Summary
Background
The incidence and short‐term outcome of anaemia in inflammatory bowel disease (IBD) are largely unknown.
Aim
To determine the incidence, prevalence and clinical outcome of anaemia in terms of resolution of anaemia within 12 months. We also planned to assess risk factors for anaemia in IBD.
Methods
A random sample of 342 patients was obtained from the population‐based IBD cohort of Örebro University Hospital, Sweden, consisting of 1405 patients diagnosed between 1963 and 2010. Haemoglobin measurements recorded from 1 January 2011 to 31 December 2013 were extracted from the Clinical Chemistry data system.
Results
In Crohn's disease, the incidence rate of anaemia was 19.3 (95% CI: 15.4‐23.7) per 100 person‐years and the prevalence was 28.7% (CI: 22.0‐36.2), compared with 12.9 (CI: 9.8‐16.5) and 16.5% (CI: 11.2‐22.9) for ulcerative colitis. Crohn's disease was associated with an increased incidence (OR = 1.60; CI: 1.02‐2.51) and prevalence of anaemia (OR = 2.04; CI: 1.20‐3.46) compared to ulcerative colitis. Stricturing disease phenotype in Crohn's disease (HR = 2.59; CI: 1.00‐6.79) and extensive disease in ulcerative colitis (HR = 2.40; CI: 1.10‐5.36) were associated with an increased risk of anaemia. Despite a higher probability of receiving specific therapy within 3 months from the diagnosis of anaemia, Crohn's disease patients had a worse outcome in terms of resolution of anaemia within 12 months (56% vs 75%; P = 0.03).
Conclusions
Anaemia is a common manifestation of IBD even beyond the first years after the diagnosis of IBD. Crohn's disease is associated with both an increased risk and a worse outcome.
1. INTRODUCTION
The two main forms of inflammatory bowel disease (IBD) are Crohn's disease and ulcerative colitis. These are chronic inflammatory diseases that mainly affect the gastrointestinal tract, although systemic complications that may involve almost any organ system are common in both disorders.1, 2 Anaemia appears to be the most common extraintestinal manifestation of IBD and has been associated with a wide range of complications such as impaired quality of life, an increased rate of hospital admissions and even mortality.3, 4, 5, 6
However, despite numerous studies, the occurrence rate of anaemia in IBD is still uncertain, as most of the data have come from selected study populations such as patients at tertiary referral centres or patients admitted to hospital.5, 7, 8, 9, 10 In these populations, the prevalence appears to be high in patients admitted to hospital (≈ 70%) and in newly diagnosed patients (≈ 65%), whereas the occurrence appears to be lower in out‐patients (≈ 20%) and in patients with an established IBD (≈ 35%).5, 7, 8, 9, 10 A high prevalence of anaemia at the time of diagnosis has been confirmed by recent population‐based studies,11, 12, 13, 14 whereas there are unfortunately few population‐based data on the occurrence of anaemia in patients with established IBD.12, 15 Furthermore, the vast majority of previous studies have only investigated the prevalence of anaemia, and to our knowledge, no general population‐based study has been performed to determine the incidence of anaemia in IBD.16 The main causes of anaemia in IBD are iron deficiency and anaemia of chronic disease.5, 17 The treatment of anaemia includes both supplementation of deficiencies and adjustment of IBD therapy.18, 19, 20 Efficient therapeutic options such as dextran‐free intravenous iron preparations and erythropoietin have been available over the past 20 years.20, 21 Even so, the extent to which these new therapies have been implemented in clinical management and also the outcome of anaemia in real‐world clinical practice, in terms of resolution of anaemia within 12 months, remain largely unknown.
The aim of this study was to determine the mean annual incidence of anaemia in an unselected cohort of patients with established IBD, to assess the prevalence of anaemia and to determine whether the occurrence and outcome differ between patients with Crohn's disease and those with ulcerative colitis, or depending on disease phenotype.
2. MATERIALS AND METHODS
2.1. Study population
In this retrospective cohort study, a random sample of patients (n = 342) diagnosed with Crohn's disease and ulcerative colitis during the period 1963‐2010 was identified from the population‐based IBD cohort of Örebro University Hospital (n = 1405).22, 23, 24 The cohort and the study region have already been described in detail.22, 23, 24 Briefly, the primary catchment area of Örebro University Hospital is located in central Sweden. The region had 189 603 inhabitants in 2010.25 The age‐standardised incidence rate of Crohn's disease was 6.7 per 100 000 inhabitants in 2006‐2010 and the corresponding figure for ulcerative colitis was 18.1 per 100 000 inhabitants.22, 23 There are no private gastroenterologists in the region, and all individuals with verified or suspected IBD are referred to the university hospital. All laboratory data collected at primary healthcare clinics and in hospital settings are prospectively stored in the digital laboratory information and management system of the Department of Clinical Chemistry.
The study was approved by the Uppsala Regional Ethics Committee in 2010 (approval number: 2010/304 and 2010/304/1).
