Abstract
The number and self‐renewal capacity of hematopoietic stem cells (HSCs) are tightly regulated at different developmental stages. Many pathways have been implicated in regulating HSC development in cell autonomous manners; however, it remains unclear how HSCs sense and integrate developmental cues. In this study, we identified an extrinsic mechanism by which HSC number and functions are regulated during mouse puberty. We found that the HSC number in postnatal bone marrow reached homeostasis at 4 weeks after birth. Luteinizing hormone, but not downstream sex hormones, was involved in regulating HSC homeostasis during this period. Expression of luteinizing hormone receptor (Lhcgr) is highly restricted in HSCs and multipotent progenitor cells in the hematopoietic hierarchy. When Lhcgr was deleted, HSCs continued to expand even after 4 weeks after birth, leading to abnormally elevated hematopoiesis and leukocytosis. In a murine acute myeloid leukemia model, leukemia development was significantly accelerated upon Lhcgr deletion. Together, our work reveals an extrinsic counting mechanism that restricts HSC expansion during development and is physiologically important for maintaining normal hematopoiesis and inhibiting leukemogenesis.
Keywords: hematopoiesis, hematopoietic stem cell, luteinizing hormone, luteinizing hormone receptor, puberty
Subject Categories: Cancer, Physiology, Stem Cells
Introduction
Hematopoietic stem cells (HSCs) are self‐renewable multipotent progenitor cells that give rise to all blood cell lineages during development and after injury. Throughout life, HSCs undergo sequential changes in several key properties, such as transcriptional profiles, self‐renewal capacities, cycling status, and lineage output potentialities (Copley & Eaves, 2013). Aorta‐gonad‐mesonephros (AGM) region is a source of pre‐HSCs at embryonic day 10.5 (E10.5) in mice. These pre‐HSCs migrate to placenta and fetal liver at E11.5. Fetal liver serves as the main organ for HSC expansion and maturation until bone marrow hematopoiesis is established (Mikkola & Orkin, 2006). Most fetal HSCs are cycling while retaining self‐renewal ability, in which they must undergo symmetric cell division to expand the HSC pool (Lessard et al, 2004). HSCs move to bone marrow where they still remain rapidly cycling until 3 weeks after birth (Bowie et al, 2006, 2007). HSCs in adult bone marrow are quiescent and divide rarely to maintain an appropriate pool size (Wilson & Trumpp, 2006).
Cell autonomous mechanisms are involved in the regulation of developmental changes of HSCs during development. Several genes have been demonstrated to be required for the self‐renewal of fetal but not adult HSCs. The transcription factor Sox17 is expressed in fetal, but not in adult, HSCs and is required for the maintenance of fetal and neonatal HSCs (Kim et al, 2007). Ectopic expression of Sox17 in adult HSCs is sufficient to confer increased self‐renewal potential and the expression of fetal HSC genes (He et al, 2011). Conversely, many other genes have been shown to be only required for the functionalities of adult HSCs, including Bmi1 (Park et al, 2003), Gfi (Hock et al, 2004a), Tel/Etv6 (Hock et al, 2004b), and C/EBPa (Ye et al, 2013). Notably, the Lin28b‐let‐7 axis has recently been identified as a master regulator of developmentally timed changes in HSC programs with Hmga2 serving as its specific downstream modulator of HSC self‐renewal potential. Another fundamental question regarding developmental changes in HSC properties is whether extrinsic factors are also involved in the timing, onset, and/or maintenance of such changes.
Extrinsic factors are known to regulate HSC homeostasis. This is best exemplified in adult bone marrow where multiple stromal cells cooperatively maintain HSCs. Endothelial cells and Lepr+ perivascular cells play key roles in maintaining HSCs by secreting niche factors, including SCF and CXCL12 (Ding et al, 2012; Ding & Morrison, 2013; Greenbaum et al, 2013). Other cell types in the bone marrow, such as osteoblasts (Calvi et al, 2003; Zhang et al, 2003), macrophages (Chow et al, 2011; Christopher et al, 2011), and megakaryocytes (Bruns et al, 2014; Zhao et al, 2014), have also been implicated in HSC maintenance via direct or indirect mechanisms. In fetal liver, the local portal vessels create a niche that supports HSC expansion (Khan et al, 2016). Besides local microenvironment, upon infection, systemic IFNa promotes the exit of HSCs out of the dormant stage, partially through its receptor IFNAR on HSCs (Essers et al, 2009). However, it remains undetermined whether there are systemic factors that play a direct role in HSC maintenance during development.
Systemic hormones, especially sex hormones, have been implicated in the regulation of the hematopoietic system. Sex steroid ablation in males by castration (Wilson et al, 1995; Ellis et al, 2001) or in females by ovariectomy (Erben et al, 1998) enhances B lymphopoiesis in rodents. Consistent with this, deletion of estrogen receptor (Thurmond et al, 2000; Erlandsson et al, 2001) or androgen receptor (Wilhelmson et al, 2015) also increases B lymphocytes. Sex hormones also regulate upstream hematopoietic progenitors. Ovariectomy or estrogen receptor alpha (Esr1) deletion increases the numbers of short‐term HSCs and lymphoid progenitors in the bone marrow (Thurmond et al, 2000; Medina et al, 2001; Li et al, 2013). Castration is shown to increase the number of functional HSCs in middle‐aged male mice (Khong et al, 2015), although the receptors of progesterone and androgen are absent from HSCs (Nakada et al, 2014; Mierzejewska et al, 2015), raising the possibility of an indirect effect. Estrogen increases HSC proliferation in female mice and during pregnancy in an Esr1‐dependent manner (Nakada et al, 2014), demonstrating a direct effect of sex hormone on the stem cell compartment. Nevertheless, Esr1 deletion does not have an effect on HSC self‐renewal in the bone marrow (Nakada et al, 2014; Sanchez‐Aguilera et al, 2014).
