Abstract
Background
Adolescence is a transition period characterized by heightened emotional reactivity, which for some sets the stage for emerging depressive symptoms. Prior studies suggest that adolescent depression is associated with deviant cortical and subcortical brain structure. Longitudinal studies are, however, currently scarce, but critical to detect which adolescents are at risk for developing depressive symptoms.
Methods
In this longitudinal study, a community sample of 205 participants underwent magnetic resonance imaging (MRI) in three biennial waves (522 scans) spanning 5 years across ages 8–25 years. Depressive symptomatology was assessed using self‐report at the third time point. Mixed models were used to examine the relations between structural brain development, specifically regional change in cortical thickness, surface area and subcortical volumes (hippocampus and amygdala), and depressive symptoms.
Results
Accelerated frontal lobe cortical thinning was observed in adolescents who developed depressive symptoms at the third time point. This effect remained after controlling for parent‐reported affective problems at the first time point. Moreover, the effect was driven by specific lateral orbitofrontal and precentral regions. In addition, differential developmental trajectories of parietal cortical thickness and surface area in several regions were found for participants reporting higher depressive symptomatology, but these results did not survive correction for multiple comparisons. Volumes or developmental volume changes in hippocampus or amygdala were not related to depressive symptoms.
Conclusions
This study showed that emerging depression is associated with cortical thinning in frontal regions within individuals. These findings move beyond detecting cross‐sectional correlations and set the stage for early detection, which may inform future intervention.
Keywords: Adolescence, brain development, longitudinal, depression, MRI, cerebral cortex
Introduction
Adolescence is a time period characterized by dramatic changes in the body and in behavior, including heightened emotional reactivity and emergence of social‐affective sensitivities (Dahl & Gunnar, 2009). During this transition from childhood to adulthood, there is a strong increase in the incidence rate of mental health problems (Paus, Keshavan, & Giedd, 2008). Approximately 12% of adolescents experience a depressive episode by the age of 18 (Merikangas et al., 2010). Adolescent onset of depression and subclinical symptoms are strong predictors of major depressive disorder (MDD) in adulthood (Fergusson, Horwood, Ridder, & Beautrais, 2005; Klein, Shankman, Lewinsohn, & Seeley, 2009; Pine, Cohen, Cohen, & Brook, 1999). Cross‐sectional studies have indicated that depression symptomatology in adolescence is associated with aberrant cortical and subcortical brain structure (Caetano et al., 2007; Pannekoek, van der Werff, et al., 2014; Rosso et al., 2005; Shad, Muddasani, & Rao, 2012). It is currently unknown how these abnormalities emerge over adolescence, a question for which longitudinal studies are pivotal.
Longitudinal magnetic resonance imaging (MRI) studies in normative samples have shown considerable changes in brain structure across adolescent development. Cortical gray matter volumes generally decrease from childhood to adulthood (Mills, Goddings, Clasen, Giedd, & Blakemore, 2014; Mills et al., 2016). Even though the underlying mechanisms of developmental changes observed in structural MRI are still not known (Paus, 2013; Paus et al., 2008), cortical thinning likely involves changes in a number of various neuronal (e.g. pyramidal cells, interneurons) and nonneural (astrocytes, oligodendrocytes) cell types (Shin et al., 2017). Nonetheless, one central process is presumed to be intracortical myelination (Grydeland, Walhovd, Tamnes, Westlye, & Fjell, 2013; Whitaker et al., 2016). Furthermore, the distinct components of cortical morphology, thickness, and surface area show different developmental trajectories (Tamnes et al., 2017; Wierenga, Langen, Oranje, & Durston, 2014).
Although structural developmental changes are observed across the whole cortex during adolescence, prefrontal cortical (PFC) regions enabling, for example, cognitive control appear to have especially protracted developmental trajectories (Gogtay et al., 2004; Tamnes et al., 2013). Some studies also suggest that the specific subcortical structure that is important for reward and emotion develops earlier than PFC regions (Mills et al., 2014). It has been speculated that such regional differences in brain maturation timing, in conjunction with contextual influences, may partly explain the rise of mental health problems, including depression (Nelson, Leibenluft, McClure, & Pine, 2005). However, such an understanding has been challenged and can, for example, not explain why depression rates persist into adulthood (Davey, Yücel, & Allen, 2008; Pfeifer & Allen, 2012). Current knowledge on the relations between brain structure and depression is mainly based on cross‐sectional studies. Prior studies of adolescents with MDD have found reduced volume of hippocampus (Jaworska et al., 2016) and PFC (Pannekoek, van der Werff, et al., 2014; Shad et al., 2012), although not consistently across all studies (Shad et al., 2012). Interestingly, similar results have been found in relation to subthreshold depression or depressive symptomatology in typically developing adolescents, including reduced hippocampus (Koolschijn, van IJzendoorn, Bakermans‐Kranenburg, & Crone, 2013), caudate, anterior cingulate cortex (ACC), and PFC volumes (Vulser et al., 2015). However, how these relationships emerge over adolescence is not well understood.
To date, only few longitudinal studies have examined the relation between gray matter development and depression during adolescence; however, the results are inconsistent. Whittle et al. (2014) showed an association between depression onset and attenuated growth in volume of the hippocampus, amygdala, and putamen, whereas Papmeyer et al. (2016) did not find any relations between subcortical development and depression onset. For cortical development, one longitudinal study showed that childhood depression was associated with accelerated global cortical thinning during adolescence (Luby et al., 2016), while another study indicated that anxious/depressed symptoms in a healthy sample was associated with attenuated thinning in right ventromedial PFC (Ducharme et al., 2014). The inconsistencies may be related to different age ranges, samples, and assessment of depression, as well as brain structure measures investigated. The present study extends previous studies by including a large community sample across a wide age range spanning from late childhood to early adulthood and by investigating the relation between structural brain development and both the emergence of depression and the degree of depressive symptomatology.
