Abstract
The present longitudinal study is the first to investigate the association between human breast milk cortisol and infant crying over the first three months of life. Higher concentrations of breast milk cortisol were expected to be differentially associated with fussing and crying in boys and girls. At 2, 6, and 12 weeks of infant age, mothers (N = 70) collected a morning sample of their milk and kept a 3‐day diary to measure infant fussing and crying. Cortisol was extracted and quantified from milk samples. Results showed that breast milk cortisol concentrations increased from 2 weeks through 12 weeks of infant age. Milk cortisol was unrelated to the total duration, frequency, and bout length of infant fussing and crying for both boys and girls. Directions for future research aiming to extend our knowledge on the biology of milk cortisol in relation to infant behavior and development are discussed.
Keywords: breast milk cortisol, breastfeeding, humans, infant crying, lactational programming
1. INTRODUCTION
Research shows that breastfeeding carries many benefits for infant health and development. For example, a longer duration of breastfeeding has been related to fewer infant illnesses (Pettigrew et al., 2003; Thomas, 2014) and better cognitive development, even beyond infancy and childhood (Agostoni et al., 2009; Evenhouse & Reilley, 2005; Huang, Peters, Vaughn, & Witko, 2014). Less research has been devoted to the possible effects of biological constituents of breast milk on offspring phenotype. Breast milk contains, among others, water, protein, carbohydrates, immune factors, and several hormones (Miller et al., 2013). The present study focuses on the hormone cortisol, the primary glucocorticoid in humans (Glynn et al., 2007). Cortisol serves numerous functions, orchestrating aspects of the physiological stress response, immunity, and metabolism. For example, cortisol concentrations increase when humans are confronted with an acute psychological or biological stressor and the hypothalamic‐pituitary‐adrenal (HPA)‐axis is activated (Dickerson & Kemeny, 2004). Furthermore, cortisol concentrations are also involved in the routine metabolism of proteins, carbohydrates, and fats (Hinde et al., 2014). Earlier research has shown that in animals, concentrations of glucocorticoids in maternal milk are related to offspring behavior (Hinde, 2013; Sullivan, Hinde, Mendoza, & Capitanio, 2011). The current study investigated whether breast milk cortisol is related to behavior, more specifically fussing and crying, in human infants.
During pregnancy, a mother's physiology can impact the developing fetus (Beijers, Buitelaar, & de Weerth, 2014). Maternal physiology is thought to relay information about the environment to the fetus, and thereby prepare the infant for the time after birth. The fetus can thus start to develop a behavioral profile matching the environment it will likely live in, a process also referred to as fetal programming (Del Giudice, 2012; Frankenhuis & de Weerth, 2013). After birth and during lactation, mothers can still transfer physiological signals to the infant through the biological constituents of breast milk (Hinde et al., 2014), including cortisol. Cortisol concentrations are transferred from plasma to breast milk, as there is no mammary synthesis of cortisol (Hamosh, 2001). In line with the fetal programming hypothesis, it might be argued that, when the environment is stressful, mothers could physiologically signal this information to their infants via breast milk cortisol, and infants could subsequently adjust their behavioral phenotype to their (future) environment. That is, mothers with higher milk cortisol concentrations might be experiencing more stressors in their life, and milk cortisol would partly underlie the maternal organization of biobehavioral processes of the offspring (Dettmer et al., 2017). This hypothesis is also referred to as the lactational programming hypothesis (Hinde, 2013). The lactational programming hypothesis is the idea that biological constituents of breast milk influence metabolic and neurobiological development which in turn influence offspring phenotype and behavior (Hinde, 2013). This hypothesis is supported by studies showing that early infancy is a sensitive period in which the brain shows large plasticity and openness to environmental influences (Kolb & Gibb, 2011). Additionally, especially early in life, the infant's intestinal tract has a high number of glucocorticoid receptors (Allen‐Blevis, Sela, & Hinde, 2015). Hence, milk cortisol that reaches the infants' intestines can easily cross the intestinal epithelial barrier (Hinde et al., 2014), as well as later on pass the blood‐brain barrier (Glynn et al., 2007). In the brain, cortisol seems to specifically bind to receptors in the limbic region (Owen, Andrews, & Matthews, 2005), a brain area involved in, among others, the regulation of emotions and behavior (Grey, Davis, Sandman, & Glynn, 2013).
In stressful environments, infants are suggested to signal needs to the caregivers, as caregivers are less likely to be involved with and available for their children when confronted with stress (Baker, 2014). To ensure being fed and taken care of, young infants are expected to trigger caregivers’ attention by fussing and crying. Indeed, in some contexts, fussing and crying may be particularly appropriate and even life‐saving for the infant (Barr, 2000). This hypothesis is in line with findings of de Vries (1984), who showed that in an environment with extreme draught and hunger, fussy infants survived more often than easy infants. Hence, and based on the lactational programming hypothesis (Hinde, 2013), higher breast milk cortisol concentrations are expected to be related to more fussing and crying behavior in infants. It is important to note, however, that even though more infant fussing and crying might be considered adaptive in stressful environments, excessive fussing and crying is energetically costly, and may be deviating energy from other developmental purposes, including physical growth and health (De Lauzon‐Guillain et al., 2012). Also, excessive infant fussing and crying may in some cases be indicating a regulatory problem that will persist into childhood (Bilgin & Wolke, 2017). Moreover, excessive infant fussing and crying causes considerable concern, distress, and exhaustion in parents (de Weerth, Fuentes, & de Vos, 2013), and in extreme cases can lead to child abuse and neglect (Reijneveld, van der Wal, Brugman, Hira Sing, & Verloove‐Vanhorick, 2004). Thus, even though more fussing and crying might be adaptive in stressful environments, excessive fussing and crying may be associated with negative outcomes for both the infant and the parents.
To date, no research has been published on the link between breast milk cortisol and infant fussing and crying. However, previous animal research indicates that a link exists between breast milk glucocorticoids and offspring behavior. Higher glucocorticoid concentrations in breast milk were related to reduced anxiety and improved learning in rat offspring (Catalani et al., 2002, 2000 ). In monkey offspring, higher cortisol concentrations were related to more frequent play and social behavior (Dettmer et al., 2017). Also, in rhesus monkeys, breast milk cortisol concentrations were positively related to more confident temperament, but only in male offspring (Sullivan et al., 2011). However, in a following, more extensive study in the same primate colony, breast milk cortisol concentrations were positively related to more nervous and less confident temperament in both male and female offspring (Hinde et al., 2014). In this study, for female offspring, this more nervous and less confident temperament was related to absolute concentrations of breast milk cortisol, whereas for male offspring, this type of temperament was related to an increase in cortisol concentrations from 1 to 3–4 months.
Only a few human studies have investigated the link between breast milk cortisol and infant behavior. In the first study on breastfeeding mother–infant pairs, higher cortisol concentrations in maternal plasma—an indicator of cortisol concentrations in breast milk—were related to more mother reported fearful temperament in 2‐month‐old infants (Glynn et al., 2007). A later study from the same research group on 3‐month‐old infants showed that higher cortisol concentrations in breast milk predicted more reported temperamental negative affectivity (including sadness and fear), but only in female infants (Grey et al., 2013). Finally, a recent study found that higher breast milk cortisol concentrations at 2.5 months postpartum were associated with higher induced fear reactivity in 8‐month‐old infant girls, but not boys (Nolvi et al, 2017).
