Abstract
Ginseng is commonly used as a medicinal herb for memory and concentration and general well-being. Drug-induced liver injury (DILI) is one of the most challenging disorders and trending events in the United States which are related to body building and weight loss supplements. Currently, herbal and dietary supplementation is the second most common cause of DILI. Here, we report on a 45-year-old healthy Chinese woman who presented with dull intermittent left upper quadrant abdomen pain for a month. Upon thorough history taking, she had been taking ginseng tea and supplementation for her menopausal symptoms for almost 3 months. Physical examination was unremarkable except mild tenderness in left upper quadrant of the abdomen. Liver function test showed aspartate transaminase (AST) 717 U/L, alanine transaminase (ALT) 343 U/L, total bilirubin 5 mg/dL, direct bilirubin 3.3 mg/dL, alkaline phosphatase 182 U/L, with international normalized ratio (INR) 1.2. Prior liver enzymes (6 months earlier) showed AST 21 U/L, ALT 18 U/L, total bilirubin 0.8 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 34 U/L, with INR 0.7. Viral serology for acute hepatitis B, C, E, cytomegalovirus, Epstein-Barr virus, and varicella zoster virus was negative. She was immune to hepatitis A. Her antinuclear antibody was positive. Her anti-Smith antibody, anti-smooth muscle antibody, HFE gene mutation, ceruloplasmin, alpha-1 antitrypsin serologies were within normal references. An abdomen sonogram showed fatty infiltration. Liver biopsy showed moderate to severe portal inflammation and marked lobular disarray. Portal and lobular inflammatory infiltrates consisted of a mixture of histiocytes, lymphocytes, plasma cells, eosinophils, and neutrophils with centrilobular necrosis and focal bridging necrosis, and necro-inflammation. After 6 weeks of follow-up, the patient improved physically, and the abdomen pain resolved. Ginseng has been widely used in the Chinese community as medicinal herb for a variety of conditions for decades. However, proper research has never been done regarding its pharmacokinetics, efficacy, and safety issues. In our case report, the idiosyncratic DILI resulted from ingestion of ginseng as herbal supplementation for premenopausal symptoms. Physicians should be aware of and suspect DILI in any patient with acute liver injury, and patients should be reminded that all medications and supplements have a potential to cause DILI.
Keywords: Ginseng, Drug-induced liver injury, Herbal and dietary supplementation, Gastroenterology
Introduction
Ginseng in Hokkien Chinese means the root with a characteristic forked shape that resembles a human's leg. It is the root of plants in the genus Panax composed of ginsenosides and gintonin. The use of ginseng as a medicinal herb began as early as in 196 AD in China. In 1596, Li Shizhen described it as “superior tonic” for convalescent and chronically ill patients [1]. For centuries, it has famously been used for its possible effects on memory and concentration, for general well-being, physical stamina, as immune booster, to slow the aging process and relieve various health problems particularly erectile dysfunction, and menopausal hot flashes. The well-known side effect is bleeding, headache, insomnia, mild gastric problems [2]. However, there are little proper research data in the literature regarding its biological effects. Significant adverse effects have been reported with the concomitant use of warfarin [3].
Drug-induced liver injury (DILI) is a rare adverse drug reaction to either antimicrobials or herbal and dietary supplementation (HDS). It is one of the most challenging disorders and trending events in the United States related to body building and weight loss supplements. Those supplements are not subjected to thorough testing for safety and efficacy unlike prescription medications.
Currently, HDS is the second most common cause of DILI [4]. In the United States, no population-based research has been reported to recognize the true prevalence and incidence of HDS-induced DILI. HDS are subject to variation depending on location and circumstances for growth, methods of manufacture, contents of ingredients and concentration in the capsules or tablets or packages. Often, the products may contain ingredients that are not identical on the listed label due to contamination or adulteration. The most important thing is no regulations or requirements for premarketing safety analysis before distribution on the markets. Here, we present a case of DILI resulting from ingestion of ginseng as herbal supplementation for premenopausal symptoms.
