Abstract
Aim
Intravenous sedatives used in the paediatric intensive care unit (PICU) need to be tapered after prolonged use to prevent iatrogenic withdrawal syndrome (IWS). We evaluated the occurrence of IWS and the levels of sedation before and after conversion from intravenous midazolam to oral lorazepam.
Methods
This was a retrospective, observational, single cohort study of children under the age of 18 admitted to the PICU of the Erasmus MC‐Sophia Children's Hospital, Rotterdam, The Netherlands, between January 2013 and December 2014. The outcome parameters were the Sophia Observation withdrawal Symptoms (SOS) scale scores and COMFORT Behaviour scale scores before and after conversion.
Results
Of the 79 patients who were weaned, 32 and 39 had before and after SOS scores and 77 had COMFORT‐B scores. IWS was reported in 15 of 79 patients (19.0%) during the 48 hours before the start of lorazepam and 17 of 79 patients (21.5%) during the 48 hours after treatment started. Oversedation was seen in 16 of 79 patients (20.3%) during the 24 hours before substitution and in 30 of 79 patients (38.0%) during the 24 hours after substitution.
Conclusion
The weaning protocol was not able to prevent IWS in all patients, but converting from intravenous midazolam to oral lorazepam did not increase the incidence.
Keywords: Lorazepam, Midazolam, Paediatric intensive care, Sedation, Withdrawal
Abbreviations
- COMFORT‐B
COMFORT‐behaviour scale
- IWS
Iatrogenic withdrawal syndrome
- NISS
Nurses Interpretation of Sedation Score
- PICU
Paediatric intensive care unit
- SOS
Sophia Observation withdrawal Symptoms scale
Key notes.
Intravenous sedatives used in the paediatric intensive care unit (PICU) need to be tapered after prolonged use to prevent iatrogenic withdrawal syndrome (IWS).
We evaluated IWS and sedation before and after conversion from intravenous midazolam to oral lorazepam in patients under 18 years of age.
The weaning protocol was not always able to prevent IWS, but converting from intravenous midazolam to oral lorazepam did not increase the incidence.
Introduction
Most children admitted to a paediatric intensive care unit (PICU) receive intravenous sedatives and analgesics to relieve anxiety, distress and pain and to tolerate mechanical ventilation and other PICU‐related procedures. The most commonly used sedatives and analgesics in paediatrics are midazolam and opioids 1. Unfortunately, these drugs can cause iatrogenic withdrawal syndrome (IWS) after prolonged use 2.
To prevent IWS, a protocolled approach to taper the drugs and to regularly monitor withdrawal symptoms and sedation levels is recommended. Intravenously administered medication can be switched to oral dosage forms, to facilitate gradual weaning without the need for cardiorespiratory monitoring required for intravenous sedation and to omit the need for intravenous access. Treatment can then be continued outside the PICU, and removing intravenous access lowers the risk of infection. Oral lorazepam has a long half‐life in children, with a median of 17 hours (range 8–53 hours), which prevents large fluctuations in plasma concentrations, and also has a lack of active metabolites. That is why it is often used off‐label as a substitute for intravenous midazolam 2, 3, 4.
In our local weaning protocol, the calculation of the initial dose of oral lorazepam was based on a conversion factor proposed by Tobias et al. 5, which assumed that lorazepam was twice as potent as midazolam and had a six‐time longer half‐life, based on adult data. This lorazepam starting dose is calculated irrespective of the potential impact of maturation of lorazepam and midazolam metabolism due to age or other factors influencing drug exposure, such as critical illness. In addition to this, the bioavailability of oral lorazepam in children is unknown and, therefore, no correction is possible for a potential incomplete bioavailability. In summary, this means that the current dosage of lorazepam for weaning of midazolam may not be optimal. At the time of our study, no clinical data on the conversion from midazolam to lorazepam in PICU settings were available in the literature.
Due to this limited information, the aim of this study was to evaluate the occurrence of IWS and the level of sedation before and after conversion from intravenous midazolam to oral lorazepam. We also wanted to assess the safety of our current midazolam to lorazepam conversion protocol.