In sum, only a few studies examined links between human breast milk cortisol and infant behavior and found that higher cortisol concentrations were related to more infant temperamental negativity (Glynn et al., 2007; Grey et al., 2013; Nolvi et al., 2017). The present study aims to longitudinally investigate associations between breast milk cortisol and infant fussing and crying. Note that the link between breast milk cortisol and infant fussing and crying behavior may be bi‐directional in nature, as it is also possible that infant fussing and crying predicts more maternal stress, and subsequently, higher cortisol in breast milk. Infant crying has been found to impair maternal psychological well‐being and decrease sleep quality (Barr et al., 2014; Brand, Furlano, Sidler, Schulz, & Holsboer‐Trachsler, 2014), which in turn may be related to more cortisol in breast milk. In the current study, infant fussing and crying was measured at 2, 6, and 12 weeks of infant age, because these ages represent, respectively, the start, peak, and decrease of the normal developmental crying curve (Barr, Trent, & Cross, 2006). Advantages of the longitudinal design are the ability to examine the stability of breast milk cortisol over time and to investigate the associations of milk cortisol with infant fussing and crying over time. We expected higher concentrations of breast milk cortisol to be related to higher levels of infant fussing and crying. Furthermore, because of the indications that breast milk cortisol may predict offspring behavior differentially for male and female offspring (Dettmer et al., 2017; Grey et al., 2013; Nolvi et al., 2017), we explored sex differences in the link between breast milk cortisol and infant fussing and crying.
2. METHOD
2.1. Participants
Participants were part of the BINGO study (Dutch acronym for Biological Influences on Baby's Health and Development), a longitudinal study aimed to identify prenatal and early postnatal predictors of infant health and development. This study was approved by the ethical committee of the Faculty of Social Sciences of the Radboud University [ECSW2014‐1003‐189]. Participants signed up via the project's website, or folders that were handed out in midwife practices, pregnancy courses, and baby stores in the region Nijmegen‐Arnhem (Netherlands). Participants received a voucher for 20€ and two presents for the infant.
Initial exclusion criteria were drug use, excessive alcohol use (i.e., alcohol dependency), insufficient knowledge of the Dutch language, and an unhealthy, complicated pregnancy. Nulliparity was not requested. Eighty‐eight eligible expectant mothers signed the informed consent form and subsequently participated in this project. Postnatal exclusion criteria were: complications during pregnancy (after initial contact), gestational age at birth <37 weeks, birth weight <2,500 g, 5‐min Apgar score <7, and congenital malformations. Five infants were born between 35 and 37 weeks of pregnancy. As these infants were otherwise healthy and fit the inclusion criteria, they were not excluded. Statistical analyses with and without these five infants rendered comparable results. One infant was born prematurely (week 32), and one infant was born with brain damage; both were excluded from further participation. Five families stopped participation after birth due to personal reasons. There were no differences in educational level, maternal age, gestational age at birth, or infant birth weight between participating and nonparticipating mothers. Of the 81 remaining mothers, 77 mothers started breastfeeding after birth and were therefore included in the current study. See Figure 1 for a flow chart of the sample.
Figure 1.
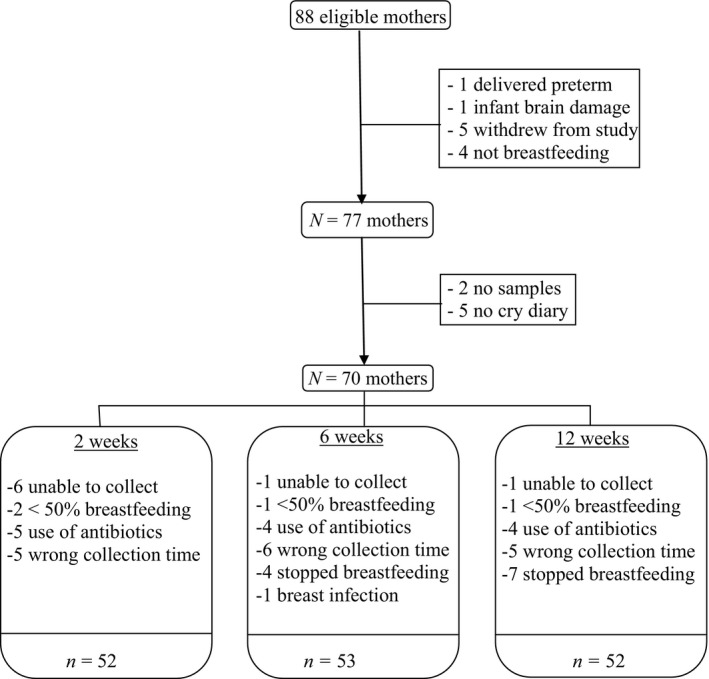
Flow chart of the sample
2.2. Procedure
Mothers were asked, and reminded the day before by email, to collect the following data on the day after the infant reached the age of 2 weeks (M age = 14.63 days; SD = 1.60), 6 weeks (M age = 43.46 days; SD = 4.79), and 12 weeks (M age = 85.38 days; SD = 2.31):
2.2.1. Breast milk
Mothers collected approximately 20 ml of the first breast milk in the morning (Mtime = 08:41, SDtime = 3:03) in small, sterile cups by hand expression. The average time between waking up and collection time of breast milk sampling was 79.92 min (SD = 170.52 min). Three mothers reported to have problems with hand expression, and they collected milk via a breast pump machine. Cortisol levels between mothers who collected by hand (M = 12.12, SD = 10.20) and mothers who collected by machine (M = 10.95, SD = 7.74) did not differ (F = 0.08, p=0.782).
Mothers were asked to wash their hands, breasts, and nipples before collection, and, in the case of the breast pump, to first boil the parts that came in contact with the milk. Mothers noted the date, time of waking, time of collection, whether they were or had been ill and/or taken medication the preceding week, and if so, which medication. The mothers collected the milk before feeding the infant. Samples were stored in the mothers' freezers and collected with a portable freezer after the last sample was taken (approximately when the infant was 13 weeks of age). At the Radboud University, all samples were stored in a freezer at −80°C, and subsequently sent by temperature‐controlled shipment to the Utrecht University Medical Centre. About 4 ml was needed for cortisol analysis.
2.2.2. Infant fussing and crying
Infant fussing and crying was assessed using the Baby Day Diary (Barr, Kramer, Boisjoly, McVey‐White, & Pless, 1988) for three consecutive days at each time point. Each day, mothers reported the occurrence of the following infant behaviors: fussing, crying, unsoothable crying, sleeping, feeding, and being awake without crying. This was indicated with lines/symbols assigned to each behavior on time bars. Each 24‐hr period was represented on a sheet of paper with four horizontal 6‐hr time bars, subdivided into periods of five minutes (Barr et al., 1988). Mothers were asked to retrospectively fill in this diary every two to three hours. The measurement of fussing and crying in the Baby Day Diary is known to be valid, and to produce data comparable to actual audio recordings (r = 0.90; Barr et al., 1988). The Baby Day Diary has been extensively used in other research (e.g., de Weerth, Fuentes, Puylaert, & de Vos, 2013; de Weerth, Fuentes, & de Vos, 2013; Korja et al., 2014; Kusaka, Ohgi, Shigemori, & Fujimoto, 2008; Radesky et al., 2013). The breast milk sample was collected on one of the three days the Baby Day Diary was filled in.
