Graphical abstract

Keywords: Cissus sicyoides, Hydroalcoholic extract, Toxicity
Highlights
-
•
The Cissus sicyoides species has several pharmacological activities.
-
•
The ethanolic extract from the leaves of Cissus caused few blood changes at the 40.5 mg/kg dose in the acute study.
-
•
In the chronic study, EHA showed no toxic effects at the popular dose (4.5 mg/kg).
-
•
Only the 13.5 mg/kg dose in the chronic trial showed blood changes.
Abstract
Cissus sicyoides (Cs) has been traditionally used to treat diabetes and belongs to the family Vitaceae, and is known as “vegetable insulin". This study aimed to evaluate the acute and chronic non-clinical toxicity of hydroalcoholic extract from the leaves of Cissus sicyoides (EHA-Cs). The acute test was performed in Wistar rats, administering a single dose of 40.5 mg/kg. Behavioral parameters for pharmacological screening were observed to detect signs of Central Nervous System activity; consumption of daily food and water, and weight evaluation. After day 14, the animals were euthanized and blood samples were collected for laboratory analyses of hematological and biochemical parameters. The chronic tests were administered in doses of 4.5, 13.5 and 40.5 mg/kg. The same parameters were observed together with body temperature, glucose, exploration activity (test on the open field), and motor activity (diagnostic tests on the Rota-rod). For the group given the highest dosage during the study, histopathological examinations of vital organs were performed. For acute toxicity, there were no CNS level effects, changes in water and food consumption, or hematologic parameters. However, there was a significant decrease in weight gain for the treated females. Biochemical analyses of the treated animals presented increased levels of AST (aspartate aminotransferase) in females, uric acid levels in females and males, and amylase in males. In the chronic toxicity tests, water consumption was higher for females (at the dosages of 13.5 and 40.5 mg/kg) and for males (at 40.5 mg/kg). At the dosages of 4.5 and 13.5 mg/kg, feed consumption increased for females, while for males it decreased along with weight gain. Blood analysis presented an increase in albumin and changes in erythrocytes and hemoglobin for males (at the dose of 13.5 mg/kg). Glycemia in females (13.5 mg/kg dose) was significantly less, presenting only slight drops at the other doses. The changes were reversible in the satellite group. EHA-Cs revealed a relatively low toxicity profile (at the popular use dose), and only small changes in hematological and biochemical parameters at the dose of 13.5 mg/kg (3x the popular use dosage). In addition, EHA-Cs did not promote histological changes in vital organs such as the heart, lungs, liver and kidneys.
1. Introduction
For millennia, natural medicinal products have been widely used to promote and restore health. Currently, there is a growing interest in the use of medicinal herbs and their extracts [1,2]. This is due to their many bioactive principles which being compatible with classical medicine can act as an aid in primary health care. According to the World Health Organization (WHO), 80% of developing country populations depend on medicinal plants as their only access to health care [3,4].
In Brazil, according to the National Pharmacological-Toxicity Information System (SINITOX), in 2013 there were 442 cases of poisonings using plants. In this context, beyond the existence of pharmacological and clinical trials proving the effectiveness of this product type, a certain guarantee of security; corroborating knowledge in relation to their possible toxic effects, side effects, drug-plant interactions, contra-indications and mutagenicity become relevant [[4], [5], [6], [7]].
Cissus L., recognized as the largest genus of the family Vitaceae, consists of 300 to 400 species of vines [8]. In Brazil, Cissus sicyoides L. has many popular names like: “cipó-pucá”, “tinta-dos-gentios”, “uva-brava” and “cortina-japonesa” [9,10]. It is an American Tropical climbing vine, which originates from the Dominican Republic; it is distributed to a large degree in Brazil, especially in the state of Maranhão, and the Amazon Region [[11], [12], [13]].
A tea made from the aerial parts of Cissus sicyoides L. is commonly used in folk medicine: to modulate (lower) glycemia [14], as an anti-hypertensive, and as an anti-thermic [8]. Other biological activities have been reported such as diaphoretic activity, and its use in treating heart diseases [13]. It presents anticonvulsant action, and for this reason is used in epileptic seizures and in stroke [15]. In Mexican popular medicine, an alcoholic infusion or aqueous solution of the stems and leaves is used for pain relief [16]. In Brazil, C. sicyoides is included among traditional medicines as a hypoglycemic. It also has anti-allergic cytostatic, antibacterial, and gastro-protective effects [12,[17], [18], [19]]. A recent study presented anti-inflammatory and anti-diarrheic activity through inhibition of PGE2 production. The anti-diarrheic effect however, may be mediated through inhibition of bowel contractions and direct activity in the smooth muscle intestinal tract [18].
In the face of such characteristics, the objective of this study was to investigate non-clinical acute and chronic toxicity of a hydroalcoholic extract of Cissus sicyoides leaves in order to evaluate its safety, in support of further clinical research.
2. Material and methods
2.1. Botanical material
Samples of C. sicyoides leaves were collected from the Garden of Medicinal Plants of the Institute for Research on Drugs and Medications (IPeFarM), of the Universidade Federal da Paraíba (UFPB). Botanical identification was performed by the UFPB botany sector. A representative sample was deposited at the Herbarium Lauro Pires Xavier at this same university: Vasconcelos s/n (JPB).
2.2. Obtaining Cissus sicyoides L. (EHA-Cs) hydroalcoholic leaf extract
The leaves were dried in an oven with air circulation at 38 °C for 72 h, and then crushed in a Harley type mill. After crushing, the leaves were macerated with 35% ethanol at ambient temperature for 72 h. The material was then filtered and submitted to a second extraction for the same period. To make the lyophilization process more efficient, the extracts were subjected to distillation at reduced pressure at 50 °C; to obtain an aqueous extract which was frozen at a temperature of -18 to -20 °C. This extract was subjected to freeze drying in an EDWARDS MODEL E2M8. At the end of the lyophilization process, the hydroalcoholic extract was dry, and when needed for use, it was dissolved in water.
2.3. Animals
Wistar rats(Ratus novergicus), albinos, adults, male and female (nulliparous and non- pregnant females), weighing between 200 and 300 g, supplied by the vivarium Prof. Thomas George (IPeFarM-UFPB) were used. The animals were grouped in cages made of polyethylene, and kept under controlled temperature conditions at 25 ± 2 °C, without the use of any medications, with free access to food (Purina pellets) and drinking water available in graduated polyethylene bottles. The animals were kept under a 12 h light-dark cycle. The use of the animals was approved by the Ethics Committee on Animal Use (CEUA-UFPB), and the "Guide for conducting non-clinical studies of toxicology and pharmacological safety necessary for drug development" was followed (ANVISA, 2013).
2.4. Acute toxicological testing (non-clinical)
To prevent the risk of acute poisoning in humans, (whether accidental or not), tests for acute toxicity are mandatory for all substances, regardless of the proposed duration for use [[20], [21], [22]]. We used Wistar rats, both genders (6 males and 6 females), divided into two groups: the control (vehicle) and the treated group. To these groups were administered a single dose of 40.5 mg/kg (9x the popular use) of the hydroalcoholic extract from the leaves of Cissus sicyoides (EHA-Cs) by oral route (gavage). After EHA-Cs administration; observations of behavioral parameters for pharmacological screening to detect signs of Central Nervous System activity at intervals of: 30, 60, 90, 120, 180 and 240 min were performed [23]. We checked the consumption of daily food and water, and animal weights were recorded on the 1 st and 14th day. After day 14, the animals were euthanized using an excess of anesthetic, and blood samples were collected for laboratory analyses of hematological and biochemical parameters.
