Abstract
Cognitive frailty, defined as the coexistence of mild cognitive impairment symptoms and physical frailty phenotype in older persons, is increasingly considered the main geriatric condition predisposing to dementia. Recent studies have demonstrated that gut microbiota may be involved in frailty physiopathology by promoting chronic inflammation and anabolic resistance. The contribution of gut microbiota to the development of cognitive impairment and dementia is less defined, even though the concept of “gut–brain axis” has been well demonstrated for other neuropsychiatric disorders. The aim of this systematic review was to summarize the current state-of-the-art literature on the gut microbiota alterations associated with cognitive frailty, mild cognitive impairment and dementia and elucidate the effects of pre- or probiotic administration on cognitive symptom modulation in animal models of aging and human beings. We identified 47 papers with original data (31 from animal studies and 16 from human studies) suitable for inclusion according to our aims. We concluded that several observational and intervention studies performed in animal models of dementia (mainly Alzheimer’s disease) support the concept of a gut–brain regulation of cognitive symptoms. Modulation of vagal activity and bacterial synthesis of substances active on host neural metabolism, inflammation and amyloid deposition are the main mechanisms involved in this physiopathologic link. Conversely, there is a substantial lack of human data, both from observational and intervention studies, preventing to formulate any clinical recommendation on this topic. Gut microbiota modulation of cognitive function represents, however, a promising area of research for identifying novel preventive and treatment strategies against dementia.
Keywords: microbiome, Alzheimer’s disease, vascular dementia, mild cognitive impairment, dysbiosis
Introduction
Cognitive frailty and dementia in the clinical setting
Cognitive frailty is a geriatric condition defined as the coexistence of the physical frailty syndrome and cognitive impairment, in the absence of a clinical diagnosis of Alzheimer’s disease (AD) or other types of dementia.1 This condition has gained great attention from the scientific community in the last decade, and is being studied more for its physiopathologic, epidemiologic and clinical aspects.2–4
Several studies, which have been comprehensively reviewed by Panza et al, support the concept that cognitive frailty is a clinical entity distinct from the classical construct of the “frailty syndrome”, in spite of a significant overlap between the two conditions.3 The frailty concept, operationalized according to either the Fried phenotype model or the Rockwood deficit accumulation model, is, in fact, mainly centered on measures of physical performance and presence of functional deficits.5 However, population-based studies have shown that, among community-dwelling older individuals diagnosed with frailty, those carrying signs of mild cognitive impairment (MCI) have poorer outcomes, supporting cognitive frailty as a distinct physiopathologic and clinical syndrome.6,7
As such, clinical tools for frailty risk assessment are increasingly incorporating cognition and measures of cognitive function,8 supporting the need for a combined assessment of motoric and cognitive function for frailty identification in real-world clinical settings.9 Disentangling physical and cognitive frailty phenotypes may be particularly challenging in clinical practice, since motoric and cognitive symptoms may coexist. Older community dwellers with cognitive impairment often have low gait speed, which represents one of the frailty phenotype criteria.10 Low gait speed is, in fact, the main motoric manifestation of cognitive frailty.11
Even if there is an international consensus on cognitive frailty definition,1 the operative diagnostic criteria are not completely standardized across clinical and epidemiologic studies. The critical issues concern both the evaluation of cognitive symptoms (diagnosis of MCI or clinical dementia rating scale score of 0.5) and of frailty (Fried criteria or a modified version of Cardiovascular Health Study criteria). The criteria used in the Italian Longitudinal Study on Aging7 are reported in Table 1. In this study, MCI was diagnosed using a battery of neuropsychological tests and not the clinical dementia rating scale,7 in line with the most recent consensus recommendations.12 However, in other studies, a Mini-Mental State Examination test score ≤25 was used as a simplified method for assessing cognitive symptoms.13
Table 1.
Summary of the clinical criteria for cognitive frailty assessment
| Presence of mild cognitive impairment (all the following criteria must be fulfilled) |
| • Cognitive concern with a change in cognition reported by the patient or caregivers |
| • Objective evidence of impairment in one or more cognitive domains, including memory, at neuropsychological tests |
| • Preservation of independence in functional abilities |
| • No evidence of dementia |
|
|
| Absence of an identifiable cause of cognitive impairment in personal history |
|
|
| Presence of frailty phenotype according to the Fried criteria modified by the Cardiovascular Health Study criteria |
| (At least three out of five criteria must be fulfilled) |
| • Unintentional weight loss >5 kg in the last year, with affirmative response to the question, “Do you think that your clothes are wide?” |
| • Exhaustion, defined as Geriatric Depression Scale-30 score ≥10 and negative answer to the question, “Do you feel full of energy?” |
| • Weakness with inability to stand from a chair unaided and without using the arms |
| • Slowness (time ≥7 seconds to walk 5 m) |
| • Low physical activity (patient reporting being inactive or performing only light physical activity) |
In cross-sectional studies, cognitive frailty was associated with higher prevalence of obesity, low functional performance and disability than physical frailty.13–15 In longitudinal studies, it also predicted several patient-centered outcomes, including disability, malnutrition, diagnosis of dementia, hospital admission and length of stay, nursing home admission and mortality.11,16–21
Cognitive frailty may be reversible, particularly in those patients where cognitive frailty is diagnosed based only on subjective cognitive decline (clinical dementia rating scale score 0.5) in the absence of a certified MCI.7 Early identification of this condition, through measurement of reliable biomarkers, is thus pivotal for secondary prevention of dementia.22,23 Understanding the pathophysiology of cognitive frailty represents a priority of geriatric research and could help elucidate some important mechanisms of dementia development.
Frailty and microbiota
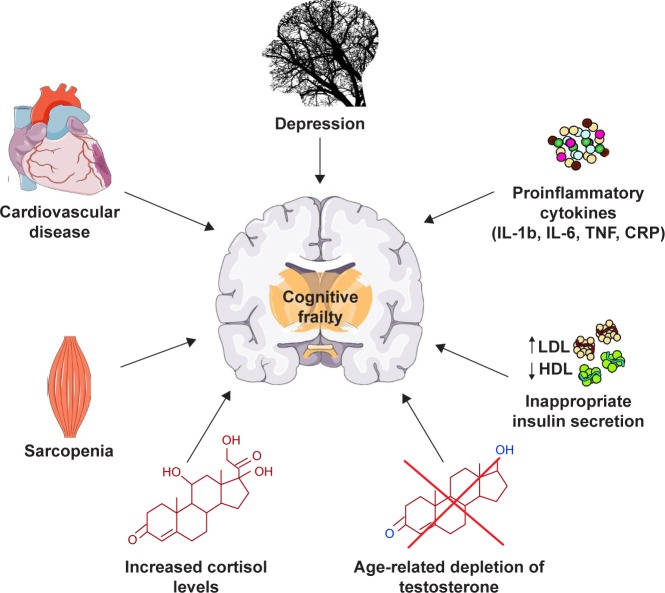
The main factors possibly involved in the onset of cognitive frailty are specific personality traits, depression, cardiac dysfunction, cardiovascular diseases, sarcopenia, dyslipidemia, insulin resistance, malnutrition and its precursor anorexia of aging, chronic inflammation and hormonal dysregulation including hypogonadism and hypovitaminosis D (Figure 1).3 Interestingly, most of these factors have been associated with alterations in the composition of the gut microbiota, that is, the ensemble of bacteria, fungi, viruses, protozoa and archaea symbiotically living in the distal human gastrointestinal tract.24
Figure 1.
Schematic overview of the possible conditions and physiopathologic alterations associated with the onset and progression of cognitive frailty.
Abbreviations: CRP, C-reactive protein; HDL, high-density lipoprotein; IL, interleukin; LDL, low-density lipoprotein; TNF, tumor necrosis factor.