3. MEASUREMENTS
3.1. Breast milk cortisol
Breast milk samples were analyzed in the University Medical Centre in Utrecht, the Netherlands. Samples were centrifuged with a force of 21.000 g, and the fatty layer was discarded. Then, d4‐cortisol was added as an internal standard, and cortisol was extracted with methyl tertiary butyl ether. Cortisol was quantified by Liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Calibrator solutions were prepared from Sigma‐Aldrich cortisol preparations. The UHPLC‐MS/MS system consisted of a Dionex Ultimate 3000 UHPLC system coupled with a TSQ Quantiva mass spectrometer (ThermoElectron Corp, West Palm Beach, FL). A Hypersil Gold 50×2.1 mm 1.9 µm column was used with a methanol/water gradient containing ammoniumformiate and formic acid for the UHPLC separation. Day‐to‐day imprecision for cortisol was 1.9% at 6 nmol/L (n = 6), LLQ is 1.0 nmol/L for cortisol.
3.2. Infant fussing and crying
The diary data were prepared for analysis by noting the number of times fussing, crying, and unsoothable crying occurred (i.e., frequency), and how long the behaviors lasted (i.e., bout length). No distinction was made between fussing, crying and unsoothable crying in the analyses, and the behavior is henceforth referred to as “crying.” This was done for each of the three days separately and then the mean daily frequency and bout length were calculated. The multiplication of the mean daily frequency and bout length rendered the mean total duration of crying. This lead to three outcome measures for crying: total duration (mean duration in minutes per 24 hr), frequency (mean number of episodes per 24 hr), and bout (mean bout length of each episode) (Fujiwara, Barr, Brant, & Barr, 2011).
Although fussing, crying, and unsoothable crying represent gradations in the intensity of crying that may be differentially displayed in different types of situations, in this study they were combined. This is common practice in research on young infants (Wolke, Bilgin, & Smara, 2017) who are not yet using fussing in an instrumental manner. Furthermore, the original research on the Baby Day Diary (Barr et al., 1988) showed that negative vocalizations tended to cluster together and that omitting fussing resulted in missing an important sign of infant distress. Also, by doing this, we reduced the number of outcome variables, hence reducing chance capitalization, and we obtained a robust measure of fussing and crying that was more in line with the general temperament measures used in the three earlier human studies on milk cortisol and behavior, namely negative affectivity, fearfulness, and fear reactivity (Grey et al., 2013; Glynn et al., 2007; Nolvi et al., 2017). It is important to note here that analyses in which fussing was excluded from the combined crying variable rendered comparable results.
3.3. Control variables
In line with Grey et al. (2013), Glynn et al. (2007), and Hinde et al. (2014), we controlled for maternal educational level and parity. Though infants with a birth weight lower than 2,500 g were excluded, birth weight was also included as a control variable because it can reflect infant vulnerability and predict both infant crying behavior (Milidou, Søndergaard, Jensen, Olsen, & Henriksen, 2014) and maternal stress (Halpern, Brand, & Malone, 2001). Furthermore, taking cortisol circadian variations into account and in line with previous research (Glynn et al., 2007; Grey et al., 2013; Nolvi et al., 2017), we controlled for collection time of breast milk sampling. Additionally, we controlled for the time interval between waking up and collection time of breast milk sampling.
Pearson correlations showed no significant relation between infant sex and number of Baby Day Diary assessments (r = −0.06, p = 0.629), nor between infant sex and number of breast milk assessments (r = −0.07, p = 0.596). There were also no significant relations between breast milk cortisol concentrations and the number of Baby Day Diary assessments (r = −0.09, p = 0.274), and breast milk cortisol concentrations and number of breast milk cortisol assessments (r = −0.05, p = 0.553). Number of Baby Day Diary and breast milk cortisol assessments were thus not included as control variables.
3.4. Missing data
An overview of sample flow and missing data is provided in Figure 1. Of the initial 77 breastfeeding mothers, two mothers did not return any breast milk samples and five mothers did not complete the crying diaries. Subsequently, we excluded samples of mothers reporting to breastfeed for less than 50% of the time, as we speculated that potential effects of breast milk cortisol would only be detectable if infants received substantial amounts of breastfeeding. Additionally, we excluded samples of mothers reporting illness and/or antibiotic use. Samples were also excluded when they were collected before 4:00 a.m. or after 12:00 p.m. since recent research indicated that cortisol in breast milk follows a circadian rhythm similar to cortisol in saliva (Pundir et al., 2017; van der Voorn et al., 2016).
At two weeks of infant age, breast milk samples were missing due to the following reasons: not able to collect (enough) breast milk (n = 6), breastfeeding for less than half of the feedings (n = 2), use of antibiotics (n = 5), and collection before 4:00 a.m. or after 12:00 p.m. (n = 5). Additionally, two mothers did not fill in the crying diary at two weeks of age.
At six weeks of infant age, breast milk samples were missing due to inability to collect breast milk (n = 1), use of antibiotics (n = 4), breast infection (n = 1), stopped breastfeeding (n = 4), use of formula for more than 50% of the time (n = 1), and collection before 4 a.m. or after 12 p.m. (n = 6). Additionally, one mother did not fill in the crying diary at six weeks of age.
At 12 weeks of infant age, breast milk samples were missing due to inability to collect (enough) breast milk (n = 1), use of antibiotics (n = 4), stopped breastfeeding (n = 7), use of formula for more than 50% of the time (n = 1), and collection before 4 a.m. or after 12 p.m. (n = 5).
In total, 68 mothers filled in the crying diary at all time points, one mother missed one time point and one mother only filled in the crying diary once. With respect to milk samples, due to missing samples and exclusion criteria, we were able to include all three breast milk cortisol samples for 41 mothers, two for 12 mothers, and one for 10 mothers. As multilevel analyses are robust for missing data (Tabachnik & Fidell, 2007), families with missing data at one or two of the time points were still included in the overall analysis and no imputation was needed. Thus, the total number of participants in the analyses is 70.
3.5. Statistical analyses
Two values for milk cortisol were outside the range of ±3 SD and were subsequently replaced with the highest cortisol value inside the range. Deleting these outliers instead of replacing them led to comparable results.
First, to investigate the stability of milk cortisol over time, multilevel models with time were used. To investigate whether breast milk cortisol is related to infant crying, three separate analyses were done; one for crying total duration, one for crying frequency, and one for crying bout. Due to the longitudinal nature of our design, multilevel (hierarchical) linear modeling (MLM), also known as mixed model analysis, was used. MLM is robust for missing data and is unaffected by unequal sample sizes (Tabachnik & Fidell, 2007). Therefore, there was no need to control for the fact that not every mother collected a breast milk sample at each time point, and we could run the analyses on the full data set (N = 70).
MLM is conveyed as a set of regression equations. First, the intercepts‐only model (a model without variables) is run in order to check whether a multilevel model is required, by means of the intraclass correlation. The intraclass correlations were 0.24 for crying total duration, 0.59 for crying frequency, and 0.72 for crying bout, which shows that multilevel analyses were appropriate. The participant was the level 2 identifier, and the outcome measure and other measures were the level 1 variables. Second, following Tabachnik and Fidell (2007), a build‐up strategy was used. To the intercept‐only model, variables are added one at a time. After each addition, the −2 log likelihood ratio scale after generalized least square estimation is examined. The −2 log likelihood is a determinant of model fit. If model fit increases, the added variable is kept. If model fit decreases, the added variable is cut from the model.
Linear time and quadratic time were entered first into the model, with linear time considered as a random factor. The time model that best improved the model fit was retained. Then, the control variables parity, infant sex, maternal educational level, birth weight, collection time, and the time interval between waking up and collection time of breast milk sampling were added one by one.
Thereafter, breast milk cortisol was added to the model. Then, interaction terms between breast milk cortisol and time, and between breast milk cortisol and quadratic time were added to examine the associations of breast milk cortisol with crying over time. Finally, the interaction term between infant sex and breast milk cortisol was added to examine sex differences. The final, best fitting models for crying total duration, crying frequency and crying bout are presented in the results. Final models were checked in regression analyses for normality of the residuals; residuals showed nearly normality. See Table 1 for Durbin–Watson indices and Variance inflation factors. Analyses were done using SPSS®, version 22.0.0.1 (IBM Corporation, Armonk NY, USA) for Windows®.
