Abstract
Objectives
Exact symmetry and perfect balance between opposite jaw halves, as well as between antagonistic teeth, is not frequently observed in natural masticatory systems. Research results show that asymmetry in our body, skull, and jaws is often related to genetic, epigenetic, environmental and individual ontogenetic factors. Our study aims to provide evidence for a significant link between masticatory asymmetry and occlusal contact between antagonist teeth by testing the hypothesis that tooth inclination is one of the mechanisms driving distribution of wear in masticatory phases in addition to dietary and cultural habits.
Materials and Methods
The present work investigates the relationship between dental macrowear patterns and tooth inclinations on a sample of complete maxillary and mandibular 3D models of dental arches from 19 young and adult Yuendumu Aboriginal individuals. The analysis was carried out on first molars (M1) from all quadrants. Occlusal Fingerprint Analysis was used for the quantification of macrowear patterns, and 2D cross‐sectional geometric analysis was carried out to investigate asymmetry in dental arches.
Results
The asymmetry is highly variable on both arches, and it is associated with differences in the inclination of upper M1 crowns. Each molar has variable inclination (buccal/lingual) which influence tooth to tooth contact, producing greater or lesser variation in wear pattern. Interindividual variability of morphological variation of the occlusal relationship has to be considered in macrowear analysis.
Discussion
Our results suggest that overall asymmetry in the masticatory apparatus in modern humans affects occlusal contact areas between antagonist teeth influencing macrowear and chewing efficiency during ontogeny.
Keywords: asymmetry, dental function, palatal arch, swallowing, tooth wear
1. INTRODUCTION
Tooth wear is a physiological and adaptive phenomenon of dental tissue loss (Benazzi et al., 2013). In anatomically modern humans, numerous factors contribute to creating and modifying wear on the occlusal surface of teeth. Such factors comprise, but are not limited to, tooth position in the dental arch (Molnar & Molnar, 1990), diet (El Zaatari, Grine, Ungar, & Hublin, 2011; El Zaatari & Hublin, 2014, El Zaatari, 2008, 2010; Fiorenza et al., 2011a; Fiorenza, Benazzi, & Kullmer, 2011b; Fiorenza, 2015; Hinton, 1982; Molnar, 1972; Smith, 1984), endogenous and exogenous chemical factors (Grippo, Simring, & Schreiner, 2004), bruxism (Sameera, Singh, & Nitya, 2017), paramasticatory activities (Fiorenza et al., 2011a; Fiorenza & Kullmer, 2013, 2015), and cultural practices such as dental treatment (Bennike & Alexandersen, 2003; Coppa et al., 2006; Lozano, Subirà, Aparicio, Lorenzo, & Gómez‐Merino, 2013; Ortiz, Torres Pino, & Orellana González, 2016; Oxilia et al., 2017, 2015; Ricci et al., 2016; Schwartz, Brauer, & Gordon‐Larsen, 1995; Seidel, Colten, Thibodeau, & Aghajanian, 2005; Turner, 2004; White, Degusta, & Richards, 1997) or food processing (Fiorenza, Benazzi, Oxilia, & Kullmer, 2018). The aforementioned factors have been extensively used to investigate dietary habits and behavioral patterns across prehistoric populations and extinct human species (El Zaatari et al., 2011; Fiorenza et al., 2011a,b, 2018; Fiorenza & Kullmer, 2013, 2015; Hinton, 1982; Molnar, 1972; Smith, 1984), behavioral patterns of historic and modern hunter‐gatherers (Hinton, 1982; Kaidonis, Townsend, & Richards, 1993; Molnar, 1971, 1972), predominant occlusal movements performed during masticatory and paramasticatory activities (Kullmer et al., 2009; Kullmer, Schulz, & Benazzi, 2012; Oxilia et al., 2017, 2015), and the biomechanical effects of occlusal loading (Benazzi, Grosse, Gruppioni, Weber, & Kullmer, 2014; Benazzi, Kullmer, Grosse, & Weber, 2011; Benazzi, Nguyen, Kullmer, & Hublin, 2015; Benazzi, Nguyen, Kullmer, & Kupczik, 2016; Dejak, Młotkowski, & Romanowicz, 2003) and the functional restoration of fossil dental arches (Benazzi, Kullmer, Schulz, Gruppioni, & Weber, 2013c; Kullmer et al., 2013).
Nevertheless, the presence of asymmetry in masticatory systems may also offer additional insights to explain the distribution of tooth wear. Asymmetry in maxilla emerges as the result of many potential factors including tongue movements involved in swallowing (Anagnostara, Stoeckli, Weber, & Kollias, 2001; Hartl, Albiter, Kolb, Luboinski, & Sigal, 2003; Lear, Flanagan, & Moorrees, 1965; Mosier, Liu, Maldjian, Shah, & Modi, 1999; Palmer et al., 2008; Pameijer, Glickman, & Roeber, 1970), speech, and postural stability, all of which influence the alteration of both upper and lower jaw morphology (Alghadir, Zafar, & Iqbal, 2015; Hiiemae & Palmer 2003; Hori et al., 2013; Palmer, Hiiemae, & Liu 1997). In particular, by pushing upwards, the tongue exerts a pressure on the palate that is then transmitted through maxillary bones and potentially resulting in tooth inclination (Proffit 1978). At the same time, this alteration involves the palate, vomer and the sphenoid bones (Brodie 1946; Fishman 1969; Kapoor, Sharma, & Grover, 1979; Rakosi 1978).
As far as the mandible is concerned, there are three main forces responsible for tooth inclination: a) lingual force (the muscles of the tongue); b) buccal force (M. buccinator and M. masseter); and c) occlusal force (loading during mastication) (Koc, Dogan, & Bek, 2010). Initially, mandibular molars erupt lingually. They then move buccally due to tongue pressure and M. masseter function (Janson, Bombonatti, Cruz, Hassunuma, & Del Santo, 2004). Finally, the molars reach a balance position (Masumoto, Hayashi, Kawamura, Tanaka, & Kasai, 2001) that will change during life due to the pressure of tongue and other muscles. It can be safely assumed that occlusal contacts between antagonist teeth with varying inclinations will result in macrowear patterns that are specific for that occlusion (Kasai & Kawamura 2001).
At present, however, there has been no substantial contribution to exploring the effect of asymmetry on tooth wear. In this preliminary work, therefore we aim to assess whether the impact of nonpathological asymmetry on wear patterns can represent an additional explanatory variable for the emergence and distribution of dental wear. More specifically, by using digital casts of upper and lower dental arches of Aboriginal individuals from Yuendumu (Australia; Brown, Townsend, Pinkerton, & Rogers, 2011), we provide evidence of linkage between dental macrowear and tooth inclination in first molars (M1), showing that structural factors such as bone asymmetry are responsible for the development of wear pattern in addition to the already explored external factors.