Changes in the gut microbiota composition have been recently associated with several human diseases and conditions, involving not only the gastroenteric system but also other distant organs.25 During aging, gut microbiota composition physiologically faces reduced species richness and increased interindividual variability.26 In 2012, Claesson et al demonstrated that these alterations are particularly observed in older subjects with physical frailty and living in nursing home, partly because of reduced mobility and variety in dietary habits.27
Since then, other studies have confirmed that the fecal microbiota of older individuals with physical frailty, mobility limitations and multimorbidity experience lower biodiversity and reduced representation of taxa with purported beneficial activity for the host physiology.28–32 In fact, many microbial taxa, underrepresented in frail older persons, may produce metabolic mediators, such as short-chain fatty acids (SCFAs), modulating inflammation and improving insulin sensitivity and anabolic responses in the host physiology.33
On these grounds, some research groups have recently hypothesized an involvement of the gut microbiota in the physiopathology of physical frailty and age-related sarcopenia.34–36 A “gut–muscle axis” may, in fact, exist, with microbial mediators influencing skeletal muscle anabolism and, in turn, physical exercise favoring gut microbiota biodiversity.34–36
On the other side, the characteristics of gut microbiota in patients with cognitive frailty and their possible involvement in dementia are someway less defined. The presence of a gut–brain crosstalk has been, however, demonstrated in intestinal diseases,37 cirrhosis38 and psychiatric disorders.39 Thus, an influence of gut microbiota in the physiopathology of cognitive frailty, MCI and dementia can be hypothesized.40
Aims
The aim of the present review is to summarize the current state-of-the-art literature on the gut microbiota alterations associated with cognitive frailty, MCI and dementia in animal models of aging and older people. It also aims to clarify the role of microbiota modifications, through administration of pre- or probiotics, in modulating the course of cognitive symptoms in both settings.
Materials and methods
A systematic literature search was performed on public databases (PubMed, Scopus). All articles published from January 1, 2004, to April 30, 2018, were considered for inclusion. The literature published before this interval was not considered since it was the period before next-generation sequencing techniques boosted the research on gut microbiota. Search queries were the following: “cognitive frailty” AND microbiota OR microbiome; “mild cognitive impairment” AND microbiota OR microbiome; “cognitive impairment” AND microbiota OR microbiome; dementia AND microbiota OR microbiome; Alzheimer AND microbiota OR microbiome; Alzheimer’s AND microbiota OR microbiome; “gut-brain axis” AND aging; frailty AND microbiota OR microbiome; “cognitive impairment” AND probiotics; dementia AND probiotics; Alzheimer AND probiotics; Alzheimer’s AND probiotics; frailty AND probiotics.
The results for each query were individually checked for relevance within the scopes of the present review by reading titles and abstracts. Duplicate papers, that is, papers retrieved with different queries, were considered only once. Papers were excluded if the topic was not adherent to the theme of gut microbiota composition in patients or animal models with cognitive frailty, MCI or dementia. Papers with main text not in English language were excluded as well. Reviews were not included in the systematic literature analysis. However, they are considered and cited in the “Discussion” section if they provided original contributions in terms of hypotheses on the possible role of gut microbiota in modulating cognitive function and dementia physiopathology and the underlying mechanisms. Thus, only papers with original data on animal models or human patients were included in the results, and the full text was analyzed to retrieve the relevant information.
Results
Literature search
A total of 312 papers fulfilled the search queries and were thus considered for inclusion in this review. However, 206 of them were excluded since the topic did not fall within the scope of the present review, or because the main text was not in English language. Another 59 papers were review articles and were considered only for literature discussion. Thus, 47 original papers (16 with data from human subjects and 31 with data from animal models) were included in the present analysis. Studies on animal models and human beings were considered separately. Moreover, a distinction between observational/descriptive studies, not implying the administration of pre- or probiotics and exploring only the physiopathologic aspects of gut–brain axis, and intervention studies, with administration of pre- or probiotics, was also made.
Observational/descriptive studies on animal models
In aging mice, deficits in spatial memory and increases in anxious behavior may arise, indicating progression toward a neurodegenerative disorder similar to human dementia. In a observational study considering mice of different ages, these symptoms were correlated with increased gut permeability, increased systemic inflammation and overrepresentation of several bacterial taxa in the cecal microbiota, including Porphyromonadaceae, Odoribacter, Butyricimonas, Clostridium and Oxalobacter, a microbiome profile resembling to that of murine inflammatory bowel disease.41 The presence of gut microbiota dysbiosis also negatively influenced the immunological response and clinical outcomes of experimental mouse models of stroke, resulting in worse motoric and cognitive symptoms than those of mice with a healthy microbiota profile.42
Experimental models of mice genetically prone to AD, such as the transgenic APP/PS1 breed, generally have a normal gut microbiota profile in the young age, but a shift toward lower microbiota biodiversity and overrepresentation of taxa with proinflammatory activity, such as Odoribacter, Helicobacter and Sutterella, occurs with aging, according to two different studies.43,44 These mice also show dramatically increased gut mucosa permeability, with several bacteria-derived membrane vesicles that can be found in the blood and could influence amyloid plaque deposition in the brain.45 Interestingly, amyloid deposition was also observed in the gut tissue of murine AD models and it correlated with dysbiosis.46 In a recent observational study, mice genetically prone to AD exhibited a distinct fecal microbiota composition than healthy controls, with reduced biodiversity and altered functionality.47 Especially, the production of SCFAs by the gut microbiota was reduced, with possible negative effects on amyloid deposition in both the brain and the gut.47
Absence of the physiological bacterial diversity between cecum and colon, overrepresentation of Firmicutes and decreased microbial metabolism of unsaturated fatty acids and choline, worsened by high-fat diets, were also demonstrated in 3xtg mice, another transgenic model of dementia.48 Even in Drosophila models of dementia, the presence of intestinal dysbiosis was associated with worsened inflammation and activation of brain neurodegeneration pathways mediated by TNF-JNK.49
Other studies investigated the effects of induced gut microbiota dysbiosis on cognitive function and gut–brain signaling of mouse models of dementia.50–54 The results, summarized in Table 2, highlight the role of gut microbiota in regulating neuroinflammation, cerebral amyloid deposition and clearance, hippocampal synthesis of mediators or receptors involved in neurotransmission.50–54 Bacterial metabolites and circulating miRNAs have been hypothesized as playing a pivotal role in this gut–brain crosstalk.52,53 Interestingly, antibiotic-induced dysbiosis may not necessarily imply unfavorable consequences on brain pathology. In fact, Minter et al showed that antibiotic administration to mice genetically prone to AD during their postnatal development resulted in reduced deposition of Aβ amyloid in the brain.54
Table 2.
Summary of animal studies investigating the effects of induced gut microbiota dysbiosis on the host physiology and cognitive function
| First author, journal, year (ref) | Animal model | Exposure | Outcomes measured | Main results | Proposed mechanisms of intestinal microbiota involvement in dementia physiopathology |
|---|---|---|---|---|---|
| Harach, Sci Rep, 201750 | APPPS1 transgenic mice | Germ-free mice obtained by IVF and bred in aseptic environment | Cerebral amyloid deposition | Lower cerebral amyloid deposition in germ-free mice than controls; increased amyloid deposition in germ- free mice receiving fecal microbiota transplantation from controls | Gastrointestinal bacteria may promote subclinical neural inflammation facilitating amyloid deposition |
| Minter, Sci Rep, 201651 | APPSWE/PS1∆E9 transgenic mice | Long-term broad- spectrum combinatorial antibiotic therapy | Aβ amyloid deposition Soluble Aβ levels Cerebral glial reactivity Circulating cytokine and chemokine levels | Antibiotic-treated mice showed higher circulating cytokine and chemokine, reduced cerebral Aβ plaque deposition, increased glial activity and Aβ circulating levels | Gut dysbiosis may facilitate neuroinflammation with positive consequences in the acute phase (reduced Aβ plaque deposition) and detrimental consequences in the long term |
| Fröhlich, Brain Behav Immun, 201652 | Adult mice | Intragastric treatment with multiple antibiotics | Metabolic profile of the colon Microbial circulating metabolites Expression of neuronal signaling molecules Cognitive behavior | Gut microbiota dysbiosis associated with decreased colonic and circulating microbial metabolites, reduced neural signaling-related molecules in the brain, increased cytokine representation in the amygdala and hippocampus and impaired novel object recognition memory | Microbial metabolite depletion in the gut may act as endocrine messengers, altering neural signaling molecule (BDNF, NMDA receptor, serotonin transporter, NPY) synthesis particularly in the amygdala and hippocampus |
| Cui, BBA Mol Basis Dis, 201753 | C57BL/6J mice | Total abdominal irradiation | Intestinal and circulating miRNAs Brain BDNF expression Gut microbiota composition | Irradiation-induced cognitive deficits, gut microbiota dysbiosis, overexpression of circulating miR-34a-5p and reduced expression of hippocampal BDNF Injection of miR-34a-5p antagonists restored BDNF production and gut microbiota eubiosis | Gut microbiota may mediate cognitive function through miRNA expression (particularly miR-34a-5p) – a bidirectional gut–brain crosstalk mediated by miRNAs may exist |
| Minter, Sci Rep, 201754 | APPSWE/PS1∆E9transgenic mice | Postnatal antibiotic treatment | Gut microbiota composition Brain Aβ deposition Foxp3+ Treg cells in blood and brain Serum and cerebrospinal fluid inflammation | Postnatal antibiotic treatment induced stable perturbations in gut microbiota diversity, with reduced species richness and increased representation of Lachnospiraceae, upregulation of circulating and brain Foxp3+ Treg cells and reduced deposition of brain Aβ amyloid | Gut microbiota may mediate neuroinflammation through production of butyrate and differentiation of T-cells in to Foxp3+ Treg cells |
Abbreviations: BDNF, brain-derived neurotrophic factor; NMDA, N-methyl-D-aspartic acid; Treg, T regulatory; IVF, in vitro fertilization; NPY, neuropeptide Y.