Luteinizing hormone is secreted by the pituitary gland, beginning at the onset of puberty, which promotes the maturation of the reproductive system in both males and females. In this study, we provided evidence that bone marrow HSCs are a direct target of LH and that deletion of the LH receptor (encoded by Lhcgr) has a direct effect on HSC self‐renewal. Our data suggested that LH signaling acts like a brake of HSC overexpansion in postnatal bone marrow, which ensure a proper HSC count for normal hematopoiesis in adulthood.
Results
Bone marrow HSCs continuously expanded until 4 weeks after birth
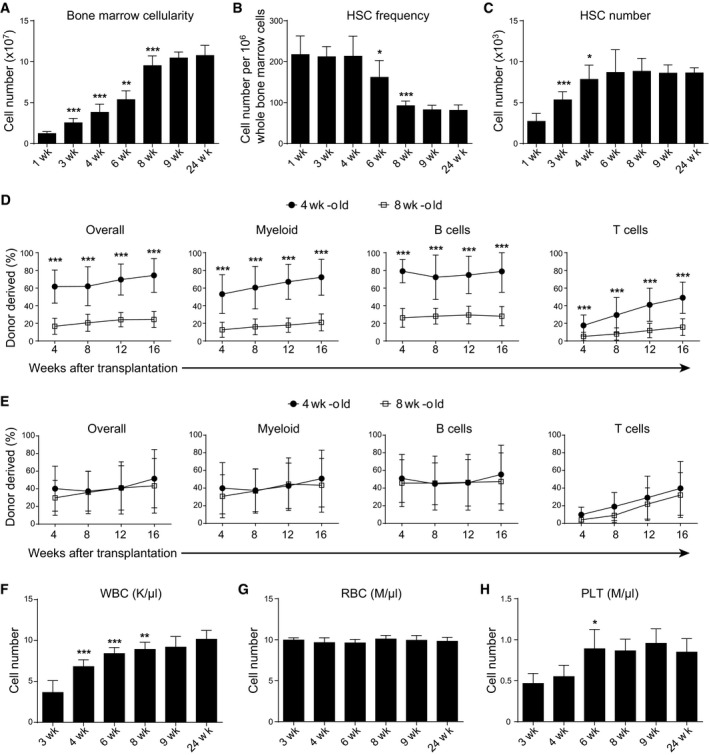
We were interested in understanding the mechanisms that regulate the developmental changes of HSCs in postnatal bone marrow. To this end, we first quantified bone marrow cellularity and HSC number in the bone marrow from mice at different time points after birth. At 1 week after birth, there were 12 ± 2.5 millions whole bone marrow (WBM) cells in two tibias and two femurs (Fig 1A). The bone marrow cellularity gradually increased while the mice grew up (Fig 1A). At 8 weeks after birth, this number reached 95 ± 12 millions and became essentially stable throughout young adulthood (Fig 1A). As measured by flow cytometry, the frequency of CD150+CD48−Lineage−Sca‐1+c‐kit+ HSCs (Kiel et al, 2005) was 217 ± 45 per million WBM cells at 1 week after birth and did not show significant reduction until 4 weeks after birth (Fig 1B). At 8 weeks after birth, the HSC frequency decreased to 93 ± 11 per million WBM cells and became basically stable throughout young adulthood (Fig 1B). The overall HSC number was 2.7 ± 1.0 thousands at 1 week after birth, progressively increased to 7.8 ± 1.7 thousands at 4 weeks after birth and then no longer changed during young adulthood (Fig 1C). Thus, the bone marrow cellularity reaches homeostasis at 8 weeks after birth, whereas the HSC number reaches homeostasis at 4 weeks after birth.
Figure 1. The HSC number in the bone marrow achieved homeostasis at 4 weeks after birth.
-
A–CThe bone marrow cellularity (A), CD150+CD48−Lineage−Sca‐1+c‐kit+ HSC frequency (B), and number (C) in the bone marrow of wild‐type mice at indicated weeks after birth. All data reflected mean ± SD of two tibias plus two femurs (n = 6 mice/age from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance of differences between consecutive ages (*P < 0.05, **P < 0.01, ***P < 0.001).
-
D, ECompetitive reconstitution assay in which 300,000 of donor‐derived bone marrow cells (D) or 0.5% of donor‐derived bone marrow cells (E) from 4‐ or 8‐week‐old mice were transplanted along with 500,000 recipient‐type competitor cells into irradiated recipient mice (n = 11–12 recipient mice/genotype from three independent experiments). Data represented mean ± SD. The statistical significance of differences was assessed using two‐way ANOVAs (***P < 0.001).
-
F–HPeripheral white blood cell count (WBC, F), red blood cell count (RBC, G), and platelet count (PLT, H) of wild‐type mice at indicated weeks after birth. All data reflect mean ± SD (n = 5 mice/time point from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance of differences between consecutive ages (*P < 0.05, **P < 0.01, ***P < 0.001).
To compare the frequency of functional HSCs between bone marrows from 4‐ and 8‐week‐old mice, we transplanted equal amount (300,000) of bone marrow cells from 4‐ or 8‐week‐old mice, together with 500,000 recipient‐type competitor bone marrow cells, into lethally irradiated recipient mice. The results showed that bone marrow cells from 4‐week‐old mice gave significantly higher levels of donor cell reconstitution in all major hematopoietic lineages than those from 8‐week‐old mice (Fig 1D and Appendix Fig S1A). This was consistent with the observation that phenotypic HSC frequency in the bone marrow was significantly higher in 4‐week‐old mice than in 8‐week‐old mice (Fig 1B).
