Abstract
This study focuses on how the body schema develops during the first months of life, by investigating infants’ motor responses to localized vibrotactile stimulation on their limbs. Vibrotactile stimulation was provided by small buzzers that were attached to the infants’ four limbs one at a time. Four age groups were compared cross‐sectionally (3‐, 4‐, 5‐, and 6‐month‐olds). We show that before they actually reach for the buzzer, which, according to previous studies, occurs around 7–8 months of age, infants demonstrate emerging knowledge about their body's configuration by producing specific movement patterns associated with the stimulated body area. At 3 months, infants responded with an increase in general activity when the buzzer was placed on the body, independently of the vibrator's location. Differentiated topographical awareness of the body seemed to appear around 5 months, with specific responses resulting from stimulation of the hands emerging first, followed by the differentiation of movement patterns associated with the stimulation of the feet. Qualitative analyses revealed specific movement types reliably associated with each stimulated location by 6 months of age, possibly preparing infants’ ability to actually reach for the vibrating target. We discuss this result in relation to newborns’ ability to learn specific movement patterns through intersensory contingency.
Statement of contribution.
what is already known on infants’ sensorimotor knowledge about their own bodies
3‐month‐olds readily learn to produce specific limb movements to obtain a desired effect (movement of a mobile).
infants detect temporal and spatial correspondences between events involving their own body and visual events.
what the present study adds
until 4–5 months of age, infants mostly produce general motor responses to localized touch.
this is because in the present study, infants could not rely on immediate contingent feedback.
we propose a cephalocaudal developmental trend of topographic differentiation of body areas.
Keywords: early body schema, limb movement differentiation, motor development, movement coordination, topographical body knowledge, touch
Background
In order to perform everyday tasks, we rely on our knowledge about the spatial configuration of our body parts, guiding these actions without the necessity of conscious monitoring. This internal model of our body's structure, along with implicit knowledge about the consequences of this configuration on touch, vision, and movement, has broadly been referred to as body schema in the literature (Graziano & Botvinick, 2002). The present study aimed at highlighting one aspect of this functional representation, during the first months of life, by investigating infants’ motor responses to localized vibrotactile stimulation on their limbs.
Before presenting our research, we will first review existing research on infants’ awareness of their own bodies, on their representations of the body, on the early topographic differentiation of limb movements, and on how infants come to represent their body in external space.
Early body mapping: Movement coordination in foetuses and newborns
Early awareness of the body is evidenced by hand‐to‐mouth coordination observable at birth and even before. Foetuses frequently bring their hand to their mouth and anticipate hand‐to‐mouth contact by opening their mouth beforehand (Myowa‐Yamakoshi & Takeshita, 2006). The kinematic pattern of these movements is distinguishable from that of movements directed towards the eye, indicating that foetuses plan these actions based on their sensory consequences (Zoia et al., 2007). By birth, infants can accurately bring their hands towards their mouth (Lew & Butterworth, 1997; Rochat, 1993). Further evidence for movement coordination at birth is provided by the systematic arm movements observed in neonates when given the opportunity to visually explore their hand in a beam of light (van der Meer, van der Weel, & Lee, 1995). Together, these findings suggest that infants are born with rudimentary knowledge about the topographical arrangement of focal regions on the body, such as the hands or the mouth.
Topographic arrangement of early body representations
Perceptual studies have typically used the preferential looking paradigm to explore how infants detect temporal and spatial correspondences between events involving their own body (self‐generated movement or external stimulation) and events presented visually. Bahrick and Watson (1985) showed that 5‐month‐old infants are sensitive to the absence of temporal contingency between their own leg movements and the accompanying video image of their leg. In addition to temporal contingency, infants also detect correspondence between the direction of their self‐generated leg movements and that of visually presented leg movements at 3 months of age (Rochat & Morgan, 1995). More recently, newborns have been shown to detect temporal and topographical correspondences between tactile stimulation of their own face and a video display of an infant's face being stroked (Filippetti, Johnson, Lloyd‐Fox, Dragovic, & Farroni, 2013; Filippetti, Orioli, Johnson, & Farroni, 2015).
Relatively few studies have looked at the neural representation of the body in the infant brain. Recent work that used electroencephalography found specific somatotopic response patterns to tactile stimulation of the hands and feet in 7‐month‐olds (Saby, Meltzoff, & Marshall, 2015). An investigation using functional near‐infrared spectroscopy revealed differential temporal lobe activity in 5‐month‐olds according to whether they viewed displays of their face that were temporally contingent with their movements or delayed displays (Filippetti, Lloyd‐Fox, Longo, Farroni, & Johnson, 2015).
The above studies on early body representations therefore show that the face area is possibly represented topographically already in neonates and that representations of the hands and the feet are already in place around 7 months.
Topographic differentiation of limb movements
Further evidence concerning infants’ awareness about their own bodies is provided by studies that used the mobile paradigm (Rovee & Rovee, 1969), in which a mobile hanging above the crib is connected to one of the baby's feet or hands with a ribbon. Although these studies were originally designed to explore the development of self‐agency, they additionally provide evidence for a topographical differentiation between the connected and unconnected legs in 3‐ to 4‐month‐old infants, who kick the contingent leg faster and harder than the non‐contingent leg (Heathcock, Bhat, Lobo, & Galloway, 2005; Rovee‐Collier, Morrongiello, Aron, & Kupersmidt, 1978). Watanabe and Taga (2006) focused on developmental changes in infants’ arm movements in the mobile paradigm and found that while 3‐ and 4‐month‐olds produced increasingly specific movements of the connected arm to activate the mobile, 2‐month‐olds produced general movements with all their limbs. In a subsequent study, the same authors (Watanabe & Taga, 2009) found that arm‐based learning emerges earlier than leg‐based learning, suggesting that early motor learning develops in a cephalocaudal pattern.
It appears that infants’ discovery of their own body through self‐touch also follows this pattern. In a study by Thomas, Karl, and Whishaw (2014), the authors documented early spontaneous self‐touching behaviour by analysing video recordings from birth to 6 months. They found that in younger infants, self‐touching targets included upper body locations such as the head and trunk. As infants aged, targets became more caudal and included the hips, then the legs, and eventually the feet.
In summary, studies suggest that infants develop specific responses to contingent stimulation gradually over several months. Before 3 months of age, infants are more likely to respond with general movement patterns, with all limbs increasing their activity when only one limb is connected to a mobile. From 3 and 4 months of age, infants begin to produce movement patterns that are more specific to the connected limb and this specification seems to appear for upper body locations first, followed by lower body locations.