Observational studies on humans
The role of gut microbiota alterations in driving cognitive symptoms of human subjects was first studied in the context of advanced cirrhosis. In the fecal microbiota of a small group of patients with hepatic encephalopathy, Bajaj et al found overrepresentation of Enterobacteriaceae and Fusobacteriaceae, and identified some taxa, namely Alcaligenaceae and Porphyromonadaceae, whose relative abundance was positively correlated with cognitive impairment measured with neuropsychological tests.55 In a later case–control study, the same authors found that cognitive performance of elderly patients was negatively correlated with the relative abundance of Enterobacteriaceae and Porphyromonadaceae and positively correlated with the Lactobacillales relative abundance, irrespective of the presence of cirrhosis.56 Finally, liver transplantation proved to be able to reduce gut microbiota dysbiosis associated with advanced cirrhosis, with a simultaneous improvement in cognitive symptoms.57 The presence of a gut–brain axis modulating cognitive function in gastrointestinal and liver diseases is also supported by the observation that rifaximin administration is associated with cognition improvement in cirrhosis,58 and that irritable bowel syndrome, a functional disorder implying mild gut microbiota dysbiosis, is associated with an increased risk of dementia.59
Most studies exploring the putative role of gut microbiota in the pathogenesis of dementia did not include metagenomics profiling of fecal microbiota, but were focused on the detection of microbial metabolites in blood or brain tissue samples. For example, Xu and Wang analyzed public anonymous databases of genetic and metabolic data to identify microbial metabolites associated with cognitive decline in AD.60 They found that three of them, that is, mannitol, succinic acid and 3,4-dihyfroxybenzeneacetic acid, are particularly overrepresented in subjects with AD, suggesting the presence of a different gut microbiota profile.60 In 59 patients with overt dementia or MCI, Andreadou et al demonstrated high serum and cerebrospinal fluid levels of rhamnolipids, representing exotoxins and virulence factors produced by Gram-negative bacteria.61 Similarly, the presence of Gram-negative bacteria–derived lipopolysaccharide (LPS) was recently detected in postmortem hippocampal and temporal neocortex tissue extracts from six patients with AD.62 These findings allow to hypothesize the presence of a distinct gut microbiota signature in AD, characterized by dysbiosis with overrepresentation of Gram-negative pathogens.
Interestingly, in another study performed on 20 patients and 13 controls, polymerase chain reaction analysis of fecal samples allowed to identify genome sequences from Clostridium spp. specific of patients with AD.63 These sequences encoded for NADH:ubiquinone oxidoreductase, an enzyme involved in aromatic amino acid production.63 High levels of these amino acids in the brain may induce cell death and neurodegeneration, and thus, the gut microbiota functionality may directly influence the AD physiopathology through this pathway.
A comprehensive microbiota analysis was performed on fecal samples from patients with dementia in only four studies. Araos et al64 used a 16S rRNS microbial profiling approach and found an extreme reduction of microbiota biodiversity, particularly in those subjects with colonization with Clostridium difficile. Cattaneo et al65 instead compared the fecal microbiota composition of 40 amyloid-positive patients with cognitive impairment, 33 amyloid-negative patients with cognitive impairment and 10 healthy controls using a quantitative-polymerase chain reaction approach. They found lower abundance of Eubacterium rectale and higher abundance of Escherichia/Shigella in amyloid-positive patients, and these gut microbiota differences were correlated with the systemic inflammatory profile. Thus, the authors hypothesized a role of the gut microbiota in regulating amyloid deposition in the brain through modulation of inflammation.65 Vogt et al analyzed fecal samples from 25 patients with AD and 25 controls, demonstrating a reduced representation of Firmicutes and Bifidobacteria and an increased representation of Bacteroidetes in the fecal microbiota of demented patients.66 They also showed that the cerebrospinal fluid levels of AD markers, such as Aβ42/Aβ40 and YKL-40, were significantly correlated with the relative abundance of Bacteroides and Blautia in a positive way and of Dialister and Turicibacter in a negative way.66 The presence of gut microbiota dysbiosis in patients with AD was also recently confirmed by Zhuang et al, who analyzed fecal samples from 43 patients with AD and 43 controls and demonstrated similar alterations, including overexpression of Bacteroides.67
Moreover, Qjan et al recently demonstrated that the relative abundance of Bifidobacterium, Butyricicoccus and Clostridium XIVb in gut microbiota was negatively correlated with the presence of cognitive impairment in patients with Parkinson’s disease, a neurodegenerative condition often correlated with dementia.68
Intervention studies on animals
Probiotics were administered to animal models of neuroinflammation, AD or vascular dementia in eleven studies,69–79 whose results have been summarized in Table 3. In all these studies, the administration of single bacterial taxa or probiotic blend determined reduced decline of cognitive function, measured in mice mainly with the Morris water maze test, which specifically explores spatial memory tasks. Multiple tests of mouse cognitive function were performed in only one study,74 where a probiotic blend containing nine bacterial strains was administered to transgenic AD mice. The probiotic intervention showed improved performance in all the cognitive tests and also reduced anxiety-like behaviors.74 From a physiopathologic point of view, probiotic interventions showed improvements, or reduced deterioration, in many other aspects of dementia, including cortical and hippocampal expression of genes involved in neural plasticity and inflammation control, excitatory postsynaptic potentials, activation of molecular pathways protecting against apoptosis (such as BDNF-p13K/Akt), histological signs of cell damage and amyloid deposition, and neurotransmitter and cytokine levels (Table 3). Overall, these experiments support the concept of a gut–brain axis in dementia, modulating brain histology and function through influence in gene expression and inflammation control.
Table 3.