2.2. Data collection
Data on clinical characteristics according to the Montreal classification,26 ongoing medication, previous surgery, visits to the out‐patient clinic and hospital admissions were extracted from the IBD cohort database of Örebro University Hospital and from medical records. The following categories were used for anti‐inflammatory therapy at baseline and during follow‐up: oral 5‐aminosalicylates (balsalazide, mesalazine, olsalazine and sulphasalazine), systemic corticosteroids (betamethasone and prednisolone), immunomodulators (azathioprine, mercaptopurine, tioguanine and methotrexate) and tumour necrosis factor‐alpha inhibitors (anti‐TNF: infliximab, adalimumab, golimumab and certolizumab). Information on supplementation therapy administered within 3 months of diagnosis of anaemia was extracted and categorised as follows: oral iron treatment, intravenous iron therapy and folic acid or vitamin B12 supplementation. Furthermore, information on blood transfusions, erythropoietin use and changes in the anti‐inflammatory treatment within 3 months of the diagnosis of anaemia was assessed. Prospectively collected laboratory data including all haemoglobin levels during the study period from 1 January 2011 to 31 December 2013 were extracted from the laboratory information and management system of the Department of Clinical Chemistry, Örebro University Hospital. Laboratory data recorded within ±3 months from the diagnosis of anaemia was used to determine the cause of anaemia in the incident cases. Iron deficiency anaemia and anaemia of chronic disease were defined according to the European evidence‐based consensus document.18 Patients were censored in the event of death, emigration or at the end of follow‐up on 31 December 2013.
2.3. Outcome measures
Anaemia was defined according to the World Health Organisation (WHO) as a haemoglobin level <120 g/L in nonpregnant women and <130 g/L in men.27 The period prevalence estimate was based on haemoglobin measurements performed during 2013. The duration from 1 January 2011 to first diagnosis of anaemia was transformed into patient‐years and used in the incidence rate analysis. In patients with an episode of anaemia during the study period, haemoglobin levels recorded at 6 and 12 months (± 2 months) after the episode were used to investigate the proportion that had a resolution of anaemia at each time point.
2.4. Statistics
Applying the incidence rate estimates in anaemia of 13.0 per 100 person‐years and 7.5 per 100 person‐years in patients with Crohn's disease and ulcerative colitis, respectively,12 we needed to include 342 patients in order to detect a difference between the two diagnoses with 80% power and a type‐1 error rate of 5%, and a random sample of patients was identified from the entire Örebro University hospital IBD cohort (n = 1,405). Age and disease duration are presented as median (interquartile range [IQR]). The incidence of anaemia was assumed to follow a Poisson distribution and the incidence rate with 95% confidence interval (CI) was calculated accordingly. The prevalence with 95% CI was calculated assuming a binomial distribution. Logistic regression with adjustments for sex and age as a continuous variable was used to evaluate possible differences in the incidence and prevalence of anaemia between Crohn's disease and ulcerative colitis. Since patients with immunomodulators and/or anti‐TNF therapy are monitored with regular blood sampling including haemoglobin measurement every third month at Örebro University Hospital,28, 29 sensitivity analyses with patients on these therapies excluded were performed in order to avoid surveillance bias. Similarly, in the assessment of risk factors for anaemia, patients with immunomodulators and/or anti‐TNF therapy were excluded. Additionally, as haemoglobin is routinely measured at out‐patient clinic visits to the Department of Gastroenterology, the comparisons between patients with Crohn's disease and those with ulcerative colitis were confirmed by supplementary analyses in which only patients with out‐patient clinic visits during the study period were included. Life tables and survival plots depicting the cumulative probability of anaemia were constructed using Kaplan‐Meier analysis. Univariable and multivariable Cox proportional hazard regression models were constructed to evaluate risk factors for anaemia in Crohn's disease and ulcerative colitis separately. Covariates (sex, age, disease duration, past bowel resection and disease phenotype according to the Montreal classification) were selected on the basis of their possible associations with the outcome. Age at baseline was not entered into the final models, as a high colinearity with disease duration was observed. The χ2‐test was used to evaluate potential differences in the treatment and outcome of anaemia between patients with Crohn's disease and those with ulcerative colitis and also between males and females. All tests were two‐tailed and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, 2013).
3. RESULTS
A random sample, consisting of 342 IBD patients (Crohn's disease, n = 171; ulcerative colitis, n = 171) who were resident in the catchment area of Örebro University Hospital on 31 December 2010, was taken from the entire Örebro University Hospital IBD cohort.22, 23, 24 Information on demographics and clinical characteristics of the study cohort is presented in Table 1. During the study period (1 January 2011 until 31 December 2013), 11 patients were lost to follow‐up (death, n = 4; emigration, n = 7). Most of the patients (236; 69%) had at least one visit to the out‐patient clinic and 51 (15%) were admitted to hospital during the study period. Overall, 332 (97%) of the 342 patients included had a haemoglobin measurement available during follow‐up, and a total of 3,438 haemoglobin levels were recorded with a median of 6 (IQR: 2‐13) samples per individual.
Table 1.