Representing the body in external space
Around 6 months of age, infants start to rely on external (for instance, visual) cues in order to localize touch on their body (Begum Ali, Spence, & Bremner, 2015). The importance of external reference frames has been shown by studies that investigated the effects of limb crossing on tactile localization. Bremner, Mareschal, Lloyd‐Fox, and Spence (2008) looked at infants’ first correct unilateral hand movement and orienting responses to hand stimulation in a crossed posture and observed that at 10 months, infants’ responses were modulated by the posture, whereas 6‐month‐olds performed equivalently. In a later study, responses to stimuli at crossed feet were modulated in 6‐month‐olds, but not in 4‐month‐old infants (Begum Ali et al., 2015). The authors interpreted these results as indicating that the use of external or spatial information localizing touch develops between 4 and 6 months.
The present study
As seen above, previous tasks used to assess body knowledge during early infancy have been largely perceptual, requiring visual responses (Bahrick & Watson, 1985; Filippetti, Orioli, et al., 2015; Rochat & Morgan, 1995). In other studies where the task required a motor response, as with different versions of the mobile paradigm, the results mainly indicate how infants learn to produce specific responses to obtain a desired effect. The studies that investigated responses to crossed limbs assessed motor responses, but did not differentiate between different types of responses. Also, most previous studies have focused on one or two age groups and have not looked at how body knowledge develops over a longer period with one and the same paradigm. It is therefore important at present to explore body mapping in infants by using the same task across several age groups, a task that involves several body areas and does not require specific motor behaviours that must be learnt during the course of the experiment.
We aimed to fill this gap with a paradigm in which we provide localized vibrotactile stimulation to infants aged 3–6 months in order to study how they produce movements either when orienting towards and exploring the impinging stimulus or in preparation to remove it. We chose 3 months as the lower age boundary because past research suggests that from this age, infants begin to produce specific movement patterns in the mobile paradigm (Watanabe & Taga, 2009) and also become increasingly systematic in the exploration of their own body and the perceptual consequences of self‐produced action (Rochat & Striano, 2000).
First, we hypothesized that younger infants would mostly produce general responses to stimulation and would not produce differentiated movement patterns according to stimulus location (Watanabe & Taga, 2006, 2009). We thus expected that at 3 months, infants would respond to stimulation with patterns that involve the whole body rather than by responding with a movement pattern that can be clearly associated with the location of the stimulus (for instance, rather than moving only the stimulated limb, they would move all four limbs in response to the buzzer). Second, we hypothesized that well before they can effectively act on the impinging stimulus by retrieving it (Leed, 2014), infants would produce other behavioural responses that indicate knowledge about where their body is stimulated, as reported, for instance, in the studies by Bremner et al. (2008) and Begum Ali et al. (2015). We expected infants to demonstrate functional knowledge about the body's configuration by producing specific orienting responses or movement and activity patterns associated with the stimulated body area (Watanabe & Taga, 2006, 2009). Third, we hypothesized that differentiated movement patterns associated with stimulus location would emerge from about 4 months of age, first for the upper body locations, namely the hands, followed by differentiated movement patterns associated with foot stimulation (Thomas et al., 2014; Watanabe & Taga, 2009), with infants gradually becoming more and more successful at localizing the buzzer.
Method
Participants
The participants were eleven 3‐month‐olds (seven girls and four boys, age range = 83–103 days), 10 4‐month‐olds (fur girls and six boys, age range = 116–131 days), twelve 5‐month‐olds (six girls and six boys, age range = 140–157 days), and ten 6‐month‐olds (five girls and five boys, age range = 174–185 days). Thus, the total sample consisted of 43 children (22 girls and 21 boys) of ages ranging from 83 to 185 days. An additional nine infants were tested, but had to be excluded due to fussiness, sleepiness, torticollis at birth, or parental intervention. Infants were recruited from a list of local families who had expressed interest in participating in studies in infant development. Families were middle to upper class. All parents gave their written informed consent before their infant participated in the study.
Materials and procedure
We attached a vibrating buzzer with double‐sided tape to four areas of the child's body. The buzzer (1 cm Ø) was custom‐made and consisted of a button battery attached to a pancake motor, with a rotation speed of 70 Hz, comparable to that found in baby teethers.
The children were seated supine in an infant seat during the whole session. Trials were recorded on video. The buzzer was first placed on the parent so they were comfortable with the task. A 1‐min baseline period followed during which the infants’ spontaneous movements were recorded (baseline). Next, the vibrating buzzer was attached to one of the areas of interest, on the top of the limb: left hand, right hand, left foot, and right foot for a total of four trials (stimulation conditions). The order of location (hands or feet first) and side (left or right first) was randomized. While placing the buzzer on the child, the experimenter also approached her hand towards the same location on the side not receiving the target with a second buzzer, which she did not attach to the body. This way, the visual cues were approximately identical for the two sides. We wished to provide tactile stimulation only in the area of interest and therefore did not place non‐vibrating buzzers on the other limbs. The buzzers were silent; therefore, any auditory discrepancies could be avoided. Each trial lasted until 35 s elapsed, at which point the experimenter removed the buzzer and attached it to the next location. If the infant removed the buzzer within 35 s, then the trial ended with the removal (Figure 1).
Figure 1.

As shown on the left, a small vibrating pancake style motor or buzzer was attached one at a time to each of the areas of interest, on the top of the limb: left hand, right hand, left foot, and right foot for a total of four trials (LH, RH, LF, and RF conditions). The order of location (hands or feet first) and side (left or right first) was randomized across the four trials for each participant. The buzzers, as illustrated on the right, were small (approximately 5 mm in diameter) and had a rated rotation speed of 70 Hz (3VDC) and were encased in soft material. The small tab kept the target from vibrating when it was not applied to a body location. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Coding of limb activity for analysis
For qualitative analyses, we collapsed hand and foot stimulation conditions across sides and compared the resulting three conditions (hand stimulation, foot stimulation, and baseline conditions). For quantitative analyses, we looked at the effect of stimulation on each limb's activity by comparing the activity of the stimulated limb to the activities of the three other, non‐stimulated limbs (in this case the four grouping conditions were labelled: stimulated limb, contralateral limb, opposite end ipsilateral limb, and opposite end contralateral limb – we used the term ‘opposite end’ to designate the distinction between upper and lower body).