2.5. Chronic (non-clinical) toxicological testing
For chronic toxicological testing, the animals were divided into 4 groups; 20 animals per group (10 males and 10 females), and the substance was administered on a daily basis in different doses to the several groups of animals for a period of 3 months [22,24]. The groups were distributed as follows: the control group was treated daily with the water vehicle used in the EHA-Cs solution, and the other three groups were treated daily, by oral route (gavage) with doses of 4.5 mg/kg (popular usage), 13.5 mg/kg (3x the popular use) or 40.5 mg/kg (9x the popular use) of the EHA-Cs; all were treated for a period of 13 weeks (90 days). The doses were selected according to the popular use, 3x the popular use, and 9x the popular use, being normally promoted doses that people traditionally use. We evaluated parameters such as: body temperature, water consumption and intake, weight gain, glucose, exploration activity (test on the open field), and motor activity (diagnostic test on the Rota-rod), the hematological and biochemical parameters, and histopathological examinations of vital organs were performed for the group given the highest dosage during the study. After the 90 days of administration, the animals were sacrificed (separating thirty percent of the male and female animals which had been treated with all doses evaluated). This group of animals was kept alive for 30 days after the end of the tests, to assess reversibility of possible toxic effects, being the satellite group.
2.6. Blood laboratory tests
Laboratory tests of biochemical parameters were performed on the serum samples. The dosages of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (AL), creatine kinase (CK), lactate dehydrogenase (LDH), total proteins, albumin, globulin, glucose, triglycerides, total cholesterol, urea, creatinine and uric acid were evaluated in an automated biochemical analyzer COBAS MIRA PLUS® - ROCHE. For laboratory analysis of hematological parameters; red series (erythrogram), and white series (leukogram), and platelet counts determinations were performed on a COBAS ARGOS 50® - ROCHE cell analyzer. Blood smears were stained automatically with HEMATEL 200, and analyzed using an Olympus microscope for confirmation and control of cell counts [25].
2.7. Anatomopathological study
The organs were examined macroscopically, by resections of the lung, heart, liver and kidneys. The tissue sections of excised organs were fixed in formalin (buffered 10% formaldehyde solution) and after 24 h, were resected for histopathological processing: dehydration with increasing series of alcohol (70–100%), diaphanization in xylol, impregnation and inclusion in paraffin according to the usual methods [26]. In a microtome, the tissue fragments were sectioned at a thickness of 3.0 μM and subsequently submitted to hematoxylin-eosin and Masson's tri-chrome staining, and then examined under an optical microscope.
2.8. Statistical analysis
The results obtained from the experiments were analyzed with the GraphPad Prism 6.0® software, San Diego, CA, USA, using the ANOVA test followed by the post-Tukey test or the non-paired Student's t-test / Mann-Whitney, for two column analyses. The values were expressed as mean ± standard error of the mean (S.E.M.), or standard deviation of the mean (S.D.), and considered significant when p < 0.05.
3. Results
In the acute test and during pharmacological screening, no behavioral changes indicating CNS level effects were observed in the treated animals. We did observe that the treated females exhibited a significant reduction in weight gain (Table 1), this without water and food consumption differences in relation to the controls (Table 2). The males presented no changes in these parameters. The hematological analyses presented no statistically significant changes in either sex (Table 4). Biochemical analysis of the treated animals’ blood (both sexes) presented high uric acid in relation to the controls, and reduced amylase in males, with increased AST in females (Table 3).
Table 1.
Evaluation of weight gain (difference between initial weight and final weight) of Wistar rats, male and female, treated with a single dose of 40.5 mg/kg of the EHA of Cissus sicyoides during the acute toxicity test. The values are expressed as mean ± S.E.M. (N = 6). Student's t-test. *p ≤ 0.05. *Significant compared between treated and controls.
| Control | 40.5 mg/kg | |
|---|---|---|
| Females | 11.0 ± 2.1 | 3.3 ± 1.6* |
| Males | 23.3 ± 6.9 | 22.8 ± 7.1 |
Table 2.
Consumption of water and rations of Wistar rats, male and female, treated with a single dose of 40.5 mg/kg of the EHA of Cissus sicyoides during the acute toxicity test. The values are expressed as mean ± S.E.M. (N = 6). Student's t-test. *p ≤ 0.05. *Significant compared between treated and controls.
| Control | 40.5 mg/kg | |
|---|---|---|
| Males | ||
| Water (mL) | 211.9 ± 7.4 | 225.8 ± 5.0 |
| Feed (g) | 130.8 ± 2.3 | 137.1 ± 2.4 |
| Females | ||
| Water (mL) | 173.1 ± 4.9 | 188.5 ± 6.2 |
| Feed (g) | 101.8 ± 1.7 | 102.5 ± 1.9 |
Table 4.
Hematologic parameters obtained from the serum of Wistar ratstreated with a single dose of 40.5 mg/kg EHA of Cissus sicyoides during the acute toxicity test. The values are expressed as mean ± S.E.M. (N = 6). Student's t-test/Mann-Whitney. * p ≤ 0.05. *Significant compared between treated and controls.
| Control | 40.5 mg/kg | |
|---|---|---|
| Males | ||
| Erythrocyte(106/mm3) | 6.9 ± 0.7 | 8.4 ± 0.3 |
| Hemoglobin (g/dL) | 15.7 ± 0.2 | 16.5 ± 0.4 |
| Hematocrit (%) | 29.4 ± 2.8 | 47.3 ± 1.6 |
| VCM (3)μ | 42.0 ± 0.3 | 56.2 ± 0.7 |
| HCM (g)μμ | 20.3 ± 0.2 | 19.6 ± 0.2 |
| MCHC (%) | 42.5 ± 0.2 | 34.9 ± 0.4 |
| Leukocytes (10/mm) | 6.3 ± 0.5 | 5.71 ± 0.9 |
| Neutrophils (%) | 26.7 ± 1.5 | 21.5 ± 4.4 |
| Eosinophils (%) | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Lymphocytes (%) | 66.7 ± 1.6 | 72.0 ± 4.4 |
| Monocytes (%) | 5.7 ± 0.3 | 5.5 ± 0.7 |
| Platelets (10/mm) | 730.6 ± 33.1 | 560.8 ± 107.5 |
| Females | ||
| Erythrocyte(106/mm3) | 7.5 ± 0.2 | 7.0 ± 0.1 |
| Hemoglobin (g/dL) | 14.5 ± 0.3 | 14.5 ± 0.2 |
| Hematocrit (%) | 34.6 ± 0.2 | 41.2 ± 0.7 |
| VCM (3)μ | 47.3 ± 0.9 | 56.4 ± 0.3 |
| HCM (g)μμ | 19.5 ± 0.1 | 19.8 ± 0.2 |
| MCHC (%) | 43.6 ± 0.5 | 35.2 ± 0.3 |
| Leukocytes (10/mm) | 4.8 ± 0.3 | 3.5 ± 0.3 |
| Neutrophils (%) | 26.4 ± 1.9 | 22.7 ± 1.9 |
| Eosinophils (%) | 1.0 ± 0.1 | 1.3 ± 0.9 |
| Lymphocytes (%) | 68.9 ± 1.8 | 68.0 ± 3.0 |
| Monocytes (%) | 3.3 ± 1.3 | 8.0 ± 0.6 |
| Platelets (10/mm) | 616.1 ± 37.9 | 845.7 ± 124.3 |
Table 3.
