Abstract
Oncolytic adenoviral immunotherapy activates the innate immune system with subsequent induction of adaptive tumor‐specific immune responses to fight cancer. Hence, oncolytic viruses do not only eradicate cancer cells by direct lysis, but also generate antitumor immune response, allowing for long‐lasting cancer control and tumor reduction. Their therapeutic effect can be further enhanced by arming the oncolytic adenovirus with costimulatory transgenes and/or coadministration with other antitumor therapies. ONCOS‐102 has already been found to be well tolerated and efficacious against some types of treatment‐refractory tumors, including mesothelin‐positive ovarian cancer (NCT01598129). It induced local and systemic CD8+ T‐cell immunity and upregulated programmed death ligand 1. These results strongly advocate the use of ONCOS‐102 in combination with other therapeutic strategies in advanced and refractory tumors, especially those expressing the mesothelin antigen. The in vivo work presented herein describes the ability of the oncolytic adenovirus ONCOS‐102 to induce mesothelin‐specific T‐cells after the administration of the virus in bagg albino (BALB/c) mice with mesothelin‐positive tumors. We also demonstrate the effectiveness of the interferon‐γ the enzyme‐linked immunospot (ELISPOT) assay to detect the induction of T‐cells recognizing mesothelin, hexon, and E1A antigens in ONCOS‐102‐treated mesothelioma‐bearing BALB/c mice. Thus, the ELISPOT assay could be useful to monitor the progress of therapy with ONCOS‐102.
Keywords: granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), mesothelin, mesothelioma, oncolytic adenovirus, ONCOS‐102
1. INTRODUCTION
Malignant mesothelioma is a fatal form of cancer, which is difficult to diagnose and cure.1, 2 Its primary cause is exposure to asbestos, and it has a long latency period, sometimes as long as 20 years. Mesothelin is a tumor antigen that is normally present on the mesothelial cells lining the pleura, peritoneum, pericardium, and tunica vaginalis.3 Mesothelin antigen is highly expressed in several cancers, including malignant mesothelioma, ovarian, and lung adenocarcinoma. Therefore, it could potentially be used as a tumor marker or as an antigenic target of vaccines.4 No effective therapeutic modalities exist for malignant mesothelioma apart from surgical resection in 10% to 15% of the patients. In the advanced disease, chemotherapy has a marginal effect and the prognosis is extremely poor.5 Hence, there is an urgent need for new and more effective therapies. Oncolytic adenoviruses are promising immunotherapeutic agents for advanced and treatment‐refractory cancer patients. Their antitumor activity is based on the direct lysis of cancer cells and the induction of systemic antitumor immunity.6, 7, 8, 9 Oncolysis leads to the release of tumor epitopes that can be processed by antigen‐presenting cells10, 11, 12, 13, 14, 15, 16, 17, 18 to activate antigen‐specific CD4+ and CD8+ T‐cell responses. Immunogenic cell death leads to changes in cell surface structure, such as exposure of calreticulin in the outer plasma membrane and subsequent release of high‐mobility group box 1 protein and adenosine triphosphate.19 Activated CD8+ T‐cells can expand into cytotoxic effector cells and infiltrate tumors where they mediate antitumor immunity after antigen recognition.
Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) mediates antitumor effects by mobilizing and maturing dendritic cells as well as increasing the activity of cytotoxic T‐cells.15, 16, 17 Systemic use of recombinant GM‐CSF is associated with well‐described harmful toxicities. Also, this route of administration may not result in an adequate local concentration of GM‐CSF in the tumors.20 Thus, engineering the viral genome with the gene encoding GM‐CSF reduces the risk of toxicities, ensuring desired local levels of GM‐CSF to improve the induction of antitumor immunity.
Ranki et al16, 17 reported the results of a Phase I study (NCT01598129) in which ONCOS‐102 (Ad5/3‐Δ24‐GM‐CSF) was well tolerated and induced local and systemic CD8+ T‐cell immunity in patients with treatment‐refractory and immune‐cell poor solid tumors. They also observed an upregulation of programmed death ligand 1 (PD‐L1) after treatment with ONCOS‐102, suggesting that combination of ONCOS‐102 with checkpoint inhibitors, including PD‐1/PD‐L1 inhibitors, could be beneficial against such refractory tumors.