We used generalized estimating equations (GEE) analyses to explore the effect of stimulation condition and age on infants’ behavioural responses. This method is particularly adapted to data clustered within the subject and does not require the outcome variable to have a particular distribution, benefiting data sets where the distribution is difficult to verify due to small sample size. For this same reason, we did not plan to conduct power analyses. The sample sizes we used are comparable to literature in the field (see, for instance, Bremner et al., 2008, Begum Ali et al., 2015 or Filippetti, Orioli, et al., 2015). We used Bonferroni procedures to adjust for subsequent multiple comparisons.
Qualitative coding
We established the following behavioural categories based on preliminary pilot observations of infants’ responses:
Retrieving the buzzer: The infant makes a clear attempt to pick the buzzer off, touching the buzzer directly with the fingers, grasping it, or performing a pinch movement.
Touching the buzzer: The infant touched the buzzer with a hand or a foot; little surprisingly, children used a foot only to touch the other foot.
Reciprocal hand touch: The infant brought one hand in contact with the other (rubbing hands or grasping one hand with the other), without fingering or exploring the buzzer.
Foot touch: The infant brought one foot in contact with the other (rubbing feet) or grasped one or both feet with the hands, without touching or exploring the buzzer.
Visual exploration of the hand: The infant explored the hand visually by bringing the hand into field of vision and clearly directing gaze at hand.
Qualitative coding was binary and trial by trial, for each of the four categories, infants were scored 1 if the behaviour was produced during the trial and 0 if the behaviour was absent. Two research assistants coded the videos (the inter‐rater agreement between them was substantial, κ = .78).
Quantitative coding
Kinovea version 0.8.15 was used to place within each video frame a marking cursor on the infants’ hands and feet, which could then be tracked by the software in order to calculate the distances travelled (in pixels) by each limb in the two‐dimensional plane of the video display. Two research assistants coded the videos (the inter‐rater agreement between them was high, rICC = .906).
As the software inevitably detected small displacements even when the marked location was judged as stationary by the coders, the data were filtered and only displacements of three pixels or more were considered as limb activity. Next, we calculated for each trial and each coded limb the percentage of time during which the limb was active (limb activity) and used this measure for all further analyses.
Note that movement data were obtained by calculating the distances travelled by each limb in the two‐dimensional plane of the video display. This method is less precise than three‐dimensional movement capture systems, as one plane of movement is necessarily lost. Given that the camera filmed the lying infant from directly above, all four limbs were equally affected by this limitation. We therefore assume that when we compare the limbs’ movements among them, proportions are not affected (Figure 2).
Figure 2.
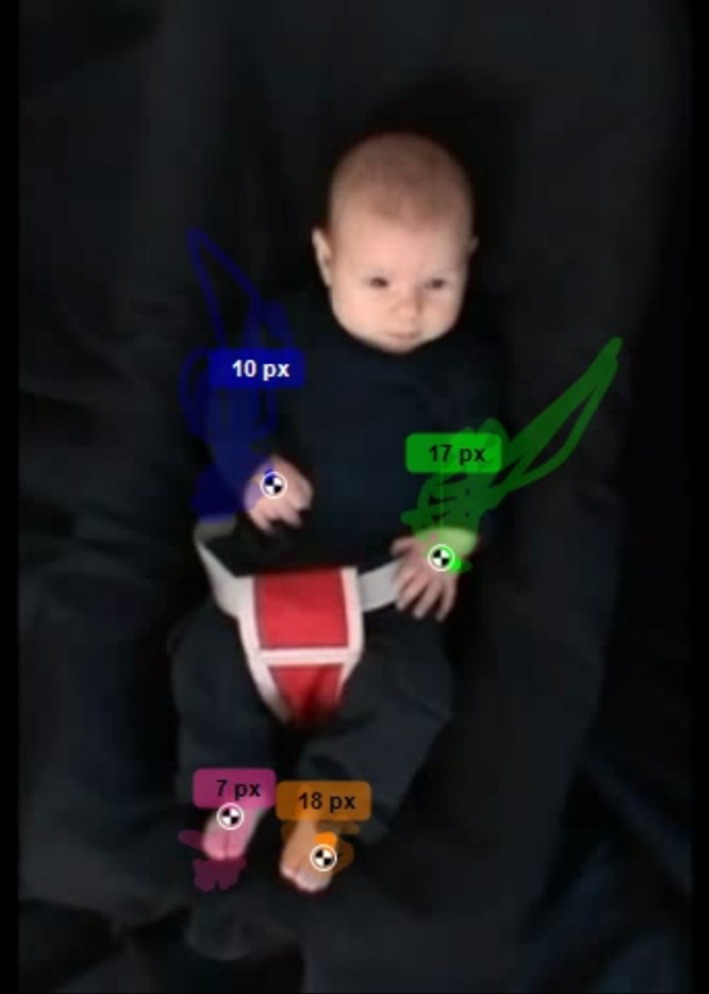
Screenshot of Kinovea in use for coding an infants’ limb activity. The coloured rectangles above each limb indicate the number of pixels traveled by the limb. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Results
Qualitative analyses
Generalized estimating equations analyses (N = 43) were used to predict the effect of stimulation condition (dependent within‐subject variable: hand stimulation, foot stimulation, and baseline where appropriate) and age (independent between‐subjects variable: 3, 4, 5, and 6 months) on each behavioural category (dependent binary variables: retrieving the buzzer, touching the buzzer, reciprocal hand touch, foot touch, and visual exploration of the hand).
Retrieving the buzzer
We found a significant main effect of age (Wald χ2 = 7.2, df = 3, p = .03). Pairwise comparisons showed that at 6 months infants retrieved the buzzer significantly more (13% of trials) than at 3 months (0% of trials, p = .02). Still, only few infants made a clear attempt to retrieve the buzzer (5% of all stimulation trials, all ages collapsed).
Touching the buzzer
We found a significant main effect of age (Wald χ2 = 16.310, df = 3, p = .001). Pairwise comparisons (Figure 3) revealed that 5‐ and 6‐month‐olds were significantly more likely to touch the buzzer than 3‐month‐olds (5 months: 10% of trials, 6 months: 20% of trials; 3 months: 0% of trials, p = .03 and p = .043, respectively). Stimulation condition did not have an effect on the frequency of buzzer touches and we did not find an interaction between the two predictors either, meaning that once infants located the buzzer, they did so regardless of where the buzzer was attached.
Figure 3.
