Abstract
Post hepatectomy liver failure (PHLF) comprises of a conundrum of symptoms and signs following major hepatic resections. The pathophysiology essentially revolves around disruption of the normal hepatocyte regeneration and disturbed liver homeostasis. Prompt identification of the pre-operative predictors of PHLF in the form of biochemical parameters and imaging features are of paramount importance for any hepatic surgeon and forms the cornerstone of its management. Treatment revolves around a goal-directed resuscitation of the systemic organ failure. Auxiliary support systems such as liver dialysis devices and stem cell therapy are still under investigational trials for treatment of the same. Orthotopic liver transplantation (OLT) is the last resort in most cases not responding to other measures.
Keywords: Hepatectomy, PHLF, Regeneration, Liver transplantation, Complications
Highlights
-
•
Understanding the mechanics and pathophysiology of post hepatectomy liver failure and its implications in management.
-
•
Preventive strategies essential for all liver surgeons.
-
•
Overview of assessing clinical severity and management algorithm of PHLF.
1. Introduction
Owing to their complex nature and the high degree of surgical expertise mandated, liver resections are often fraught with a relatively greater incidence of post-operative complications. With the advent of sophisticated critical care in most high volume the mortality associated with hepatectomy has come down to less than 5%, with a significant proportion still being contributed by Post hepatectomy liver failure (PHLF) [1]. The incidence reported in the literature is variable, ranging from 0.7 to 35% [2]. It varies according the underlying pre-operative status of the liver and the underlying pathology requiring liver resection, among the many other factors. This is evident from the literature quoting an incidence of 5–15% in patients undergoing partial hepatectomy in a cirrhotic liver to 0.9–5% in patients undergoing hepatectomy for colorectal liver metastases [[3], [4], [5]]. There has also been geographical variation in the incidence of PHLF with the data from East Asian countries reporting an incidence as low as 1–2% [6].
2. Definition of PHLF – controversies and the consensus
There have been numerous versions regarding the objective definition of PHLF and till date no standardised concise definition has been formulated. However, due to the complexity of calculation of the former and the lack of specificity of the latter in determining PHLF, these have not been standardised in. Balzan et al. proposed the “50-50” criteria for defining PHLF (serum bilirubin >50μL/L and Prothrombin time <50% of normal on POD 5) [7]. This was predictive of 60-day mortality in 59% of cases in a series of 775 hepatectomies. Another criterion proposed by Mullen et al. (Peak bilirubin level >120 μmol/L) was found predictive of 90-day mortality in a series of 1059 liver resections [8]. However, these definitions relied only on the laboratory values and not considering the clinical parameters of severity. In 2011, the International Study Group of Liver Surgeries (ISGLS) proposed a consensus on defining PHLF using laboratory and clinical parameters [9]. The consensus had defined PHLF as “A post-operatively acquired deterioration in the ability of the liver (in patients with normal and abnormal liver function) to maintain its synthetic, excretory and detoxifying function, characterised by increase in the INR and hyperbilirubinemia on or after post-operative day 5”. Based on this definition PHLF was stratified into three grades of severity (A, B and C). The peri-operative mortality ranged from 0% for grade A to 54% for grade C, with grade B requiring non-invasive intensive care management alone.
3. Pathophysiology – dynamics of liver regeneration and PHLF
In a normally functioning liver, the process of regeneration usually starts in the first two weeks after hepatectomy and is usually completed by three months. The initial phase of regeneration is characterised by an increased expression of the transcription factors (motifs) like c-fos, c-jun and c-myc [10]. An important role in regeneration is played by the liver sinusoidal endothelial cells (LSECs), which release the chemical mediator nitric oxide (NO) [11]. Following partial hepatectomy, there is sudden increase in the portal pressure due to the reduction of the vascular bed volume and increase in the portal flow per gram of tissue. This generates sheer stress on the vascular endothelium. The LSECs are activated to release NO and other hepatotrophic factors. NO has been proposed to act by downregulation of S-adenosyl methionine (SAM) synthesis, thereby promoting the expression of cyclin D1 and D2. This in turn sensitises the hepatocytes to the other Hepatocyte growth factors (HGFs) [12]. Animal experiments have shown the role of NO agonist in increasing hepatocyte proliferation at 24 h after 85% partial hepatectomy [13]. However, shear stress alone is not an effective factor in induction of hepatocyte regeneration. Mortensen et al. have shown in their experiments on pigs that arterio-portal anastomosis subsequent to ipsilateral portal vein ligation was not associated with regeneration on the arterialised side [14]. Also, excessive shear stress has been associated with endothelial cell necrosis and subsequent oxidative damage to the regenerating hepatocytes. Ryan et al. have shown the role of IL-6 in inducing transcription by binding to the hepatocyte receptors [15]. Factors like lipopolysaccharide (LPS), bacterial endotoxins have also been shown to play a role in this process by binding to the Toll like receptors, causing downstream activation of IL-6 and TNF α [16,17]. Kawasaki et al. have shown the role of serotonin in proliferation of LSECs and tissue remodelling [18]. Lesurtel et al. have shown the role of platelets in hepatocyte regeneration [19]. They also found the association of thrombocytosis with increased survival after 90% partial hepatectomy in mice experiments, primarily through the activation of STAT and Akt pathways. Use of thrombocytopenic agents like Busulfan and Clopidogrel were found to alter hepatocyte proliferation.
