Abstract
Recently, immune checkpoint inhibitors have revolutionized cancer care by enhancing anti-tumor immunity. However, by virtue of stimulating the immune system, they can lead to immune-related adverse events (irAEs). Neurologic irAEs are uncommon but are becoming increasingly recognized and can be quite serious or even fatal. Furthermore, central nervous system (CNS) manifestations may be difficult to distinguish from CNS metastases, posing management challenges. Here, we describe a patient who developed exacerbation of sarcoidosis leading to CNS involvement following dual checkpoint blockade with nivolumab and ipilimumab for metastatic melanoma and review the relevant literature.
Keywords: Immunotherapy, Immune-related adverse event, Immune checkpoint inhibitor, Neurosarcoidosis, Nivolumab, Ipilimumab
Introduction
Immune checkpoint inhibitors (ICPIs) have drastically improved patient outcomes in a variety of malignancies and continue to have expanding indications. ICPIs function to enhance anti-tumor immunity by de-regulating the immune system and consequently can lead to immune-related adverse events (irAEs). Neurologic toxicities, once thought to be rare, have become increasingly recognized. In fact, the combined use of the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor with programmed cell death protein 1 (PD-1) inhibitors can cause neurologic irAEs in 12$ of patients [1]. Here, we describe a rare case of presumed neurosarcoidosis following dual checkpoint inhibition for metastatic melanoma, which was initially concerning for leptomeningeal metastasis and review the pertinent literature.
Case Report
A 68-year-old Caucasian man with known metastatic melanoma to an axillary lymph node and evidence of metabolically active mediastinal/hilar lymph nodes on staging positron emission tomography-computed tomography (PET-CT) was treated with 2 cycles of ipilimumab/nivolumab. This was discontinued early due to multiple adverse events including rash, transaminitis, thrombocytopenia, and biopsy-proven immune-mediated colitis requiring prednisone and 1 dose of infliximab. He was then monitored off therapy with serial PET-CTs. At 4 months following treatment cessation, a PET-CT was concerning for worsened metabolically active mediastinal/hilar lymphadenopathy. Simultaneously, the patient also developed hypercalcemia of unknown etiology. These changes prompted a mediastinal lymph node biopsy and pathological analysis demonstrated noncaseating granulomata; confirming the suspicion of pulmonary sarcoidosis. The patient did not receive sarcoidosis treatment as he was without pulmonary symptoms and there was concern that the addition of corticosteroids could interfere with remaining ICPI anti-tumor activity.
Approximately 10 months following treatment with ipilimumab/nivolumab, the patient was admitted to the hospital after developing 2 weeks of progressive headaches, vision changes, and word-finding difficulty. Neurologic examination was significant for a right incongruent homonymous hemianopia, finger agnosia, acalculia, left-right disorientation, and alexia. Magnetic resonance imaging (MRI) of the brain revealed subtle leptomeningeal enhancement and T2-weighted fluid-attenuated inversion recovery (FLAIR) signal abnormality within the left occipital pole and overlying left parietal lobe (Fig. 1a, b). Given this presentation, there was initial concern for leptomeningeal metastases, but a neurologic irAE was also considered. The patient underwent a lumbar puncture and cerebral spinal fluid (CSF) analysis demonstrated an elevated protein of 75 mg/dL (15–50 mg/dL), 13 white blood cells, and normal glucose. CSF cytological examination was also negative. During the hospitalization, the patient experienced a secondary generalized tonic-clonic seizure and was started on levetiracetam. After initiating treatment with dexamethasone, he had partial symptomatic improvement and was discharged home.
Fig. 1.
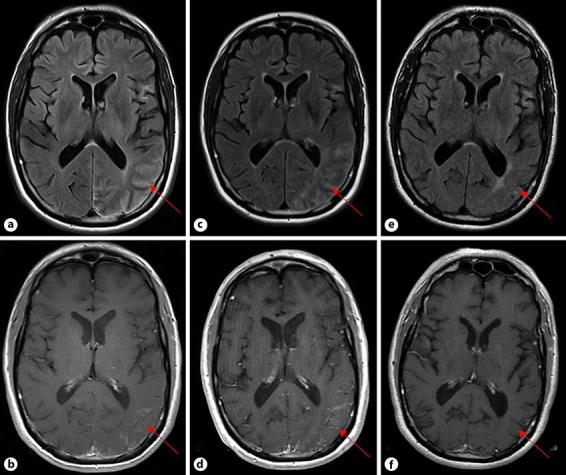
Changes on axial MRI over time. a FLAIR image showing signal abnormalities in the left temporal, parietal, and occipital lobes at the time of neurological presentation. b T1+ contrast image demonstrating leptomeningeal enhancement within the left occipital and parietal lobes at the time of neurological presentation. c FLAIR image showing increased signal abnormalities 3.5 months after presentation. d T1+ contrast image demonstrating increased leptomeningeal enhancement 3.5 months after presentation. e FLAIR image showing significant reduction in signal abnormality 22 months after presentation. f T1+ contrast image demonstrating resolution of leptomeningeal enhancement 22 months after presentation.