We have earlier reported synergistic antitumor efficacy of ONCOS‐102 in combination with standard of care chemotherapy (pemetrexed, cisplatin, and carboplatin) in a xenograft BALB/c model of human malignant mesothelioma. The synergism observed in this preclinical study gives hope for combinations of ONCOS‐102 with first‐line chemotherapy in patients suffering from malignant mesothelioma.19
In this study, we show the induction of T‐cells specific for mesothelin, hexon, and E1A antigens after the treatment of mesothelioma‐bearing BALB/c mice with ONCOS‐102 and the effectiveness of the interferon (IFN)‐γ ELISPOT in detecting this response.
2. MATERIALS AND METHODS
2.1. Materials and reagents
Murine mesothelioma cell line AB12 (HPA Culture Collections, Sigma‐Aldrich; Cell Bank Australia reference number: CBA0146) was cultured in Roswell Park Memorial Institute (RPMI) 1640 (ThermoFisher Scientific, Bleiswijk, Netherlands) (with 2 mM l‐Glutamine [ThermoFisher Scientific, Bleiswijk, Netherlands] + 25 mM 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid [HEPES], ThermoFisher Scientific, NY) +5% fetal calf serum (FCS), ThermoFisher Scientific, NY. Cells were incubated at 37°C with 5% CO2 and harvested using 0.05% Trypsin/EDTA (ThermoFisher Scientific, Bleiswijk, Netherlands) at 37°C for 5 minutes. The construction and characterization of chimeric oncolytic adenovirus coding for human GM‐CSF (ONCOS‐102) has been described previously.14, 19, 20 ONCOS‐102 was produced by Biovian (Turku, Finland) according to good laboratory practice and stored at −80°C until use.
2.2. Mesothelioma xenograft model
All animal experiments were approved by the Ethics Committee for Animal Experimentation from the Biomedical Research Institute of Bellvitge (IDIBELL). Animals were housed in the IDIBELL Animal Core Facility (AAALAC Unit 1155). Mice (BALB/c) were exposed to crocidolite asbestos through intraperitoneal injection, resulting in tumor formation. Then, cultures were established from malignant mesothelial cells obtained from ascites fluid (Cell Bank Australia). Mesothelioma murine cell line AB12 (positive for mesothelin antigen) was implanted intraperitoneally (5 × 105 cells/200 µL) in BALB/c mice (2 groups: 1 treated with ONCOS‐102 and the other with phosphate‐buffered saline; n = 6 mice). Repeated intraperitoneal injections of 1 × 1011 oncolytic adenoviral particles/200 µL were given on days 0, 3, and 6 after tumor formation. Tumor size was measured with caliper on 2 dimensions on day 20. The longest and shortest diameter were recorded, and the tumor volume was calculated using a formula of 0.52 × length × (width)2.
2.3. IFN‐γ ELISPOT
At endpoint (day 20), spleens were harvested from untreated (phosphate‐buffered saline) and ONCOS‐12‐treated BALB/c mice. Splenocytes were isolated to determine counts of T‐cells responding to mesothelin, human adenovirus 5 E1A, and hexon peptides by secretion of IFN‐γ. Harvested splenocytes were stimulated with peptide pools of the complete murine mesothelin protein sequence, human adenovirus 5 E1A, and hexon proteins. IFN‐γ production by T‐cells was evaluated by using IFN‐γ ELISPOT (Abcam, Cambridge, UK). A single‐cell suspension of 2.5 × 105 splenocytes/well was plated in RPMI medium including 200 ng of peptide. After incubating overnight at 37°C and 5% CO2, plates were washed and stained with biotinylated anti‐mouse IFN‐γ and incubated for 2 hours, followed by streptavidin conjugate enzyme. The spots were counted using the ELISpot Reader (AID, Strasberg, Germany).
2.4. Statistical analysis
Statistical significance was analyzed by using the Mann‐Whitney test. All statistical analysis, calculations and tests were performed using GraphPad Prism 7 (GraphPad Software, San Diego, CA). Results are presented as mean ± standard deviation. All P values were 2 sided and considered statistically significant when ≤.05.
3. RESULTS AND DISCUSSION
Oncolytic adenoviruses are immunotherapeutic agents with the ability to prime and boost immune responses, leading to the development of anticancer immunity.15 ONCOS‐102 is an engineered adenovirus (Ad5/3) that codes for GM‐CSF. Its chimeric 5/3 capsid contains a fiber with a c‐terminal knob derived from serotype 3, which binds to tumor‐associated desmoglein 2 receptor instead of the coxsackie‐adenovirus receptor, which is found to be downregulated in advanced tumors.7 The 24‐bp deletion in the Rb binding site of the E1A gene causes the virus to replicate selectively in cells with p16‐Rb pathway defects, which includes most cancers.20 ONCOS‐102 causes immunogenic cancer cell death19 and the subsequent release of tumor antigens to be processed by antigen‐presenting cells, resulting in the priming of tumor‐specific immunity. This effect may be further enhanced by combination therapies11, 21 and by immunostimulatory transgenes coded by the adenovirus.