The aim of this study was to examine the relations between developmental trajectories of cortical and subcortical gray matter structure with depression. We used data from our accelerated longitudinal study (Braintime) where we followed a community sample, spanning from late childhood to early adulthood, over a period of 5 years. Depressive symptoms were assessed using self‐report at the third time point. Affective problems reported by parents were assessed at all time points. Given that depressive symptoms are often not directly observable for parents and others, it is generally assumed that self‐report provides a more reliable estimate of depressive symptoms than parent report (Waters, Stewart‐Brown, & Fitzpatrick, 2003). Therefore, self‐reported depression was the main interest of this study. MRI data were obtained at three time points (2 years in between each time point), which allowed for modeling of individual developmental trajectories. Moreover, we investigated both regional cortical thickness and surface area, which related to different patterns of impairments across neuropsychiatric disorders (Fan et al., 2013; Luby et al., 2016; Mensen et al., 2017), as well as volume of hippocampus and amygdala. Our main cortical analyses were performed for five lobes: frontal, temporal, parietal, occipital, and cingulate. Based on previous findings (Ducharme et al., 2014; Luby et al., 2016; Whittle et al., 2014), we hypothesized that accelerated thinning in PFC and attenuated development of hippocampal and amygdala volumes would be associated with the emergence of depression and depressive symptomatology in adolescence.
Methods and materials
Participants and procedure
Participants were recruited from a community sample of children, adolescents, and young adults through local schools and advertisement across Leiden, the Netherlands. This study was part of a large accelerated longitudinal research project, Braintime (Braams & Crone, 2016; Braams, van Duijvenvoorde, Peper, & Crone, 2015; Peters, Van Duijvenvoorde, Koolschijn, & Crone, 2016), focusing on cognitive and affective development from childhood to adulthood. An initial sample of 299 participants (ages 8–29; 146 male participants) was recruited to participate at the first time point (TP1; for more detailed information on the complete sample, including information on participant loss, see Peters & Crone, 2017; Schreuders et al., in press). All participants were fluent in Dutch, right‐handed, and had normal or corrected‐to‐normal vision, and none reported neurological or mental health problems or use of psychotropic medication at TP1 based on self‐report.
The final sample for the current study consisted of 205 adolescents (96 males) for whom self‐report Beck Depression Inventory (BDI) data at TP3 were available and who had at least one structural MRI scan of sufficient quality (Table 1). Our sample was a representative subsample of the initial sample of 299 participants (see supplementary results in Appendix S1). All participants were invited to participate in three consecutive waves of assessment and neuroimaging approximately every 2 years. All participants had an estimated IQ > 80 (assessed using two subtest of age‐appropriate Wechsler Intelligence Scales; TP1: Similarities and Block Patterns; TP2: Picture Completion and Vocabulary; TP3: no measurement). Depressive symptoms were assessed at TP3 using self‐report. Affective problems using parent report were assessed at all time points. At each time point, informed consent was obtained from participants or from parents in case of minors. The study was approved by the Institutional Review Board at Leiden University Medical Center. Participants received a financial reimbursement for their participation in the study.
Table 1.
Sample characteristics for each wave
| TP1 | TP2 | TP3 | |
|---|---|---|---|
| Age, mean (SD) | 13.55 (2.50) | 15.72 (2.43) | 17.67 (2.47) |
| Age range | 8.98–20.25 | 11.03–21.49 | 13.11–24.68 |
| MRI scans available | 204 | 182 | 184 |
| MRI scan of sufficient quality | 177 (79 males) | 170 (81 males) | 175 (80 males) |
| IQ indication, mean (SD) | 110 (9.76) | 108 (10.08) | |
| Parent‐report affective problems (CBCL), mean (SD) | 2.31 (2.56)a | ||
| Parent‐report affective problems (CBCL), range | 0–15 | ||
| Self‐report depressive symptoms (BDI), mean (SD) | 8.02 (7.42) | ||
| Self‐report depressive symptoms (BDI), range | 0–49 | ||
| BDI severity | |||
| No (BDI: 0–9) | n = 144 (75 males) | ||
| Boundary (BDI: 10–12) | n = 28 (13 males) | ||
| Mild to severe (BDI ≥ 14) | n = 33 (8 males) | ||
CBCL information was available for 188 participants.
Materials
Depressive symptoms – self‐report
The BDI‐II (Beck, Steer, & Brown, 1996) was used to assess depressive symptoms of adolescents between 13 and 25 years old. The BDI is a widely used and well‐validated questionnaire consisting of 21 items referring to the past 2 weeks. Participants are asked to rate each item on a 4‐point Likert scale, with higher scores indicating greater severity of depressive symptoms. In this study, the item assessing suicidal thoughts was not administered to participants, resulting in a total of 20 administered items and a scoring range of 0‐60. The internal consistency of the BDI in the current sample was good (Cronbach's α = .90).
Affective problems
The Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000) is a parent‐report screening instrument that was used to assess levels of affective problems of children (age 6‐17) in the previous 2 months. The affective problems scale consists of 13 items related to mood problems and parents were asked to rate each statement on a 3‐point scale ranging from ‘not true’ to ‘very true/often true’. Internal consistency of the scale was sufficient in this study (Cronbach's α = .71). We used the raw scores in the analyses, given that we were interested in natural variation in the data, whereas T‐scores adjust the natural distribution of the data.
Image acquisition and analysis
All scanning was performed on the same 3 Tesla Philips Achieva whole‐body scanner, with a standard 32‐channel head coil. T1‐weighted anatomical scans were obtained at each time point (TR = 9.8 ms, TE = 4.6 ms, flip angle = 8°, 140 slices, 0.875 mm × 0.875 mm × 1.2 mm, FOV = 224 × 177 × 168 mm). Scan time for this sequence was 4 min 56 s. A radiologist reviewed all T1‐weighted scans and no anomalous findings were reported.