Biochemical parameters obtained from the serum of Wistar ratstreated with a single dose of 40.5 mg/kg of EHA of Cissus sicyoides during the acute toxicity test. The values are expressed as mean ± S.E.M. (N = 6). Student's t-test/Mann-Whitney. * p ≤ 0.05. *Significant compared between treated and controls.
| Control | 40.5 mg/kg | |
|---|---|---|
| Males | ||
| Glucose (mg/dL) | 117.4 ± 2.8 | 117.0 ± 8.1 |
| Urea (mg/dL) | 45.2 ± 2.3 | 35.6 ± 2.3 |
| Serum creatinine (mg/dL) | 0.5 ± 0.1 | 0.6 ± 0.0 |
| Cholesterol (mg/dL) | 62.2 ± 2.4 | 62.4 ± 3.2 |
| Triglycerides (mg/dL) | 107.0 ± 16.6 | 137.6 ± 9.6 |
| Uric Acid (mg/dL) | 0.5 ± 0.2 | 1.6 ± 0.2* |
| AST (U/L) | 152.0 ± 6.5 | 171.6 ± 0.7 |
| ALT (U/L) | 60.9 ± 2.4 | 66.8 ± 7.11 |
| FAL (U/L) | 127.3 ± 3.2 | 149.2 ± 9.1 |
| CK (U/L) | 3732 ± 830.3 | 10284 ± 5198 |
| LDH (U/L) | 1353 ± 64.3 | 1363 ± 411.5 |
| Amylase (U/dL) | 758.7 ± 42.6 | 480.0 ± 14.1* |
| Protein (g/dL) | 6.10 ± 0.1 | 6.3 ± 0.1 |
| Albumin (g/dL) | 2.6 ± 0.1 | 2.6 ± 0.0 |
| Globulin (g/dL) | 3.9 ± 0.2 | 3.7 ± 0.1 |
| Females | ||
| Glucose (mg/dL) | 104.0 ± 2.2 | 109.3 ± 9.9 |
| Urea (mg/dL) | 40.2 ± 1.9 | 45.3 ± 2.5 |
| Serum creatinine (mg/dL) | 0.4 ± 0.0 | 0.6 ± 0.0 |
| Cholesterol (mg/dL) | 59.8 ± 2.2 | 61.5 ± 4.4 |
| Triglycerides (mg/dL) | 101.1 ± 8.9 | 108.0 ± 21.0 |
| Uric Acid (mg/dL) | 0.9 ± 0.1 | 1.7 ± 0.1* |
| AST (U/L) | 171.5 ± 24.8 | 208.5 ± 23.9* |
| ALT (U/L) | 53.8 ± 2.8 | 65.5 ± 6.2 |
| ALP (U/L) | 91.0 ± 13.5 | 123.8 ± 15.6 |
| CK (U/L) | 3547 ± 1037 | 17785 ± 8735 |
| LDH (U/L) | 1869 ± 278.1 | 975 ± 244.7 |
| Amylase (U/dL) | 670.7 ± 119.1 | 296.3 ± 7.4 |
| Protein (g/dL) | 6.51 ± 0.1 | 6.8 ± 0.1 |
| Albumin (g/dL) | 3.0 ± 0.1 | 2.87 ± 0.1 |
| Globulin (g/dL) | 4.23 ± 0.2 | 3.92 ± 0.1 |
The non-clinical chronic toxicological testing trial was conducted for a period of 90 days and analyzed using parameters such as: behavioral screening, body temperature, blood glucose, consumption of water and food, weight gain, hematology, blood biochemistry and histopathology. The extract was administered by oral route in differing doses: the popular use dose (4.5 mg/kg), 3x the dose of popular use (13.5 mg/kg), and 9x the dose of popular use (40.5 mg/kg). Body temperatures remained normal throughout the test, varying from 36.1 to 38.0 °C for the males, and from 36.8 to 38.1 °C for the females, indicating that the metabolism/regulation ratio was maintained during prolonged EHA-Cs treatment. The males presented no significant changes in blood glucose; however, females presented a significant decrease of blood glucose on the 15th and 75th days of evaluation at the dose of 13.5 mg/kg (3x the popular dose) (Table 5). Only this treated group showed reduced glycemia as compared to the controls. This study is insufficient to affirm and corroborate that Cissus sicyoides presents hypoglycemic effect. The main objective was to evaluate its possible toxic effects. To assess behavioral changes involving the central nervous system effects we used: the open field, evaluating exploratory activity through spontaneous movement (ambulation), the number of self-cleaning behaviors (grooming), raises (rearing), and the number feces (as an index of emotion) [27,28]. During the tests, the females presented no changes. The treated males, at doses of 4.5 mg/kg and 40.5 mg/kg, presented alterations in ambulation on the 15th day (Table 6). Tests on the diagnostic Roto-rod were performed for evaluation of abnormalities and no significant differences were found for either sex, with a conclusion that the EHA-Cs doses administered under test presented no stimulating effects on the nervous system.
Table 5.
Effect of chronic administration of different doses of the EHA of Cissus sicyoides on the glycemia of animals. The values are expressed as mean ± S.E.M. (N = 4). One-way ANOVA/Tukey. *p ≤ 0.05. *Significant compared between treated and controls.
| Control | 4.5 mg/kg | 13.5 mg/kg | 40.5 mg/kg | |
|---|---|---|---|---|
| Males | ||||
| 15 days | 92.3 ± 4.0 | 88.0 ± 4.4 | 78.0 ± 0.9 | 81.0 ± 4.0 |
| 30 days | 84.3 ± 2.8 | 85.3 ± 2.1 | 82.3 ± 2.9 | 84.8 ± 4.2 |
| 45 days | 83.0 ± 3.0 | 79.0 ± 3.0 | 81.0 ± 3.0 | 74.0 ± 2.0 |
| 60 days | 86.0 ± 2.4 | 85.0 ± 1.8 | 87.3 ± 3.1 | 82.0 ± 3.0 |
| 75 days | 86.0 ± 3.0 | 84.0 ± 3.5 | 82.0 ± 2.7 | 77.0 ± 2.7 |
| 90 days | 86.0 ± 4.0 | 85.0 ± 1.0 | 90.0 ± 3.0 | 90.3 ± 3.4 |
| Females | ||||
| 15 days | 92.0 ± 5.5 | 94.0 ± 1.0 | 73.5 ± 2.9* | 80.3 ± 1.8 |
| 30 days | 83.8 ± 4.1 | 84.0 ± 1.8 | 78.3 ± 3.9 | 80.5 ± 3.0 |
| 45 days | 83.0 ± 4.0 | 78.0 ± 2.7 | 71.0 ± 2.0 | 74.0 ± 3.0 |
| 60 days | 85.3 ± 1.9 | 79.0 ± 4.0 | 82.8 ± 3.1 | 84.5 ± 2.5 |
| 75 days | 88.0 ± 3.0 | 84.8 ± 1.3 | 77.3 ± 2.9* | 79.8 ± 1.9 |
| 90 days | 85.0 ± 4.0 | 86.8 ± 1.8 | 84.0 ± 4.0 | 83.0 ± 2.0 |
Table 6.