To compare the absolute numbers of functional HSCs between bone marrows from 4‐ and 8‐week‐old mice, we transplanted equal percentage (0.5%) of whole bone marrow cells from 4‐ or 8‐week‐old mice, together with 500,000 recipient‐type competitor bone marrow cells, into lethally irradiated recipient mice. In this scenario, the bone marrow cells from 4‐ and 8‐week‐old mice displayed comparable levels of long‐term hematopoietic reconstitution activity in the recipient mice (Fig 1E). These data suggested that the bone marrows from 4‐ and 8‐week‐old mice contain equal amounts of functional HSCs, consistent with the flow cytometric analysis of phenotypic HSCs (Fig 1C).
To understand how HSC number in the bone marrow correlates with blood production in the periphery, we measured the complete blood counts of white blood cells (WBCs), red blood cells (RBCs), and platelet (PLT). The WBC counts correlated well with bone marrow cellularity (Fig 1A and F), which reached homeostasis at 8 weeks after birth. In contrast, the RBC count did not show any significant changes from 3 to 24 weeks after birth (Fig 1G). The PLT count reached homeostasis at around 6 weeks after birth (Fig 1H).
We also checked the expression of CD34 on HSCs during development and found a trend toward reduction from 3 to 8 weeks after birth (Appendix Fig S1B and C).
Many sex hormone receptors were expressed by hematopoietic stem and/or progenitor cells
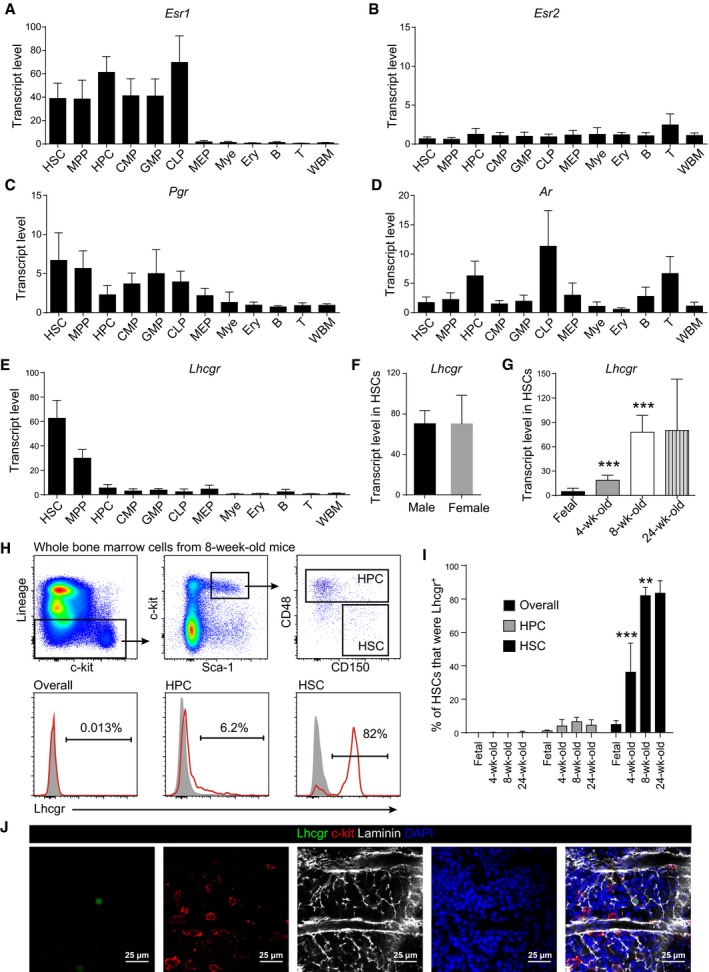
The HSC number in the bone marrow stopped expansion at 4 weeks after birth, which happens to be at the onset of mouse puberty. This raised the possibility that sex hormones play a role in regulating HSC homeostasis. To test this, we first examined the expression of sex hormone receptors in the hematopoietic hierarchy. Estrogen receptor α (encoded by Esr1) was highly expressed by HSCs, multipotent progenitors (MPPs; Kiel et al, 2005), hematopoietic progenitor cells (HPCs; Ding & Morrison, 2013), common myeloid progenitors (CMPs; Akashi et al, 2000), granulocyte–monocyte progenitors (GMPs; Akashi et al, 2000), and common lymphoid progenitors (CLPs; Kondo et al, 1997), but not megakaryocyte–erythroid progenitors (MEPs; Akashi et al, 2000), erythroid, myeloid, B cells, or T cells (Fig 2A, Appendix Fig S2A–C). In contrast, estrogen receptor β (encoded by Esr2) showed basal expression in all tested cell populations (Fig 2B). Progesterone receptor (Pgr) was lowly expressed by HSCs, MPPs, and some restricted progenitors, but not by terminally differentiated hematopoietic lineages (Fig 2C). Androgen receptor (Ar) was not expressed by HSCs or MPPs, but by HPCs and CLPs (Fig 2D).
Figure 2. Expression of sex hormone receptors and luteinizing hormone receptor in the hematopoietic system.
-
A–DQuantitative real‐time PCR analyses of the transcript levels (normalized to β‐actin) of Esr1 (A), Esr2 (B), Pgr (C), and Ar (D) in indicated cell populations relative to unfractionated whole bone marrow cells (WBM) in 8‐ to 12‐week‐old mice. The relative transcript level in WBM was set as 1. Data represent mean ± SD (n = 3 mice from three independent experiments).