Methods
Design and study population
A retrospective, single center, cohort study was performed to evaluate the move from intravenous midazolam to oral lorazepam to keep patients comfortable and prevent IWS. Our study population was admitted to the level five PICU of the Erasmus MC ‐ Sophia Children's Hospital, Rotterdam, The Netherlands, between January 2013 and December 2014. Patients were selected from our Critical Care Suite electronic patient data management system (Picis Clinical Solutions SA, Barcelona, Spain), when they had received oral lorazepam following intravenous midazolam. The exclusion criteria were the use of midazolam and lorazepam for epilepsy or delirium, when the latter had been diagnosed by a trained psychiatrist, or for other reasons such as incidental sleep medication. The medical ethics committee of the hospital waived the need for institutional review board approval and informed consent according to the Dutch law on Medical Human Research.
IWS and sedation scores
To achieve optimal weaning, it is necessary to monitor symptoms of IWS from benzodiazepines and opioids and to monitor the level of sedation. These are assessed using the Sophia Observation withdrawal Symptoms (SOS) scale to determine IWS and the COMFORT behaviour (COMFORT‐B) scale to assess the level of sedation 6, 7, 8. The SOS scale consists of 15 items representing signs and symptoms of opioid and/or benzodiazepine withdrawal, including changes in heart and respiratory rate and signs of discomfort. IWS scoring is initiated at start of weaning and performed at eight‐hour intervals, when the occurrence of IWS is suspected, and to evaluate any interventions that were made to treat IWS. The COMFORT‐B scale consists of six behavioural items and is applied in combination with the Nurses Interpretation of Sedation Score (NISS) 6 from the start of mechanical ventilation. It has been validated to assess the level of sedation in ventilated and nonventilated children. Scoring is performed by the attending nurses at eight‐hour intervals and if there are signs of distress or increasing discomfort. It continues until discharge from the PICU or until all sedative medication has been stopped.
Weaning protocol
Weaning of sedative and analgesic medication is initiated as soon as the patient's underlying condition and pathology improve, their electrolytes are within normal range and they are cardiovascularly stable. The protocol for weaning of continuous opioids and sedatives implemented at our PICU starts with decreasing continuous infusion rates of the drugs and the intervals depend on the preceding length of treatment. Infusion rates are decreased, one drug at a time, by 10% of the initial rate. This occurs every 24 hours when the patient has received the drug for six to nine days and every 48 hours when they have received the drug for 10 days or more. The intravenous medication is converted to an effect‐equivalent dose of oral medication within the same therapeutic class when the patient is due to be discharged to the general ward without cardiorespiratory monitoring, when intravenous access is no longer required or available or when prolonged weaning is expected. The initial daily dose of oral lorazepam is calculated by dividing the daily dose of midazolam by 12. This conversion is based on the lorazepam and midazolam ratio for half‐life (6:1) and its relative potency (2:1) in adults 5. This lorazepam dose is administered orally four times a day, and the intravenous midazolam is tapered over 24 hours as shown in Figure 1. Lorazepam is subsequently tapered in steps of 10% of the initial dose every 24 or 48 hours. If there are withdrawal symptoms, indicated by an SOS score of four or more, a rescue dose of 0.1 mg/kg midazolam is administered or the oral lorazepam dose is increased to the previous strength. If applicable, opioids and other sedatives, such as morphine, fentanyl, clonidine and pentobarbital, are also converted to oral alternatives in a similar manner, for example methadone, clonidine per os and phenobarbital, preferably with a minimum of 48 hours between conversions. They are tapered according to the same principles.
Figure 1.

Tapering of midazolam after substitution with oral lorazepam. The intravenous midazolam dose is halved after the second administration of lorazepam, again halved after the third administration of lorazepam and ceased after the fourth administration of lorazepam (24 hours after switch). The first dose of lorazepam is calculated upon the last infusion rate of midazolam.
Medication
Intravenous midazolam was administered using a Perfusor FM syringe pump (B Braun Medical, Oss, The Netherlands), in concentrations of 1 or 5 mg/mL dissolved in 5% glucose, which were prepared by the pharmacy. Oral midazolam for rescue administrations was available as an extemporaneous liquid of 1 mg/mL. Oral lorazepam was administered as either commercial tablets, extemporaneous capsules of 0.1 mg or a 4 mg/mL commercial injection fluid that was administered orally. Solid dosage forms were usually dispersed in water and administered through a feeding tube.
