Summary
Viscoelastic assays such as TEG ® and ROTEM ® are increasingly used to guide transfusion of blood products. The EXTEM assay maximum clot firmness (MCF) is a ROTEM measure available after 25–29 min used to guide early decisions. EXTEM A10, the clot firmness at 10 min, is an accepted early surrogate, but investigators differ on whether A5, the clot firmness at 5 min, is acceptable. We re‐examined this in a retrospective observational analysis of 1146 trauma patients in one centre who had ROTEM data recorded. A5 and A10 both correlated well with maximum clot firmness, with Pearson coefficients of r = 0.92 and r = 0.96, respectively. The correlations of A5, A10 and maximum clot firmness with requirement for massive transfusion were all similarly high, with c‐stats of 0.87, 0.89 and 0.90, respectively. The correlations with mortality were also similar but weaker, with c‐stats of 0.67, 0.69 and 0.69, respectively. Using a previously validated cut‐off of A5 < 35 mm to predict massive transfusion gave a sensitivity of 95%, specificity 83%, positive predictive value 9.3% and negative predictive value 100%. Using a value of A5 < 29 mm, for a pragmatic positive predictive value of 20%, gave a sensitivity of 67%, specificity 95% and negative predictive value 99%. Whether aiming for a high sensitivity or a strong predictive value, A5 was non‐inferior to A10 and actually missed fewer cases needing massive transfusion. A5 has similar utility to both A10 and maximum clot firmness as an early measure of clot firmness, and a low A5 value is strongly predictive of the need for massive transfusion.
Keywords: bleeding; massive transfusion; ROTEM; trauma, coagulation
Introduction
In the past 15 years, point‐of‐care viscoelastic testing of blood coagulation, such as thromboelastography (TEG®, Haemonetics Corp, Braintree, MA, USA) and rotational thromboelastometry (ROTEM®, Tem International GmbH, Munich, Germany), has played an increasingly prominent role in the diagnosis and management of the acute coagulopathy of trauma and traumatic bleeding 1. In particular, it has been used before laboratory tests were available to guide early decisions on blood product transfusion 2, including triggering a massive transfusion protocol 3. The functional nature of the tests allows rapid detection of coagulation defects 4, 5, as well as early differentiation of treatable pathologies such as clotting factor deficiency, platelet depletion or dysfunction, and fibrinolysis 6, 7. This has been recognised in the Association of Anaesthetists of Great Britain and Ireland (AAGBI) guidelines on the use of blood components 8.
A reduced maximum clot firmness (MCF) has been used as a trigger for administration of blood products 9, but it can take up to 25–29 min to obtain this measurement 10. For this reason, some researchers in trauma 10, 11 and in peri‐operative medicine 12, 13, 14 have investigated whether clot firmness at 5 min (A5) or 10 min (A10) are acceptable substitutes. Meyer et al. found A10, but not A5, to correlate better with laboratory tests than MCF 10, and they suggested that early clot amplitude measurements may in fact ‘reflect a more dynamic part of the haemostatic process’ than MCF.
Using an equivalent but larger database of consecutive trauma patients from a regional trauma centre who had ROTEM measurements, we performed a similar analysis on the utility of A5 and A10. Our thesis was that (1) the first available clot firmness measure A5 would correlate with MCF in a similar fashion to A10 and (2) the early clot firmness measures, A5 and A10, would predict the requirement for massive transfusion in a similar way to MCF.
Methods
This research was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre. Patient consent was deemed unnecessary, as this was an observational study and patients received standard care for the time. Data were anonymised before analysis.
Viscoelastic coagulation testing using a single ROTEM test on admission was performed as a standard of care, in addition to traditional coagulation tests, for all trauma patients between August 2011 and March 2013. These data were recorded along with other clinical information including in‐hospital mortality, Injury Severity Score 2005 (ISS 05) and massive transfusion (defined as 10 units of packed red blood cells within 24 h) 15, 16.
We focused on A5, A10 and MCF using the EXTEM assay 10, 11, 12, 13. We used scatter plots and Spearman correlation coefficients to examine the relationship of A5 and A10 with MCF. We also compared the correlation between these measures and transfusion requirements and mortality using receiver operating characteristic curves and c‐stat values.