Whole‐brain volumetric segmentation and cortical surface reconstruction were performed using the longitudinal pipeline of FreeSurfer 5.3 (Fischl, 2012; Reuter, Schmansky, Rosas, & Fischl, 2012), which is documented and freely available online (http://surfer.nmr.mgh.harvard.edu; see also supplementary methods in Appendix S1). For each scan, volumetric estimates for the hippocampus and amygdala were extracted per hemisphere. Parcellation of the cerebral cortex into 31 gyral regions was performed using the Desikan‐Killiany‐Tourville atlas (Klein & Tourville, 2012), and cortical thickness and surface area were estimated per region. The five standard lobes were calculated by combining the related regions based on the DKT atlas (see supplementary methods in Appendix S1). All measures were then averaged across the two hemispheres and combined into lobes.
Initially, 569 scans from 205 participants with available BDI data at TP3 were successfully processed via the longitudinal pipeline. Detailed postprocessing quality control (QC) in the form of visual inspection focusing both on overall image quality and the accuracy of reconstructed surfaces and segmentations was then performed on all scans by trained operators. Scans judged to be of poor quality were excluded and remaining scans from participants with one or more excluded scans were reprocessed through the longitudinal pipeline. This QC procedure was repeated until only acceptable scans were included in the processing (note that single time points were also processed longitudinally). No manual editing was performed. The QC procedure resulted in the exclusion of 47 scans from 41 participants. This yielded a final dataset consisting of 522 scans from 205 participants (Table 1); 131 participants had scans from three time points, 55 participants had scans from two time points, and 19 participants had one scan (mean number of scans per participants = 2.55).
Statistical analyses
Statistical analyses were performed using SPSS 23.0 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and R 3.2.0 (R Core Team, 2014). To investigate the relation between developmental trajectories of gray matter with emerging depression, we used mixed models, performed using the nlme package (Pinheiro, Bates, DebRoy, & Sarkar, 2014) in R. The advantage of a multilevel approach is that it allows for hierarchy within data, such as observed in longitudinal data. That is, time points are nested within participants and mixed models can account for this data dependency. Another advantage is that mixed models can handle missing data. We tested (a) general patterns of age‐related change and (b) whether depression could additionally explain variance in brain changes in each measures (see supplementary methods in Appendix S1 for model selection procedure). To do so, we divided the participants into two groups: High‐depression symptoms (BDI ≥ 14, n = 33) and no depression symptoms (BDI ≤ 9, n = 144). Note that we excluded participants with a BDI score between 10 and 13, a subclinical range (Beck et al., 1996). For significant results (corrected for multiple comparisons) in the analyses on cortical measures, we additionally tested which specific regions within the lobes were driving the effect. Next, if there was a corrected significant effect of Depression group on development of our brain measures, we performed a follow‐up test to examine whether this main or interaction effect with Depression group could be explained by depressive symptoms at TP1 by controlling for parent‐reported affective problems. We did not test for sex differences given the relatively small number of participants in the high BDI group. Next, we retested these relations using depression symptoms as a continuous variable. The advantage of this approach is that we can test if there is a relation between brain development and depression severity. Moreover, using continuous scores, we have more statistical power compared with our analysis using depression groups. We additionally tested whether including interactions with sex (i.e. Age × Sex; BDI continuous × Sex; Age × BDI continuous × Sex) improved model fit of describing brain development.
Results were corrected for multiple comparisons using a Bonferroni procedure adjusted for correlated variables (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm; Perneger, 1998; Sankoh, Huque, & Dubey, 1997), yielding a significance level for α (2‐sided adjusted) = .023.
Results
Depressive symptomatology
We first tested whether there was a relation between self‐reported depressive symptoms at TP3 and age at TP3. Correlation analyses revealed a small association on trend level (r = −.14, p = .051). A t‐test comparison showed that females reported more depressive symptoms (M = 9.37, SD = 8.91) compared with males (M = 6.49, SD = 4.85; t 203 = 2.92, p = .004, d = .40). Correlation analyses of parent‐reported affective problems at TP1 showed no relation with age (r = −.02, p = .710). There were no differences in affective problems at TP1 between males and females (t 186 = 0.38, p = .520). Furthermore, there was a relatively small, but significant correlation between parent‐reported affective problems at TP1 and self‐reported depressive symptoms at TP3 (r = .16, p = .025).
Next, we examined whether the two depression groups (low vs. high) differed on age, sex, and parent‐reported affective problems at TP1. Age was not different for the two depression groups (t 175 = 1.55, p = .123). In the high‐depression group, there were relatively more females than in the low‐depression group (χ 2 = 8.36, p = .003). Finally, participants in the high‐depression group scored higher on parent‐reported affective problems at TP1 (M = 3.44, SD = 2.44) compared with participants in the low‐depression group (M = 2.07, SD = 2.44; t 158 = −2.70, p < .010).
Cortical thickness
Mixed model analyses showed that cortical thickness decreased with age in all five lobes (Tables S1 and S2). For the frontal, temporal, parietal, and cingulate cortices, this age pattern followed a cubic curve. For the occipital lobe, a linear model fitted best.
Next, we examined the relation between cortical development and depression (Table S2). The results revealed a significant linear Age × Depression group interaction (B = −0.573, p = .005) for the frontal lobe that was still significant after correction for multiple comparisons. There was an accelerated decline of cortical thickness in the frontal lobe for participants within the Depression group (Figure 1). This interaction effect also remained after controlling for parent‐reported affective problems at TP1 (Age [linear] × Depression group, B = −0.423, p = .030). Follow‐up analyses on specific regions within the frontal lobe revealed that this effect was driven by four regions: precentral (Age [linear] × Depression group, B = −0.446, p = .033), paracentral (Age [linear] × Depression group, B = −0.705, p = .006), pars orbitalis (Age [linear] × Depression group, B = −0.758, p = .018), and lateral orbitofrontal (OFC; Age [linear] × Depression group, B = −0.696, p = .008; Figure 2).
Figure 1.