2. MATERIALS AND METHODS
2.1. Sample
The Yuendumu collection consists of measurements, radiographs, family data, and 1717 sets of dental casts representing 446 individuals that were produced from alginate impressions (Brown et al., 2011). This is one of the most widely studied dental collections in the world (with over 250 scientific publications), and represent an ideal sample for the present study. The Yuendumu collection was created from a unique longitudinal research project in which data on dentition and growth of Aboriginal children and young adults from Yuendumu (Northern Territory, Australia) were collected annually between 1951 and 1971. This indigenous population was at an early stage of transition from a nomadic and hunter‐gatherer way of life to a more settled existence (Campbell & Barrett 1954), with limited contacts with Europeans (Brown et al., 2011). Their dentition was mostly characterized by a normal occlusion (or Angle Class I; Barrett, 1969; Beyron 1964; Nakahara, Takahashi, Kameda, Kameda, & Townsend, 1998a, 1998b, 1999; Nakahara, Takahashi, & Townsend, 1997) and alternate intercuspation (Brown, Abbott, & Burgess, 1987; Corruccini, 1990; Richards & Brown 1986) with little evidence of pathological conditions (Campbell, 1923), such as caries (Barrett & Williamson, 1972; Brown, 1974), tooth crowding (Björk & Helm 1969, Helm, 1979), malocclusions (Corruccini, 1990), molar agenesis (Macintosh & Barker, 1978), periodontal diseases (Barrett & Williamson, 1972), and asymmetry (Campbell, 1925; Proffit, 1975; Proffit, McGlone, & Barrett, 1975; Townsend & Brown, 1980). Dental wear of Aboriginal people is characterized by evidence of non‐masticatory activity (Barrett, 1958; Barrett & Williamson, 1972, Clement, Hillson, de la Torre, & Townsend, 2009) bruxism (Barrett, 1960) and traces of function in eccentric jaw positions (Kaidonis et al., 1993).
The sample used in this study consists of complete casts of maxillary and mandibular dental arches belonging to 19 adult and subadult individuals (Table 1), all of which are characterized by slight or moderate M1s wear (up to wear stage 2 on Smith 1984 (Supporting Information Figure S1). The sample comprises younger individuals (ca. 30 years old and below), because older Australian aboriginals show an increase in tooth wear (stage 3–5; Smith, 1984), with extended dentine areas that compromise the identification of macrowear patterns. The number of selected individuals was further reduced by the exclusion of all damaged samples.
Table 1.
Individuals analyzed from the Yuendumu Aboriginal group
| Mandible | Maxilla | |||
|---|---|---|---|---|
| Specimen | Age | Sex | Dentition | Dentition |
| 751 | 8 | Male | Mixed | Mixed |
| 640 | 9 | Male | Mixed | Mixed |
| 247 | 10 | Male | Mixed | Mixed |
| 716 | 10 | Male | Mixed | Mixed |
| 869 | 10 | Male | Mixed | Mixed |
| 288 | 11 | Male | Mixed | Mixed |
| 634 | 11 | Female | Mixed | Mixed |
| 859 | 12 | Male | Permanent | Permanent |
| 359 | 15 | Male | Permanent | Permanent |
| 251 | 16 | Male | Permanent | Permanent |
| 183 | 17 | Male | Permanent | Permanent |
| 305 | 17 | Male | Permanent | Permanent |
| 307 | 17 | Male | Permanent | Permanent |
| 549 | 17 | Female | Permanent | Permanent |
| 243 | 19 | Male | Permanent | Permanent |
| 294 | 21 | Female | Permanent | Permanent |
| 421 | 26 | Male | Permanent | Permanent |
| 338 | 29 | Male | Permanent | Permanent |
| 466 | 30 | Male | Permanent | Permanent |
2.2. Digital acquisition of upper and lower arches derived from dental casts
We digitized the specimens from dental cast collection using a white‐light scanning system with a xy resolution of 45 μm based on structured‐light technology (smartSCAN3D C‐5, Breuckmann, GmbH). Collection and alignment of scan‐data was carried out using the integrated scanning software optoCAT (Breuckmann, GmbH). Generated virtual 3D models were further post‐processed using PolyWorks® V12 (InnovMetric Software Inc.), a 3D metrology software package. The limited size and high resolution of the polygonal models allowed us to avoid the use of smoothing on the meshes and therefore to preserve the original surface. Each raw data polygonal model was imported into the IMEditTM module where topology errors, artifacts, and degenerate/duplicate triangles were manually identified and removed.
For each specimen, macrowear pattern of left/right maxillary/mandibular first molars (i.e., 76 M1s), tooth inclination at the level of M1s, and quantification of jaw asymmetry were obtained as detailed below.
2.3. Macrowear pattern of the M1s
M1 wear areas were manually outlined on each digital 3D surface models and were labeled according to the wear area terminology and numbering system created by Kullmer et al., (2009). Areas that were affected by wear were then grouped into their respective masticatory cycle phases, that is, phase I buccal areas (1, 1.1, 2, 2.1, 3, 4), phase II (9, 10, 11, 12, 13, 10.1), and phase I lingual areas (5, 6, 7, 8) (Kay & Hiiemae, 1974). The relative wear area of each chewing phase was computed by summing the absolute areas (in mm2) belonging to the same phase, and dividing this sum with to the total occlusal wear area. The resulting values (proportions) were visually represented by the ternary plot, which is a diagram that describes the proportions of three variables (in this specific case represented by the relative areas of Phase II, and Buccal and Lingual Phase I) which have to sum to 1 or 100%.
2.4. M1 crown inclination
A reference plane (hereafter called “RP”) was identified on virtual models. The xy‐plane of a Cartesian coordinate system was transformed parallel to the RP. The RP was obtained by observing: (i) the apex of the septum between the central incisors; and (ii) two points marked respectively on the left and right hypoconid (distobuccal cusp of mandibular M1s) and metacone (distobuccal cusp of maxillary M1s) (Figure 1a,e).
Figure 1.
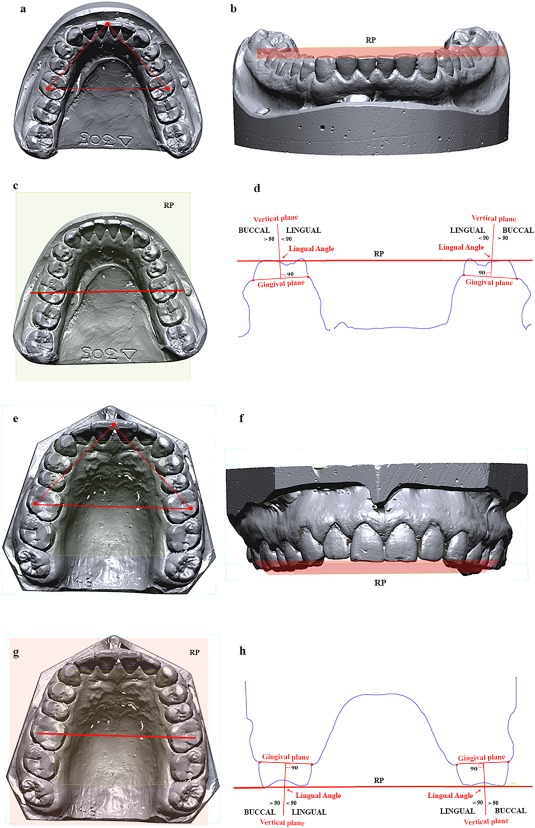
Occlusal reference plane. Three anatomical points were identified on the occlusal surface (a, e) and the plane crated form them was taken as reference plane (RP) (b, f). Afterward a plane was draw between Hypoconid (c) and Metacone (g) of first molars perpendicular to the RP. A cross‐section of the entire virtual model was obtained (d, h) in order to calculate the inclinations of alveolar bone (vertical plane perpendicular to the gingival plane) in relation to RP
Then, a plane perpendicular to the RP (Figure 1b,f), passing through the hypoconid and metacone (Figure 1c,g), was created in order to obtain a cross‐section of dental arch (Figure 1d,h). On the cross‐section, a line was drawn for each M1 between the buccal and lingual gingival sulcus (hereafter called “Gingival Line”). Finally, further an additional line was located at the cross‐section perpendicular to the Gingival Line (hereafter called “Vertical Line”). The lingual angle measured at the cross‐section between the Vertical Line and the RP was used to establish the buccal or lingual inclination of the alveolar arch at the level of M1s (Figure 1d,h). This measurement is taken as a proxy for the inclination of the M1 crowns. An angle higher or lower than 90° suggests a lingual or buccal inclination of the M1 crowns respectively.
