total, and lobectomy.
Statistical Analysis
Descriptive statistics (mean and standard deviation for continuous variables; frequency and proportion for categorical variables) are presented. Continuous variables among the 3 insurance groups were compared using unpaired, 2-tailed t-test, whereas categorical variables were compared using Pearson’s chi-square test. With regard to income, patients were stratified into four categories: <$30000, $30000–$34999, $35000–$45999, and >$46000.
Outcome was examined in an as-treated analysis. Logistic regression models were fitted in order to evaluate the effect of insurance status on receiving surgery, radiation, and/or chemotherapy adjusting for age, sex, race, ethnicity, Charlson-Deyo comorbidity score, facility type, and facility location. We tested an interaction term in the regression model to assess the impact of region on receiving each of the treatment modalities. ANOVA analysis was used to evaluate the statistical significance of the interaction term as per Harrell.11 If the interaction term was significant, subgroup analysis stratified by region was subsequently performed. Kaplan–Meier survival curves for insurance status were constructed and compared using both the log-rank and Wilcoxon–Breslow tests. The latter method was included as it places more weight on earlier events and is thus more sensitive to survival differences for diseases with high mortality rates, such as GBM.12 The effect of insurance status (using private insurance as the reference category) and other covariates on hazard of death was evaluated in univariate Cox regression analysis. Assumptions of proportional hazards were evaluated by examining the Schoenfeld residuals and the log-log plots of survival against time. When significant interactions were found, those interaction terms were included in the final model at time-dependent covariates. A multivariable Cox regression model was subsequently built in order to evaluate the adjusted effect of insurance coverage on survival after controlling for age, sex, race, ethnicity, Charlson-Deyo comorbidity score, treatment received, and facility type and location. Collinearity among all independent variables was evaluated with the variance inflation factor. Given that the amount of missing data for the variables included in the model was very small, we performed complete-case analysis.
Statistical analysis was performed using R Statistical Computing software (https://www.R-project.org/). The R survival package by Therneau and colleagues was used for the survival analysis.13P-values < 0.05 were considered statistically significant.
Results
Baseline Characteristics
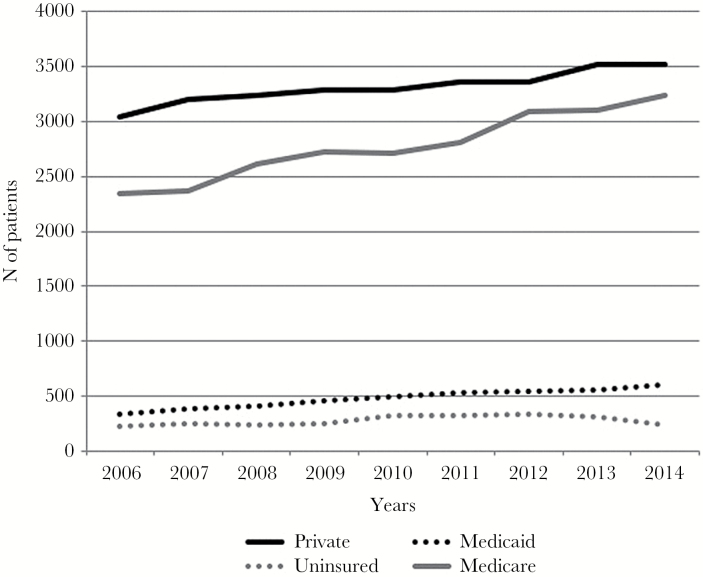
The NCDB was queried for histologically verified cases of GBM between 2006 and 2014. A total of 61614 patients were analyzed: 29811 (48.4%) with private insurance, 24994 (40.6%) Medicare patients, 4318 (7.0%) with Medicaid, and 2491 (4.0%) uninsured. Insurance trends of all patients in the database are shown in Figure 1. Most patients were privately insured and the number of patients with private insurance and Medicare rose over the study period. The number of Medicaid patients also rose slightly, while the number of uninsured patients remained largely stable with a modest drop between 2013 and 2014.
Fig. 1.
Trends of patient population in the database.