Data collection
Data were extracted from the electronic medical records. The clinical and demographic parameters that were retrieved included age, sex, diagnosis, cumulative doses and duration of midazolam and lorazepam therapy, analgesic and sedative co‐medication and the patient's destination after their discharge from the PICU.
Outcomes
The SOS scores were retrieved to determine the incidence of withdrawal from 48 hours before substitution to 48 hours after substitution. A cut‐off score of at least four was defined as withdrawal. The COMFORT‐B scores and NISS were analysed from 48 hours before substitution to 48 hours after substitution to determine the level of sedation. COMFORT‐B scores of ≥23 or 11–22 with a NISS of one were regarded as undersedation, COMFORT‐B scores of 11–22 with a NISS of two were regarded as adequate sedation and COMFORT‐B scores of ≤10 or 11–22 with a NISS of three were regarded as oversedation. Similarly, the number of rescue dosages of midazolam and other sedatives were compared from 48 hours before to 48 hours after substitution. The frequency and severity of apnoeas and the need for flumazenil during the 48 hours after start of lorazepam were used to assess the safety of the conversion. Apnoeas were registered manually in the patient data management system by the attending physician or nurse as part of standard care. The agreement of the actual midazolam to lorazepam conversion with the conversion protocol was assessed with respect to the dose calculation of lorazepam and the tapering of midazolam within 24 hours after conversion.
Analysis
Data were analysed using IBM SPSS statistics version 21.0 (IBM Corporation, New York, NY, USA). Demographic and clinical data were processed using descriptive statistics. The number of rescue administrations of midazolam and other sedatives before and after substitution were compared using a paired‐sample t‐test.
Results
During the 24‐month study period between January 2013 and December 2014, 111 cases met the inclusion criterion for oral lorazepam use after intravenous midazolam therapy. After excluding three patients who started lorazepam in 2012, 20 patients who received lorazepam for other purposes than weaning, and excluding multiple occasions within one subject (n = 9), 79 cases were included for further analysis. The patient characteristics are listed in Table 1.
Table 1.
Patient characteristics (n = 79)
| Parameter | n | % |
|---|---|---|
| Sex | ||
| Male | 37 | 46.8 |
| Female | 42 | 53.2 |
| Age, median (months) (IQR) | 5.3 (1.7–19.8) | |
| Age | ||
| 0–27 days | 13 | 16.5 |
| 28 days–11 months | 40 | 50.6 |
| 12–23 months | 8 | 10.1 |
| 2–11 years | 16 | 20.2 |
| 12–18 years | 2 | 2.5 |
| Weight, median (kg) (IQR) | 5.5 (3.6–10.0) | |
| Reason for PICU admission | ||
| Cardiac | 30 | 28.0 |
| Noncardiac surgical | 4 | 5.1 |
| Neurological | 1 | 1.3 |
| Infection/respiratory | 19 | 24.1 |
| Trauma | 2 | 2.5 |
| Congenital | 9 | 11.4 |
| Other | 14 | 17.7 |
| Ventilation | 79 | 100 |
| ECMO therapy | 7 | 8.9 |
| Transfer after PICU | ||
| Home | 7 | 8.9 |
| Other hospital | 18 | 22.8 |
| Other department | 45 | 57.0 |
| Mortality | 9 | 11.4 |
| Median length of PICU stay | ||
| Days (range) | 32 (4–183) | |
ECMO = Extracorporeal membrane oxygenation; IQR = interquartile range; PICU = paediatric intensive care unit.
At the point of the midazolam to lorazepam switch, the median duration of midazolam infusion, from the day of admittance to the Sophia Children's Hospital, was 12 days (range 1–69), and the median cumulative dose was 46.5 mg/kg (range 0.47–287). We also noted that 23 patients were still on invasive ventilation, and 11 patients had received midazolam at infusion rates that were higher than 0.35 mg/kg/h during their admission. Further information on the patients’ sedative treatment during PICU admission is summarised in Table 2.
Table 2.