Clinical characteristics of patients with inflammatory bowel disease
| Crohn's disease n = 171 | Ulcerative colitis n = 171 | |
|---|---|---|
| Age, years; median (IQR) | 57 (41‐66) | 57 (40‐67) |
| Female sex; n (%) | 92 (54) | 73 (43) |
| Disease duration, years; median (IQR) | 20 (9‐32) | 16 (8‐28) |
| Location at baseline | ||
| L1 ileal; n (%) | 49 (29) | |
| L2 colonic; n (%) | 49 (29) | |
| L3 ileocolonic; n (%) | 72 (41) | |
| L4 isolated upper disease; n (%) | 1 (1) | |
| Behaviour at baseline | ||
| B1 nonstricturing, nonpenetrating; n (%) | 78 (45) | |
| B2 stricturing; n (%) | 71 (42) | |
| B3 penetrating; n (%) | 22 (13) | |
| Extent at baseline | ||
| E1 proctitis; n (%) | 39 (23) | |
| E2 left‐sided colitis; n (%) | 51 (30) | |
| E3 extensive colitis; n (%) | 79 (46) | |
| Unknown; n (%) | 2 (1) | |
| Treatment at baseline | ||
| None; n (%) | 91 (53) | 68 (40) |
| 5‐aminosalicylic acids; n (%) | 21 (12) | 60 (35) |
| Corticosteroids; n (%) | 7 (4) | 11 (6) |
| Immunomodulatorsa; n (%) | 46 (27) | 28 (17) |
| Anti‐TNF; n (%) | 6 (4) | 4 (2) |
| Past bowel resection; n (%) | 99 (58) | 26 (15) |
Immunomodulators: azathioprine, mercaptopurine or methotrexate.
3.1. Incidence rate
Altogether, 124 patients (36%) had an episode of anaemia during follow‐up, corresponding to a mean annual incidence rate of 15.9 (95% CI: 13.4‐18.7) per 100 person‐years. For Crohn's disease, the incidence rate was 19.3 (95% CI: 15.4‐23.7) per 100 person‐years, as compared to 12.9 (95% CI: 9.8‐16.5) per 100 person‐years for ulcerative colitis (Figure 1). In the logistic regression model, Crohn's disease was associated with an increased risk of anaemia compared to ulcerative colitis after adjustments for age and sex (adjusted odds ratio [OR] = 1.60; 95% CI: 1.02–2.51). Correspondingly, in the sensitivity analyses that were performed to minimise the influence of surveillance bias, Crohn's disease was associated with an increased risk of anaemia in both the model where patients treated with immunomodulators and/or anti‐TNF agents were excluded (adjusted OR = 1.89; 95% CI: 1.10‐3.24) and in the model only including patients who had an out‐patient clinic visit during follow‐up (adjusted OR = 1.69; 95% CI: 1.00‐2.86).
Figure 1.
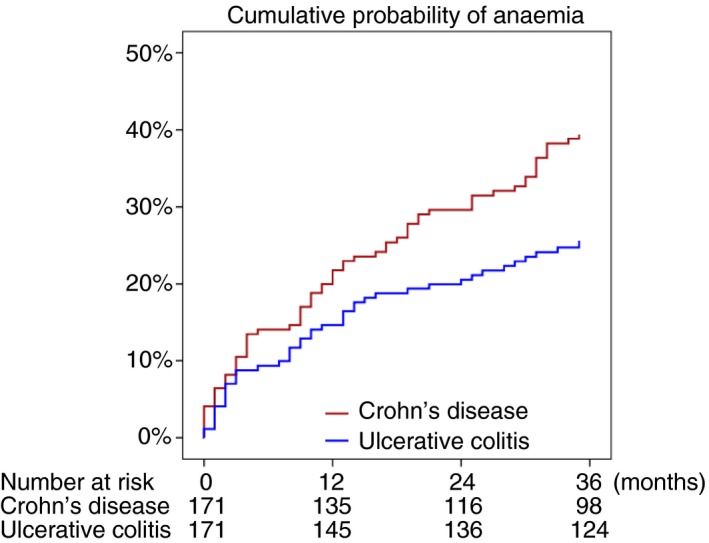
Kaplan‐Meier plot showing the cumulative probability of anaemia in patients with Crohn's disease and ulcerative colitis
3.2. Prevalence of anaemia in 2013
At the beginning of 2013, four Crohn's disease patients and one ulcerative colitis patient were lost to follow‐up (emigration, n = 3; or death, n = 2). Of the remaining 337 patients, 266 (79%) had a haemoglobin measurement recorded during 2013. Overall, 76 patients were diagnosed with anaemia from 1 January 2013 to 31 December 2013, which corresponds to a period prevalence of 22.6% (95% CI: 18.2‐27.4). For Crohn's disease, the prevalence was 28.7% (95% CI: 22.0‐36.2) as compared to 16.5% (95% CI: 11.2‐22.9) for ulcerative colitis. In the logistic regression model, Crohn's disease was associated with an increased prevalence of anaemia after adjustment for age and sex (adjusted OR = 2.04; 95% CI: 1.20‐3.46). The association between Crohn's disease and increased prevalence of anaemia remained in both the model in which patients treated with immunomodulators and/or anti‐TNF agents were excluded (adjusted OR = 1.96; 95% CI: 1.03‐3.37) and in the model that only included patients with out‐patient clinic visits during the study period (adjusted OR = 2.25; 95% CI: 1.22‐4.14).