2.5. Quantifying palatal arch asymmetry
The cross‐sections described above were also used to quantify the asymmetry of the palatal arch. In detail, for both mandibular and maxillary cross‐sections, the midpoint (MP) between the left and right M1 lingual gingival sulcus was computed (Figure 2). The midpoint MP was then projected on the parallel reference plane (PRP), thus obtaining a new point called PMP (projected midpoint). The line passing through MP and PMP was used to split the palatal arch in a left and right half‐component (Figure 2). The relative area of each half‐component (Figure 2) was calculated by dividing the absolute area of each half side by the total palatal area.
Figure 2.
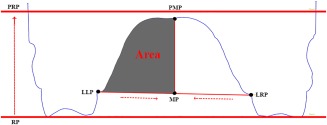
Cross‐section of upper jaw. Points used to calculate the relative areas of the palatal arch. (RP = reference plane; PRP = projected reference plane; LLP = lingual left point; LRP = lingual right point; MP = middle point; PMP = projected middle point)
2.6. Measures of statistical association
Considering the small size of the present sample (n = 19), nonparametric tests were used to formally assess the presence of significant associations between variables. More in detail, Mann‐Whitney‐Wilcoxon signed‐rank test was used on paired observations to identify significant differences in wear patterns across the masticatory phases of each dental arch.
The potential relationship among teeth inclinations was analyzed using Spearman rank correlation coefficient (ρ). The same statistical analysis was performed to test for significant relationship between tooth inclination and macrowear in each masticatory phase of each quadrant. The latter consist of: upper right (UR), upper left (UL), lower right (LR), and lower left (LL). For significant cases identified through correlation, we assessed the explanatory power of tooth inclination with respect to wear patterns through an ordinary least squares (OLS) linear regression analysis. Segregation based on age was further explored by computing a Principal Component Analysis (PCA) based on alveolar inclinations, and by performing AMOVA (Analysis of Molecular Variance; Excoffier, Smouse, & Quattro, 1992) based on interindividual, pairwise Euclidean distances computed on the same alveolar inclinations.
Finally, the presence of significant differences in the distribution of tooth inclination values across classes of palatal asymmetry (symmetric, predominant left, and predominant right) at each position (UR, UL, LR, and LL) was explored using a Kruskal‐Wallis test. All analyses were performed in R version 3.4.3 (R Core Team, 2017).
3. RESULTS
3.1. Macrowear pattern phase distribution of the M1s
The relative proportion (percentage) of the three masticatory phases identified on the M1s were graphically represented in the ternary plots (Figure 3). Overall distributions of mandibular and maxillary M1s overlap (Figure 3a), even though the latter (black circle) is more scattered (Figure 3b) than the former (red circle) (Figure 3c). Indeed, significant differences between antagonists (Mann‐Whitney‐Wilcoxon signed‐rank test) were obtained for buccal and lingual phase I of both the left (p value = 0.04) and right (p value = 0.03) M1s (Table 2).
Figure 3.
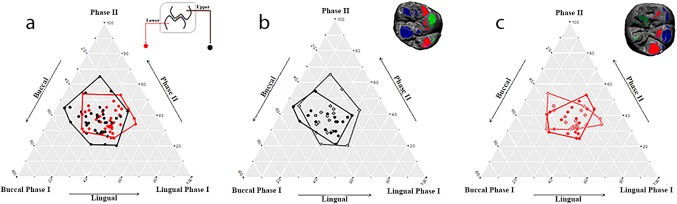
Ternary diagrams showing the proportions (in %) of relative wear areas of buccal phase I areas, lingual phase I areas, and phase II areas, which are positioned in an equilateral triangle. Each base of the triangle represents a ratio of 0% while the vertices correspond to a percentage of 100%. (a) Relation between upper (black) and lower (red) molars. (b) Relation between right (filled points) and left (empty points) upper molars. (c) Relationship between right (filled points) and left (empty points) lower molars
Table 2.
Relationship between masticatory phases of each dental arch measured using Mann‐Whitney‐Wilcoxon signed‐rank test for two‐sample, paired study design (T = test statistic; α = 0.05)
| Phase II | Buccal phase I | Lingual phase I | ||||
|---|---|---|---|---|---|---|
| T | p | T | p | T | p | |
| UL—UR | 109.5 | 0.3 | 64 | 0.6 | 76 | 0.7 |
| UL—LL | 102 | 0.8 | 134 | 0.04 | 44 | 0.04 |
| LR—UR | 103.5 | 0.75 | 43 | 0.03 | 131 | 0.05 |
| LR—LL | 68 | 0.46 | 107.5 | 0.63 | 88 | 0.93 |
UL = upper left; UR = upper right; LL = lower left; LR = lower right.
When maxillary and mandibular M1s of the same individual are considered separately, there are no significant differences in the pattern of masticatory phases between left and right side (Table 2). Overall, we observed that right maxillary M1s (points) are more variable than left maxillary M1s (circles) (Figure 3b), while such difference was not observed for mandibular molars (Figure 3c).
3.2. M1 crown inclination
The inclination of maxillary and mandibular M1 crowns are listed in Supporting Information Table S1. Even though we observe variability between opposite (left and right; Supporting Information Table S2a) and antagonist teeth (Supporting Information Table S2b), the only significant relationship emerges between tooth inclinations of opposite sides of the upper (ρ = 0.55, p value = 0.016) and lower (ρ = 0.76, p value = 0.00016) dental arch respectively (Table 3).
Table 3.
Potential relationship among teeth inclinations expressed as Spearman rank correlation coefficients (ρ)
| ρ | p value | |
|---|---|---|
| UR∼UL | 0.55 | 0.016 |
| UR∼LR | −0.18 | 0.46 |
| UR∼LL | −0.17 | 0.46 |
| UL∼LR | 0.2 | 0.39 |
| UL∼LL | 0 | 1 |
| LR∼LL | 0.76 | 0.00016 |
UL = upper left; UR = upper right; LL = lower left; LR = lower right.
Significant values in bold (α = 0.05).
3.3. Relationship between alveolar inclination and dental wear development
The relationship between molar inclination and wear patterns in each masticatory phase at each position is shown in Table 4. Significant correlations are identified only for the right side, in both the upper and lower arch (quadrants 1 and 4). More specifically, significant negative relationship is identified for phase II in UR position and for buccal phase I in LR position. On the other hand, lingual phase I of the right side always exhibits positive correlation with tooth inclination.