Table 1.
Regression statistics
| Crying total duration | Crying frequency | Crying bout | |
|---|---|---|---|
| Durbin‐Watson indexa | 1.793 | 1.463 | 1.542 |
| VIFb | |||
| Birthweight infant | 1.070 | 1.110 | 1.130 |
| Education | 1.038 | 1.038 | 1.043 |
| Sex infant | na | 1.044 | 1.044 |
| Collection time | 5.355 | 5.384 | 5.448 |
| Interval between waking and collection | 5.208 | 5.215 | 5.217 |
| Milk cortisol | 1.063 | 1.064 | 2.807 |
| Interaction infant SexXCortisol | na | na | na |
Notes. aDurbin‐Watson statistic is a measure of autocorrelation in residuals, and is always between 0 and 4. A value of 2 means no autocorrelation, values approaching 0 indicate positive and values approaching 4 indicate negative autocorrelation.
bVariance inflation factors (VIF) indicate how much the variance of the estimated regression coefficients are inflated. VIF = 1 means not correlated, VIF 1–5 means moderately correlated, VIF 5–10 means highly correlated.
4. RESULTS
4.1. Preliminary analyses
On average, breast milk cortisol levels increased from 2 weeks through 12 weeks of infant age (see Table 2).
Table 2.
Demographic statistics for maternal and infant characteristics, separately for boys and girls
| M ( SD ) |
Boys (n = 36) M ( SD ) |
Girls (n = 34) M ( SD ) |
|
|---|---|---|---|
| Age of mother | 31.81 ( 3.67) | ||
| Educational level mother | |||
| College | n = 21 (30%) | ||
| University | n = 37 (53%) | ||
| Cortisol (nmol/L) | |||
| 2 weeks | 10.06 (9.72), n = 52 | 9.59 (9.03), n = 27 | 10.56 (10.57), n = 25 |
| 6 weeks | 12.26 (9.07), n = 53 | 12.62 (7.25), n = 28 | 11.87 (10.89), n = 25 |
| 12 weeks | 13.24 (8.82), n = 52 | 12.36 (8.34), n = 23 | 13.95 (9.26), n = 29 |
| Parity | First n = 58, Second n = 12 | ||
| Gestational age at birth | 39.89 (1.53), n = 70 | 40.05 (1.51), n = 36 | 39.72 (1.55), n = 34 |
| Birth weight (in grams) | 3,544.23 (414.053), n = 66 | 3,613.26 (462.20), n = 34 | 3,470.88 (348.25), n = 32 |
| Crying bouta , b | |||
| 2 weeks | 17.03 (14.76), n = 68 | 14.84 (8.74), n = 35 | 19.35 (19.09), n = 33 |
| 6 weeks | 15.63 (12.73), n = 69 | 13.90 (6.99), n = 36 | 17.52 (16.84), n = 33 |
| 12 weeks | 13.60 (11.03), n = 70 | 12.17 (7.20), n = 36 | 15.12 (13.96), n = 34 |
| Crying frequencyc | |||
| 2 weeks * | 9.25 (4.26), n = 68 | 10.35 (4.58), n = 35 | 8.08 (3.61), n = 33 |
| 6 weeks * | 9.96 (4.58), n = 69 | 11.55 (4.87), n = 36 | 8.22 (3.56), n = 33 |
| 12 weeks † | 7.36 (4.13), n = 70 | 8.17 (4.35), n = 36 | 6.50 (3.76), n = 34 |
| Crying durationd | |||
| 2 weeks | 131.85 (64.83), n = 68 | 135.14 (59.76), n = 35 | 128.35 (70.58), n = 33 |
| 6 weeks | 134.56 (62.88), n = 69 | 145.83 (61.45), n = 36 | 122.27 (63.02), n = 33 |
| 12 weeks | 89.23 (48.21), n = 69 | 92.36 (48.98), n = 36 | 85.82 (47.88), n = 33 |
Notes. For t tests for the difference between female and male infants: † p <0.10, *p <0.05.
Crying levels are in the normal range for healthy infants of these ages (Wolke et al., 2017).
Mean bout length of each crying episode in minutes.
Mean number of episodes per 24 hr.
Mean duration in minutes per 24 hr.
To examine stability or changes in breast milk cortisol levels over time, multilevel analyses with time and quadratic time were performed (see Table 3). The time model proved a better fit; time was significant (t = 2.23, p=0.028).
Table 3.
Mixed model analysis for breast milk cortisol across time
| Estimate | Standard error | p | |
|---|---|---|---|
| Intercept | 9.65 | 1.35 | <0.001 |
| Time | 0.34 | 0.15 | 0.028 |
| Deviance | 1,040.345 | ||
Note. N = 70.
Bivariate Pearson correlations, not presented here, showed modest correlations (r's ranging between −0.41 and 0.68), between crying duration, frequency and bout indicating that they measure different constructs, justifying the separate analyses. Table 4 presents the bivariate Pearson correlations between milk cortisol and infant crying variables. Breast milk cortisol levels at 2 weeks were positively related to breast milk cortisol levels at 6 weeks (r = 0.36, p = 0.009), and positively related to cortisol levels at 12 weeks (r = 0.54, p < 0.001). Breast milk cortisol levels at 6 weeks were not related to breast milk cortisol levels at 12 weeks (r = 0.06, p = 0.670). Finally, milk cortisol at 6 weeks was positively correlated to crying duration at 2 weeks (r = 0.35, p < 0.05) and negatively correlated to crying bout at 12 weeks (r = −0.32, p < 0.05).
Table 4.
Correlations between breast milk cortisol and infant fussing and crying
|
Whole sample Cortisol |
Male infants Cortisol |
Female infants Cortisol |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 weeks | 6 weeks | 12 weeks | 2 weeks | 6 weeks | 12 weeks | 2 weeks | 6 weeks | 12 weeks | |
| Cortisol | |||||||||
| 2 weeks | 1 | 0.36 * * | 0.54 * * | 1 | 0.51 | 0.68 * * | 1 | 0.30 | 0.47 * |
| 6 weeks | 1 | 0.06 | 1 | 0.46 * | 1 | −0.10 | |||
| 12 weeks | 1 | 1 | 1 | ||||||
| Crying bout | |||||||||
| 2 weeks | −0.17 | 0.10 | −0.12 | −0.25 | −0.24 | −0.41 | −0.17 | 0.35 | 0.06 |
| 6 weeks | −0.11 | 0.20 | −0.20 | −0.19 | −0.19 | −0.45 * | −0.07 | 0.47 * | −0.03 |
| 12 weeks | −0.03 | −0.32 * | 0.04 | −0.13 | −0.17 | −0.39 | 0.05 | 0.44 * * | 0.32 |
| Crying frequency | |||||||||
| 2 weeks | 0.18 | 0.14 | 0.15 | 0.21 | 0.20 | 0.25 | 0.21 | 0.09 | 0.14 |
| 6 weeks | 0.18 | 0.14 | 0.13 | 0.17 | 0.16 | 0.34 | 0.27 | 0.12 | 0.05 |
| 12 weeks | 0.11 | 0.11 | 0.00 | 0.15 | 0.11 | 0.07 | 0.12 | 0.10 | −0.02 |
| Crying duration | |||||||||
| 2 weeks | 0.02 | 0.35 * | −0.02 | −0.07 | 0.21 | −0.24 | 0.08 | 0.46 * | 0.16 |
| 6 weeks | 0.10 | 0.12 | −0.06 | −0.03 | −0.01 | −0.21 | 0.24 | 0.22 | 0.11 |
| 12 weeks | 0.01 | 0.01 | −0.12 | 0.01 | −0.03 | −0.31 | 0.03 | 0.04 | 0.04 |
Notes. Correlations shown are based on the full data set and pairwise deletion.
