Abstract
Background
To overcome challenges with traditional response assessment in anti-angiogenic agents, the current study uses T1 subtraction maps to quantify volumetric radiographic response in monotherapy with cabozantinib, an orally bioavailable tyrosine kinase inhibitor with activity against vascular endothelial growth factor receptor 2 (VEGFR2), hepatocyte growth factor receptor (MET), and AXL, in an open-label, phase II trial in patients with recurrent glioblastoma (GBM) (NCT00704288).
Methods
A total of 108 patients with adequate imaging data and confirmed recurrent GBM were included in this retrospective study from a phase II multicenter trial of cabozantinib monotherapy (XL184-201) at either 100 mg (N = 87) or 140 mg (N = 21) per day. Contrast enhanced T1-weighted digital subtraction maps were used to define volume of contrast-enhancing tumor at baseline and subsequent follow-up time points. Volumetric radiographic response (>65% reduction in contrast-enhancing tumor volume from pretreatment baseline tumor volume sustained for more than 4 wk) was tested as an independent predictor of overall survival (OS).
Results
Volumetric response rate for all therapeutic doses was 38.9% (41.4% and 28.6% for 100 mg and 140 mg doses, respectively). A log-linear association between baseline tumor volume and OS (P = 0.0006) and a linear correlation between initial change in tumor volume and OS (P = 0.0256) were observed. A significant difference in OS was observed between responders (median OS = 20.6 mo) and nonresponders (median OS = 8.0 mo) (hazard ratio [HR] = 0.3050, P < 0.0001). Multivariable analyses showed that continuous measures of baseline tumor volume (HR = 1.0233, P < 0.0001) and volumetric response (HR = 0.2240, P < 0.0001) were independent predictors of OS.
Conclusions
T1 subtraction maps provide value in determining response in recurrent GBM treated with cabozantinib and correlated with survival benefit.
Keywords: cabozantinib, GBM, XL184, recurrent glioblastoma, T1 subtraction
Importance of the study
GBM is the most common malignant brain tumor in adults, and treatment options for patients with recurrent GBM are significantly limited. Cabozantinib is an oral inhibitor of tyrosine kinases, including VEGFR2, MET, and AXL, which are implicated in the pathophysiology of GBM. Previous studies examining radiographic response in anti-VEGF therapies have been challenging due to reduction in vascular permeability; however, T1 subtraction maps may overcome these challenges and allow for more accurate estimates of tumor burden. The current study demonstrates that patients with recurrent GBM who exhibit a significant and sustained reduction in contrast-enhancing tumor volume on T1 subtraction maps following treatment with cabozantinib exhibit a significant survival advantage. This suggests that T1 subtraction may be a better way of estimating tumor burden and that early changes may be a surrogate for clinical activity of cabozantinib.
Glioblastoma (GBM) is the most common malignant brain tumor in adults and accounts for more than 54% of all gliomas and 45% of all malignant primary brain and CNS tumors.1 Median survival for non-elderly patients with GBM is only around 14 months,2 with fewer than 10% of patients surviving beyond 5 years following initial diagnosis.3 Standard of care for newly diagnosed GBM patients includes maximum surgical resection followed by radiotherapy plus concomitant temozolomide2 until tumor recurrence or relapse. Upon recurrence, however, very few effective therapeutic options exist. Thus, there continues to be an unmet need for drug development in the setting of recurrent GBM.