Table 4.
Relationship between tooth inclination of each position and the relative masticatory phases measured as Spearman rank correlation coefficient (rho)
| Phase II | Buccal phase I | Lingual phase I | ||||
|---|---|---|---|---|---|---|
| ρ | p value | ρ | p value | ρ | p value | |
| UR | −0.65 | 0.0026 | −0.38 | 0.1 | 0.73 | 0.00035 |
| UL | −0.09 | 0.7 | 0.14 | 0.55 | 0.006 | 0.98 |
| LR | 0.18 | 0.44 | −0.56 | 0.011 | 0.52 | 0.025 |
| LL | −0.056 | 0.82 | −0.19 | 0.439 | 0.14 | 0.55 |
UL = upper left; UR = upper right; LL = lower left; LR = lower right.
Significant values in bold (α = 0.05).
The coefficient of determination (R 2) obtained through OLS regression for the same cases, and the relative F statistic, suggest that variability in tooth inclination is one of the mechanisms driving the distribution of wear in masticatory phases, and the significance of the obtained values suggests that these results may be also generalized in a broader sample (Table 5).
Table 5.
Coefficient of determination (R 2) and the relative F statistic produced by linear regression to infer the proportion of variability in wear masticatory phases that could be explained by tooth inclination
| R 2 | p value | F statistic for 1 and 17 df | |
|---|---|---|---|
| UR_Phase II ∼ UR inclination | 0.32 | 0.006 | 9.579 |
| UR_Lingual Phase l ∼ UR inclination | 0.42 | 0.0016 | 14.09 |
| LR_Buccal Phase I∼ LR inclination | 0.33 | 0.006 | 9.881 |
| LR_Lingual Phase I∼ LR inclination | 0.17 | 0.05 | 4.654 |
UL = upper left; UR = upper right; LL = lower left; LR = lower right.
As far as the impact of age is concerned, we computed a PCA on all four inclination values and color‐coded individuals based on age classes (“mixed” for subadults and “permanent” for adults; Figure 4, Supporting Information Table S3, S4, and S5). The first two principal components explain 91% of the total variance and show a certain level of segregation between the two age groups, with the majority of subadults grouped in the right side of the graph while adult individuals tend to be grouped in the left half. Subadults exhibit most of their variability on PC1 (with the exception of specimen 288), while adult individuals—with the exception of two outliers (specimens 359, 243) —seem to vary predominantly along PC2. A closer inspection of variable loadings (Supporting Information Table S3) suggests that PC1 may indicate variability in mandibular inclinations, while PC2 refers to maxillary variability.
Figure 4.
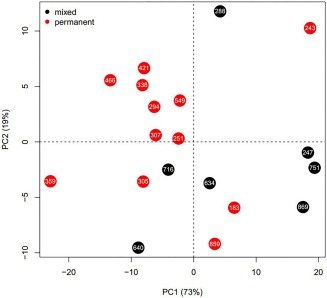
Principal component analysis (PCA) based on alveolar inclinations. Individuals are color‐coded according to their dentition (mixed = red; permanent = black). Horizontal axis represents PC1 (describing 73% of the total variance) while vertical axis represents PC2 (capturing 19% of the total variance)
Results of AMOVA based on the same grouping show that age differences between mixed and permanent dentition explain about 12% of the total variability in tooth inclination in the current sample (Ф ST = 0.12, p value = 0.032).
3.4. Relationship between palatal arch and tooth inclination
The values of the relative palatal areas (Supporting Information Table S6) show only six individuals with no difference (50%) between each half. The Kruskal‐Wallis test performed to preliminary explore the possible relationship between palatal arch asymmetry and tooth inclination yielded no significant results (Table 6), suggesting that—at least in the present sample—tooth inclination may not be directly linked to palatal asymmetry.
Table 6.
Results of Kruskal‐Wallis test assessing whether the distribution of alveolar inclinations is significantly different between classes of palatal asymmetry
| Chi‐squared | df | p value | |
|---|---|---|---|
| UR | 3.4829 | 2 | 0.175 |
| UL | 2.2418 | 2 | 0.326 |
| LR | 5.1052 | 2 | 0.078 |
| LL | 3.1766 | 2 | 0.2043 |
UL = upper left; UR = upper right; LL = lower left; LR = lower right.
The latter consist of: (1) symmetric halves; (2) asymmetry with predominant right half; and (3) asymmetry with predominant left half.
4. DISCUSSION
The results described in this study provide evidence that tooth inclination (lingual or buccal) has an impact on the distribution of dental wear. Upper and lower tooth inclinations can produce an increase in tooth wear areas. When tooth inclination presents with an angle greater than 90 degree (buccal tendency) there is a general increase of the area interested by Lingual Phase I, while an angle of less than 90 degrees (lingual tendency) tends to increase wear area of the buccal slope.
Percentage values of dental masticatory phases show differences in wear between antagonistic molars (Table 2). In this respect, it is recommended to separately analyze the areas of wear of maxillary and mandible molars for comparative group studies in order to reduce variability. Whether this effect depends on particular features of the present sample or on sample size will need to be further tested in the future with larger samples.
Tooth inclination values exhibit a high degree of interindividual variability when considering the whole of the sample (Supporting Information Table S2). Subadults exhibit a more pronounced mandibular variability, while adults tend to vary more in the inclination of maxillary teeth (Figure 4). Although the impact of age on tooth inclination in the present sample is only moderate (∼10% of the total variance), results point to a stronger correlation between maxillary dentition and age, as opposed to a more negligible effect of ageing on the mandible. Correlation among inclinations of right and left sides of upper molars is generally lower than the relationship documented between mandible molars of both sides. In view of these results, upper and lower jaws seem to be two separate and yet interdependent elements, and the lack of covariance between upper and lower jaws (Supporting Information Table S2, S7, and S8) is also supported by the absence of statistical significance (Table 3). The most relevant result consists of a significant correlation between tooth inclination and tooth wear. Such a relationship is particularly strong on the right side of the present group of individuals. This result can be explained based on the characteristic mode of occlusion observed among Australian Aborigines called: “X‐occlusion” or “alternate intercuspation” (Barrett, 1953). Barrett (1953) carried out a study on dental morphology in Yuendumu Aborigenal people, showing that upper and lower teeth could join in maximum contact on either left or right side, but not on both sides at the same time. The latter observation could suggest that Aboriginal individuals probably had a tendency to occlude properly on the right side, producing an increase in occlusal force and thus a more localized evidence of the relationship between wear development and tooth inclination (Table 4). This condition is considered as a malocclusion by orthodontists. Nevertheless, it does not prevent mastication and provides a much wider range of lateral or rotational jaw movements, which can prove to be advantageous when grinding during chewing hard and tough food entailed by the diet of nomadic hunter‐gatherers (Brown et al., 2011).
In the present study no statistically significant relationship between palatal asymmetry and tooth inclination could be documented, possibly because of the joint effect of a small sample size and the absence of parafunctional, pathological conditions. The latter element in particular can increase asymmetry during palatal bone development due to incorrect tongue posture and pressure (Alghadir et al., 2015; Hiiemae & Palmer, 2003; Hori et al., 2013; Palmer et al., 1997).