Case Report
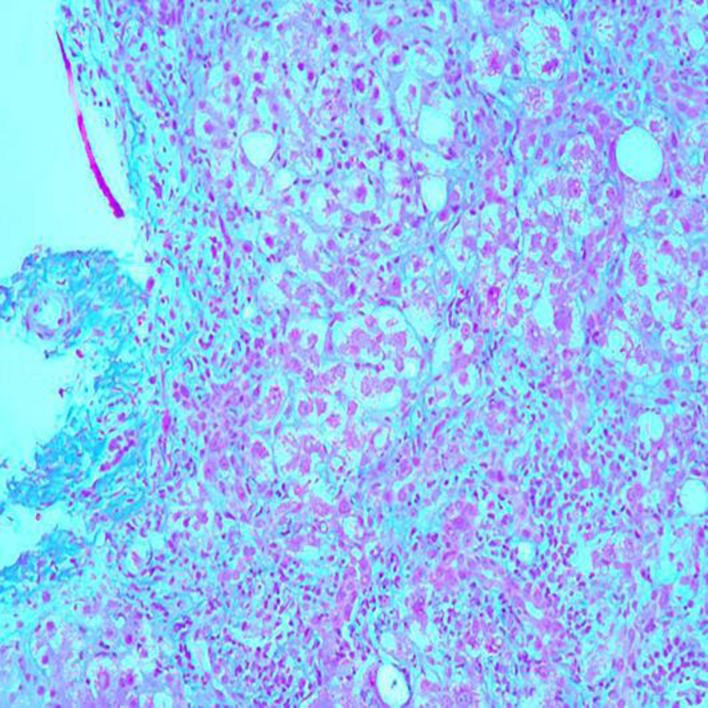
A 45-year-old healthy Chinese woman presented with dull intermittent left upper quadrant abdomen pain for a month. The abdomen pain was localized, nonradiating, and there were no aggravating or relieving factors. It was associated with fatigue, loss of appetite, and nausea. However, the patient denied jaundice, high-colored urine, pale-colored stool, pruritus, fullness after meals, or significant loss of weight. Past medical and social history was not pertinent. Upon thorough history taking, she had been taking ginseng tea and supplementation for her menopausal symptoms for almost 3 months. General examination showed no jaundice, clubbing, spider telangiectasia, or scratch marks. Physical examination was unremarkable except mild tenderness in left upper quadrant of the abdomen. Liver function test showed aspartate transaminase (AST) 717 U/L, alanine transaminase (ALT) 343 U/L, total bilirubin 5 mg/dL, direct bilirubin 3.3 mg/dL, alkaline phosphatase 182 U/L, with international normalized ratio (INR) 1.2. Prior liver enzymes (6 months earlier) showed AST 21 U/L, ALT 18 U/L, total bilirubin 0.8 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 34 U/L, with INR 0.7. Viral serology for acute hepatitis B, C, E, cytomegalovirus, Epstein-Barr virus, and varicella zoster virus was negative. She was immune to hepatitis A. Her antinuclear antibody was positive. Her anti-Smith antibody, anti-smooth muscle antibody, HFE gene mutation, ceruloplasmin, alpha-1 antitrypsin serologies were within normal references. An abdomen sonogram revealed that the right lobe of the liver was 13.3 cm in length. The hepatic parenchyma was increased in echotexture. There was no mass or intrahepatic biliary ductal dilatation. There was normal direction of flow in the hepatic vessels. The gallbladder is distended. There was no wall thickening, stone, or pericholecystic fluid. There was no sonographic Murphy's sign. The common bile duct measures 5.0 mm in diameter which is within normal limits. The pancreatic head and visualized body was normal in appearance. There was no ascites. The echogenic appearance of the hepatic parenchyma was compatible with fatty infiltration (Fig. 1). Computed tomography of the abdomen showed fat containing umbilical hernia without inflammatory changes and bilateral fat containing indirect inguinal hernias (Fig. 2). Liver biopsy showed moderate to severe portal inflammation and marked lobular disarray (Fig. 3). Portal and lobular inflammatory infiltrates consisted of a mixture of histiocytes, lymphocytes, plasma cells, eosinophils, and neutrophils (Fig. 4). The lobular disarray is characterized by ballooning degeneration of hepatocytes, centrilobular necrosis and focal bridging necrosis, and necroinflammation (Fig. 5). Cholestasis as well as mild steatosis were also seen. After 6 weeks of follow-up, liver enzymes showed AST 28 U/L, ALT 20 U/L, total bilirubin 1.1 mg/dL, direct bilirubin 0.5 mg/dL, alkaline phosphatase 39 U/L, with INR 0.9. The patient improved physically, and the abdomen pain resolved. Repeated abdomen sonogram showed a normal hepatic parenchyma, and repeated hepatic function tests showed within-normal reference values after 6 months.