3.3. Risk factors for anaemia
We created separate Cox proportional hazard regression models to identify risk factors for anaemia in Crohn's disease and in ulcerative colitis. In order to avoid surveillance bias due to regular monitoring of patients treated with immunomodulators and/or anti‐TNF agents with blood sampling (including haemoglobin) every third month,28, 29 these patients were excluded from the analyses. In Crohn's disease, patients with stricturing disease behaviour had a higher risk of anaemia than patients with inflammatory phenotype, after making adjustments for potential confounders (adjusted HR = 2.59; 95% CI: 1.00‐6.79) (Table 2). In ulcerative colitis, extensive disease was associated with an increased risk of anaemia as compared to proctitis (adjusted HR = 2.40; 95% CI: 1.10‐5.36) (Table 3).
Table 2.
Risk factors for anaemia in Crohn's diseasea
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Female sex | 0.82 (0.52‐1.31) | 0.41 | 0.86 (0.53‐1.27) | 0.52 |
| Duration of disease | 1.00 (0.98‐1.01) | 0.50 | 0.99 (0.96‐1.01) | 0.14 |
| Disease locationb | ||||
| L1, terminal ileum | Reference | Reference | ||
| L2, colonic | 0.82 (0.43‐1.56) | 0.56 | 1.00 (0.47‐2.12) | 0.99 |
| L3, ileocolonic | 1.26 (0.73‐2.20) | 0.41 | 1.52 (0.85‐2.73) | 0.16 |
| Disease behaviour | ||||
| B1 nonstricturing, nonpenetrating | Reference | Reference | ||
| B2 stricturing | 1.63 (0.98‐2.70) | 0.06 | 2.59 (1.00‐6.79) | 0.05 |
| B3 penetrating | 1.27 (0.60‐2.71) | 0.54 | 2.04 (0.63‐6.56) | 0.23 |
| Past bowel resection | 1.25 (0.77‐2.03) | 0.36 | 1.67 (0.61‐4.58) | 0.32 |
Patients receiving immunomodulators and/or anti‐TNF therapy were excluded from the analysis.
The only patient with isolated upper gastrointestinal disease was excluded.
Table 3.
Risk factors for anaemia in ulcerative colitisa
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Female sex | 1.22 (0.71‐2.09) | 0.48 | 1.23 (0.71‐2.13) | 0.45 |
| Duration of disease | 0.99 (0.96‐1.01) | 0.21 | 0.98 (0.95‐1.00) | 0.06 |
| Extent of disease | ||||
| E1, proctitis | Reference | Reference | ||
| E2, left‐sided colitis | 1.41 (0.59‐3.41) | 0.44 | 1.50 (0.61‐3.66) | 0.38 |
| E3, extensive colitis | 2.32 (1.07‐5.03) | 0.03 | 2.40 (1.10‐5.36) | 0.03 |
| Past colectomy | 1.57 (0.81‐3.04) | 0.19 | 1.66 (0.80‐3.44) | 0.17 |
Patients receiving immunomodulators and/or anti‐TNF therapy were excluded from the analysis.
3.4. Causes, treatment and outcome of anaemia
The median haemoglobin levels at diagnosis of anaemia were 117 g/L (IQR: 112‐123) in Crohn's disease and 118 g/L (IQR: 115‐126) in ulcerative colitis. The corresponding figures were 124 g/L (IQR: 117‐128) and 115 g/L (IQR: 110‐118) in males and females, respectively. In both Crohn's disease and ulcerative colitis, the main causes of anaemia were iron deficiency and anaemia of chronic disease while other causes such as vitamin B12 or folate acid deficiency were uncommon (Table 4). Within 3 months of the diagnosis of anaemia, 51 (41%) of the 124 patients who were diagnosed with anaemia during the study period received supplementation therapy (oral iron, n = 18; iron intravenously, n = 22; oral folic acid, n = 3; or oral vitamin B12, n = 8) and eight patients received blood transfusions. Similarly, 42 patients (34%) underwent intensified anti‐inflammatory treatment (introduction or dose optimisation of oral 5‐aminosalicylates, n = 8; immunomodulators, n = 15; anti‐TNF, n = 4; leucocyte apheresis, n = 1; or corticosteroids, n = 14). However, none of the patients were treated with erythropoietin and 42 (36%) of the patients who were diagnosed with anaemia received no treatment at all within 3 months of the diagnosis. In 123 of 124 patients, repeated measurements of haemoglobin were performed at 6 and 12 months after the diagnosis of anaemia. At 6 months from diagnosis, 65 (52%) had a normal haemoglobin level restored and the corresponding figure at 12 months was 80 (65%). Within 3 months of diagnosis, 51 of 71 Crohn's disease patients (72%) received specific treatment (either supplementation or anti‐inflammatory therapy), as compared to 29 of 53 ulcerative colitis patients (55%; P = 0.05). However, 40 of 71 Crohn's disease patients (56%) and 39 of 53 ulcerative colitis patients (75%) had a resolution of anaemia within 12 months (P = 0.03). Correspondingly, 44 of 60 female IBD patients (73%) and 36 of 64 male IBD patients (56%) received specific therapy (P = 0.05) and the proportions of patients that had a resolution of anaemia within 12 months were 45 of 60 females (75%) and 34 of 64 males (54%; P = 0.02). When restricting the analysis by only including patients who received iron supplementation (Crohn's disease, n = 27; ulcerative colitis, n = 13), the 12‐month resolution rates remained very similar (Crohn's disease, n = 14 [52%]; ulcerative colitis, n = 10 [77%]) although the difference between the two diagnoses did not remain statistically significant (p = 0.12). The overall 12‐month resolution rate was 24/40 (60%) in patients receiving iron supplementation and the rates were similar in patients treated with oral iron supplementation (61%) or iron intravenously (59%).
