Abstract
Perineuronal satellitosis, the microanatomical clustering of glioma cells around neurons in the tumor microenvironment, has been recognized as a histopathological hallmark of high-grade gliomas since the seminal observations of Scherer in the 1930s. In this review, we explore the emerging understanding that neuron‒glioma cell interactions regulate malignancy and that neuronal activity is a critical determinant of glioma growth and progression. Elucidation of the interplay between normal and malignant neural circuitry is critical to realizing the promise of effective therapies for these seemingly intractable diseases. Here, we review current knowledge regarding the role of neuronal activity in the glioma microenvironment and highlight critical knowledge gaps in this burgeoning research space.
Keywords: glioma, tumor microenvironment, neurons, neuronal activity
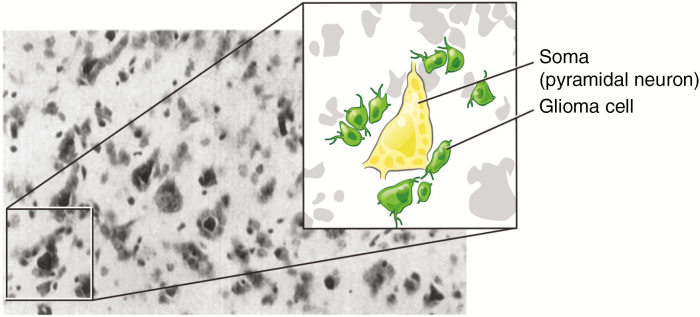
Over 80 years ago, a neuropathologist working in Belgium painstakingly described the structures and cytological features of a class of brain tumors known as gliomas. Dr. H. J. Scherer noted the predilection of glioma cells to grow along and around normal neurons, referring to the phenomenon as “precocious perineural growth” (Fig. 1).1 Were the glioma cells under Scherer’s microscope simply clustering around abundant neuronal soma, an observation now referred to as “perineuronal satellitosis,” and following paths of least resistance along axons as they invaded distant brain regions, or were they actively engaged in a signaling relationship with normal neural circuitry? Our conceptual understanding of cancer has evolved a great deal in 80 years and now includes an appreciation for the importance of microenvironmental influences on cancer cell behavior. Neuronal regulation of cancer cells has recently emerged as a fundamentally important aspect of brain tumor biology. Here, we discuss new insights and evolving concepts regarding the relationship between glioma cells and neurons in tumor growth and progression.
Fig. 1 .
An image from Dr. Scherer’s 19381 analysis depicts clusters of glioma cells around neuronal soma. Magnified and schematized inset added for clarity. Neuronal soma, yellow. Glioma cells, green.
Electrical Activity as a Component of the Tumor Microenvironment
Among the most common primary brain tumors in adults and children, gliomas are so named because their constituent cells are morphologically and molecularly reminiscent of normal macroglial cells. A subset of these neoplasms, the high-grade gliomas, carry the worst prognoses and are the principal cause of death among patients with brain tumors. These tumors include adult glioblastoma, anaplastic astrocytoma, anaplastic oligodendroglioma, pediatric glioblastoma, and H3K27M-mutant diffuse midline gliomas of childhood2 such as diffuse intrinsic pontine glioma (DIPG). The growth of gliomas around and along neurons is not a unique trait of neoplastic cells, but rather reflects a shared behavior with normal neural cell types such as oligodendrocytes and their precursors. Ample evidence from the literature illustrates that healthy glial cells and glial precursors participate in bidirectional and mutually beneficial relationships with nearby neurons. Accumulating data support a model in which glioma cells utilize and exploit normal neuronal signaling mechanisms to promote their own survival and growth. Here, we review our current knowledge of the mechanisms by which neurons in the microenvironment regulate glioma growth, and discuss the emerging therapeutic avenues to target newly understood microenvironmental dependencies in these devastating cancers.
Gliomas exist within and are regulated by a unique and dynamic microenvironment, the properties of which change throughout postnatal neurodevelopment and during adult neural plasticity. As one example, diffuse midline gliomas chiefly arise during a temporally restricted window in early to middle childhood and within the signaling environment of developing midline (diencephalic, rhombencephalic, and lower neural tube–derived) structures. In contrast, glioblastomas of adulthood chiefly grow within the (telencephalic) cerebrum. The differences between these two brain ecosystems are many and include cell type composition, signaling molecules, developmental state, and profiles of axonal myelination. Understanding microenvironmental determinants of glioma growth and progression is therefore a focus of current research efforts aimed at uncovering new targets and strategies for treating these seemingly intractable diseases. There has been a recent accumulation of knowledge regarding interactions of glioma cells with astrocytes, endothelial cells, immune cells, and neural precursor cells.3–8 While these relationships are no doubt integral to glioma pathobiology, emerging research now suggests that interactions with neurons and the direct and indirect consequences of neuronal activity represent critically important determinants of glioma cell behavior.