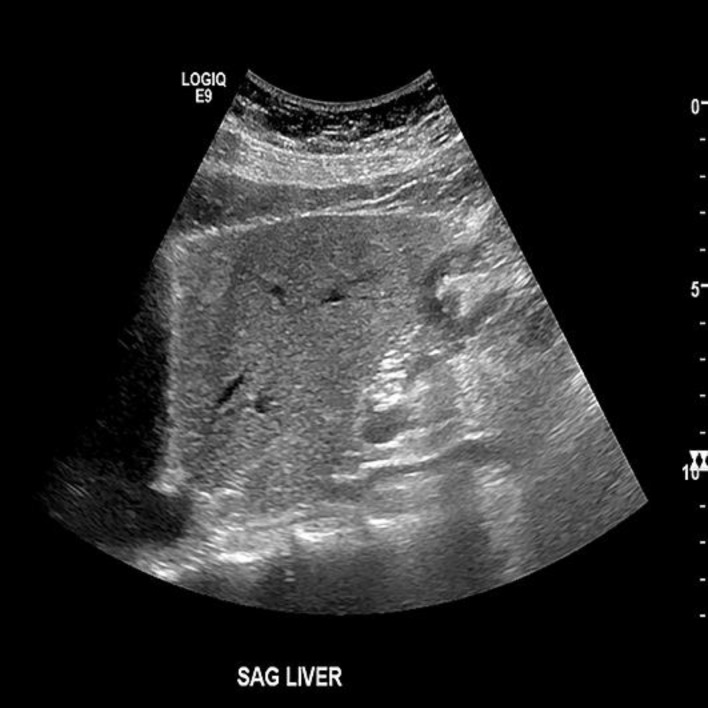
Fig. 1.
An abdomen sonogram showed fatty liver. There was no intrahepatic biliary ductal dilatation, no wall thickening, stone, or pericholecystic fluid. There was no sonographic Murphy's sign. The pancreatic head and visualized body was normal in appearance. There was no ascites.
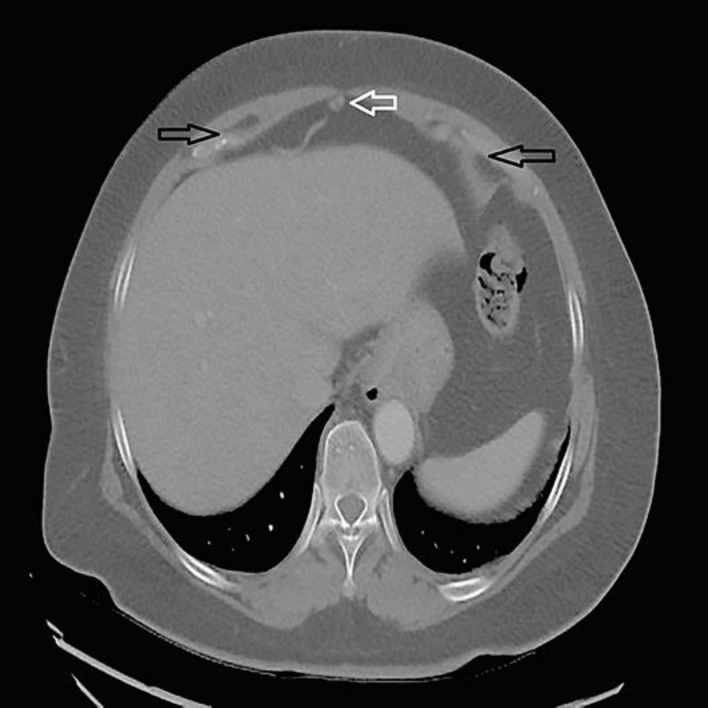
Fig. 2.