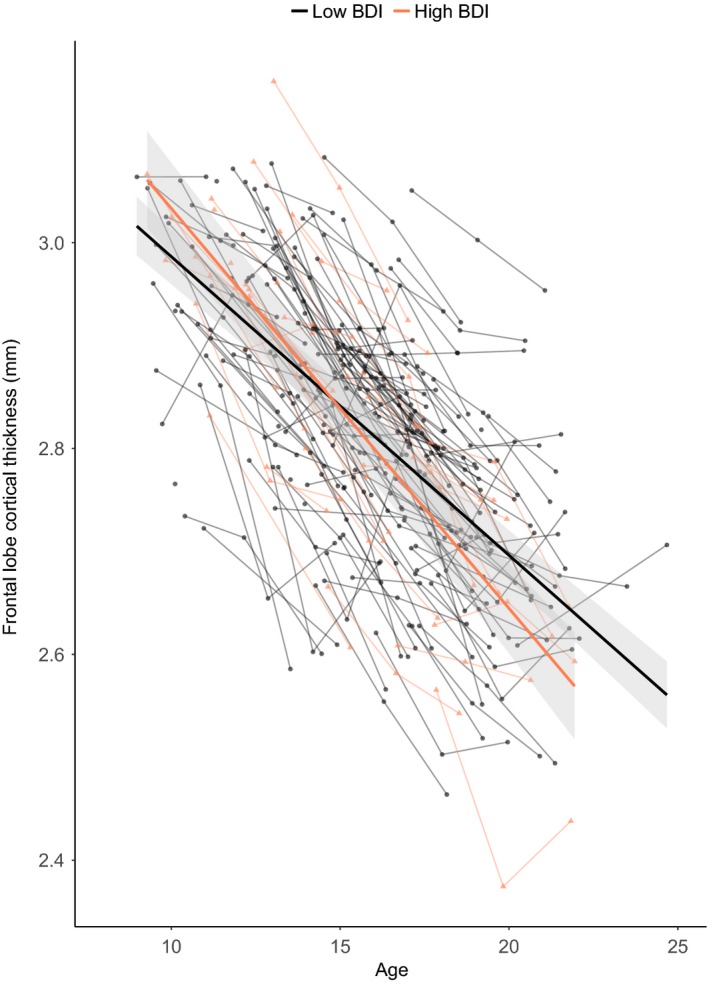
Developmental trajectories for cortical thickness of the frontal lobe. Mean cortical thickness (y‐axis) by age in years (x‐axis) is shown for participants in the high‐depression group (red) and low‐depression group (gray) based on the optimal fitting model. The shade represents 95% confidence interval. Individual participants are represented by individual lines. Participants measured once are represented by dots
Figure 2.
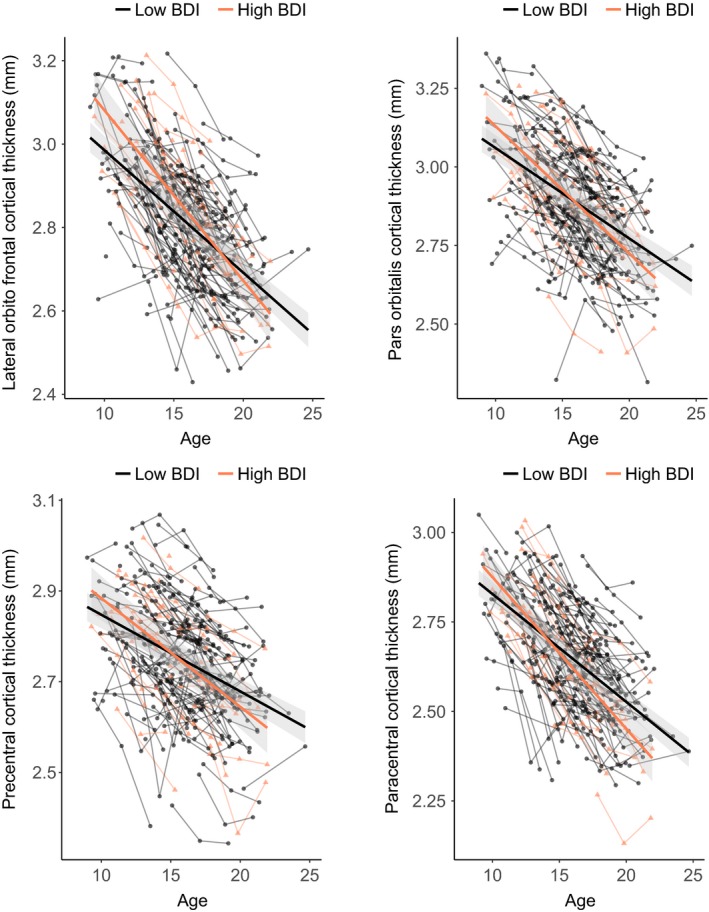
Developmental trajectories for cortical thickness of (A) lateral orbitofrontal cortex, (B) pars orbitalis, (C) precentral, and (D) paracentral. Mean cortical thickness (y‐axis) by age in years (x‐axis) is shown for participants in the high‐depression group (red) and low‐depression group (gray) based on the optimal fitting model. The shade represents 95% confidence interval. Individual participants are represented by individual lines. Participants measured once are represented by dots
Furthermore, we found a linear Age × Depression group interaction for the parietal lobe (B = −0.372, p = .037), with accelerated decline of cortical thickness for participants within the high‐depression group (Figure S1a). However, this finding did not survive correction for multiple comparisons.
Moreover, there was a main effect of Depression group on cortical thickness in the occipital lobe (B = −0.038, p = .030). Participants within the depression group showed smaller cortical thickness of the occipital lobe (Figure S1B), but this effect did not survive correction for multiple comparisons either.
Surface area
Mixed model analyses revealed that cubic age terms best described the developmental trajectory of cortical surface area for all five lobes (Tables S1 and S3). Depression group was associated with the developmental trajectory of surface area in the frontal (Age [cubic] × Depression group, B = −2,724.670, p = .045), temporal (Age [linear] × Depression group, B = −3,210.140, p = .026), parietal (Age [cubic] × Depression group, B = −2,812.840, p = .024), and occipital lobes (Age [cubic] × Depression group, B = −2,155.049, p = .045; Table S3 and Figure S2); however, none of these results survived correction for multiple comparisons. There were no significant main effects of Depression group.