Because our results suggest that tooth inclination has an impact on macrowear pattern, we must inquire about the processes that are responsible for such asymmetry in the masticatory apparatus. In this respect, elements of particular interest can be the specific role of tongue during important steps of human evolution, such as changes due to locomotion, speech and dietary habits.
As far as the first point is concerned, tongue thrust is a common kind of orofacial myofunctional disorder (OMD) where constant pressure from resting or incorrectly thrusting the tongue away from the hard palate may push teeth out of place and that pressure may later prevent teeth from erupting. The correct posture of the tongue (Van Dyck et al., 2016) seems one of the most promising solutions to this problem, further stressing the importance of tongue in the rehabilitation from oral disorders. Therefore, it could be possible that the physiological asymmetries in the masticatory system of the studied human group is attributable to the posture of the tongue which, in addition to all other muscles involved in swallowing, exerts a force that is absorbed by the hard palate (Anagnostara et al., 2001; Hartl et al., 2003; Lear et al., 1965; Mosier et al., 1999; Palmer et al., 2008; Pameijer et al., 1970). When swallowing takes place correctly, the tip of the tongue presses firmly against the roof of the mouth or hard palate, which is located slightly behind the front teeth. During an incorrect deglutition, the tip and/or sides of the tongue press against or spread between the teeth producing loading asymmetries and affecting bone asymmetry (Matsuov & Palmer, 2008; Van Dyck et al., 2016).
Considering biocultural developments involved in human evolution, on the pathway towards Homo sapiens food energy content and quality increased over time, while physical stiffness and toughness were reduced through cultural adaptation (Demes & Creel, 1988), in particular with the development of more and more sophisticated external food preparation techniques (Brace, Smith, & Hunt, 1991; Hinton, 1982; Kaidonis et al., 1993; Lieberman, Krovitz, Devlin, Yates, & St. Clair, 2004; Lucas et al., 2013; Mariotti et al., 2015; Molnar, 1971, 1972; Shipman & Rose, 1983; Smith, 1984; Stratus, 1989; Toth, 1985, Watson, 2008). This general trend led to a reduction of biomechanical loading and forces in our masticatory system during food ingestion and dental processing (Zink & Lieberman, 2016). From a biological perspective, it is likely that through the continuing reduction of biomechanical pressure on our masticatory system, the human organism reacted with a dimensional reduction in the system itself, favoring an increased variability in the emergence of asymmetry during the process of development, growth and remodeling of the entire masticatory apparatus.
The analysis of this specific masticatory context opens a new perspective on dental macrowear development. Endogenous factors such as dental inclination (Table 4) are to be added to the already known, multiple exogenous variables (such as food and environment abrasiveness, as well as cultural adaptation) in order to obtain a comprehensive model for the appearance of occlusal wear patterns.
The role of asymmetry in the masticatory apparatus and its change over time due to the development of various cultural and dietary habits should therefore receive more consideration both in modern dentistry and dental anthropology. In the first case, comparative studies of tongue posture and tooth inclination would be helpful to enhance knowledge on parafunctional influences in jaw asymmetry, on the relationship between alveolar inclination and crown orientation, and on how individual wear areas are produced by loading and pressure during occlusion and swallowing.
As far as dental anthropology is concerned, future studies on dental macrowear would benefit from adding asymmetry to the already known explanatory variables for the distribution of dental wear, that is, cultural and dietary habits. A more comprehensive evaluation of tooth inclination could also facilitate a better understanding of the mechanisms driving the formation of paramasticatory wear areas (Fiorenza et al., 2011b).
A more holistic view that harmonizes mouth functionality with the rest of the human body (through postcranial posture, chewing, and functional deglutition) is therefore desirable in future occlusal research in order to understand the development of asymmetry in our masticatory system, and to evaluate individual patient situations in dental rehabilitation.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1
ACKNOWLEDGMENTS
This study was supported by the Faculty of Medicine, Nursing and Health Sciences at Monash University through the Strategic Grant Scheme 2016 (Grant SGS16–0344) and by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 724046 – SUCCESS and grant agreement no.639286, HIDDEN FOODS); http://www.erc-success.eu/, http://www.hiddenfoods.eu. We also gratefully acknowledge the financial support from the United States Public Health Service Research Grant DE02034‐07 from the National Institute of Dental Research, National Institute of Health, Bethesda, Maryland, that ensured the continuity of the longitudinal growth study at Yuendumu when dental models were collected. The use of Yuendumu casts for this study is covered by the approval from the Human Ethics Research Committee, University of Adelaide (H‐27–1990).
Oxilia G, Bortolini E, Martini S, et al. The physiological linkage between molar inclination and dental macrowear pattern. Am J Phys Anthropol. 2018;166:941–951. 10.1002/ajpa.23476
Funding information Faculty of Medicine, Nursing and Health Sciences at Monash University through the Strategic Grant Scheme 2016, Grant Number: SGS16‐0344; European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme, Grant Number: 724046, 639286; United States Public Health Service Research (National Institute of Dental Research, National Institute of Health, Bethesda, Maryland), Grant Number: DE02034‐07
REFERENCES
- Alghadir, A. H. , Zafar, H. , & Iqbal, Z. A. (2015). Effect of tongue position on postural stability during quiet standing in healthy young males. Somatosensory & Motor Research, 32, 183–186. [DOI] [PubMed] [Google Scholar]
- Anagnostara, A. , Stoeckli, S. , Weber, O. M. , & Kollias, S. S. (2001). Evaluation of the anatomical and functional properties of deglutition with various kinetic high‐speed MRI sequences. Journal of Magnetic Resonance Imaging, 14, 194–199. [DOI] [PubMed] [Google Scholar]
- Barrett, M. J. (1953). X‐occlusion. The Dental Magazine and Oral Topics, 70, 279. [PubMed] [Google Scholar]
- Barrett, M. J. (1958). Dental observations on Australian Aborigines: Continuously changing functional occlusion. Australian Dental Journal, 3, 39–52. [Google Scholar]