Demographic characteristics of the study subjects are shown in Table 1. Mean age was 57.7 years overall, with mean ages for privately insured, Medicare, Medicaid, and uninsured patients of 55.2, 72.5, 50.0, and 53.2, respectively (P < 0.001). Caucasians represented 90.5% of patients in the study and 91.1% of the privately insured, 92.7% of Medicare patients, 78.2% of Medicaid patients, and 82.4% of the uninsured (P < 0.001). Patients with private insurance and Medicare tended to have higher income compared with Medicaid patients and the uninsured; 49.6% of privately insured patients and 40.4% of Medicare recipients had income over $46000 compared with 28.1% of Medicaid recipients and 32.0% of those uninsured (P < 0.001). The average distance from residence to treatment facility was 50.4 miles for privately insured patients, 43.3 miles for Medicare recipients, 32.8 miles for Medicaid recipients, and 43.3 miles for the uninsured (P < 0.001).
Table 1.
Baseline demographics and hospital characteristics
| Variable | Available Data (all = 61 614) | Private Insurance N = 29 811 | Medicare N = 24 994 | Medicaid N = 4318 | Uninsured N = 2491 | P-value |
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 61 614 | 55.2 (11.6) | 72.5 (8.35) | 50.0 (15.4) | 53.2 (11.9) | <0.001 |
| Female sex, n | 61 614 | 12 012 (40.3%) | 11 507 (46.0%) | 1737 (40.2%) | 965 (38.7%) | <0.001 |
| Race, n | 61 614 | <0.001 | ||||
| White | 27 169 (91.1%) | 23 163 (92.7%) | 3378 (78.2%) | 2053 (82.4%) | ||
| African American | 1475 (4.95%) | 1106 (4.43%) | 604 (14.0%) | 262 (10.5%) | ||
| Other | 1167 (3.91%) | 725 (2.90%) | 336 (7.78%) | 176 (7.07%) | ||
| Hispanic ethnicity, n | 61 614 | <0.001 | ||||
| Yes | 1274 (4.27%) | 927 (3.71%) | 732 (17.0%) | 421 (16.9%) | ||
| Unknown | 1526 (5.12%) | 1397 (5.59%) | 162 (3.75%) | 88 (3.53%) | ||
| Charlson index, n | 61 614 | <0.001 | ||||
| 0 | 23 171 (77.7%) | 16 154 (64.6%) | 3179 (73.6%) | 1862 (74.7%) | ||
| 1 | 4099 (13.7%) | 5278 (21.1%) | 664 (15.4%) | 357 (14.3%) | ||
| 2 | 2541 (8.52%) | 3562 (14.3%) | 475 (11.0%) | 272 (10.9%) | ||
| Income, n | 58 750 | <0.001 | ||||
| Less than $30 000 | 2511 (8.86%) | 2879 (12.1%) | 892 (21.5%) | 387 (16.4%) | ||
| $30 000–$34 999 | 4188 (14.8%) | 4570 (19.1%) | 896 (21.6%) | 492 (20.9%) | ||
| $35 000–$45 999 | 7579 (26.7%) | 6788 (28.4%) | 1198 (28.8%) | 722 (30.6%) | ||
| $46 000 + | 14 077 (49.6%) | 9648 (40.4%) | 1168 (28.1%) | 755 (32.0%) | ||
| Distance traveled, miles, mean (SD) | 60 233 | 50.4 (173) | 43.3 (150) | 32.8 (103) | 43.3 (133) | <0.001 |
| Facility characteristics | ||||||
| Facility type, n | 58 026 | <0.001 | ||||
| Community Cancer Program | 1265 (4.61%) | 1549 (6.22%) | 212 (6.09%) | 101 (4.63%) | ||
| Comprehensive Community Cancer Program | 9671 (35.2%) | 10 230 (41.1%) | 1086 (31.2%) | 821 (37.7%) | ||
| Academic/Research Program | 13 500 (49.1%) | 10 341 (41.5%) | 1803 (51.8%) | 973 (44.6%) | ||
| Integrative Network Cancer Care program | 3031 (11.0%) | 2779 (11.2%) | 379 (10.9%) | 285 (13.1%) | ||
| Facility region location, n | 61 614 | <0.001 | ||||
| Midwest | 6787 (22.8%) | 6228 (24.9%) | 754 (17.5%) | 428 (17.2%) | ||
| Northeast | 5805 (19.5%) | 5116 (20.5%) | 734 (17.0%) | 287 (11.5%) | ||
| South | 9483 (31.8%) | 9266 (37.1%) | 1090 (25.2%) | 1108 (44.5%) | ||
| West | 7736 (26.0%) | 4384 (17.5%) | 1740 (40.3%) | 668 (26.8%) | ||
Bold denotes statistical significance.