Summary of animal intervention studies investigating the effects of probiotic formulations on dementia-related outcomes
| First author, journal, year (ref) | Animal model | Exposure | Duration | Dementia-related outcomes measured | Main results |
|---|---|---|---|---|---|
| Davari, Neuroscience, 201369 | Rats with streptozocin-induced diabetes | Probiotic blend with Lactobacillus acidophilus, Bifidobacterium lactis, Lactobacillus fermentum vs placebo | 2 months | Spatial learning tasks (Morris water maze test) Basic and potentiated EPSPs in the CA1 area of hippocampus | Improvement in spatial learning tasks Maintenance of basic and potentiated EPSPs (decline observed in controls) |
| Distrutti, PLoS One, 201470 | Young and aged male Wistar rats | VSL#3 probiotic blend vs maple syrup (control) | 6 weeks | EPSPs in dentate gyrus after long-term potentiation shock test Expression of cortical tissue genes detected by microarray analysis | Improvement of cortical expression of genes involved in inflammation modulation and neural plasticity (BDNF, synapsin among others) Attenuation of the age-related deficit in EPSPs after long- term potentiation shock test |
| Liu, Biomed Res Int, 201571 | Mice with vascular dementia induced by a permanent unilateral right common carotid artery occlusion | Clostridium butyricum | 6 weeks | Spatial learning tasks (Morris water maze test) Histological signs of neuronal apoptosis in hippocampal tissue BDNF-P13K/Akt pathway protein expression | Improvement in spatial learning tasks Reduced signs of hippocampal neuron apoptosis Activation of BDNF-P13K/Akt pathway-related proteins |
| Liang, Neuroscience, 201572 | Sprague Dawley rats subjected to restraint stress | Lactobacillus helveticus NS8 | 3 weeks | Anxiety behaviors and memory tasks Plasma cortisol, ACTH and cytokine levels BDNF mRNA expression in prefrontal cortex and hippocampus Brain monoamine neurotransmitters | Reduction of anxiety behaviors and memory dysfunction induced by stress Lower plasma cortisol and ACTH levels, higher IL-10 levels Higher hippocampal BDNF expression |
| Sun, Brain Res, 201673 | Mice with streptozocin-induced diabetes subjected to 30 minutes of bilateral common carotid artery occlusion | C. butyricum | 6 weeks | Spatial learning tasks (Morris water maze test) Hippocampal neuron damage and apoptosis Brain expression of Akt, phospho-Akt, caspase-3 | Attenuation of decline in spatial learning tasks Attenuation of hippocampal neuron apoptosis Increased expression of Akt, phosphor-Akt and caspase-3, limiting apoptosis in hippocampal neurons |
| Bonfili, Sci Rep, 201774 | 3xTg-AD transgenic mice with early-stage Alzheimer’s disease | SLAB51 probiotic | 4 months | Multiple tests of | Reduced decline in |
| blend containing nine strains (bifidobacteria, lactobacilli and Streptococcus thermophilus) | locomotor activity, recognition memory, fear-motivated reactions, anxiety-related behaviors Amyloid brain deposition Proteasome activity in brain homogenates | performance at cognitive and anxiety-related tests Reduced amyloid accumulation Increased expression of neuronal proteolytic pathways | |||
| Nimgampalle, J Clin Diagn Res, 201775 | Albino rats with D-galactose induced Alzheimer’s disease | Lactobacillus plantarum | 2 months | Spatial learning tasks (Morris water maze test) Gross behavioral activity Brain neuron degeneration Brain acetylcholine level | Improved spatial learning tasks and gross behavior activity Reduced neuron degeneration Maintenance of acetylcholine levels |
| Kobayashi, Sci Rep, 201776 | ddY mice with Aβ injection-induced Alzheimer’s disease | Bifidobacterium breve strain A1 | 6 days | Alternation behavior in Y maze test Latency time in passive avoidance test Hippocampal neuron gene expression | Prevention of decline in cognitive dysfunction measured with alternation behavior and passive avoidance test Reversal of Aβ-induced change in hippocampal gene expression (reduced expression of proinflammatory genes) |
| Musa, J Dairy Res, 201777 | ICR mice with neuroinflammation induced by lipopolysaccharide brain injection | Lactic acid bacteria probiotic blend derived from fresh cow milk | 28 days | Spatial learning tasks (Morris water maze test) Acetylcholine, antioxidant and proinflammatory cytokine levels in brain lysate | Attenuation of neuroinflammation-induced decline in spatial learning tasks Increase of acetylcholine and antioxidant brain levels, reduction of proinflammatory cytokines |
| Chunchai, J Neuroinflammation, 201878 | Male Wistar rats fed with high-fat diet- induced dementia | Lactobacillus paracasei | 12 weeks | Spatial learning tasks (Morris water maze test) Microglial activation Brain mitochondrial function Hippocampal plasticity, oxidative stress, apoptosis | Improvement of high-fat diet- induced impairment in spatial learning tasks Reduced microglial activation Improved brain mitochondrial dysfunction Restored hippocampal plasticity and reduced oxidative stress and apoptosis |
| Athari Nik Azm, Appl Physiol Nutr Metab, 201879 | Mice with Aβ injection-induced Alzheimer’s disease | Probiotic blend with L. acidophilus, L. fermentum, B. lactis, Bifodobacterium longum | 8 weeks | Spatial learning tasks (Morris water maze test) Hippocampal oxidative stress biomarkers | Reduced decline in spatial learning tasks Improvement in oxidative stress biomarkers (malondialdehyde, superoxide dismutase) |
Abbreviations: ACTH, adrenocorticotropic hormone; BDNF, brain-derived neurotrophic factor; EPSP, excitatory postsynaptic potential; IL-10, interleukin-10.
In other six intervention studies,79–84 the effects of gut microbiota on animal cognitive function or dementia-related outcomes were explored by administering prebiotics, that is, functional foods or nutrition supplements inducing the selective growth of specific gut microbiota populations. The results of these studies are summarized in Table 4. The presence of a gut–brain axis modulating dementia physiopathology was first demonstrated with the circumstance that urolithins, gut microbiota-derived metabolites from grape seed ellagitannins, can improve β-amyloid aggregation and signs of cognitive impairment in animal models.80,81 Then, the administration of other prebiotics, including teasaponin, fructo-oligosaccharides, baicalein and xylo-oligosaccharides, proved effective in preventing the advancement of cognitive symptoms in mouse models of AD or vascular dementia.79,82–84
Table 4.
Summary of animal intervention studies investigating the effects of prebiotic formulations or functional foods on dementia-related outcomes
| First author, journal, year (ref) | Animal model | Exposure | Duration | Dementia-related outcomes measured | Main results |
|---|---|---|---|---|---|
| Wang, Mol Nutr Food Res, 201580 | Male Sprague Dawley rats | Grape seed polyphenol extract | 11 days | Brain concentration of phenolic acids generated by gut microbiota metabolism of grape seed anthocyanidins In vitro β-amyloid aggregation | Elevated phenolic compound concentrations in the brain In vitro inhibition of β-amyloid aggregation induced by the same phenolic compound concentrations |
| Yuan, ACS Chem Neurosci, 201681 | Transgenic Caenorhabditis elegans with AD induced by brain β-amyloid injection | Pomegranate extracts containing ellagitannins; extracts containing urolithins (gut microbiota-derived ellagitannin metabolites) | 20 hours | Survival and speed of mobility decline | Reduced decline of mobility in worms treated with urolithins, but not in worms treated with pomegranate extracts No effect on survival time |
| Wang, Sci Rep, 201782 | C57BL/6J male mice with high- fat diet-induced obesity and cognitive decline | Teasaponin (terpenic glycoside derived from tea) | 6 weeks | Object recognition performance Recognition memory assessed with discrimination index BDNF levels in the hippocampus | Reduced decline of recognition memory and object recognition performance Decreased decline in BDNF expression in the hippocampus |
| Chen, Front Aging Neurosci, 201783 | Rats with D-galactose and β-amyloid administration- induced AD | Fructo-oligosaccharides from Morinda officinalis | 4 weeks | Spatial learning tasks (Morris water maze test) Brain levels of cytokines, antioxidants, and neurotransmitters Brain tissue histology, including amyloid deposition | Improved spatial learning tasks Improved oxidative stress, inflammation and synthesis of neurotransmitters Reduced neuronal apoptosis and amyloid deposition |
| Gao, ACS Chem Neurosci, 201884 | Transgenic senescence- accelerated mouse prone 8 (SAMP8) | Baicalein (flavonoid derived from Scutellariae baicalensis Georgi roots) | 4 weeks | Spatial learning tasks (Morris water maze test) Olfactory memory Novel object recognition ability Brain cortical levels of proinflammatory cytokines | Improved spatial learning abilities, olfactory memory and object recognition memory Reduced expression of proinflammatory cytokines in the brain cortex |
| Chunchai, J Neuroinflammation, 201878 | Male Wistar rats fed with high-fat diet-induced dementia | Xylo-oligosaccharide formulas | 12 weeks | Spatial learning tasks (Morris water maze test) Microglial activation Brain mitochondrial function Hippocampal plasticity, oxidative stress, apoptosis | Improvement of impairment in spatial learning tasks Reduced microglial activation Improved brain mitochondrial dysfunction Restored hippocampal plasticity, reduced oxidative stress and apoptosis |
Abbreviations: AD, Alzheimer’s disease; BDNF, brain-derived neurotrophic factor.