We induced mesothelioma in BALB/c mice by injecting the murine mesothelioma cell line AB12, which is positive for mesothelin antigen. IFN‐γ ELISPOT was performed with splenocytes from untreated or ONCOS‐102‐treated mice to determine the specificity of tumor‐related T‐cells for the antigen mesothelin. Splenocytes from the vehicle (phosphate‐buffered saline)‐treated mice were negative for tumor antigen‐specific T‐cells, whereas those from ONCOS‐102‐treated mice showed induction of tumor‐specific T‐cells.
Murine splenocytes from untreated and ONCOS‐102‐treated mice were examined in IFN‐γ ELISPOT for the frequency of T‐cells specific for mesothelin, hexon, and E1A. As expected, positive controls, phorbol 12‐myristate 13‐acetate (PMA), and ionomycin showed high frequencies of T‐cells secreting IFN‐γ in both untreated and ONCOS‐102‐treated mice.
In this study, we have demonstrated the activation of mesothelin‐specific T‐cells in a preclinical setting after the treatment of mesothelioma tumors in BALB/c mice with the oncolytic adenovirus, ONCOS‐102 (Figure 1).
Figure 1.
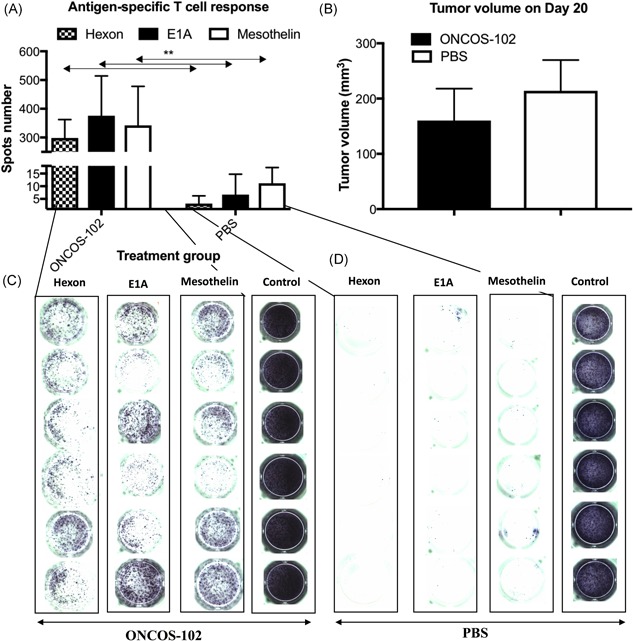
IFN‐γ ELISPOT. (A) Antigen‐specific T‐cell response. IFN‐γ ELISPOT was performed with splenocytes from untreated and ONCOS‐102‐treated mice to determine the specificity of tumor‐related T‐cells for the antigen mesothelin tumor treated with ONCOS‐102. (B) Mesothelioma murine cell line AB12 was implanted intraperitoneally (5 × 105 cells/200 µL) in BALB/c mice (2 groups: 1 treated with ONCOS‐102 and the other with PBS; n = 6 mice). Repeated intraperitoneal injections of 1 × 1011 ONCOS‐102 particles/200 µL were given on days 0, 3, and 6 after tumor formation. Tumor size was measured with a caliper on 2 dimensions on day 20. The longest and shortest diameter were recorded, and the tumor volume was calculated using a formula of 0.52 × length × (width)2. (C) Left panels for the tumor treated with ONCOS‐102 and (D) PBS, respectively, stimulated with hexon pool, E1A pool (haplotype b), mesothelin pool, PMA, and Ionomycin, respectively (positive control). Error bars, mean ± SD: *p < .05, **p < .01, ***p < .001. BALB/c, bagg albino; ELISPOT, enzyme‐linked immunospot; IFN, interferon; PBS, phosphate‐buffered saline; PMA, phorbol 12‐myristate 13‐acetate; SD, standard deviation
Hexon and E1A peptide were selected to evaluate the response against the injected adenovirus. Mice are not permissive to human adenovirus replication, and the most immunogenic protein is the early protein E1A, contrary to humans where hexon is the main target for T‐cell.22, 23 Leen et al. reported a panel of CD4+ and CD8+ T‐cell epitopes that could be used to prime antigen‐specific T‐cells and challenge adoptively transferred T‐cells in vivo. These epitopes span conserved regions of the hexon protein and would be useful to monitor immune response before and after immunotherapy.24 As the capsid of ONCOS‐102 contains hexon protein, which plays an important role in virus entry into cells, and E1A protein, which binds to pRb/p300 family of histone acetyltransferases and induces p53‐dependent apoptosis in cancer cells5; they were used to stimulate the splenocytes in ELISPOT.