Table 4.
Classification of anaemia in patients with inflammatory bowel disease
|
Crohn's disease (n = 71) n (%) |
Ulcerative colitis (n = 53) n (%) |
|
|---|---|---|
| Absolute iron deficiencya | ||
| S‐ferritin <30 μg/L | 25 (35) | 17 (32) |
| Anaemia of chronic disease with functional iron deficiencyb | ||
| P‐CRP >2 mg/L or f‐calprotectin >250 and s‐ferritin 30‐100 ng/mL | 14 (20) | 8 (15) |
| Anaemia of chronic disease | ||
| P‐CRP >2 mg/L or f‐calprotectin >250 and S‐ferritin >100 ng/mL | 6 (8) | 7 (13) |
| Folate acid deficiencyc | ||
| S‐folate <7 nmol/L | 1 (1) | 2 (3) |
| Vitamin B12 deficiency | ||
| S‐vitamin B12 < 140 pmol/L | 3 (4) | 1 (2) |
| Anaemia of unknown cause | ||
| Microcytic (MCV < 82 fL) | 1 (1) | 2 (4) |
| Normocytic (MCV 82‐98 fL) | 13 (18) | 9 (17) |
| Macrocytic (MCV > 98 fL) | 3 (4) | 2 (4) |
| No data available | 5 (7) | 5 (9) |
Two patients with Crohn′s disease and one patient with ulcerative also had folate acid deficiency and one Crohn′s disease patient also had Vitamin B12 deficiency.
One patient with Crohn′s disease also had folate acid deficiency and one had vitamin B12 deficiency.
The Crohn′s disease patient with folate acid deficiency also had vitamin B12 deficiency.
4. DISCUSSION
In this general population‐based IBD cohort, we found a mean annual incidence rate of anaemia of 15.9 per 100 person‐years and a prevalence of 22.6%. Considering the long median disease duration of the cohort (19 years), our novel data indicate that anaemia is a common manifestation of IBD even beyond the first years after diagnosis. Both the incidence and the prevalence of anaemia were higher in Crohn's disease than in ulcerative colitis. Interestingly, we found that Crohn's disease was associated with a worse prognosis in terms of resolution of anaemia within 12 months, despite that the probability of receiving specific therapy was higher in Crohn's disease.
In previous studies of the epidemiology of anaemia in IBD, the prevalence differed considerably depending on the study population and on the definition of anaemia.7, 8, 30, 31 In the current study, the WHO criteria for anaemia were used.27 In order to minimise the influence of selection bias, we used a random sample from the entire Örebro University Hospital population‐based IBD cohort, which consists of all the patients who were diagnosed with Crohn's disease and ulcerative colitis in the geographic area during 1963‐2010.22, 23 Most of the patients included in the current study (69%) had at least one visit to the out‐patient clinic of the Department of Gastroenterology, and some patients were admitted to hospital during the 3‐year study period (15%). However, 29% of the patients did not have any visits to the out‐patient clinic or hospital admissions during follow‐up. Even so, the vast majority (97%) of the patients included had haemoglobin measurements recorded in the laboratory information and management system of the Department of Clinical Chemistry, since some patients may be monitored by laboratory tests, that is, haemoglobin, CRP and f‐calprotectin; and follow‐up over the phone or had haemoglobin measurements because of other reasons. In contrast to previous studies addressing the occurrence of anaemia in IBD, we collected all haemoglobin measurements recorded during follow‐up (n = 3,438) and assessed both the incidence and the period prevalence of anaemia. In the IBSEN study, the prevalence of anaemia at 10 years from diagnosis was 13.0% in Crohn's disease and 7.5% in ulcerative colitis.12 The corresponding figures in a population‐based study from southern Sweden were 9% and 5%, for Crohn's disease and ulcerative colitis, respectively.15 However, in both these studies, the prevalence estimates were based on haemoglobin levels recorded at scheduled follow‐up appointments at the out‐patient clinic.