Gliomas are named for their morphological similarity to normal macroglial cells. While the cell of origin for gliomas remains an open point of debate and may differ between glioma subtypes, mounting evidence supports the idea that many glioma types originate from neural stem or precursor cells of the oligodendroglial lineage.9–18 Identifying a cell of origin for glioma has important implications for understanding the pathophysiology of the disease. As discussed below, neurons regulate the proliferation, differentiation, and function of normal glial cells in an activity-dependent manner, suggesting that these regulatory mechanisms may be co-opted for the promotion of tumor growth and progression.
Activity-Dependent Regulation of Neural Development, Plasticity, and Cancer
Activity of the nervous system is a powerful regulator of numerous aspects of neurodevelopment and ongoing neural plasticity, from neural stem cell proliferation, survival, and differentiation in the developing and mature nervous system to refinement and plasticity of synaptic connectivity.19–23 It has been known for some time that early in neurodevelopment, neurotransmitters regulate neural precursor cell proliferation and differentiation through nonsynaptic depolarization.24 A more recent report demonstrated that the orderly transition from multipolar-to-bipolar migration of neocortical neuroblasts is instructed by transient glutamatergic synaptic communication from subplate neurons to neuroblasts.25 In the adult brain, proliferation and differentiation of stem cells in the subgranular zone of the dentate gyrus is coupled to excitation of neighboring neurons.19 Neurogenesis in the postnatal subventricular zone, another stem cell niche in the brain, is regulated by the activity of a population of cholinergic neurons that project to the subventricular zone.26 In the peripheral nervous system, axons regulate Schwann cell proliferation and survival and influence peripheral nerve myelination.27,28
We now recognize that neuronal activity also robustly regulates central nervous system glial precursor proliferation as part of a process known as myelin plasticity: activity-regulated, adaptive changes to myelination that represent a newly recognized dimension along which experience modulates brain structure and thus function. Researchers in the early 1990s first studied the rat optic nerve as a model system to identify an influence of electrical activity on normal oligodendrocyte progenitor cells (OPCs).29 In this landmark study, silencing neuronal activity surgically by transection of the optic nerve or chemically with sodium channel blockade effectively and specifically inhibited local proliferation of OPCs. Since OPCs terminally differentiate into myelinating oligodendrocytes, it was reasonable to extrapolate from these studies that neuronal activity may allow for dynamic and adaptive modulation of circuit myelination, a concept supported by numerous in vitro studies30,31 and studies correlating experience with changes in myelin-forming cells.32–34 Direct in vivo evidence for this concept was enabled by the recent advent of optogenetic techniques that allow for minimally invasive, cell type–specific experimental manipulation of neuronal activity in awake, behaving mice.35–37 These in vivo optogenetic studies revealed that neuronal activity of glutamatergic projection neurons in the premotor cortex elicit circuit-specific mitogenic responses from NPCs, pre-OPCs, and OPCs, ultimately resulting in differentiation of some progeny to functionally mature oligodendrocytes and adaptive changes to myelin microstructure of the involved circuit.38 The influences of neuronal activity on OPC proliferation, oligodendrogenesis, and adaptive myelin changes have now been replicated in additional model systems, including volitional motor behavior during motor learning39 and elevation of neuronal activity in the somatosensory cortex by chemogenetic means.40
If neuronal activity promotes the proliferation of normal glial precursor cells that represent candidate cells of origin for high-grade glioma, do high-grade glioma cells similarly respond to neuronal activity? Indeed, in vivo optogenetic stimulation of premotor cortex neuronal activity also leads to increased proliferation of patient-derived pediatric cortical glioblastoma xenografts in the premotor circuit.41 The proliferative response of glioma cells in this study was restricted to the stimulated premotor circuit, indicating circuit specificity of the growth-promoting effects of neuronal activity. Collectively, these data demonstrate that the parallels between human glioma cells and normal glial precursor cells extend to their proliferative responses to neuronal activity in the brain and define neurons as an important component of the glioma microenvironment.41
Molecular Mechanisms of Neural Regulation of Glioma Growth
An Unexpected Dependency on Neuroligin-3
While the molecular mechanism by which neuronal activity induces proliferation of normal OPCs remains an open question, several have been proposed and include activity-regulated neurotransmitters and neurotrophic factors.42 In contrast, the mechanisms regulating glioma cell response to elevated neuronal activity are better understood. Glioma cell proliferation assays using conditioned medium from mouse brain slices with varying levels of neuronal activity demonstrated a robust proliferative effect of activity-regulated secreted factors in a range of clinically and molecularly distinct high-grade glioma types, including pediatric glioblastoma, DIPG, adult glioblastoma, and adult anaplastic oligodendroglioma.41 Biochemical and proteomic analyses of this conditioned medium followed by candidate sufficiency testing revealed 3 activity-regulated glioma mitogens: brain-derived neurotrophic factor (BDNF), 78-kDa glucose-regulated protein (GRP78), and the synaptic cell adhesion protein neuroligin-3 (NLGN3).41 Neuroligin-3, which classically binds presynaptic neurexin proteins, plays important roles in the maturation and proper functioning of synapses.43 However, this interesting synaptic protein was not previously known to regulate cell cycle progression in any context.