Intervention studies on humans
Only four papers reporting results of studies where probiotics were administered to human subjects fulfilled the search criteria.85–88 In three of them, healthy volunteers were involved, with a randomized, placebo-controlled study design.85–87 Conversely, probiotics were administered to older persons with dementia in only one small study, but cognitive outcomes were not assessed.88
In the earliest of these intervention studies,85 132 volunteers aged on average 61.8 years were randomized to receive milk drink containing Lactobacillus casei Shirota or milk drink alone, in order to verify the effect on mood symptoms. The intervention group did actually experience improved mood, but unexpectedly exhibited decreased performance in memory tasks.85 On the other hand, Allen et al showed that the administration of Bifidobacterium longum 1,714 to 22 healthy young volunteers induced subtle, but significant, improvements in hippocampus-dependent visuospatial memory tasks and electroencephalographic profile.86 However, the same research group later reported that the administration of Lactobacillus rhamnosus to 29 young male volunteers did not improve electroencephalographic parameters and cognitive outcomes, including visuospatial memory and attention switching tasks, after an 8-week treatment.87
Discussion
Most of the existing research exploring the possible interaction between gut microbiota composition and cognitive function has been performed in animal models. Human studies were mostly performed in subjects with an established diagnosis of dementia, and outside the geriatric constructs of cognitive frailty and MCI. In this context, there is a substantial gap between animal studies, indicating an established involvement of gut microbiota in modulating several physiopathologic aspects of dementia, and clinical studies, performed mainly on small samples and lacking a comprehensive profiling of gut microbiota composition and functionality.89
The reasons of this gap are not completely clear. The reduced availability of metagenomics lab research platforms in clinical contexts represents probably the main one. Despite the massive boost of scientific literature on gut microbiota, next-generation sequencing analyses of fecal samples for microbial profiling have entered clinical research only recently, and many aspects of the gut microbiota function in modulation of human diseases are still poorly investigated.24 Additionally, research on human subjects with cognitive impairment or dementia may imply several critical issues, from the ethical and methodological point of view, preventing their participation in research protocols with several laboratory analyses. Older patients with dementia may also have complex clinical pictures, with multimorbidity, polypharmacy, functional dependence, malnutrition, constipation and geriatric syndromes, which are very difficult to consider as covariates in microbiome research.24 Finally, dementia has long been considered as an “organ” disease, while modern evidence supports its definition as a “systemic disease”, where peripheral physiopathologic phenomena may also have an important role in its development and course.90
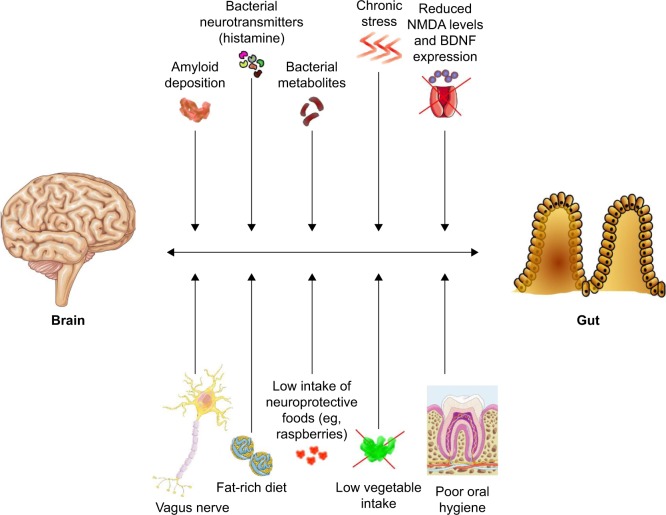
Therefore, the knowledge of the physiopathologic role of gut microbiota composition in modulating the onset and clinical course of chronic diseases is much more established for other neuropsychiatric disorders such as Parkinson disease.91,92 Age-related degenerative diseases, however, share several physiopathologic pathways,93 so that a role of gut microbiota in the development and clinical course of AD is highly plausible, even if not yet comprehensively investigated. In fact, studies exploring the possible contribution of infective agents in AD onset have mainly focused on the central nervous system or systemic infections, but not on microbiota.94 A synthetic graphical overview of the factors possibly involved in the dementia “gut–brain axis” is presented in Figure 2.
Figure 2.
Synthetic graphical overview of the possible factors and mechanisms involved in the putative “gut–brain axis” of dementia physiopathology.
Abbreviations: BDNF, brain-derived neurotrophic factor; NMDA, N-methyl-D-aspartic acid.
Several authors have, however, underlined the circumstance that many gut bacterial taxa can produce amyloid proteins.95,96 These proteins could be phagocytized in enteric immune system cells and then delivered to the central nervous system,95 or directly enter the systemic circulation as a consequence of a “leaky gut”, that is, altered mucosal permeability induced by microbiota dysbiosis.96 Diet could also have a relevant influence in inducing gut microbiota alterations predisposing to amyloid production and delivery to the brain.97 Bacterial amyloid proteins could then influence β-amyloid deposition in the brain with two different pathways: induction of neuroinflammation or molecular mimicry, that is, misfolding of neuronal proteins through cross-seeding.98
In aging individuals, several stressors, such as dietary variations, acute or chronic illness, gastrointestinal infections, constipation and pharmacotherapy, can contribute to induce gut microbiota dysbiosis.24,31,99,100 This phenomenon, especially when associated with microbiota composition instability, can promote systemic inflammation through the absorption of bacterial toxins such as LPS. The resulting proinflammatory cytokine production can result in brain microglia activation, promoting neuroinflammation, neuronal apoptosis and β-amyloid deposition.101 Interestingly, some gut bacteria, such as cyanobacteria, can produce neurotoxins (saxitoxin and anatoxin-α) that may be involved in neurodegeneration as well.102
Gut microbiota can influence the physiopathology of AD also through the synthesis of metabolites that, once absorbed in the systemic circulation, can modulate neural function.103 These metabolites, extensively reviewed by Alkasir et al, may have either neuroprotective or neurotoxic effects.104 For example, Lactobacillus, Lactococcus, Streptococcus and Enterococcus may produce histamine, which acts as a neurotransmitter and an important modulator of neuroinflammation through reduction of tumor necrosis factor-alpha expression in the brain.103,104 Similarly, other bacterial taxa, including Bacillus, Lactobacillus and Bifidobacterium, are able to synthesize neurotransmitters involved in memory and learning function regulation, such as gamma-aminobutyric acid, serotonin, norepinephrine and acetylcholine.103,104 Clostridium spp. may also produce indole-3-propionic acid, a relevant antioxidant for neurons.103,104
Conversely, β-N-methylamino-alanine, a metabolite produced by cyanobacteria, has been linked with intraneuronal protein misfolding, and thus could contribute to β-amyloid deposition.102 Metabolites derived from gut microbiota tryptophan metabolism may also promote neuronal oxidative stress, cell death, tau phosphorylation and tangle formation.103
Among the most interesting putative microbial mediators regulating brain function, SCFAs, and particularly butyrate, derived from carbohydrate fermentation, have demonstrated a strong systemic and neural anti-inflammatory activity, promoting also the synthesis of several neurotransmitters and hormones.104,105 In fact, a large body of scientific literature has demonstrated that the administration of butyrate to experimental mouse models of neurological diseases resulted in significant modulation of gene expression and improvement in molecular pathways of neuron damage.105 Interestingly, gut microbiota-derived butyrate has been recently proposed as one of the main mediators of a putative gut–muscle axis regulating the onset of physical frailty and sarcopenia in older individuals.34
The importance of the microbiota in modulating systemic inflammation and neuroinflammation leading to the development and progression of AD has been also underlined by the circumstance that AD patients may have oral microbiota overgrowth. This finding allowed to hypothesize a role of periodontitis in the pathogenesis of AD.106,107
Other authors have also emphasized the possible role of the vagal nerve in transducing gut microbiota signals to the brain.108,109 This mechanism has been particularly studied in situations of stress, where gut microbiota dysbiosis can promote neurohormonal alterations sustaining inflammation and promoting apoptosis.108,109 These pathways may represent the physiopathologic substrate linking irritable bowel syndrome-associated dysbiosis with the risk of dementia.56,110
The role of diet in these mechanisms is still poorly understood, but probably of utmost importance. Dietary patterns are strongly involved in the risk of developing AD and in its course, once the disease has established.111 Diets with patterns similar to Mediterranean and Dietary Approaches to Stop Hypertension diets have been associated with a reduced risk of AD and milder disease course, while Western-style diet represents an important risk factor.111 Among nutrients, saturated fatty acid consumption is associated with increased risk of AD, while an adequate dietary intake of polyphenols, unsaturated fatty acids and vitamins A, E and C may have a protective effect against the development of dementia.111,112 Interestingly, diet represents one of the main environmental factors shaping gut microbiota composition.24 The variety and nutrient composition of diet, and particularly long-term dietary habits, may, in fact, influence the gut microbiota biodiversity and shape the overall microbial community.113,114 For example, in healthy adults, dietary habits were significantly associated with specific gut microbiota composition patterns, the so-called “enterotypes”.115 Similarly, in older individuals, the variety and pattern of food consumption were associated with a different trend toward gut microbiota dysbiosis.27
