Abstract
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the two most common skin cancers found in humans. These cancers can acquire drug resistance and pose considerable medical burdens to clinics and patients if left untreated. Two recent studies show that active Hippo signaling plays a critical role in initiating BCC and SCC tumorigenesis, providing new opportunities to develop therapies against these skin malignancies.
Subject Categories: Cancer, Signal Transduction
Maintenance of the skin barrier is a complex process requiring intricate signaling. When these signaling processes are disrupted—usually through environmental insults, i.e., sun exposure, chronic inflammation, wounding—the barrier can be compromised, increasing the risks of dehydration, infection, irritation/inflammation, and cancer. The two most common types of cancer—both of the skin and amongst all cancers—are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). BCC is an invasive cutaneous tumor characterized by its histological appearance which resembles that of the basal layer of the epidermis. SCC, on the other hand, presents itself as scaly lesions and is more aggressive, prone to recurrence and metastasis.
Basal cell carcinoma and squamous cell carcinoma are both thought to be caused by homeostatic signaling pathway(s) gone awry. One such signaling pathway that regulates skin barrier function—which is then exploited during skin tumorigenesis—is the Hippo pathway and its downstream effectors Yes‐associated protein (Yap) and transcriptional co‐activator with PDZ‐binding motif (Taz; Varelas, 2014). The Hippo pathway was initially identified through a screen for regulators of organ size in Drosophila and is an evolutionarily conserved signaling pathway that coordinates cell proliferation, apoptosis, and differentiation. Several decades’ of research has demonstrated that Hippo signaling and its downstream effectors Taz/Yap are influenced by extracellular cues such as cytoskeletal changes and mechanical stress, which guide the formation and maintenance of tissues/organs.
In a tenuous balancing act, Hippo signaling promotes the expansion and subsequent differentiation of progenitor populations for homeostasis. There is extensive evidence showing that Hippo signaling controls skin development and the sensation of cell crowding and crowd control. Hippo/Yap signaling is sensitive to cytoskeletal dynamics to generate a stratified skin epithelium, skin barrier, and hair follicle morphogenesis (Zhang et al, 2011). Upon moderate epidermal wounding, Yap/Taz localizes to the wound area(s) and stimulates stem cells to assist in re‐epithelialization necessary for healing (Lee et al, 2014). This same ability to drive widespread proliferation can result in unregulated skin expansion and formation of tissue overgrowths found in BCC and SCC diagnoses. Dysregulation of Hippo signaling has been heavily implicated in human cancers (Moroishi et al, 2015). In BCC, Yap/Taz is oncogenic; hyperactivation works in concert with other signaling pathways (such as Hedgehog, Wnt) to exacerbate disease (Youssef et al, 2008; Akladios et al, 2017).
There are less data on the role of how Hippo signaling regulates nonmelanoma cancers in particular. In a related study (Zanconato et al, 2015), Yap/Taz transcriptional activity was found to drive proliferation not only in cancer cell lines but also in human tumors. Elevated transcriptional Yap/Taz signatures via the Ap‐1 transcriptional program correlated with tumor aggressiveness, while in vivo studies of Yap/Taz deficiency revealed an inhibition of papilloma formation despite potent chemical carcinogenesis.
Now, Debaugnies et al (2018) and Maglic et al (2018) use well‐thought‐out experiments and sophisticated molecular genetics to evaluate the functional role(s) of Yap/Taz in the pathogenesis of both SCC and BCC. Their work capitalizes on the previous work, but expands the role(s) of Yap/Taz in mammalian BCC and SCC tumors to highlight opportunities to develop more advanced nonmelanoma therapies.
The current studies demonstrate that the Hippo co‐activators Yap/Taz are upregulated in human BCC and SCC, and induce similar gene signatures in both skin cancer subtypes (see Fig 1). Maglic et al (2018) delve deeper into the molecular role for Yap/Taz by taking advantage of ChIP‐SEQ to show Yap must directly interact with DNA‐binding transcription factor Tead in BCC expansion. Maglic et al (2018) then explored how cellular localization might influence disease progression, and found nuclear Yap/Taz association yields more invasive tumors. This phenomenon is not unique to BCC; Debaugnies et al (2018) also observed activated Yap in invasive SCC and similar Yap gene expression profile (in relation to BCC).
Figure 1. Aberrant Hippo signaling and downstream YAP/TAZ nuclear signaling are required for mammalian nonmelanoma skin cancers.
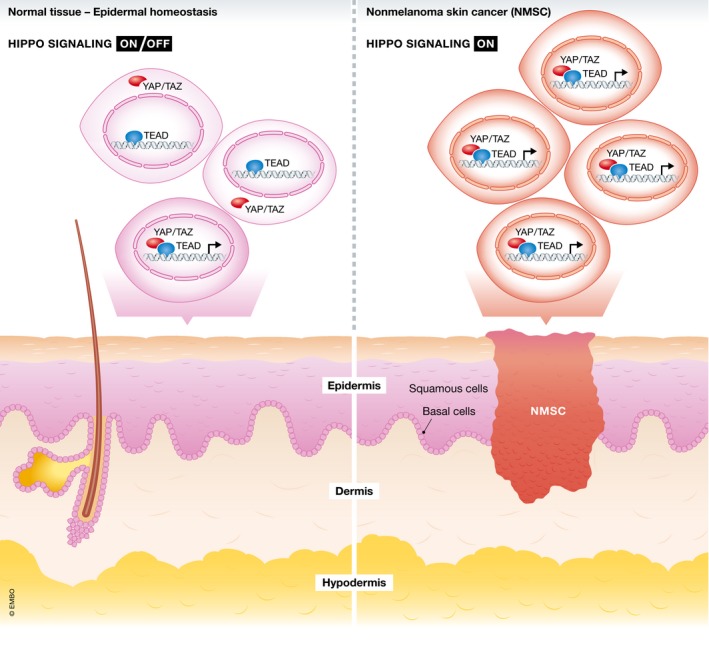
In normal tissue (left), skin cells temporarily express nuclear YAP/TAZ in order to expand cell population to maintain homeostasis or to facilitate wound healing. However, overactive Hippo signaling initiates basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) (right) due to YAP/TAZ overexpression, nuclear localization, and their interaction with TEAD DNA‐binding transcription factors.