The study ended when the loan of the ROTEM machines finished. There was no formal power calculation to determine sample size. We used SAS 9.3 (SAS Institute, Cary, NC, USA) for statistical analysis and significance calculations.
Results
Table 1 shows the characteristics of patients enrolled in the study. Major trauma, defined as ISS ≥ 15, was recorded in 635 (55%) of the patients.
Table 1.
Characteristics of 1146 patients included in the study. Values are median (IQR [range]) or number (proportion)
| Age; years | 41 (26–58 [13–96]) |
| Injury severity score | 17 (9–26 [1–75]) |
| Sex; male | 837 (73.0%) |
| Penetrating trauma | 196 (17.0%) |
| Mechanism | |
| Motor vehicle driver/ passenger | 387 (33.8%) |
| Pedestrian/ cyclist | 207 (18.1%) |
| Fall | 269 (23.5%) |
| Industrial (excluding falls) | 31 (2.7%) |
| Stabbing | 142 (12.4%) |
| Gunshot wound | 45 (3.9%) |
| Other assault | 44 (3.8%) |
| Other | 22 (1.9%) |
| Died in first 24 h | 97 (8.5%) |
| Any transfusion in first 24 h | 172 (15.0%) |
| Massive transfusion in first 24 h (> 9 units packed red blood cells) | 21 (1.8%) |
| Time, injury to hospital arrival; h | 1.2 (0.8–4.9 [0.05–24]) |
| Time, hospital arrival to ROTEM; min | 40 (32–51 [2.4–390]). |
| Systolic arterial pressure; mmHg | 142 (126–160 [0–250]) |
| Platelet count; ×109.l−1 | 231 (192–275 [12–545]) |
| INR | 1.07 (1.00–1.17 [0.86–8.63]) |
Figures 1 and 2 show the correlation of A5:MCF and A10:MCF, which were both linear and strongly positive. The Pearson correlation coefficients were 0.92 for A5:MCF, and 0.96 for A10:MCF.
Figure 1.
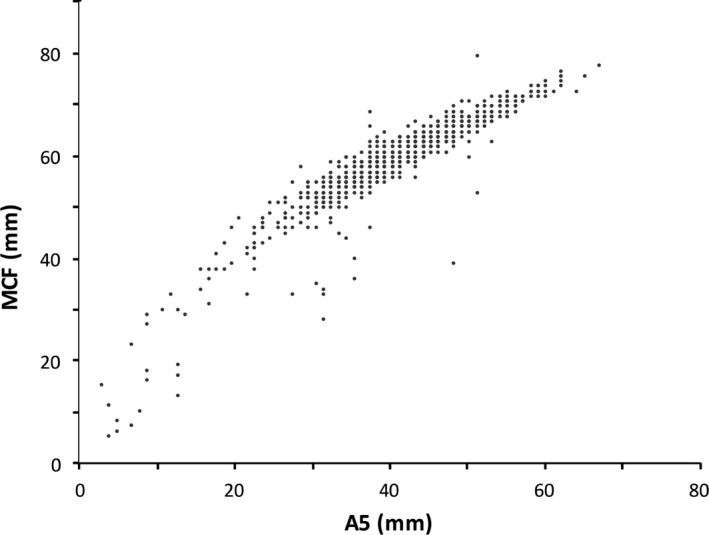
Correlation of A5 and MCF.
Figure 2.
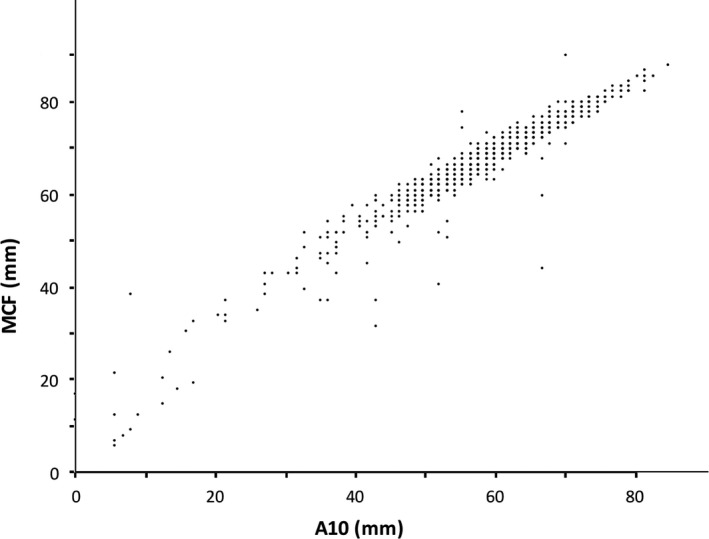
Correlation of A10 and MCF.
