Abstract
Purpose
Obesity is a serious problem, which is now a worldwide health problem. Kaempferia parviflora extract (KPE) exhibits anti-obesity effects in animals. However, as no clinical trials have evaluated the anti-obesity effects of KPE in humans, we examined the effects of KPE in reducing abdominal fat in overweight and preobese Japanese subjects.
Materials and methods
A 12-week, single-center, randomized, double-blind, placebo-controlled clinical trial was conducted. Seventy-six subjects (males and females aged 20 to <65 years) with a body mass index ≥24 and <30 kg/m2 were randomly assigned into two groups. The subjects in each group ingested one capsule of placebo or active KPE (containing 150 mg of KPE) once daily for 12 weeks. The primary outcome was reduction in visceral fat area as determined by computed tomography scanning. The key secondary outcomes were reductions in subcutaneous fat area and total fat area. Subgroup analysis was also performed in healthy subjects without dyslipidemia, hypertension, or hyperglycemia. The safety of KPE ingestion was also evaluated.
Results
Compared with the placebo group, the active KPE group exhibited significant reduction in abdominal fat area (visceral, subcutaneous, and total fat) and triglyceride levels after 12 weeks. Subgroup analyses demonstrated a significant reduction in abdominal fat area and triglyceride levels in healthy subjects compared with the placebo group after 12 weeks. Neither group exhibited adverse events related to the test foods or clinically relevant abnormal changes in physical, biochemical, or hematologic parameters, or in urinalysis results and medical interview.
Conclusion
Daily ingestion of KPE safely reduces body fat, particularly abdominal fat, in Japanese overweight and preobese subjects.
Keywords: healthy subjects, metabolic disorder, obesity, randomized clinical trial, triglyceride, visceral fat
Introduction
Obesity is a serious global health problem. In Japan, >30% of adult men and 20% of adult women are categorized as preobese or obese, based on a body mass index (BMI) of ≥25 kg/m2. Obesity is a significant risk factor for development of metabolic disorders such as insulin resistance, hyperlipidemia, and hypertension, which in turn are risk factors for cardiovascular diseases.1–3 Obesity develops when there is an imbalance between energy intake and expenditure and is characterized by increased body weight resulting from excessive lipid accumulation.4 Accumulation of excessive fat in adipocytes increases the risk of developing type 2 diabetes associated with insulin resistance.5,6 Strategies to prevent and improve obesity, particularly by decreasing visceral fat accumulation, are thus important in the control of these metabolic diseases. Various so-called functional foods or natural components for preventing or improving obesity have been developed recently. Several of these foods reportedly inhibit the development of obesity, and some have been used to achieve body fat loss.7–10
Kaempferia parviflora Wall. ex Baker (KP), also known as Krachai-Dam or black ginger in Thailand, is a plant belonging to the family Zingiberaceae. KP rhizomes are used in folk medicine to improve blood flow and treat inflammatory, allergic, and gastrointestinal disorders. KP also reportedly exhibits beneficial activities, such as anticholinesterase,11 antiinflammatory,12–14 spasmolytic,15 gastric ulcer amelioration,16 antioxidative,17 and vasodilatory18 effects in addition to regulation of adipocyte differentiation.19,20 Dietary supplementation with KP extract (KPE) also reportedly suppresses body weight increases, body fat accumulation, and glucose intolerance in obese mice.21–23 Other reports suggest that oral intake of KPE increases energy expenditure and fat utilization.23–25 Collectively, these findings suggest that KPE could be used to reduce body fat in humans; however, there are no human clinical data to support this hypothesis.
The World Health Organization defines obesity as a BMI ≥30 kg/m2 and preobesity as a BMI ≥25 but <30 kg/m2.26 The Japan Society for the Study of Obesity has classified persons with a BMI between 23 and 25 kg/m2 as “overweight” and those with a BMI between 25 and 30 kg/m2 as “obese level 1 (preobese)”. The overall goal of the present study was to investigate whether dietary KPE ingestion reduces abdominal fat in Japanese overweight and preobese subjects. We hypothesized that dietary KPE ingestion would reduce visceral fat in healthy subjects with no metabolic abnormalities due to increased lipolysis and energy expenditure. We, therefore, also performed subgroup analyses of healthy subjects without elevated triglycerides (TGs), reduced high-density-lipoprotein cholesterol (HDL-Cho), elevated blood pressure, and elevated fasting blood glucose (FBG). In addition, we evaluated the safety of KPE ingestion by monitoring adverse events.
Materials and methods
Study design
A randomized, double-blind, placebo-controlled study designed to evaluate the efficacy and safety of KPE ingestion was carried out in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. The study protocol and all related documents were approved by the Miyawaki Orthopedics Clinic Institutional Review Board (Hokkaido, Japan; date of approval: February 20, 2017; approval No: 16211) – an agency not involved in this study – to ensure that approval for the study was unbiased and that there were no conflicts of interest. Appropriate explanations were provided to subjects regarding the study’s purpose, content, procedures, methods, and potential adverse reactions. All subjects gave their signed written informed consent before participating in the study. The study was conducted at the Fukuhara Clinic (Hokkaido, Japan) between April 2017 and July 2017 under control of the principal investigator. The data were analyzed from September to December, 2017. The study is registered under clinical trial registration number UMIN000026291. A data monitoring manager assured accuracy of data collection and inputs.
Subjects
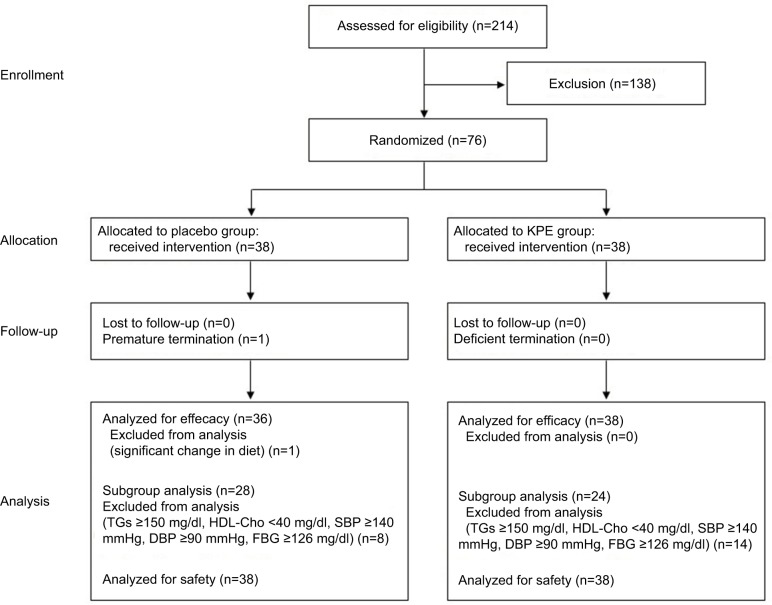
Subjects were Japanese adults (men and women) aged 20 to <65 years with a BMI ≥24 kg/m2 and <30 kg/m2. In total, 214 subjects (men: n=103, women: n=111) agreed to participate and were enrolled by the New Drug Research Center, Inc. (Tokyo, Japan). The exclusion criteria were as follows: history of severe illness affecting the heart, liver, kidney, or digestive organs; meeting the diagnostic criteria for metabolic syndrome (visceral fat area [VFA] ≥100 cm2 plus fewer than two of the following: elevated TGs and/or reduced HDL-Cho, elevated blood pressure, and elevated FBG)27; underwent medical treatment or surgery within 2 months before this study; use of drugs affecting carbohydrate metabolism and lipid metabolism; use of antihypertensive drugs; continual use of oral medications and foods affecting body fat or lipid metabolism; possible pregnancy, pregnancy, and lactation; heavy alcohol consumption; difficulty in collecting blood; donated ≥200 mL of blood within 1 month before this study; constipated for >5days; shift worker; allergic to the test foods; participation in another clinical study; any other reason for ineligibility as judged by the principal investigator. Based on these criteria, 138 subjects were excluded from the study. Figure 1 shows a flowchart for the study.