In addition to uncovering an unexpected role for NLGN3 in glioma proliferation, these studies elucidated previously unknown proteolytic processing of this protein. A type 1 membrane protein, NLGN3 straddles the cell membrane with an N-terminal ectodomain and a C-terminal cytoplasmic domain. In the course of identifying proteins responsible for the mitogenic response observed in glioma cells, it became evident that despite abundant spectral counts for the N-terminal portion of NLGN3 by mass spectrometry, peptides corresponding to the C-terminus were notably absent. This discrepancy in peptide coverage suggested that NLGN3 could be proteolytically cleaved at the cell surface, releasing the N-terminal ectodomain into the extracellular space and, in the context of glioma, into the tumor microenvironment. Bioinformatic analysis of peptide sequences near the transmembrane domain of NLGN3 pointed toward the metalloprotease family of proteins as likely cleavage enzymes. Subsequent pharmacological and genetic mouse modeling assays identified a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) as the enzyme primarily responsible for cleavage of NLGN3, a result that is logically consistent with previous research implicating the same enzyme in activity-dependent cleavage of NLGN1.44 Given the position of NLGN3 within postsynaptic specializations, it is perhaps not surprising that neurons were found to be one cellular source of cleaved NLGN3. Intriguingly, another postsynaptic cell type in the brain, the OPC, was also found to contribute substantially to the production of cleaved NLGN3 in the brain.45 Once cleaved by ADAM10 and released into the extracellular space, soluble NLGN3 engages an unidentified glioma cell surface receptor, activates numerous oncogenic signaling pathways (discussed below), and promotes proliferation.41,45
Seeking to isolate and clarify the relative contribution of activity-regulated NLGN3 secretion into the glioma microenvironment toward tumor growth and progression, the NLGN3 knockout mouse was crossed onto an immunodeficient background amenable to orthotopic xenografting of human patient–derived high-grade glioma cells.45 Given the mitogenic influence of NLGN3 in vitro, some growth defects were expected in the human glioma cells implanted in the NLGN3 knockout brain microenvironment. Unexpectedly, while human glioma cells engrafted and survived, xenografts in the NLGN3-deficient brain did not expand over the course of several months in many cases. In other words, the ability of glioma cells to thrive in the brain is unexpectedly reliant on microenvironmental NLGN3. This dependency on NLGN3 was conserved across molecularly and clinically distinct glioma subtypes, including adult and pediatric glioblastoma and DIPG, suggesting a fundamental characteristic of gliomas that may supersede specific genetic mutations in regulating survival and growth. The importance of this observation for glioma cell biology is underscored by the fact that this dependency was not shared by patient-derived breast cancer brain metastasis xenografts, which grew equivalently in the brains of NLGN3−/− and wild-type mice.45 Collectively, these data support a model in which gliomas are uniquely dependent on NLGN3 exposure in the brain microenvironment.