At a microscopic level, continuous cellular exchange between hepatocytes and LSECs is crucial for augmentation of liver function after resection to avoid PHLF [20]. After partial hepatectomy, there is dysregulation of the Kupffer cells with hyposecretion of PGE2 and hypersecretion of TNF α leading to irreversible cell damage by apoptosis. Therefore, overactivation of the inflammatory mediators is not always conducive to liver regeneration. Hence, hyper-mitogenic stimulation alone is not the goal for therapy in PHLF. Activation of mediators like HGF may improve post-operative function in partial hepatectomy in the presence of underlying liver cirrhosis. But, some studies have shown overactivation of HGF to be associated with oncogenesis. Petrowsky et al. have further supported this hypothesis by showing no benefit of administering Pentoxifylline (inhibitor of TNF α and promoter of HGF) after partial hepatectomy on the clinical and laboratory parameters [21]. Belghiti et al. found a higher morbidity among the living donors undergoing major partial hepatectomies with resultant excess regeneration (46.8% v/s 21.8%) [22]. Therefore, the management of PHLF needs to be directed at preservation of residual hepatocyte function and microvascular organisation and not immediate recovery of the total liver volume.
4. Risk factors of PHLF
The predictive risk factors of PHLF can be categorised into: Patient related, Liver related and Surgery related.
4.1. Patient related
-
1.
Age: The effect of ageing on liver functions is unclear and is vaguely elucidated to be related to factors such as reduced capacity to produce acute phase reactants, and decrease in basal and taurocholate-stimulated bile flow [23]. In a study on 775 patients, Balzan et al. found age>65 years to be an independent predictor of mortality post hepatectomy [7]. Kim et al., in their study on 279 patients undergoing partial hepatectomy reported no correlation of age with the post-operative outcome [24].
-
2.
Metabolic factors: Role of insulin as a potent hepatotrophic factor (stimulation of IGF and HGFs) has been quoted widely [25]. Bucher reported a higher incidence of hepatic atrophy with insulin depletion in their study on animal models [26]. In a series of 104 patients undergoing major liver resections (>/ = 3 major liver segments), Schindl et al. reported a direct correlation of BMI with incidence of PHLF [27]. Similarly, Fan et al. demonstrated a correlation of malnutrition with higher incidence of PHLF in their prospective series of 124 patients undergoing hepatectomy [28].
-
3.
Sepsis: The mechanisms proposed are: action of endotoxin on the Kupffer cells causing impairment of the cytokines necessary for regeneration and by affecting the internal milieu of hepatocytes disrupting the transport mechanisms of the regenerative cytokines and cells [29].
-
4.
Miscellaneous: Other factors such as hyperbilirubinemia, renal insufficiency, cardiopulmonary compromise and thrombocytopenia have also been linked to high incidence of PHLF [30]. (CASH and SOS mentioned in liver related)
4.2. Liver related
-
1.
Hepatic steatosis: The effect of steatosis is perhaps explained by the higher incidence of ischemia-reperfusion injury in a steatotic liver due to altered sinusoidal microcirculation. In a recent study comparing 174 patients with steatohepatitis versus normal liver, the authors concluded a higher incidence of hepatic decompensation and 90-day post-operative morbidity (56.9% vs 37.3%; p = 0.008) and surgical hepatic complications (19.6% vs 8.8%; p = 0.04) among the former [31].
-
2.
Chemotherapeutic agents: In a study of 248 patients receiving neo-adjuvant chemotherapy for CRLM, Vauthey et al. found a greater incidence of SOS compared to the chemotherapy naïve livers (18.9% vs 1.9%; p < 0.001) [32]. The same group of authors also found more fatty infiltration on liver biopsy of patients having received irinotecan based chemotherapy (20.2% vs 4.4%; p < 0.001). In a prospective study of 173 patients undergoing hepatectomy for CRLM, Mehta et al. found a higher rate of biliary complications (16%) and sinusoidal dilatation (56.2% vs 23%) in the group receiving preoperative oxaliplatin based chemotherapy [33]. Preoperative parameters like aspartate aminotransferase to platelet ratio (APRI) and splenic volumetry have been suggested as effective predictors of PHLF especially in the setting of oxaliplatin induced SOS [34].
-
3.
Extent of cirrhosis/fibrosis: Capussotti et al. reported a lower in-hospital mortality in patients with Child's A cirrhosis undergoing hepatectomy versus Child's B/C (4.7% vs 21%; p < 0.05) [35]. Pre-operative assessment criteria developed by Makucchi et al., which includes presence of ascites, bilirubin level and ICG clearance may be used as a tool for guiding decision to take up for major liver resections [36].
4.3. Surgery related factors
-
1.
Intraoperative blood loss and Blood transfusions: Excess intra-operative blood loss (>1200 ml) is associated with intravascular fluid shifts that may induce bacterial translocation with resultant systemic inflammation and coagulopathy, predisposing to PHLF. In a study on 1056 patients undergoing hepatectomy, Imamura et al. found a strong association between intra-operative blood loss (>1000 ml) and incidence of post-operative complications [37].
-
2.
Dissection techniques: Intra-operative events such as vascular resections or IVC repair have been associated with higher incidence of PHLF [38]. Also, excessive dissection of tissues around the portal triad and hepatoduodenal ligament have shown a higher chance of developing PHLF.
-
3.
Remnant liver volume: The earliest description of ‘small for size syndrome’ (SFSS) dates to 1996, when Emond et al. defined this entity as graft recipient weight ratio (GRWR) less than 0.8–1.0 or less than 30–50% of standard/estimated liver volumes [39]. SFSS exerts its deleterious effect on the liver parenchyma by causing hemodynamic changes in the form of increase in portal pressure with resultant increase in intra-sinusoidal pressures and hepatocyte damage (see Pathophysiology). Hence, two important determinants for hepatectomy are: a) Future liver remnant (FLR) volume/standardised liver volume (SLV) ratio; preferably >20%, and b) Body weight ratio of liver volume; 0.5 set as the threshold value. These have been found to be highly predictive of PHLF [40].