Computed tomography of the abdomen showed fat-containing umbilical hernia without inflammatory changes and bilateral fat-containing indirect inguinal hernias.
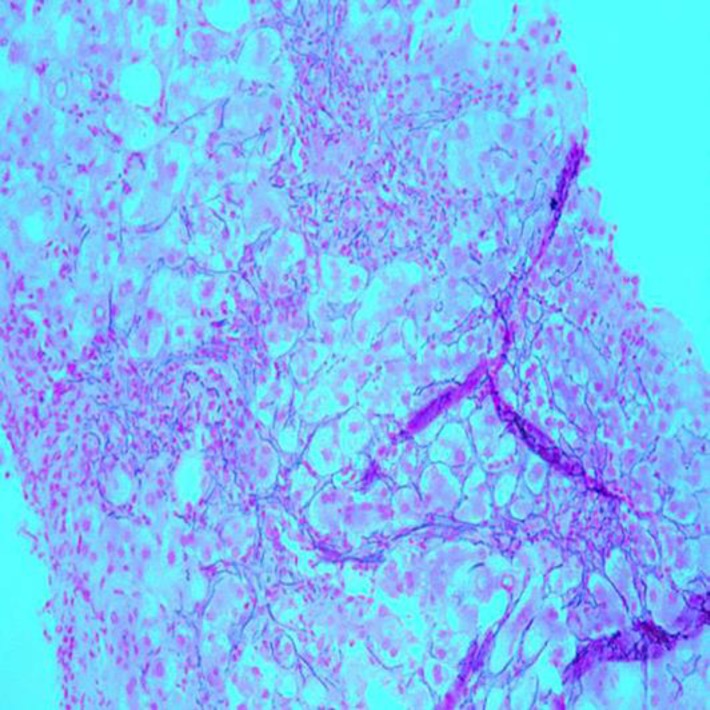
Fig. 3.
Histopathologic image demonstrating disarray of liver cell plates with a loss of cohesion between hepatocytes. Liver biopsy. Reticulin stain. ×100.
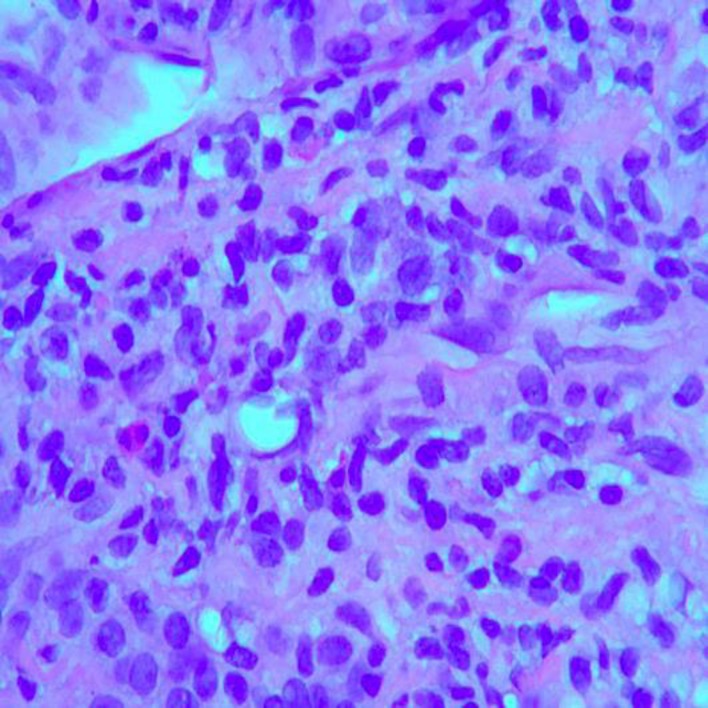
Fig. 4.