-
E–GQuantitative real‐time PCR analysis of Lhcgr transcript level (normalized to β‐actin) relative to unfractionated whole bone marrow cells (WBM). Panel (E): different hematopoietic cell populations; panel (F): different sexes; panel (G): different ages. The relative transcript level in WBM was set as 1. Data represented mean ± SD (n = 6 mice from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance of differences between consecutive ages (***P < 0.001).
-
H, IFlow cytometric analysis of Lhcgr expression in pre‐fixed and permeabilized bone marrow cells. Panel (H) shows the gating strategy for quantifying the percentages of Lhcgr+ cells in different cell populations. The gray peaks in the histograms in (H) were isotype controls. Panel (I) shows the quantification and statistical analysis results (n = 6 mice/age from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance of differences between consecutive ages (**P < 0.01, ***P < 0.001).
-
JConfocal imaging of femur sections stained with anti‐Lhcgr, anti‐c‐kit, and anti‐laminin antibodies (n = 3 mice from three independent experiments).
Lhcgr highly restricted its expression to HSCs/MPPs in the hematopoietic system
Luteinizing hormone (LH) is also associated with the onset of puberty. It is essential for gonadal development in both male and female mice (Lei et al, 2001; Zhang et al, 2001; Huhtaniemi et al, 2002; Yarram et al, 2003). Administration of LH‐releasing hormone agonists increased lymphocyte number under homeostasis and enhanced lymphopoietic recovery following bone marrow transplantation (Mann et al, 1994; Goldberg et al, 2009), suggesting a potential role of LH in the hematopoietic system. However, we found that Lhcgr was not expressed by lymphocytes or lymphoid progenitors, but rather by the primitive HSCs and MPPs in adult mice (Fig 2E). The expression of Lhcgr by HSCs did not show sexual dimorphism in 8‐week‐old mice (Fig 2F). These data implied a potential effect of LH on HSCs.
To investigate whether the Lhcgr expression changes during development, we purified HSCs from fetal liver (E16.5) and postnatal bone marrows at various stages after birth and compared their transcript levels of Lhcgr. The fetal liver HSCs expressed basal level of Lhcgr (Fig 2G). The expression level of Lhcgr in bone marrow HSCs at 4 weeks after birth was significantly higher than that of fetal HSCs (Fig 2G). The Lhcgr expression kept increasing until 8 weeks after birth (Fig 2G). We were also able to detect the expression of Lhcgr protein on bone marrow HSCs by flow cytometric analysis of fixed and permeabilized bone marrow cells. Few WBM cells or HPCs expressed Lhcgr protein during development (Fig 2H and I). In contrast, Lhcgr increased its expression in HSCs during development and became stable at around 8 weeks after birth (Fig 2H and I). Confocal imaging of femur sections from 8‐week‐old mice with anti‐Lhcgr antibody detected rare staining that colocalized with c‐kit+ cells and were surrounded by laminin+ vessels (Fig 2J). These data suggested that the expression of Lhcgr on HSCs is activated after birth and peaks after sexual maturation.
Ovariectomy or castration did not affect HSC self‐renewal in the bone marrow
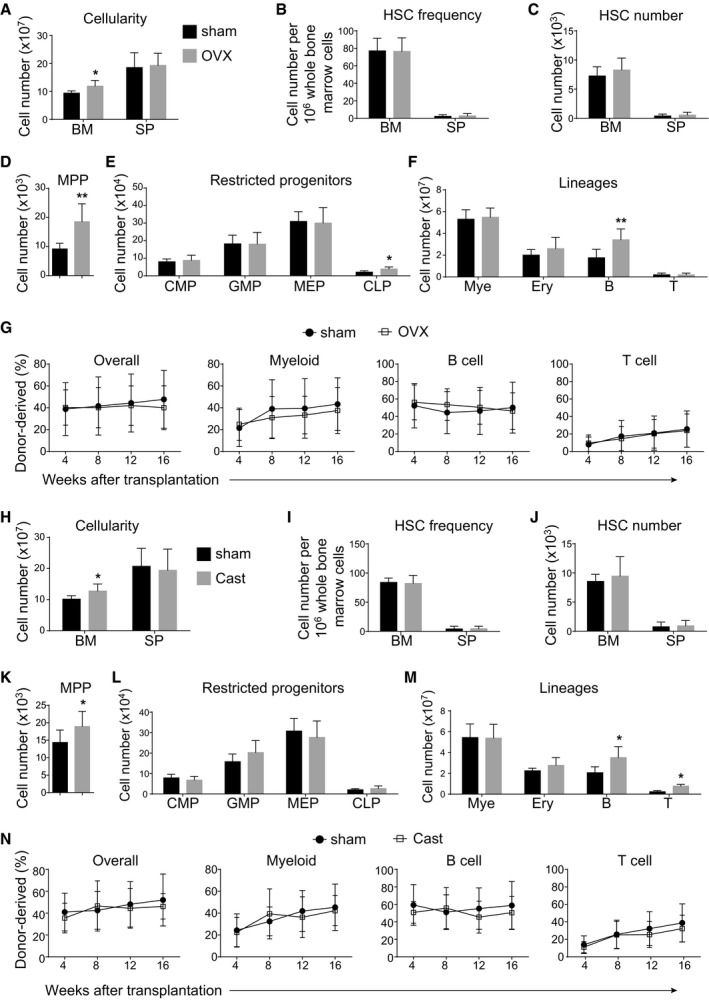
To directly test whether sex hormones regulate HSC homeostasis in the bone marrow, we performed ovariectomy surgery on 8‐week‐old female mice to block female sex steroid secretion. At 8 weeks after the surgery, we did not observe significant differences between ovariectomized and shammed groups in terms of spleen cellularity (Fig 3A), HSC frequency (Fig 3B), and number (Fig 3C) in the bone marrow or spleen. The ovariectomized mice had higher bone marrow cellularity (Fig 3A), more MPPs (Fig 3D), CLPs (Fig 3E), and B cells (Fig 3F) in the bone marrow than shammed mice. Bone marrow cells from ovariectomized mice were indistinguishable from control cells in their capacity to give long‐term multilineage reconstitution of irradiated mice (Fig 3G). Similar results were also obtained from male mice after castration (Fig 3H–N). Therefore, in line with previous studies (Erben et al, 1998; Li et al, 2013), our data indicated that sex steroid ablation increases MPPs and lymphopoiesis but not HSCs in the bone marrow.