Mechanistic insight into the consequences of NLGN3 binding to glioma cells was garnered by detailed phosphoproteomic studies which demonstrated that NLGN3 stimulates numerous oncogenic signaling cascades in the glioma cell, with early activation of focal adhesion kinase and downstream activation not only of phosphatidylinositol-3 kinase (PI3K)–mammalian target of rapamycin but also the Src and Ras pathways. In addition to these signaling events, transient NLGN3 exposure also induces numerous gene expression changes in the glioma cell. Perhaps the most intriguing changes include upregulated expression of numerous synapse-associated genes. NLGN3 exposure strongly increases NLGN3 mRNA and protein expression by glioma cells in a feed-forward, potentially autocrine/paracrine loop.41 Of note, NLGN3 expression was inversely correlated with overall survival in adult glioblastoma patients, emphasizing the clinical significance of this mechanism in human disease. In addition to this feed-forward expression of NLGN3, several glutamate receptor subunit genes and the BDNF receptor gene neurotrophic receptor tyrosine kinase 1 (NTRK2) are upregulated following NLGN3 exposure in glioma.45 Alongside increases in synapse-related genes, NLGN3 also leads to increased expression of tweety homologue 1, a protein that was reported to regulate tumor microtube network formation in adult high-grade astrocytomas.46,47 While the functional implications of these transcriptional changes require further clarification, the complex downstream consequences of activity-regulated NLGN3 release into the tumor microenvironment highlight our nascent molecular understanding of the roles played by this crucial molecule. Altogether, these findings further the concept that neuronal activity represents an integral component of the glioma microenvironment, highlighting an understudied topic that is ripe for investigation.
While there is much to learn about the mechanisms that account for the observed NLGN3 dependency in glioma, this activity-regulated molecule nevertheless represents an important therapeutic target. Leveraging the new insight that the ADAM10 metalloprotease mediates release of soluble NLGN3 into the tumor microenvironment, recent work has demonstrated that inhibiting ADAM10 prevents NLGN3 release and dramatically reduces the growth of patient-derived high-grade glioma orthotopic xenografts, suggesting a new therapeutic strategy targeting this key neuron–glioma interaction.45
Neurotrophins
As mentioned previously, BDNF was implicated alongside NLGN3 as contributing to activity-dependent glioma proliferation, suggesting a role for neurotrophins in glioma cell survival and growth.41 Neurotrophins, a family of growth factor proteins expressed in the nervous system, promote broad aspects of neural function, survival, and maturation.48 Accordingly, the diverse activities of neurotrophins are engaged throughout development and during adult neural plasticity. Of particular relevance to the present discussion, neurotrophins also influence the proliferation, differentiation, and function of OPCs during development.49–51 This particular role is linked to neuronal activity, as BDNF expression is regulated in an activity-dependent manner52,53 and can be secreted in response to depolarization.54,55 Mechanistically, BDNF initiates a signaling cascade upon binding to the high affinity tropomyosin-related kinase B (TrkB) (NTRK2) receptor on the cell surface.56 In vitro evidence supports the idea that BDNF-TrkB signaling is involved in promoting proliferation, survival, and migration of high-grade glioma cells.57,58 In further support of a role for BDNF-TrkB signaling in the glioma microenvironment, many human gliomas, particularly astrocytomas, express neurotrophin genes and their receptors31,57,59,60 and exhibit mutations in Trk genes. Among Trk gene alterations in brain tumors are fusion proteins involving NTRK1, NTRK2, and NTRK3 in pediatric high-grade glioma,61 pilocytic astrocytoma,62 and less commonly in adult glioblastoma.63 In one study, these chimeric proteins were identified as fusions between the C-terminal kinase domains of NTRK1–3 and domains from 5 N-terminal partners, a pattern that appeared to activate oncogenic BDNF signaling cascades in these cells.61 In addition to Trk fusions, about half of DIPGs in one sample cohort harbor genomic amplifications of NTRK1 and/or NTRK2, implying increased gene expression and therefore pathway activity in these cells.64 This early research highlights the need to clarify whether gliomas harboring NTRK fusions or amplifications are dependent upon or differentially responsive to activity-regulated neurotrophins in the microenvironment. Collectively, these data suggest that activity-regulated neurotrophin signaling in glioma is potentially targetable, although the efficacy of disrupting this particular signaling network for glioma therapy remains to be defined experimentally.
Neurotransmitters
Given that glioma growth and progression are impacted by neuronal activity-regulated release of protein factors into the microenvironment, it is reasonable to speculate that neurotransmitters may also regulate aspects of glioma cell behavior. This supposition has recently gained traction as several reports have begun to interrogate glioma cell responses to neurotransmitter exposure. One curious and potentially illuminating observation comes from a retrospective analysis demonstrating that patients with a history of long-term therapy with tricyclic antidepressants also display a reduced incidence of glioma. These drugs are known to have broad effects, but are thought to act primarily via reuptake inhibition of serotonin and norepinephrine.65 Although a correlative study, this phenomenon may nevertheless indicate an unappreciated causal link between neurotransmitters and glioma cell behavior. One group provided experimental support for this concept by treating low-grade glioma-bearing mice with the tricyclic antidepressant imipramine, resulting in prolonged survival, reduced tumor cell proliferation, and a lower rate of progression to high-grade lesions.66 Although apparently initiated by blockade of neurotransmitter reuptake, the mechanism of action in these studies also appeared to involve downstream regulation of autophagy, ultimately leading to programmed cell death of glioma cells.