Number of ambulations in the field experiment, rats treated with different doses of the EHA of Cissus sicyoides during the test. The values are expressed as mean ± S.E.M. (N = 5). One-way ANOVA/Tukey. *p ≤ 0.05. *Significant compared between treated and controls.
| Control | 4.5 mg/kg | 13.5 mg/kg | 40.5 mg/kg | |
|---|---|---|---|---|
| Males | ||||
| 15 days | 36.0 ± 2.9 | 19.0 ± 5.1* | 28.0 ± 2.6 | 18.0 ± 3.6* |
| 30 days | 24.8 ± 2.4 | 20.6 ± 5.2 | 18.2 ± 2.9 | 20.8 ± 2.1 |
| 45 days | 14.8 ± 3.5 | 13.6 ± 3.0 | 12.0 ± 3.8 | 13.6 ± 2.1 |
| 60 days | 21.2 ± 4.3 | 23.4 ± 2.5 | 19.8 ± 3.0 | 12.8 ± 2.5 |
| 75 days | 21.2 ± 2.3 | 18.4 ± 2.1 | 13.4 ± 2.5 | 13.4 ± 2.0 |
| 90 days | 16.2 ± 3.2 | 14.6 ± 3.8 | 18.6 ± 2.5 | 9.8 ± 2.4 |
| Females | ||||
| 15 days | 31.2 ± 2.4 | 27.2 ± 3.1 | 34.8 ± 6.0 | 27.6 ± 3.1 |
| 30 days | 18.8 ± 3.5 | 14.4 ± 1.9 | 24.2 ± 5.5 | 22.4 ± 5.0 |
| 45 days | 20.0 ± 3.0 | 16.0 ± 4.0 | 22.0 ± 4.0 | 11.0 ± 2.0 |
| 60 days | 24.6 ± 1.0 | 22.2 ± 5.5 | 17.4 ± 4.7 | 16.8 ± 2.5 |
| 75 days | 22.0 ± 2.9 | 14.0 ± 3.7 | 14.6 ± 2.8 | 18.4 ± 3.5 |
| 90 days | 12.0 ± 2.0 | 15.0 ± 2.0 | 12.0 ± 2.0 | 14.0 ± 1.0 |
The hematological and biochemical parameter analyses for the treated groups (with three doses), and in the satellite groups, found no significant changes for the females. The only biochemical alteration observed was an increase of albumin in males at the dose of 13.5 mg/kg (Table 7). The elevation was reversible, i.e., the albumin did not increase in the satellite group. Hematological abnormalities were detected in an elevation of erythrocytes and hemoglobin in males at the dose of 13.5 mg/kg (Table 8). Yet this was reversible in the satellite group.
Table 7.
Biochemical parameters obtained from the serum of rats treated with different doses of the EHA of Cissus sicyoides during the chronic toxicity trial. The values are expressed as mean ± S.E.M. (N = 4). One-way ANOVA/Kruskal-Wallis/Dunn's. * p ≤ 0.05. *Significant compared between treated and controls.
| Control | 4.5 mg/kg | 13.5 mg/kg | 40.5 mg/kg | 4.5 mg/kg (Satellites) |
13.5 mg/kg (Satellites) |
40.5 mg/kg (Satellites) |
|
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Glucose (mg/dL) | 117.7 ± 8.9 | 119.7 ± 9.1 | 128.0 ± 8.7 | 132.7 ± 5.4 | 121.7 ± 15.1 | 122.0 ± 7.7 | 132.3 ± 18.8 |
| Urea (mg/dL) | 42.0 ± 1.5 | 38.0 ± 3.5 | 42.0 ± 3.5 | 38.0 ± 2.7 | 34.3 ± 0.9 | 41.0 ± 2.0 | 39.3 ± 3.4 |
| Serum creatinine (mg/dL) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| Cholesterol (mg/dL) | 52.7 ± 6.5 | 55.7 ± 0.7 | 59.3 ± 2.8 | 60.3 ± 1.8 | 56.7 ± 4.9 | 64.0 ± 7.2 | 57.3 ± 2.3 |
| Triglycerides (mg/dL) | 107.0 ± 16.6 | 109.3 ± 8.3 | 116.0 ± 7.9 | 105.0 ± 18.9 | 91.3 ± 13.9 | 106.3 ± 8.4 | 104.0 ± 15.1 |
| Uric Acid (mg/dL) | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.0 | 0.7 ± 0.3 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| AST (U/L) | 124.0 ± 10.3 | 119.3 ± 1.2 | 170.0 ± 4.0 | 118.7 ± 3.4 | 233.7 ± 92.2 | 121.7 ± 7.9 | 114.3 ± 1.2 |
| ALT (U/L) | 56.3 ± 2.3 | 55.7 ± 5.5 | 67.0 ± 0.6 | 59.0 ± 3.8 | 112.0 ± 44.5 | 66.0 ± 4.9 | 67.7 ± 9.2 |
| ALP (U/L) | 127.3 ± 31.1 | 11.7 ± 16.8 | 136.7 ± 5.8 | 96.7 ± 7.3 | 113.7 ± 2.9 | 117.3 ± 6.4 | 135.5 ± 24.3 |
| CK (U/L) | 3732 ± 830.3 | 3469 ± 177.4 | 9059 ± 833.6 | 3861 ± 86.2 | 2428 ± 737.6 | 3518 ± 1190 | 2625 ± 609 |
| LDH (U/L) | 1353 ± 64.3 | 1057 ± 204 | 2667 ± 206.7 | 1480 ± 163.2 | 1895 ± 239.6 | 920.7 ± 144.1 | 899.3 ± 234.7 |
| Amylase (U/dL) | 758.7 ± 42.6 | 933.7 ± 41.2 | 951.0 ± 16.1 | 832.0 ± 24.5 | 734.3 ± 74.4 | 856.7 ± 132.9 | 760.7 ± 11.2 |
| Protein (g/dL) | 6.5 ± 0.2 | 6.7 ± 0.0 | 6.7 ± 0.1 | 6.3 ± 0.0 | 6.7 ± 0.2 | 7.6 ± 0.2 | 6.9 ± 0.4 |
| Albumin (g/dL) | 2.6 ± 0.1 | 2.9 ± 0.0 | 3.1 ± 0.1* | 2.8 ± 0.1 | 2.8 ± 0.1 | 3.1 ± 0.1 | 2.7 ± 0.2 |
| Globulin (g/dL) | 3.9 ± 0.2 | 3.8 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 | 4.0 ± 0.1 | 4.5 ± 0.1 | 4.2 ± 0.3 |
| Calcium (mEq/L) | 10.0 ± 0.1 | 10.1 ± 0.2 | 9.6 ± 0.3 | 9.5 ± 0.1 | 9.3 ± 0.1 | 9.5 ± 0.1 | 9.6 ± 0.4 |
| Magnesium (mEq/L) | 2.3 ± 0.2 | 2.2 ± 0.0 | 2.3 ± 0.1 | 2.0 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 |
| Females | |||||||