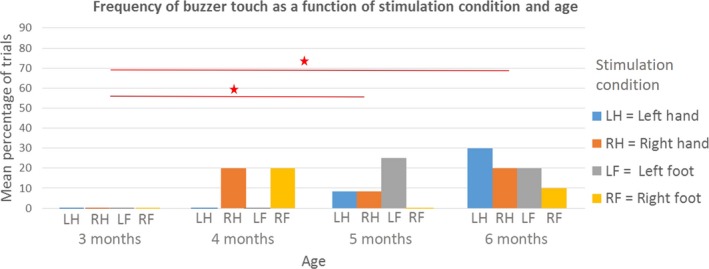
The effect of stimulus location on the mean percentage of trials where infants touched the buzzer either with a hand or with a foot. By 5 and 6 months of age, infants were significantly more likely to touch the buzzer than 3‐month‐olds, all conditions collapsed. Stimulation condition did not have an effect on the frequency of retrievals, meaning that once infants located the buzzer and touched it, they did so regardless of where the buzzer was attached. Within each age group, significant differences between means are marked with an asterisk (∗ p < 0.05; ∗∗ p < 0.01), as calculated with pairwise comparisons following the generalized estimating equations (GEE) procedure. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Reciprocal hand touch
We found significant main effects of both stimulation condition (Wald χ2 = 556, df = 2, p < .001) and age (Wald χ2 = 181, df = 3, p < .001). Pairwise comparisons (Figure 4) revealed that at 3 months, reciprocal hand touch was more frequent in the hand stimulation conditions (30% of trials) than in the baseline condition (0% of trials, p = .021). At 4 and 5 months, infants brought their hands together more frequently in the hand stimulation conditions (4 months: 40% of trials, 5 months: 33% of trials) than in the foot stimulation conditions (4 months: 0% of trials, p = .004, 5 months: 0% of trials, p = .007). We did not find an interaction between the two predictors.
Figure 4.
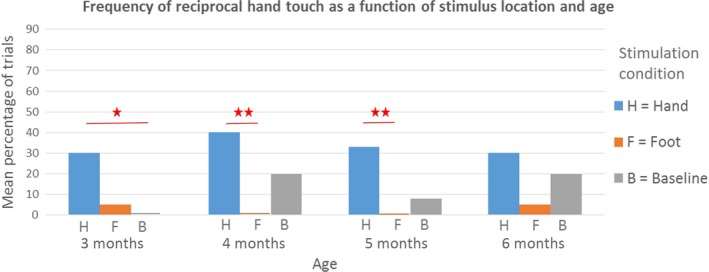
The effect of stimulus location on the mean percentage of trials where infants touched one hand with the other. At 3 months, infants were significantly more likely to touch their hands in the hand stimulation conditions than in the baseline condition. At 4 and 5 months, infants touched their hands more in the hand stimulation conditions than in the foot stimulation conditions. At these ages, infants began to touch their hands spontaneously in the baseline condition as well, it seems therefore that this spontaneous behaviour was inhibited in the foot conditions at this age, yielding the significant difference between hand and feet conditions. At 6 months, differences between conditions disappear, as infants begin to touch the buzzer directly, as shown in Figure 3 above. Within each age group, significant differences between means are marked with an asterisk (∗p < 0.05; ∗∗p < 0.01), as calculated with pairwise comparisons following the generalized estimating equations (GEE) procedure. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Foot touch
We found a significant main effect of stimulation condition (Wald χ2 = 8.481, df = 2, p = .014), independently of age (Wald χ2 = 6.603, df = 3, p = .092) as well as an interaction between the effects of stimulation condition and age (Wald χ2 = 28.424, df = 6, p < .001). Pairwise comparisons (Figure 5) revealed that at 4, 5, and 6 months, but not at 3 months, infants touched their feet significantly more in the foot stimulation conditions (4 months: 80% of trials, 5 months: 58% of trials, 6 months: 70% of trials) than in the baseline condition (4 months: 30% of trials, 5 months: 33% of trials, 6 months: 20% of trials, p < .001, p = .034, and p < .001, respectively) or in the hand stimulation conditions (4 months: 25% of trials, 5 months: 37% of trials, 6 months: 10% of trials, p < .001, p = .007, and p < .001, respectively).
Figure 5.
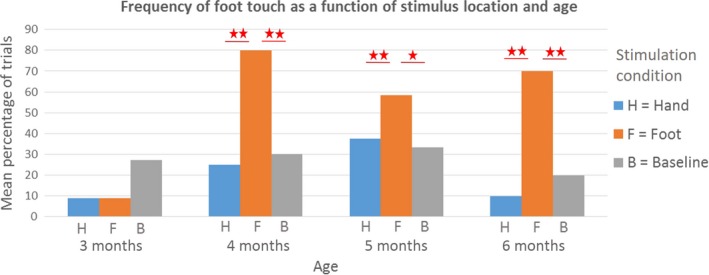
The effect of stimulus location on the frequency of foot touches, either with the hands or the other foot. At 4, 5, and 6 months, but not at 3 months, infants touched their feet significantly more in the foot stimulation conditions than in the baseline condition or the hand stimulation conditions. In response to stimulation, foot touch appears later than hand touch, which can already be observed at 3 months, as shown in Figure 4 above. Within each age group, significant differences between means are marked with an asterisk (∗ p < 0.05; ∗∗ p < 0.01), as calculated with pairwise comparisons following the generalized estimating equations (GEE) procedure. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Visual exploration of hand
We found a significant main effect of stimulation condition (Wald χ2 = 6.2, df = 2, p = .013). Pairwise comparisons showed that infants, all ages collapsed, looked at their hand significantly more in hand stimulation conditions (16% of trials) than in the baseline condition (0%, p = .02) or in foot stimulation conditions (5%, p = .03). Still, only few infants looked at their hand across trials (6% of all trials). In each case, the infant looked at the stimulated hand.
Quantitative analyses
We compared the activity of the stimulated limb itself (stimulated limb) to the activity of each of the three non‐stimulated limbs (contralateral limb, opposite end ipsilateral limb, and opposite end contralateral limb) during the same trial, as well as to the average activity of the four limbs in the baseline condition (baseline whole body activity). For example, when the stimulated limb was the right hand, the left (non‐stimulated) hand was coded as the contralateral limb, the right foot (non‐stimulated) as the ipsilateral limb at the opposite end of the body, and the left foot (non‐stimulated) as the contralateral limb at the opposite end of the body.
We performed a GEE analysis (N = 43) for the effect of stimulation and age (3, 4, 5, and 6 months) on limb activity (stimulated limb, contralateral limb, opposite end ipsilateral limb, opposite end contralateral limb, and as compared to baseline whole body activity). We found significant main effects of stimulation (Wald χ2 = 22.19, df = 4, p < .001) and age (Wald χ2 = 14.16, df = 3, p = .003). We also found significant interactions between the two factors (Wald χ2 = 30.22, df = 12, p = .003). This suggests that limb activity depended on whether the limb was stimulated or not and that this effect differed across age groups.