GBM is a highly vascularized tumor, which is thought to result from overproduction of pro-angiogenic growth factors, including vascular epithelial growth factor (VEGF).4,5 However, targeted inhibition of VEGF alone using bevacizumab, a humanized monoclonal antibody for VEGF-A, has shown only limited efficacy.6–10 Preclinical data suggest that overexpression of hepatocyte growth factor receptor (MET) in GBM may aid in eventual resistance to bevacizumab,11,12 suggesting that targeted inhibition of both MET and VEGF may overcome these challenges. Further, studies in a variety of tumor types have suggested that chronic inhibition of VEGF results in upregulation of both MET and AXL,13,14 and inhibition of AXL during anti-VEGF therapy also increases efficacy through prolonging resistance.15,16 Further, both MET12,17–19 and AXL20–23 have been implicated in GBM pathogenesis and are associated with poor prognosis in patients with GBM. Thus, inhibition of MET, AXL, and VEGF may have a significant impact on outcomes in patients with recurrent GBM.
Cabozantinib is an oral tyrosine kinase inhibitor (TKI) with activity against VEGF receptors, MET, and AXL.24,25 Preclinical data have shown suppression of MET and VEGF receptor 2 (VEGFR2) signaling with improved survival in a mouse xenograft model,26 and clinical trials in solid tumors have demonstrated tumor regression.27–29 A recent open-label, phase II trial in patients with recurrent GBM has shown some evidence of clinical activity30,31; however, radiographic response evaluation in patients receiving anti-VEGF therapy can be particularly challenging due to the dramatic changes in contrast enhancement from decreased vascular permeability regardless of antitumor activity.32–37 Previous studies have shown that digital subtraction of pre- from postcontrast T1-weighted images, or “T1 subtraction maps,” improves visualization and volumetric quantification of subtly enhancing tumor. Additionally, early changes in contrast-enhancing tumor volume defined using T1 subtraction maps have been shown to predict long-term clinical outcome in recurrent GBM treated with bevacizumab.38 Therefore, in the current study we examine whether early radiographic response defined using quantitative tumor volume estimates from T1 subtraction maps could predict overall survival (OS) in an open-label, phase II, multicenter clinical trial of bevacizumab-naïve recurrent GBM patients treated with cabozantinib (NCT00704288).
Methods
Patients
Included in the current retrospective study were 152 patients who had not previously failed anti-angiogenic therapy (ie, bevacizumab, cediranib, or pazopanib) of the 222 total patients enrolled in XL184-201, a multicenter (8 sites), phase II, open-label, uncontrolled study of cabozantinib, a TKI with principal targets of MET, VEGFR2, AXL, and RET, at doses of 140 or 100 mg (free base equivalent weight, oral, daily) in patients with recurrent GBM at first or second relapse. Of these patients, a total of 108 (N = 87 for 100 mg and N = 21 for 140 mg) had serial MR images (at least 3 to confirm response) with sufficient quality available for volumetric analyses. The median age for these patients was 56.5 years (range = 21–73) and, of the patients included, approximately 65% were male. All patients included had Karnofsky performance status of more than 70 at baseline. The study spanned from June 2008 through July 2014. In all patients, initial standard radiation therapy and chemotherapy (concurrent radiation therapy and temozolomide treatment) failed, and radiation therapy (or previous investigational drugs) had been completed more than 12 weeks previously. Baseline images were obtained within 14 days prior to treatment according to study guidelines, and follow-up imaging was performed every 6 to 8 weeks until disease progression. The current imaging analysis was performed retrospectively using data from the study sponsor (Exelixis). All participants in XL184-201 signed institutional review board–approved informed consent at their respective institutions prior to enrolling in the multicenter clinical trial. Specific inclusion and exclusion criteria for this trial can be found at clinicaltrials.gov/ct2/show/NCT00704288.
Magnetic Resonance Imaging
Anatomic MR images were acquired for all patients in the current study using a 1.5T or 3T clinical MR scanner using pulse sequences supplied by their respective manufacturers and according to their local standard-of-care protocols. Standard anatomic images consisted of precontrast, 2D axial T1-weighted fast spin-echo (repetition time (msec)/echo time (msec) = 400–3209/3.6– 21.9; slice thickness = 3–6.5 mm; intersection gap = 0–2.5 mm; number of averages = 1–2; matrix size = 176–512 × 256–512; and field of view = 24–25.6 cm) along with T2-weighted fast spin-echo and fluid-attenuated inversion-recovery (FLAIR) images. In addition, parameter matched axial 2D T1-weighted fast spin-echo images enhanced with gadopentetate dimeglumine (Magnevist, Berlex), 0.1 mmol/kg, were acquired shortly after contrast material injection, followed by MPRAGE (3D magnetization-prepared rapid acquisition gradient-echo) sequences in most patients.