Figure 1.
Flowchart illustrating the phases of the study.
Abbreviations: Cho, cholesterol; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL, high-density lipoprotein; KPE, Kaempferia parviflora extract; SBP, systolic blood pressure; TGs, triglycerides.
Test food
Two types of capsules were prepared: “active” capsules containing KPE powder™ (Maruzen Pharmaceuticals, Hiroshima, Japan) and placebo capsules without KPE. Active and placebo capsules were indistinguishable in terms of flavor and appearance, including color, size, and packaging. The composition and nutritional content of both types of capsule are shown in Table 1. Standardization and conformity of the KPE used in the active capsules were assured by implementation of strict in-process controls during manufacture and complete analytical control of the resulting dry extract. The KPE used in the active capsules included standardized polymethoxyflavone (>8%), a total of six compounds analyzed by high-performance liquid chromatography: 5,7-dimethoxy-flavone; 3,5,7-trimethoxyflavone; 5,7,4′-trimethoxyflavone; 3,5,7,4′-tetramethoxyflavone; 5,7,3′,4′-tetramethoxyflavone; and 3,5,7,3′,4′-pentamethoxyflavone.
Table 1.
Composition and nutritional content of test foods
| Food characteristics | Serving size: 1 capsule (308 mg)
|
|
|---|---|---|
| Placebo | KPE | |
| Composition | ||
| KPE (mg) | 0.0 | 150.0 |
| Dextrin (mg) | 242.5 | 92.5 |
| Calcium stearate (mg) | 2.5 | 2.5 |
| Gelatin cap (mg) | 63.0 | 63.0 |
| Nutritional content per capsule | ||
| Energy (kcal) | 1.16 | 1.18 |
| Protein (g) | 0.06 | 0.07 |
| Carbohydrate (g) | 0.23 | 0.21 |
| Fat (g) | 0.00 | 0.01 |
Abbreviation: KPE, Kaempferia parviflora extract.
Randomization and blinding
Randomization was performed by Statcom Company Limited (Tokyo, Japan) using a computer-generated permuted block randomization scheme. After stratifying by age, gender, and VFA, eligible participants were randomized to active KPE group or placebo group, with an allocation ratio of 1:1. Allocation manager who had no contact with the subjects, outcome evaluator, and data collector generated a random number sequence list. The random sequence was kept by a designated person who was in charge of assignment of the test foods to subjects. Allocation manager and the designated person who was in charge of assignment were not involved in the study design, subject recruitment, assessment, data collection, intervention, or analysis. All subjects and investigators were blinded to the group assignment. The randomization code was opened after the dataset was carefully checked, cleaned, and fixed.
Study schedule
Each subject received one capsule per day for 12 weeks, and they visited the clinic for assessments and measurements on Weeks 0 (0W, at the start of intake), 4 (4W), 8 (8W), and 12 (12W). The subjects were instructed to drink only water after 9:00 pm on the day before the visit until the examination was completed on the following day. On the day of examination, smoking was prohibited until the examination was completed. During the study period, subjects were instructed not to use oral medications, dietary supplements, or functional foods affecting body fat or lipid metabolism, and we instructed the subjects not to significantly change their daily routines or lifestyle compared with before initiation of the study. Physical parameters (height, body weight, BMI, waist circumference, body temperature, blood pressure, and heart rate), hematologic parameters, blood biochemical parameters, urinalysis, and abdominal fat area were assessed for all subjects.
Target sample size
Based on another preliminary human study, capsules containing 150 mg of KPE were predicted to reduce body fat by 2.5% (SD 4.4%) compared with the placebo capsules (−0.5% [SD 4.4%]). To detect a difference with a power of 80% at a significance level of α=0.05, the number of subjects required per group was estimated as 35. To account for an expected dropout rate of 10%, the number of subjects required per group was estimated as 38.
Measurement of abdominal fat area
Subjects underwent computed tomography (CT) scanning of the abdominal transverse section at the umbilical position using a Robusto-Ei scanner (Hitachi Medico, Tokyo, Japan). Abdominal body fat area (VFA and subcutaneous fat area [SFA]) values were calculated based on analysis of the abdominal CT scan using visceral fat measurement software (Fat Scan™ Ver. 3.0, N2 Systems Inc., Osaka, Japan). The abdominal total fat area (TFA) was calculated as the sum of the VFA and SFA. In consideration of the risk of radiation exposure, subjects underwent CT scans only at the initial screening and at 0W, 8W, and 12W. The primary outcome was reduction in VFA at 8W and 12W. The key secondary outcomes were reductions in TFA and SFA at 8W and 12W.
Physical and blood examinations and urinalysis
Height was measured only at the screening examination. Body weight, BMI, waist and hip circumference, body temperature, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were measured at each examination. BMI was calculated as body weight (in kg)/height (in m2). Blood pressure and heart rate were measured after rest for about 5 minutes with the subject in the sitting position.
The following fasting blood sample parameters were measured: white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin, hematocrit, platelets, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration for hematology; and total protein, albumin, total bilirubin (T-bil), aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, alkaline phosphatase, γ-glutamyltranspeptidase, creatine phosphokinase, total cholesterol, HDL-Cho, low-density-lipoprotein cholesterol (LDL-Cho), TGs, FBG, hemoglobin A1c (HbA1c), uric acid, blood urea nitrogen (BUN), creatinine, sodium (Na), chloride (Cl), potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) for biochemical analyses. All blood analyses were performed by a commercial clinical laboratory (SRL Inc., Tokyo, Japan).
The semiquantitative analyses of urine protein, urine glucose, urobilinogen, bilirubin, ketone bodies, and urine occult blood were performed using fasting urine samples. Urine pH was also measured. All urinalyses were performed by a commercial clinical laboratory (SRL Inc.).
Dietary record and physical activity
Subjects recorded the content of their daily meals, snacks, and beverages (except for water), as well as the number of steps counted using a pedometer. Values of dietary and physical activity were recorded on a paper for 3 days prior to each visit at 0W, 4W, 8W, and 12W. Based on the meal survey, a nutritionist calculated each subject’s energy intake. The daily average number of steps counted was calculated as physical activity. Subjects also recorded the time of test food ingestion, subjective symptoms, and daily activities, including eating habits, exercise habits, alcohol intake, and drug intake. The rate of test food intake was calculated based on subject’s daily paper record.