Sedative treatment characteristics during PICU admission (n = 79)
| Parameter | Median (range) | Unit |
|---|---|---|
| Median dose per patient | ||
| Midazolama | 130 (30–393) | mcg/kg/h |
| Lorazepamb | 0.30 (0.08–2.76) | mg/kg/d |
| Cumulative dose | ||
| Midazolamc | 46.5 (0.47–287) | mg/kg |
| Lorazepam | 1.42 (0.08–79.32) | mg/kg |
| Maximum infusion rate before subsitution | ||
| Midazolam | 300 (12–1000) | mcg/kg/h |
| Duration of infusion until substitution | ||
| Midazolam | 12 (1–69) | days |
| Duration of midazolam therapy until substitutiond | n | % |
|---|---|---|
| <5 days | 3 | 3.8 |
| 5–10 days | 16 | 20.3 |
| >10 days | 60 | 75.9 |
| Duration of lorazepam taper | Days (range) | |
|---|---|---|
| Lorazepame | 22 (3–97) | (n = 45) |
| Fixed‐interval and continuous sedative and analgesic co‐medication | n | % |
|---|---|---|
| Alimemazine po | 10 | 13 |
| Clonidine | ||
| iv | 41 | 52 |
| po | 23 | 29 |
| Esketamine iv | 26 | 33 |
| Fentanyl iv | 9 | 11 |
| Methadone po | 16 | 20 |
| Morphine iv | 73 | 92 |
| Pentobarbital iv | 3 | 4 |
| Propofol iv | 19 | 24 |
po = orally; iv = intravenous; PICU = paediatric intensive care unit.
Throughout PICU admission.
Starting dose at substitution.
Until substitution.
Midazolam therapy was calculated from the first administration to the last administration in the Sophia Children's hospital. The short administration of one day is due to the transfer from another hospital.
n = 45. Total lorazepam duration, including use at home. Only the patients with complete postclinical duration were used to calculate the median.
The SOS scores were available for 32 of 79 (40.5%) of the patients in the 48 hours before substitution and 39 of 79 (49.4%) of the patients in the 48 hours after substitution. The median score per patient before the start of lorazepam ranged between 0 and 9.0, with 15 patients (19.0%) having one or more SOS score of at least four, indicating IWS. After the start of lorazepam, the median score per patient ranged between 0 and 5.0, with 17 patients (21.5%) having one or more SOS score of at least four. In eight of these 17 patients, the morphine infusion rates were decreased during the 96 hours around conversion. Figure 2 shows the range of the highest SOS score per patient within our study period. Seven patients experienced IWS both before and after substitution, and 11 patients experienced both oversedation and IWS in the 48 hours after substitution.
Figure 2.
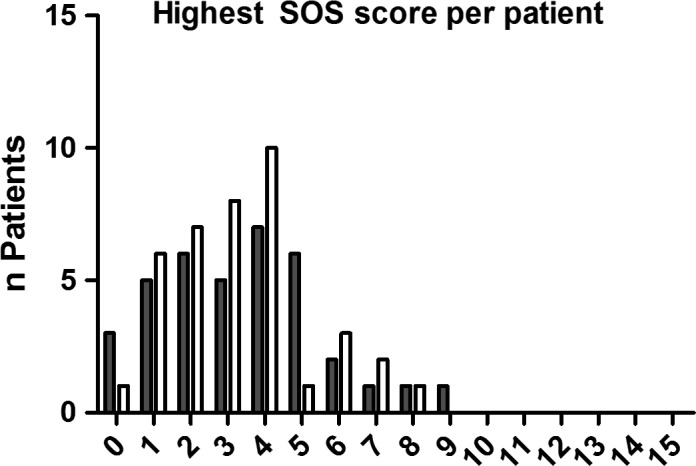
Distribution of the highest SOS score per patient during the first 48 hours before substitution (grey bars) and 48 hours after substation (open bars) of iv midazolam with oral lorazepam. Maximum score is 15, with scores ≥4 indicating withdrawal.
COMFORT‐B scores were available for 77 of 79 patients (97.5%). All the available scores are shown in Figure 3, with a median of three scores per patient per day. From a total of 1122 COMFORT‐B scores, 136 incidences of oversedation and 150 incidences of undersedation were determined, in combination with the NISS, during the 96‐hour study period. Only 44 of the incidences of undersedation were accounted for by COMFORT‐B scores of at least 23 and the other 106 by a COMFORT‐B score between 11 and 22 and a NISS of one.
Figure 3.

Histograms of available COMFORT‐B scores during the four different study periods. The window between the dotted lines show scores that are regarded as adequate sedation, while lower scores (≤10) are regarded as oversedation and higher scores (≥23) as undersedation.