A repeat lumbar puncture 2 weeks later showed resolving CSF abnormalities and CSF cytologic examination was again negative. Dexamethasone was slowly tapered over 3 months and upon discontinuation, the patient experienced headache recurrence and worsening neurological symptoms. A repeat brain MRI at this time revealed progression of leptomeningeal enhancement and FLAIR signal abnormalities (Fig. 1c, d). As a result, the patient received infliximab (5 mg/kg) every 4–6 weeks for presumed neurosarcoidosis. However, he developed a severe hypersensitivity reaction during his third infusion and was transitioned to oral methotrexate (12.5 mg weekly). His neurologic symptoms continued to resolve and repeat brain MRIs demonstrated marked improvement (Fig. 1e, f). Currently, nearly 2 years after the original neurologic presentation, the patient has made nearly a full neurological recovery, is tapering methotrexate, and continues to be stable from an oncologic perspective.
Discussion
10–40$ of patients with melanoma develop central nervous system (CNS) metastases, and thus, any new neurological symptom in patients with melanoma is concerning [2]. Moreover, melanoma metastases to the leptomeninges portend a dismal prognosis with overall survival estimates of approximately 3 months [3]. Since the advent of ICPIs, the differential diagnosis for leptomeningeal enhancement in patients with cancer has broadened beyond leptomeningeal carcinomatosis and infectious etiologies. Specifically, ICPIs can cause irAEs such as aseptic meningitis and encephalitis, which may mimic leptomeningeal metastases. ICPI-associated aseptic meningitis typically presents between the first and seventh week after initiating therapy and the usual presentation includes headache, stiff neck, and fever [4]. ICPI-related encephalitis has been reported to occur in 0.1–0.2$ of patients; has a more variable presentation including headache, altered mental status, focal deficits, and seizures; can be fatal and is and less likely to have leptomeningeal enhancement compared to aseptic meningitis [4, 5]. In recognition that ICPI-induced neurologic toxicities are more prevalent than previously believed, the American Society of Clinical Oncology addressed management of specific neurological complications in their 2018 practice guidelines for management of irAEs, including aseptic meningitis and encephalitis [6]. A CSF analysis should be conducted to evaluate for evidence of inflammation, and for the detection of malignant cells in order to help distinguish neoplastic meningitis from neurological irAEs. However, the sensitivity of CSF cytological analysis is low, failing to detect malignant cells in up to 45$ of patients [7]. Therefore, repeating CSF cytology evaluation may be warranted, just as in our case.
This case represents an alternative etiology of leptomeningeal enhancement in a cancer patient treated with ICPIs. We hypothesize that the patient may have had preexisting subclinical sarcoidosis prior to ipilimumab/nivolumab treatment as evident by the detection of hilar/mediastinal lymphadenopathy on the staging PET-CT, but only after exposure to ICPIs did progression of sarcoidosis and subsequent CNS involvement occur. One initially puzzling component to this case was that the patient's neurologic symptoms did not develop until nearly 10 months following ICPI cessation. However, this is consistent with other reports of irAEs occurring up to 1 year after starting immune checkpoint blockade [8].
Sarcoidosis is a multi-organ inflammatory disease characterized by noncaseating granulomata, which most often affects the lungs and frequently involves the lymphatics, skin, and eyes. Infrequently, sarcoidosis can involve the nervous system with up to 5–10$ of patients developing neurologic complications [9]. The most common neurologic manifestation are cranial mononeuropathies, neuroendocrine dysfunction, encephalopathy, vasculopathy, seizures, hydrocephalus, acute or chronic meningitis, myelopathy, and peripheral nerve abnormalities [10]. The pathophysiology of sarcoidosis is not completely understood but sarcoid-related granulomata are composed of macrophage-derived cells and activated T cells. Cytokine profiles are consistent with a highly polarized type 1 T helper cell cytokine response, including factors such as interferon gamma and tumor necrosis factor alpha (TNFα) [11]. It is worth noting that TNFα inhibitors, like infliximab, are effective in treating both neurosarcoidosis and certain ICPI irAEs [6, 12].