Evidence for the importance of neurotransmitters to glioma biology extends to high-grade lesions such as glioblastoma, the cells of which are known to express dopamine receptors.67,68 To test the functional relevance of these receptors, one group performed a proliferation screen across 3 patient-derived glioma cell cultures that were exposed to a panel of neurotransmitter agonists, antagonists, and reuptake inhibitors. The screen revealed that pharmacologic blockade of dopamine receptor D4 selectively and effectively inhibited glioma cell proliferation via disruption of autophagy and downstream induction of apoptosis,67 a mechanism reminiscent of but distinct from that observed in the low-grade glioma imipramine study.
The results described above highlight the relevance of specific molecules to glioma biology, but it is unclear whether this trend will expand to include other neurotransmitters. For instance, glioma cells also express functional serotonin receptors, but whether serotonergic signaling impacts glioma cell behavior is less clear.69 One retrospective study found that increased serotonin levels as a result of selective serotonin reuptake inhibitor therapy did not impact survival in patients with glioblastoma and comorbid depression.70 One important caveat from such studies is that in vivo pharmacologic manipulation of neurotransmitter signaling is likely to alter both cell autonomous effects on glioma and non-cell autonomous effects on neuronal activity. Therefore, careful interrogation and dissection of neurotransmitter signaling in the glioma microenvironment should be an area of concerted research effort.
The functional relevance of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the mature CNS, to glioma regulation is also an area in need of investigation. One study demonstrated that primary low-grade astrocytomas and oligodendrogliomas express functional GABAA receptors, and though the function of such signaling is largely unclear, it is notable that higher-grade gliomas did not express GABAA receptors in that study. In addition, the authors found that glioma-derived cell culture lines failed to exhibit functional GABAA receptor activity in vitro.71
Normally, GABAergic inputs to OPCs inhibit their proliferation and promote differentiation to mature oligodendrocytes,72 but it is unknown whether glioma cells respond to GABA in a similar manner. This raises an interesting and unanswered question regarding the role that GABAergic interneurons may play in regulating glioma. Elucidating the activity of GABA signaling in glioma growth and progression may therefore reveal additional therapeutic targets for these incurable diseases.
Both normal glial precursors and glioma cells express glutamate receptors.73,74 In addition, nonsynaptic secretion of glutamate by glioblastoma cells has been reported in vitro75 and further supported by in vivo studies describing increased extracellular glutamate levels in brain tissue in physical proximity to glioblastomas.76 Glutamate has been shown to augment glioblastoma cell survival, growth, and migration via calcium influx-mediated activation of PI3K-Akt signaling through AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors.74,77,78 In line with these observations, elevated glutamate secretion from gliomas is positively correlated with tumor growth in mammalian models.78 Collectively, nonsynaptic glutamate secretion by glioma cells appears to function in an autocrine/paracrine fashion in promoting tumor growth, survival, and progression. Neuronal glutamate release could further augment glioma progression, although the extent to which neuron-derived glutamate contributes to glioma progression—and whether neuronal glutamate release would signal in the same way as glioma-derived glutamate—has not yet been tested.