In terms of facility type, the majority of patients in each insurance category were managed at academic/research programs, although privately insured patients (49.1%) and Medicaid patients (51.8%) were more likely to receive care at academic programs relative to Medicare patients (41.5%) and the uninsured (44.6%) (P < 0.001). Comprehensive community cancer programs were the second most utilized treatment facility, but the pattern of utilization was starkly different compared with that seen at academic programs. Medicare and uninsured patients represented 41.1% and 37.7% of patients, respectively, versus privately insured and Medicaid patients, who represented 35.2% and 31.2%, respectively (P < 0.001). There were regional variations in insurance coverage, with 44.5% of uninsured patients from the South compared with only 11.5% from the Northeast; 40.3% of Medicaid patients hailed from Western states (P < 0.001).
Lastly, O6-methylguanine-DNA methyltransferase (MGMT) methylation was slightly higher in Medicare patients (42.9%) versus privately insured (39.1%), Medicaid (37.3%), and uninsured patients (39.9%), although data were available for only 5506 patients (P = 0.03). Lesion number and location were clinically similar among patients. These data are summarized in Table 2.
Table 2.
Tumor and treatment characteristics
| Variable | Available Data (all = ) |
Private Insurance N = 29 811 |
Medicare N = 24 994 |
Medicaid N = 4318 |
Uninsured N = 2491 |
P-value |
|---|---|---|---|---|---|---|
| Tumor characteristics | ||||||
| MGMT methylation* | 5506 | 1184 (39.1%) | 847 (42.9%) | 133 (37.3%) | 57 (39.9%) | 0.03 |
| Number of lesions | 31 358 | |||||
| Unifocal | 12 182 (82.6%) | 10 479 (81.0%) | 1968 (83.3%) | 1118 (84.7%) | <0.001 | |
| Multifocal | 2563 (17.4%) | 2452 (19.0%) | 394 (16.7%) | 202 (15.3%) | ||
| Location | 61 614 | <0.001 | ||||
| Frontal lobe | 8367 (28.1%) | 6508 (26.0%) | 1221 (28.3%) | 748 (30.0%) | ||
| Parietal lobe | 4550 (15.3%) | 4011 (16.0%) | 636 (14.7%) | 384 (15.4%) | ||
| Temporal lobe | 7413 (24.9%) | 6399 (25.6%) | 953 (22.1%) | 523 (21.0%) | ||
| Occipital lobe | 1188 (3.99%) | 1159 (4.64%) | 146 (3.38%) | 88 (3.53%) | ||
| Cerebrum | 1245 (4.18%) | 131 (0.52%) | 221 (5.12%) | 103 (4.13%) | ||
| Brainstem | 196 (0.66%) | 51 (0.20%) | 54 (1.25%) | 18 (0.72%) | ||
| Cerebellum | 163 (0.55%) | 131 (0.52%) | 40 (0.93%) | 15 (0.60%) | ||
| Ventricles | 127 (0.43%) | 58 (0.23%) | 30 (0.69%) | 20 (0.80%) | ||
| Overlapping | 4121 (13.8%) | 3668 (14.7%) | 637 (14.8%) | 332 (13.3%) | ||
| Unspecified | 2441 (8.19%) | 2180 (8.72%) | 380 (8.80%) | 260 (10.4%) | ||
*Available data, N = 5506.
Bold denotes statistical significance.
Access to Treatment
Access to and the nature of treatment were significantly different among the groups, as shown in Table 3. Mean time to commence treatment was 7.8 days for privately insured, 8.8 days among Medicare, 8.5 days among Medicaid, and 7.1 days among uninsured patients (P = 0.028). Privately insured patients were more likely to receive therapy of any modality (96.4%) compared with Medicare, Medicaid, and uninsured patients (87.7%, 93.9%, and 92.0%, respectively; P < 0.001). Medicare (12.7%) and uninsured (8.48%) patients were more likely to receive no therapy at all compared with privately insured (3.73%) and Medicaid (6.35%) patients (P < 0.001). When the 3 main treatment modalities (surgery, radiation, and chemotherapy) were analyzed separately, privately insured patients were more likely to receive each individual modality of treatment, and these differences were statistically significant (Table 3). Surgery was performed in 83.9% of privately insured, 72.7% of Medicare, 80.7% of Medicaid, and 80.9% of uninsured patients (P < 0.001). The nature and extent of surgery also slightly varied with insurance type. Medicare and uninsured patients were slightly more likely to receive biopsies (24.7% and 25.6%, respectively) compared with privately insured (21.6%) and Medicaid (21.9%) patients (P < 0.001). Subtotal resections were more common among Medicaid patients (35.6%) versus privately insured (31.8%), Medicare (31.4%), or uninsured (31.0%) patients (P < 0.001). On the contrary, gross total resections were more common among privately insured (34.6%) versus Medicare (32.1%), Medicaid (30.1%), or uninsured (28.4%) patients ((P < 0.001). Radiotherapy was performed in 81.6%, 65.3%, 74.8%, and 68.0% of private, Medicare, Medicaid, and uninsured patients, respectively (P < 0.001). Chemotherapy was performed in 80.8%, 60.5%, 70.5%, and 64.5% of private, Medicare, Medicaid, and uninsured patients, respectively.