Contrast-Enhanced T1-Weighted Digital Subtraction Maps
Contrast-enhanced T1-weighted subtraction maps (Fig. 1) were created using parameter matched pre- and postcontrast axial 2D T1-weighted images and techniques previously described.38 First, linear registration was performed between all images, including contrast-enhanced T1-weighted images and T2-weighted and/or FLAIR images to non-enhanced T1-weighted images using a 12 degree-of-freedom transformation and a correlation coefficient cost function in FSL (FLIRT; FMRIB Software Library, Oxford, England; http://www.fmrib.ox.ac.uk/fsl/). Next, “Gaussian normalization” of image intensity for both non-enhanced and contrast-enhanced T1-weighted images was performed using custom c-code and bash scripts, courtesy of the National Institute of Mental Health Magnetoencephalography Core Facility (3dNormalize; NIMH MEG Core, kurage.nimh.nih.gov/meglab/Meg/3dNormalize), which normalizes image intensity by dividing each voxel by the standard deviation of the image intensity from the whole brain [SNor(x,y,z) = S (x,y,z)/σWB], where S is raw image signal intensity, Nor is normalized, x,y,z are voxel coordinates, and WB is whole brain. Next, bias field correction was performed (FAST segmentation; FLIRT; FMRIB Software Library, Oxford, England; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST) and voxel-by-voxel subtraction between normalized non-enhanced and contrast-enhanced T1-weighted images was performed. Image voxels with a positive (greater than zero) before-to-after change in normalized contrast enhancement signal intensity (ie, voxels increasing in MR signal after contrast agent administration) within T2-weighted FLAIR hyperintense regions were isolated to create the final T1 subtraction maps by thresholding the T2-weighted FLAIR images based on relative signal intensity (ie, intersection of binary mask created by empirical thresholding by FLAIR hyperintensity and positive contrast from T1 subtraction). Estimates of tumor volume included areas of contrast enhancement on T1 subtraction maps including central necrosis (defined as being enclosed by contiguous, positive enhancing disease). A team of trained lab technologists created initial segmentations, and all final volumes were reviewed by a single investigator (B.M.E.) who was blinded to other relevant metrics until study completion. Volumetric response was defined as a sustained decrease of enhancing volume greater than 65% for at least 4 weeks, as described previously.39–41
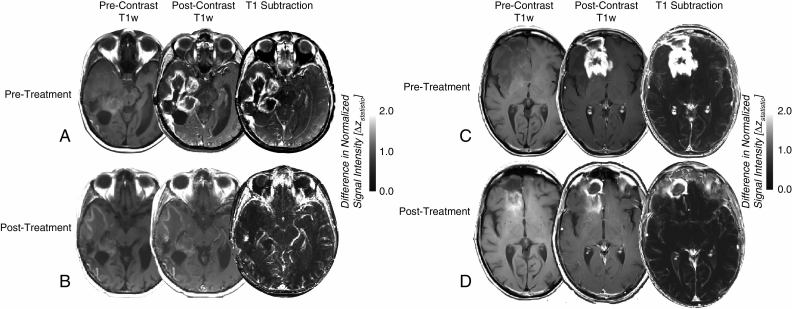
Fig. 1.