5. Morphological and biochemical assessment of patients
These can be categorised into: Qualitative and Quantitative assessment.
5.1. Qualitative assessment
-
1.Liver function scoring systems:
-
•Child Turcotte Pugh (CTP) scoring: Patients at the extreme end (late Child's B or C) are usually not candidates for liver resection due to high incidence of PHLF. However, studies have reported a low predictability of this scoring [41].
-
•Model for end stage liver disease (MELD): In a series of 2056 patients, Hyder et al. have reported a higher risk of mortality and PHLF with MELD>10 (p < 0.001) [42]. However, Rahbari et al. reported a sensitivity of only 51% and 70% of MELD score for predicting morbidity and mortality respectively [43].
-
•50-50 criteria: Balzan et al. showed that PT<50% (INR>1.7) and serum bilirubin>50 μmol/L (>2.9 mg/dL) on day 5 of surgery was associated with 59% risk of early post-operative mortality versus 1.2% risk in cases where these criteria were not fulfilled (p < 0.001) [7]. The accuracy of this criteria to predict the in-hospital mortality was reported to be around 97.7%. Kim et al. proposed a modification of the 50-50 criteria to PT<65% with bilirubin >38 μmol/L on POD 5 [44].
-
•ISGLS grading: Rahbari et al. validated this grading in a study of 807 patients undergoing hepatectomy and found it to be an independent predictor of mortality [9]. However, Skrzypezyk et al. compared this grading with the 50-50 criteria and snap bilirubin levels (see below) and found it to be least predictive of PHLF (PPV 49.2% vs 78.9% vs 65% respectively) [45].
-
•Snap bilirubin levels: In a study of 1059 patients undergoing major hepatectomy, peak bilirubin levels (>7 mg/dL) was found to be a good independent predictor of complications and 90-day morbidity and mortality [8]. The authors reported a sensitivity and specificity of greater than 90%.
-
•Hyder et al. risk score: A scoring system combining Clavien-Dindo grade, INR, bilirubin and creatinine levels on day 3 of surgery [42]. The authors found a linear association of the risk of mortality with increasing numerical scores. Scores>/ = 11 had a sensitivity and specificity of 83.3% and 98.9% respectively to predict the risk of PHLF.
-
•
-
2.Metabolic excretion tests (Table 1):
-
•Indocyanine green retention rate (ICG-R15): Zipprich et al. have reported a superior predictable accuracy of ICG-R15 for PHLF when compared to both CTP classification and MELD score [46]. In a series of 101 patients with Child's A and B cirrhosis, Fan et al. reported a cut-off value 14% for ICG-R15 to segregate patients with high morbidity from those with low morbidity (p < 0.05) [47]. Others have reported a cut off value of 17% for the same [48]. Makucchi et al. proposed a limited resection for ICG-R15 of >30% versus resection of >/ = 4 segments for a normal ICG-R15 (<15%) [36]. Carino et al. found that in the setting of pre-operative serum bilirubin of >17μmol//L, a pre-operative ICG plasma disappearance rate (PDR) of less than 17.6%/min had a positive predictive value of 75% and a negative predictive value of 90% for post hepatectomy liver dysfunction [49].
-
•Other metabolic tests: There are various other tests for metabolic functioning of the liver such as Aminopyrine breath test, Caffeine and Lidocaine clearance tests that are markers of microsomal function and Galactose elimination test that is a marker of cytosolic function of the liver. Also, there are tests such as the LiMax breath test (using methacetin injection), that have the ability to predict postoperative liver function status even in fibrotic livers [50]. Hoekstra et al. have shown a better representation of the disease severity by Tc99 m tagged Galactosyl asialoglycoprotein in 9–20% of the patients underestimated by ICG clearance test [51].
-
•
-
3.Role of contrast enhanced MRI scan:
- MRI finds its role as an attractive tool of assessing functional liver remnant using liver-specific contrast agents. The most promising in this regard has been Gadoxetic acid. It is a gadolinium-based paramagnetic agent taken up rapidly by the functional hepatocytes after IV injection and excreted rapidly into the canalicular system by the membrane receptors. Recent studies have proved a superiority of preoperative relative liver enhancement (RLE) over both 50-50 criteria and ISGLS grading system in terms of predicting probability of PHLF [52].
Table 1.
Metabolic assessment of liver.
|
5.2. Quantitative assessment
CT volumetry is an effective tool used to assess the resection volumes of the liver parenchyma preoperatively by digital contouring of the liver. A liver attenuation (in Hounsefeld units) lower than the splenic attenuation is an indicator of fatty infiltration or steatosis. This is expressed mathematically as the Liver attenuation index (LAI). Values less than +5 or more negative values indicate higher degrees of fat infiltration [53].
There is no uniform consensus to define the volume of the future remnant liver (FLR) to achieve a safe liver resection. Most have proposed a threshold ranging from 25 to 40% for a safe hepatectomy [54].
Based on the colorectal liver metastasis resection consensus guidelines (2006), the acceptable FLR has been stated to be >20% of Total liver volume (TLV) in normal livers, >30% in the presence of steatosis and >40% in the presence of fibrosis/cirrhosis [55]. This has been further validated by Kishi et al. in their study on 301 patients undergoing right hepatectomy, where FLR <20% was found to be the strongest predictor of developing PHLF [56].
6. Prevention of PHLF
Most strategies of preventing PHLF aim at augmenting the volumes of the future remnant liver (FLR) by modulating the porto-splenic circulation. The current methods and strategies of preventing and minimising the risk of PHLF are summarised in Table 2.
-
1.