Table 3.
Treatment-related variables
| Variable | Available Data (all = ) |
Private Insurance N = 29 811 |
Medicare N = 24 994 |
Medicaid N = 4318 |
Uninsured N = 2491 |
P-value |
|---|---|---|---|---|---|---|
| Receive any treatment, n | 61 341 | 28 635 (96.4%) | 21 794 (87.7%) | 4037 (93.9%) | 2277 (92.0%) | <0.001 |
| TIme to first treatment, mean (SD) | 56 048 | 7.80 (19.8) | 8.82 (20.1) | 8.49 (24.3) | 7.11 (17.2) | 0.028 |
| Surgery, n | 61 585 | 24 989 (83.9%) | 18 158 (72.7%) | 3484 (80.7%) | 2015 (80.9%) | <0.001 |
| Extent of resection | 27 555 | <0.001 | ||||
| Biopsy | 2835 (21.6%) | 2480 (24.7%) | 448 (21.9%) | 297 (25.6%) | ||
| Subtotal | 4170 (31.8%) | 3153 (31.4%) | 729 (35.6%) | 360 (31.0%) | ||
| Gross total | 27 555 | 4537 (34.6%) | 3225 (32.1%) | 616 (30.1%) | 330 (28.4%) | |
| Lobectomy | 1373 (10.5%) | 1034 (10.3%) | 216 (10.5%) | 151 (13.0%) | ||
| Not specified | 186 (1.42%) | 160 (1.59%) | 40 (1.95%) | 22 (1.90%) | ||
| Radiotherapy, n | 61 269 | 24 185 (81.6%) | 16 239 (65.3%) | 3212 (74.8%) | 1675 (68.0%) | <0.001 |
| Chemotherapy, n | 59 683 | 23 397 (80.8%) | 14 646 (60.5%) | 2936 (70.5%) | 1518 (64.5%) | <0.001 |
| Treatment rejection,* n | ||||||
| Surgery | 61 408 | 91 (0.31%) | 369 (1.48%) | 32 (0.74%) | 23 (0.93%) | <0.001 |
| Radiation | 60 220 | 498 (1.70%) | 1334 (5.49%) | 154 (3.65%) | 97 (4.02%) | <0.001 |
| Chemotherapy | 61 207 | 539 (1.82%) | 1297 (5.22%) | 152 (3.54%) | 96 (3.91%) | <0.001 |
| Treatment regimen received, n | 59 541 | <0.001 | ||||
| Nothing | 1078 (3.73%) | 3057 (12.7%) | 264 (6.35%) | 199 (8.48%) | ||
| Chemo alone | 138 (0.48%) | 139 (0.58%) | 19 (0.46%) | 6 (0.26%) | ||
| Radiation alone | 348 (1.20%) | 806 (3.34%) | 79 (1.90%) | 59 (2.51%) | ||
| Surgery alone | 2980 (10.3%) | 4209 (17.4%) | 597 (14.4%) | 434 (18.5%) | ||
| Radiation and chemo | 3125 (10.8%) | 2647 (11.0%) | 450 (10.8%) | 194 (8.26%) | ||
| Surgery and chemo | 683 (2.36%) | 632 (2.62%) | 96 (2.31%) | 57 (2.43%) | ||
| Surgery and radiation | 1136 (3.93%) | 1430 (5.93%) | 281 (6.76%) | 139 (5.92%) | ||
| Surgery, radio and chemo | 19 414 (67.2%) | 11 215 (46.5%) | 2370 (57.0%) | 1260 (53.7%) | ||
*Documented when treatment was recommended by the patient’s physician, but it was refused by the patient, the patient’s family member, or the patient’s guardian AND it was noted in patient record.
Bold denotes statistical significance.