Precontrast T1-weighted images, postcontrast T1-weighted images, and contrast-enhanced T1-weighted digital subtraction maps in 2 representative recurrent GBM patients with a sustained volumetric response following treatment with 100 mg of cabozantinib. (A) Pretreatment and (B) posttreatment MRI scans showing precontrast T1-weighted images (left column), postcontrast T1-weighted images (middle column), and T1 subtraction maps (right column) in a 62-year-old patient with an OS of 16.8 months following treatment. (C) Pretreatment and (D) posttreatment MRI scans showing precontrast T1-weighted images, postcontrast T1-weighted images, and T1 subtraction maps in a 57-year-old patient who was still alive (censored) 20.5 months following treatment. Note that in both cases, as in many patients exhibiting a durable response, we observed significant T1 shortening on precontrast T1-weighted images following therapy, which resulted in a significant decrease in true contrast-enhancing tumor burden following digital subtraction.
Statistical Analysis
A log-linear regression model was created to examine the association between baseline enhancing tumor volume and OS [OS = a•log10(Volume)+b] in patients who were not censored (82 of 108). Similarly, a linear model was used to explore the influence of initial changes (ie, posttreatment volume minus pretreatment volume) in enhancing volume and OS (OS = a•(Change in Volume)+b) in patients who were not censored. Univariate, log-rank analysis on Kaplan–Meier data was performed to compare durable volumetric radiographic responders (as defined by a sustained decrease of enhancing volume >65% for at least 4 wk) with nonresponders for patients treated with 100 mg or 140 mg and pooled patients from both doses. A multivariable Cox regression model including age, baseline tumor volume, treatment dose, and radiographic response was used to predict OS. All statistical tests were performed using GraphPad Prism v7.0c or Stata v12.
Results
T1 subtraction maps provided clear benefit for delineating contrast-enhancing tumor from surrounding tissue in patients treated with cabozantinib. Fig. 1 illustrates representative images from 2 patients with recurrent GBM who experienced a sustained volumetric response using T1 subtraction maps after treatment with cabozantinib at a dose of 100 mg. In both of these cases, as with many patients who experienced volumetric response, a significant level of T1 shortening (hyperintensity) was observed on precontrast T1-weighted images following initial treatment (Fig. 1B, D). After T1 subtraction it was apparent that most of the observed residual contrast enhancement on postcontrast T1-weighted images following therapy was likely attributed to precontrast T1 shortening, resulting in a relatively small volume of posttreatment contrast enhancement tumor burden.
Approximately 38.9% of patients (42 of 108) with recurrent GBM treated with cabozantinib at either dose experienced a durable volumetric radiographic response, as defined by a sustained decrease on enhancing volume greater than 65% for at least 4 weeks (Table 1). In patients treated with 100 mg of cabozantinib (N = 87) and patients treated with 140 mg of cabozantinib (N = 21), 41.4% (36 of 87) and 28.6% (6 of 21), respectively, experienced a confirmed volumetric response.
Table 1.
Analysis of OS by durable volumetric response (VR)
| 100 mg (N = 87) | 140 mg (N = 21) | All (N = 108) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | VR (N = 36) |
Non-VR (N = 51) |
Total (N = 87) |
VR (N = 6) |
Non-VR (N = 15) |
Total (N = 21) |
VR (N = 42) |
Non-VR (N = 66) |
Total (N = 108) |
| Deaths, n (%) | 22 (61.1%) | 41 (80.4%) | 63 (72.4%) | 4 (66.7%) | 15 (100%) | 19 (90.5%) | 26 (61.9%) | 56 (84.8%) | 82 (75.9%) |
| Censored, n (%) | 14 (38.9%) | 10 (19.6%) | 24 (27.6%) | 2 (33.3%) | 0 (0%) | 2 (9.5%) | 16 (38.1%) | 10 (15.2%) | 26 (24.1%) |
| Median OS, mo | 16.8 | 8.3 | 12.1 | 34.4 | 5.9 | 6.9 | 20.6 | 8.0 | 11.0 |
N = number of subjects in the specified population.