Subgroup analyses
To evaluate the effects of KPE in healthy subjects with no metabolic abnormalities, we performed subgroup analyses. The following diagnostic criteria are used to diagnose metabolic syndrome in Japan: elevated TGs (≥150 mg/dL), reduced HDL-Cho (<40 mg/dL), elevated SBP (≥140 mmHg), elevated DBP (≥90 mmHg), and elevated FBG (≥126 mg/dL). In the subgroup analyses, we evaluated the effect of KPE in healthy subjects with BMI ≥24 and <30 kg/m2, TGs <150 mg/dL, HDL-Cho ≥40 mg/dL, SBP <140 mmHg, DBP <90 mmHg, and FBG ≤125 mg/dL.
Statistical analysis
Data are expressed as mean values with SD. All randomized (intention-to-treat [ITT]), per-protocol analyses (PPS) and subgroup analyses were performed. The data are reported ITT for the background of subjects and safety evaluation and PPS for the efficacy evaluation.
An F-test was used to assess homoscedasticity between the placebo and active food groups. If homoscedasticity was confirmed, the Student’s t-test was used, whereas Aspin–Welch’s t-test was used for comparisons if the variances were unequal. In addition, Wilcoxon’s rank sum test was used for comparisons between the placebo and active food groups in urine semiquantitative analyses. For primary and secondary endpoints, the effect of two factors (time and group) and time-by-group interactions were analyzed by repeated measures analysis of variance (ANOVA) using values obtained during the test food ingestion period. The level of statistical significance was set at p<0.05. Statistical analyses were performed using Microsoft Excel® 2013 (Microsoft Corp., Redmond, WA, USA) and SAS® 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
After exclusion of 138 subjects in the screening examination based on the abovementioned exclusion criteria, 76 subjects (men=16, women=60) were randomized into the KPE and placebo groups (38 subjects per group). One subject subsequently dropped out of the study because of personal issues not related to the study. Of the 75 subjects who completed the study, 1 was excluded from the efficacy evaluation before the randomization code was opened due to failure to follow compliance rules (engaged in a significant change in lifestyle). Consequently, the efficacy statistical analyses were conducted on 74 subjects (38 subjects in the KPE group and 36 subjects in the placebo group). The baseline characteristics of the subjects are shown in Table 2. No significant differences in baseline parameters were observed between the KPE and placebo groups. Table 3 shows daily average values for parameters associated with energy intake and physical activity. No significant differences in any of these parameters were observed between the two groups throughout the study. The rate of test food intake was 99.6% (94.0%–100.0%) for the KPE group and 99.5% (92.9%–100.0%) for the placebo group, with no between-group differences (p=0.89).
Table 2.
Characteristics of the subjects at baseline
| Characteristics | Placebo group (n=38) | KPE group (n=38) |
|---|---|---|
| Age (years) | 49.5±9.3 | 50.2±8.0 |
| Height (cm) | 159.45±6.28 | 159.0±8.28 |
| Body weight (kg) | 69.01±5.44 | 68.81±7.21 |
| BMI (kg/m2) | 27.14±1.47 | 27.19±1.42 |
| Waist circumference (cm) | 94.27±5.67 | 94.38±5.51 |
| SBP (mmHg) | 125.0±14.9 | 127.0±13.1 |
| DBP (mmHg) | 76.1±10.8 | 75.9±9.1 |
| HR (bpm) | 68.0±10.3 | 66.7±7.4 |
| Abdominal fat area | ||
| Visceral (cm2) | 90.30±19.45 | 90.23±19.22 |
| Subcutaneous (cm2) | 258.13±72.16 | 257.30±69.43 |
| Total (cm2) | 348.43±71.63 | 347.54±70.95 |
| Cholesterol | ||
| Total-Cho (mg/dL) | 221.4±28.4 | 219.7±26.4 |
| HDL-Cho (mg/dL) | 71.4±14.2 | 68.8±15.5 |
| LDL-Cho (mg/dL) | 139.0±29.3 | 137.7±25.4 |
Notes: Values are presented as mean±SD. There were no significant differences between the groups.
Abbreviations: BMI, body mass index; bpm, beats per minute; Cho, cholesterol; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HR, heart rate; KPE, Kaempferia parviflora extract; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Table 3.
Changes in energy intake and physical activity after daily intake of test foods
| Measurements | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|---|
| Energy intake (kcal) | Placebo | 1760.3±311.8 | 1787.5±379.8 | 1765.2±343.8 | 1690.6±359.7 |
| KPE | 1745.2±331.4 | 1737.5±341.6 | 1711.4±370.4 | 1672.5±338.6 | |
| Steps (number) | Placebo | 7300.9±2953.1 | 8194.6±3254.3 | 8021.2±3761.7 | 8145.8±4195.2 |
| KPE | 6620.2±3929.0 | 7558.2±4048.6 | 7568.5±3915.5 | 8083.1±4020.7 |
Notes: Data are presented as mean±SD. There were no significant differences between the groups. Energy intake was calculated based on the meal survey. The start of intake is represented as 0 weeks.
Abbreviation: KPE, Kaempferia parviflora extract.
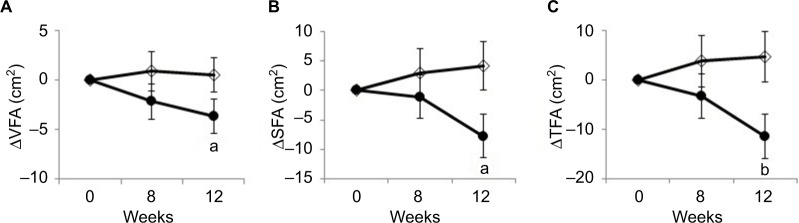
Figure 2 shows changes in VFA, SFA, and TFA compared with baseline. Changes in VFA were −3.67±9.20 and 0.50±8.62 cm2 in the KPE group and placebo group, respectively. Changes in SFA were −7.79±19.69 and 4.21±21.42 cm2 in the KPE group and placebo group, respectively. Changes in TFA were −11.46±23.38 and 4.71±25.84 cm2 in the KPE group and placebo group, respectively. The decrease in VFA, SFA, and TFA at 12W in the KPE group was significantly greater than that in the placebo group. The result of repeated measures ANOVA indicated a significant group effect in terms of TFA (p=0.028) and a significant time-by-group interaction between SFA and TFA (p=0.038 and p=0.038), respectively. The results of assessments of physical parameters (body weight, BMI, and lipid and glucose metabolism blood parameters) are shown in Table 4. FBG was higher in the KPE group compared with the placebo group at 0W. The change in FBG from 0W was significantly lower at 12W in the KPE group compared with the placebo group. Moreover, the decrease in TGs in the KPE group was significantly greater than that observed in the placebo group after 12W. There were no significant differences between the two groups in terms of the other parameters.