Together, these studies add further to our understanding of a well‐characterized, but underappreciated mechanism by which nonmelanoma tumors use Hippo signaling to progress from neoplasia to aggressive skin growths. Importantly, both studies suggest a molecular weakness that could be exploited to possibly treat such malignancies. In particular, Maglic used cell culture models to highlight known inhibitors of Hippo/AP1 targets to slow proliferation based on what was gleaned from their extensive molecular analyses. In addition, these molecular experiments also implicated the activation of c‐Jun (part of the AP1 complex) in BCC tumorigenesis, ultimately contributing to tumor formation. BCC cell lines were then used to probe the role of the Jnk‐Jun pathway activity using SP600125, a Jnk1/2/3 inhibitor. BCC cell proliferation was completed abolished with this treatment, further implicating the role of c‐Jun to propagate BCC initiation, while also highlighting a new opportunity to develop JNK‐JUN inhibitors in the fight against BCC.
Debaugnies et al (2018) propose extending these studies to include additional Hippo signaling members such as the Tead transcription factors in skin tumorigenesis by transgenic manipulation in the skin epithelium. Taken together with the Maglic et al (2018) findings, presumably it will be possible to block growth of BCC and SCC with Yap/Taz/Tead manipulation, but further molecular characterization will be required to understand the extent of inputs and outputs of this circuit in skin cancers.
While the findings of Zanconato et al (2015) initially showed the relevance of Hippo signaling in driving basal cell tumor growth, the current studies went well beyond this work to show that both BCC and SCC tumors rely on Hippo/Yap signaling for disease progression. Furthermore, the extensive molecular characterization performed by Maglic et al (2018) not only shed light on the basic mechanisms of the Hippo signaling pathway, but also provide novel insights into the physical interactions of Yap/Taz with the genome to exert their effects on the transcriptional program of cancer.
Targeting Hippo signaling would provide a dual approach to combating NMSC, as there have been cases of mixed tumors; SCC presents in advanced BCC (Ransohoff et al, 2015). The observations of Debaugnies et al (2018) and Maglic et al (2018) offer promising results for targeting YAP/TAZ in both of these skin cancer subtypes. Considering the parallels of Hippo signaling in BCC and SCC as a result of these two reports, it would be interesting to see whether the JNK inhibitor used in Maglic et al (2018) might also function to reduce tumor cell proliferation in an SCC model. Moreover, this approach could also be extended to melanoma studies, where there is growing literature showing Yap/Taz activity may contribute to its malignancy (Moroishi et al, 2015; Andl et al, 2017).
In conclusion, the work generated by Debaugnies et al (2018) and Maglic et al (2018) will be fundamental in optimizing pharmaceutical treatments of BCC and SCC rather than relying on surgical excision as the standard method of care. The successful characterization of Hippo signaling in nonmelanoma cancers will likely spur great interest to generate small molecules targeted against Yap/Taz that can be effectively used in a clinical setting.
The EMBO Journal (2018) e99921 30037822
See also: https://doi.org/10.15252/embj.201798642 (September 2018) and
https://doi.org/10.15252/embr.201845809 (July 2018)
References
- Akladios B, Mendoza Reinoso V, Cain JE, Wang T, Lambie DL, Watkins DN, Beverdam A (2017) Positive regulatory interactions between YAP and Hedgehog signalling in skin homeostasis and BCC development in mouse skin in vivo . PLoS One 12: e0183178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Zhou L, Yang K, Kadekaro AL, Zhang Y (2017) YAP and WWTR1: new targets for skin cancer treatment. Cancer Lett 396: 30–41 [DOI] [PubMed] [Google Scholar]
- Debaugnies M, Sánchez‐Danés A, Rorive S, Raphaë M, Liagre M, Parent MA, Brisebarre A, Salmon I, Blanpain C (2018) YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep 19: e45809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Byun MR, Furutani‐Seiki M, Hong JH, Jung HS (2014) YAP and TAZ regulate skin wound healing. J Invest Dermatol 134: 518–525 [DOI] [PubMed] [Google Scholar]
- Maglic D, Schlegelmilch K, Dost A, Panero R, Dill M, Calogero R, Camargo F (2018) YAP‐TEAD signaling promotes Basal Cell Carcinoma development via c‐JUN/AP1 axis. EMBO J 37: e98642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL (2015) The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 15: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff KJ, Tang JY, Sarin KY (2015) Squamous change in basal‐cell carcinoma with drug resistance. N Engl J Med 373: 1079–1082 [DOI] [PubMed] [Google Scholar]
- Varelas X (2014) The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141: 1614–1626 [DOI] [PubMed] [Google Scholar]
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Epstein EH (2008) Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 8: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S (2015) Genome‐wide association between YAP/TAZ/TEAD and AP‐1 at enhancers drives oncogenic growth. Nat Cell Biol 17: 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E (2011) Yes‐associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA 108: 2270–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]