In reviewing safety data of patients with predisposition for autoimmune disorders receiving cancer immunotherapy, it became apparent that autoimmune disorders are not a contraindication for ICPI therapy. One recent study demonstrated that 75$ of 123 patients with preexisting autoimmune disorders had exacerbation of their autoimmune disease and/or development of irAEs [12]. Fortunately, in most instances, complications were managed successfully by corticosteroids alone, but 16$ required other immunosuppression, and 3 patients died of related events. Additionally, there are also a handful of reports describing patients with known sarcoidosis who later received ICPIs. Gaughan [13] summarized 2 series that included 5 patients with preexisting sarcoidosis treated with ICPIs and of which 2 patients experienced sarcoidosis exacerbations that were treated with corticosteroids.
We conclude that our patient's presentation was most consistent with an ICPI-related exacerbation of sarcoidosis resulting in CNS involvement that had a partial response to corticosteroids but required additional immunosuppression. In the appropriate clinical setting, neurosarcoidosis may be a possible explanation for leptomeningeal enhancement in cancer patients treated with checkpoint blockade. As neurologic irAEs can be aggressive and even fatal, and TNFα inhibitors are already indicated for several ICPI irAEs and have efficacy in neurosarcoidosis, we recommend earlier rather than later consideration of inflixumab when there is a high suspicion for ICPI-related neurosarcoidosis.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
Drs. Dunn-Pirio and Shah declare no conflicts of interest. Dr. Eckstein receives honoraria from Biogen Idec and Genzyme.
Author Contributions
Dr. Shah: acquisition of data, analysis and interpretation, drafting the manuscript for intellectual content. Dr. Dunn-Pirio: acquisition of data, analysis and interpretation, drafting the manuscript for intellectual content. Dr. Eckstein: critical revisions of the manuscript for important intellectual content, study supervision.
References
- 1.Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. 2017 Mar;73:1–8. doi: 10.1016/j.ejca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Chiarion-Sileni V, Murr R, Pigozzo J, Sarti S, Tomassi O, Romanini A. Brain metastases from malignant melanoma. Forum (Genova) 2003;13((2)):170–82. [PubMed] [Google Scholar]
- 3.Oinino S, Rodrigues I, Boulanger T, Andre C, Bonneterre J, Zairi F, et al. Prognosis of leptomeningeal metastases from melanoma: A case series of 28 patients. J Clin Oncol. 2017;35((15_suppl)) [Google Scholar]
- 4.Astaras C, de Micheli R, Moura B, Hundsberger T, Hottinger AF. Neurological adverse events associated with immune checkpoint inhibitors: diagnosis and management. Curr Neurol Neurosci Rep. 2018 Feb;18((1)):3. doi: 10.1007/s11910-018-0810-1. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017 Jun;22((6)):709–18. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. National Comprehensive Cancer Network Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018 Jun;36((17)):1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leal T, Chang JE, Mehta M, Robins HI. Leptomeningeal metastasis: challenges in diagnosis and treatment. Curr Cancer Ther Rev. 2011 Nov;7((4)):319–27. doi: 10.2174/157339411797642597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016 Feb;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Stern BJ, Krumholz A, Johns C, Scott P, Nissim J. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985 Sep;42((9)):909–17. doi: 10.1001/archneur.1985.04060080095022. [DOI] [PubMed] [Google Scholar]
- 10.Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol. 2017 May;264((5)):1023–8. doi: 10.1007/s00415-016-8336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ES, Moller DR. Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol. 2011 Jul;7((8)):457–67. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 12.Gelfand JM, Bradshaw MJ, Stern BJ, Clifford DB, Wang Y, Cho TA, et al. Infliximab for the treatment of CNS sarcoidosis: A multi-institutional series. Neurology. 2017 Nov;89((20)):2092–100. doi: 10.1212/WNL.0000000000004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaughan EM. Sarcoidosis, malignancy and immune checkpoint blockade. Immunotherapy. 2017 Oct;9((13)):1051–3. doi: 10.2217/imt-2017-0128. [DOI] [PubMed] [Google Scholar]