Subsequent pairwise comparisons (Figure 6) revealed that at 3 months, when any limb was stimulated, the activity of all four limbs (M Stimulated = 60.20; M Contralateral = 59.34; M OppositeIpsilateral = 55.55; M OppositeContralateral = 60.05) increased significantly as compared to baseline whole body activity (M BaselineWholeBody = 43.45, p = .002, p = .003, p = .05, and p = .007, respectively). In other words, when any limb was stimulated, the activity of all four limbs increased as compared to the average activity of the four limbs in the baseline condition.
Figure 6.
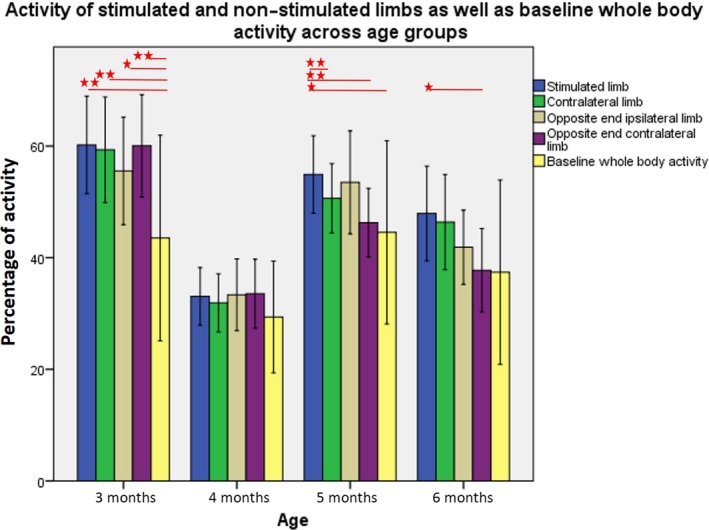
Mean percentage of activity of stimulated limb as compared to the three non‐stimulated limbs and whole body activity at baseline (averaged activity of the four limbs) across age groups. In case of hand stimulations, ‘opposite end limb’ refers to the feet, and in case of foot stimulations, it refers to the hands. Within each age group, significant differences between means are marked with an asterisk (*p < 0.05; ∗∗ p < 0.01), as calculated with pairwise comparisons following the generalized estimating equations (GEE) procedure. [Colour figure can be viewed at http://wileyonlinelibrary.com]
At 4 months, we did not find significant differences in limb activity across conditions. This may be explained by the fact that this age corresponds to the onset of object‐directed reaching, an important transition in the development of infants’ voluntary activity.
At 5 months, the activity of the stimulated limb (M Stimulated = 54.92) was significantly greater than baseline whole body activity (M BaselineWholeBody = 44.44, p = .047). The stimulated limb was also more active than the contralateral limb (M Contralateral = 50.65, p = .001) or the opposite end contralateral limb (M OppositeContralateral = 46.27, p = .003).
At 6 months, the stimulated limb was more active (M Stimulated = 47.93) than the opposite end contralateral limb (M OppositeContralateral = 37.75, p = .044).
See the Appendix for the comparison of each limb's activity under stimulation to its own baseline activity recorded before the stimulation trials.
Discussion
In this study, we explored 3‐ to 6‐month‐old infants’ motor responses to localized vibrotactile stimulation. Such orienting paradigms have the advantage of providing direct information concerning the development of functional representations of the body in comparison with tasks that use the preferential looking paradigm or contingent reinforcement.
Our first hypothesis was that well before they can actually take the vibrating stimulus off their bodies, infants would demonstrate functional knowledge about their body's configuration by producing specific movement and activity patterns associated with the stimulated body area. Indeed, although only five of the 43 infants retrieved the buzzer, infants showed a variety of other responses that could be associated with stimulus location. Infants started to produce movement patterns associated with the stimulated body area from 3 months of age, when they were significantly more likely to bring one hand in contact with the other when any of the hands were stimulated as compared to baseline. At 4 and 5 months, the same pattern persisted for the hands and appeared also for the feet. Infants started to produce more specific responses by directly touching the buzzer from 5 months, with 5‐ and 6‐month‐olds touching it significantly more frequently than 3‐month‐olds, who did not touch the buzzer. Quantitative analyses revealed specific activity patterns from 5 months, when the activity of the stimulated limb was greater than that of the contralateral limbs as well as the average baseline activity of the four limbs. These observations support our hypothesis of gradual development leading to actual grasping behaviour, since well before the appearance of precise movements towards the target, we captured movement patterns associated with stimulus location.
Our second hypothesis was that younger infants would produce general responses to stimulation and would not produce differentiated movement patterns according to stimulus location (Watanabe & Taga, 2006, 2009). As discussed above, qualitative analyses showed that at 3 months of age infants contacted their hands more when either hand or, more generally, upper body locations were stimulated. This response, however, was not specific to the precise location of stimulation (which hand was stimulated) and no further movement patterns could be associated with stimulus location at this age. Furthermore, 3‐month‐old infants’ responses to stimulation were even more clearly generalized with respect to movement quantity. Comparing the four limbs’ activity within the same stimulation trial, we observed unspecific responses; that is, all four limbs increased their activity in response to tactile stimulation, not only the stimulated limb. Thus, the data supported our second hypothesis, as 3‐month‐old infants’ motor responses to tactile stimulation were general, meaning that infants produced the same movements mostly and moved their limbs equally, regardless of stimulus location.
Thirdly, we hypothesized that differentiated movement patterns associated with stimulus location would emerge from about 4 months of age, first for the upper body locations, namely the hands, followed by differentiated movement patterns associated with foot stimulation (Thomas et al., 2014; Watanabe & Taga, 2009), with infants gradually becoming more and more successful at localizing the buzzer. Movement patterns associated with hand stimulation (reciprocal hand touch) appeared already at 3 months. The developmental trend from responsiveness of the upper towards that of the lower body locations was confirmed, as responsiveness to hand stimulation was followed a month later by responsiveness to foot stimulation from 4 months of age. Infants started to produce more specific responses by directly touching the buzzer from 5 months. Regarding movement quantity, differentiation of the activity of the stimulated limb appeared at 5 months, when the stimulated limb was more active than the contralateral limbs and produced more movement than the average baseline activity of the four limbs.