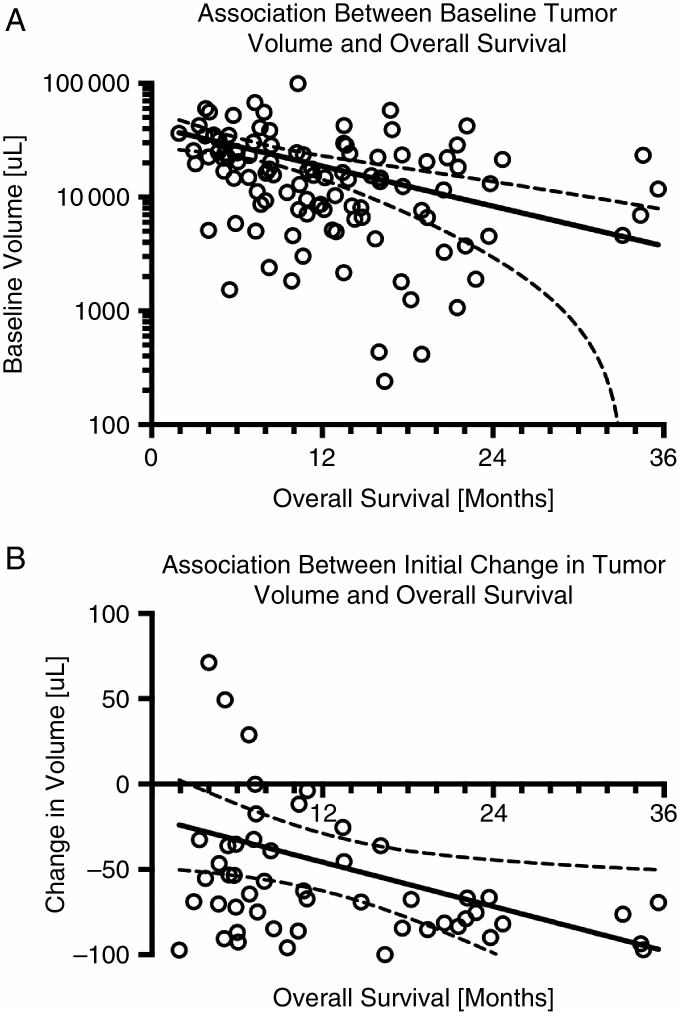
As reported previously, baseline enhancing tumor volume is an independent and significant prognostic factor for OS in patients treated with cabozantinib.42 A reexamination of this previous data suggests that a simple log-linear model can be used to predict OS given only the volume of contrast-enhancing tumor prior to treatment (Fig. 2A; OS = −4.7•log10(Volume) + 31.7 mo; P = 0.0006). Examination of the initial change in contrast-enhancing tumor volume following administration of cabozantinib (Fig. 2B) suggests a linear relationship between the degree of tumor shrinkage and OS benefit (OS = −0.04•(Change in Volume) + 10.6 mo; P = 0.0256).
Fig. 2.
Log-linear or linear associations between baseline or change in contrast-enhancing tumor burden and OS following treatment with cabozantinib in recurrent GBM. (A) Log-linear (OS = a•log10(Volume)+b) association between baseline tumor volume and OS. (B) Linear (OS = a•(Change in Volume) + b) association between initial change in tumor volume and OS. Solid lines represent best fit line after log-linear or linear regression and dashed lines represent 95% CIs for the respective model fits.