Figure 2.
Changes in abdominal fat area after daily intake of test food.
Notes: Values are presented as mean±standard error. Changes in VFA (A), SFA (B), and TFA (C) are shown as closed circles (KPE group) and open diamonds (Placebo group). Significant differences are observed between the groups (ap<0.05; bp<0.01).
Abbreviations: KPE, Kaempferia parviflora extract; SFA, subcutaneous fat area; TFA, total fat area; VFA, visceral fat area.
Table 4.
Changes in physical parameters after daily intake of test food
| Measurements | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks | |
|---|---|---|---|---|---|---|
| Body weight (kg) | Placebo | Values | 69.12±5.54 | 69.14±5.47 | 68.89±5.42 | 68.86±5.57 |
| Δ | 0.02±0.60 | −0.22±0.91 | −0.26±0.96 | |||
| KPE | Values | 68.63±7.14 | 68.63±7.24 | 68.33±7.16 | 68.14±7.18 | |
| Δ | 0.00±0.75 | −0.54±0.97 | −0.48±1.13 | |||
| BMI (kg/m2) | Placebo | Values | 27.16±1.52 | 27.17±1.57 | 27.07±1.56 | 27.06±1.58 |
| Δ | 0.01±0.24 | −0.09±0.37 | −0.10±0.37 | |||
| KPE | Values | 27.09±1.35 | 27.09±1.35 | 26.92±1.29 | 26.90±1.38 | |
| Δ | 0.01±0.29 | −0.22±0.38 | −0.19±0.45 | |||
| Total-Cho (mg/dL) | Placebo | Values | 211.9±29.9 | 209.4±27.2 | 215.6±28.4 | 215.1±28.4 |
| Δ | −2.5±15.4 | 3.6±18.5 | 3.1±20.3 | |||
| KPE | Values | 219.2±25.8 | 214.3±24.7 | 217.1±26.8 | 218.9±28.0 | |
| Δ | −4.9±14.8 | −1.9±18.5 | −0.3±16.0 | |||
| HDL-Cho (mg/dL) | Placebo | Values | 67.7±14.3 | 65.6±14.0 | 65.7±14.2 | 63.1±13.4 |
| Δ | −2.1±6.1 | −2.0±6.0 | −4.6±6.2 | |||
| KPE | Values | 67.6±16.2 | 65.3±15.2 | 64.3±15.8 | 63.1±13.7 | |
| Δ | −2.2±5.5 | −3.0±7.7 | −4.5±6.5 | |||
| LDL-Cho (mg/dL) | Placebo | Values | 130.5±26.4 | 127.1±27.6 | 133.9±30.6 | 132.2±25.5 |
| Δ | −3.4±15.4 | 3.4±18.0 | 1.7±16.9 | |||
| KPE | Values | 135.9±24.9 | 132.0±22.2 | 134.5±25.3 | 133.8±25.2 | |
| Δ | −3.9±11.9 | −1.5±16.4 | −2.1±13.2 | |||
| TGs (mg/dL) | Placebo | Values | 83.2±35.5 | 86.9±31.7 | 90.0±40.7 | 88.4±32.3 |
| Δ | 3.7±27.4 | 6.8±35.8 | 5.2±21.9 | |||
| KPE | Values | 102.6±67.0 | 91.6±36.9 | 98.5±57.7 | 92.3±54.9 | |
| Δ | −10.9±45.7 | −5.6±29.5 | −10.3±37.8a | |||
| FBG (mg/dL) | Placebo | Values | 85.7±7.3 | 86.5±7.3 | 86.5±7.7 | 88.6±9.2 |
| Δ | 0.8±4.9 | 0.8±6.2 | 2.9±6.6 | |||
| KPE | Values | 89.2±7.3a | 87.7±6.8 | 88.9±7.1 | 88.9±7.9 | |
| Δ | −1.5±4.9a | −0.4±6.0 | −0.3±5.3a | |||
| HbA1c (%) | Placebo | Values | 5.39±0.33 | Not measured | Not measured | 5.44±0.32 |
| Δ | 0.06±0.15 | |||||
| KPE | Values | 5.44±0.26 | Not measured | Not measured | 5.48±0.25 | |
| Δ | 0.04±0.19 |
Notes: Values are presented as mean±SD. Changes from the start of intake (0 weeks) are represented as delta (Δ). There were significant differences for the placebo group according to the Student’s t-test (aP<0.05).
Abbreviations: BMI, body mass index; Cho, cholesterol; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; KPE, Kaempferia parviflora extract; LDL, low-density lipoprotein; TGs, triglycerides.
We also performed subgroup analyses of healthy subjects corresponding to BMI ≥24 and <30 kg/m2, TGs <150 mg/dL, HDL-Cho ≥40 mg/dL, SBP <140 mmHg, DBP <90 mmHg, and FBG ≤125 mg/dL. Table 5 shows the changes in physical parameters after daily intake of the test food in these healthy subjects. Healthy subjects who ingested KPE exhibited significantly greater decreases in VFA, SFA, and TFA compared with healthy subjects who ingested the placebo. Moreover, no significant difference in TGs was observed between the placebo and KPE groups at 0W, but the KPE group exhibited a greater decrease in TGs levels at 12W compared with the placebo group.
Table 5.
Changes in physical parameters in healthy subjects (excluding subjects with dyslipidemia, hypertension, and hyperglycemia)
| Measurements | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks | |
|---|---|---|---|---|---|---|
| Body weight (kg) | Placebo | Values | 69.78±5.65 | 69.76±5.62 | 69.48±28.4 | 69.55±5.76 |
| Δ | −0.01±0.11 | −0.29±0.16 | −0.22±0.19 | |||
| KPE | Values | 66.35±6.45a | 66.42±6.45 | 66.18±6.55 | 65.92±6.44a | |
| Δ | 0.07±0.15 | −0.46±0.23 | −0.44±0.25 | |||
| BMI (kg/m2) | Placebo | Values | 27.43±1.37 | 27.43±1.43 | 27.31±1.40 | 27.35±1.45 |
| Δ | 0.00±0.05 | −0.12±0.06 | −0.09±0.07 | |||
| KPE | Values | 27.09±1.45 | 27.13±1.45 | 26.99±1.34 | 26.91±1.47 | |
| Δ | 0.04±0.06 | −0.20±0.09 | −0.18±0.10 | |||
| VFA (cm2) | Placebo | Values | 88.33±20.78 | Not measured | 88.55±21.95 | 89.08±19.18 |
| Δ | 0.22±1.85 | 0.75±1.77 | ||||
| KPE | Values | 84.72±18.26 | Not measured | 83.32±18.06 | 80.42±18.30 | |
| Δ | −2.17±1.70 | −4.30±1.40a | ||||
| SFA (cm2) | Placebo | Values | 276.21±68.29 | Not measured | 277.29±73.74 | 279.57±73.72 |
| Δ | 1.08±4.20 | 3.36±4.40 | ||||
| KPE | Values | 263.96±67.76 | Not measured | 267.96±63.89 | 254.43±59.51 | |
| Δ | 2.02±4.74 | −9.53±4.56a | ||||
| TFA (m2) | Placebo | Values | 364.54±72.51 | Not measured | 365.84±81.04 | 368.65±78.20 |
| Δ | 1.30±5.00 | 4.11±5.39 | ||||
| KPE | Values | 348.68±71.67 | Not measured | 351.28±64.90 | 334.85±62.79 | |
| Δ | −0.15±5.78 | −13.83±5.13a | ||||
| TGs (mg/dL) | Placebo | Values | 75.46±23.67 | 81.36±24.56 | 87.54±41.53 | 85.11±29.16 |
| Δ | 5.89±3.77 | 12.07±5.72 | 9.64±3.59 | |||
| KPE | Values | 70.83±21.30 | 79.38±27.13 | 74.26±25.54 | 69.21±24.59a | |
| Δ | 8.54±4.66 | 2.43±3.57 | −1.63±4.71 |
Notes: Values are presented as mean±SD. There were significant differences from the placebo group according to the Student’s t-test (ap<0.05).