Interestingly, at 4 months, we did not find significant differences in limb activity across stimulation conditions. This may be explained by the fact that at this age, with the onset of reaching, the motor system is reorganized and refined (Bushnell & Boudreau, 1993; Corbetta, Thurman, Wiener, Guan, & Williams, 2014). This developmental trend of progressive specification of motor responses possibly fits into a wider developmental process of gradual motor specialization over the first year of life, as shown by a recent study that documented a substantial decrease in extraneous movements of the non‐acting limb accompanying unimanual object‐directed reaching between 9 and 12 months of age (D'Souza, Cowie, Karmiloff‐Smith, & Bremner, 2016). The cephalocaudal progress of this development is also in line with earlier studies showing that specific movement patterns appear for the hands first, and about a month later for the feet (Watanabe & Taga, 2009).
Finally, our results show that only few infants looked at their hand across trials (6% of all trials), indicating that infants below 6 months of age did not use visual information to localize touch. Similarly, Bremner et al. (2008) report that infants show much less robust visual orienting to tactile stimuli at 6.5 months of age as compared to 10 months.
All in all, our results differ in three respects from those reported in earlier studies. First, we observed that localized responses occur later in development for contexts in which infants cannot rely on contingent feedback related to their limb activity. Thus, it has been shown that newborns produce systematic arm movements in order to visually explore their hand and arm when it is illuminated (van der Meer et al., 1995) and studies using the mobile paradigm have found a differentiation in activity between the connected and unconnected legs as early as 3–4 months (Heathcock et al., 2005; Rovee‐Collier et al., 1978; Watanabe & Taga, 2006). In our study, however, where infants did not have the possibility to learn about the relationship of stimulation and body through self‐directed contingency, specific responses to stimulation appeared only from 5 months of age. Similar to Watanabe and Taga's (2009) findings with 2‐month‐olds, we observed that 3‐month‐old infants produced general movements with all their limbs when any of the four limbs was stimulated. This shows that earlier studies revealed more about infants’ ability to learn specific means‐end actions than about infants’ underlying body knowledge. Second, studies on body representation during the first months of life suggest that the privileged areas of the face and mouth are topographically arranged already in foetuses (Myowa‐Yamakoshi & Takeshita, 2006; Zoia et al., 2007) and that even newborns are able to distinguish topographically congruent from incongruent stimulation in the face area (Filippetti, Orioli, et al., 2015; Filippetti, Lloyd‐Fox, et al., 2015). Note that these studies did not look at the functional aspect of body representations, namely the ability to give motor responses to stimulation of these body areas. We show that specific responses for hands and feet appear later, at 3 months for the hands and 4 months for the feet. The hands’ developmental advantage over the feet may be linked to differences in tactile sensitivity between these areas, with the fingertips and facial skin being particularly receptive to touch (Weinstein, 1968). Finally, in the present study, we analysed infants’ active orienting and motor responses, in contrast to perceptual studies where looking time was used as a measure to explore infants’ body representations. These latter studies highlighted the important role of spatial orientation and directionality from at least 3 months of age by showing discrimination of congruent and incongruent direction of leg movements (Rochat & Morgan, 1995) and detection of differences in canonical left/right directionality (Schmuckler, 1996). Our present results confirm the differentiation of responses in the upper/lower directionality from about 3 months; however, response differentiation along the left/right axis appeared only from 5 months of age.
One limitation of the present study is that it does not allow us to differentiate between infants’ ability to localize vibrotactile stimulation (sensory map) and their ability to perform motor responses specifically associated with the stimulated area (motor map). It is possible that even before 3 months of age, when they do not perform specific motor responses to vibrotactile stimulation in our experiments, infants sense precisely where they are stimulated, but are unable to act upon this sensory information. One way to disentangle to two would be to explore neural responses to localized tactile stimulation during the very first months of life. To date, the neurorepresentational studies on early sensory body mapping have been conducted with infants older than 6 months of age (Saby et al., 2015). A second limitation to our study is that we have explored responses only to stimulation on the four limbs. In order to obtain a more comprehensive picture of how infants respond to tactile stimulation of their body and how they use their gradually developing body knowledge, further studies are needed in which other areas (ears, elbow, trunk, knees, etc.) are stimulated (see Hoffmann et al., 2017). Third, the present study does not allow us to explore the possible mechanisms that might generate the observed behaviour and that might underlie infants’ early learning about their body. The roles of everyday tactile stimulation (through feeding or cuddling), self‐touch, and vision still need to be assessed.
In conclusion, we have shown that at 3 months of age, infants respond to vibrotactile stimulation in an undifferentiated way, by increasing movements of the whole body. Response to touch becomes specific around 5 months and by 7–8 months infants become able to perform precise movements in order to act upon the impinging stimulus (Leed, 2014). These results are particularly relevant regarding infants’ early body knowledge as well as the effect of social touch.
Acknowledgments
E.S., J.F., and J.K.O. gratefully acknowledge the support of ERC Grant 323674 ‘FEEL’ and FET Open Grant 713010 ‘GOAL‐Robots’. T.H. was supported by the Emmy Noether Grant He 6368/1‐1 by the German Research Foundation (DFG). M.H. was supported by a Marie Curie Intra European Fellowship (iCub Body Schema 625727) within the 7th European Community Framework Programme and the Czech Science Foundation under Project GA17‐15697Y. J.L. was supported in part by the National Institutes of Health Award 5R01HD067581. We would like to thank Zsófia Hodován for her precious assistance in data coding. Our special thanks to all parents and infants who participated in this study.
Comparison of the activity of stimulated limbs to their baseline activity
The quantitative analyses presented in the main body of this article compared the four limbs’ activity during one limb's stimulation, within the same trial. We were also interested in comparing each limb's activity under stimulation to its own baseline activity recorded before the stimulation trials. Thus, for each limb, we performed a GEE analysis for the effect of stimulation condition (RH, LH, RF, LF, and baseline) and age (3, 4, 5, and 6 months) on limb activity (percentage of the trial spent in activity of the observed limb). Figure A1 shows the mean percentage of activity of each limb across stimulation conditions and age groups.
Figure A1.