Portal vein embolization (PVE):
The molecular dynamics involve induction of growth factors with redistribution of the portal flow and release of substances like nitric oxide (discussed in pathophysiology). PVE was first described in early 1980s by Kinoshita and later by Makuuchi et al. [57]. However, the main goal of PVE is to cause an augmentation of the functional capacity of remnant liver. The current guidelines recommend PVE for patients with underlying cirrhosis and an anticipated FLR of </ = 40% or normal liver function with an intended FLR of <20% [58].
Absence of parenchymal hypertrophy after PVE may be explained by the presence of porto-systemic shunts or by the existence of stagnant or pre-existing hepato-fugal portal flow prior to PVE. It is recommended to perform CT volumetry 3–4 weeks after PVE to assess the degree of hypertrophy. Capussotti et al. have reported an FLR hypertrophy of 30–40% in 4–6 weeks in more than 80% of patients, thereby making them suitable for a hepatectomy 6 weeks after the procedure [59].
PVE causes stasis in the portal flow, which leads to increased arterial flow in the embolised segments (Hepatic arterial buffer response), which may in turn cause increase in the size of the tumor. However, PVE preceded by Trans-arterial chemoembolization (TACE) may prevent this by causing tumor necrosis. This forms the rationale of TACE followed by PVE in treatment of HCC. The tumors may also be associated with formation of arterioportal shunts. TACE targets these shunts and contributes further to tumor necrosis. In a group of 36 patients with HCC, with 18 patients undergoing TACE three weeks prior to PVE, Ogata et al. reported a higher mean increase in FLR following TACE followed by PVE versus PVE alone (12.5% vs 8.4%; p < 0.002) [60].
-
2.
PVE with two-staged hepatectomy:
This is an approach utilised for bilobar colorectal liver metastases. The first stage involves one or more minor liver resection(s) in the remnant liver with or without a local ablative therapy. This is followed by occluding the contralateral portal vein, either by ligation or by PVE. The rationale behind this approach is attempting to prevent the tumor growth in the FLR induced by portal ligation and decreasing the technical difficulty associated with a major partial hepatectomy.
In a series by Narita et al. in 36 patients undergoing partial hepatectomy, the 5-yr overall survival was reported to be 32%, with an overall median survival of 39.6 months [61].
-
3.
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS):
This innovative approach involves associating the ligation of right portal vein with complete transection (partitioning) of the liver. The first stage of the procedure involves ligation of the right portal vein with parenchymal transection without resection. As opposed to the conventional hepatectomy, the diseased part of the liver is left in situ and remains vascularised by the right hepatic artery only. The second part of the procedure is performed 7–15 days later and involves removing the diseased part of the liver by simply sectioning the remaining vascular and biliary pedicles. This allows a significant hypertrophy of the FLR. It was first described by Schnitzbauer et al., in 2012, who in a series of 25 patients reported an enlargement of seg II and III of up to 74% in 9 days, which was much superior to other described methods [62]. This approach finds its superiority over other conventional portal vein ligation methods in saving time and thereby, avoiding the risk of tumor progression and its applicability in the presence of thrombus in right portal vein. In a series of 134 patients, Pandanaboyana et al. reported an additional increment of FLR volume by 17% in comparison to PVE alone [63]. However, the procedure requires a higher technical expertise because of its attendant operative morbidity (16–64%) and perioperative mortality (12–23%).
-
4.
Hepatic venous outflow reconstruction:
Proper anatomical repositioning of the remnant liver parenchyma in the right hypochondrium and ensuring an adequate venous outflow by avoiding venous kinks and ruling out congestion on table are essential in preventing PHLF. Mise et al. studied the relationship of possibility of venous reconstruction with preoperative ICG-R15 and liver volume [64]. They proposed the following criteria mandating hepatic venous reconstruction: a) ICG-R15 < 10% with non-congested liver remnant (NCLR) of <40% of TLV, or b) ICG-R15 10–20% with NCLR of <50% of the TLV.
-
5.
In-situ hypothermic liver perfusion:
Hoti et al. proposed that decreasing the cellular activity with hypothermia (22–26 deg Celsius) improved the tolerance to ischemia and caused a reduction in ischemia-reperfusion injury [65]. Based on these findings, this approach of in-situ hypothermia may be planned in cases where the total vascular exclusion (TVE) is expected for a duration of >60 min. Azoulay et al. reported a better ischemic tolerance and better post-operative renal and hepatic functions with this method, when compared to a standard TVE of any duration [66].
-
6.
Ischemic preconditioning:
Ischemic preconditioning strategies (Direct and Remote) have been shown to reduce the effect of ischemic reperfusion injury (IRI) by subjecting the liver to brief periods of ischemia prior to resection. Direct ischemic preconditioning (IPC) has been mostly applied experimentally on animal models. Most of the authors have reported a detrimental of this on the portal hemodynamics [67]. However, in remote ischemic preconditioning (RIPC), a remote organ is subjected to the ischemic stimulus prior to the target organ. Kanoria et al. studied this in a series of 16 patients randomised to RIPC and sham control groups [68]. RIPC was induced through 10 min cycles of alternate ischemia and reperfusion to the lower limb. The authors reported a lower level of transaminases immediately post-resection (ALT: 43% lower; AST: 50% lower; p < 0.001) along with an improved clearance of ICG in the group subjected to RIPC. Alchera et al. had evaluated the means of pharmacologic preconditioning by stimulation of the adenosine A2a receptors (Apadenoson, ALT-313); employed mostly on the animal models [69]. Therefore, this could pave a path for further research into the role of this strategy in decreasing the incidence of PHLF in patients undergoing major hepatectomies.
-
7.