Figure 3. Sex steroid ablation did not affect hematopoiesis or HSC homeostasis in adult bone marrow.
-
A–FBone marrow cellularity (A), HSC frequency (B), and numbers of HSCs (C), MPPs (D), restricted progenitors (E) and hematopoietic lineages (F) in the bone marrow (BM, two tibias + two femurs) and spleen (SP) from female mice at 8 weeks after sham or ovariectomy surgery (OVX). All data reflected mean ± SD (n = 6 mice/treatment from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance between ovariectomized and shammed groups (*P < 0.05, **P < 0.01).
-
GCompetitive reconstitution assay in which 300,000 of donor‐derived bone marrow cells from female mice at 8 weeks after sham or ovariectomy surgery were transplanted along with 300,000 recipient‐type competitor cells into irradiated recipient mice (n = 12 recipient mice/genotype from three independent experiments). Data represented mean ± SD. The statistical significance of differences was assessed using two‐way ANOVAs.
-
H–MBone marrow cellularity (H), HSC frequency (I), and numbers of HSCs (J), MPPs (K), restricted progenitors (L), and hematopoietic lineages (M) in the bone marrow (two tibias + two femurs) from male mice at 8 weeks after sham or castration surgery (Cast). All data reflected mean ± SD (n = 6 mice/treatment from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance between castrated and shammed groups (*P < 0.05).
-
NCompetitive reconstitution assay in which 300,000 of donor‐derived bone marrow cells from male mice at 8 weeks after sham or castration surgery were transplanted along with 300,000 recipient‐type competitor cells into irradiated recipient mice (n = 12 recipient mice/genotype from three independent experiments). Data represented mean ± SD. The statistical significance of differences was assessed using two‐way ANOVAs.
Consistent with previous study (Nakada et al, 2014), ovariectomy, but not castration, significantly reduced the incorporation of BrdU by HSCs after 10‐day BrdU administration (Appendix Fig S3A–C). Thus, estrogen regulates the cell cycle but not the self‐renewal of HSCs in the bone marrow.
We also performed ovariectomy or castration surgery on 4‐week‐old mice. At 8 weeks after the surgery, we still did not observe significant changes to the HSC numbers in the bone marrow and spleen (Appendix Fig S3D–G), suggesting that sex hormones are dispensable for HSC self‐renewal during puberty.
Lhcgr deletion increased HSC number and hematopoiesis in the bone marrow of 8‐week‐old, but not 4‐week‐old mice
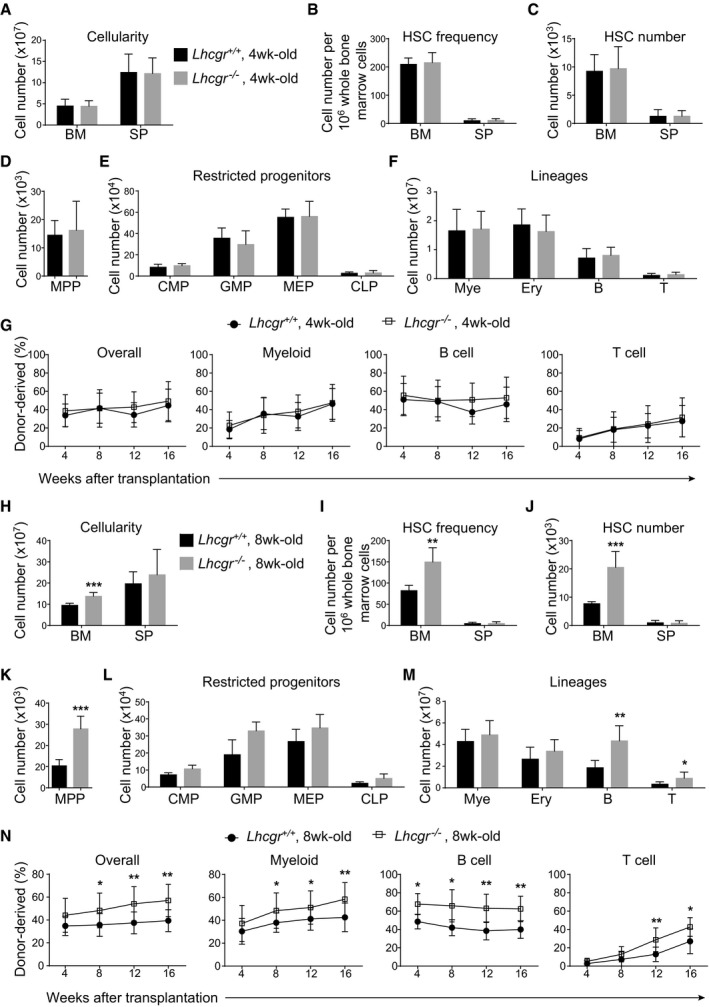
Using Lhcgr −/− mice (Zhang et al, 2001), we examined the effects of Lhcgr deletion on HSC self‐renewal and hematopoiesis in the bone marrow before and after sexual maturation. At 4 weeks after birth, the Lhcgr −/− mice had normal bone marrow and spleen cellularity (Fig 4A), normal HSC frequencies (Fig 4B), and numbers (Fig 4C) in the bone marrow and spleen and normal numbers of MPPs (Fig 4D), committed progenitors (Fig 4E), and mature cells (Fig 4F) in the bone marrow. The capacity of long‐term hematopoietic reconstitution capacity of bone marrow cells from Lhcgr −/− mice at 4 weeks after birth was not significantly different from that from control mice (Fig 4G). Together, these data suggested that Lhcgr is not required for HSC self‐renewal and hematopoiesis in the bone marrow of juvenile mice.