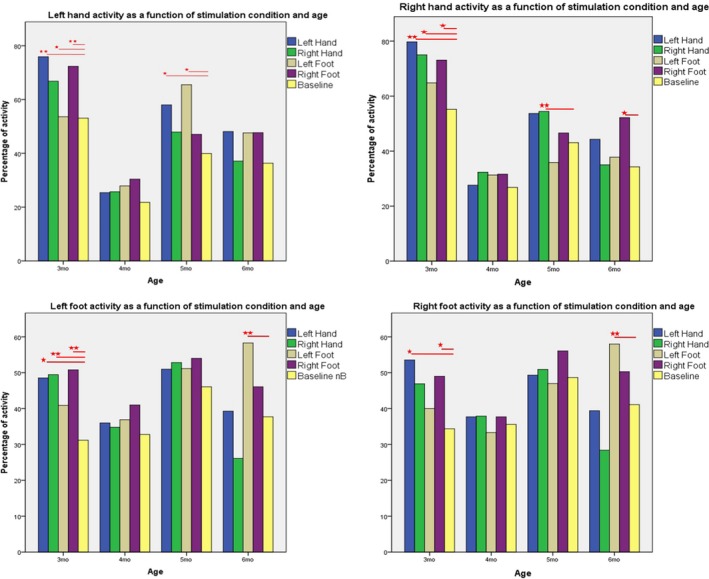
Mean percentage of activity of each limb across stimulation conditions and age groups. Within each age group, significant differences between means are marked with an asterisk (∗ p < 0.05; ∗∗ p < 0.01), as calculated with pairwise comparisons following the generalized estimating equations (GEE) procedure. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Left hand
The GEE analysis (N = 43) revealed significant main effects of stimulation condition (Wald χ2 = 20.21, df = 4, p < .000) and age (Wald χ2 = 26.30, df = 3, p < .001) on the activity of the left hand.
Subsequent pairwise comparisons revealed that at 3 months, the activity of the left hand increased significantly compared to baseline (M LH/Basel = 53.09) in the left hand (M LH/LHStim = 75.91), right hand (M LH/RHStim = 66.82), and right foot (M LH/RFStim = 72.36) stimulation conditions (p = .001, p = .019, and p = .003, respectively). Thus, the activity of the left hand increased significantly in three conditions of four.
At 4 months, the activity of the left hand did not change significantly across conditions.
By 5 months, infants moved their left hand significantly more than baseline (M LH/Basel = 39.92) when the left hand (M LH/LHStim = 58, p = .027) or the left foot (M LH/LFStim = 66, p = .053) was stimulated.
At 6 months, the activity of the left hand did not change significantly across conditions. This may be due to the fact that by this age, responses became specific in their quality, rather than in quantity.
Right hand
The GEE analysis (N = 43) revealed significant main effects of stimulation condition (Wald χ2 = 10.95, df = 4, p = .027) and age (Wald χ2 = 14.16, df = 3, p = .003) on the activity of the right hand. We also found significant interactions between the two factors (Wald χ2 = 26.02, df = 12, p = .011).
Subsequent pairwise comparisons revealed that at 3 months, the activity of the right hand increased significantly compared to baseline (M RH/Basel = 43.45) in the left hand (M RH/LHStim = 64.43), right hand (M RH/RHStim = 59.55), and right foot (M RH/RFStim = 61.32) stimulation conditions (p = .008, p = .031, and p = .05, respectively). Thus, at this age, the right hand responded in the same way as the left, increasing its activity significantly in three of four conditions.
At 4 months, just like for the left hand, the activity of the right hand did not change significantly across conditions.
Again, selective response appeared at 5 months, when infants moved their right hand significantly more than baseline (M RH/Basel = 43.08) only when the right hand was stimulated (M RH/RHStim = 54.42, p = .008).
At 6 months, infants moved their right hand significantly more than baseline (M RH/Basel = 34.30) only when the right foot was stimulated (M RH/RFStim = 52.20, p = .05).
Left foot
The GEE analysis (N = 43) revealed a significant main effect of stimulation condition (Wald χ2 = 23.16, df = 4, p < .000) on the activity of the left foot. We also found a significant interaction between stimulation condition and age (Wald χ2 = 21.08, df = 12, p = .049).
Subsequent pairwise comparisons revealed that at 3 months, the activity of the left foot increased significantly compared to baseline (M LF/Basel = 31.18) in the left hand (M LF/LHStim = 48.55), right hand (M LF/RHStim = 49.45), and right foot (M LF/RFStim = (50.82) stimulation conditions (p = .011, p = .002, and p < .000, respectively). In other words, the activity of the left foot increased significantly in three conditions of four.
At 4 and 5 months, the activity of the left foot did not change significantly across conditions.
At 6 months, infants moved their left foot significantly more than baseline (M LF/Basel = 37.7) only when the left foot was stimulated (M LF/LFStim = 58.3, p < .000).
Right foot
The GEE analysis (N = 43) revealed a significant main effect of stimulation condition (Wald χ2 = 11.31, df = 4, p = .023) on the activity of the right foot. We also found a significant interaction between stimulation condition and age (Wald χ2 = 23.52, df = 12, p = .024).
Subsequent pairwise comparisons revealed that at 3 months, the activity of the right foot increased significantly compared to baseline (M RF/Basel = 34.36) in the left hand (M RF/LHStim = 53.55) and right foot (M RF/RFStim = 49) stimulation conditions (p = .001 and p = .028, respectively). In other words, the activity of the right foot increased significantly in two conditions of four.
At 4 and 5 months, the activity of the right foot did not change significantly across conditions.
At 6 months, infants moved their right foot significantly more than baseline (M RF/Basel = 41.1) only when the left foot was stimulated (M RF/LFStim = 58, p = .001).
Summary
We can therefore conclude that at 5 months, the right hand emerged as the most responsive, increasing its activity compared to its own baseline only when it was stimulated and not in other conditions. The activity of the left hand also increased its specificity by this age and increased its activity compared to its own baseline when it was stimulated, but also when the left foot was stimulated, showing that response to stimulation of the left hand was more generalized than that of the right hand at this age. By 6 months of age, the left foot also produced a specific activity pattern, increasing its activity compared to its own baseline only when it was stimulated. The right foot responded less selectively, increasing its activity when the left foot was stimulated.