Histopathologic image demonstrating a mixed inflammatory cellular infiltrate in the parenchyma with numerous lymphocytes and plasma cells, as well as occasional eosinophils. Liver biopsy. HE stain. ×400.
Fig. 5.
Histopathologic image demonstrating extensive portal and periportal fibrosis. Liver biopsy. Trichrome stain. ×100.
Discussion
Generally, DILI can be classified into intrinsic, idiosyncratic, and chronic. Intrinsic DILI is defined as hepatotoxicity with potential to affect all the individuals to varying degrees. Adverse reaction is typically stereotypic and dose dependent. Acetaminophen is a well-known cause of intrinsic DILI. Idiosyncratic DILI is defined as hepatotoxicity affecting only rare susceptible individuals. Adverse reaction is not dose dependent and more varied in latency, duration of presentation, and its clinical course. Chronic DILI is defined as failure to return of liver enzymes or bilirubin to pre-DILI baseline and/or other signs and symptoms of impending liver diseases such as ascites, portal hypertension, or hepatic encephalopathy 6 months after onset of DILI.
Antimicrobials and medications that act on the central nervous system are the common causes of DILI in children, while certain medications such as minocycline, diclofenac, and methyldopa are more common in DILI in females. DILI due to nevirapine is more common in female HIV patients with higher CD4 count [5]. Variables such as age, gender, diabetic mellitus, and alcohol consumption may increase the risk for DILI in a drug-specific manner.
Thorough and pertinent history plays a crucial role as DILI is a diagnosis of exclusion. Generally, DILI occurs within 6 months of initiation of new medications or supplementations. Acute hepatitis C and E are well-known masqueraders for DILI mimicking 1.3 and 3$ of all DILI cases, respectively [6]. Autoimmune hepatitis, viral, and metabolic causes should be considered as a differential diagnosis for DILI.
In clinical practice, to assess and evaluate the pattern of liver injury, the R value can be used, while for mortality risk, Hy's Law can be applied. The R value is defined as serum ALT/upper limit of normal divided by serum alkaline phosphatase/upper limit of normal. R < 2 can be labeled as cholestatic DILI, R 2–5 as mixed DILI and R > 5 as hepatocellular DILI. However, the same offending agent can present with variable laboratory profiles and clinical presentation in each DILI patient. Hy's Law was made by Hyman Zimmerman, who suggested a mortality risk of 1 in 10 if 3 criteria are met: serum AST or ALT more than 3 times than upper normal values, serum total bilirubin more than 2 times than upper normal values, and no other reasons can be found to explain the combination of increased aminotransferases and bilirubin or other preexisting or acute liver disease [7].
For causality assessment for suspected DILI, the RUCAM and the Maria and Victorino system can be used at bedside or in clinic [8, 9]. RUCAM has a summed score from −9 to +10, and higher scores indicate higher likelihood of DILI. The score system is also divided into hepatocellular injuries versus cholestatic or mixed injuries. However, RUCAM is not recommended to use as the main diagnostic tool alone due to its low retest reliability and poor validation.