Mice treated with the virus generated specific T‐cells against hexon and E1A antigen, as can be seen in Figure 1, in which the signal was detected from only ONCOS‐102‐treated mice. This is not surprising as viral capsid components such as hexon,22 penton, and fiber25 play important roles in establishing adaptive immune responses against the adenovirus. Optimal antiadenoviral response requires both CD4+ and CD8+ T‐cell responses. Leen et al24 found that immune response to hexon protein was dominated by CD4+ T‐cells directed against multiple major histocompatibility complex II epitopes. CD8+ reactivity was found less frequently and recognized fewer epitopes presented by fewer class I molecules. However, Leen et al reported the identification of a panel of CD4+ and CD8+ T‐cell epitopes that stimulated both CD4+ and CD8+ T‐cells and hence could be used in vaccination studies to prime antigen‐specific T‐cells, could serve as antigens to challenge adoptively transferred T‐cells in vivo, and could help in monitoring before and after immunotherapy.
As the murine mesothelioma cell line, AB12, was implanted in the BALB/c mice, mesothelin peptide was used to stimulate splenocytes in ELISPOT. High frequency of mesothelin‐specific T‐cells was seen but only in splenocytes from ONCOS‐102‐treated mice. Repeated intraperitoneal treatment with oncolytic adenovirus ONCOS‐102 in an immunocompetent animal model of BALB/c mice bearing mesothelioma tumor shows the ability to control the tumor growth over the control. T‐cells recognizing the mesothelin antigen expressed on mesothelioma cells are stimulated by ONCOS‐102. Also, Vassilev et al15 reported systemic induction of many tumor‐specific CD8+ T‐cell populations alongside infiltration of CD8+ lymphocytes into a tumor‐infiltrating lymphocyte negative, chemotherapy refractory patient's ovarian cancer. In this patient, CD8+ cells were isolated from peripheral blood mononuclear cells and analyzed with IFN‐γ ELISPOT for specificity for cancer‐testis antigens, NY‐ESO‐1, MAGE‐A1, MAGE‐A3, and a differentiation antigen, mesothelin, reported to be widely expressed in ovarian carcinoma. Mesothelin‐specific CD8+ T‐cells were more numerous than the other T‐cell populations. Thus, ONCOS‐102 treatment primed the immune system and facilitated tumor antigen presentation to cytotoxic cells. However, levels of antigen‐specific T‐cells decreased in late pools of CD8+ T‐cells, leading to the speculation that the concomitant or sequential treatment with an immune checkpoint inhibitor would be beneficial when treating with ONCOS‐102. Furthermore, here too, as CD8+ T‐cells are negatively regulated by PD‐1/PD‐L1 interactions within tumors, the rise in levels of CD8+ T‐cells after ONCOS‐102 treatment suggests that PD‐1 inhibition could be beneficial in combination with ONCOS‐102.15
The ability of ONCOS‐102 to cause immunogenic cell death and antitumor immune response, especially directed against mesothelin antigen, suggests that increased effect may be achieved by combining with other therapeutic agents to treat tumors that lack tumor‐infiltrating lymphocytes and that express mesothelin on the cell surface. Our IFN‐γ ELISPOT results suggest that the clear and significant increase in the counts of T‐cells directed against mesothelin, hexon, and E1A could validate the IFN‐γ ELISPOT assay to monitor the efficacy and progress of treatment with ONCOS‐102 alone or in combination therapies.
CONFLICTS OF INTEREST
L Kuryk, A‐S W Møller, M Jaderberg, and S Pesonen are employees and/or shareholders in Targovax Oy in Finland and Targovax ASA in Norway. V Cerullo and S Pesonen are employees and/or shareholders in Valo Therapeutics.
AUTHORS’ CONTRIBUTIONS
All authors have made contribution to this paper and all authors have approved the final article.
ACKNOWLEDGMENTS
The work of L Kuryk was supported by Marie Curie Innovative Training Network (ITN) grant, ADVance (FP7‐290002), funded by the European Commission.