*p<0.05, * *p<0.01.
4.2. Multilevel analyses
4.2.1. Crying total duration
The best fitting multilevel model for infant crying total duration showed a significant association between maternal educational level and crying duration (p = 0.041). Infants from mothers with higher educational levels cried longer, in total, than infants from mothers with lower educational levels. There was no significant association between milk cortisol and infant crying total duration. Finally, there were no significant relations between our other control variables (i.e., parity, infant sex, birth weight, collection time, and time from waking to collection), or any of the interaction terms and infant crying total duration (see Table 5).
Table 5.
Estimates for the best fitting model for fussing and crying total duration, frequency, and bout
| Total duration | Frequency | Bout | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Fixed effects | |||||||||
| Intercept | 19.37 | 71.68 | 0.788 | 6.62 | 5.37 | 0.222 | 5.71 | 9.72 | 0.559 |
| Time | 3.34 | 5.66 | 0.557 | 0.49 | 0.25 | 0.060 | −0.37 | 0.15 | 0.016 |
| Time quadratic | −0.59 | 0.39 | 0.132 | −0.05 | 0.02 | 0.006 | na | na | na |
| Education | 8.71 | 4.14 | 0.041 | 0.93 | 0.36 | 0.012 | −0.50 | 0.54 | 0.367 |
| Birthweight infant | 0.00 | 0.01 | 0.904 | −0.00 | 0.00 | 0.509 | 0.00 | 0.00 | 0.173 |
| Collection time | 0.00 | 0.00 | 0.128 | −0.00 | 0.00 | 0.911 | 0.00 | 0.00 | 0.228 |
| Interval between waking and collection | 0.01 | 0.09 | 0.892 | 0.00 | 0.00 | 0.886 | −0.01 | 0.01 | 0.567 |
| Cortisol | 0.25 | 0.55 | 0.652 | 0.03 | 0.03 | 0.264 | −0.15 | 0.12 | 0.189 |
| Sex infanta | na | na | na | −2.82 | 0.98 | 0.006 | 3.13 | 1.46 | 0.037 |
| Interaction sex infant × cortisol | na | na | na | na | na | na | 0.23 | 0.15 | 0.113 |
| Deviance | 1,452.327 | 699.009 | 924.534 | ||||||
Notes. SE = standard error, na = not included in the final model; N = 70.
0 = male, 1 = female.
4.2.2. Crying frequency
The best fitting multilevel model for crying frequency showed a significant association between quadratic time (p = 0.006), infant sex (p = 0.006), maternal educational level (p = 0.012), and crying frequency. In line with the normative development of infant crying (Barr et al., 2006), crying frequency significantly increased from time 1 to time 2, and decreased from time 2 to time 3. Overall, female infants cried significantly less frequently than male infants. Infants from mothers with higher educational levels cried more frequently than infants from mothers with lower educational levels. There was no significant relation between breast milk cortisol and infant crying frequency. Finally, no significant associations between any of our other control variables (i.e., parity, birth weight, collection time, and time from waking to collection), or any of the interaction terms and infant crying frequency were found (see Table 5).
4.2.3. Crying bout
The best fitting multilevel model for crying bout length showed a significant association between time (p = 0.016) and crying bout length. On average, crying bout length decreased over time. There was no significant relation between milk cortisol and infant crying bout length (p = 0.189). Furthermore, there was a significant association between infant sex (p = 0.037) and crying bout length, indicating that female infants tended to have longer crying bouts than male infants. Finally, there were no significant relations between our control variables (i.e., parity, maternal educational level, birth weight, collection time, and time from waking to collection), quadratic time, or any of the interaction terms and crying bout length (see Table 5).
5. DISCUSSION
The present study aimed to longitudinally investigate the association between breast milk cortisol and infant crying during the first twelve postnatal weeks. Higher concentrations of milk cortisol were expected to be related to higher levels of infant fussing and crying. Furthermore, we explored sex differences in the link between milk cortisol and infant fussing and crying. Results showed that milk cortisol concentrations increased from 2 weeks through 12 weeks of infant age. Milk cortisol concentrations were unrelated to the total duration, frequency, and bout length of infant fussing and crying for both boys and girls.
The results are not in line with the two previous studies that showed higher breast milk cortisol concentrations to be related to more infant temperamental negativity (Grey et al., 2013; Nolvi et al., 2017). Based on these studies, we expected milk cortisol to be related to infant fussing and crying. There are several possible explanations for the current study's lack of relations between milk cortisol and infant fussing and crying in the first three months postpartum. First, differences in the designs and measures used may explain the differences in results. Although in this study fussing and crying over three 72‐hr periods were assessed within the first 3 months, the Grey et al. (2013) study used maternal reports of temperamental negative affectivity (including sadness and fear) at 3 months and the Nolvi et al. (2017) study observed laboratory‐induced fear reactivity at 8 months. This variation in designs and outcome measures may be behind the differences in results between the three studies. Also, while Nolvi et al. (2017) found a relation between milk cortisol and girls’ fear reactivity in a laboratory setting, but no relation between milk cortisol and maternal reported fearfulness, Grey et al. (2013) did find a relation between milk cortisol and maternal reported infant negative affectivity. This positive relation between milk cortisol and negative affectivity seemed to be strongest for the fear subscale of negative affectivity (Grey et al., 2013). Thus, it is possible that milk cortisol is more specifically related to child fearfulness instead of to more general measures of fussing and crying, as were assessed in the current study. Clearly, more research is needed in this field before conclusions on relations between milk cortisol and infant behavior can be drawn.
A second explanation for the lack of relation between breast milk cortisol and infant fussing and crying might be related to our restriction of milk collection to the morning. Just as in saliva, cortisol follows a circadian rhythm in milk, with high concentrations in the morning and a decline throughout the day (Pundir et al., 2017; van der Voorn et al., 2016). Our restriction of milk collection to the morning, when milk cortisol is naturally high, might explain why we found no association between breast milk cortisol and infant behavior. In laboratory stressors, greater cortisol reactivity is obtained when tests are conducted in the afternoon (Dickerson & Kemeny, 2004). Thus, if our assumption that higher milk cortisol reflects environmental stressors of the mother is true, differences between mothers might be most notable in milk samples collected in the afternoon or evening. Additionally, future research into the relations between milk cortisol and infant behavior should take several milk samples throughout the day, including afternoon and evening samples, to calculate the total cortisol output an infant is exposed to, as also suggested by Finken et al. (2017).
The longitudinal design of the present study allowed us to describe the time course and stability of cortisol concentrations in breast milk throughout the first three months. On average, milk cortisol levels increased from 2 weeks through 12 weeks of infant age. One explanation for this interesting finding is that many mothers in the Netherlands, given the length of maternity leave, transition back to work around 10–12 weeks postpartum after giving birth. This transition poses many stressors for mothers, including readjusting to work, combining working and family life, and getting used to being separated from the infant (Costigan, Cox, & Cauce, 2003; Nichols & Roux, 2004; Wiese & Heidemeier, 2012). Although transitioning back to work also brings positive emotions, research indicates that mothers perceive it primarily as stressful (Nichols & Roux, 2004). Transitioning back to work, and/or the anticipation thereof, might thus have led to increasing milk cortisol concentrations over the first three months of lactation.