Abbreviations: BMI, body mass index; KPE, Kaempferia parviflora extract; SFA, subcutaneous fat area; TFA, total fat area; TGs, triglycerides; VFA, visceral fat area.
The safety of KPE was evaluated in the 76 subjects participated in this study (analysis of ITT). Table 6 shows the changes in physical assessment parameters in the safety evaluation. There were no significant differences observed between groups in terms of physical parameters (SBP, DBP, and HR).
Table 6.
Changes in blood pressure and heart rate after daily intake of test food
| Measurements | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|---|
| SBP (mmHg) | Placebo | 123.9±15.1 | 120.4±16.0 | 122.5±15.9 | 117.5±14.0 |
| KPE | 125.7±10.7 | 120.8±12.7 | 124.0±14.1 | 118.3±11.7 | |
| DBP (mmHg) | Placebo | 74.9±11.6 | 71.6±12.8 | 73.3±11.7 | 68.8±11.4 |
| KPE | 74.6±9.0 | 70.8±9.2 | 72.3±9.2 | 67.7±10.8 | |
| HR (bpm) | Placebo | 67.2±9.1 | 68.0±8.5 | 66.3±8.2 | 71.3±8.9 |
| KPE | 66.1±7.6 | 67.1±7.3 | 66.4±7.7 | 70.0±8.1 |
Notes: Values are presented as the mean±SD. There were no significant differences between the groups.
Abbreviations: bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; KPE, Kaempferia parviflora extract; SBP, systolic blood pressure.
Tables 7 and 8 show the results of biochemical analyses. Significant differences in K levels were observed between the two groups at all time points except 0W, but all values were within the reference range. Significant differences in Cl levels were observed between the two groups at 0W. No significant differences in any other parameters were observed between the two groups throughout the study. Values of all biochemical parameters were within the range of physiologic variation. Table 9 shows the results of hematologic analyses. There were no significant differences in any of the hematologic parameters between the two groups at any time point, and values of all hematologic parameters were within reference ranges.
Table 7.
Changes in biochemical parameters after daily intake of test food
| Measurements | Standard range | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|---|---|
| TP (g/dL) | 6.7–8.3 | Placebo | 7.40±0.28 | 7.32±0.28 | 7.41±0.34 | 7.25±0.32 |
| KPE | 7.53±0.37 | 7.43±0.40 | 7.45±0.35 | 7.35±0.37 | ||
| Albumin (g/dL) | 3.8–5.2 | Placebo | 4.38±0.27 | 4.25±0.21 | 4.22±0.24 | 4.17±0.24 |
| KPE | 4.42±0.27 | 4.28±0.22 | 4.27±0.25 | 4.17±0.25 | ||
| T-bil (mg/dL) | 0.3–1.2 | Placebo | 0.76±0.26 | 0.78±0.26 | 0.74±0.27 | 0.78±0.28 |
| KPE | 0.78±0.26 | 0.78±0.28 | 0.80±0.26 | 0.80±0.26 | ||
| AST (U/L) | 10–40 | Placebo | 21.6±4.4 | 21.0±4.6 | 21.9±5.5 | 21.0±4.1 |
| KPE | 20.7±4.5 | 20.5±4.8 | 21.0±4.9 | 20.4±4.7 | ||
| ALT (U/L) | 5–40 | Placebo | 22.3±9.3 | 21.4±9.2 | 22.8±10.5 | 21.1±9.6 |
| KPE | 20.4±7.3 | 21.9±12.7 | 19.8±7.2 | 18.9±8.3 | ||
| γ-GTP (U/L) | M: 70 | Placebo | 29.2±22.3 | 30.2±30.3 | 29.7±23.5 | 28.6±21.7 |
| F: 30 | KPE | 26.1±16.3 | 27.0±18.8 | 24.4±15.7 | 24.6±17.3 | |
| LDH (U/L) | 115–245 | Placebo | 179.8±29.4 | 180.9±30.0 | 183.5±35.1 | 186.5±28.6 |
| KPE | 182.9±28.6 | 181.7±30.3 | 180.4±30.5 | 196.0±30.9 | ||
| ALP (U/L) | 115–359 | Placebo | 198.2±42.4 | 196.1±46.9 | 193.4±42.4 | 191.8±46.5 |
| KPE | 211.7±68.4 | 208.4±65.5 | 203.7±59.8 | 201.1±63.2 | ||
| Uric acid (mg/dL) | M: 3.7–7.0 | Placebo | 4.96±1.31 | 5.07±1.39 | 5.29±1.31 | 5.16±1.43 |
| F: 2.5–7.0 | KPE | 4.91±1.22 | 5.06±1.46 | 4.99±1.13 | 4.99±1.12 | |
| BUN (mg/dL) | 8.0–22.0 | Placebo | 13.16±3.35 | 12.92±3.20 | 13.69±3.29 | 13.72±3.24 |
| KPE | 11.99±3.42 | 13.10±3.42 | 12.52±3.05 | 12.41±3.74 | ||
| Creatinine (mg/dL) | M: 0.61–1.04 | Placebo | 0.642±0.098 | 0.625±0.113 | 0.677±0.085 | 0.664±0.090 |
| F: 0.47–0.79 | KPE | 0.651±0.144 | 0.637±0.151 | 0.677±0.145 | 0.674±0.140 |
Notes: Values are presented as mean±SD. There were no significant differences between the groups.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; γ-GTP, γ-glutamyltranspeptidase; KPE, Kaempferia parviflora extract; LDH, lactate dehydrogenase; T-bil, total bilirubin; TP, total protein.
Table 8.