Splenectomy:
Normally, spleen contributes to 25–30% of the total portal flow, which rises to 50% in the setting of portal hypertension. Ito et al. proposed the following mechanisms of decreasing the risk of ischemia-reperfusion injury by splenectomy: a) reduction of portal flow and pressure thereby, decreasing the endothelial injury, b) increasing the level of heme oxygenase-1 protein with anti-oxidant effects and, c) reducing the portal flow with increase in arterial flow (Hepatic arterial buffer response). Arakawa et al. reported an improved survival with reduced hepatocyte damage after splenectomy in rats undergoing up to 90% partial hepatectomy [70].
-
8.
Portocaval shunt:
Portocaval shunt has been described in the setting of an anticipated SFSS and in the setting of a small Graft recipient weight ratio (GRWR) leading to SFSS, to decrease the risk of PHLF. Another attractive strategy is the creation of a hemi-portocaval shunt (HPCS) in patients with anticipated low GRWR and resultant SFSS. The right or the left portal vein is permanently shunted to the ventral aspect of IVC in an end-side fashion with a PDS 6/0 running suture. In a series of 13 patients undergoing adult-adult LDLT, Troisis et al. found a significant reduction of portal vein flow among the HPCS group compared to the group without graft inflow modulation (190 ± 70 ml/min/100 g liver v/s 401 ± 225 ml/min/100 g liver; p < 0.001) [71]. But, irrespective of the technique used, portal flow modulation has an attractive future direction in prevention of PHLF.
-
9.
Pharmacologic:
Owing to its vasoconstrictor (down-regulation of endothelin-1 receptor) and anti-oxidant (up-regulation of heme-oxygenase-1) properties, somatostatin has been a suggested as an experimental agent for prevention of PHLF. Xu et al. demonstrated a superior graft survival and improved liver functions in animal model of post OLT small for size syndrome [72].
Table 2.
Prevention of PHLF (strategies).
|
7. Treatment of PHLF
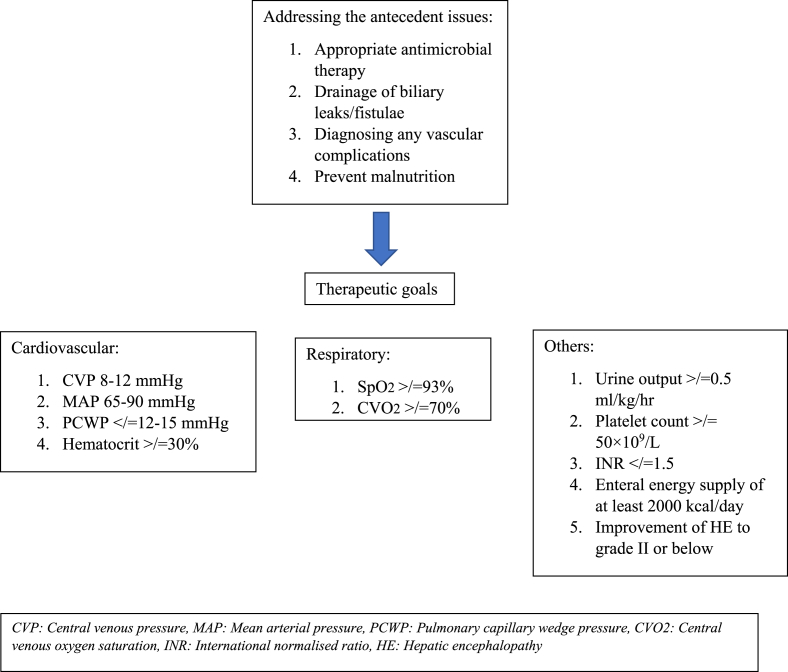
The principles of managing PHLF follow similar protocol as managing an acute liver failure. The treatment essentially focusses on an early recognition and Goal-directed resuscitation and has been outlined in Fig. 1.
Fig. 1.
Management protocol for PHLF.
In case of diagnostic uncertainty even at the end of 2 weeks after hepatectomy, with progressive deterioration of the clinical and biochemical parameters (isolated refractory hyperbilirubinemia, rise in Alkaline phosphatase), a liver biopsy is warranted to rule out intra-hepatic cholestasis. This is associated with very high mortality [73].
Liver support systems: The liver support systems have brought some hope in the management of PHLF, since they act as a bridge between development of acute liver failure to liver transplantation. There are three main types:
-
•
Molecular adsorbent recirculating system (MARS): Most of the studies have found a biochemical improvement in patients with PHLF with the use of MARS, although the subsequent outcome was poor due to development of other morbid complications. Van de Kerkhoeve et al. in series of 5 patients found clinical improvement in 3 and biochemical improvement in all 5, with the use of MARS [74]. However, lack of definitive evidence of its use in the management of PHLF limits the applicability of MARS in the management of PHLF presently.
-
•
Modified fractionated plasma separation and adsorption (Prometheus): The albumin bound toxins are eliminated through an albumin bound permeable membrane and the detoxified albumin is subsequently returned to the patient. It has a superior detoxifying efficacy compared to MARS, although there is lack of conclusive evidence to support its use in the management of PHLF.
-
•
Bio-artificial liver and extra-corporeal liver assist device: The first bioartificial liver (BAL) was developed by Matsumara in 2001. These essential comprise of embedded hepatocytes within a matrix composed of collagen etc. In addition to detoxification, they also address the metabolic and synthetic functions of the liver, thereby suggesting some superiority over the conventional liver dialysis systems listed above. Studies have been conducted to assess its efficacy in acute liver failure, which have shown some promising results [75]. However, there is lack of any evidence to demonstrate its use in PHLF in the present date.
The most definitive management of PHLF remains Liver transplantation. However, due to graft shortage, high risk:benefit ratio and high cost, the indications remain marginal, especially in developing countries.