Figure 4. Lhcgr deletion increased HSC number and hematopoiesis in the bone marrow of 8‐week‐old but not 4‐week‐old mice.
-
A–FBone marrow cellularity (A), HSC frequency (B) and numbers of HSCs (C), MPPs (D), restricted progenitors (E), and hematopoietic lineages (F) in the bone marrow (two tibias + two femurs) from paired 4‐week‐old Lhcgr −/− and control mice. All data reflect mean ± SD (n = 6 mice/genotype from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice.
-
GCompetitive reconstitution assay in which 300,000 of donor‐derived bone marrow cells from 4‐week‐old Lhcgr −/− or control mice were transplanted along with 300,000 recipient‐type competitor cells into irradiated recipient mice (n = 12 recipient mice/genotype from three independent experiments). Data represented mean ± SD. The statistical significance of differences between Lhcgr −/− and control mice was assessed using two‐way ANOVAs.
-
H–MBone marrow cellularity (H), HSC frequency (I) and numbers of HSCs (J), MPPs (K), restricted progenitors (L), and hematopoietic lineages (M) in the bone marrow (two tibias + two femurs) from paired 8‐week‐old Lhcgr −/− and control mice. Data from male and female mice did not significantly differ from each other and were therefore pooled together. All data reflected mean ± SD (n = 6 mice/genotype from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice (*P < 0.05, **P < 0.01, ***P < 0.001).
-
NCompetitive reconstitution assay in which 300,000 of donor bone marrow cells from 8‐week‐old Lhcgr −/− or control mice were transplanted along with 300,000 recipient‐type competitor cells into irradiated recipient mice (n = 12 recipient mice/genotype from three independent experiments). Data represented mean ± SD. The statistical significance of differences between Lhcgr −/− and control mice was assessed using two‐way ANOVAs (*P < 0.05, **P < 0.01).
At 8 weeks after birth, the Lhcgr −/− mice showed significantly higher bone marrow cellularity (Fig 4H) and HSC frequency in the bone marrow (Fig 4I) than littermate controls. The overall number of HSCs in the bone marrow of 8‐week‐old Lhcgr −/− mice was approximately 2.7‐fold as high as normal level (Fig 4J). The 8‐week‐old Lhcgr −/− mice also had significantly higher numbers of MPPs, B cells, and T cells in the bone marrow than controls (Fig 4K–M). Notably, compared to controls, bone marrow cells from 8‐week‐old Lhcgr −/− mice conferred significantly higher levels of donor cell reconstitution in all major hematopoietic lineages upon transplantation into recipient mice (Fig 4N). These data demonstrated that LH signaling downregulates HSC expansion in the bone marrow during sexual maturation.
We then investigated whether Lhcgr deficiency had any effects on the bone marrow microenvironment that is known to regulate HSC maintenance (Morrison & Scadden, 2014). By flow cytometric analysis of enzymatically dissociated bone marrow cells, we found that 8‐week‐old Lhcgr −/− mice had normal frequencies of CD45/Ter119−VE‐cadherin+ endothelial cells (Fig 5A and B) and CD45/Ter119−PDGFRα+ perivascular cells (Fig 5C and D). By confocal imaging of 50‐μm‐thick femur sections (Fig 5E), we found that Lhcgr −/− mice were indistinguishable from control mice in terms of the densities of CD41+ megakaryocytes (Fig 5F), VE‐cadherinbrightLaminindim sinusoids (Fig 5G), and VE‐cadherindimLamininbright arterioles (Fig 5H). Lhcgr deletion did not have a significant effect on the expression of Scf and Cxcl12, two of the key niche factors, in endothelial cells and perivascular cells (Fig 5I and J).
Figure 5. Lhcgr deletion did not affect HSC niche function.