Gliomas Increase Neuronal Activity
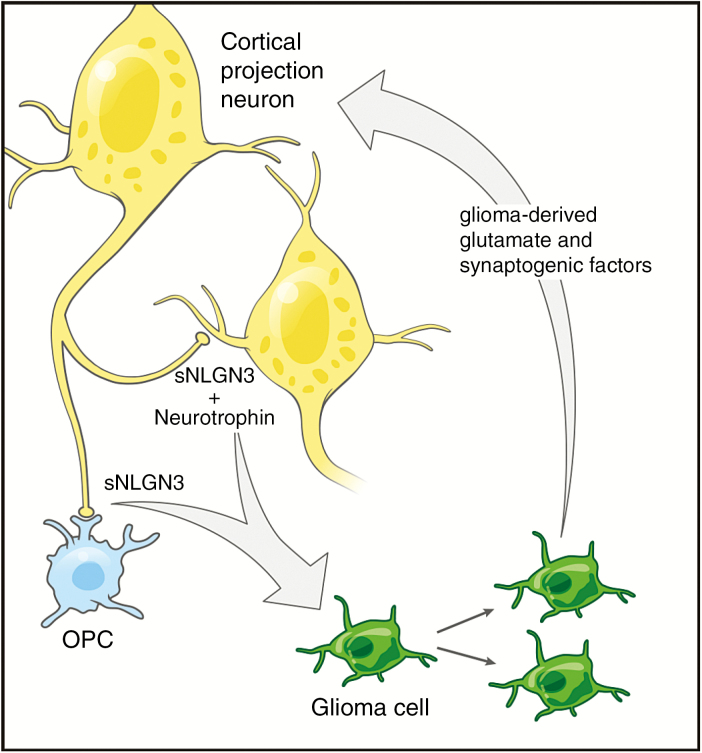
Accumulating research now suggests that the influence of active neurons on glioma cells may actually represent one facet of a bidirectional relationship. For example, glutamate secretion by glioma cells contributes to hyperexcitability of neural circuits in the glioma microenvironment, including promotion of seizure activity.79,80 This suggests that gliomas may enhance their own growth and progression not only via direct autocrine/paracrine effects of nonsynaptic secreted glutamate, but also by enhancing local neuronal activity, thereby resulting in greater release of activity-dependent mitogens by other cell types in the microenvironment (Fig. 2). This may play a role in the common, yet functionally enigmatic clinical feature of seizure activity among human glioma patients.
Fig. 2.
Schematic illustrating a bidirectional relationship between normal neural cells and their malignant glioma counterparts. Activity-regulated factors including a secreted form of neuroligin-3 (sNLGN3) from normal OPCs (blue) and neurons (yellow) and neurotrophins such as BDNF fuel the growth of glioma. Glioma cells (green), in turn, secrete glutamate and synaptogenic factors that increase neuronal excitability and probability of firing action potentials.
Increased cortical excitability may also manifest as gliomas progress and cancer cells evolve to promote synaptogenesis. A recent elegant study leveraged fluorescence activated cell sorting screening of cell-surface markers to separate astrocytes into 5 functionally diverse subgroups. One of these populations was particularly proficient in supporting synaptogenesis between neurons in a co-culture system. The authors then interrogated human gliomas for correlates of these astrocyte subgroups and found that the synaptogenic signature was represented among malignant cells.81 Using 2 autochthonous mouse models of glioblastoma, the authors showed that the subpopulation of glioma cells exhibiting synaptogenic properties emerged as tumors progressed. This phenomenon correlated with the onset of clinical seizure activity in the tumor-bearing mice, suggesting that increased and/or altered synaptogenesis due to shifting composition of tumor cell phenotypes may underlie the cortical hyperexcitability seen in later-stage gliomas in this model.81 In addition, this increased excitability was positively associated with migratory behavior of the glioma cells, suggesting a possible link between neuronal activity and promotion of infiltration, though this association has yet to be formally tested. Thus, more work is needed to determine the extent to which seizure activity promotes glioma growth. In addition, as clinically apparent seizures are more common in individuals with low-grade astrocytomas and oligodendrogliomas than those with high-grade gliomas, it remains to be determined whether low-grade gliomas promote cortical hyperexcitability via similar mechanisms. The influence of neuronal activity on low-grade gliomas also remains to be demonstrated.
Conclusion
The neural regulation of gliomas is now coming into focus and fits a developing paradigm in which neuronal activity promotes the growth and progression of several cancer types, including colon, gastric, prostate, pancreas, and skin,82–87 as previously reviewed.88 Furthermore, as glioma cells induce remodeling of the microenvironment to promote hyperexcitability of local circuits, the resultant increase in neuronal activity may in turn contribute to various activity-dependent mechanisms of tumor growth and progression. While research is needed to elucidate the nature and details of many of these mechanisms, the regulation of normal glial precursor cells by neuronal activity during development and plasticity suggests parallel interactions between neurons and glioma cells. A better understanding of the degree to which glioma cells may co-opt or diverge from physiological activity-dependent processes may elucidate promising new therapeutic approaches for these devastating cancers.
Funding
This work was supported by the National Institutes of Neurological Disorders and Stroke (R01NS092597 to M.M.), National Cancer Institute (CA09302 TO S.G.), Child Health Research Institute at Stanford (M.M.), and the Anne T. and Robert M. Bass Endowed Faculty Scholarship in Pediatric Cancer and Blood Diseases (M.M.).
Acknowledgments
Special thanks to Sigrid Knemeyer for illustrations.
Conflict of interest statement. The authors have no conflicts of interest to report.