Future trends in management: Strategies such as hepatocyte transplantation and intra-splenic injection of immortalised human primary hepatocytes have been studied in various animal experiments. However, there is lack of any evidence regarding their efficacy in humans so far. Furst et al. demonstrated a gain of parenchymal growth after PVE related to the portal injection of CD133 + cells in the non-embolised sectors in a series of 11 patients [76]. Similarly, Yamanka et al. found some efficacy of Olprione, a phosphodiesterase inhibitor with vasodilating properties by demonstrating reduction in endothelial damage and hepatocyte apoptosis in murine models [77]. However, these strategies are largely experimental in the present date and are yet to be validated for clinical use in humans.
8. Conclusion
PHLF is an extremely morbid clinical problem in patients undergoing hepatectomies. Strategies for augmenting the remnant liver function such as PVE, ALPPS are essential for preventing PHLF. The treatment of PHLF mainly involves a goal directed therapy to restore the cardiac, respiratory and renal hemodynamic status. Auxiliary liver support systems such as MARS and Bio-assist devices have shown some effectiveness in the management of PHLF. In certain selected cases, listing patients early for OLT has been found to be beneficial. However, the mainstay of its management centres on adequate preoperative preparation and meticulous intra-operative surgical techniques to reduce the incidence of this complication.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
Not applicable.
Sources of funding
None.
Author contribution
Samrat Ray: Prepared the manuscript. Gathered all the data for preparing the review. Naimish N Mehta: Supervised the preparation of the manuscript. Did the first editing. Ankush Golhar: Collected data in collaboration with the first author. Samiran Nundy: Chief supervisor. Final correction and approval of the manuscript.
Conflicts of interest
None.
Research registration number
NA.
Guarantor
Samrat Ray.
Acknowledgements
Dr Shailendra Lalwani, Dr Vivek Mangla and Dr Siddharth Mehrotra for their valuable inputs.
References
- 1.Zheng Y., Yang H., He L. Reassessment of different criteria for diagnosing post-hepatectomy liver failure: a single-center study of 1683 hepatectomy. Oncotarget. 2017;8:89269–89277. doi: 10.18632/oncotarget.19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreckenbach T., Liese J., Bechstein W.O., Moench C. Posthepatectomy liver failure. Dig. Surg. 2012;29:79–85. doi: 10.1159/000335741. [DOI] [PubMed] [Google Scholar]
- 3.Figueras J., Valls C., Rafecas A., Fabregat J., Ramos E., Jaurrieta E. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br. J. Surg. 2001;88:980–985. doi: 10.1046/j.0007-1323.2001.01821.x. [DOI] [PubMed] [Google Scholar]
- 4.Finch R.J.B., Malik H.Z., Hamady Z.Z.R. Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br. J. Surg. 2007;94:1242–1248. doi: 10.1002/bjs.5640. [DOI] [PubMed] [Google Scholar]
- 5.Karanjia N.D., Lordan J.T., Quiney N., Fawcett W.J., Worthington T.R., Remington J. A comparison of right and extended right hepatectomy with all other hepatic resections for colorectal liver metastases: a ten-year study. Eur. J. Surg. Oncol. 2009;35:65–70. doi: 10.1016/j.ejso.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Ren Z., Xu Y., Zhu S. Indocyanine green retention test avoiding liver failure after hepatectomy for hepatolithiasis. Hepato-Gastroenterology. 2012;59:782–784. doi: 10.5754/hge11453. [DOI] [PubMed] [Google Scholar]
- 7.Balzan S., Belghiti J., Farges O. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann. Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullen J.T., Ribero D., Reddy S.K. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J. Am. Coll. Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 62–4. [DOI] [PubMed] [Google Scholar]
- 9.Rahbari N.N., Garden O.J., Padbury R. Posthepatectomy liver failure: a definition and grading by the international study group of liver surgery (ISGLS) Surgery (St Louis) 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Michalopoulos G.K. Liver regeneration. J. Cell. Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu Z., Bozorgzadeh A., Pierce R.H., Kurtis J., Crispe I.N., Orloff M.S. TLR-dependent cross talk between human Kupffer cells and NK cells. J. Exp. Med. 2008;205:233–244. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto K., Yoshitomi H., Rossant J., Zaret K.S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 13.Cantré D., Schuett H., Hildebrandt A. Nitric oxide reduces organ injury and enhances regeneration of reduced-size livers by increasing hepatic arterial flow. Br. J. Surg. 2008;95:785–792. doi: 10.1002/bjs.6139. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen K.E., Conley L.N., Nygaard I. Increased sinusoidal flow is not the primary stimulus to liver regeneration. Comp. Hepatol. 2010;9:2. doi: 10.1186/1476-5926-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan C.J., Guest J., Harper A.M., Blumgart L.H. Liver blood flow measurements in the portacavally transposed rat before and after partial hepatectomy. Br. J. Exp. Pathol. 1978;59:111–115. [PMC free article] [PubMed] [Google Scholar]
- 16.Taub R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto T., Liu Z., Murase N. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403–411. doi: 10.1002/hep.510290244. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki T., Murata S., Takahashi K. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J. Hepatol. 2010;53:648–654. doi: 10.1016/j.jhep.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Lesurtel M., Graf R., Aleil B. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 20.Wack K. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33:363–378. doi: 10.1053/jhep.2001.21998. [DOI] [PubMed] [Google Scholar]
- 21.Petrowsky H., Breitenstein S., Slankamenac K. Effects of pentoxifylline on liver regeneration. Ann. Surg. 2010;252:813–822. doi: 10.1097/SLA.0b013e3181fcbc5e. [DOI] [PubMed] [Google Scholar]