Liver-related mortality from acute liver failure (ALF) in the setting of DILI occurs only in a few cases and usually in the first 6 months. ALF due to DILI has a slower speed of disease evolution compared to that due to acetaminophen. Those with ALF due to DILI are prone to develop ascites, infection, and hepatorenal syndrome, and causes of mortality are due to systemic infections and/or cerebral edema [10]. In terms of predictors of liver transplantation, the Model for End-Stage Liver Disease (MELD) score and advanced coma grade are used on admission, and the higher the score, the poorer the outcomes. Comparing different scoring systems and assessments, Sequential Organ Failure Assessment (SOFA) is reportedly the best, but is limited to DILI due to acetaminophen [11].
The mainstay treatment for DILI is prompt stopping of the offending agent or medication. No approved antidotes are available for idiosyncratic DILI with/without ALF. Most clinicians use diphenhydramine and hydroxyzine for symptomatic treatment of pruritus. N-acetyl cysteine can be considered in adults with early stage ALF but is not recommended for children with severe DILI with ALF.
Liver biopsy is recommended if autoimmune hepatitis is a concern, there is persistent rise in liver biochemistries or signs of worsening liver function despite stopping the suspected agents, peak ALT level has not decreased by > 50$ at the end of 30–60 days of unresolved acute hepatocellular DILI and at 180 days for cholestatic or mixed DILI, and the patient is evaluated for chronic liver disease and chronic DILI. Acute DILI can progress to chronic DILI in 15–20$ of the cases [12]. However, a guideline for liver biopsy for patients with chronic methotrexate has been established [13].
For decades, ginseng has been widely used in the Chinese community as medicinal herb for a variety of conditions. However, proper research has never been done regarding its pharmacokinetics, efficacy, and safety issues. In our case report, idiosyncratic DILI resulted from ingestion of ginseng as herbal supplementation for premenopausal symptoms. Physicians should be aware of and suspect DILI in any patient with acute liver injury, and patients should be reminded that all medications and supplements have a potential to cause DILI.
Statement of Ethics
The patient's consent has been obtained.
Disclosure Statement
The authors declare no conflicts of interest.
Author Contributions
Dr. Kyawzaw Lin contributed equally in gathering information, writing introduction, case report, and discussion. Dr. Aung Naing Lin, Dr. Sandar Linn and Dr. Pwint Phyu Hlaing contributed to the editing of the manuscript. Dr. Viswanath Vasudevan and Dr. Madhavi Reddy provided the optimal patient care and management of the case.
References
- 1.Mahady GB, Fong HH, Farnsworth NR. Botanical Dietary Supplements: Quality, Safety and Efficacy. Lisse, Swets & Zeitlinger. 2001
- 2.Kim Young-Sook, Jung-Yoon Woo, Chang-Kyun Han, Il-Moo Chang. Safety Analysis of Panax Ginseng in Randomized Clinical Trials: A Systematic Review. Medicines (Basel) 2015 Jun;8;2((2)):106–126. doi: 10.3390/medicines2020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurley BJ, Fifer EK, Gardner Z. Pharmacokinetic herb-drug interactions (part 2): drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 2012 Sep;78((13)):1490–514. doi: 10.1055/s-0031-1298331. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Drug Induced Liver Injury Network (DILIN) Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008 Dec;135((6)):1924–34. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005 Jun;4((6)):489–99. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 6.Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, et al. Drug-Induced Liver Injury Network (DILIN) Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011 Nov;141((5)):1665–72.e1. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006 Apr;15((4)):241–3. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 8.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993 Nov;46((11)):1331–6. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 9.Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997 Sep;26((3)):664–9. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 10.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. U.S. Acute Liver Failure Study Group Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002 Dec;137((12)):947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cholongitas E, Theocharidou E, Vasianopoulou P, Betrosian A, Shaw S, Patch D, et al. Comparison of the sequential organ failure assessment score with the King's College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transpl. 2012 Apr;18((4)):405–12. doi: 10.1002/lt.23370. [DOI] [PubMed] [Google Scholar]
- 12.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ, Practice Parameters Committee of the American College of Gastroenterology ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014 Jul;109((7)):950–66. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 13.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008 Jun;59((6)):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]