Relation with depression severity
Next, we tested whether the Age × Depression group effects described above were also present using depression symptomatology as a continuous variable. For cortical thickness, the results revealed a corrected significant interaction effect between linear Age and Depression severity for the frontal lobe (B = −0.033, p = .003). There was also a significant interaction effect between linear Age and Depression severity for the parietal lobe (B = −0.028, p = .005). For the occipital lobe thickness, we found an uncorrected significant main effect of depression severity (B = −0.002, p = .028). For surface area of frontal and parietal cortex, we found corrected significant interaction effects between Age (cubic) and Depression severity (B = −188.37, p = .009 and B = −151.71, p = .016, respectively). For cortical surface area of occipital and temporal lobes, the age (cubic) by Depression severity interaction effect was not significant (ps > .20). Including interaction effects with sex into the models did not improve model fit (Table S4).
Hippocampus and amygdala
Hippocampal volume showed no relation with age. For the amygdala, we found an inverted‐U shape developmental trajectory (quadratic). We did not find significant main effects of Depression group or an interaction effects between Depression group and age for hippocampus or amygdala volume (Table S5).
Discussion
Structural brain development during adolescence has been fairly well characterized using longitudinal methods, but it remained largely unknown if these trajectories are different for adolescents who developed depressive symptoms. We tested this using a large longitudinal brain imaging dataset with three waves in a community sample of 8‐ to 25‐year olds. The study yielded three main findings. First, depressive symptoms were associated with accelerated frontal lobe cortical thinning. This pattern was evident at the group level – comparing participants reporting no depressive symptoms to participants reporting levels within the clinical range – as well as with BDI score as a continuous variable, and also while controlling for parent‐reported affective problems at inclusion. Second, there were also relations between depressive symptoms and the developmental trajectory of cortical thickness in the parietal lobe and surface area in several regions, yet these findings did not survive correction for multiple comparisons. Third, the results were specific for cortical development, as we did not find any relationships between depressive symptoms and development of hippocampus or amygdala volume.
Our main finding was that depressive symptoms were associated with accelerated thinning in the frontal lobe, compared with the normative development of cortical thinning. Speculatively, these findings might be explained as a need to mature faster, but also longitudinal data do not allow for causal inferences. Even though accelerated cortical thinning in adolescence has been related to positive behavior outcomes like better cognitive performance (Shaw et al., 2006), the current study indicates that faster maturation can also be detrimental. In follow‐up analyses, we found that specific lateral orbitofrontal and precentral regions showed the strongest relation between accelerated cortical thinning and depression symptomatology. Lateral orbitofrontal regions and their connections with, for example, the amygdala (Von Der Heide, Skipper, Klobusicky, & Olson, 2013) are thought to be important for regulating emotional arousal which is related to depressive symptomatology, such as flattened affect (Jenkins et al., 2016). Hence, earlier maturation of PFC regions may indicate excessive cognitive control over emotional tendencies; however, this remains speculative. Interestingly, functional connectivity studies have found that circuitries involving frontal‐parietal regions are affected in adolescents with depression (Pannekoek, Werff, et al., 2014). Moreover, our findings cannot be explained by affective problems at TP1, suggesting that these altered brain trajectories occur vis‐à‐vis emergence of depressive symptoms. An intriguing question for future research is how negative or stress‐related experiences, and in particular specific depression‐related experience, relate to developmental changes in cortical thickness. Accumulating evidence suggests that stressful life events play a central role in the onset of depression (Spinhoven et al., 2010). Moreover, childhood adversity has been linked to deleterious effects on brain development (Jensen et al., 2015; McLaughlin, Sheridan, & Lambert, 2014).
Our result of accelerated cortical thinning in the frontal lobe is at least partly in line with a previous longitudinal study, which showed a relation between early childhood depression and global reduction in cortical thickness from late childhood to early adolescence (Luby et al., 2016). Other longitudinal studies examining specific ROIs within the PFC have shown attenuated growth (Ducharme et al., 2014) or no relation between cortical thickness development and depression (Schmaal et al., 2017; Whittle et al., 2014). These inconsistencies within the few available longitudinal datasets might be explained by differences in participant sampling (high‐risk individuals vs. community samples) and age range. This study, therefore, provides important new evidence for accelerated cortical thinning in adolescents who develop depressive symptoms in a normative sample.
Furthermore, there seems to be an interaction between developmental trajectory for cortical thickness in the parietal lobe and surface area in widespread regions and depression; however, these findings did not survive correction for multiple comparisons and, therefore, need to be confirmed in future research. Prior meta‐analyses on cross‐sectional data showed lower surface area in regions of the PFC in adolescent patients with MDD (Schmaal, Hibar, et al., 2016). Moreover, a recent longitudinal study found that lower ACC surface area was associated with depressive symptomatology in young girls only (Schmaal et al., 2017). Our finding seem to be in contrast with these two previous studies; however, this might be due to relatively small samples, differences in sex distribution, and age of onset of depression symptoms.
Aside from cortical development, this study also tested whether depression was associated with development of hippocampus and amygdala. Prior studies in both adults and adolescents have shown volumetric deviances in these structures between participants with and without depression or depressive symptomatology (Koolschijn et al., 2013; Schmaal, Veltman, et al., 2016; Whittle et al., 2014), but not consistently across studies (Papmeyer et al., 2016). Contrary to our expectations, we observed no effect of depressive symptoms on volume or volume change for the hippocampus or the amygdala. Prior research suggested that reduction in hippocampal volume is related to duration and recurrence of depression (Sheline, Sanghavi, Mintun, & Gado, 1999; Treadway et al., 2015). Future research should, thus, examine these brain regions over longer time periods. Furthermore, depression‐related correlations may occur in subregions of the hippocampus or the amygdala (Treadway et al., 2015), leading to inconsistent findings across studies.