Figure 3 shows the receiver operating characteristic curves for the ROTEM measurements in prediction of mortality. The c‐stat values for correlation with mortality were A5 0.67, A10 0.69 and MCF 0.69.
Figure 3.
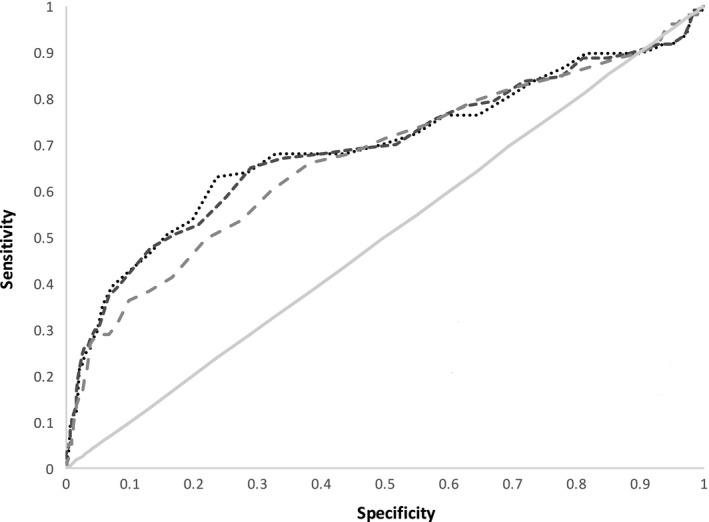
Receiver operating characteristic curves for A5, A10 and MCF vs. in‐hospital mortality. Grey solid line – null effect; dotted line – A5; short dashes – A10; long dashes – MCF.
Figure 4 shows the receiver operating characteristic curves for the ROTEM measurements in prediction of massive transfusion. The c‐stat values for correlation with massive transfusion were A5 0.87, A10 0.89 and MCF 0.90.
Figure 4.
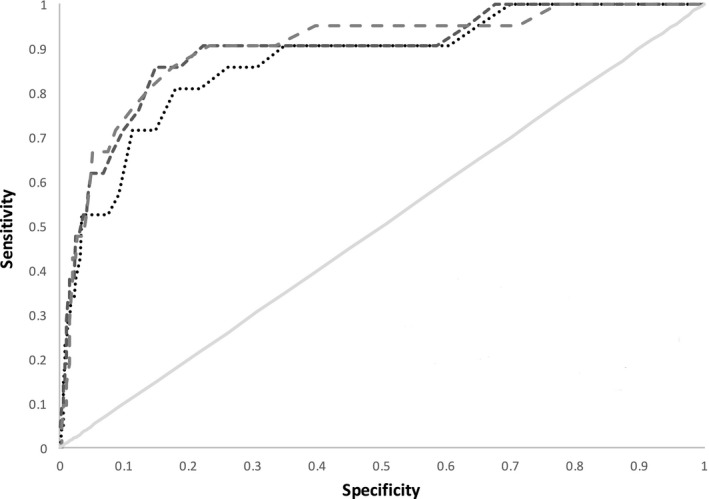
Receiver operating characteristic curves for A5, A10 and MCF vs. massive transfusion. Grey solid line – null effect; dotted line – A5; short dashes – A10; long dashes – MCF.
We wished to establish whether cases of massive transfusion would be missed if A5 were used as a predictor instead of A10, which might be affected by the cut‐off values used. Appendix 1 shows the effects of changing cut‐off values for A5 and A10 in predicting massive transfusion.