Changes in blood electrolyte levels after daily intake of test food
| Measurements | Standard range | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|---|---|
| Na (mEq/L) | 136–147 | Placebo | 140.4±1.5 | 140.6±1.5 | 140.3±1.6 | 140.4±1.3 |
| KPE | 140.5±1.9 | 140.4±2.0 | 139.9±1.7 | 140.5±1.7 | ||
| K (mEq/L) | 3.6–5.0 | Placebo | 4.04±0.28 | 4.04±0.24 | 4.13±0.29 | 3.99±0.25 |
| KPE | 3.98±0.28 | 3.90±0.25a | 3.97±0.21b | 3.85±0.24a | ||
| Cl (mEq/L) | 98–109 | Placebo | 103.8±1.5 | 103.9±1.5 | 104.0±1.5 | 105.0±1.7 |
| KPE | 102.9±2.0a | 103.3±2.1 | 103.5±1.9 | 104.5±1.8 | ||
| Ca (mg/L) | 8.5–10.2 | Placebo | 9.19±0.27 | 9.19±0.30 | 9.36±0.23 | 9.26±0.24 |
| KPE | 9.30±0.24 | 9.28±0.25 | 9.40±0.26 | 9.23±0.21 | ||
| Mg (mg/L) | 1.8–2.6 | Placebo | 2.22±0.14 | 2.16±0.12 | 2.22±0.13 | 2.19±0.14 |
| KPE | 2.21±0.14 | 2.18±0.14 | 2.23±0.13 | 2.20±0.14 | ||
| Fe (μg/L) | M: 54–200 | Placebo | 116.8±43.5 | 114.1±44.8 | 107.6±38.1 | 109.0±40.8 |
| F: 48–154 | KPE | 107.5±25.8 | 109.3±38.3 | 114.7±43.3 | 100.2±34.1 |
Notes: Values are presented as mean±SD. There were significant differences between the groups (ap<0.05; bp<0.01).
Abbreviations: Ca, calcium; Cl, chloride; Fe, iron; K, potassium; KPE, Kaempferia parviflora extract; Mg, magnesium; Na, sodium.
Table 9.
Changes in hematologic parameters after daily intake of test food
| Measurements | Standard range | Group | 0 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|---|---|---|
| WBC (/μL) | M: 3900–9800 | Placebo | 5666±1425 | 5627±1291 | 5459±1242 | 5670±1262 |
| F: 3500–9100 | KPE | 5595±1359 | 5634±1414 | 5670±1474 | 5800±1562 | |
| RBC (×104/μL) | M: 427–570 | Placebo | 463.2±25.4 | 465.5±27.0 | 468.1±28.2 | 460.0±27.7 |
| F: 376–500 | KPE | 465.4±46.3 | 458.1±46.8 | 471.7±43.7 | 456.9±45.0 | |
| Hb (g/dL) | M: 13.5–17.6 | Placebo | 13.61±1.15 | 13.46±1.20 | 13.86±1.35 | 13.61±1.33 |
| F: 11.3–15.2 | KPE | 13.56±1.15 | 13.38±1.07 | 13.83±1.15 | 13.36±1.17 | |
| Ht (%) | M: 39.8–51.8 | Placebo | 42.28±2.86 | 42.12±3.21 | 43.09±3.54 | 43.01±3.48 |
| F: 33.4–44.9 | KPE | 42.14±3.31 | 41.89±3.08 | 42.89±3.16 | 42.35±3.27 | |
| Plts (×104/μL) | M: 13.1–36.2 | Placebo | 27.29±5.35 | 27.57±4.76 | 27.10±4.56 | 26.60±5.56 |
| F: 13.0–36.9 | KPE | 27.88±5.01 | 27.09±5.59 | 27.33±5.53 | 26.78±5.78 | |
| MCV (fL) | M: 82.7–101.6 | Placebo | 91.32±4.73 | 92.29±4.93 | 92.05±5.10 | 93.46±4.76 |
| F: 79.0–100.0 | KPE | 90.86±4.70 | 91.80±4.79 | 91.19±4.65 | 92.99±4.79 | |
| MCH (pg) | M: 28.0–34.6 | Placebo | 29.37±1.91 | 29.49±1.90 | 29.59±1.97 | 29.56±1.98 |
| F: 26.3–34.3 | KPE | 29.22±1.54 | 29.31±1.56 | 29.38±1.59 | 29.33±1.62 | |
| MCHC (%) | M: 31.6–36.6 | Placebo | 32.15±0.91 | 31.94±0.89 | 32.15±0.90 | 31.62±1.20 |
| F: 30.7–36.6 | KPE | 32.17±0.62 | 31.93±0.69 | 32.22±0.67 | 31.53±0.102 |
Notes: Values are presented as mean±SD. There were no significant differences between the groups.
Abbreviations: F, female; Hb, hemoglobin; Ht, hematocrit; KPE, Kaempferia parviflora extract; M, male; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; Plts, platelets; RBC, red blood cell; WBC, white blood cell.
The following adverse events were recorded in the KPE group: common cold in six cases; abdominal pain in two cases; and influenza, zinc deficiency, menopause, tooth ache, and cystitis in one case each. Adverse events reported in the placebo group included common cold in two cases and influenza, allergy to pollen, chest pain, herpes zoster, abdominal pain, and constipation in one case each. All adverse events reported involved symptoms that could have occurred regardless of participation in this study. The adverse events were transient, and there was no observed aggravation due to continuous ingestion of the test food in either group. There were no clinically problematic abnormalities or findings resulting from the physical assessments (Table 6), biochemical and hematologic analyses (Tables 7–9), urinalysis (Table 10), or medical interview conducted by the investigator, which included auscultation and percussion.
Table 10.
Changes in urinalysis parameters after daily intake of test food
| Measurements | Standard range | Group | 0 weeks
|
12 weeks
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| – | ± | + | – | ± | + | |||||||
| Urine protein | (–) | Placebo | 34 | 4 | 0 | 29 | 8 | 0 | ||||
| KPE | 34 | 4 | 0 | 28 | 9 | 1 | ||||||
| Urine glucose | (–) | Placebo | 38 | 0 | 0 | 37 | 0 | 0 | ||||
| KPE | 38 | 0 | 0 | 38 | 0 | 0 | ||||||
| Urobilinogen | (±) | Placebo | 0 | 38 | 0 | 0 | 37 | 0 | ||||
| KPE | 0 | 38 | 0 | 0 | 38 | 0 | ||||||
| Bilirubin | (–) | Placebo | 38 | 0 | 0 | 37 | 0 | 0 | ||||
| KPE | 38 | 0 | 0 | 38 | 0 | 0 | ||||||
| – | ± | + | 2+ | 3+ | – | ± | + | 2+ | 3+ | |||
| Ketone bodies | (–) | Placebo | 36 | 0 | 2 | 0 | 0 | 37 | 0 | 0 | 0 | 0 |
| KPE | 38 | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 1 | 0 | ||
| Occult blood | (–) | Placebo | 31 | 0 | 3 | 1 | 3 | 33 | 0 | 2 | 1 | 1 |
| KPE | 31 | 0 | 5 | 0 | 2 | 34 | 0 | 4 | 0 | 0 | ||
| Specific gravity | 1.005–1.030 | Placebo | 1.0153±0.0068 | 1.0183± | 0.0072 | |||||||
| KPE | 1.0160±0.0062 | 1.0170± | 0.0081 | |||||||||
| pH | 5.0–7.5 | Placebo | 6.25±0.75 | 6.34±0.84 | ||||||||
| KPE | 6.39±0.70 | 6.37±0.66 | ||||||||||
Notes: Urine protein, urine glucose, urobilinogen, bilirubin, ketone bodies, and occult blood: values indicate the number of subjects. Status is indicated as follows: −, negative; ±, false-positive; +, positive (mild); 2+, positive (moderate); 3+, positive (serious). Specific gravity and pH: values are presented as mean±SD. There were no significant differences between the groups.