- Barrett, M. J. (1960). Parafunctions and tooth attrition. In D. Lipke & U. Posselt (Eds.), Parafunctions of the masticatory system (bruxism) The Journal of the Western Society of Periodontology/Periodontal, 8, 133–148. [Google Scholar]
- Barrett, M. J. (1969). Functioning occlusion. Annals of the Australian College of Dental Surgeons, 2, 68–80. [PubMed] [Google Scholar]
- Barrett, M. J. , & Williamson, J. J. (1972). Oral health of Australian Aborigines: Survey methods and prevalence of dental caries. Australian Dental Journal, 17, 37–50. [DOI] [PubMed] [Google Scholar]
- Benazzi, S. , Grosse, I. R. , Gruppioni, G. , Weber, G. W. , & Kullmer, O. (2014). Comparison of occlusal loading conditions in a lower second premolar using three‐dimensional finite element analysis. Clinical Oral Investigations, 18, 369–375. [DOI] [PubMed] [Google Scholar]
- Benazzi, S. , Kullmer, O. , Grosse, I. R. , & Weber, G. W. (2011). Using occlusal wear information and finite element analysis to investigate stress distributions in human molars. Journal of Anatomy, 219, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzi, S. , Kullmer, O. , Schulz, D. , Gruppioni, G. , & Weber, G. W. (2013c). Individual tooth macrowear pattern guides the reconstruction of Sts 52 (Australopithecus africanus) dental arches. American Journal of Physical Anthropology, 150, 324–329. [DOI] [PubMed] [Google Scholar]
- Benazzi, S. , Nguyen, H. N. , Kullmer, O. , & Hublin, J. J. (2015). Exploring the biomechanics of taurodontism. Journal of Anatomy, 226, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzi, S. , Nguyen, H. N. , Kullmer, O. , & Kupczik, K. (2016). Dynamic modelling of tooth deformation using occlusal kinematics and finite element analysis. PLoS One, 11, e0152663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzi, S. , Nguyen, H. N. , Schulz, D. , Grosse, I. R. , Gruppioni, G. , Hublin, J. J. , & Kullmer, O. (2013). The evolutionary paradox of tooth wear: Simply destruction or inevitable adaptation? PLoS One, 8, e62263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennike, P. , & Alexandersen, V. (2003). Dental modification in the past In Iregren E. & Larsson L. (Eds.), A tooth for a tooth (pp. 85–100). Lund: University of Lund. [Google Scholar]
- Beyron, H. (1964). Occlusal relations and mastication in Australian Aborigines. Acta Odontologica Scandinavica, 22, 597–678. [DOI] [PubMed] [Google Scholar]
- Björk, A. , & Helm, S. (1969). Need for orthodontic treatment as reflected in the prevalence of malocclusion in various ethnic groups. Acta Society Medica Scandinavica (Supplementum), 1, 209–214. [Google Scholar]
- Brace, C. L. , Smith, S. L. , & Hunt, K. D. (1991). What big teeth you had Grandma! Human tooth size, past and present In Kelley M. A. & Larsen C. S. (Eds.), Advances in dental anthropology (pp. 33–57). New York: Wiley‐Liss. [Google Scholar]
- Brodie, A. G. (1946). “Facial Pattern”. A theme on variation. The Angle Orthodontist, 16, 75–86. [Google Scholar]
- Brown, T. (1974). Dental decay in Aborigines In Hetzel B. S., Dobbin M., Lippman M., & Eggleston E. (Eds.), Better health for aborigines (pp. 97–101). St. Lucia: The University of Queensland Press. [Google Scholar]
- Brown, T. , Abbott, A. , & Burgess, V. B. (1987). Longitudinal study of dental arch relationships in Australian Aboriginals with reference to alternate intercuspation. American Journal of Physical Anthropology, 72, 49–57. [DOI] [PubMed] [Google Scholar]
- Brown, T. , Townsend, G. C. , Pinkerton, S. K. , & Rogers, J. R. (2011). Yuendumu: Legacy of a longitudinal growth study in Central Australia. Adelaide: University of Adelaide Press. [Google Scholar]
- Campbell, T. D. (1923). Dentition and palate of the Australian aboriginal from observations on the skull: A study in physical anthropology and dental pathology. DDSc Thesis, Th University of Adelaide.
- Campbell, T. D. (1925). Dentition and palate of the Australian aboriginal. Adelaide: Hassell Press. [Google Scholar]
- Campbell, T. D. , & Barrett, M. J. (1954). So Thy Did Eat. Documentary Film, The University of Adelaide.
- Clement, A. , Hillson, S. , de la Torre, I. , & Townsend, G. (2009). Tooth use in Aboriginal Australia. Archives of Internal Medicine, 11, 37–40. [Google Scholar]
- Coppa, A. , Bondioli, L. , Cucina, A. , Frayer, D. W. , Jarrige, C. , Jarrige, J.‐F. , … Macchiarelli, R. (2006). Early Neolithic tradition of dentistry. Nature, 440, 755–756. [DOI] [PubMed] [Google Scholar]
- Corruccini, R. S. (1990). Australian aboriginal tooth succession, interproximal attrition, and Begg's theory. American Journal of Orthodontics and Dentofacial Orthopedics, 97, 349–357. [DOI] [PubMed] [Google Scholar]
- Dejak, B. , Młotkowski, A. , & Romanowicz, M. (2003). Finite element analysis of stresses in molars during clenching and mastication. Journal of Prosthetic Dentistry, 90, 591–597. [DOI] [PubMed] [Google Scholar]
- Demes, B. , & Creel, N. (1988). Bite force, diet, and cranial morphology of fossil hominids. Journal of Human Evolution, 17, 657–670. [Google Scholar]
- El Zaatari, S. (2008). Occlusal molar microwear and the diets of the Ipiutak and Tigara populations (Point Hope) with comparisons to the Aleut and Arikara. Journal of Archaeological Science, 35, 2517–2522. [Google Scholar]
- El Zaatari, S. (2010). Occlusal microwear texture analysis and the diets of historical/prehistoric hunter‐gatherers. International Journal of Osteoarchaeolgy, 20, 67–87. [Google Scholar]
- El Zaatari, S. , Grine, F. E. , Ungar, P. S. , & Hublin, J. J. (2011). Ecogeographis variation in Neandertal dietary habits: Evidence from occlusal molar microwear texture analysis. Journal of Human Evolution, 61, 411–424. [DOI] [PubMed] [Google Scholar]
- El Zaatari, S. , & Hublin, J. J. (2014). Diet of upper Paleolithic modern humans: Evidence from microwear texture analysis. American Journal of Physical Anthropology, 153, 570–581. [DOI] [PubMed] [Google Scholar]
- Excoffier, L. , Smouse, P. E. , & Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics, 131, 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza, L. (2015). Reconstructing diet and behaviour of Neanderthals from Central Italy through dental macrowear analysis. Journal of Archaeological Science, 93, 1–15. [DOI] [PubMed] [Google Scholar]
- Fiorenza, L. , Benazzi, S. , & Kullmer, O. (2011b). Para‐masticatory wear facets and their functional significance in hunter‐gatherer maxillary molars. Journal of Archaeological Science, 38, 2182–2189. [Google Scholar]
- Fiorenza, L. , Benazzi, S. , Oxilia, G. , & Kullmer, O. (2018). Functional relationship between dental macrowear and diet in Late Pleistocene and recent modern human populations. International Journal of Osteoarchaeology, 1–9, 10.1002/oa.2642. [DOI] [Google Scholar]