Discussion
The strong correlation of both A5 and A10 with MCF indicate that either of these early clotting measures is acceptable as an early substitute in decision making. However, A5 and A10 need not be seen only as surrogates for MCF. To our knowledge, although the correlation of A5 and A10 with MCF has been studied 11, MCF has not yet been demonstrated as superior to A5 or A10 for predicting clinical outcome.
Generally models are considered reasonable when c‐stat exceeds 0.7, and strong if it exceeds 0.8 17. Although not exactly the same, the c‐stat for A5, A10 and MCF vs. massive transfusion all indicate similarly strong models; in other words, all three clot firmness measures are similarly and strongly predictive of the requirement for massive transfusion. Despite the slight variance in c‐stat values, in no case did using A5 miss cases of massive transfusion, compared with A10. In fact, depending on the cut‐off used, A5 identified some cases of massive transfusion that would have been missed using A10.
Although the c‐stats for A5, A10 and MCF vs. mortality do not indicate a strong model, the values are similar. Therefore, even if these measures were combined with others as part of a multivariate model or scoring system, there would be little or no advantage in using MCF compared with A5 or A10.
There is some evidence that TEG and ROTEM are useful in predicting transfusion requirements and survival 5, 18, 19, 20, and in guiding resuscitation 2, 21, 22, 23, 24, 25, with some reports of favourable outcomes 26, 27. However, the most recent Cochrane review in 2015 concluded “…evidence strongly suggests that at present these tests should only be used for research” 28. Although algorithms have been developed to aid decision making based on ROTEM measures 29, there remains a question mark over the appropriate diagnostic thresholds to use. One review 30 noted that the best‐designed study in terms of predicting transfusion using ROTEM measures was by Davenport et al. in 2011 31, using a cut‐off value of A5 < 35 mm.
The appropriate cut‐off value for A5 in fact depends on what weight it is given in the decision‐making process. Our data show a high sensitivity and specificity for the previously published cut‐off value of A5 < 35 mm 29, but the positive predictive value at 9% is low. This may be an appropriate threshold to inform a multi‐variate analysis, or add support to triggering a massive transfusion protocol in view of an overall clinical picture. However, given the resource implications, a lower threshold of A5 < 29 mm with a positive predictive value of 20%, or A5 < 30 mm with a positive predictive value of 18%, may be more pragmatic if triggering a massive transfusion protocol purely on the basis of one ROTEM measure.
In summary, ROTEM EXTEM A5 is as useful clinically as A10 and MCF in making early treatment decisions in bleeding following trauma, for example, triggering a massive transfusion protocol. This is in line with the results of a recent international multi‐centre prospective study 32. A5 is a useful early measure of clot firmness, and with appropriate selection of the cut‐off value, can be strongly predictive of requirement for massive transfusion.
Acknowledgements
Dedicated to the memory of P. Veigas. Our thanks to S. Trpcic for organisational assistance, and to DRDC (Canadian military research branch) for loan of the ROTEM machines. This research was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre (fully affiliated with the University of Toronto), Project Identification Number 038‐2015. Original data collection was part‐funded by a grant to SR from the Academic Health Science Centres – Alternative Funding Plan Innovation Fund 2011–2012. No competing interests declared.
Table A1.