| Glucose (mg/dL) | 101.3 ± 5.2 | 105.7 ± 1.9 | 123.3 ± 5.8 | 122.3 ± 10.4 | 106.3 ± 7.8 | 134.3 ± 13.3 | 114.0 ± 9.5 |
| Urea (mg/dL) | 41.0 ± 3.6 | 41.3 ± 4.3 | 42.7 ± 3.5 | 41.0 ± 5.0 | 68.3 ± 25.4 | 40.3 ± 13.2 | 114.0 ± 9.5 |
| Serum creatinine (mg/dL) | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| Cholesterol (mg/dL) | 84.0 ± 20.1 | 64.3 ± 6.8 | 73.7 ± 5.0 | 62.3 ± 8.7 | 79.3 ± 8.1 | 80.7 ± 3.5 | 78.3 ± 4.4 |
| Triglycerides (mg/dL) | 132.7 ± 28 | 112.3 ± 14.5 | 95.3 ± 5.9 | 74.6 ± 10.9 | 141.7 ± 18.9 | 88.3 ± 12.4 | 108.0 ± 14.2 |
| Uric Acid (mg/dL) | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.2 | 0.5 ± 0.0 |
| AST (U/L) | 151.7 ± 34.3 | 143.0 ± 19.1 | 158.3 ± 14.4 | 150.0 ± 26.6 | 123.0 ± 15.9 | 118.0 ± 5.8 | 118.0 ± 7.2 |
| ALT (U/L) | 67.0 ± 5.8 | 59.3 ± 3.2 | 66.6 ± 6.1 | 60.0 ± 13.5 | 61.3 ± 7.5 | 58.0 ± 7.9 | 56.7 ± 1.5 |
| FAL (U/L) | 91.0 ± 13.5 | 74.3 ± 12.4 | 85.0 ± 28.0 | 86.0 ± 41.0 | 74.3 ± 16.3 | 77.3 ± 13 | 55.3 ± 6.9 |
| CK (U/L) | 3547 ± 1037 | 3134 ± 63.3 | 6976 ± 1844 | 4045 ± 26.5 | 4658 ± 2350 | 5018 ± 372 | 2110 ± 690.7 |
| LDH (U/L) | 1679 ± 40.2 | 1490 ± 284.3 | 2057 ± 360 | 1745 ± 341.8 | 1305 ± 129.8 | 987 ± 138.2 | 1007 ± 117.4 |
| Amylase (U/dL) | 670.7 ± 119.1 | 597.3 ± 42.0 | 699.0 ± 42.9 | 750.7 ± 16.3 | 610.7 ± 42.7 | 695.7 ± 23.7 | 538.3 ± 27.2 |
| Protein (g/dL) | 7.4 ± 0.2 | 6.9 ± 0.2 | 7.0 ± 0.2 | 6.5 ± 0.3 | 7.4 ± 0.2 | 7.4 ± 0.3 | 7.2 ± 0.3 |
| Albumin (g/dL) | 3.1 ± 0.7 | 3.1 ± 0.1 | 3.4 ± 0.1 | 2.7 ± 0.0 | 3.3 ± 0.3 | 3.4 ± 0.2 | 3.4 ± 0.1 |
| Globulin (g/dL) | 4.2 ± 0.2 | 3.8 ± 0.3 | 3.6 ± 0.2 | 3.7 ± 0.3 | 4.1 ± 0.1 | 3.9 ± 0.2 | 3.8 ± 0.3 |
| Calcium (mEq/L) | 9.9 ± 0.0 | 10.0 ± 0.7 | 10.1 ± 0.3 | 9.1 ± 0.1 | 9.5 ± 0.2 | 9.8 ± 0.4 | 9.5 ± 0.1 |
| Magnesium (mEq/L) | 2.4 ± 0.0 | 2.3 ± 0.0 | 2.3 ± 0.1 | 2.1 ± 0.0 | 2.1 ± 0.1 | 0.4 ± 0.2 | 2.4 ± 0.1 |
Table 8.
Hematological parameters of rats treated with different doses of the EHA of Cissus sicyoides during the chronic toxicity trial. The values are expressed as mean ± S.E.M. (N = 4). One-way ANOVA/Kruskal-Wallis/Dunn's. *p ≤ 0.05. *Significant compared between treated and controls.
| Control | 4.5 mg/kg | 13.5 mg/kg | 40.5 mg/kg | 4.5 mg/kg (Satellites) |
13.5 mg/kg (Satellites) |
40.5 mg/kg (Satellites) | |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| Erythrocyte (106/mm3) |
7.0 ± 0.7 | 7.8 ± 0.3 | 8.8 ± 0.2* | 8.4 ± 0.7 | 8.7 ± 0.2 | 8.6 ± 0.3 | 9.2 ± 0.3 |
| Hemoglobin(g/dL) | 12.5 ± 1.2 | 14.5 ± 0.3 | 16.2 ± 0.2* | 15.4 ± 0.3 | 16.8 ± 0.4 | 15.2 ± 0.2 | 15.2 ± 0.4 |
| Hematocrit (%) | 29.4 ± 2.8 | 33.7 ± 0.6 | 37.9 ± 0.8 | 33.5 ± 0.9 | 37.4 ± 0.8 | 37.5 ± 0.9 | 39.1 ± 0.8 |
| VCM (3)μ | 42.3 ± 0.3 | 43.0 ± 0.6 | 43.0 ± 0.0 | 43.6 ± 0.7 | 43.3 ± 1.2 | 44.0 ± 0.6 | 44.0 ± 0.4 |
| HCM (g)μμ | 17.9 ± 0.1 | 18.5 ± 0.4 | 18.5 ± 0.2 | 18.4 ± 0.3 | 17.1 ± 0.3 | 17.7 ± 0.3 | 16.9 ± 0.3 |
| MCHC (%) | 42.5 ± 0.2 | 42.9 ± 0.3 | 42.9 ± 0.3 | 42.8 ± 1.0 | 39.4 ± 0.5 | 40.5 ± 0.3 | 38.5 ± 0.5 |
| Leukocytes (10/mm) |
4.9 ± 0.5 | 5.4 ± 1.4 | 5.5 ± 0.7 | 5.2 ± 0.2 | 9.1 ± 0.6 | 8.2 ± 1.3 | 5.7 ± 1.0 |
| Neutrophils (%) | 33.7 ± 5.2 | 27.7 ± 0.9 | 38.7 ± 2.9 | 39.3 ± 5.7 | 31.7 ± 6.6 | 35.3 ± 1.9 | 32.25 ± 3.47 |
| Eosinophils (%) | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.3 | 0.3 ± 0.3 | 1.3 ± 0.9 | 2.0 ± 0.4 |
| Lymphocytes (%) | 61.0 ± 4.6 | 68.0 ± 0.6 | 58.7 ± 3.2 | 56.7 ± 5.7 | 63.0 ± 4.6 | 60.0 ± 2.9 | 63.0 ± 3.8 |
| Monocytes (%) | 4.3 ± 0.7 | 3.3 ± 1.5 | 1.7 ± 0.3 | 2.7 ± 0.3 | 5.0 ± 1.7 | 3.3 ± 0.9 | 2.8 ± 0.3 |
| Platelets (10/mm) | 730.3 ± 56.9 | 685.0 ± 47.7 | 760.7 ± 59.4 | 812.0 ± 73.8 | 707.3 ± 14.5 | 738.0 ± 11.2 | 789.0 ± 49.1 |
| Females | |||||||
| Erythrocyte(106/mm3) | 7.4 ± 0.2 | 6.9 ± 0.5 | 7.8 ± 0.4 | 7.9 ± 0.1 | 7.4 ± 0.1 | 8.0 ± 0.2 | 8.6 ± 0.2 |
| Hemoglobin(g/dL) | 15.1 ± 0.1 | 13.4 ± 0.6 | 15.4 ± 0.6 | 16.3 ± 0.2 | 14.1 ± 0.2 | 15.5 ± 0.3 | 15.9 ± 0.4 |
| Hematocrit (%) | 34.6 ± 0.2 | 32.1 ± 1.9 | 35.7 ± 1.6 | 37.5 ± 0.3 | 34.1 ± 0.4 | 37.4 ± 1.1 | 40.3 ± 1.0 |
| VCM (3)μ | 43.3 ± 0.9 | 46.3 ± 0.7 | 45.3 ± 0.3 | 47.3 ± 0.3 | 46.3 ± 0.9 | 46.3 ± 0.7 | 46.5 ± 0.5 |
| HCM (g)μμ | 20.6 ± 0.5 | 19.5 ± 0.5 | 19.6 ± 0.2 | 20.5 ± 0.2 | 19.1 ± 0.5 | 18.9 ± 0.4 | 18.4 ± 0.2 |