Privately insured patients were more likely to receive the full Stupp protocol with surgery, radiation, and chemotherapy (67.2%) compared with Medicare (46.5%), Medicaid (57.0%), and uninsured (53.7%) patients (P < 0.001). Surgery as monotherapy was performed in 10.3% of privately insured, 17.4% of Medicare, 14.4% of Medicaid, and 18.5% of uninsured patients (P < 0.001). Other combinations and the rates are shown in Table 3.
We assessed whether differences in treatment rates were due to rejection of recommended treatment by patients. The overall rate of treatment rejection was low but significantly higher in Medicare patients than in patients from any other category. Patient rejection for surgery occurred in 1.48% of Medicare versus 0.31% of privately insured, 0.74% of Medicaid, and 0.93% of uninsured patients (P < 0.001). Rejection of radiotherapy occurred in 5.49% of Medicare versus 1.70% of privately insured, 3.65% of Medicaid, and 4.02% of uninsured patients (P < 0.001). Rejection of chemotherapy occurred in 5.22% of Medicare versus 1.82% of privately insured, 3.54% Medicaid, and 3.91% of uninsured patients (P < 0.001).
The differences seen on univariate analysis remained statistically significant on multivariable modeling after controlling for potentially confounding variables with a single exception (Table 4): the odds of receiving surgery for Medicare patients was no longer different relative to patients with private insurance (odds ratio [OR]: 1.03, 95% CI: 0.97–1.09, P = 0.27). The odds of surgery for Medicaid and uninsured patients were 0.72 (95% CI: 0.66–0.79) and 0.77 (95% CI: 0.69–0.87), respectively (P < 0.001). The multivariable odds of receiving radiotherapy was 0.91 (95% CI: 0.86–0.96), 0.62 (95% CI: 0.57–0.68), and 0.47 (95% CI: 0.43–0.52) for Medicare, Medicaid, and uninsured patients, respectively (all P < 0.001). Finally, the odds of receiving chemotherapy were 0.94 (95% CI: 0.89–0.99), 0.53 (95% CI: 0.49–0.57), and 0.42 (95% CI: 0.38–0.46) for Medicare, Medicaid, and uninsured patients, respectively (all P < 0.001).
Table 4.
Results of univariate and multivariable logistic regression analysis for receiving treatment
| Variable* | Univariate | Multivariable** | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Surgery | ||||
| Medicare | 0.51 (0.49–0.53) | <0.001 | 1.03 (0.97–1.09) | 0.31 |
| Uninsured | 0.81 (0.73–0.90) | <0.001 | 0.77 (0.69–0.87) | <0.001 |
| Medicaid | 0.81 (0.74–0.87) | <0.001 | 0.72 (0.66–0.79) | <0.001 |
| Radiotherapy | ||||
| Medicare | 0.43 (0.41–0.44) | <0.001 | 0.91 (0.86–0.96) | <0.001 |
| Uninsured | 0.48 (0.44–0.53) | <0.001 | 0.47 (0.43–0.52) | <0.001 |
| Medicaid | 0.67 (0.62–0.73) | <0.001 | 0.62 (0.57–0.68) | <0.001 |
| Chemotherapy | ||||
| Medicare | 0.37 (0.35–0.38) | <0.001 | 0.94 (0.89–0.99) | 0.02 |
| Uninsured | 0.43 (0.40–0.47) | <0.001 | 0.41 (0.38–0.46) | <0.001 |
| Medicaid | 0.57 (0.53–0.61) | <0.001 | 0.53 (0.49–0.57) | <0.001 |
*Reference category is private insurance.
**Control for age, sex, race, ethnicity, distance traveled, Charlson comorbidity score, facility type, and facility location.
Bold denotes statistical significance.
The impact of geographic region on outcome was addressed by including an interaction term for region in the logistic regression model. The associated P-values using the likelihood ratio method were 0.19 (surgery), 0.07 (chemotherapy), and <0.001 (radiotherapy). Subgroup analysis for radiotherapy revealed lower odds for radiotherapy for uninsured and Medicaid patients in all regions, whereas Medicare patients had significantly lower odds of receiving radiotherapy only in the Midwest (OR: 0.84, 95% CI: 0.75–0.95, P = 0.005). The results of the subgroup analysis by region are summarized in Supplementary Table S2.