As suggested by the findings of studies summarized in Table 4, gut microbiota may be strongly involved in the association between diet and dementia development. Healthy dietary habits promote a gut microbiota composition with anti-inflammatory activity.116 Conversely, high-fat diets may promote gut microbiota dysbiosis, and thus support systemic inflammation.117 Moreover, healthy gut microbiota may be fundamental for the transformation of dietary nutrients into active compounds exerting beneficial effects on the brain. Polyphenols represent a prototype of this possible mechanism.118 In fact, red raspberry-derived anthocyanins and ellagitannins need specific gut microbiota metabolism to be transformed into urolithins,119 which may exert important neuroprotective effects.80,81 Similarly, curcumin has recently emerged as a promising modulator of the gut microbiota, showing neuroprotective effects.120 In fact, its administration is able to induce increased expression of taxa with anti-inflammatory properties in gut microbiota, attenuating the systemic LPS absorption associated with Western-style diet.120 Moreover, curcumin metabolites produced by the microbiota itself can exert independent antioxidant activity, particularly against the central nervous system,121 and inhibit tau protein aggregation in vitro.122 In an open-label intervention study, curcumin administration to patients with MCI resulted in improvement in cognitive symptoms, making it a very promising target for novel drug development.123
Another critical issue of the existing studies on the microbiota–dementia relationship is the circumstance that most of them have been performed on animal models or patients with AD type of dementia. Very few of them have been performed in the context of vascular dementia, and none on other types of dementia, despite the clinical and physiopathologic relevance of this classification. However, the gut–brain axis also mediates the neuroinflammatory response after a vascular injury, such as ischemic stroke.124 Additionally, diabetes mellitus and obesity, which are among the main conditions predisposing to vascular dementia, are both associated with gut microbiota alterations and/or dysbiosis.125,126 Thus, the gut microbiota may also be involved in vascular cognitive impairment, mainly through modulation of local neural and systemic inflammation and insulin sensitivity.127
The results of animal intervention studies (Tables 3 and 4) support the assumption that manipulation of gut microbiota can improve cognitive function. This is a very promising field of preventive and therapeutic intervention,125,128 although the few human studies performed up to date have given disappointing results.85–87 However, there is increasing evidence indicating that some cerebral functions, and particularly mood and anxiety, can be ameliorated in subjects with psychiatric conditions by the administration of probiotics.129 The word “psychobiotics” has been coined to design this specific function of probiotics.130 More studies are needed in the future to assess the effects of these preparations on cognitive functions of both healthy individuals and older patients with dementia.125,130
Conclusion
The existence of a gut–brain axis involved in dementia is supported by several observational and intervention studies performed in animal models. As such, a physiopathologic role of gut microbiota alterations in cognitive frailty, MCI and dementia of older people has been hypothesized by many authors, particularly for AD. However, very few investigators have explored this hypothesis with clinical studies. Gut microbiota modulation represents a very interesting area of research for developing novel preventive or curative strategies against cognitive impairment, but, at the current state of the art, no recommendations for clinical practice can be made.
Acknowledgments
Part of the images is distributed under Creative Commons Licence and can be freely available at the following links: https://smart.servier.com/131 and https://pixabay.com.132
Footnotes
Author contributions
Conception and design of the work: Andrea Ticinesi, Antonio Nouvenne, Fulvio Lauretani, and Tiziana Meschi. Acquisition of data: Andrea Ticinesi, Antonio Nouvenne, and Beatrice Prati. Data analysis: Andrea Ticinesi, Antonio Nouvenne, and Beatrice Prati. Interpretation of data for the work: Andrea Ticinesi, Claudio Tana, and Fulvio Lauretani. Manuscript drafting: Andrea Ticinesi and Claudio Tana. Revision of the manuscript for important content: Fulvio Lauretani and Tiziana Meschi. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–734. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 2.Panza F, Seripa D, Solfrizzi V, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47(4):793–813. doi: 10.3233/JAD-150358. [DOI] [PubMed] [Google Scholar]
- 3.Panza F, Lozupone M, Solfrizzi V, et al. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J Alzheimers Dis. 2018;62(3):993–1012. doi: 10.3233/JAD-170963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto T, Sakurai T, Ono R, et al. Epidemiological and clinical significance of cognitive frailty: a mini review. Ageing Res Rev. 2018;44:1–7. doi: 10.1016/j.arr.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng L, Nyunt MS, Gao Q, et al. Physical frailty, cognitive impairment, and the risk of neurocognitive disorder in the Singapore Longitudinal Ageing Study. J Gerontol A Biol Sci Med Sci. 2017;72(3):369–375. doi: 10.1093/gerona/glw050. [DOI] [PubMed] [Google Scholar]
- 7.Solfrizzi V, Scafato E, Lozupone M, et al. Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: The Italian Longitudinal Study on Aging. Am J Geriatr Psychiatry. 2017;25(11):1236–1248. doi: 10.1016/j.jagp.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Vella Azzopardi R, Beyer I, Vermeiren S, et al. Increasing use of cognitive measures in the operational definition of frailty – a systematic review. Ageing Res Rev. 2018;43:10–16. doi: 10.1016/j.arr.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Lauretani F, Meschi T, Ticinesi A, Maggio M. “Brain–muscle loop” in the fragility of older persons: from pathophysiology to new organizing models. Aging Clin Exp Res. 2017;29(6):1305–1311. doi: 10.1007/s40520-017-0729-4. [DOI] [PubMed] [Google Scholar]
- 10.Fougère B, Daumas M, Lilamand M, et al. Association between frailty and cognitive impairment: cross-sectional data from Toulouse frailty day hospital. J Am Med Dir Assoc. 2017;18(11):990.e1–990.e5. doi: 10.1016/j.jamda.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Montero-Odasso MM, Barnes B, Speechley M, et al. Disentangling cognitive-frailty: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2016;71(11):1476–1482. doi: 10.1093/gerona/glw044. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roppolo M, Mulasso A, Rabaglietti E. Cognitive frailty in Italian community-dwelling older adults: prevalence rate and its association with disability. J Nutr Health Aging. 2017;21(6):631–636. doi: 10.1007/s12603-016-0828-5. [DOI] [PubMed] [Google Scholar]
- 14.Shimada H, Makizako H, Lee S, et al. Impact of cognitive frailty on daily activities in older persons. J Nutr Health Aging. 2016;20(7):729–735. doi: 10.1007/s12603-016-0685-2. [DOI] [PubMed] [Google Scholar]
- 15.Rietman ML, van der ADL, van Oostrom SH, et al. The association between BMI and different frailty domains: a U-shaped curve? J Nutr Health Aging. 2018;22(1):8–15. doi: 10.1007/s12603-016-0854-3. [DOI] [PubMed] [Google Scholar]
- 16.Doi T, Shimada H, Makizako H, et al. Mild cognitive impairment, slow gait, and risk of disability: a prospective study. J Am Med Dir Assoc. 2015;16(12):1082–1086. doi: 10.1016/j.jamda.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Solfrizzi V, Scafato E, Seripa D, et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian Longitudinal Study on Aging. J Am Med Dir Assoc. 2017;18(1):89.e1–89.e8. doi: 10.1016/j.jamda.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Yu R, Morley JE, Kwok T, Leung J, Cheung O, Woo J. The effects of combinations of cognitive impairment and pre-frailty on adverse outcomes from a prospective community-based cohort study of older Chinese people. Front Med. 2018;5:50. doi: 10.3389/fmed.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Han L, Gahbauer EA, Allore HG, Gill TM. Joint trajectories of cognition and frailty and associated burden of patient-reported outcomes. J Am Med Dir Assoc. 2018;19(4):304–309. doi: 10.1016/j.jamda.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chye L, Wei K, Nyunt MSZ, Gao Q, Wee SL, Tp N. Strong relationship between malnutrition and cognitive frailty in the Singapore Longitudinal Ageing Studies (SLAS-1 and SLAS-2) J Prev Alzheimers Dis. 2018;5(2):142–148. doi: 10.14283/jpad.2017.46. [DOI] [PubMed] [Google Scholar]
- 21.Liu L-K, Lee W-J, Wu Y-H, et al. Cognitive frailty and its association with all-cause mortality among community-dwelling older adults in Taiwan: results from I-Lan Longitudinal Aging Study. Rejuvenation Res. 2018 Apr 12; doi: 10.1089/rej.2017.2038. Epub. [DOI] [PubMed] [Google Scholar]