We found that 28.7% of Crohn's disease patients and 16.5% of patients with ulcerative colitis had an episode of anaemia during 1 year. Similarly, the mean annual incidence rate was 19.3 per 100 person‐years in Crohn's disease as compared to 12.9 per 100 person‐years in ulcerative colitis, suggesting that the magnitude of anaemia in patients with established IBD has previously been underestimated. Both the incidence and the prevalence of anaemia were significantly higher in Crohn's disease than in ulcerative colitis. However, in the cohort, a higher proportion of Crohn's disease patients were treated with immunomodulators and/or anti‐TNF agents. As patients with those therapies are monitored with blood sampling (including haemoglobin levels) every third month, we confirmed the increased occurrence of anaemia in Crohn's disease by performing a sensitivity analysis with patients on immunomodulators and/or anti‐TNF agents excluded and also with an analysis in which only patients with out‐patient clinic visits were included (haemoglobin is routinely measured in association with visits to the out‐patient clinic at the Department of Gastroenterology).
Interestingly, our data indicate that Crohn's disease patients have a worse prognosis in terms of resolution of anaemia than patients with ulcerative colitis. Although the probability of receiving specific therapy was higher in Crohn's disease, only 56% had a resolution of anaemia within 12 months—as compared to 75% of patients with ulcerative colitis (P = 0.03). When restricting the analysis by only including patients who received iron supplementation (n = 40), the figures were very similar, although the association did not remain statistically significant.
It has been proposed that intravenous iron has a superior effect and a better gastrointestinal tolerability in IBD,19, 30, 32 while other studies found no difference in clinical effectiveness and tolerability between oral and intravenous iron supplementation.33, 34 In the current study, the outcome of anaemia at 12 months did not differ by route of iron administration. However, these findings should be interpreted with caution since the study was designed to assess differences in the occurrence of anaemia between subgroups of IBD patients, and unfortunately, it was underpowered to examine anaemia outcomes.
Previous studies have proposed that disease activity is the most important risk factor for anaemia in IBD,12, 35, 36 and one possible explanation for the observed worse 12‐month outcome in Crohn's disease patients is that these patients generally have a higher burden of systemic inflammation, with impaired iron uptake, and they are more refractory to anti‐inflammatory treatment than ulcerative colitis patients.9 However, no accurate measure of disease severity was available to confirm this hypothesis, since the data capture of this study was retrospective.
Similar to a recent European multicentre study of patients with newly diagnosed IBD, we found that Crohn's disease patients with stricturing disease behaviour had an increased risk of anaemia as compared to patients with an inflammatory phenotype, and that extensive disease was associated with an increased risk of anaemia in ulcerative colitis.11 In contrast, we could not confirm that there was an increased risk in Crohn's disease patients with a colonic disease location,11, 14 although the predictors of anaemia at the time of diagnosis of IBD may be different from the risk factors in the long term.
Despite current guidelines,37 only 64% of the patients in this cohort received specific therapy within 3 months of the diagnosis of anaemia. Unfortunately, this is in line with other studies reflecting real‐life IBD practices.11, 13, 14, 18 In a recent cross‐sectional study, Voegtlin et al reported that only 40% and 43% of IBS patients with anaemia in Switzerland, in private practice and university hospitals, respectively, received supplementation therapy.38 The fact that IBD patients with anaemia suffer from impaired quality of life, with an increased risk of hospital admission, and that anaemia in IBD has been associated with increased mortality makes these figures somewhat astonishing.4, 5 Intriguingly, in the current cohort, female sex was associated with a better chance of receiving specific therapy and also with a higher probability of resolution of anaemia within 12 months. A possible explanation for this unexpected finding may be different in healthcare‐seeking behaviour, depending on sex.39
This was an observational study, and one limitation was that information regarding pregnancy in the patients may have been incomplete. The WHO definition of anaemia in pregnant women is haemoglobin <110 mg/L, as compared to <120 mg/L in women who are not pregnant. Another limitation was that 21% of patients had no haemoglobin measurement during 2013 for use in the prevalence analysis. Attrition analysed did not reveal any significant differences by sex, age, disease duration, IBD diagnosis or phenotype between patients with and patients without haemoglobin samples in 2013, whereas missing values were more common in patients without IBD therapy and also in patients who had no visits to the out‐patient clinic during follow‐up. This suggests that patients with missing data were mainly cases with mild or indolent disease and with a low risk of anaemia. However, the possibility cannot be excluded that our prevalence estimate was slightly lower than the true prevalence, since patients without data were considered to be non‐anaemic. In addition, information on smoking habits was incomplete.