Furthermore, breast milk cortisol concentrations at 2 weeks were positively and significantly related to breast milk cortisol concentrations at 6 and 12 weeks, suggesting some intra‐individual stability over time. Yet breast milk cortisol levels at 6 weeks were not related to breast milk cortisol concentrations at 12 weeks. As the link between milk cortisol and infant fussing and crying behavior might be bidirectional, it is possible that infant fussing and crying leads to more maternal stress (Barr et al., 2014; Brand et al., 2014; Fujiwara et al., 2011), and subsequently, higher milk cortisol concentrations. Attributed to a relatively small sample size, we were unable to employ a cross‐lagged panel design to shed more light on the bidirectional relations between crying behavior and milk cortisol concentrations. However, at around 6 weeks of age, infants are at the peak of the normal developmental crying curve (Barr et al., 2006), and this increased crying might have influenced maternal milk cortisol concentrations at 6 weeks, explaining the lack of a relation between milk cortisol concentrations at 6 weeks and at 12 weeks. Nevertheless, previous research did not find an association between maternal perceived stress, anxiety, and depression and breast milk cortisol concentrations (Grey et al., 2013; Nolvi et al., 2017). Hence, more research is needed to investigate milk cortisol stability and its determinants, including maternal psychological complaints.
6. FUTURE DIRECTIONS
There are several suggestions for future investigations. First, in line with the lactational programming hypothesis, we hypothesized that the mother, via her milk, transfers environmental signals to the infant who in turn adapts his or her behavior accordingly. However, this process has been challenged by the idea that the mother not simply signals information about the environment, but also fine‐tunes these signals. Wells (2014) suggested that maternal physiology is capable of smoothing over short‐term signals which buffer against ecological stresses. In line with this idea, future research could further examine when, and under which conditions, ecological stresses are reflected in maternal milk cortisol concentrations, and also when, and under which conditions, maternal milk cortisol concentrations predict child outcomes.
Second, research on breast milk cortisol is as yet scarce. Fundamental research on the biology of lactational programming is needed to better understand potential mechanisms underlying hypothesized effects of milk cortisol. For example, research focusing on possible changes in intestinal permeability and receptor expression in the infants' intestines could help disentangle underlying mechanisms. Animal research has shown that especially early in life, the infant's intestinal tract has a high number of glucocorticoid receptors, which have been assumed to create an intersection between the gut‐brain axis and the hypothalamic‐pituitary‐adrenal (HPA) axis (Allen‐Blevis et al., 2015). Intestinal glucocorticoid receptors decline after the mother is no longer breastfeeding, suggesting a special role for milk‐borne cortisol on offspring behavior (Hinde et al., 2014), as milk cortisol would bind to these receptors producing effects that are likely to influence infant behavior (Allen‐Blevis et al., 2015). In future (animal) research, it would be interesting to examine whether breast milk cortisol has an effect on infant biobehavioral regulation, and whether intestinal permeability and receptor expression determine a certain window of sensitivity to breast milk cortisol.
Third, other infant outcomes and their relation with breast milk cortisol should be investigated. Next to temperament and infant negativity, we recommend examining the relation between breast milk cortisol and other developmental outcomes influenced by the limbic system, including long‐term memory and learning. A variety of animal and human research has already shown that high exposure to cortisol, albeit in the blood stream, can impair explicit learning and plasticity in the limbic system (reviewed in Sapolsky, 2003). Furthermore, animal studies showed that higher cortisol concentrations in breast milk were related to improved learning in rat offspring (Catalani et al., 2002).
Fourth, another direction for future research is related to possible influences of breast milk cortisol on infant physical health. Hinde et al. (2014) suggested that milk cortisol binding to infants' intestinal tract cortisol receptors influences the maturation of the gastrointestinal tract. A healthy development of the gastrointestinal tract in turn, is related to the infant's developing immune system and health (Bäckhed, 2011; Dimmitt et al., 2010; Sudo et al., 2004). For example, a recent study found that 3‐month‐old infants exposed to higher breast milk cortisol concentrations showed lower body mass index percentile gains over the first two postnatal years and lower body mass index percentile than infants exposed to lower levels of cortisol (Hahn‐Holbrook, Bao Le, Chung, Davis, & Glynn, 2016). Future research should therefore also focus on the relation between breast milk cortisol and infant physical health, including illnesses and gut microbiota.
Fifth, on a more general note, future studies on relations between breast milk cortisol and infant outcomes, including biobehavioral regulation, cognitive outcomes, and physical health, would also benefit from taking milk volumes into account. Milk volumes are expected to vary across the first three months of life, as a function of infant mass, gastrointestinal capacity, and behavioral development (Hinde, 2013; Hinde et al., 2014). Thus, even if milk cortisol concentrations were more or less stable, since volume is increasing, the total transfer of cortisol to the infant may be actually increasing.
Lastly, as our current longitudinal study only spans a short period of time and previous research is mostly cross‐sectional, future research should also investigate whether early breast milk cortisol relates to offspring behavior at later ages. Consistent with the idea of lactational programming, the effects of early breast milk cortisol exposure might extend to, or even become clearer, in childhood or adulthood.
7. STRENGTHS AND LIMITATIONS
A strong point of this study is its longitudinal nature. Mothers collected breast milk samples and filled in infant behavior diaries for three consecutive days when the infant was 2, 6, and 12 weeks of age. The current study also has limitations to note. First, even though the diary used to measure infant crying and fussing behavior has been shown to be a valid maternal self‐report measure that is comparable to audio recordings (Barr, et al., 1988), it might still be possible that mothers over‐ or underreported infant crying and fussing behavior. Furthermore, the cry diary uses 5‐min intervals, so the diary‐recorded crying bout might also reflect intermittent clusters of crying behaviors (Barr, Paterson, Macmartin, Lehtonen, & Young, 2005). Future research should therefore also include audio recordings or observations. Second, although the study controlled for time of day effects by only including morning samples and controlling for collection time, only one sample was collected at each assessment moment. As discussed above, multiple daily samples may provide additional and more reliable information on breast milk cortisol and related lactational programming processes. Finally, it is important to note that, due to our exclusion criteria or because mothers stopped breastfeeding, only 41 of the 70 mothers could be included at all three time points. As our multilevel analyses are robust for missing data, and unequal sample sizes pose no problems (Tabachnik & Fidell, 2007), this pattern did not affect our results. However, future studies should find ways to decrease missingness in order to improve overall sample size.
8. CONCLUSION
While the health benefits of breast milk are long known, the role that biological constituents present in breast milk may have in programming child development are just beginning to be uncovered. The present longitudinal study is the first to investigate the association between human breast milk cortisol and infant crying over the first three months of life. On average, milk cortisol levels increased from 2 weeks through 12 weeks of infant age. No evidence was found for relations between milk cortisol and total duration, frequency, and bout length of fussing and crying. As the research on breast milk cortisol and lactational programming is in its initial phases, future studies, preferably with larger populations, are highly needed to replicate and extend these findings.
CONFLICT OF INTEREST
There is no conflict of interest to declare.