Survival Analysis
Overall, median survival was 14.8 months for privately insured patients, 5.8 months for Medicare patients, 12.6 months for Medicaid patients, and 12.5 months for the uninsured (Wilcoxon and log-rank P < 0.001) as shown in Supplementary Figure S1. When patients receiving the full Stupp protocol were held as the reference group, patients who underwent surgery alone (hazard ratio [HR] 1.89, 95% CI: 1.83–195, P < 0.001), those who underwent surgery with radiation (HR 1.60, 95% CI: 1.54–1.68, P < 0.001), and those who underwent surgery with chemotherapy (HR 1.24, 95% CI: 1.16–1.32, P < 0.001) had significantly higher risk of death (Table 5). Furthermore, compared with private insurance, Medicaid (HR 1.10, 95% CI: 1.05–1.16, P < 0.001), uninsured (HR 1.06, 95% CI: 1.001–1.13, P = 0.048), and Medicare patients (HR 1.11, 95% CI: 1.09–1.16, P < 0.001) had significantly worse survival after adjusting for age, sex, race, Charlson comorbidity index, region, and treatment facility type.
Table 5.
Results of multivariable Cox proportional hazards regression analysis*
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Insurance type | ||
| Private | Ref | Ref |
| Medicare | 1.11 (1.09–1.16) | <0.001 |
| Uninsured | 1.06 (1.001–1.13) | 0.048 |
| Medicaid | 1.10 (1.05–1.16) | <0.001 |
| Treatment type | ||
| Surgery-radiation-chemotherapy | Ref | Ref |
| Surgery alone | 1.89 (1.83–1.95) | <0.001 |
| Surgery and radiation | 1.60 (1.54–1.68) | <0.001 |
| Surgery and chemotherapy | 1.24 (1.16–1.32) | <0.001 |
*Adjusting for age, sex, race, distance, traveled, Charlson comorbidity index, region, and type of facility.
Bold denotes statistical significance.
Discussion
Herein we present an analysis of patients in the United States undergoing treatment for GBM, as reported to a large national database (NCDB), with attention paid to patients’ insurance status. Demographic factors, treatment paradigms, and outcomes have been correlated with SES in other malignancies, and we sought to investigate whether this pattern held true in GBM.14–18 Unlike data shown in multiple other malignancies, where poverty and low SES are associated with increased incidence, there are some data suggesting that a higher SES correlates with an increased GBM risk.2 However, the impact of SES on survival for patients with GBM is unclear. A single-institution study showed no impact of SES on patient survival, although in the study SES did not predict insurance status or employment.3 A recent Australian study comparing factors associated with long- versus short-term survival found that only participation in a clinical trial predicted survival >2 years and no statistically significant socioeconomic factors were identified.4 In West Scotland, where access to and timing of care is regulated by the UK’s National Health Service, median overall survival for GBM patients improved following the introduction of the Stupp protocol from 10.7 to 15.3 months. There was concomitant improvement in 2-year survival from 12% to 19% with age, greater extent of surgical resection, and postoperative chemoradiotherapy independently associated with improved survival.5
As the standard of care for GBM involves surgery and intensive postsurgical treatment with radiation and chemotherapy, we hypothesized that those patients with less access to care would have a lower likelihood of receiving full standard treatment and, as a result, experience worse outcomes. Specifically, we surmise that differences in outcome are less likely the result of intrinsic socioeconomic conditions per se, and more likely a direct result of inadequate access to health care, a less ambiguous concept to quantify.19 We put forth insurance status as an effective surrogate for “access” to care that captures some socioeconomic disparities, given the complex insurance landscape in the US with a preponderance of employer-provided private insurance; Medicaid, which offers assistance to low-income citizens; Medicare, which is available primarily to those over 65 years of age; and large numbers of uninsured patients given the absence of universal coverage.
Medicare, Medicaid, and uninsured patients were significantly less likely to receive any given treatment modality and less likely to complete the full Stupp protocol than privately insured patients. These differences (with the exception of Medicare patients receiving any surgery) remained significant on multivariable analysis controlling for differences in patient populations, including age and comorbid MGMT status (for the small proportion of cases where MGMT data were available). Completion of the full protocol was associated with longer overall survival. Privately insured patients traveled farthest for care, likely reflecting the luxury of choice and/or distance from tertiary centers, which tend to be located in urban areas. Consistent with this assumption, Medicaid patients traveled the shortest distance. The time to get to treatment was not clinically significant among the groups.