| MCHC (%) | 43.6 ± 0.5 | 42.1 ± 0.7 | 43.1 ± 0.4 | 43.6 ± 0.3 | 41.3 ± 0.1 | 40.6 ± 0.5 | 39.4 ± 0.4 |
| Leukocytes (10/mm) | 4.4 ± 0.4 | 3.3 ± 0.3 | 4.8 ± 1.0 | 5.0 ± 1.5 | 7.3 ± 1.6 | 5.2 ± 1.1 | 3.9 ± 0.3 |
| Neutrophils (%) | 25.0 ± 1.2 | 26.0 ± 3.2 | 26.7 ± 1.7 | 30.7 ± 1.2 | 37.0 ± 3.2 | 23.3 ± 2.9 | 23.3 ± 4.9 |
| Eosinophils (%) | 1.0 ± 0.6 | 1.0 ± 0.0 | 1.0 ± 0.6 | 1.3 ± 0.3 | 1.3 ± 0.3 | 0.3 ± 0.3 | 1.2 ± 0.2 |
| Lymphocytes (%) | 70.7 ± 0.3 | 71.0 ± 3.6 | 70.0 ± 1.5 | 64.0 ± 0.0 | 58.3 ± 2.6 | 71.3 ± 3.5 | 72.4 ± 3.4 |
| Monocytes (%) | 3.3 ± 1.3 | 2.0 ± 0.6 | 2.3 ± 0.3 | 4.0 ± 1.0 | 3.3 ± 0.9 | 4.7 ± 0.3 | 3.4 ± 0.8 |
| Platelets(10/mm) | 757.7 ± 80.4 | 843.7 ± 84.7 | 720.7 ± 125.9 | 687.3 ± 47.4 | 781.3 ± 58.7 | 656.7 ± 94.0 | 612.0 ± 32.0 |
The anatomopathological study included both sexes, submitted to a Cissus sicyoides (EHA-Cs) dose of 40.5 mg/kg. The heart, lungs, liver and kidneys of all the animals were evaluated. These components presented no macroscopic anatomical changes, with only minimal statistically insignificant variations in weight (Table 9).
Table 9.
Organ weight of animals treated with different doses of the EHA of Cissus sicyoides during chronic treatment. The values are expressed as mean ± S.E.M. (N = 4). One-way ANOVA/Tukey. *p ≤ 0.05. *Significant compared between treated and controls.
| Control | 4.5 mg/kg | 13.5 mg/kg | 40.5 mg/kg | |
|---|---|---|---|---|
| Males | ||||
| Heart | 1.18 ± 0.09 | 1.23 ± 0.02 | 1.21 ± 0.08 | 1.33 ± 0.09 |
| Liver | 7.99 ± 0.11 | 9.29 ± 0.80 | 8.94 ± 0.32 | 8.45 ± 0.34 |
| Kidneys | 2.11 ± 0.007 | 2.15 ± 0.052 | 2.09 ± 0.104 | 2.04 ± 0.075 |
| Females | ||||
| Heart | 0.86 ± 0.07 | 1.12 ± 0.064 | 1.09 ± 0.076 | 1.11 ± 0.088 |
| Liver | 6.42 ± 1.22 | 6.57 ± 0.33 | 7.07 ± 0.35 | 6.57 ± 0.46 |
| Kidneys | 1.57 ± 0.20 | 1.68 ± 0.08 | 1.74 ± 0.09 | 1.70 ± 0.06 |
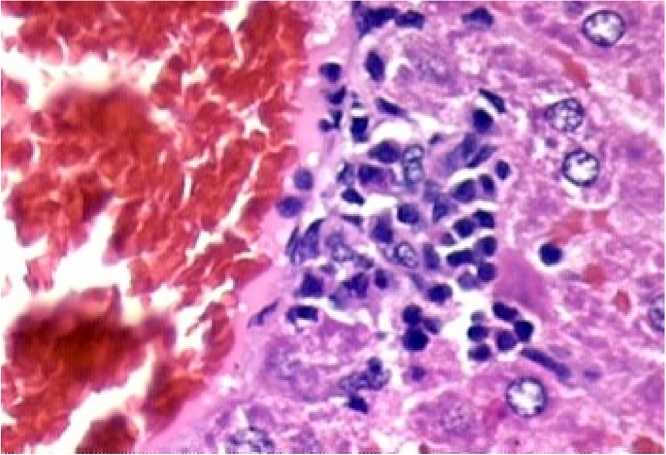
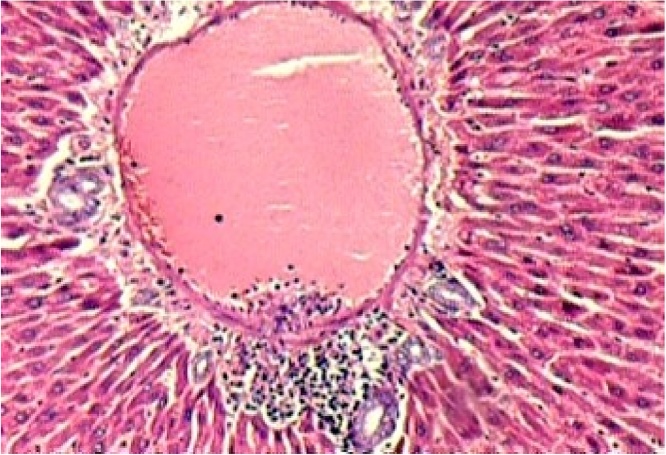
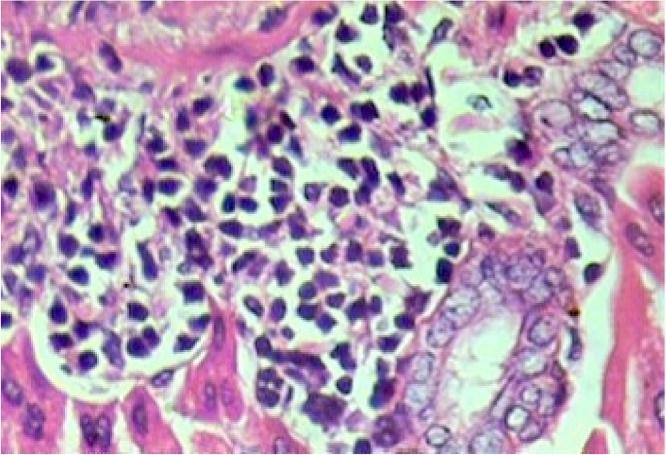
Histopathological evaluation of the animal organs: the heart and kidneys presented no particularities. Yet histopathological examination of the lungs (Fig. 5), and liver (Fig. 1, Fig. 2, Fig. 3, Fig. 4) presented significant though minor changes. In both sexes, a microscopic study of the lungs (Fig. 5) presented lobular parenchymal architecture with well distributed bronchial and bronchiolar elements. The alveolar tissue was represented by thin walled alveolar structures, exhibiting normal pneumocyte covering; the septal matrix was both lacking and weak, with congested capillaries, without, however, a sign of recent or older hemorrhages. In the peri-bronchial connective tissue, the presence of follicular, non-encapsulated lymphoid aggregates was found in close association with peri-bronchial venules and with the bronchiolar mucosa, and represented evidence of regular local BALT (bronchi associated lymphoid tissue) connections. Finally, in the samples analyzed, no atypical epithelial cell stigmas were observed. With regard to the histological liver study (Fig. 1, Fig. 2, Fig. 3, Fig. 4), preservation of the lobular architecture was observed with regular distribution of vascular-biliary atresia, and terminal hepatic vein portal triads in all samples. The portal spaces were host to a mild focally distributed lymphocytic influx. In four of the six animals in this study, an occurrence of small and isolated outbreaks of hepato-cytolysis (lobular necrosis) associated with exudation of mononuclear cells was noted. Signs of mild reactivity were represented by Kupffer cell hyperplasia. In addition, the presence of venule and sinusoidal congestion, in discreet degrees in zone 3 was observed. Although all tissues have some ability to metabolize drugs, the liver is the main organ that performs this function, and probably due to this factor, the liver is the organ most affected by the toxic effects of certain substances, even though they affect all systems and organs [29]. The results of this study demonstrate that the hydroalcoholic extract of the leaves of Cissus sicyoides, does not present chronic non-clinical toxicological effects at the popular dose of 4.5 mg/kg.