Hechler C, Beijers R, Riksen‐Walraven JM, de Weerth C, . Are cortisol concentrations in human breast milk associated with infant crying?. Developmental Psychobiology. 2018;60:639–650. 10.1002/dev.21761
REFERENCES
- Agostoni, C. , Braegger, C. , Decsi, T. , Kolacek, S. , Koletzko, B. , Fleischer Michaelsen, K. , … van Goudoever, J. (2009). Breast‐feeding: A commentary by the ESPGHAN committee on nutrition. Journal of Pediatric Gastroenterology & Nutrition, 49, 112–25. 10.1097/MPG.0b013e31819f1e05 [DOI] [PubMed] [Google Scholar]
- Allen‐Blevis, C. R. , Sela, D. A. , & Hinde, K. (2015). Milk bioactives manipulate microbes to mediate parent‐offspring conflict. Evolution, Medicine, and Public Health, 1, 106–21. 10.1093/emph/eov007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed, F. (2011). Programming of host metabolism by the gut microbiota. Annals of Nutrition and Metabolism, 58, S44–S52. 10.1159/000328042 [DOI] [PubMed] [Google Scholar]
- Baker, C. E. (2014). African American fathers’ depression and stress as predictors of father involvement during early childhood. Journal of Black Psychology, 40, 311–33. 10.1177/0095798413486480 [DOI] [Google Scholar]
- Barr, R. G. (2000). Excessive crying In Sameroff A. J., Lewis M., & Miller S. M. (Eds.), Handbook of developmental psychopathology (pp. 327–50). New York, NY: Springer Science+Business Media LLC. [Google Scholar]
- Barr, R. G. , Fairbrother, N. , Pauwels, J. , Green, J. , Chen, M. , & Brant, R. (2014). Maternal frustration, emotional and behavioural responses to prolonged infant crying. Infant Behavior and Development, 37, 652–64. 10.1016/j.infbeh.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Barr, R. G. , Kramer, M. S. , Boisjoly, C. , McVey‐White, L. , & Pless, I. B. (1988). Parental diary of infant cry and fuss behaviour. Archives of Disease in Childhood, 63, 380–7. 10.1136/adc.63.4.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, R. G. , Paterson, J. A. , MacMartin, L. M. , Lehtonen, L. , & Young, S. N. (2005). Prolonged and unsoothable crying bouts in infants with and without colic. Developmental and Behavioral Pediatrics, 26, 14–23. 0196-206X/00/2601-0014 [PubMed] [Google Scholar]
- Barr, R. G. , Trent, R. B. , & Cross, J. (2006). Age‐related incidence curve of hospitalized Shaken Baby Syndrome cases: Convergent evidence for crying as a trigger to shaking. Child Abuse & Neglect, 30, 7–16. 10.1016/j.chiabu.2005.06.009 [DOI] [PubMed] [Google Scholar]
- Beijers, R. , Buitelaar, J. K. , & de Weerth, C. (2014). Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: Beyond the HPA axis. European Child & Adolescent Psychiatry, 23, 943–56. 10.1007/s00787-014-0566-3 [DOI] [PubMed] [Google Scholar]
- Bilgin, A. , & Wolke, D. (2017). Development of comorbid crying, sleeping, feeding problems across infancy: Neurodevelopmental vulnerability and parenting. Early Human Development, 109, 37–43. 10.1016/j.earlhumdev.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Brand, S. , Furlano, R. , Sidler, M. , Schulz, J. , & Holsboer‐Trachsler, E. (2014). Associations between infants' crying, sleep and cortisol secretion and mother's sleep and well‐being. Neuropsychobiology, 69, 39–51. 10.1159/000356968 [DOI] [PubMed] [Google Scholar]
- Catalani, A. , Casolini, P. , Cigliana, G. , Scaccianoce, S. , Consoli, C. , Cinque, C. , … Angelucci, L. (2002). Maternal corticosterone influences behavior, stress response and corticosteroid receptors in the female rat. Pharmacology, Biochemistry, and Behavior, 73, 105–14. 10.1016/S0091-3057(02)00755-4 [DOI] [PubMed] [Google Scholar]
- Catalani, A. , Casolini, P. , Scaccianoce, S. , Patacchioli, F. R. , Spinozzi, P. , & Angelucci, L. (2000). Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience, 100, 319–25. 10.1016/S0306-4522(00)00277-3 [DOI] [PubMed] [Google Scholar]
- Costigan, C. L. , Cox, M. J. , & Cauce, A. M. (2003). Work‐parenting linkages among dual‐earner couples at the transition to parenthood. Journal of Family Psychology, 17(3), 397–408. 10.1037/0893-3200.17.3.397 [DOI] [PubMed] [Google Scholar]
- de Lauzon‐Guillain, B. , Wijndaele, K. , Clark, M. , Acerini, C. , Hughes, I. A. , Dunger, D. B. , … Wells, J. C. (2012). Breastfeeding and infant temperament at age three months. PLOS One, 10.1371/journal.pone.0029326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, M. W. (1984). Temperament and infant mortality among the Masai of East Africa. American Journal of Psychiatry, 141, 1189–94. [DOI] [PubMed] [Google Scholar]
- de Weerth, C. , Fuentes, S. , & de Vos, W. (2013). Crying in infants: On the possible role of intestinal microbiota in the development of colic. Gut Microbes, 4(5), 416–21. 10.4161/gmic.26041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth, C. , Fuentes, S. , Puylaert, P. , & de Vos, W. M. (2013). Intestinal microbiota of infants with colic: Development and specific signatures. Pediatrics, 131, e550–e8. 10.1542/peds.2012-1449 [DOI] [PubMed] [Google Scholar]
- Del Giudice, M. (2012). Fetal programming by maternal stress: Insight from a conflict perspective. Psychoneuroendocrinology, 37, 1614–29. 10.1016/j.psyneuen.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Dettmer, A. M. , Murphy, A. M. , Guitarra, D. , Slonecker, E. , Suomi, S. J. , Rosenberg, K. L. , … Hinde, K. (2017). Cortisol in neonatal mother's milk predicts later infant social and cognitive functioning in rhesus monkeys. Child Development, 89(2), 525–38. 10.1111/cdev.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, S. S. , & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–91. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dimmitt, R. A. , Staley, E. M. , Chuang, G. , Tanner, S. M. , Soltau, T. D. , & Lorenz, R. G. (2010). Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. Journal of Pediatric Gastroenterology and Nutrition, 51, 262–73. 10.1097/MPG.0b013e3181e1a114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenhouse, E. , & Reilley, S. (2005). Improved estimates of the benefits of breastfeeding using sibling comparisons to reduce selection bias. Health Service Research, 40, 1781–802. 10.1111/j.1475-6773.2005.00453.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finken, M. J. J. , van der Voorn, B. , Hollanders, J. J. , Dijkstra, L. , Toorop, A. A. , & Rotteveel, J. (2017). Cortisol in human milk: The good, the bad, or the ugly? Obesity, 25(7), 1153 10.1002/oby.21882 [DOI] [PubMed] [Google Scholar]
- Frankenhuis, W. E. , & de Weerth, C. (2013). Does early‐life exposure to stress shape or impair cognition? Current Directions in Psychological Science, 22, 407–12. 10.1177/0963721413484324 [DOI] [Google Scholar]