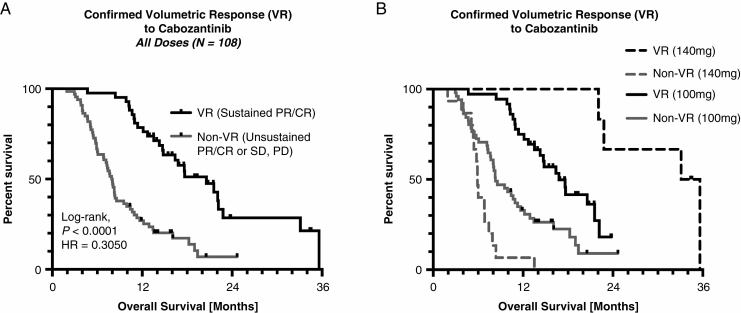
Univariate log-rank analysis suggested a significant survival advantage in patients exhibiting a confirmed volumetric response (median OS = 20.6 mo) compared with patients who did not respond (median OS = 8.0 mo) when pooling patients from both dose levels (Fig. 3A; hazard ratio [HR] = 0.3050, P < 0.0001). When splitting patients by dose, significant survival differences still remained between volumetric responders and nonresponders (Fig. 3B). In particular, patients treated with either 100 mg or 140 mg of cabozantinib who exhibited a sustained volumetric response had a significantly longer OS (HR = 0.3842 and HR = 0.1132, respectively; P < 0.0001). Additionally, patients who responded to 140 mg had a significantly longer OS compared with responders treated with 100 mg (HR = 0.3448, P = 0.0211), suggesting there may be dose-dependent survival benefit for patients with recurrent GBM treated with cabozantinib. Multivariable Cox regression showed that continuous baseline tumor volume (HR = 1.0233, P < 0.0001) and confirmed volumetric response (HR = 0.2240, P < 0.0001) were independent predictors of OS, while age (HR = 1.0038, P = 0.7022) and dose level (HR = 0.9885, P = 0.1368) were not statistically significant independent predictors when accounting for these other covariates (Table 2).
Fig. 3.
Kaplan–Meier plots showing the association between confirmed volumetric response (VR) and OS in recurrent GBM patients treated with cabozantinib. (A) Kaplan–Meier plots demonstrating a significant survival advantage in patients with a confirmed VR treated with cabozantinib pooled across both 100 mg and 140 mg dose levels (log-rank, P < 0.0001, HR = 0.3050). (B) Kaplan–Meier survival plots showing a significant survival advantage in patients with confirmed VR treated with 100 mg and 140 mg. Note that patients with confirmed response treated with 140 mg had a significantly longer OS compared with volumetric responders treated with 100 mg (log-rank, P = 0.0211).
Table 2.
Multivariate Cox regression model results including age, treatment dose (100 mg vs. 140 mg), baseline contrast-enhancing lesion volume, and confirmed volumetric responders versus nonresponders
| Variable | Coefficient | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Age | 0.0038 ± 0.0100 | 1.0038 | (0.9844–1.0236) | 0.7022 |
| (Continuous) | ||||
| Dose | 0.0115 ± 0.0077 | 1.0116 | (0.9964–1.0270) | 0.1368 |
| (100 mg or 140 mg) | ||||
| Pretreatment volume | 0.0231 ± 0.0055 | 1.0233 | (1.0123–1.0345) | < 0.0001 |
| [uL] (Continuous) | ||||
| Responders vs nonresponders | -1.4963 ± 0.2662 | 0.2240 | (0.1329–0.3774) | < 0.0001 |
Discussion
The current study indicates that T1 subtraction maps provide equivalent (eg, Fig. 1A, C) or improved (eg, Fig. 1B, D) visualization and detailed demarcation for quantitation of enhancing disease burden in GBM. Results from the current trial provide additional support for the use of T1 subtraction maps to estimate change in contrast-enhancing tumor volume as an early surrogate of treatment efficacy in recurrent GBM based on initial response, similar to those reported previously after treatment with bevacizumab.38 A recent study by Gahrmann et al43 examining bevacizumab therapy in the BELOB trial suggests that T1 subtraction maps provided only equivalent performance for identifying time to progression compared with postcontrast T1-weighted images alone. This suggests that the largest added value of T1 subtraction in recurrent GBM may be in assessment of early response, rather than early disease progression, in anti-angiogenic therapies. Importantly, the current study indicates that patients with a large and sustained reduction in contrast-enhancing tumor volume following cabozantinib therapy will experience more than twice the survival benefit compared with patients who do not. This large and clinically meaningful difference in OS implies that early changes in contrast-enhancing tumor volume using T1 subtraction may be a robust imaging biomarker for OS benefit in recurrent GBM.