Another weakness of this study was that some patients (n = 10; 8%) had no thorough workup to characterise the type of anaemia. However, similar to the results of a previous study on the main causes of anaemia in Scandinavian outpatients with IBD, we found that the majority of patients suffered from iron deficiency and/or anaemia of chronic disease, and that just about 5% could be attributed to vitamin B12 or folic acid deficiency.17
An additional weakness of the study was that no data on the prevalence of anaemia in the background population was available. In a recent general population‐based study from Norway, the crude prevalence of anaemia was 2.8% in men and 6.3% in women.40
The main strength of this study was the random inclusion of patients from our general population‐based cohort consisting of IBD patients of all ages, comprising both recently diagnosed patients and patients with a long history of IBD, living in a well‐defined geographical area. By reporting both the incidence of anaemia, which conveys information about the risk over time; the prevalence, which demonstrates how widespread the condition is; and also the clinical outcome of anaemia, our study indicates that the occurrence of anaemia in patients with established IBD may previously been underestimated.12, 15
In conclusion, we found a high incidence and prevalence of anaemia in a general population‐based IBD cohort. Considering the long median disease duration of the cohort (19 years), our novel data indicate that the magnitude of anaemia in patients with established IBD previously has been underestimated. Crohn's disease was associated with both an increased risk of anaemia and a worse prognosis in terms of resolution of anaemia within 12 months. The risk factors for anaemia, that is, stricturing disease behaviour in Crohn's disease and extensive disease in ulcerative colitis, should be recognised and current evidence‐based guidelines on the management of anaemia in IBD should be better implemented in clinical practice.18
AUTHORSHIP
Guarantor of the article: Carl Eriksson.
Author contributions: CE, IH, JH and SM planned and conducted the study. CE, IH and YZ collected the data. CE, IH and OB performed the data analyses. CE, CT, IH, JH, OB, NN and SM interpreted the data. CE, IH and JH drafted the manuscript. CE, CT, IH, JH and SM revised the manuscript.
All authors approved the final version of the manuscript.
ACKNOWLEDGEMENT
We thank Åsa Ekblom for assistance with data collection. Conference presentation: none.
Declaration of personal interests: CE: Received a research grant from the Swedish government's Agreement on Medical Training and Research and a speaker's fee from Takeda. CT: Served as a speaker for Dr Falk Pharma, Tillotts Pharma, Ferring, MSD and AstraZeneca. IH: Nothing to declare. JH: Received research grants from the Swedish Research Council, the Örebro University Hospital Research Foundation, the Swedish Foundation for Strategic Research and speaker's and/or consultancy fees from Abbvie, Celgene, Hospira, Janssen, Medivir, MSD, Pfizer, Shire, Takeda, Tillotts Pharma and Vifor Pharma. OB: Nothing to declare. NN: Received research grants from the Örebro University Hospital Research Foundation and consultancy fees from Abbvie, MSD and Norgine. SM: Nothing to declare. YZ: Nothing to declare.
Eriksson C, Henriksson I, Brus O, et al. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: a population‐based cohort study. Aliment Pharmacol Ther. 2018;48:638–645. 10.1111/apt.14920
Funding information This work was founded in full by the Swedish government's Agreement on Medical Training and Research [OLL‐549221].
The Handling Editor for this article was Dr Nicholas Kennedy, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population‐based study. Am J Gastroenterol. 2001;96:1116‐1122. [DOI] [PubMed] [Google Scholar]
- 2. Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110‐119. [DOI] [PubMed] [Google Scholar]
- 3. Cucino C, Sonnenberg A. Cause of death in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:250‐255. [DOI] [PubMed] [Google Scholar]
- 4. Danese S, Hoffman C, Vel S, et al. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn's and Ulcerative Colitis Association's online survey. Eur J Gastroenterol Hepatol. 2014;26:1385‐1391. [DOI] [PubMed] [Google Scholar]
- 5. Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123‐130. [DOI] [PubMed] [Google Scholar]
- 7. Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):44‐49. [DOI] [PubMed] [Google Scholar]
- 8. Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther. 2006;24:1507‐1523. [DOI] [PubMed] [Google Scholar]
- 9. Bergamaschi G, Di Sabatino A, Albertini R, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti‐tumor necrosis factor‐alpha treatment. Haematologica. 2010;95:199‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomollon F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659‐4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burisch J, Vegh Z, Katsanos KH, et al. Occurrence of Anaemia in the First Year of Inflammatory Bowel Disease in a European Population‐based Inception Cohort‐An ECCO‐EpiCom Study. Journal of Crohn's & colitis. 2017;11:1213‐1222. [DOI] [PubMed] [Google Scholar]
- 12. Hoivik ML, Reinisch W, Cvancarova M, Moum B. Anaemia in inflammatory bowel disease: a population‐based 10‐year follow‐up. Aliment Pharmacol Ther. 2014;39:69‐76. [DOI] [PubMed] [Google Scholar]