- 22.Ruan Q, D’Onofrio G, Sancarlo D, et al. Emerging biomarkers and screening for cognitive frailty. Aging Clin Exp Res. 2017;29(6):1075–1086. doi: 10.1007/s40520-017-0741-8. [DOI] [PubMed] [Google Scholar]
- 23.Panza F, Lozupone M, Solfrizzi V, et al. Cognitive frailty: a potential target for secondary prevention of dementia. Expert Opin Drug Metab Toxicol. 2017;13(10):1023–1027. doi: 10.1080/17425255.2017.1372424. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt TSB, Raes J, Bork P. The human gut microbiome: from association to modulation. Cell. 2018;172(6):1198–1215. doi: 10.1016/j.cell.2018.02.044. [DOI] [PubMed] [Google Scholar]
- 25.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. 2017;35:36–45. doi: 10.1016/j.arr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170–182. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson MA, Jeffery IB, Beaumont M, et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maffei VJ, Kim S, Blanchard E, et al. Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci. 2017;72(11):1474–1482. doi: 10.1093/gerona/glx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ticinesi A, Milani C, Lauretani F, et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7(1):11102. doi: 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haran JP, Bucci V, Dutta P, Ward D, Mccormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67(1):40–51. doi: 10.1099/jmm.0.000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.di Sabatino A, Lenti MV, Cammalleri L, Corazza GR, Pilotto A. Frailty and the gut. Dig Liver Dis. 2018 Mar 16; doi: 10.1016/j.dld.2018.03.010. Epub. [DOI] [PubMed] [Google Scholar]
- 34.Ticinesi A, Lauretani F, Milani C, et al. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: is there a gut–muscle axis? Nutrients. 2017;9(12):1303. doi: 10.3390/nu9121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grosicki GJ, Fielding RA, Lustgarten MS. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut–muscle axis. Calcif Tissue Int. 2018;102(4):433–442. doi: 10.1007/s00223-017-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picca A, Fanelli F, Calvani R, et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm. 2018;2018:7026198. doi: 10.1155/2018/7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonaz BL, Bernstein CN. Brain–gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144(1):36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5(3):397–403. doi: 10.4161/gmic.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: a review. Brain Behav Immun. 2017;66:9–17. doi: 10.1016/j.bbi.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Calvani R, Picca A, Lo Monaco MR, Landi F, Bernabei R, Marzetti E. Of microbes and minds: a narrative review on the second brain aging. Front Med. 2018;5:53. doi: 10.3389/fmed.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott KA, Ida M, Peterson VL, et al. Revisiting Metchnikoff: age-related alterations in microbiota–gut–brain axis in the mouse. Brain Behav Immun. 2017;65:20–32. doi: 10.1016/j.bbi.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Sadler R, Singh V, Benakis C, et al. Microbiota differences between commercial breeders impacts the post-stroke immune response. Brain Behav Immun. 2017;66:23–30. doi: 10.1016/j.bbi.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Shen L, Liu L, Ji HF. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis. 2017;56(1):385–390. doi: 10.3233/JAD-160884. [DOI] [PubMed] [Google Scholar]
- 44.Bauerl C, Collado MC, Diaz Cuevas A, Vina J, Perez Martinez G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett Appl Microbiol. 2018 Mar 25; doi: 10.1111/lam.12882. Epub. [DOI] [PubMed] [Google Scholar]
- 45.Park J-Y, Choi J, Lee Y, et al. Metagenome analysis of bodily micro-biota in a mouse model of Alzheimer disease using bacteria-derived membrane vesicles in blood. Exp Neurobiol. 2017;26(6):369–379. doi: 10.5607/en.2017.26.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandscheid C, Schuck F, Reinhardt S, et al. Altered gut microbiome composition and tryptic activity of the 5xFAD Alzheimer’s mouse model. J Alzheimers Dis. 2017;56(2):775–788. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Wang Y, Xiayu X, et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2017;60(4):1241–1257. doi: 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- 48.Sanguinetti E, Collado MC, Marrachelli VG, et al. Microbiome–metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci Rep. 2018;8(1):4907. doi: 10.1038/s41598-018-23261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sc W, Cao ZS, Chang KM, Juang JL. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease of Drosophila. Nat Comm. 2017;8:24. doi: 10.1038/s41467-017-00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harach T, Marungruang N, Duthilleul N, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6(1):30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fröhlich EE, Farzi A, Mayerhofer R, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota–brain communication. Brain Behav Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui M, Xiao H, Li Y, et al. Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim Biophys Acta. 1863;2017:2333–2341. doi: 10.1016/j.bbadis.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minter MR, Hinterleitner R, Meisel M, et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1∆E9 murine model of Alzheimer’s disease. Sci Rep. 2017;7(1):10411. doi: 10.1038/s41598-017-11047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajaj JS, Ridlon JM, Hylemon PB, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302(1):G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajaj JS, Ahluwalia V, Steinberg JL, et al. Elderly patients have an altered gut–brain axis regardless of the presence of cirrhosis. Sci Rep. 2016;6(1):38481. doi: 10.1038/srep38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bajaj JS, Fagan A, Sikaroodi M, et al. Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver Transpl. 2017;23(7):907–914. doi: 10.1002/lt.24754. [DOI] [PubMed] [Google Scholar]
- 58.Ahluwalia V, Wade JB, Heuman DM, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with rifaximin in cirrhosis: implications for the gut–liver–brain axis. Metab Brain Dis. 2014;29(4):1017–1025. doi: 10.1007/s11011-014-9507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C-H, Lin C-L, Kao C-H. Irritable bowel syndrome is associated with an increased risk of dementia: a nationwide population-based study. PLoS One. 2016;11(1):e0144589. doi: 10.1371/journal.pone.0144589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu R, Wang Q. Towards understanding brain–gut–microbiome connections in Alzheimer’s disease. BMC Syst Biol. 2016;10(S3):63. doi: 10.1186/s12918-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andreadou E, Pantazaki AA, Daniilidou M, Tsolaki M. Rhamnolipids, microbial virulence factors, in Alzheimer’s disease. J Alzheimers Dis. 2017;59(1):209–222. doi: 10.3233/JAD-161020. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Jaber V, Lukiw WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 2017;7:318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paley EL, Merkulova-Rainon T, Faynboym A, Shestopalov VI, Aksenoff I. Geographical distribution and diversity of gut microbial NADH:ubiquinone oxidoreductase sequence associated with Alzheimer’s disease. J Alzheimers Dis. 2018;61(4):1531–1540. doi: 10.3233/JAD-170764. [DOI] [PubMed] [Google Scholar]
- 64.Araos R, Andreatos N, Ugalde J, et al. Fecal microbiome among nursing home residents with advanced dementia and Clostridium difficile. Dig Dis Sci. 2018 Mar 28; doi: 10.1007/s10620-018-5030-7. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Vogt NM, Kerby RL, Dill-Mcfarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang ZQ, Shen LL, Li WW, et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis. 2018;63(4):1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 68.Qjan Y, Yang Y, Xu S, et al. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun. 2018 Mar 2; doi: 10.1016/j.bbi.2018.02.016. Epub. [DOI] [PubMed] [Google Scholar]
- 69.Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome–gut–brain axis. Neuroscience. 2013;240:287–296. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 70.Distrutti E, O’Reilly J-A, Mcdonald C, et al. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9(9):e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Sun J, Wang F, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang S, Wang T, Hu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 73.Sun J, Wang F, Ling Z, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 74.Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nimgampalle M, Kuna Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J Clin Diagn Res. 2017;11(8):KC01–KC05. doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi Y, Sugahara H, Shimada K, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep. 2017;7(1):13510. doi: 10.1038/s41598-017-13368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musa NH, Mani V, Lim SM, Vidyadaran S, Abdul Majeed AB, Ramasamy K. Lactobacilli-fermented cow’s milk attenuated lipopoly-saccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J Dairy Res. 2017;84(04):488–495. doi: 10.1017/S0022029917000620. [DOI] [PubMed] [Google Scholar]