Medicare patients are older by definition, and age may affect GBM outcome in many important ways. Older patients have lower life expectancy at baseline, often present with lower KPS, and often are poorer surgical candidates. Furthermore, older age may legitimately change the treatment paradigm for some patients as new data continue to emerge to suggest, for example, that limited chemotherapy and radiotherapy regimens may provide equivalent survival with reduction in treatment-related morbidity, and thus older patients may opt out of pursuing aggressive measures or have comorbid health concerns precluding aggressive intervention.6–9 Nonetheless, on multivariable analysis, Medicare patients were as likely to undergo surgery as privately insured patients. However, privately insured patients were more likely to undergo gross total resection, whereas Medicaid and uninsured patients were more likely to undergo biopsy or subtotal resections. These differences remained significant after controlling for facility type. There is no evidence to support significant differences in the size or location of the lesions across the groups, so the reason for the disparate extent of resection remains unclear. These observations are further validated by the most recent report by the Central Brain Tumor Registry of the United States.20
That there is any distinction in patient treatment and outcome based on insurance type bears important consideration given the idiosyncrasies of the US health care system. Health care spending in the United States outstrips that of other high-income member nations of the Organisation for Economic Co-operation and Development. The US spent 17.1% of its gross domestic product (GDP) on health care in 2013, 50% higher than France (11.6% of GDP), the second highest spender on health care and almost twice that of the UK (8.8% of GDP). This is in spite of relatively fewer physicians per capita, and such spending appears to yield suboptimal benefit, with the US lagging behind nations that spend significantly less on health care in critical metrics such as life expectancy, outcomes with chronic diseases, and even outcomes with specific malignancies.21,22
The comparatively unique health insurance landscape in the US is suggested as a potential cause of these discrepancies.23 While many developed nations have government-sponsored health care, patients in the United States under age 65 are most often covered by private insurance through their employer, with those unable to obtain such insurance left uninsured or covered by state-sponsored programs for those with limited income (Medicaid). These disparate mechanisms of coverage confer differential access to health care, as not all providers or facilities accept all types of insurance, while differences in the out-of-pocket costs may significantly impact decision making regarding plan of care.
In our analysis of GBM patients in the NCDB, the majority were covered by either private insurance or Medicare. Only 6.8% of patients in our study were uninsured, a significantly lower number than the estimated 20.4% of uninsured US citizens under 65 years of age in 2013.24 This likely has to do with the demographic characteristics of GBM as a disease, affecting predominantly older individuals (covered by Medicare) and whites (a subpopulation with higher average SES and a higher likelihood of having private health insurance). Indeed, in our study population, most patients were between the fifth and seventh decades of life, and the overwhelming majority of privately insured patients were white, whereas black or Hispanic patients were more likely to be uninsured or have Medicaid coverage. Similar distribution has been reported in prior SEER papers as well.6–8
These findings have significant implications for caring for patients with GBM. Given that the overall survival in GBM is quite poor and that the current treatment paradigm of surgery, chemotherapy, and radiation remains the only proven modality for prolonging survival, factors impeding patient access to care can have a direct and meaningful impact on life expectancy. Such a discrepancy has broad social implications as the health insurance landscape continues to evolve and underscores the idea that simply being insured in some form does not necessarily imply equivalent access to care, even in the case of a catastrophic diagnosis.
Limitations
This study has several limitations as well. First, it is retrospective in nature and there is a risk for coding misclassification. Second, we analyzed patients by insurance status at the time of diagnosis; patients might change insurance during the course of their treatment. However, given that treatment plan for GBM is crafted at the time of diagnosis and the median survival for GBM is very short (ie, ~15 months), the risk of misclassifying patients and its impact on our findings is mitigated. Third, the mix of patients in the cohort was heavily skewed toward patients with either private insurance or Medicare, while comparably fewer uninsured or Medicaid patients were included. The term “private insurance” includes a disparate array of specific insurance types with high variability in terms of services covered and actual costs to the insured, and these differences are not captured in the database. Fourth, there is a risk for residual confounding despite conducting multivariable regression analysis. Given the limited availability of baseline demographics and characteristics (age, sex, race, and Charlson comorbidity score) in the NCDB, it is unlikely that propensity score analysis would have conferred different estimates of effect compared with multivariable logistic regression. In addition, propensity score analysis increases the risk of discarding statistical information (ie, decreases sample size) in attempts to create identical groups.25 Regardless of these limitations, we present evidence that patients with less robust medical insurance are provided with fewer treatment options and that they have poorer overall survival when controlling for relevant demographic factors.