- 22.Belghiti J., Liddo G., Raut V. “Inherent limitations” in donors: control matched study of consequences following a right hepatectomy for living donation and benign liver lesions. Ann. Surg. 2012;255:528–533. doi: 10.1097/SLA.0b013e3182472152. [DOI] [PubMed] [Google Scholar]
- 23.Timchenko N.A. Aging and liver regeneration. Trends Endocrinol. Metabol. 2009;20:171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Kim J.M., Cho B.I., Kwon C.H. Hepatectomy is a reasonable option for older patients with hepatocellular carcinoma. Am. J. Surg. 2015;209:391–397. doi: 10.1016/j.amjsurg.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Little S.A., Jarnagin W.R., DeMatteo R.P., Blumgart L.H., Fong Y. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J. Gastrointest. Surg. 2002;6:88–94. doi: 10.1016/s1091-255x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 26.N.L. Bucher. Insulin, glucagon, and the liver. Adv. Enzym. Regul.;15:221-230. [DOI] [PubMed]
- 27.Schindl M.J., Redhead D.N., Fearon K.C. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan S.T., Lo C.M., Lai E.C., Chu K.M., Liu C.L., Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N. Engl. J. Med. 1994;331:1547–1552. doi: 10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
- 29.Kaibori M., Inoue T., Sakakura Y. Impairment of activation of hepatocyte growth factor precursor into its mature form in rats with liver cirrhosis. J. Surg. Res. 2002;106:108–114. doi: 10.1006/jsre.2002.6438. [DOI] [PubMed] [Google Scholar]
- 30.Robinson S.M., Wilson C.H., Burt A.D., Manas D.M., White S.A. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann. Surg Oncol. 2012;19:4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy S.K., Marsh J.W., Varley P.R. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case–control study. Hepatology. 2012;56:2221–2230. doi: 10.1002/hep.25935. [DOI] [PubMed] [Google Scholar]
- 32.Vauthey J.N., Pawlik T.M., Ribero D. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J. Clin. Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 33.Mehta N.N., Ravikumar R., Coldham C.A. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2008;34:782–786. doi: 10.1016/j.ejso.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Overman M.J., Maru D.M., Charnsangavej C. Oxaliplatin mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J. Clin. Oncol. 2010;28:2549–2555. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 35.Capussotti L., Muratore A., Amisano M., Polastri R., Bouzari H., Massucco P. Liver resection for hepatocellular carcinoma on cirrhosis: analysis of mortality, morbidity and survival–a European single center experience. Eur. J. Surg. Oncol. 2005;31:986–993. doi: 10.1016/j.ejso.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Makuuchi M., Kosuge T., Takayama T. Surgery for small liver cancers. Semin. Surg. Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 37.Imamura H., Seyama Y., Kokudo N. One thousand fifty-six hepatectomies without mortality in 8 years. Arch. Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. discussion 206. [DOI] [PubMed] [Google Scholar]
- 38.Fujii Y., Shimada H., Endo I. Risk factors of posthepatectomy liver failure after portal vein embolization. J Hepatobiliary Pancreat. Surg. 2003;10:226–232. doi: 10.1007/s00534-002-0820-9. [DOI] [PubMed] [Google Scholar]
- 39.Emond J.C., Renz J.F., Ferrell L.D. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann. Surg. 1996;224:544–552. doi: 10.1097/00000658-199610000-00012. discussion 52-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C., Mi K., Wen T.-F., Yan L.-N., Li B. Safety of patients with a graft to body weight ratio less than 0.8% in living donor liver transplantation using right hepatic lobe without middle hepatic vein. Hepato-Gastroenterology. 2012;59:469–472. doi: 10.5754/hge11217. [DOI] [PubMed] [Google Scholar]
- 41.van den Broek M.A., Olde Damink S.W., Dejong C.H. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 42.Hyder O., Pulitano C., Firoozmand A. A risk model to predict 90-day mortality among patients undergoing hepatic resection. J. Am. Coll. Surg. 2013;216:1049–1056. doi: 10.1016/j.jamcollsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahbari N.N., Reissfelder C., Koch M. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann. Surg Oncol. 2011;18:3640–3649. doi: 10.1245/s10434-011-1829-6. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.H., Kang D.R., Lee J.G. Early predictor of mortality due to irreversible posthepatectomy liver failure in patients with hepatocellular carcinoma. World J. Surg. 2013;37:1028–1033. doi: 10.1007/s00268-013-1959-z. [DOI] [PubMed] [Google Scholar]
- 45.Skrzypczyk C., Truant S., Duhamel A. Relevance of the ISGLS definition of posthepatectomy liver failure in early prediction of poor outcome after liver resection: study on 680 hepatectomies. Ann. Surg. 2014;260:865–870. doi: 10.1097/SLA.0000000000000944. discussion 70. [DOI] [PubMed] [Google Scholar]
- 46.Zipprich A., Kuss O., Rogowski S. Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut. 2010;59:963–968. doi: 10.1136/gut.2010.208595. [DOI] [PubMed] [Google Scholar]
- 47.Fan S.T., Lai E.C., Lo C.M., Ng I.O., Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch. Surg. 1995;130:198–203. doi: 10.1001/archsurg.1995.01430020088017. [DOI] [PubMed] [Google Scholar]
- 48.Lam C.M., Fan S.T., Lo C.M., Wong J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br. J. Surg. 1999;86:1012–1017. doi: 10.1046/j.1365-2168.1999.01204.x. [DOI] [PubMed] [Google Scholar]
- 49.de Liguori Carino N., O'Reilly D.A., Dajani K. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur. J. Surg. Oncol. 2009;35:957–962. doi: 10.1016/j.ejso.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Stockmann M., Lock J.F., Riecke B. Prediction of postoperative outcome after hepatectomy with a new bedside test for maximal liver function capacity. Ann. Surg. 2009;250:119–125. doi: 10.1097/SLA.0b013e3181ad85b5. [DOI] [PubMed] [Google Scholar]