- 78.Chunchai T, Thunapong W, Yasom S, et al. Decreased microglial activation through gut–brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation. 2018;15(1):11. doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Athari Nik Azm S, Djazayeri A, Safa M, et al. Lactobacillus and bifidobacterium ameliorate memory and learning deficits and oxidative stress in Aβ (1–42) injected rats. Appl Physiol Nutr Metab. 2018 Feb 20; doi: 10.1139/apnm-2017-0648. Epub. [DOI] [PubMed] [Google Scholar]
- 80.Wang D, Ho L, Faith J, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res. 2015;59(6):1025–1040. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan T, Ma H, Liu W, et al. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitanningut microbial derived metabolites. ACS Chem Neurosci. 2016;7(1):26–33. doi: 10.1021/acschemneuro.5b00260. [DOI] [PubMed] [Google Scholar]
- 82.Wang S, Huang X-F, Zhang P, et al. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci Rep. 2017;7(1):12203. doi: 10.1038/s41598-017-12156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen D, Yang X, Yang J, et al. Prebiotic effect of fructooligosaccharides from Morinda officinalis on Alzheimer’s disease in rodent models by targeting the microbiota–gut–brain axis. Front Aging Neurosci. 2017;9:403. doi: 10.3389/fnagi.2017.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao L, Li J, Zhou Y, Huang X, Qin X, Du G. Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8. ACS Chem Neurosci. 2018;9(7):1714–1724. doi: 10.1021/acschemneuro.8b00074. [DOI] [PubMed] [Google Scholar]
- 85.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61(3):355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 86.Allen AP, Hutch W, Borre YE, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6(11):e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 88.Carlsson M, Gustafson Y, Haglin L, Eriksson S. The feasibility of serving liquid yoghurt supplemented with probiotic bacteria, Lactobacillus rhamnosus LB 21, and Lactococcus lactis L1A – a pilot study among old people with dementia in a residential care facility. J Nutr Health Aging. 2009;13(9):813–819. doi: 10.1007/s12603-009-0218-3. [DOI] [PubMed] [Google Scholar]
- 89.Mancuso C, Santangelo R. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol Res. 2018;29:329–336. doi: 10.1016/j.phrs.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Morris JK, Honea RA, Vidoni ED, Swerdlow RH, Burns JM. Is Alzheimer’s disease a systemic disease? Biochim Biophys Acta. 1842;2014(9):1340–1349. doi: 10.1016/j.bbadis.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: a review. Ann Neurol. 2017;81(3):369–382. doi: 10.1002/ana.24901. [DOI] [PubMed] [Google Scholar]
- 92.Dinan TG, Cryan JF. Gut insticts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Franceschi C, Garagnani P, Morsiani C, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med. 2018;5:61. doi: 10.3389/fmed.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer’s disease. J Alzheimers Dis. 2016;51(4):979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedland RP, Chapman MR. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017;13(12):e1006654. doi: 10.1371/journal.ppat.1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Köhler C, Maes M, Slyepchenko A, et al. The gut–brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des. 2016;22(40):6152–6166. doi: 10.2174/1381612822666160907093807. [DOI] [PubMed] [Google Scholar]
- 97.Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr Rev. 2016;74(10):624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 98.Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45(2):349–362. doi: 10.3233/JAD-142841. [DOI] [PubMed] [Google Scholar]
- 99.Ticinesi A, Milani C, Gerritsen J, et al. Gut microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: a metagenomic study. Sci Rep. 2016;6:25945. doi: 10.1038/srep25945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mancabelli L, Milani C, Lugli GA, et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci Rep. 2017;7(1):9879. doi: 10.1038/s41598-017-10663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li CQ, Zheng Q, Wang Q, Zeng QP. Biotic/abiotic stress-driven Alzheimer’s disease. Front Cell Neurosci. 2016;10:269. doi: 10.3389/fncel.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Front Cell Neurosci. 2013;7:153. doi: 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Westfall S, Lomis N, Kahouli I, et al. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8(2):90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota–gut–brain axis? Neurochem Int. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 106.Shoemark DK, Allen SJ. The microbiome and disease: reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J Alzheimers Dis. 2015;43(3):725–738. doi: 10.3233/JAD-141170. [DOI] [PubMed] [Google Scholar]
- 107.Harding A, Gonder U, Robinson SJ, Crean S, Singhrao SK. Exploring the association between Alzheimer’s disease, oral health, microbial endocrinology and nutrition. Front Aging Neurosci. 2017;9:398. doi: 10.3389/fnagi.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leung K, Thuret S. Gut microbiota: a modulator of brain plasticity and cognitive function in ageing. Healthcare (Basel) 2015;3(4):898–916. doi: 10.3390/healthcare3040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prenderville JA, Kennedy PJ, Dinan TG, Cryan JF. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 2015;38(1):13–25. doi: 10.1016/j.tins.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Daulatzai MA. Chronic functional bowel syndrome enhances gut–brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res. 2014;39(4):624–644. doi: 10.1007/s11064-014-1266-6. [DOI] [PubMed] [Google Scholar]
- 111.Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59(3):815–849. doi: 10.3233/JAD-170248. [DOI] [PubMed] [Google Scholar]
- 112.Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut–brain interactions. Mech Ageing Dev. 2014;136–137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 113.Zhang N, Ju Z, Zuo T. Time for food: the impact of diet on gut microbiota and human health. Nutrition. 2018;51–52:80–85. doi: 10.1016/j.nut.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Milani C, Ferrario C, Turroni F, et al. The human gut microbiota and its interactive connections to diet. J Hum Nutr Diet. 2016;29(5):539–546. doi: 10.1111/jhn.12371. [DOI] [PubMed] [Google Scholar]
- 115.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russo R, Cristiano C, Avagliano C, et al. Gut–brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem. 2017;24:1–22. doi: 10.2174/0929867324666170216113756. [DOI] [PubMed] [Google Scholar]
- 118.Pasinetti GM, Singh R, Westfall S, Herman F, Faith J, Ho L. The role of the gut microbiota in the metabolism of polyphenols as characterized by gnotobiotic mice. J Alzheimers Dis. 2018;63(2):409–421. doi: 10.3233/JAD-171151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ludwig IA, Mena P, Calani L, et al. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic Biol Med. 2015;89:758–769. doi: 10.1016/j.freeradbiomed.2015.10.400. [DOI] [PubMed] [Google Scholar]
- 120.Shen L, Ji HF. Bidirectional interactions between dietary curcumin and gut microbiota. Crit Rev Food Sci Nutr. 2018:1–28. doi: 10.1080/10408398.2018.1478388. [DOI] [PubMed] [Google Scholar]
- 121.Yan FS, Sun JL, Xie WH, Shen L, Ji HF. Neuroprotective effects and mechanisms of curcumin–Cu(II) and –Zn(II) complexes systems and their pharmacological implications. Nutrients. 2018;10(1):28. doi: 10.3390/nu10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rane JS, Bhaumik P, Panda D. Curcumin inhibits tau aggregation and disintegrates preformed tau filaments in vitro. J Alzheimers Dis. 2017;60(3):999–1014. doi: 10.3233/JAD-170351. [DOI] [PubMed] [Google Scholar]
- 123.Tabira T, Kawamura N. A study of a supplement containing huperzine a and curcumin in dementia patients and individuals with mild cognitive impairment. J Alzheimers Dis. 2018;63(1):75–78. doi: 10.3233/JAD-171154. [DOI] [PubMed] [Google Scholar]
- 124.Singh V, Roth S, Llovera G, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Solas M, Milagro FI, Ramírez MJ, Martínez JA. Inflammation and gut–brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87–92. doi: 10.1016/j.coph.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 126.Xu Y, Zhou H, Zhu Q. The impact of microbiota–gut–brain axis on diabetic cognition impairment. Front Aging Neurosci. 2017;9:106. doi: 10.3389/fnagi.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li S, Shao Y, Li K, et al. Vascular cognitive impairment and the gut microbiota. J Alzheimers Dis. 2018;63(4):1209–1222. doi: 10.3233/JAD-171103. [DOI] [PubMed] [Google Scholar]
- 128.Zheng X, Zhang X, Kang A, Ran C, Wang G, Hao H. Thinking outside the brain for cognitive improvement: is peripheral immunomodulation on the way? Neuropharmacology. 2015;96:94–104. doi: 10.1016/j.neuropharm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J Neurogastroenterol Motil. 2016;22(4):589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016;39(11):763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. smart.servier.com [homepage on the Internet] 3000 free medical images to illustrate your publications and PowerPoint presentations. 2018. [Accessed on May 20th 2018].
- 132. pixabay.com [homepage on the Internet] 2018. [Accessed on May 20th 2018].