- Fiorenza, L. , Benazzi, S. , Tausch, J. , Kullmer, O. , Bromage, T. G. , & Schrenk, F. (2011a). Molar macrowear reveals Neanderthal eco‐geographic dietary variation. PLoS ONE, 6, e14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza, L. , & Kullmer, O. (2013). Dental wear and cultural behaviour in middle Paleolithic humans from the near east. American Journal of Physical Anthropology, 152, 107–117. [DOI] [PubMed] [Google Scholar]
- Fiorenza, L. , & Kullmer, O. (2015). Dental wear patterns in early modern humans from Skhul and Qafzeh: A response to Sarig and Tillier. Homo: Internationale Zeitschrift Fur Die Vergleichende Forschung Am Menschen, 66, 414–419. [DOI] [PubMed] [Google Scholar]
- Fishman, L. S. (1969). Postural and dimensional changes in the tongue from rest position to occlusion. The Angle Orthodontist, 39, 109–113. [DOI] [PubMed] [Google Scholar]
- Grippo, J. O. , Simring, M. , & Schreiner, S. (2004). Attrition, abrasion, corrosion and abreaction revisited: A new perspective on tooth surface lesions. The Journal of the American Dental Association, 135, 1109–1118. [DOI] [PubMed] [Google Scholar]
- Hartl, D. M. , Albiter, M. , Kolb, F. , Luboinski, B. , & Sigal, R. (2003). Morphologic parameters of normal swallowing events using singleshot fast spin echo dynamic MRI. Dysphagia, 18, 255–262. [DOI] [PubMed] [Google Scholar]
- Helm, S. (1979). Etiology and treatment need of malocclusion. Journal (Canadian Dental Association)), 45, 673–676. [PubMed] [Google Scholar]
- Hiiemae, K. M. , & Palmer, J. B. (2003). Tongue movements in feeding and speech. Review. Critical Reviews in Oral Biology & Medicine, 14, 413–429. [DOI] [PubMed] [Google Scholar]
- Hinton, R. (1982). Differences in interproximal and occlusal tooth wear among prehistoric Tennessee Indians: implications for masticatory function. American Journal of Physical Anthropology, 57, 103–115. [DOI] [PubMed] [Google Scholar]
- Hori, K. , Taniguchi, H. , Hayashi, H. , Magara, J. , Minagi, Y. , Li, Q. , … Inoue, M. (2013). Role of tongue pressure production in oropharyngeal swallow biomechanics. Physiological Reports, 1, e00167–e00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson, G. , Bombonatti, R. , Cruz, K. S. , Hassunuma, C. Y. , & Del Santo, M. J. (2004). Buccolingual inclinations of posterior teeth in subjects with different facial patterns. American Journal of Orthodontics and Dentofacial Orthopedics, 125, 316–322. [DOI] [PubMed] [Google Scholar]
- Kaidonis, J. A. , Townsend, G. C. , & Richards, L. C. (1993). Nature and frequency of dental wear facets in an Australian Aboriginal population. Journal of Oral Rehabilitation, 20, 333–340. [DOI] [PubMed] [Google Scholar]
- Kapoor, D. N. , Sharma, V. P. , & Grover, C. M. (1979). Dentofacial pattern of tongue thrusters—A cephalometric study. Journal of the Indian Dental Association, 51, 295–297. [Google Scholar]
- Kasai, K. , & Kawamura, A. (2001). Correlation between buccolingual inclination and wear of mandibular teeth in ancient and modern Japanese. Archives of Oral Biology, 46, 269–273. [DOI] [PubMed] [Google Scholar]
- Kay, R. F. , & Hiiemae, K. M. (1974). Jaw movement and tooth use in recent and fossil primates. American Journal of Physical Anthropology, 40, 227–256. [DOI] [PubMed] [Google Scholar]
- Koc, D. , Dogan, A. , & Bek, B. (2010). Bite force and influential factors on bite force measurements: A literature review. European Journal of Dentistry, 4, 223–232. [PMC free article] [PubMed] [Google Scholar]
- Kullmer, O. , Benazzi, S. , Fiorenza, L. , Schulz, D. , Bacso, S ., & Winzen, O . (2009). Occlusal Fingerprint Analysis (OFA) – Quantification of tooth wear pattern. American Journal of Physical Anthropology, 139, 600–605. [DOI] [PubMed] [Google Scholar]
- Kullmer, O. , Benazzi, S. , Schulz, D. , Gunz, P. , Kordos, L. , & Begun, D. R. (2013). Dental arch restoration using tooth macrowear patterns with application to Rudapithecus hungaricus, from the late Miocene of Rudabánya, Hungary. Journal of Human Evolution, 64, 151–160. [DOI] [PubMed] [Google Scholar]
- Kullmer, O. , Schulz, D. , & Benazzi, S. (2012). An experimental approach to evaluate the correspondence between wear facet position and occlusal movements. The Anatomical Record, 295, 846–852. [DOI] [PubMed] [Google Scholar]
- Lear, C. S. , Flanagan, J. B. , & Moorrees, C. F. (1965). The frequency of deglutition in man. Archives of Oral Biology, 10, 83–89. [DOI] [PubMed] [Google Scholar]
- Lieberman, D. E. , Krovitz, G. , Devlin, M. , Yates, F. , & St. Clair, M. (2004). Effects of food processing on masticatory strain and craniofacial growth in a retro gnathic face. Journal of Human Evolution, 46, 655–677. DOI 10.1016/j.jhevol.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Lozano, M. , Subirà, M. , Aparicio, J. , Lorenzo, C. , & Gómez‐Merino, G. (2013). Toothpicking and periodontal disease in a Neanderthal specimen from Cova Foradà Site (Valencia, Spain). PLoS One, 8, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, P. W. , Omar, R. , Al‐Fadhalah, K. , Almusallam, A. S. , Henry, A. G. , Michael, S. , … Atkins, A. G. (2013). Mechanisms and causes of wear in tooth enamel: Implications for hominin diets. Journal of the Royal Society Interface, 10, 20120923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh, N. W. G. , & Barker, B. C. W. (1978). The Tabon Cave mandible. Archeology & Physical Anthropology in Oceania, 13, 143–166. [Google Scholar]
- Maier, W. , & Schneck, G. (1981). Konstruktionsmorphologische Untersuchungen am Gebiß der hominoiden Primaten. Zeitschrift Für Morphologie Und Anthropologie, 72, 127–169. [PubMed] [Google Scholar]
- Mariotti Lippi, M. , Foggi, B. , Aranguren, B. , Ronchitelli, A. , & Revedin, A. (2015). Multistep food plant processing at Grotta Paglicci (Southern Italy) around 32,600 cal B.P. Proceedings of the National Academy of Sciences of the United States of America, 112, 12075–12080. [DOI] [PMC free article] [PubMed]
- Masumoto, T. , Hayashi, I. , Kawamura, A. , Tanaka, K. , & Kasai, K. (2001). Relationships among facial type, buccolingual molar inclination, and cortical bone thickness of the mandible. European Journal of Orthodontics, 23, 15–23. [DOI] [PubMed] [Google Scholar]
- Matsuov, K. , & Palmer, J. B. (2008). Anatomy and physiology of feeding and swallowing—Normal and abnormal. Physical Medicine & Rehabilitation Clinics of North America, 19, 691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, S. (1971). Human tooth wear, tooth function and cultural variability. American Journal of Physical Anthropology, 34, 175–189. [DOI] [PubMed] [Google Scholar]
- Molnar, S. (1972). Tooth wear and culture: A survey of tooth functions among some prehistoric populations. Current Anthropology, 13, 511–526. [Google Scholar]