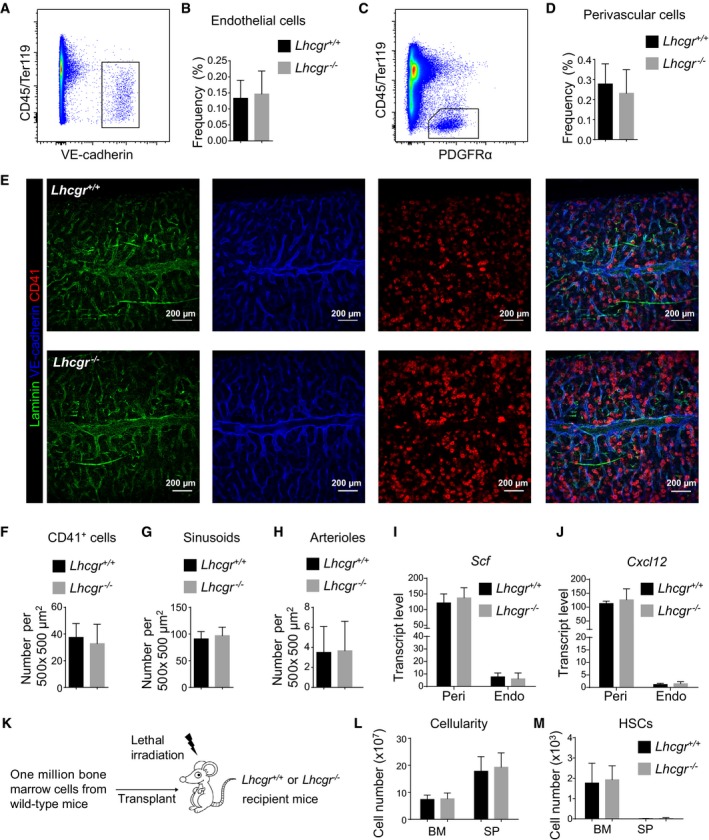
-
A–DFlow cytometric analyses showed that Lhcgr deletion did not alter the frequency of CD45/Ter119−VE‐cadherin+ endothelial cells (A,B) or CD45/Ter119−PDGFRα+ perivascular cells (C,D) in the bone marrow. Panels (A) and (C) show the gating strategies for analyzing endothelial cells and perivascular cells, respectively, by flow cytometry. Panels (B) and (D) show the quantification results for endothelial cells and perivascular cells, respectively (n = 6 mice from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice.
-
E–HConfocal imaging of thick bone marrow sections (50 μm) showed no significant changes to the frequencies of CD41+ megakaryocytes (F), VE‐cadherinbrightLaminindim sinusoids (G) or VE‐cadherindimLamininbright arterioles (H). Representative confocal images are shown in (E) (n = 6 mice/genotype from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice.
-
I, JQuantitative real‐time PCR analyses of the transcript levels (normalized to β‐actin) of Scf (I) and Cxcl12 (J) in perivascular cells (Peri) and endothelial cells (Endo). Data represented mean ± SD (n = 3 mice from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice.
-
K–MBone marrow and spleen cellularity (L) and HSC numbers in the bone marrow and spleen (M) from 12‐week‐old Lhcgr −/− and control mice at 4 weeks after lethal irradiation and transplantation with one million bone marrow cells from wild‐type mice. Transplantation was performed as depicted in (K) (n = 6 mice/genotype from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice.
Next, we did transplantation assay in which one million bone marrow cells from wild‐type mice were transplanted into lethally irradiated Lhcgr −/− mice and their littermate controls. The results showed that neither the bone marrow cellularity nor the HSC numbers in those recipient mice were affected by Lhcgr deletion (Fig 5K–M). These results further supported the idea that lack of Lhcgr does not have a significant effect on the bone marrow microenvironment that maintains HSCs.
We traced the cycled cells by treating the mice with BrdU. As expected, most HSCs were BrdU+ in 4‐week‐old mice (Appendix Fig S4A and B). In contrast, in 8‐week‐old mice, most HSCs were BrdU− (Appendix Fig S4A and B). For Lhcgr‐deficient mice, the trend was similar to that in wild‐type mice (Appendix Fig S4A and B). These results excluded the possibility that Lhcgr‐null HSCs do not acquire quiescence during puberty. In addition, we also found that Lhcgr‐null HSCs were even more quiescent than wild‐type HSCs in 8‐week‐old female mice (Appendix Fig S4B). This is likely due to the essential role of LH in estrogen signaling, which is known to mediate a sexual dimorphic effect on HSC proliferation (Nakada et al, 2014; Appendix Fig S3A–C).
Lhcgr regulated HSC expansion independent of sex hormones
In both sexes, LH stimulates secretion of sex steroids from the gonads. To test whether the regulation of HSC self‐renewal by Lhcgr depends on downstream sex steroids, we sought out to investigate the effects of Lhcgr deletion on HSC self‐renewal in sex steroid‐ablated mice. At 8 weeks after ovariectomy of 4‐week‐old females, the Lhcgr −/− mice had significantly higher bone marrow cellularity (Fig 6A) and significantly higher HSC frequency and number in the bone marrow (Fig 6B and C) than Lhcgr +/+ mice. Similarly, at 8 weeks after castration of 4‐week‐old males, the Lhcgr −/− mice had significantly higher bone marrow and spleen cellularity (Fig 6D) and significantly higher HSC frequency and number in the bone marrow (Fig 6E and F) than Lhcgr +/+ male mice. These data suggested that sex hormones are dispensable for the regulation of HSC expansion by Lhcgr.
Figure 6. HSCs are likely a direct target of LH in the bone marrow.
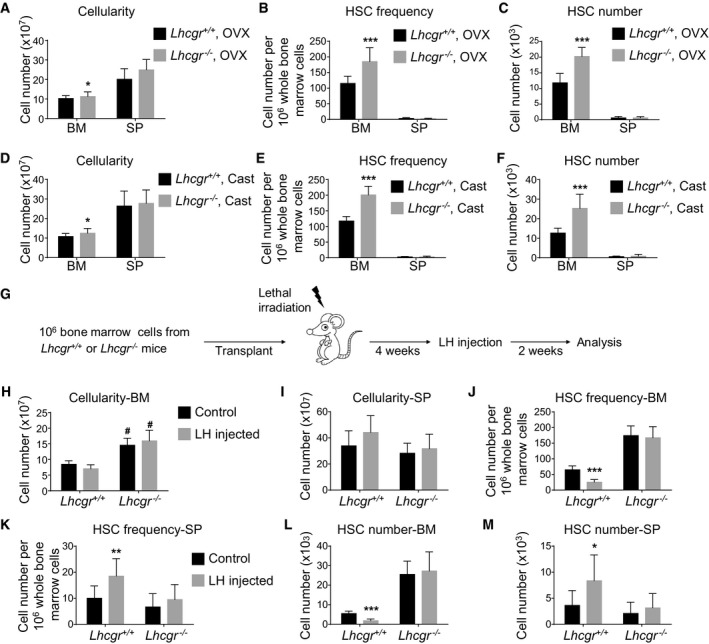
-
A–CBone marrow cellularity (A), HSC frequency (B), and number (C) in the bone marrow from Lhcgr −/− and control mice at 8 weeks after ovariectomy (OVX) (n = 6 mice/genotype from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice (*P < 0.05, ***P < 0.001).