References
- 1. Scherer HJ. Structural development in gliomas. Am J Cancer. 1938;34(3):333–351). [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. [DOI] [PubMed] [Google Scholar]
- 4. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin EY, Cooper DD, Abbott KL et al. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell. 2017;170(5):845–859 e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pyonteck SM, Akkari L, Schuhmacher AJ et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silver DJ, Siebzehnrubl FA, Schildts MJ et al. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J Neurosci. 2013;33(39):15603–15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galvao RP, Kasina A, McNeill RS et al. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc Natl Acad Sci U S A. 2014;111(40):E4214–E4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu C, Sage JC, Miller MR et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monje M, Mitra SS, Freret ME et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108(11):4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagaraja S, Vitanza NA, Woo PJ et al. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell. 2017; 31(5):635–652 e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tirosh I, Venteicher AS, Hebert C et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature. 2016;539(7628):309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Yang J, Zheng H et al. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15(6):514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venteicher AS, Tirosh I, Hebert C et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355(6332):eaai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Filbin MG, Tirosh I, Hovestadt V et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science. 2018;360(6386):331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee DY, Gianino SM, Gutmann DH. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alcantara Llaguno SR, Wang Z, Sun D et al. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell. 2015;28(4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–552. [DOI] [PubMed] [Google Scholar]
- 20. Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. [DOI] [PubMed] [Google Scholar]
- 21. Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schafer DP, Lehrman EK, Kautzman AG et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26(6):1003–1017. [DOI] [PubMed] [Google Scholar]
- 24. LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15(6):1287–1298. [DOI] [PubMed] [Google Scholar]
- 25. Ohtaka-Maruyama C, Okamoto M, Endo K et al. Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science. 2018;360(6386):313–317. [DOI] [PubMed] [Google Scholar]
- 26. Paez-Gonzalez P, Asrican B, Rodriguez E, Kuo CT. Identification of distinct ChAT⁺ neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci. 2014;17(7):934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000;20(12):4635–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci U S A. 1995;92(5):1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129–134. [DOI] [PubMed] [Google Scholar]
- 30. Ishibashi T, Dakin KA, Stevens B et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Negorev D, Riethman H, Wechsler-Reya R, Sakamuro D, Prendergast GC, Simon D. The Bin1 gene localizes to human chromosome 2q14 by PCR analysis of somatic cell hybrids and fluorescence in situ hybridization. Genomics. 1996;33(2):329–331. [PubMed] [Google Scholar]
- 32. Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning?Hippocampus. 2006;16(3):216–224. [DOI] [PubMed] [Google Scholar]
- 33. Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takeuchi H, Sekiguchi A, Taki Y et al. Training of working memory impacts structural connectivity. J Neurosci. 2010;30(9):3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arenkiel BR, Peca J, Davison IG et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. [DOI] [PubMed] [Google Scholar]
- 37. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71(1):9–34. [DOI] [PubMed] [Google Scholar]
- 38. Gibson EM, Purger D, Mount CW et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16(3):233–238. [DOI] [PubMed] [Google Scholar]
- 40. Mitew S, Gobius I, Fenlon LR et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun. 2018;9(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Venkatesh HS, Johung TB, Caretti V et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161(4):803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mount CW, Monje M. Wrapped to adapt: experience-dependent myelination. Neuron. 2017;95(4):743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chanda S, Hale WD, Zhang B, Wernig M, Südhof TC. Unique versus redundant functions of neuroligin genes in shaping excitatory and inhibitory synapse properties. J Neurosci. 2017;37(29):6816–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki K, Hayashi Y, Nakahara S et al. Activity-dependent proteolytic cleavage of neuroligin-1. Neuron. 2012;76(2):410–422. [DOI] [PubMed] [Google Scholar]
- 45. Venkatesh HS, Tam LT, Woo PJ et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature. 2017;549(7673):533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jung E, Osswald M, Blaes J et al. Tweety-homolog 1 drives brain colonization of gliomas. J Neurosci. 2017;37(29):6837–6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osswald M, Jung E, Sahm F et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528(7580):93–98. [DOI] [PubMed] [Google Scholar]
- 48. Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. [DOI] [PubMed] [Google Scholar]