- Molnar, S. , & Molnar, I. M. (1990). Dental arch shape and tooth wear variability. American Journal of Physical Anthropology, 82, 385–395. [DOI] [PubMed] [Google Scholar]
- Mosier, K. M. , Liu, W. , Maldjian, J. A. , Shah, R. , & Modi, B. (1999). Lateralization of cortical function in swallowing: A functional MR imaging study. American Journal of Neuroradiology, 20, 1520–1526. [PMC free article] [PubMed] [Google Scholar]
- Nakahara, S. , Takahashi, M. , Kameda, T. , Kameda, A. , & Townsend, G. (1998a). Longitudinal changes in the permanent dentition of traditional Aborigines: Movements of the fist molar and the anterior dentition. Shigaku. Odontology, 86, 2–6. [Google Scholar]
- Nakahara, S. , Takahashi, M. , Kameda, T. , Kameda, A. , & Townsend, G. (1998b). Longitudinal changes in the modes of occlusion of permanent teeth—Changes in the teeth and dentition of Australian Aborigines—Part 1: Normalization of malocclusion. Shigaku. Odontology, 86, 209–230. [Google Scholar]
- Nakahara, S. , Takahashi, M. , Kameda, T. , Kameda, A. , & Townsend, G. C. (1999). Longitudinal changes in the modes of occlusion of permanent teeth—Changes in the teeth and dentition of Australian Aborigines—Part 2: Transition of normal incisal occlusion into malocclusions. Shigaku. Odontology, 87, 8–29. [Google Scholar]
- Nakahara, S. , Takahashi, M. , & Townsend, G. (1997). Modes of occlusion in humans: A comparison of traditional Aborigines and modern Japanese. Shigaku. Odontology, 85, 345–356. [Google Scholar]
- Ortiz, A. , Torres Pino, E. C. , & Orellana González, E. (2016). First evidence of pre‐Hispanic dentistry in South America—Insights from Cusco, Peru. HOMO, 67, 100–109. [DOI] [PubMed] [Google Scholar]
- Oxilia, G. , Fiorillo, F. , Boschin, F. , Boaretto, E. , Apicella, S. A. , Matteucci, C. , … Benazzi, S. (2017). The dawn of dentistry in the late upper Paleolithic: An early case of pathological intervention at Riparo Fredian. American Journal of Physical Anthropology, 163, 446–461. [DOI] [PubMed] [Google Scholar]
- Oxilia, G. , Peresani, M. , Romandini, M. , Matteucci, C. , Spiteri, C. D. , Henry, A. G. , … Benazzi, S. (2015). Earliest evidence of dental caries manipulation in the Late Upper Palaeolithic. Scientific Reports, 5, 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, J. B. , Hiiemae, K. M. , & Liu, J. (1997). Tongue–jaw linkages in feeding: A preliminary videofluorographic study. Archives of Oral Biology, 42, 429–441. [DOI] [PubMed] [Google Scholar]
- Palmer, P. M. , Jaffe, D. M. , McCulloch, T. M. , Finnegan, E. M. , Van Daele, D. J. , & Luschei, E. S. (2008). Quantitative contributions of the muscles of the tongue, floor‐of‐mouth, jaw, and velum to tongue‐to‐palate pressure generation. Journal of Speech, Language, and Hearing Research, 51, 828–835. [DOI] [PubMed] [Google Scholar]
- Pameijer, J. H. , Glickman, I. , & Roeber, F. W. (1970). Intraoral occlusal telemetry. Part IV. Tooth contact during swallowing. Journal of Prosthetic Dentistry, 24, 396–400. [DOI] [PubMed] [Google Scholar]
- Proffit, W. R. (1975). Muscle pressure and tooth position: North American Whites and Australian Aborigines. Angle Orthodontist, 45, 1–11. [DOI] [PubMed] [Google Scholar]
- Proffit, W. R. (1978). Equilibrium theory revisited: Factors influencing position of the teeth. The Angle Orthodontist, 48, 175–186. [DOI] [PubMed] [Google Scholar]
- Proffit, W. R. , McGlone, R. E. , & Barrett, M. J. (1975). Lip and tongue pressure related to dental arch and oral cavity size in Australian Aborigines. Journal of Dental Research, 54, 1161–1172. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: URL https://www.R-project.org/.
- Rakosi, T. (1978). An atlas and manual of cephalometric radiography (pp. 96–98). London: Wolf Medical Publication Limited. [Google Scholar]
- Ricci, S. , Capecchi, G. , Boschin, F. , Arrighi, S. , Ronchitelli, A. , & Condemi, S. (2016). Toothpick use among Epigravettian humans from Grotta Paglicci (Italy). International Journal of Osteoarchaeology, 26, 281–289. [Google Scholar]
- Richards, L. C. , & Brown, T. (1986). Development of the helicoidal plane. Journal of Human Evolution, 1, 385–398. [Google Scholar]
- Sameera, S. D. , Singh, D. P. , & Nitya, D. (2017). Bruxism: Its multiple causes and its effects on Dental Implants: A review. Journal of Oral Health and Craniofacial Science, 2, 057–063. [Google Scholar]
- Schwartz, J. H. , Brauer, J. , & Gordon‐Larsen, P. (1995). Tigaran (Point Hope, Alaska) tooth drilling. American Journal of Physical Anthropology, 97, 77–82. [DOI] [PubMed] [Google Scholar]
- Seidel, J. C. , Colten, R. H. , Thibodeau, E. A. , & Aghajanian, J. G. (2005). Iatrogenic molar borings in 18th and early 19th century Native American dentitions. American Journal of Physical Anthropology, 127, 7–12. [DOI] [PubMed] [Google Scholar]
- Shipman, P. , & Rose, J. (1983). Early hominid hunting, butchering and carcass‐processing behaviors: Approaches to the fossil record. Journal of Anthropological Archaeology, 2, 57–98. [Google Scholar]
- Smith, B. H. (1984). Patterns of molar wear in hunter‐gatherers and agriculturists. American Journal of Physical Anthropology, 63, 39–56. [DOI] [PubMed] [Google Scholar]
- Smith, R. J. (2009). Use and misuse of reduced major axis for line‐fitting. American Journal of Physical Anthropology, 140, 476–486. [DOI] [PubMed] [Google Scholar]
- Stratus, L. G. (1989). On early hominid use of fire. Current Anthropology, 30, 488–491. [Google Scholar]
- Toth, N. P. (1985). The Oldowan reassessed: A close look at early stone artifacts. The Journal of Archaeological Science, 12, 101–120. [Google Scholar]
- Townsend, G. C. , & Brown, T. (1980). Dental asymmetry in Australian Aboriginals. Human Biology, 52, 661–673. [PubMed] [Google Scholar]
- Turner, C. G. (2004). A second drilled tooth from prehistoric western North America. American Antiquity, 69, 356–360. [Google Scholar]
- Van Dyck, C. , Dekeyser, A. , Vantricht, E. , Manders, E. , Goeleven, A. , Fieuws, S. , & Willems, G. (2016). The effect of orofacial myofunctional treatment in children with anterior open bite and tongue dysfunction: A pilot study. European Journal of Orthodontics, 38, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, J. T. (2008). Changes in food processing and occlusal dental wear during the early agricultural period in northwest Mexico. American Journal of Physical Anthropology, 135, 92–99. [DOI] [PubMed] [Google Scholar]
- White, T. D. , Degusta, D. , & Richards, G. D. (1997). Prehistoric dentistry in the American Southwest: a drilled canine from sky Aerie, Colorado. American Journal of Physical Anthropology, 103, 409–414. [DOI] [PubMed] [Google Scholar]
- Zink, K. D. , & Lieberman, D. E. (2016). Impact of meat and lower Paleolithic food processing techniques on chewing in humans. Nature, 531, 500–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information 1