The rates of confirmed volumetric response (38.9% overall, 41.1% for 100 mg, and 28.6% for 140 mg) in the current study (N = 108) were substantially higher than the objective response rates reported for this same trial (N = 152) by an independent radiological facility using criteria from Response Assessment in Neuro-Oncology (RANO) (14.5% and 17.6% for 100 mg and 140 mg doses, respectively30). These rates were similar to response rates reported for bevacizumab using traditional postcontrast T1-weighted images and modified Macdonald or RANO criteria6,44,45 (28%–63%), yet higher than traditional chemotherapies like irinotecan.46,47 Interestingly, patients treated with cabozantinib who had long posttreatment OS had notable T1 shortening on precontrast T1-weighted images. This was in notable contrast to patients with long posttreatment OS following bevacizumab, in which significant T1 hypointensity was observed. Subsequent studies aimed at determining the source of post-cabozantinib T1 shortening in recurrent GBM may be warranted to determine whether it has biologic or prognostic significance.
Despite occurring less frequently, a volumetric response in patients treated with 140 mg appeared to impart a longer OS compared with 100 mg. Although speculative given the small sample size, this may suggest increased therapeutic efficacy with increased dose, so long as tumor shrinkage is observed early after the start of therapy. Additional studies at higher doses may be warranted provided they are safe and tolerated,30 as the 140 mg/day dose has been approved for treatment of medullary thyroid cancer based on results from the pivotal phase III trials.27,48
The current study had a few limitations that should be addressed. First, this study was retrospective and as such the conclusions were solely based on the data available for advanced image analyses wherein only 108 of the 152 patients treated in the trial had sufficient imaging for analysis.31 Additionally, lesion segmentation was not fully automated or controlled and involved multiple layers of human interpretation. Thus, some variability in lesion size measurement and response rate may have been present and unaccounted for. Lastly, the current study does not involve a formal comparison to the traditional RANO criteria, so questions still remain regarding the added value of the current technique with respect to other, more accepted approaches commonly used in clinical trials.
In conclusion, results from the current study suggest that volumetric analysis using T1 subtraction maps represents a detailed and informative assessment method to evaluate response of GBM patients treated with anti-angiogenic therapy. Results support the hypothesis that volumetric response predicts OS in patients treated with cabozantinib and suggests that cabozantinib may have clinical activity in more than one third of recurrent anti-angiogenic therapy naïve GBM patients as evidenced by a durable volumetric response using T1 subtraction maps associated with a significant survival benefit.
Funding
This work was supported by a National Brain Tumor Society Research Grant (B.M.E., T.F.C.); American Cancer Society Research Scholar Grant (RSG-15-003-01-CCE) (B.M.E.); Art of the Brain (T.F.C.); UCLA SPORE in Brain Cancer (NIH/NCI 1P50CA211015-01A1) (B.M.E., T.F.C.); NIH/NCI 1R21CA223757-01 (B.M.E.)
Acknowledgments
All authors contributed equally to data collection, editing and manuscript review, and data interpretation. B.M.E. contributed to figures, study design, data analysis, and writing.
Conflict of interest statement. B.M.E., P.Y.W., and T.F.C. are paid consultants, members of the advisory board, and research grant recipients of Roche/Genentech. D.T.A., G.M.S., and C.H. are paid employees and stockholders for Exelixis.