This study had several strengths including a three‐wave longitudinal design, a wide age range, MRI scanning at one site, and the ability to examine the full range of depression symptomatology. There are also some limitations that should be acknowledged. First, we measured depressive symptoms only at the last time point. Therefore, we could not examine whether changes in symptomatology coincided with changes in structural brain development. Note that we attempted to control for initial differences by controlling for parent‐reported affective problems at inclusion, but using a different measurement and a different informant (parent vs. participant), we cannot rule out that subclinical symptoms at TP1 affected our results. Second, we used an accelerated longitudinal design, which is common in developmental neuroimaging studies (Vijayakumar, Mills, Alexander‐Bloch, Tamnes, & Whittle, in press) and has both a cross‐sectional and longitudinal component. A major trade‐off of this research design is the missing data for each individual (Galbraith, Bowden, & Mander, 2017), with some individuals only contributed one single (cross‐sectional) data point. This drawback is most important when using small sample sizes, such as in our high BDI group. Note, however, that using BDI as continuous variable resulted in similar findings. Third, by using a community sample of adolescents, the number of participants that fell within clinical levels of BDI score was rather small (n = 33). Moreover, only four participants reported symptoms that could be classified within the severe range (BDI score ≥ 31). Future research in community samples should recruit an even larger number of participants to ensure that enough participants fall within the full range of depression severity (no symptoms to severe symptoms). Last, we had limited statistical power to examine sex differences. As expected, there were higher levels of depressive symptomatology in females compared with males, consistent with prior studies showing that depression is more prevalent among women (Kessler, Avenevoli, & Merikangas, 2001). In conclusion, this longitudinal study demonstrated that emerging depression in adolescence is associated with accelerated frontal lobe cortical thinning. Our findings emphasize the need to examine dynamic changes in brain structure and its relation to psychopathology using within‐person comparisons. Moreover, we showed that cortical structural changes are not only related to categorical classifications of depression but also to severity of symptoms. Taken together, the findings give new insight into the neurodevelopmental patterns associated with the ontogeny of depression and might have future implications for early identification and interventions for depressive symptoms in adolescence.
Key points.
Most evidence of structural brain abnormalities associated with depression in adolescence is based on cross‐sectional studies.
To examine how these brain abnormalities emerge, longitudinal studies are pivotal.
In this study, we followed a community sample of adolescents (n = 205) over a period of 5 years (522 scans), where none of the adolescents reported a depressive disorder at start of the study.
We demonstrated that emerging depression is associated with accelerated frontal lobe cortical thinning.
Our findings might have future implications for early identification and interventions for depressive symptoms in adolescence.
Supporting information
Appendix S1. Supplementary methods and results.
Table S1. BIC values for null, linear, quadratic and cubic age models to describe the relationship with age and brain measures reported in the table.
Table S2. Model parameters for the best fitting model for the five lobes of cortical thickness.
Table S3. Model parameters for the best fitting model for the five lobes of cortical surface area.
Table S4. BIC values for best age model controlling for sex and the model including sex interaction terms for thickness of frontal, parietal, and occipital lobe and surface area of temporal, frontal, parietal, and occipital lobes.
Table S5. Model parameters for the best fitting model for hippocampal and amygdala volume.
Figure S1. Developmental trajectories for cortical thickness of the parietal lobe (A) and occipital lobe (B).
Figure S2. Developmental trajectories for cortical surface area of the parietal lobe (A), temporal lobe (B), frontal lobe (C), occipital lobe (D).
Acknowledgements
This work was supported by a European Research Council (ERC) starting grant (ERC‐2010‐StG‐263234) awarded to E.A.C. and by a grant from the Research Council of Norway and the University of Oslo (FRIMEDBIO 230345) awarded to C.K.T. The authors have declared that they have no competing or potential conflicts of interest.
Conflict of interest statement: No conflicts declared.
References
- Achenbach, T.M. , & Rescorla, L.A. (2000). ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth and Families. [Google Scholar]
- Beck, A.T. , Steer, R.A. , & Brown, G.K. (1996). Beck depression inventory‐II. San Antonio, 78, 490–498. [Google Scholar]
- Braams, B.R. , & Crone, E.A. (2016). Longitudinal changes in social brain development: Processing outcomes for friend and self. Child Development, 88, 1952–1965. [DOI] [PubMed] [Google Scholar]
- Braams, B.R. , van Duijvenvoorde, A.C. , Peper, J.S. , & Crone, E.A. (2015). Longitudinal changes in adolescent risk‐taking: A comprehensive study of neural responses to rewards, pubertal development, and risk‐taking behavior. Journal of Neuroscience, 35, 7226–7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano, S.C. , Fonseca, M. , Hatch, J.P. , Olvera, R.L. , Nicoletti, M. , Hunter, K. , … & Soares, J.C. (2007). Medial temporal lobe abnormalities in pediatric unipolar depression. Neuroscience Letters, 427, 142–147. [DOI] [PubMed] [Google Scholar]
- Dahl, R.E. , & Gunnar, M.R. (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21, 1–6. [DOI] [PubMed] [Google Scholar]
- Davey, C.G. , Yücel, M. , & Allen, N.B. (2008). The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews, 32, 1–19. [DOI] [PubMed] [Google Scholar]
- Ducharme, S. , Albaugh, M.D. , Hudziak, J.J. , Botteron, K.N. , Nguyen, T.‐V. , Truong, C. , … & Byars, A.W. (2014). Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cerebral Cortex, 24, 2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Q. , Palaniyappan, L. , Tan, L. , Wang, J. , Wang, X. , Li, C. , … & Liddle, P. (2013). Surface anatomical profile of the cerebral cortex in obsessive–compulsive disorder: A study of cortical thickness, folding and surface area. Psychological Medicine, 43, 1081–1091. [DOI] [PubMed] [Google Scholar]