- 49. Tsiperson V, Huang Y, Bagayogo I et al. Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN Neuro. 2015;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31(40):14182–14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J Neurosci. 2013;33(11):4947–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60(4):610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lindholm D, Castrén E, Berzaghi M, Blöchl A, Thoenen H. Activity-dependent and hormonal regulation of neurotrophin mRNA levels in the brain–implications for neuronal plasticity. J Neurobiol. 1994;25(11):1362–1372. [DOI] [PubMed] [Google Scholar]
- 54. Androutsellis-Theotokis A, McCormack WJ, Bradford HF, Stern GM, Pliego-Rivero FB. The depolarisation-induced release of [125I]BDNF from brain tissue. Brain Res. 1996;743(1-2):40–48. [DOI] [PubMed] [Google Scholar]
- 55. Goggi J, Pullar IA, Carney SL, Bradford HF. The control of [125I]BDNF release from striatal rat brain slices. Brain Res. 2003;967(1-2):201–209. [DOI] [PubMed] [Google Scholar]
- 56. Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. [DOI] [PubMed] [Google Scholar]
- 57. Lawn S, Krishna N, Pisklakova A et al. Neurotrophin signaling via TrkB and TrkC receptors promotes the growth of brain tumor-initiating cells. J Biol Chem. 2015;290(6):3814–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiong J, Zhou L, Lim Y et al. Mature BDNF promotes the growth of glioma cells in vitro. Oncol Rep. 2013;30(6):2719–2724. [DOI] [PubMed] [Google Scholar]
- 59. Assimakopoulou M, Kondyli M, Gatzounis G, Maraziotis T, Varakis J. Neurotrophin receptors expression and JNK pathway activation in human astrocytomas. BMC Cancer. 2007;7(1):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–43. [DOI] [PubMed] [Google Scholar]
- 61. Wu G, Diaz AK, Paugh BS et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jones DT, Hutter B, Jäger N et al. ; International Cancer Genome Consortium PedBrain Tumor Project Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Frattini V, Trifonov V, Chan JM et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grasso CS, Tang Y, Truffaux N et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walker AJ, Card T, Bates TE, Muir K. Tricyclic antidepressants and the incidence of certain cancers: a study using the GPRD. Br J Cancer. 2011;104(1):193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shchors K, Massaras A, Hanahan D. Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit. Cancer Cell. 2015;28(4):456–471. [DOI] [PubMed] [Google Scholar]
- 67. Dolma S, Selvadurai HJ, Lan X et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29(6):859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li JSZ, Kozono D, Ng K, Chen CC. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014; 5(4):882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mahé C, Bernhard M, Bobirnac I et al. Functional expression of the serotonin 5-HT7 receptor in human glioblastoma cell lines. Br J Pharmacol. 2004;143(3):404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Caudill JS, Brown PD, Cerhan JH, Rummans TA. Selective serotonin reuptake inhibitors, glioblastoma multiforme, and impact on toxicities and overall survival: the mayo clinic experience. Am J Clin Oncol. 2011;34(4):385–387. [DOI] [PubMed] [Google Scholar]
- 71. Labrakakis C, Patt S, Hartmann J, Kettenmann H. Functional GABA(A) receptors on human glioma cells. Eur J Neurosci. 1998;10(1):231–238. [DOI] [PubMed] [Google Scholar]
- 72. Zonouzi M, Scafidi J, Li P et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat Neurosci. 2015;18(5):674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. V Gallo DKP, Mayer ML, Vaccarino FM. Excitatory amino acid receptors in glial progenitor cells: molecular and functional properties. Glia. 1994; 11(2):94–101. [DOI] [PubMed] [Google Scholar]
- 74. Ishiuchi S, Tsuzuki K, Yoshida Y et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8(9):971–978. [DOI] [PubMed] [Google Scholar]
- 75. Ye Z, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999; 59(17):4383–4391. [PubMed] [Google Scholar]
- 76. Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 2000;47(1):11–22. [DOI] [PubMed] [Google Scholar]
- 77. Ishiuchi S, Yoshida Y, Sugawara K et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J Neurosci. 2007;27(30):7987–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takahiro Takano JH-CL, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001; 7(9):1010–1015. [DOI] [PubMed] [Google Scholar]
- 79. Buckingham SC, Campbell SL, Haas BR et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Campbell SL, Buckingham SC, Sontheimer H. Human glioma cells induce hyperexcitability in cortical networks. Epilepsia. 2012;53(8):1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. John Lin CC, Yu K, Hatcher A et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20(3):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao CM, Hayakawa Y, Kodama Y et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Magnon C, Hall SJ, Lin J et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 84. Peterson SC, Eberl M, Vagnozzi AN et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16(4):400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stopczynski RE, Normolle DP, Hartman DJ et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res.. 2014;74(6):1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hayakawa Y, Sakitani K, Konishi M et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Renz BW, Takahashi R, Tanaka T et al. beta2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018; 33(1):75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Venkatesh H, Monje M. Neuronal activity in ontogeny and oncology. Trends Cancer. 2017;3(2):89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]