In some patients, the COMFORT‐B scores, in combination with the NISS, were outside the adequate sedation range and these are presented in Figure 4. This Figure shows that the incidence of oversedation increased after substitution with lorazepam. During the two days before substitution, 13 and 16 patients, respectively, experienced oversedation compared to 39 and 30 patients in the two days after substitution. Undersedation decreased from 28 and 21 patients before lorazepam initiation to 16 and 13 patients after the start of lorazepam.
Figure 4.

Oversedation: COMFORT‐B scores ≤10 or 11–22 with NISS = 3. Adequate sedation: COMFORT‐B scores of 11–22 with NISS = 2. Undersedation: COMFORT‐B scores ≥23 or 11–22 with NISS = 1. For study periods 1–4, respectively 7, 9, 11 and 6 children were both under‐ and oversedated.
A total of 34 patients (43.0%) received one or more rescue administrations of midazolam before substitution, compared to 19 patients (24.1%) after substitution, with a 95% confidence interval (95% CI) of −0.06 to 0.77, p = 0.096. Furthermore, 29 patients (36.7%) received rescue administrations of other sedatives before substitution compared to 21 patients (26.6%) after substitution (95% CI: −0.18 to 0.94, p = 0.178). In total, 50 patients (63.3%) received rescue administrations before substitution and 34 patients (43.0%) after substitution with a median of two administrations in both periods. During the 48‐hour postsubstitution period, 56 patients (70.9%) continued their sedative or analgesic co‐medication. Co‐medication was decreased in 44 patients and increased in three patients.
Regarding the safety of the substitution, no apnoeas were reported, and no flumazenil was prescribed during the 96 hours around the conversion.
Adherence to the conversion protocol was variable. The median midazolam/lorazepam dose ratio was 11.4 (range 1.31–22.6), and 62.0% of the ratios were between 10 and 14. In 45.6% of the patients, midazolam was tapered in a time frame of 24 hours from substitution, in agreement with the protocol. In 32.9%, intravenous midazolam was discontinued before 24 hours and in 21.5%, simultaneous administration of intravenous midazolam and oral lorazepam continued for more than 24 hours.
Discussion
Our midazolam to lorazepam switch protocol to prevent IWS appeared to be effective in the majority of patients, as no increase in the occurrence of IWS was detected. Nevertheless, at least 20% of patients still experienced withdrawal symptoms, while almost 40% showed signs of oversedation in the early stages after conversion.
Based upon the available SOS scores, the incidence of IWS was similar before and after conversion to lorazepam. A limitation is that only about half of the patients were scored for withdrawal, making the results hard to extrapolate. When we assume that the exhibition of IWS symptoms is a trigger to start collecting SOS scores, the absence of SOS scores may be seen as a sign that the patients were doing well, but this needs to be verified in a prospective setting. Furthermore, the SOS scale cannot discriminate between opioid and benzodiazepine withdrawal. This means that the reported IWS cannot unequivocally be attributed to benzodiazepine withdrawal, especially in the eight patients where morphine was tapered simultaneously. Nevertheless, we did not observe an increase in IWS after the conversion to lorazepam.
The incidence of IWS in critically ill children has been reported to range from 13 to 87% 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. This large variation was the result of small sample sizes, a large variety in often unvalidated assessment methods and nonstandardised or absent sedation protocols and weaning regimens. Identified risk factors for IWS are cumulative doses of midazolam greater than 40 mg/kg 8, 11, infusion of opioids and benzodiazepines for more than five days 8, 11, 13 and midazolam infusion rates above 0.35–0.42 mg/kg/h 18, 19. Taking into consideration, the clinical patient characteristics, such as the high cumulative doses of midazolam and long PICU stays, it becomes apparent that the patients in our cohort were at high risk for developing IWS. In our retrospective cohort, based upon the available SOS scores, IWS was diagnosed in one‐fifth of the patients, both before and after substitution.
The majority of the collected COMFORT‐B scores were within the target range for adequate sedation, with a tendency towards more oversedation postsubstitution. This could suggest supratherapeutic dosages of sedatives, especially during the first 24 hours in which midazolam and lorazepam were simultaneously administered. To put these findings into perspective, COMFORT‐B scores of nine and 10 could be the result of a comfortably asleep child with normal muscle and facial tone and is not necessarily indicative of an unsafe situation. Considering it may take a number of days to reach steady‐state plasma levels of lorazepam due to its long half‐life, it seems rational to start with lorazepam while phasing out midazolam to ensure adequate exposure. The absence of apnoeas and flumazenil administration during the study period provides evidence that the combined blood levels of benzodiazepines were not within the toxic range. It is notable that several patients experienced both oversedation and withdrawal after substitution, which illustrates the complexity of managing IWS. The comparison of rescue administrations of midazolam and other sedatives yielded no statistically significant results.