Conclusions
Insurance status and type of insurance coverage appear to impact treatments rendered for GBM, independently of other variables. Furthermore, we find that such differential access to care significantly impacts survival. Given the poor prognosis associated with GBM, any incremental improvement in survival time can be quite meaningful. Thus, in a wider effort to improve outcomes for patients with GBM, adequate access to care should play a central role in optimizing survival.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
None
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Supplementary Material
Acknowledgments
We would like to thank Ms Lindsey Sangaralingham, MPH for her assistance with the statistical analysis of the manuscript.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Field KM, Rosenthal MA, Yilmaz M, Tacey M, Drummond K. Comparison between poor and long-term survivors with glioblastoma: review of an Australian dataset. Asia Pac J Clin Oncol. 2014;10(2):153–161. [DOI] [PubMed] [Google Scholar]
- 3. Kasl RA, Brinson PR, Chambless LB. Socioeconomic status does not affect prognosis in patients with glioblastoma multiforme. Surg Neurol Int. 2016;7(Suppl 11):S282–S290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sia Y, Field K, Rosenthal M, Drummond K. Socio-demographic factors and their impact on the number of resections for patients with recurrent glioblastoma. J Clin Neurosci. 2013;20(10):1362–1365. [DOI] [PubMed] [Google Scholar]
- 5. Tseng JH, Merchant E, Tseng MY. Effects of socioeconomic and geographic variations on survival for adult glioma in England and Wales. Surg Neurol. 2006;66(3):258–263; discussion 263. [DOI] [PubMed] [Google Scholar]
- 6. Rong X, Yang W, Garzon-Muvdi T, et al. Influence of insurance status on survival of adults with glioblastoma multiforme: a population-based study. Cancer. 2016;122(20):3157–3165. [DOI] [PubMed] [Google Scholar]
- 7. Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107(1):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thumma SR, Fairbanks RK, Lamoreaux WT, et al. Effect of pretreatment clinical factors on overall survival in glioblastoma multiforme: a Surveillance Epidemiology and End Results (SEER) population analysis. World J Surg Oncol. 2012;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohanty S, Bilimoria KY. Comparing national cancer registries: the national cancer data base (NCDB) and the surveillance, epidemiology, and end results (SEER) program. J Surg Oncol. 2014;109(7):629–630. [DOI] [PubMed] [Google Scholar]
- 10. Commission on Cancer. American College of Surgeons. https://www.facs.org/quality-programs/cancer/coc. Accessed January 25, 2018. [Google Scholar]
- 11. Harrell FE. Multivariable modeling strategies. In: Frank E. Harrell, Jr, eds. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Cham: New York, Dordrecht, Heidelberg, and London: Springer International Publishing; 2015:63–102. [Google Scholar]
- 12. Kleinbaum DG, Klein M.. Survival Analysis: A Self-Learning Text. Springer Science & Business Media; 2005. [Google Scholar]
- 13. Therneau T. A Package for Survival Analysis in S. version 2.38. http://cran.r-project.org/package=survival; 2015. [Google Scholar]
- 14. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–496. [DOI] [PubMed] [Google Scholar]
- 15. Aarts MJ, Lemmens VE, Louwman MW, Kunst AE, Coebergh JW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46(15):2681–2695. [DOI] [PubMed] [Google Scholar]
- 16. Penson DF, Stoddard ML, Pasta DJ, Lubeck DP, Flanders SC, Litwin MS. The association between socioeconomic status, health insurance coverage, and quality of life in men with prostate cancer. J Clin Epidemiol. 2001;54(4):350–358. [DOI] [PubMed] [Google Scholar]
- 17. Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harari A, Li N, Yeh MW. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2014;99(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99(9):1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 20. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. [DOI] [PubMed] [Google Scholar]
- 22. Sunkara V, Hébert JR. The colorectal cancer mortality-to-incidence ratio as an indicator of global cancer screening and care. Cancer. 2015;121(10):1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wubulihasimu P, Brouwer W, van Baal P. The impact of hospital payment schemes on healthcare and mortality: evidence from hospital payment reforms in OECD countries. Health Econ. 2016;25(8):1005–1019. [DOI] [PubMed] [Google Scholar]
- 24. Cohen RA, Martinez ME. Health insurance coverage: early release of estimates from the National Health Interview Survey, January–March 2014. Natl Vital Stat Rep. 2014. https://pdfs.semanticscholar.org/6731/d57a1c79d2e1049a80092d3219f510167665.pdf. Accessed January 12, 2017. [Google Scholar]
- 25. King G, Nielsen R. Why propensity scores should not be used for matching. Copy at http://j mp/1sexgVw Download 2016. http://www.polmeth.wustl.edu/files/polmeth/psnot4.pdf. Accessed January 12, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.