References
- Bahrick, L. E. , & Watson, J. S. (1985). Detection of intermodal proprioceptive–visual contingency as a potential basis of self‐perception in infancy. Developmental Psychology, 21, 963–973. 10.1037/0012-1649.21.6.963 [DOI] [Google Scholar]
- Begum Ali, J. , Spence, C. , & Bremner, A. J. (2015). Human infants’ ability to perceive touch in external space develops postnatally. Current Biology, 25, 978–979. 10.1016/j.cub.2015.08.055 [DOI] [PubMed] [Google Scholar]
- Bremner, A. J. , Mareschal, D. , Lloyd‐Fox, S. , & Spence, C. (2008). Spatial localization of touch in the first year of life: Early influence of a visual spatial code and the development of remapping across changes in limb position. Journal of Experimental Psychology: General, 137, 149‐162. [DOI] [PubMed] [Google Scholar]
- Bushnell, E. W. , & Boudreau, J. P. (1993). Motor development and the mind: The potential role of motor abilities as a determinant of aspects of perceptual development. Child Development, 64, 1005–1021. 10.2307/1131323 [DOI] [PubMed] [Google Scholar]
- Corbetta, D. , Thurman, S. L. , Wiener, R. , Guan, Y. , & Williams, J. L. (2014). Mapping the feel of the arm with the sight of the object: On the embodied origins of infant reaching. Frontiers in Psychology, 5 576 10.3389/fpsyg.2014.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, H. , Cowie, D. , Karmiloff‐Smith, A. , & Bremner, A. J. (2016). Specialization of the motor system in infancy: From broad tuning to selectively specialized purposeful actions. Developmental Science, 20(4). 10.1111/desc.12409 [DOI] [PubMed] [Google Scholar]
- Filippetti, M. L. , Johnson, M. H. , Lloyd‐Fox, S. , Dragovic, D. , & Farroni, T. (2013). Body perception in newborns. Current Biology, 23(23), 2413–2416. 10.1016/j.cub.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippetti, M. L. , Lloyd‐Fox, S. , Longo, M. R. , Farroni, T. , & Johnson, M. H. (2015). Neural mechanisms of body awareness in infants. Cerebral Cortex, 25, 3779–3787. 10.1093/cercor/bhu261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippetti, M. L. , Orioli, G. , Johnson, M. H. , & Farroni, T. (2015). Newborn body perception: Sensitivity to spatial congruency. Infancy, 20(4), 455–465. 10.1111/infa.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano, M. S. A. , & Botvinick, M. M. (2002). How the brain represents the body: Insights from neurophysiology and psychology. Common Mechanisms in Perception and Action: Attention and Performance, 19, 136–157. https://doi.org/citeulike-article-id:10048109 [Google Scholar]
- Heathcock, J. C. , Bhat, A. N. , Lobo, M. A. , & Galloway, J. C. (2005). The relative kicking frequency of infants born full‐term and preterm during learning and short‐term and long‐term memory periods of the mobile paradigm. Physical Therapy, 85(1), 8–18. 10.1093/ptj/85.1.8 [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Chinn, L. K. , Somogyi, E. , Heed, T. , Fagard, J. , Lockman, J. J. , & O'Regan, J. K. (2017). Development of reaching to the body in early infancy: From experiments to robotic models. IEEE International Conference on Development and Learning and Epigenetic Robotics (ICDL), 112–119.
- Leed, J. (2014). Canonical Body Knowledge, Perceptuo‐motor Coordination, and Tactile Localization. Thesis Dissertation. Tulane University.
- Lew, A. R. , & Butterworth, G. (1997). The development of hand‐mouth coordination in 2‐ to 5‐month‐old infants: Similarities with reaching and grasping. Infant Behavior and Development, 20(1), 59–69. 10.1016/S0163-6383(97)90061-8 [DOI] [Google Scholar]
- van der Meer, A. L. , van der Weel, F. R. , & Lee, D. N. (1995). The functional significance of arm movements in neonates. Science, 267, 693–695. 10.1126/science.7839147 [DOI] [PubMed] [Google Scholar]
- Myowa‐Yamakoshi, M. , & Takeshita, H. (2006). Do Human fetuses anticipate self‐oriented actions? A study by four‐dimensional (4D) ultrasonography. Infancy, 10(3), 289–301. 10.1207/s15327078in1003_5 [DOI] [Google Scholar]
- Rochat, P. (1993). Hand‐mouth coordination in the newborn: Morphology, determinants, and early development of a basic act In Geert J. P. S. (Ed.), Advances in psychology, 97, pp. 265–288: Provincie, NH: Elsevier. [Google Scholar]
- Rochat, P. , & Morgan, R. (1995). Spatial determinants in the perception of self‐produced leg movements in 3‐ to 5‐month‐old infants. Developmental Psychology, 31, 626–636. 10.1037/0012-1649.31.4.626 [DOI] [Google Scholar]
- Rochat, P. , & Striano, T. (2000). Perceived self in infancy. Infant Behavior and Development, 23(3–4), 513–530. 10.1016/S0163-6383(01)00055-8 [DOI] [Google Scholar]
- Rovee, C. K. , & Rovee, D. T. (1969). Conjugate reinforcement of infant exploratory behavior. Journal of Experimental Child Psychology, 8(1), 33–39. 10.1016/0022-0965(69)90025-3 [DOI] [PubMed] [Google Scholar]
- Rovee‐Collier, C. K. , Morrongiello, B. A. , Aron, M. , & Kupersmidt, J. (1978). Topographical response differentiation and reversal in 3‐month‐old infants. Infant Behavior and Development, 1, 323–333. 10.1016/S0163-6383(78)80044-7 [DOI] [Google Scholar]
- Saby, J. N. , Meltzoff, A. N. , & Marshall, P. J. (2015). Neural body maps in human infants: Somatotopic responses to tactile stimulation in 7‐month‐olds. NeuroImage, 118, 74–78. 10.1016/j.neuroimage.2015.05.097 [DOI] [PubMed] [Google Scholar]
- Schmuckler, M. A. (1996). Visual‐proprioceptive intermodal perception in infancy. Infant Behavior and Development, 19(2), 221–232. 10.1016/S0163-6383(96)90021-1 [DOI] [Google Scholar]
- Thomas, B. L. , Karl, J. M. , & Whishaw, I. Q. (2014). Independent development of the Reach and the Grasp in spontaneous self‐touching by human infants in the first 6 months. Frontiers in Psychology, 5, 1526 10.3389/fpsyg.2014.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, H. , & Taga, G. (2006). General to specific development of movement patterns and memory for contingency between actions and events in young infants. Infant Behavior and Development, 29(3), 402–422. 10.1016/j.infbeh.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Watanabe, H. , & Taga, G. (2009). Flexibility in infant actions during arm‐ and leg‐based learning in a mobile paradigm. Infant Behavior and Development, 32(1), 79–90. 10.1016/j.infbeh.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Weinstein, S. (1968). Intensive, extensive aspects of tactile sensitivity as a function of body part, sex and laterality In Kenshalo D. & Charles C. (Eds.), The skin senses (pp. 195–222). Springfield, IL: Thomas. [Google Scholar]
- Zoia, S. , Blason, L. , D'Ottavio, G. , Bulgheroni, M. , Pezzetta, E. , Scabar, A. , & Castiello, U. (2007). Evidence of early development of action planning in the human foetus: A kinematic study. Experimental Brain Research, 176(2), 217–226. 10.1007/s00221-006-0607-3 [DOI] [PubMed] [Google Scholar]