Characteristics of populations defined by different cut‐off values for A5, where predicted event is massive transfusion (21 cases)
| A5 (mm) | Positive test | % testing positive | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV | Youden's index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 20 | 29 | 2.5 | 10 | 19 | 1106 | 11 | 0.48 | 0.98 | 0.34 | 0.99 | 0.46 |
| < 21 | 31 | 2.7 | 10 | 21 | 1104 | 11 | 0.48 | 0.98 | 0.32 | 0.99 | 0.46 |
| < 22 | 32 | 2.8 | 10 | 22 | 1103 | 11 | 0.48 | 0.98 | 0.31 | 0.99 | 0.46 |
| < 23 | 35 | 3.1 | 11 | 24 | 1101 | 10 | 0.52 | 0.98 | 0.31 | 0.99 | 0.50 |
| < 24 | 43 | 3.7 | 12 | 31 | 1094 | 9 | 0.57 | 0.97 | 0.28 | 0.99 | 0.54 |
| < 25 | 49 | 4.1 | 14 | 35 | 1090 | 7 | 0.67 | 0.97 | 0.29 | 0.99 | 0.64 |
| < 26 | 52 | 4.5 | 14 | 38 | 1087 | 7 | 0.67 | 0.97 | 0.27 | 0.99 | 0.63 |
| < 27 | 55 | 4.8 | 14 | 41 | 1084 | 7 | 0.67 | 0.96 | 0.25 | 0.99 | 0.63 |
| < 28 | 64 | 5.6 | 14 | 50 | 1075 | 7 | 0.67 | 0.96 | 0.22 | 0.99 | 0.62 |
| < 29 | 69 | 6.0 | 14 | 55 | 1070 | 7 | 0.67 | 0.95 | 0.20 | 0.99 | 0.62 |
| < 30 | 78 | 6.8 | 14 | 64 | 1061 | 7 | 0.67 | 0.94 | 0.18 | 0.99 | 0.61 |
| < 31 | 93 | 8.1 | 14 | 79 | 1046 | 7 | 0.67 | 0.93 | 0.15 | 0.99 | 0.60 |
| < 32 | 113 | 9.9 | 15 | 98 | 1027 | 6 | 0.71 | 0.91 | 0.13 | 0.99 | 0.63 |
| < 33 | 139 | 12.1 | 18 | 121 | 1004 | 3 | 0.86 | 0.89 | 0.13 | 1.00 | 0.75 |
| < 34 | 178 | 15.5 | 18 | 160 | 965 | 3 | 0.86 | 0.86 | 0.10 | 1.00 | 0.71 |
| < 35 | 214 | 18.7 | 20 | 194 | 931 | 1 | 0.95 | 0.83 | 0.09 | 1.00 | 0.78 |
| < 36 | 255 | 22.2 | 20 | 235 | 890 | 1 | 0.95 | 0.79 | 0.08 | 1.00 | 0.74 |
| < 37 | 306 | 26.7 | 21 | 285 | 840 | 0 | 1.00 | 0.75 | 0.07 | 1.00 | 0.75 |
TP, true positive; FP, false negative; TN, true negative; FN, false negative; PPV, positive predictive value, NPV, negative predictive value; Youden's index = (sensitivity + specificity) –1.
Table A2.
Characteristics of populations defined by different cut‐off values for A10, where predicted event is massive transfusion (21 cases)
| A10 | Positive test | % testing positive | TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV | Youden's index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 25 | 25 | 2.2 | 8 | 17 | 1108 | 13 | 0.38 | 0.98 | 0.32 | 0.99 | 0.37 |
| < 26 | 26 | 2.3 | 8 | 18 | 1107 | 13 | 0.38 | 0.98 | 0.31 | 0.99 | 0.36 |
| < 27 | 26 | 2.3 | 8 | 18 | 1107 | 13 | 0.38 | 0.98 | 0.31 | 0.99 | 0.36 |
| < 28 | 27 | 2.4 | 8 | 19 | 1106 | 13 | 0.38 | 0.98 | 0.30 | 0.99 | 0.36 |
| < 29 | 30 | 2.6 | 8 | 22 | 1103 | 13 | 0.38 | 0.98 | 0.27 | 0.99 | 0.36 |
| < 30 | 34 | 3.0 | 8 | 26 | 1099 | 13 | 0.38 | 0.98 | 0.24 | 0.99 | 0.36 |
| < 31 | 34 | 3.0 | 8 | 26 | 1099 | 13 | 0.38 | 0.98 | 0.24 | 0.99 | 0.36 |
| < 32 | 37 | 3.2 | 10 | 27 | 1098 | 11 | 0.48 | 0.98 | 0.27 | 0.99 | 0.45 |
| < 33 | 43 | 3.8 | 10 | 33 | 1092 | 11 | 0.48 | 0.97 | 0.23 | 0.99 | 0.45 |
| < 34 | 50 | 4.4 | 11 | 39 | 1086 | 10 | 0.52 | 0.97 | 0.22 | 0.99 | 0.49 |
| < 35 | 54 | 4.7 | 11 | 43 | 1082 | 10 | 0.52 | 0.96 | 0.20 | 0.99 | 0.49 |
| < 36 | 55 | 4.8 | 11 | 44 | 1081 | 10 | 0.52 | 0.96 | 0.20 | 0.99 | 0.48 |
| < 37 | 59 | 5.1 | 12 | 47 | 1078 | 9 | 0.57 | 0.96 | 0.20 | 0.99 | 0.53 |
| < 38 | 65 | 5.7 | 13 | 52 | 1073 | 8 | 0.62 | 0.95 | 0.20 | 0.99 | 0.57 |
| < 39 | 76 | 6.6 | 13 | 63 | 1062 | 8 | 0.62 | 0.94 | 0.17 | 0.99 | 0.56 |
| < 40 | 80 | 7.0 | 13 | 67 | 1058 | 8 | 0.62 | 0.94 | 0.16 | 0.99 | 0.56 |
| < 41 | 88 | 7.7 | 13 | 75 | 1050 | 8 | 0.62 | 0.93 | 0.15 | 0.99 | 0.55 |