References
- 1. Ostrom QT, Gittleman H, Farah P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15 (Suppl 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78(3):233–247. [DOI] [PubMed] [Google Scholar]
- 5. Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–848. [DOI] [PubMed] [Google Scholar]
- 6. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 7. Kreisl TN, Kim L, Moore K et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reardon DA, Turner S, Peters KB et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9(4):414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taal W, Oosterkamp HM, Walenkamp AM et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 10. Wick W, Gorlia T, Bendszus M et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 11. Jahangiri A, De Lay M, Miller LM et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res. 2013;19(7):1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu KV, Chang JP, Parachoniak CA et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou L, Liu XD, Sun M et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gjerdrum C, Tiron C, Høiby T et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107(3):1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye X, Li Y, Stawicki S et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29(38):5254–5264. [DOI] [PubMed] [Google Scholar]
- 16. Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer. 2012;12(10):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7(4):436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arrieta O, Garcia E, Guevara P et al. Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94(12):3210–3218. [DOI] [PubMed] [Google Scholar]
- 19. Kong DS, Song SY, Kim DH et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115(1):140–148. [DOI] [PubMed] [Google Scholar]
- 20. Hutterer M, Knyazev P, Abate A et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(1):130–138. [DOI] [PubMed] [Google Scholar]
- 21. Keating AK, Kim GK, Jones AE et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onken J, Torka R, Korsing S et al. Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget. 2016;7(9):9876–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vajkoczy P, Knyazev P, Kunkel A et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103(15):5799–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yakes FM, Chen J, Tan J et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. [DOI] [PubMed] [Google Scholar]
- 25. You WK, Sennino B, Williamson CW et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71(14):4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Navis AC, Bourgonje A, Wesseling P et al. Effects of dual targeting of tumor cells and stroma in human glioblastoma xenografts with a tyrosine kinase inhibitor against c-MET and VEGFR2. PLoS One. 2013;8(3):e58262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elisei R, Schlumberger MJ, Müller SP et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurzrock R, Sherman SI, Ball DW et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith DC, Smith MR, Sweeney C et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cloughesy TF, Drappatz J, de Groot J et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients with prior antiangiogenic therapy. Neuro Oncol. 2018;20(2):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen PY, Drappatz J, de Groot J et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018;20(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. [DOI] [PubMed] [Google Scholar]
- 33. Norden AD, Young GS, Setayesh K et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 34. Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008;112(10):2267–2273. [DOI] [PubMed] [Google Scholar]
- 35. Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008;89(1):113–118. [DOI] [PubMed] [Google Scholar]
- 36. de Groot JF, Yung WK. Bevacizumab and irinotecan in the treatment of recurrent malignant gliomas. Cancer J. 2008;14(5):279–285. [DOI] [PubMed] [Google Scholar]
- 37. Batchelor TT, Sorensen AG, di Tomaso E et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ellingson BM, Kim HJ, Woodworth DC et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chappell R, Miranpuri SS, Mehta MP. Dimension in defining tumor response. J Clin Oncol. 1998;16(3):1234. [DOI] [PubMed] [Google Scholar]
- 40. James K, Eisenhauer E, Christian M et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91(6):523–528. [DOI] [PubMed] [Google Scholar]
- 41. Shah GD, Kesari S, Xu R et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ellingson BM, Harris RJ, Woodworth DC et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gahrmann R, van den Bent M, van der Holt B et al. Comparison of 2D (RANO) and volumetric methods for assessment of recurrent glioblastoma treated with bevacizumab—a report from the BELOB trial. Neuro Oncol. 2017;19(6):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vredenburgh JJ, Desjardins A, Herndon JE II et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 45. Vredenburgh JJ, Desjardins A, Herndon JE II et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 46. Friedman HS, Petros WP, Friedman AH et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–1525. [DOI] [PubMed] [Google Scholar]
- 47. Prados MD, Lamborn K, Yung WK et al. ; North American Brain Tumor Consortium A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8(2):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choueiri TK, Escudier B, Powles T et al. ; METEOR Investigators Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]