- Fergusson, D.M. , Horwood, L.J. , Ridder, E.M. , & Beautrais, A.L. (2005). Subthreshold depression in adolescence and mental health outcomes in adulthood. Archives of General Psychiatry, 62, 66–72. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, S. , Bowden, J. , & Mander, A. (2017). Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Statistical Methods in Medical Research, 26, 374–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J.N. , Lusk, L. , Hayashi, K.M. , Greenstein, D. , Vaituzis, A.C. , … & Toga, A.W. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland, H. , Walhovd, K.B. , Tamnes, C.K. , Westlye, L.T. , & Fjell, A.M. (2013). Intracortical myelin links with performance variability across the human lifespan: Results from T1‐and T2‐weighted MRI myelin mapping and diffusion tensor imaging. Journal of Neuroscience, 33, 18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska, N. , Yücel, K. , Courtright, A. , MacMaster, F.P. , Sembo, M. , & MacQueen, G. (2016). Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: The role of comorbidity and age. Journal of Affective Disorders, 190, 726–732. [DOI] [PubMed] [Google Scholar]
- Jenkins, L.M. , Barba, A. , Campbell, M. , Lamar, M. , Shankman, S.A. , Leow, A.D. , … & Langenecker, S.A. (2016). Shared white matter alterations across emotional disorders: A voxel‐based meta‐analysis of fractional anisotropy. NeuroImage: Clinical, 12, 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, S.K. , Dickie, E.W. , Schwartz, D.H. , Evans, C.J. , Dumontheil, I. , Paus, T. , & Barker, E.D. (2015). Effect of early adversity and childhood internalizing symptoms on brain structure in young men. JAMA Pediatrics, 169, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R.C. , Avenevoli, S. , & Merikangas, K.R. (2001). Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry, 49, 1002–1014. [DOI] [PubMed] [Google Scholar]
- Klein, D.N. , Shankman, S.A. , Lewinsohn, P.M. , & Seeley, J.R. (2009). Subthreshold depressive disorder in adolescents: Predictors of escalation to full‐syndrome depressive disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 48, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, A. , & Tourville, J. (2012). 101 labeled brain images and a consistent human cortical labeling protocol. Frontiers in Neuroscience, 6, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn, P.C.M. , van IJzendoorn, M.H. , Bakermans‐Kranenburg, M.J. , & Crone, E.A. (2013). Hippocampal volume and internalizing behavior problems in adolescence. European Neuropsychopharmacology, 23, 622–628. [DOI] [PubMed] [Google Scholar]
- Luby, J.L. , Belden, A.C. , Jackson, J.J. , Lessov‐Schlaggar, C.N. , Harms, M.P. , Tillman, R. , … & Barch, D.M. (2016). Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry, 73, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K.A. , Sheridan, M.A. , & Lambert, H.K. (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensen, V.T. , Wierenga, L.M. , van Dijk, S. , Rijks, Y. , Oranje, B. , Mandl, R.C. , & Durston, S. (2017). Development of cortical thickness and surface area in autism spectrum disorder. NeuroImage: Clinical, 13, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas, K.R. , He, J.‐p. , Burstein, M. , Swanson, S.A. , Avenevoli, S. , Cui, L. , … & Swendsen, J. (2010). Lifetime prevalence of mental disorders in US adolescents: Results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS‐A). Journal of the American Academy of Child & Adolescent Psychiatry, 49, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K.L. , Goddings, A.‐L. , Clasen, L.S. , Giedd, J.N. , & Blakemore, S.‐J. (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36, 147–160. [DOI] [PubMed] [Google Scholar]
- Mills, K.L. , Goddings, A.‐L. , Herting, M.M. , Meuwese, R. , Blakemore, S.‐J. , Crone, E.A. , … & Sowell, E.R. (2016). Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage, 141, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, E.E. , Leibenluft, E. , McClure, E.B. , & Pine, D.S. (2005). The social re‐orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–174. [DOI] [PubMed] [Google Scholar]
- Pannekoek, J.N. , van der Werff, S.J. , van den Bulk, B.G. , van Lang, N.D. , Rombouts, S.A. , van Buchem, M.A. , … & van der Wee, N.J. (2014). Reduced anterior cingulate gray matter volume in treatment‐naive clinically depressed adolescents. NeuroImage: Clinical, 4, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek, J.N. , Werff, S. , Meens, P.H. , Bulk, B.G. , Jolles, D.D. , Veer, I.M. , … & Vermeiren, R.R. (2014). Aberrant resting‐state functional connectivity in limbic and salience networks in treatment‐naive clinically depressed adolescents. Journal of Child Psychology and Psychiatry, 55, 1317–1327. [DOI] [PubMed] [Google Scholar]
- Papmeyer, M. , Sussmann, J.E. , Stewart, T. , Giles, S. , Centola, J.G. , Zannias, V. , … & McIntosh, A.M. (2016). Prospective longitudinal study of subcortical brain volumes in individuals at high familial risk of mood disorders with or without subsequent onset of depression. Psychiatry Research: Neuroimaging, 248, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus, T. (2013). How environment and genes shape the adolescent brain. Hormones and Behavior, 64, 195–202. [DOI] [PubMed] [Google Scholar]
- Paus, T. , Keshavan, M. , & Giedd, J.N. (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger, T.V. (1998). What's wrong with Bonferroni adjustments. British Medical Journal, 316, 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, S. , & Crone, E.A. (2017). Increased striatal activity in adolescence benefits learning. Nature Communications, 8, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, S. , Van Duijvenvoorde, A.C. , Koolschijn, P.C.M. , & Crone, E.A. (2016). Longitudinal development of frontoparietal activity during feedback learning: Contributions of age, performance, working memory and cortical thickness. Developmental Cognitive Neuroscience, 19, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer, J.H. , & Allen, N.B. (2012). Arrested development? Reconsidering dual‐systems models of brain function in adolescence and disorders Trends in Cognitive Sciences, 16, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine, D.S. , Cohen, E. , Cohen, P. , & Brook, J. (1999). Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? American Journal of Psychiatry, 156, 133–135. [DOI] [PubMed] [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , & Sarkar, D. (2014). R Core Team (2014) Nlme: Linear and nonlinear mixed effects models. R package version 3.1‐117.