Abbreviation: KPE, Kaempferia parviflora extract.
Discussion
In the present study, we evaluated the effect of continual KPE ingestion for 12 weeks in reducing abdominal fat in subjects with a BMI ≥24 and <30 kg/m2 via an inventional trial without change in lifestyle. We found that VFA decreased significantly in the KPE group compared with the placebo group after 12W. SFA, TFA, and TGs also decreased significantly in the KPE group after 12W compared with the placebo group. The subjects were instructed to maintain their usual eating, exercise, sleeping, smoking, and drinking habits during the study period. Indeed, energy intake and physical activity did not differ between the two groups throughout the study. As the only difference between the active and placebo test food capsules was the presence of KPE, the results of our study indicate that ingestion of KPE reduces abdominal fat in overweight and preobese subjects with a BMI ≥24 and <30 kg/m2, ruling out changes in energy intake and physical activity as causing the observed effects. It was previously reported that seasonal variations in food intake, physical activity, and body weight can affect VFA, SFA, and TFA.28,29 However, in the present study, no differences were observed compared with baseline values (0W) in terms of VFA, SFA, TFA, body weight, and BMI in the placebo group. Seasonal variation thus appears to have had little influence on the results.
The results of previous rodent and in vitro studies suggested that KPE and polymethoxyflavone reduce levels of body fat.19–23 Hence, we hypothesize that the effect of KPE in reducing abdominal fat in this study is mainly due to polymethoxyflavone contained in the KPE.
Because obesity develops when energy intake exceeds energy expenditure, reducing energy intake and accelerating energy expenditure are considered important strategies for preventing obesity.3 Some food ingredients reportedly modulate energy intake and energy expenditure in humans. For example, indigestible dextrin reportedly inhibits lipid absorption, and capsinoids accelerate energy expenditure.30–32 In the present study, ingestion of KPE did not result in a change in energy intake during the ingestion period, which suggests that the anti-obesity effects of KPE involve acceleration of energy expenditure rather than inhibition of energy intake. Indeed, we previously reported that KPE enhances energy expenditure in mice via activation of sympathetic nerve activity.23 Others reported that one intake of KPE increases whole-body energy expenditure in humans.24 Hence, a KPE-induced increase in energy expenditure could have contributed to the observed reduction in abdominal fat in our study.
Obesity is associated with metabolic disorders such as hypertension, diabetes mellitus, and atherosclerosis, which are risk factors for coronary artery disease.1–3 A number of studies have reported that body fat distribution is closely linked with risk of developing metabolic disorders, and an excess of abdominal fat, especially abdominal visceral fat, can lead to these disorders, irrespective of body weight.33–35 Accordingly, reducing body fat, especially visceral fat, is critical for the prevention of metabolic disorders. Our results indicate that KPE reduces TFA by reducing VFA. Therefore, KPE may be useful for preventing obesity as well as reducing the risk of metabolic disorders and coronary artery disease. The improvements in lipid metabolism, such as a reduction of TGs and an increase of HDL-Cho, reduce the risk of cardiovascular diseases. Indeed, fibrates are used to lower elevated TGs and raise HDL-Cho levels. Previous studies have shown that fibrates reduce cardiovascular diseases.36 Although no significant differences in HDL-Cho and LDL-Cho levels were observed in this study, KPE significantly decreased serum TGs. Therefore, daily intake of KPE may prevent and/or reduce the risk of cardiovascular diseases via improvements in lipid metabolism.
Adipomyokines such as leptin, adiponectin, and irisin and gut hormones such as ghrelin and glucagon-like peptide-1 play important roles in maintaining energy homeostasis.37,38 Imbalances in the circulating levels of these hormones are reportedly related to metabolic disorder development,37,39 and ingestion of specific foods has a beneficial effect on profiles of these factors. For example, regular consumption of foods and beverages containing catechin compounds increases adiponectin levels in individuals with metabolic syndrome and type 2 diabetes.40 It is anticipated that further intensive studies examining the effect of KPE on adipomyokine levels will provide evidence for whether KPE ingestion can prevent the development of metabolic disorders.
It has been reported that KPE is not associated with any pharmacotoxic or histopathological effects in rats.41 As KPE has been reported to be safe in animals, we evaluated the safety of KPE ingestion. In this study, no serious or test foods-related adverse events occurred. Moreover, there were no clinically problematic abnormalities or findings resulting from the physical assessments, biochemical and hematologic analyses, urinalysis, or medical interview conducted by the investigator. Therefore, KPE used in this study (150 mg KPE) is considered safe over long-term ingestion.
A potential limitation of this study was its relatively short duration; as such, the potential long-term effects of KPE ingestion remain unknown. As the differences in VFA, SFA, and TFA between the active KPE and placebo groups increased over time and did not plateau by the end of the study, it is possible that the benefits afforded by KPE would increase with longer ingestion. Moreover, despite careful instruction on dietary habits and other lifestyle factors, the changes in total and macronutrient intake and daily physical activity may have differed in the two groups. These between-group differences in compliance outcomes were not significant, but they may have affected the study results.
Conclusion
The results of the present study indicate that continual ingestion of KPE reduces abdominal fat in healthy humans with a BMI ≥24 and <30 kg/m2. KPE could therefore be a useful and safe food for preventing and/or improving obesity and the development of obesity-related metabolic disorders.
Acknowledgments
The authors would like to thank the volunteers who participated in this study. We acknowledge Ms. Heather Suzuki for substantially contributing to the preparation of the manuscript, Mr. Yoshitaka Hatakeyama who oversaw assignment of the test foods to the study subjects, and Mr Fujio Matsuo who generated randomization codes and created an allocation list. We also gratefully thank the contribution of all the study staff. Maruzen Pharmaceuticals Co. Ltd. sponsored this study.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
SY, RA, YM, and HK are employees of Maruzen Pharmaceuticals Co. Ltd. IF was the principal investigator. HS, TA, and ST are employees of New Drug Research Center Inc. The authors report no other conflicts of interest in this work.