Fig. 5.
Thin Wall Lung Alveoli, Bronchus and BALT.
Fig. 1.
Liver – Focus of hepatocellular necrosis associated with the influx of lymphocytes. Reactive Kupffer cells. Hematoxylin and eosin, X400.
Fig. 2.
Liver – lobular necrosis with lymphocyte influx, in zone 3 (perivenular). Venule congestion. Hematoxylin and eosin, X400.
Fig. 3.
Liver – Portal Space - venous congestion and lymphocyte profile. Hematoxylin-eosin, X100.
Fig. 4.
Liver – Lymphocyte profile - Hematoxylin-eosin, X400.
4. Discussion
In previous toxicological bioassay screening studies in Artémia salina, it has been verified that potent bioactive components are present in EHA-Cs. However, high toxicity was also observed, presenting an average LC50 value of 930.7 g/mL [18]. Another acute study in mice with EHA-Cs, submitted to the maximum dosage of 5.0 g/kg (oral) and 2.0 g/kg (intraperitoneal route) did not report death for any animal, but did present liver disorder through increases in AST, ALT, alkaline phosphatase, and reactive hepatitis with a chronic lymphocytic profile, multifocal lobular Kupferian hyperplasia, focal collapses of the reticular connective tissue; and pulmonary changes with slight venule-capillary congestion (at the given dose), with lymphoid, follicular, and both focal and peri bronchial hyperplasia [27].
Significant changes in liver function were found. To obtain more preliminary information for the basis of the chronic toxicity tests, we performed a new EHA-Cs acute toxicological evaluation at a dose of 40.5 mg/kg (9x the dose of popular use). When studying a new substance, an important step is to investigate possible adverse reactions, which may be presented by drugs currently in therapeutic use [28].
In this present acute study, we did observe that the treated females exhibited a significant reduction in weight gain. Certain studies correlate the effect of stress on body weight and food ingestion, suggesting that stress can physiologically influence the animals [[29], [30], [31]]. This shows that the females were more sensitive to the induced pharmacological stress than the males. The resulting biochemical changes of the treated animals in uric acid, amylase and AST, though being statistically significant, were not of clinical importance, since at the dose of 40.5 mg/kg (9x the popular use) neither liver or renal toxicity profiles were presented. The principal biochemical parameters such as AST and ALT for the liver, and urea for the kidneys demonstrated that these organs were preserved in the treated animals relative to the controls.
In the chronic study, seeing that the population makes use of Cissus sicyoides (for its hypoglycemic activity), we noted that even in certain evaluations presenting no statistically significant results, blood glucose presented a slight tendency to fall. The only biochemical alteration observed was an increase of albumin in males at the dose of 13.5 mg/kg. The increase in hemoglobin in this group is commensurate with the increase in erythrocytes, since hemoglobin is contained within red blood cells responsible for gas transport, especially oxygen. Erythrocytosis consists in increasing erythrogram numbers and may be due to an actual increase in the circulating erythrocyte mass, a reduction in plasma volume (pseudo-erythrocytosis), or a combination of both mechanisms. Discrete erythrocytoses is a difficult differential diagnosis, the blood test only provides quantitative data, and clinical diagnosis is essential [[32], [33], [34]].
5. Conclusion
The non-clinical toxicological trials of the hydroalcoholic extract of the leaves of Cissus sicyoides (EHA-Cs) in rats presented few toxic effects at the popular use dosage and only small changes in hematological and biochemical parameters at the dose of 13.5 mg/kg (3x the popular usage dose). At the highest dose (40.5 mg/kg) changes in hematological and biochemical parameters occurred, which were reversed upon cessation, not causing histological changes of clinical character in the vital organs: heart, lungs, liver and kidneys.
Since the population uses the tea from the leaves of Cissus sicyoides for its hypoglycemic action, it is important to note that the EHA-Cs generated a slight but not significant decrease in fasting plasma glucose, but only the females presented a significant reduction in blood glucose at the dose of 13.5 mg/kg (3x popular usage). We conclude that the EHA-Cs possesses a relatively low toxicity profile.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgement
This research was supported by CNPq (National Council for Scientific and Technological Development).