- Reuter, M. , Schmansky, N.J. , Rosas, H.D. , & Fischl, B. (2012). Within‐subject template estimation for unbiased longitudinal image analysis. NeuroImage, 61, 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso, I.M. , Cintron, C.M. , Steingard, R.J. , Renshaw, P.F. , Young, A.D. , & Yurgelun‐Todd, D.A. (2005). Amygdala and hippocampus volumes in pediatric major depression. Biological Psychiatry, 57, 21–26. [DOI] [PubMed] [Google Scholar]
- Sankoh, A.J. , Huque, M.F. , & Dubey, S.D. (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine, 16, 2529–2542. [DOI] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. , Sämann, P. , Hall, G. , Baune, B. , Jahanshad, N. , … & Ikram, M. (2016). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22, 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D.J. , van Erp, T.G. , Sämann, P. , Frodl, T. , Jahanshad, N. , … & Niessen, W. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Molecular Psychiatry, 21, 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Yucel, M. , Ellis, R. , Vijayakumar, N. , Simmon, J.G. , Allen, N.B. , & Whittle, S. (2017). Brain structural signatures of adolescent depressive symptom trajectories: A longitudinal magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 593–601. [DOI] [PubMed] [Google Scholar]
- Schreuders, E. , Braams, B.R. , Blankenstein, N.E. , Peper, J.S. , Güroğlu, B. , & Crone, E.A. (in press). Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad, M.U. , Muddasani, S. , & Rao, U. (2012). Gray matter differences between healthy and depressed adolescents: A voxel‐based morphometry study. Journal of Child and Adolescent Psychopharmacology, 22, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Greenstein, D. , Lerch, J. , Clasen, L. , Lenroot, R. , Gogtay, N. , … & Giedd, J. (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440, 676. [DOI] [PubMed] [Google Scholar]
- Sheline, Y.I. , Sanghavi, M. , Mintun, M.A. , & Gado, M.H. (1999). Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience, 19, 5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J. , French, L. , Xu, T. , Leonard, G. , Perron, M. , Pike, G.B. , … & Paus, T. (2017). Cell‐specific gene‐expression profiles and cortical thickness in the human brain. Cerebral Cortex, 1–11. Advanced online publication. 10.1093/cercor/bhx19. [DOI] [PubMed] [Google Scholar]
- Spinhoven, P. , Elzinga, B.M. , Hovens, J.G. , Roelofs, K. , Zitman, F.G. , van Oppen, P. , & Penninx, B.W. (2010). The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. Journal of Affective Disorders, 126, 103–112. [DOI] [PubMed] [Google Scholar]
- Tamnes, C.K. , Herting, M. , Goddings, A.‐L. , Meuwese, R. , Blakemore, S.‐J. , Dahl, R.E. , … & Mills, K.L. (2017). Development of the cerebral cortex across adolescence: A multisample study of interrelated longitudinal changes in cortical volume, surface area and thickness. Journal of Neuroscience, 37, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes, C.K. , Walhovd, K.B. , Dale, A.M. , Østby, Y. , Grydeland, H. , Richardson, G. , … & Due‐Tønnessen, P. (2013). Brain development and aging: Overlapping and unique patterns of change. NeuroImage, 68, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway, M.T. , Waskom, M.L. , Dillon, D.G. , Holmes, A.J. , Park, M.T.M. , Chakravarty, M.M. , … & Fava, M. (2015). Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biological Psychiatry, 77, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar, N. , Mills, K.L. , Alexander‐Bloch, A. , Tamnes, C.K. , & Whittle, S. (in press). Structural brain development: A review of methodological approaches and best practices. Developmental Cognitive Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide, R.J. , Skipper, L.M. , Klobusicky, E. , & Olson, I.R. (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136, 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulser, H. , Lemaitre, H. , Artiges, E. , Miranda, R. , Penttilä, J. , Struve, M. , … & Goodman, R. (2015). Subthreshold depression and regional brain volumes in young community adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, E. , Stewart‐Brown, S. , & Fitzpatrick, R. (2003). Agreement between adolescent self‐report and parent reports of health and well‐being: Results of an epidemiological study. Child: Care, Health and Development, 29, 501–509. [DOI] [PubMed] [Google Scholar]
- Whitaker, K.J. , Vértes, P.E. , Romero‐Garcia, R. , Váša, F. , Moutoussis, M. , Prabhu, G. , … & Rittman, T. (2016). Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proceedings of the National Academy of Sciences of the United States of America, 113, 9105–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle, S. , Lichter, R. , Dennison, M. , Vijayakumar, N. , Schwartz, O. , Byrne, M.L. , … & McGorry, P. (2014). Structural brain development and depression onset during adolescence: A prospective longitudinal study. American Journal of Psychiatry, 171, 564–571. [DOI] [PubMed] [Google Scholar]
- Wierenga, L.M. , Langen, M. , Oranje, B. , & Durston, S. (2014). Unique developmental trajectories of cortical thickness and surface area. NeuroImage, 87, 120–126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary methods and results.
Table S1. BIC values for null, linear, quadratic and cubic age models to describe the relationship with age and brain measures reported in the table.
Table S2. Model parameters for the best fitting model for the five lobes of cortical thickness.
Table S3. Model parameters for the best fitting model for the five lobes of cortical surface area.
Table S4. BIC values for best age model controlling for sex and the model including sex interaction terms for thickness of frontal, parietal, and occipital lobe and surface area of temporal, frontal, parietal, and occipital lobes.
Table S5. Model parameters for the best fitting model for hippocampal and amygdala volume.
Figure S1. Developmental trajectories for cortical thickness of the parietal lobe (A) and occipital lobe (B).
Figure S2. Developmental trajectories for cortical surface area of the parietal lobe (A), temporal lobe (B), frontal lobe (C), occipital lobe (D).