- Fujiwara, T. , Barr, R. G. , Brant, R. , & Barr, M. (2011). Infant distress at five weeks of age and caregiver frustration. The Journal of Pediatrics, 159, 425–30. 10.1016/j.jpeds.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Glynn, L. M. , Davis, E. P. , Schetter, C. D. , Chicz‐Demet, A. , Hobel, C. J. , & Sandman, C. A. (2007). Postnatal maternal cortisol levels predict temperament in healthy breastfed infants. Early Human Development, 83, 675–81. 10.1016/j.earlhumdev.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Grey, K. R. , Davis, E. P. , Sandman, C. A. , & Glynn, L. M. (2013). Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology, 38, 1178–85. 10.1016/j.psyneuen.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn‐Holbrook, J. , Le, T. B. , Chung, A. , Davis, E. P. , & Glynn, L. (2016). Cortisol in human milk predicts child BMI. Obesity, 24, 2471–4. 10.1002/oby.21682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern, L. F. , Brand, K. L. , & Malone, A. F. (2001). Parenting stress in mothers of very‐low‐birth‐weight (VLBW) and full‐term infants: A function of infant behavioral characteristics and child‐rearing attitudes. Journal of Pediatric Psychology, 26, 93–104. 10.1093/jpepsy/26.2.93 [DOI] [PubMed] [Google Scholar]
- Hamosh, M. (2001). Bioactive factors in human milk. Pediatric Clinics of North America, 48, 69–86. 10.1016/S0031-3955(05)70286-8 [DOI] [PubMed] [Google Scholar]
- Hinde, K. (2013). Lactational programming of infant behavioral phenotype In Clancy K. B. H., Hinde K., & Rutherford's J. N. (Eds.), Building Babies: Primate development in proximate and ultimate perspective (pp. 187–207). New York, NY: Springer Science + Business Media. [Google Scholar]
- Hinde, K. , Skibiel, A. L. , Foster, A. B. , Del Rosso, L. , Mendoza, S. P. , & Capitanio, J. P. (2014). Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology, 26(1), 269–81. 10.1093/beheco/aru186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Peters, K. E. , Vaughn, M. G. , & Witko, C. (2014). Breastfeeding and trajectories of children’s cognitive development. Developmental Science, 17, 452–61. 10.1111/desc.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, B. , & Gibb, R. (2011). Brain plasticity and behaviour in the developing brain. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 20(4), 265–76. [PMC free article] [PubMed] [Google Scholar]
- Korja, R. , Huhtala, M. , Maunu, J. , Rautava, P. , Haataja, L. , Lapinleimu, H. , & Lehtonen, L. (2014). Preterm infant's early crying associated with child's behavioral problems and parents' stress. Pediatrics, 133, 1–7. 10.1542/peds.2013-1204 [DOI] [PubMed] [Google Scholar]
- Kusaka, R. , Ohgi, S. , Shigemori, K. , & Fujimoto, T. (2008). Crying and behavioural characteristics in premature infants. Journal of the Japanese Physical Therapy Association, 11, 15–21. 10.1298/jjpta.11.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milidou, I. , Søndergaard, C. , Jensen, M. S. , Olsen, J. , & Henriksen, T. B. (2014). Gestational age, small for gestational age, and infantile colic. Paediatric and Perinatal Epidemiology, 2, 138–45. 10.1111/ppe.12095 [DOI] [PubMed] [Google Scholar]
- Miller, E. M. , Aiello, M. O. , Fujita, M. , Hinde, K. , Milligan, L. , & Quinn, E. A. (2013). Field and laboratory methods in human milk research. American Journal of Human Biology, 25, 1–11. 10.1002/ajhb.22334 [DOI] [PubMed] [Google Scholar]
- Nichols, M. R. , & Roux, G. M. (2004). Maternal perspectives on postpartum return to the workplace. Journal of Obstetric, Gynecologic & Neonatal Nursing, 33(4), 463–71. 10.1177/0884217504266909 [DOI] [PubMed] [Google Scholar]
- Nolvi, S. , Uusitupa, H.‐M. , Bridgett, D. J. , Pesonen, H. , Aatsinki, A.‐K., Kataja, E.‐L., … Karlsson, L. (2017). Human milk cortisol concentration predicts experimentally induced fear reactivity: Moderation by infant sex. Developmental Science, e12625 10.1111/desc.12625 [DOI] [PubMed] [Google Scholar]
- Owen, D. , Andrews, M. H. , & Matthews, S. G. (2005). Maternal adversity, glucocorticoids and programming of neuroendocrine function and behavior. Neuroscience and Biobehavioral Reviews, 29, 209–26. 10.1016/j.neubiorev.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Pettigrew, M. M. , Khodaee, M. , Gillespie, B. , Schwartz, K. , Bobo, J. K. , & Foxman, B. (2003). Duration of breastfeeding, daycare, and physician visits among infants 6 months and younger. Annals of Epidemiology, 13, 431–5. 10.1016/S1047-2797(02)00463-5 [DOI] [PubMed] [Google Scholar]
- Pundir, S. , Wall, C. R. , Mitchell, C. J. , Thostensen, E. B. , Lai, C. T. , Geddes, D. T. , & Cameron‐Smith, D. (2017). Variation of human milk glucocorticoids over 24 hour period. Journal of Mammary Gland Biology and Neoplasia, 22, 85–92. 10.1007/s10911-017-9375-x [DOI] [PubMed] [Google Scholar]
- Radesky, J. S. , Zuckerman, B. , Silverstein, M. , Rivara, F. O. , Barr, M. , Taylor, J. A. , … Barr, R. G. (2013). Inconsolable crying and maternal postpartum depressive symptoms. Pediatrics, 131, 1–8. 10.1542/peds.2012-3316 [DOI] [PubMed] [Google Scholar]
- Reijneveld, S. A. , van der Wal, M. F. , Brugman, E. , Hira Sing, R. A. , & Verloove‐Vanhorick, S. P. (2004). Infant crying and abuse. Lancet, 364, 1340–2. 10.1016/S0140-6736(04)17191-2 [DOI] [PubMed] [Google Scholar]
- Sapolsky, R. M. (2003). Stress and plasticity in the limbic system. Neurochemical Research, 28, 1735–42. 10.1023/A:1026021307833 [DOI] [PubMed] [Google Scholar]
- Sudo, N. , Chida, Y. , Aiba, Y. , Sonoda, J. , Oyama, N. , Yu, X. N. , … Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic‐pituitary‐adrenal system for stress response in mice. The Journal of Physiology, 558, 263–75. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, E. C. , Hinde, K. , Mendoza, S. P. , & Capitanio, J. P. (2011). Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Developmental Psychobiology, 53, 96–104. 10.1002/dev.20483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnik, B. G. , & Fidell, L. S. (2007). Multilevel linear modeling In Hartman's S. (Ed.), Using multivariate statistics (pp. 781–857). Boston, MA: Pearson Education Inc. [Google Scholar]
- Thomas, D. G. (2014). Is breast best in the west of England? Use of linked routinely collected data sources to quantify the protective effect of breastfeeding against infectious gastrointestinal and respiratory illness during the first year of life. Lancet, 384, S78 10.1016/S0140-6736(14)62204-2 [DOI] [Google Scholar]
- Van der Voorn, B. , de Waard, M. , van Goudoever, J. B. , Rotteveel, J. , Heijboer, A. C. , & Finken, M. J. J. (2016). Breast‐milk cortisol and cortisone concentrations follow the diurnal rhythm of maternal hypothalamus‐pituitary‐adrenal axis activity. Journal of Nutrition, 146, 2174–9. 10.3945/jn.116.236349 [DOI] [PubMed] [Google Scholar]
- Wells, J. C. K. (2014). Adaptive variability in the duration of critical windows of plasticity: Implications for the programming of obesity. Evolution, Medicine, and Public Health, 2014(1), 109–21. 10.1093/emph/eou019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, B. S. , & Heidemeier, H. (2012). Successful return to work after maternity leave: Self‐regulatory and contextual influences. Research in Human Development, 9(4), 317–36. 10.1080/15427609.2012.729913 [DOI] [Google Scholar]
- Wolke, D. , Bilgin, A. , & Samara, M. (2017). Systematic review and meta‐analysis: Fussing and crying durations and prevalence of colic in infants. The Journal of Pediatrics, 185, 55–61. 10.1016/j.jpeds.2017.02.020 [DOI] [PubMed] [Google Scholar]