- 13. Ott C, Liebold A, Takses A, Strauch UG, Obermeier F. High prevalence but insufficient treatment of iron‐deficiency anemia in patients with inflammatory bowel disease: results of a population‐based cohort. Gastroenterology research and practice 2012;2012:595970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sjöberg D, Holmström T, Larsson M, Nielsen AL, Holmquist L, Rönnblom A. Anemia in a population‐based IBD cohort (ICURE): still high prevalence after 1 year, especially among pediatric patients. Inflamm Bowel Dis. 2014;20:2266‐2270. [DOI] [PubMed] [Google Scholar]
- 15. Rejler M, Tholstrup J, Andersson‐Gare B, Spangeus A. Low prevalence of anemia in inflammatory bowel disease: a population‐based study in Sweden. Scand J Gastroenterol. 2012;47:937‐942. [DOI] [PubMed] [Google Scholar]
- 16. Atug O, Kani HT, Banzragch M, Imeryuz N, Akin H. Incidence rate of anemia in inflammatory bowel diseases. The Turkish journal of gastroenterology. 2016;27:143‐148. [DOI] [PubMed] [Google Scholar]
- 17. Bager P, Befrits R, Wikman O, et al. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand J Gastroenterol. 2011;46:304‐309. [DOI] [PubMed] [Google Scholar]
- 18. Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. Journal of Crohn's & colitis. 2015;9:211‐222. [DOI] [PubMed] [Google Scholar]
- 19. Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator‐blind, multicentre study. Scand J Gastroenterol. 2009;44:838‐845. [DOI] [PubMed] [Google Scholar]
- 20. Schreiber S, Howaldt S, Schnoor M, et al. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N Engl J Med. 1996;334:619‐623. [DOI] [PubMed] [Google Scholar]
- 21. Bodemar G, Kechagias S, Almer S, Danielson BG. Treatment of anaemia in inflammatory bowel disease with iron sucrose. Scand J Gastroenterol. 2004;39:454‐458. [DOI] [PubMed] [Google Scholar]
- 22. Eriksson C, Cao Y, Rundquist S, et al. Changes in medical management and colectomy rates: a population‐based cohort study on the epidemiology and natural history of ulcerative colitis in Örebro, Sweden, 1963‐2010. Aliment Pharmacol Ther. 2017;46:748‐757. [DOI] [PubMed] [Google Scholar]
- 23. Zhulina Y, Udumyan R, Henriksson I, Tysk C, Montgomery S, Halfvarson J. Temporal trends in non‐stricturing and non‐penetrating behaviour at diagnosis of Crohn's disease in Örebro, Sweden: a population‐based retrospective study. Journal of Crohn's & colitis. 2014;8:1653‐1660. [DOI] [PubMed] [Google Scholar]
- 24. Zhulina Y, Udumyan R, Tysk C, Montgomery S, Halfvarson J. The changing face of Crohn's disease: a population‐based study of the natural history of Crohn's disease in Örebro, Sweden 1963‐2005. Scand J Gastroenterol. 2016;51:304‐313. [DOI] [PubMed] [Google Scholar]
- 25. Statistics Sweden . Tables on the population in Sweden. Tables on the population in Sweden. http://www.scb.se/be0101-en. Accessed January 20, 2018.
- 26. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5‐37. [PubMed] [Google Scholar]
- 28. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 2: current management. Journal of Crohn's & colitis. 2012;6:991‐1030. [DOI] [PubMed] [Google Scholar]
- 29. Gomollon F, Dignass A, Annese V, et al. 3rd European Evidence‐based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. Journal of Crohn's & colitis. 2017;11:3‐25. [DOI] [PubMed] [Google Scholar]
- 30. Stein J, Bager P, Befrits R, et al. Anaemia management in patients with inflammatory bowel disease: routine practice across nine European countries. Eur J Gastroenterol Hepatol. 2013;25:1456‐1463. [DOI] [PubMed] [Google Scholar]
- 31. Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in European countries: a systematic review and individual patient data meta‐analysis. Inflamm Bowel Dis. 2014;20:936‐945. [DOI] [PubMed] [Google Scholar]
- 32. Schroder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease–a randomized, controlled, open‐label, multicenter study. Am J Gastroenterol. 2005;100:2503‐2509. [DOI] [PubMed] [Google Scholar]
- 33. Rampton DS, Goodhand JR, Joshi NM, et al. Oral iron treatment response and predictors in anaemic adolescents and adults with IBD: a prospective controlled open‐label trial. Journal of Crohn's & colitis. 2017;11:706‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Silva AD, Tsironi E, Feakins RM, et al. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther. 2005;22:1097‐1105. [DOI] [PubMed] [Google Scholar]
- 35. Bager P, Befrits R, Wikman O, et al. High burden of iron deficiency and different types of anemia in inflammatory bowel disease outpatients in Scandinavia: a longitudinal 2‐year follow‐up study. Scand J Gastroenterol. 2013;48:1286‐1293. [DOI] [PubMed] [Google Scholar]
- 36. Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545‐1553. [DOI] [PubMed] [Google Scholar]
- 38. Voegtlin M, Vavricka SR, Schoepfer AM, et al. Prevalence of anaemia in inflammatory bowel disease in Switzerland: a cross‐sectional study in patients from private practices and university hospitals. Journal of Crohn's & colitis. 2010;4:642‐648. [DOI] [PubMed] [Google Scholar]
- 39. Malterud K, Okkes I. Gender differences in general practice consultations: methodological challenges in epidemiological research. Fam Pract. 1998;15:404‐410. [DOI] [PubMed] [Google Scholar]
- 40. Skjelbakken T, Langbakk B, Dahl IM, et al. Haemoglobin and anaemia in a gender perspective: the Tromso Study. Eur J Haematol. 2005;74:381‐388. [DOI] [PubMed] [Google Scholar]