| < 42 | 104 | 9.1 | 14 | 90 | 1035 | 7 | 0.67 | 0.92 | 0.13 | 0.99 | 0.59 |
| < 43 | 123 | 10.7 | 15 | 108 | 1017 | 6 | 0.71 | 0.90 | 0.12 | 0.99 | 0.62 |
| < 44 | 152 | 13.3 | 16 | 136 | 989 | 5 | 0.76 | 0.88 | 0.11 | 0.99 | 0.64 |
| < 45 | 183 | 16.0 | 18 | 165 | 960 | 3 | 0.86 | 0.85 | 0.10 | 1.00 | 0.71 |
| < 46 | 225 | 19.6 | 18 | 207 | 918 | 3 | 0.86 | 0.82 | 0.08 | 1.00 | 0.67 |
| < 47 | 267 | 23.3 | 19 | 248 | 877 | 2 | 0.90 | 0.78 | 0.07 | 1.00 | 0.68 |
| < 48 | 317 | 27.7 | 19 | 298 | 827 | 2 | 0.90 | 0.74 | 0.06 | 1.00 | 0.64 |
| < 49 | 361 | 31.5 | 19 | 342 | 783 | 2 | 0.90 | 0.70 | 0.05 | 1.00 | 0.60 |
| < 50 | 415 | 36.3 | 19 | 396 | 729 | 2 | 0.90 | 0.65 | 0.05 | 1.00 | 0.55 |
TP, true positive; FP, false negative; TN, true negative; FN, false negative; PPV, positive predictive value, NPV, negative predictive value; Youden's index = (sensitivity + specificity) –1.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. Journal of Trauma and Acute Care Surgery 2003; 54: 1127–30. [DOI] [PubMed] [Google Scholar]
- 2. Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Annals of Surgery 2012; 256: 476–86. [DOI] [PubMed] [Google Scholar]
- 3. Stein P, Kaserer A, Sprengel K, et al. Change of transfusion and treatment paradigm in major trauma patients. Anaesthesia 2017; 72: 1317–26. [DOI] [PubMed] [Google Scholar]
- 4. Liou DZ, Shafi H, Bloom MB, et al. Defining early trauma‐induced coagulopathy using thromboelastography. American Surgeon 2014; 80: 994–8. [PubMed] [Google Scholar]
- 5. Levrat A, Gros A, Rugeri L, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. British Journal of Anaesthesia 2008; 100: 792–7. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point‐of‐care thromboelastography. Seminars in Thrombosis and Hemostasis 2010; 36: 723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carroll RC, Craft RM, Langdon RJ, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Translational Research 2009; 154: 34–9. [DOI] [PubMed] [Google Scholar]
- 8. Klein AA, Arnold P, Bingham RM, et al. AAGBI guidelines: the use of blood components and their alternatives. Anaesthesia 2016; 71: 829–42. [DOI] [PubMed] [Google Scholar]
- 9. McDaniel LM, Etchill EW, Raval JS, Neal MD. State of the art: massive transfusion. Transfusion Medicine 2014; 24: 138–44. [DOI] [PubMed] [Google Scholar]
- 10. Meyer ASP, Meyer MA, Sørensen AM, et al. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. Journal of Trauma and Acute Care Surgery 2014; 76: 682–90. [DOI] [PubMed] [Google Scholar]
- 11. Reed MJ, Nimmo AF, McGee D, et al. Rotational thrombolelastometry produces potentially clinical useful results within 10 min in bleeding Emergency Department patients: the DEUCE study. European Journal of Emergency Medicine 2013; 20: 160–6. [DOI] [PubMed] [Google Scholar]
- 12. Song J‐G, Jeong S‐M, Jun I‐G, Lee H‐M, Hwang G‐S. Five‐minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantation. British Journal of Anaesthesia 2014; 112: 290–7. [DOI] [PubMed] [Google Scholar]
- 13. Dirkmann D, Görlinger K, Dusse F, Kottenberg E, Peters J. Early thromboelastometric variables reliably predict maximum clot firmness in patients undergoing cardiac surgery: a step towards earlier decision making. Acta Anaesthesiologica Scandinavica. 2013; 57: 594–603. [DOI] [PubMed] [Google Scholar]
- 14. Raymer JM, Flynn LM, Martin RF. Massive transfusion of blood in the surgical patient. Surgical Clinics 2012; 92: 221–34. [DOI] [PubMed] [Google Scholar]