References
- 1.Souza-Moreira T.M., Salgado H.R.N., Pietro R.C.L.R. O Brasil no contexto de controle de qualidade de plantas medicinais. Rev. Bras. Farmacogn. 2010;20(3):435–440. [Online], ISSN 0102-695X. [Google Scholar]
- 2.Giraldi M., Hanazaki N. Uso e conhecimento tradicional de plantas medicinais no Sertão do Ribeirão, Florianópolis, Santa Catarina, Brasil. Acta Bot. Bras. 2015;24(2):395–406. [online], ISSN 0102-3306. [Google Scholar]
- 3.Sachan A.K., Gupta A. A review on nanotized herbal drugs. Int. J. Pharm. Sci. Res. 2015;6(3):961–970. [Google Scholar]
- 4.Jaiswal Y., Liang Z., Zhao Z. Botanical drugs in ayurveda and traditional Chinese medicine. J. Ethnopharmacol. 2016;194(1):245–259. doi: 10.1016/j.jep.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 5.SINITOX - National Information System Tóxico-Farmacológicas . SINITOX; Rio de Janeiro, Brazil: 2013. Data from Intoxication. [Google Scholar]
- 6.Cunha C.B.A. Tese Doutorado em Química, Universidade Federal do Rio Grande do Sul; Brasil: 2005. Desenvolvimento de Procedimento Analítico para Determinação de Fármacos e Pesticidas em Amostra Aquosas Ambientais. [Google Scholar]
- 7.Alcantara R.G.L., Joaquim R.H.V.T., Sampaio S.F. Plantas medicinais: o conhecimento e uso popular. Revista de APS. 2016;18(4):470–482. [Google Scholar]
- 8.Beltrame F.L., Startoretto J.L., Bazotte R.B., Cuman R.N., Cortez D.A.G. Estudo fitoquímico e avaliação do potencial antidiabético de Cissus sicyoides L. (Vitaceae) Quim. Nova. 2001;24(6):783–785. [Google Scholar]
- 9.Abreu I.N., Pinto J.E.B.P., Grandson A., Bertolucci S.K.V., Ladeira G.C. Nitrogênio e fósforo na produção vegetal e na indução de mucilagem em plantas de insulina. Hortic. Bras. 2002;20(4):536–540. ISSN 0102-0536. [Google Scholar]
- 10.Silva G.A., Almeida-Muradian L.B., Akisue G., Iron V.O. Padronização farmacognóstica de Cissus sicyoides L. (insulina vegetal) e identificação. Rev. Bras. Farmacogn. 1996;5(1):96–112. [Google Scholar]
- 11.Garcia M.D., Altet T.O.M., Sáenz M.T., Martínez-Dominguez P.R. Anti-inflammatory activity of Agave intermixta trel. and Cissus sicyoides L., species used in the Caribbean traditional medicine. J. Ethnopharmacol. 2000;71(3):395–400. doi: 10.1016/s0378-8741(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 12.Vasconcelos T.H.C., Modesto-Filho J., Diniz M.F.F.M., Santos H.B., Aguiar F.B., Moreira P.V.L. Estudo toxicológico pré-clínico agudo com o extrato hidroalcoólico das folhas de Cissus sicyoides L. (Vitaceae) Rev. Bras. Farmacogn. 2007;17(4):583–591. [Google Scholar]
- 13.Abreu I.N., Pinto J.E.B.P., Bertolucci S.K.V., Moral G.C., Ladeira Propagação in vivo e in vitro de Cissus sicyoides, uma planta medicinal. Acta Amaz. 2003;33(1):1–7. [Google Scholar]
- 14.Doro D.L., Pessine G.L., Fields E.J.V., Nakamura C.V., Cortez R., Cortez D.A.G. Estudo fitoquímico e avaliação antimicrobiana do cissus sicyoides L. (Vitaceae) Arq. Ciênc. Saúde UNIPAR. 1997;1(1):45–47. [Google Scholar]
- 15.Silva G.A., Araujo L.C.L., Oga S., Akisue G. Estudo toxicológico e farmacológico dos extratos fluidos de Cissus sicyoides L. Rev. Bras. Farmacogn. 1996;5(2):143–155. [Google Scholar]
- 16.Garcia X., Cartas-Heredia L., Lorenzana-Jímenez M., Gijón E. Vasoconstrictor effect of Cissus sicyoides on guinea-pig aortic rings. Gen. Pharmacol. Vasc. Syst. 1997;29(3):457–462. doi: 10.1016/s0306-3623(96)00478-8. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes G., Banu J. Medicinal properties of plants from the genus Cissus: a review. J. Med. Plants. Res. 2012;6(16):3080–3086. [Google Scholar]
- 18.Beserra F.P., Santos R.C., Périco L.L., Rodrigues V.P., Kiguti L.R.A., Saldanha L.L. Cissus sicyoides: pharmacological mechanisms involved in the anti-inflammatory and antidiarrheal activities. Int. J. Mol. Sci. 2016;17(2):149. doi: 10.3390/ijms17020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias G.T., Lima C.M.B.L., Lira A.B., Ramalho J.A., Oliveira M.K., Diniz M.F.F.M. Toxicidade do extrato hidroalcoólico das folhas de Cissus sicyoides. Acta Bras. [S.E.] 2017;1(1):8–12. [Google Scholar]
- 20.Larini L. Avaliação toxicológica. In: Larini L., editor. Toxicologia. Editora Manole; São Paulo: 1999. pp. 43–58. [Google Scholar]
- 21.Barros S.B.M., Davino S.C. Avaliação da toxicidade. In: OGA S., editor. Fundamentos de Toxicologia. Ed Ateneu; São Paulo: 2003. pp. 57–67. [Google Scholar]
- 22.Brazil . Management Assessment of Safety and Effectiveness - GESEF; Brasília: 2013. The National Agency of Sanitary Surveillance – ANVISA: Guide to Conducting non-Clinical Studies of Toxicology and Safety Pharmacology Necessary for the Development of Drugs. [Google Scholar]
- 23.Almeida R.N., Falcon A.C.G.M., Diniz R.S.T., Quintans-Júnior L.J., Polari R.M., Barbosa-Filho J.M. Metodologia para avaliação de plantas com atividade no sistema nervoso central e alguns dados experimentais. Rev. Bras. Farm. 1999;80(3):72–76. [Google Scholar]
- 24.Brito . Editora da UNICAMP; Campinas, SP: 1994. Manual de ensaios toxicológicos in vivo; p. 122. Campinas, SP. [Google Scholar]
- 25.Angelucci E., Mantovani D.M.B. ITAL/SBCTA; Campinas: 1986. Minerais em alimentos: manual técnico. 131 p. [Google Scholar]
- 26.Bacha W.J., Wood L.M. Lea & Febiger; 1990. Color Atlas of Veterinary Histology. 269 p. [Google Scholar]
- 27.Almeida R.N., Oliveira T.M.L. Triagem farmacológica comportamental. In: Almeida R.N., editor. Psicofarmacologia: fundamentos práticos. Guanabara Koogan; Rio de Janeiro: 2006. pp. 131–137. [Google Scholar]
- 28.Vasconcelos T.H.C. Tese de Doutorado em Produtos Naturais. Laboratório de Tecnologia Farmacêutica, Universidade Federal da Paraíba; João Pessoa, Brasil: 2004. Ensaios toxicológicos pré-clínicos e clínicos com as folhas de Cissus sicyoides L. (VITACEAE) [Google Scholar]
- 29.Failace R. Artes Médicas; Porto Alegre: 1995. Hemograma: Manual de interpretação. [Google Scholar]
- 30.Kavvadias D., Sand P., Youdim K.A., Qaiser M.Z., Rice-Evans C., Baur R. The flavones hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. Br. J. Pharmacol. 2004;142(5):811–820. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto V.M., Mello F.B., Mello J.R.B. Avaliação toxicológica de preparação fitoterápica contendo Piper methysticum Forst Piperaceae (Kava Kava ®) sobre o desenvolvimento pré-natal em ratos Wistar. Lat. Am. J. Pharm. 2007;26(6):818–824. [Google Scholar]
- 32.Kato H., Tsuji M., Miyagawa K., Takeda K., Takeda H. Repeated exposure to stress stimuli during ethanol consumption prolongs withdrawal-induced emotional abnormality in mice. Eur. J. Pharmacol. 2013;721(1-3):29–34. doi: 10.1016/j.ejphar.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Goebel-Stengel M., Yuan P., Stengel A., Taché Y. Corticotropin-releasing factor overexpression in mice abrogates sex differences in body weight, visceral fat, and food intake response to a fast and alters levels of regulatory hormones. Biol. Sex Differ. 2017;8(2):1–14. doi: 10.1186/s13293-016-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masur J., Martz R.M., Carlini E.A. Effects of acute and chronic administration of Cannabis sativa and (-)-trastetrahydrocannabionol on the behavior of rats in open-field arena. Psychopharmacology. 1971;19(4):388–397. doi: 10.1007/BF00404383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.