The lorazepam dose calculation was based on the relative half‐life and potency of lorazepam versus midazolam, as determined in adult patients, and irrespective of individual patient characteristics. Lorazepam is primarily metabolised through conjugation with glucuronic acid by multiple hepatic UDP‐glucuronosyltransferase enzymes, to inactive metabolites. The maturation rates of involved enzyme systems differ between the subtypes, but may well extend beyond the age of two years, based upon gene expression data and in vivo experiments 20, 21. Paediatric pharmacokinetic data after the oral administration of lorazepam are unavailable. At the moment, there are insufficient data available to establish an age‐dependent conversion factor. Midazolam pharmacokinetics in paediatric patients are well studied and are highly dependent on CYP3A4 activity. High blood levels of midazolam might be caused by delayed clearance due to immature metabolism at a neonatal age 22, ongoing inflammation and critical illness 23, co‐medication, accumulation of its active metabolites after prolonged use 19 or renal insufficiency 24. None of these factors are currently considered in the dose calculation.
This retrospective analysis of a weaning strategy reflects clinical practice in patients in a complex, intensive care setting. We acknowledge that our study had several limitations. Although COMFORT‐B scores were taken regularly, we found that SOS scores were underreported. In addition, the lorazepam dose calculation in some patients was based upon the midazolam dosage rate at the moment of conversion instead of the cumulative dose of the last 24 hours, resulting in different dosing strategies. Since 2017, a lorazepam extemporaneous oral liquid of 1 mg/mL has been available 25. As a result, oral administration of injection fluid is no longer applied and capsules are no longer used. The dose conversion is now checked by the attending pharmacist. One further limitation was that the concomitant use of other central nervous depressants was common during PICU stays in our study, and this hindered the attribution of the observations to the conversion from midazolam to lorazepam.
In the past two decades, considerable progress has been made in recognising the need for weaning‐off sedation strategies in PICUs. Risk factors for the development of IWS have been identified, and scoring systems have been validated and implemented to monitor the patients. This study was the first to specifically address the use of oral lorazepam in the weaning‐off sedation strategy in PICU patients.
Conclusion
The weaning protocol for sedatives using lorazepam did not increase the incidence of IWS and appeared to be safe. A better understanding of the factors that explain variations in both pharmacokinetics and pharmacodynamics may help us to further tailor weaning strategies to the individual patient.
Finance
This research was supported by a ZonMW grant (number 113202004) awarded L M Hanff, as part of the Priority Medicines Children programme.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank Professor D Tibboel for his contributions to the manuscript.
References
- 1. Kudchadkar SR, Yaster M, Punjabi NM. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake‐up call for the pediatric critical care community. Crit Care Med 2014; 42: 1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Best KM, Boullata JI, Curley MA. Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model. Pediatr Crit Care Med 2015; 16: 175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Connell C, Ziniel S, Hartwell L, Connor J. Management of opioid and sedative weaning in pediatric congenital heart disease patients: assessing the state of practice. Dimens Crit Care Nurs 2017; 36: 116–24. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez D, Chamberlain JM, Guptill JT, Cohen‐Wolkowiez M, Harper B, Zhao J, et al. Population pharmacokinetics and exploratory pharmacodynamics of Lorazepam in pediatric status Epilepticus. Clin Pharmacokinet 2017; 56: 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tobias JD. Tolerance, withdrawal, and physical dependency after long‐term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med 2000; 28: 2122–32. [DOI] [PubMed] [Google Scholar]