- 51.Hoekstra L.T., de Graaf W., Nibourg G.A. Physiological and biochemical basis of clinical liver function tests: a review. Ann. Surg. 2013;257:27–36. doi: 10.1097/SLA.0b013e31825d5d47. [DOI] [PubMed] [Google Scholar]
- 52.Wibmer A., Prusa A.M., Nolz R., Gruenberger T., Schindl M., Ba-Ssalamah A. Liver failure after major liver resection: risk assessment by using preoperative Gadoxetic acid-enhanced 3-T MR imaging. Radiology. 2013;269:777–786. doi: 10.1148/radiol.13130210. [DOI] [PubMed] [Google Scholar]
- 53.Varma V., Mehta N., Kumaran V., Nundy S. Indications and contraindications of liver transplantation. Int J Hepatol. 2011 doi: 10.4061/2011/121862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribero D., Chun Y.S., Vauthey J.N. Standardized liver volumetry for portal vein embolization. Semin. Intervent. Radiol. 2008;25:104–109. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdalla E.K., Adam R., Bilchik A.J., Jaeck D., Vauthey J.N., Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann. Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 56.Kishi Y., Abdalla E.K., Chun Y.S. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann. Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 57.Hemming A.W., Reed A.I., Howard R.J. Preoperative portal vein embolization for extended hepatectomy. Ann. Surg. 2003;237:686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. discussion 691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.May B.J., Madoff D.C. Portal vein embolization: rationale, technique and current application. Semin. Intervent. Radiol. 2012;29:81–89. doi: 10.1055/s-0032-1312568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capussotti L., Muratore A., Baracchi F. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch. Surg. 2008;143:978–982. doi: 10.1001/archsurg.143.10.978. discussion 982. [DOI] [PubMed] [Google Scholar]
- 60.Ogata S., Belghiti J., Farges O., Varma D., Sibert A., Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br. J. Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 61.Narita M., Oussoultzoglou E., Jaeck D. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br. J. Surg. 2011;98:1463–1475. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 62.Schnitzbauer A.A., Lang S.A., Goessmann H. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right. J. Gastrointest. 2013;17:593–605. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 63.Pandanaboyana S., Bell R., Hidalgo E. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery (St Louis) 2015;157:690–698. doi: 10.1016/j.surg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Mise Y., Hasegawa K., Satou S. Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br. J. Surg. 2011;98:1742–1751. doi: 10.1002/bjs.7670. [DOI] [PubMed] [Google Scholar]
- 65.Hoti E., Salloum C., Azoulay D. Hepatic resection with in situ hypothermic perfusion is superior to other resection techniques. Dig. Surg. 2011;28:94–99. doi: 10.1159/000323817. [DOI] [PubMed] [Google Scholar]
- 66.Azoulay D., Eshkenazy R., Andreani P., Castaing D., Adam R. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann. Surg. 2005;241:277–285. doi: 10.1097/01.sla.0000152017.62778.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clavien P.A., Yadav S., Sindram D., Bentley R.C. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann. Surg. 2000;232:155–162. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanoria S., Robertson F.P., Mehta N.N., Fusai G., Sharma D., Davidson B.R. Effect of remote ischemic preconditioning on liver injury in patients undergoing major hepatectomy for colorectal liver metastasis: a pilot randomised controlled feasibility trial. World J. Surg. 2017;41:1322–1330. doi: 10.1007/s00268-016-3823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alchera E., Imarisio C., Mandili G. Pharmacological preconditioning by adenosine A2a receptor stimulation: features of the protected liver cell phenotype. BioMed Res. Int. 2015 doi: 10.1155/2015/286746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arakawa Y., Shimada M., Uchiyama H. Beneficial effects of splenectomy on massive hepatectomy model in rats. Hepatol. Res. 2009;39:391–397. doi: 10.1111/j.1872-034X.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- 71.Troisi R., Ricciardi S., Smeets P. Effects og hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am. J. Transplant. 2005;5:1397–1404. doi: 10.1111/j.1600-6143.2005.00850.x. [DOI] [PubMed] [Google Scholar]
- 72.Xu X., Man K., Zheng S.S. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transplant. 2006;12:621–627. doi: 10.1002/lt.20630. [DOI] [PubMed] [Google Scholar]
- 73.Chiarla C., Giovannini I., Giuliante F. Plasma bilirubin correlations in non-obstructive cholestasis after partial hepatectomy. Clin. Chem. Lab. Med. 2008;46:1598–1601. doi: 10.1515/CCLM.2008.321. [DOI] [PubMed] [Google Scholar]
- 74.van de Kerkhove M.P., de Jong K.P., Rijken A.M., de Pont A.C., van Gulik T.M. MARS treatment in posthepatectomy liver failure. Liver Int. 2003;23:44–51. doi: 10.1034/j.1478-3231.23.s.3.2.x. [DOI] [PubMed] [Google Scholar]
- 75.Demetriou A.A., Brown R.S., Busuttil R.W., Fair J., McGuire B.M., Rosenthal P. Prospective, randomized, multicentre, controlled trial of a bioartificial liver in treating acute liver failure. Ann. Surg. 2004;239:660–670. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furst G., Schulte am Esch J., Poll L.W. Portal vein embolization and autologous CD133+ bone marrow stem cells for liver regeneration: initial experience. Radiology. 2007;243:171–179. doi: 10.1148/radiol.2431060625. [DOI] [PubMed] [Google Scholar]
- 77.Yamanaka K., Hatano E., Narita M. Olprinone attenuates excessive shear stress through up-regulation of endothelial nitric oxide synthase in a rat excessive hepatectomy model. Liver Transplant. 2011;17:60–69. doi: 10.1002/lt.22189. [DOI] [PubMed] [Google Scholar]