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathieu P, Pibarot P, Despres JP. Metabolic syndrome: the danger signal in atherosclerosis. Vasc Health Risk Manag. 2006;2(3):285–302. doi: 10.2147/vhrm.2006.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 5.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86(Suppl 3):s867–s871. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 6.Frayn KN. Visceral fat and insulin resistance –causative or correlative? Br J Nutr. 2000;83(Suppl 1):S71–S77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- 7.Fujita H, Yokoyama K, Yoshikawa M. Classification and antihypertensive activity of angiotensin I-converting enzyme derived from food proteins. J Food Sci. 2000;65(4):564–569. [Google Scholar]
- 8.Fujita H, Yamagami T, Ohshima K. Long-term ingestion of a fermented soybean-derived touchi- extract with α-glucosidase inhibitory activity is safe and effective in human with borderline and mild type-2 diabetes. J Nutr. 2001;131(8):2105–2108. doi: 10.1093/jn/131.8.2105. [DOI] [PubMed] [Google Scholar]
- 9.Hursel R, Viechtbauer W, Dulloo AG, et al. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev. 2011;12(7):573–581. doi: 10.1111/j.1467-789X.2011.00862.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji H, Kasai M, Takeuchi H, Nakamura M, Okazaki M, Kondo K. Dietary medium-chain triacylglycerols suppress accumulation of body fat in a double blind, controlled trial in healthy men and women. J Nutr. 2001;131(11):2853–2859. doi: 10.1093/jn/131.11.2853. [DOI] [PubMed] [Google Scholar]
- 11.Sawasdee P, Sabphon C, Sitthiwongwanit D, Kokpol U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferiaparviflora. Phytother Res. 2009;23(12):1792–1794. doi: 10.1002/ptr.2858. [DOI] [PubMed] [Google Scholar]
- 12.Sae-wong C, Tansakul P, Tewtrakul S. Anti-inflammatory mechanism of Kaempferiaparviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J Ethnopharmacol. 2009;124(3):576–580. doi: 10.1016/j.jep.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 13.Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkol-wongse S, Chansuvanich N. Vasorelaxation and antispasmodic effects of Kaempferiaparviflora ethanolic extract in isolated rat organ studies. Fitoterapia. 2008;79(3):214–216. doi: 10.1016/j.fitote.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Tewtrakul S, Subhadhirasakul S. Anti-allergic activity of some selected plants in the Zingiberaceae family. J Ethnopharmacol. 2007;109(3):535–538. doi: 10.1016/j.jep.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Sae-wong C, Matsuda H, Tewtrakul S, et al. Suppressive effect of methoxyflavonoids isolated from Kaempferiaparviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J Ethnopharmacol. 2011;136(3):488–495. doi: 10.1016/j.jep.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Wattanapitayakul SK, Suwatronnakorn M, Chularojmontri L, et al. Kaempferiaparviflora ethanolic extract promoted nitric oxide production in human umbilical vein endothelial cells. J Ethnopharmacol. 2007;110(3):559–562. doi: 10.1016/j.jep.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S. Anti-gastric ulcer effect of Kaempferiaparviflora. J Ethnopharmacol. 2005;102(1):120–122. doi: 10.1016/j.jep.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, et al. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem. 2009;5(2):139–147. doi: 10.2174/157340609787582918. [DOI] [PubMed] [Google Scholar]
- 19.Horikawa T, Shimada T, Okabe Y, et al. Polymethoxyflavonoids from Kaempferiaparviflora induce adipogenesis on 3T3-L1 preadipocytes by regulating transcription factors at an early stage of differentiation. Biol Pharm Bull. 2012;35(5):686–692. doi: 10.1248/bpb.35.686. [DOI] [PubMed] [Google Scholar]
- 20.Okabe Y, Shimada T, Horikawa T, et al. Suppression of adipocyte hypertrophy by polymethoxyflavonoids isolated from Kaempferiaparviflora. Phytomedicine. 2014;21(6):800–806. doi: 10.1016/j.phymed.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Akase T, Shimada T, Terabayashi S, Ikeya Y, Sanada H, Aburada M. Antiobesity effects of Kaempferiaparviflora in spontaneously obese type II diabetic mice. J Nat Med. 2011;65(1):73–80. doi: 10.1007/s11418-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 22.Shimada T, Horikawa T, Ikeya Y, et al. Preventive effect of Kaempferiaparviflora ethyl acetate extract and its major components poly-methoxyflavonoid on metabolic diseases. Fitoterapia. 2011;82(8):1272–1278. doi: 10.1016/j.fitote.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino S, Kim M, Awa R, Kuwahara H, Kano Y, Kawada T. Kaempferiaparviflora extract increases energy consumption through activation of BAT in mice. Food Sci Nutr. 2014;2(6):634–637. doi: 10.1002/fsn3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita M, Yoneshiro T, Aita S, et al. Kaempferiaparviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J Nutr Sci Vitaminol. 2015;61(1):79–83. doi: 10.3177/jnsv.61.79. [DOI] [PubMed] [Google Scholar]
- 25.Yoshino S, Awa R, Miyake Y, et al. Effects of single oral intake of Kaempferiaparviflora extract on energy metabolism –a randomized double-blind crossover study- Jpn Pharmacol Ther. 2016;44(12):1757–1762. [Google Scholar]
- 26.WHO Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 27.Takahashi H, Mori M. Characteristics and significance of criteria for obesity disease in Japan 2011. Nihon Rinsho. 2013;71(2):257–261. [PubMed] [Google Scholar]
- 28.Ma Y, Olendzki BC, Li W, et al. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur J Clin Nutr. 2006;60(4):519–528. doi: 10.1038/sj.ejcn.1602346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kajimoto O, Kajimoto Y, Yabune M, et al. Tea catechins with a galloyl moiety reduce body weight and fat. J Health Sci. 2005;51(2):161–171. [Google Scholar]
- 30.Kishimoto Y, Oga H, Tagami H, Okuma K, Gordon DT. Suppressive effect of resistant maltodextrin on postprandial blood triacylglycerol elevation. Eur J Nutr. 2007;46(3):133–138. doi: 10.1007/s00394-007-0643-1. [DOI] [PubMed] [Google Scholar]
- 31.Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol. 2013;24(1):71–77. doi: 10.1097/MOL.0b013e32835a4f40. [DOI] [PubMed] [Google Scholar]
- 32.Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imbeault P, Lemieux S, Prudhomme D, et al. Relationship of visceral adipose tissue to metabolic risk factors for coronary heart disease: is there a contribution of subcutaneous fat cell hypertrophy? Metabolism. 1999;48(3):355–362. doi: 10.1016/s0026-0495(99)90085-9. [DOI] [PubMed] [Google Scholar]
- 34.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 35.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 36.Adkins JC, Faulds D. Micronised fenofibrate: a review of its pharmacodynamics properties and clinical efficacy in the management of dyslipidemia. Drugs. 1997;54(4):615–633. doi: 10.2165/00003495-199754040-00007. [DOI] [PubMed] [Google Scholar]
- 37.Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463–500. doi: 10.1210/er.2012-1041. [DOI] [PubMed] [Google Scholar]
- 38.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444(7121):854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 39.Crujeiras AB, Zulet MA, Lopez-Legarrea P, et al. Association between circulating irisin levels and the promotion of weight-lowering program in obese patients. Metabolism. 2014;63(4):520–531. doi: 10.1016/j.metabol.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Nagao T, Meguro S, Hase T, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2009;17(2):310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 41.Songpol C, Pranee C, Aimmanas A, Anudep R. Chronic study of Kaempferia parviflora Wall ex. Extract. Thai J Vet Med. 2010;40(4):377–383. [Google Scholar]