- 6. Ista E, van Dijk M, Tibboel D, de Hoog M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT “behavior” scale. Pediatr Crit Care Med 2005; 6: 58–63. [DOI] [PubMed] [Google Scholar]
- 7. Ista E, van Dijk M, de Hoog M, Tibboel D, Duivenvoorden HJ. Construction of the Sophia Observation withdrawal Symptoms‐scale (SOS) for critically ill children. Intensive Care Med 2009; 35: 1075–81. [DOI] [PubMed] [Google Scholar]
- 8. Ista E, de Hoog M, Tibboel D, Duivenvoorden HJ, van Dijk M. Psychometric evaluation of the Sophia Observation withdrawal symptoms scale in critically ill children. Pediatr Crit Care Med 2013; 14: 761–9. [DOI] [PubMed] [Google Scholar]
- 9. Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit Care Med 1999; 27: 196–9. [DOI] [PubMed] [Google Scholar]
- 10. Hughes J, Gill A, Leach HJ, Nunn AJ, Billingham I, Ratcliffe J, et al. A prospective study of the adverse effects of midazolam on withdrawal in critically ill children. Acta Paediatr 1994; 83: 1194–9. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez‐Carrion F, Gaboli M, Gonzalez‐Celador R, Gomez de Quero‐Masia P, Fernandez‐de Miguel S, Murga‐Herrera V, et al. Withdrawal syndrome in the pediatric intensive care unit. Incidence and risk factors. Med Intensiva 2013; 37: 67–74. [DOI] [PubMed] [Google Scholar]
- 12. Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med 1994; 22: 763–7. [DOI] [PubMed] [Google Scholar]
- 13. Franck LS, Scoppettuolo LA, Wypij D, Curley MA. Validity and generalizability of the Withdrawal Assessment Tool‐1 (WAT‐1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain 2012; 153: 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MA. The Withdrawal Assessment Tool‐1 (WAT‐1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med 2008; 9: 573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neunhoeffer F, Kumpf M, Renk H, Hanelt M, Berneck N, Bosk A, et al. Nurse‐driven pediatric analgesia and sedation protocol reduces withdrawal symptoms in critically ill medical pediatric patients. Paediatr Anaesth 2015; 25: 786–94. [DOI] [PubMed] [Google Scholar]
- 16. Dominguez KD, Crowley MR, Coleman DM, Katz RW, Wilkins DG, Kelly HW. Withdrawal from lorazepam in critically ill children. Ann Pharmacother 2006; 40: 1035–9. [DOI] [PubMed] [Google Scholar]
- 17. Bowens CD, Thompson JA, Thompson MT, Breitzka RL, Thompson DG, Sheeran PW. A trial of methadone tapering schedules in pediatric intensive care unit patients exposed to prolonged sedative infusions. Pediatr Crit Care Med 2011; 12: 504–11. [DOI] [PubMed] [Google Scholar]
- 18. Amigoni A, Vettore E, Brugnolaro V, Brugnaro L, Gaffo D, Masola M, et al. High doses of benzodiazepine predict analgesic and sedative drug withdrawal syndrome in paediatric intensive care patients. Acta Paediatr 2014; 103: e538–43. [DOI] [PubMed] [Google Scholar]
- 19. da Silva PS, Reis ME, Fonseca TS, Fonseca MC. Opioid and Benzodiazepine Withdrawal Syndrome in PICU Patients: which Risk Factors Matter? J Addict Med 2016; 10: 110–6. [DOI] [PubMed] [Google Scholar]
- 20. Divakaran K, Hines RN, McCarver DG. Human hepatic UGT2B15 developmental expression. Toxicol Sci 2014; 141: 292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, et al. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut 2002; 50: 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Wildt SN, Kearns GL, Murry DJ, Koren G, van den Anker JN. Ontogeny of midazolam glucuronidation in preterm infants. Eur J Clin Pharmacol 2010; 66: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vet NJ, de Hoog M, Tibboel D, de Wildt SN. The effect of critical illness and inflammation on midazolam therapy in children. Pediatr Crit Care Med 2012; 13: e48–50. [DOI] [PubMed] [Google Scholar]
- 24. Bauer TM, Ritz R, Haberthur C, Ha HR, Hunkeler W, Sleight AJ, et al. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995; 346: 145–7. [DOI] [PubMed] [Google Scholar]
- 25. van der Vossen AC, van der Velde I, Smeets OS, Postma DJ, Eckhardt M, Vermes A, et al. Formulating a poorly water soluble drug into an oral solution suitable for paediatric patients; lorazepam as a model drug. Eur J Pharm Sci 2017; 100: 205–10. [DOI] [PubMed] [Google Scholar]