Kuryk L, Møller A‐SW, Garofalo M, et al. Antitumor‐specific T‐cell responses induced by oncolytic adenovirus ONCOS‐102 in peritoneal mesothelioma mouse model. J Med Virol. 2018;90:1669–1673. 10.1002/jmv.25229
References
REFERENCES
- 1. Moore AJ, Parker RJ, Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis. 2008;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tipu SA, Ahmed I, Ishtiaq S. Malignant mesothelioma. Pak J Med Sci. 2013;29(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okio H, Kazu S, Masahiro M. Diagnostic biomarker of asbestos‐related mesothelioma: example of translational research. Cancer Sci. 2007;98(8):1147‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44(1):46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotova S, Wong RM, Cameron RB. New and emerging therapeutic options for malignant pleural mesothelioma: review of early clinical trials. Cancer Manag Res. 2015;7:51‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerullo V, Vähä‐Koskela M, Hemminki A. Oncolytic adenoviruses: a potent form of tumor immunovirotherapy. Oncoimmunology. 2012;1(6):979‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howells A, Marelli G, Lemoine NR, Wang Y. Oncolytic viruses‐interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol. 2017;7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh PK, Doley J, Kumar GR, Sahoo AP, Tiwari AK. Oncolytic viruses & their specific targeting to tumour cells. Indian J Med Res. 2012;136:571‐584. [PMC free article] [PubMed] [Google Scholar]
- 10. Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Hum Gene Ther. 2014;25(4):265‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuryk L. Strategies to Enhance Efficacy of Oncolytic Virotherapy [dissertation]. Helsinki, Finland: University of Helsinki, Faculty of Pharmacy, Doctoral Programme in Biomedicine; 2016.
- 12. Garofalo M, Iovine B, Kuryk L, et al. Oncolytic adenovirus loaded with L‐carnosine as novel strategy to enhance the anti‐tumor activity. Mol Cancer Ther. 2016;15(4):651‐660. [DOI] [PubMed] [Google Scholar]
- 13. Capasso C, Hirvinen M, Garofalo M, et al. Oncolytic adenoviruses coated with MHC‐I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology. 2016;5(4):e1105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuryk L, Vassilev L, Ranki T, et al. Toxicological and bio‐distribution profile of a GM‐CSF‐expressing, double‐targeted, chimeric oncolytic adenovirus ONCOS‐102 ‐ Support for clinical studies on advanced cancer treatment. PLoS One. 2017;12(8):e0182715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vassilev L, Ranki T, Joensuu T, et al. Repeated intratumoral administration of ONCOS‐102 leads to systemic antitumor CD8 T‐cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology. 2015;4(7):e1017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranki T, Pesonen S, Hemminki A, et al. Phase I study with ONCOS‐102 for the treatment of solid tumors ‐ an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer. 2016;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ranki T, Joensuu T, Jäger E, et al. Local treatment of a pleural mesothelioma tumor with ONCOS‐102 induces a systemic antitumor CD8 T‐cell response, prominent infiltration of CD8 lymphocytes and Th1 type polarization. Oncoimmunology. 2014;3(10):e958937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pesonen S, Diaconu I, Kangasniemi L, et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L‐expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72(7):1621‐1631. [DOI] [PubMed] [Google Scholar]
- 19. Kuryk L, Haavisto E, Garofalo M, et al. Synergistic anti‐tumor efficacy of immunogenic adenovirus ONCOS‐102 (Ad5/3‐D24‐GM‐CSF) and standard of care chemotherapy in preclinical mesothelioma model. Int J Cancer. 2016;139(8):1883‐1893. [DOI] [PubMed] [Google Scholar]
- 20. Koski A, Kangasniemi L, Escutenaire S, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18(10):1874‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siurala M, Bramante S, Vassilev L, et al. Oncolytic adenovirus and doxorubicin‐based chemotherapy results in synergistic antitumor activity against soft‐tissue sarcoma. Int J Cancer. 2015;136(4):945‐954. [DOI] [PubMed] [Google Scholar]
- 22. Sumida SM, Truitt DM, Lemckert AAC, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174(11):7179‐7185. [DOI] [PubMed] [Google Scholar]
- 23. Gürlevik E, Woller N, Strüver N, et al. Selectivity of oncolytic viral replication prevents antiviral immune response and toxicity, but does not improve antitumoral immunity. Mol Ther. 2010;18(11):1972‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leen AM, Christin A, Khalil M, et al. Identification of hexon‐specific CD4 and CD8 T‐cell epitopes for vaccine and immunotherapy. J Virol. 2008;82(1):546‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu B, Dong J, Wang C, et al. Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology. 2013;437(2):118‐123. [DOI] [PubMed] [Google Scholar]