- 15. Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical Care 2016; 20: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keller WK, Dillihunt RC, Fenner HA, et al. Rating the severity of tissue damage. I. The abbreviated scale. Journal of the American Medical Association 1971; 215: 277–80. [DOI] [PubMed] [Google Scholar]
- 17. Hosmer DW, Lemeshow S. Applied Logistic Regression, 2nd edn New York: Wiley, 2000. [Google Scholar]
- 18. da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NKJ. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Critical Care 2014; 18: 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz‐Summercorn AC, Cuffolo G, Hossain MA, Wilde M. The use of rapid thromboelastogram for trauma mortality prediction. American Journal of Surgery 2014; 208: 316. [DOI] [PubMed] [Google Scholar]
- 20. Theusinger OM, Wanner GA, Emmert MY, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM®) is associated with higher mortality in patients with severe trauma. Anesthesia and Analgesia 2011; 113: 1003–12. [DOI] [PubMed] [Google Scholar]
- 21. Keene DD, Nordmann GR, Woolley T. Rotational thromboelastometry‐guided trauma resuscitation. Current Opinion in Critical Care 2013; 19: 605–12. [DOI] [PubMed] [Google Scholar]
- 22. Schöchl H, Nienaber U, Hofer G, et al. Goal‐directed coagulation management of major trauma patients using thromboelastometry (ROTEM®)‐guided administration of fibrinogen concentrate and prothrombin complex concentrate. Critical Care 2010; 14: R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haas T, Görlinger K, Grassetto A, et al. Thromboelastometry for guiding bleeding management of the critically ill patient: a systematic review of the literature. Minerva Anestesiologica 2014; 80: 1320–35. [PubMed] [Google Scholar]
- 24. Spahn DR. TEG®‐ or ROTEM®‐based individualized goal‐directed coagulation algorithms: don't wait – act now!. Critical Care 2014; 18: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grassetto A, de Nardin M, Ganzerla B, et al. ROTEM®‐guided coagulation factor concentrate therapy in trauma: 2‐year experience in Venice, Italy. Critical Care 2012; 16: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tapia NM, Chang A, Norman M, et al. TEG‐guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. Journal of Trauma and Acute Care Surgery 2013; 74: 378–86. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez E, Moore EE, Moore HB, et al. Goal‐directed hemostatic resuscitation of trauma‐induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Annals of Surgery 2016; 263: 1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma‐induced coagulopathy in adult trauma patients with bleeding. Cochrane Database of Systematic Reviews 2015; 2: CD010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lier H, Vorweg M, Hanke A, Görlinger K. Thromboelastometry guided therapy of severe bleeding. Essener Runde Algorithm. Haemostaseologie 2013; 33: 51–61. [DOI] [PubMed] [Google Scholar]
- 30. Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2016; 24: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davenport R, Manson J, De'Ath H, et al. Functional definition and characterization of acute traumatic coagulopathy. Critical Care Medicine 2011; 39: 2652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hagemo JS, Christiaans SC, Stanworth SJ, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Critical Care 2015; 19: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]