-
D–FBone marrow cellularity (D), HSC frequency (E), and number (F) in the bone marrow from Lhcgr −/− and control mice at 8 weeks after castration (Cast) (n = 6 mice/genotype from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice (*P < 0.05, ***P < 0.001).
-
G–MCellularity of bone marrow (H) and spleen (I), HSC frequencies in the bone marrow (J) and spleen (K), and HSC numbers in the bone marrow (L) and spleen (M) of wild‐type mice that had been lethally irradiated and transplanted with donor bone marrow cells from Lhcgr −/− or control mice. Transplantation and LH injection were performed as depicted in (G) (n = 6 mice/condition/genotype from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance. * represents difference between groups with and without LH injection; # represents difference between Lhcgr −/− and control mice (*P < 0.05, #P < 0.05, **P < 0.01, ***P < 0.001).
Hematopoietic cell‐specific Lhcgr deletion blocked the effects of LH on HSCs
To test whether HSCs are a direct target of LH, we generated chimeric mice with hematopoietic cell‐specific Lhcgr deletion. We transplanted one million bone marrow cells from Lhcgr −/− or Lhcgr +/+ mice into lethally irradiated wild‐type mice. At 4 weeks after transplantation, those recipient mice received recombinant LH administration for 14 consecutive days before analysis (Fig 6G). In the mice transplanted with Lhcgr +/+ bone marrow cells (control group), 2‐week LH treatment did not affect the bone marrow or spleen cellularity (Fig 6H and I), but significantly reduced HSC number in the bone marrow (Fig 6J and K) and increased HSC number in the spleen (Fig 6L and M). These data suggested that LH administration depletes HSCs from the bone marrow.
When LH was administered to lethally irradiated mice that were transplanted with Lhcgr −/− bone marrow cells, there were no significant changes to any hematopoietic parameters, including bone marrow and spleen cellularity (Fig 6H and I), HSC frequencies, and numbers in the bone marrow and spleen (Fig 6J–M). These data indicate that Lhcgr expression on hematopoietic cells is required for the action of LH on HSC self‐renewal. Notably, mice receiving bone marrow cells from Lhcgr −/− mice had significantly higher bone marrow cellularity than controls, irrespective of LH injection (Fig 6H), supporting the idea that Lhcgr deficiency affects HSC numbers in a cell‐intrinsic way.
Lhcgr deletion led to leukocytosis in the peripheral blood
We investigated how altered HSC number and hematopoiesis in the bone marrow affected blood production in Lhcgr −/− mice. The results showed no significant difference of WBC counts between Lhcgr −/− and Lhcgr +/+ mice at 3 or 4 weeks after birth (Fig 7A). However, the WBC counts in Lhcgr −/− mice were constantly higher than that in Lhcgr +/+ mice at 6 weeks after birth (Fig 7A). At 8 weeks after birth and thereafter, WBC counts in Lhcgr −/− mice went beyond 20,000/μl, over twofold as high as normal level (Fig 7A). The increased WBC counts in Lhcgr −/− mice were evident in all white blood cell lineages, including neutrophils (Fig 7D), lymphocytes (Fig 7E), monocytes (Fig 7F), eosinophils (Fig 7G), and basophils (Fig 7H). In contrast, the RBC and PLT counts in the peripheral blood were comparable between Lhcgr −/− and Lhcgr +/+ mice from 3 to 24 weeks after birth (Fig 7B and C). Therefore, Lhcgr deletion leads to excessive white blood cell production that mimics leukocytosis symptom.
Figure 7. Lhcgr deletion led to leukocytosis in the peripheral blood.
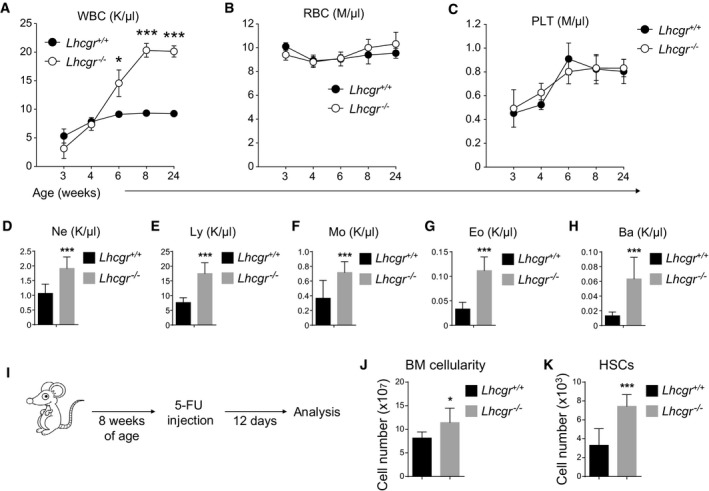
-
A–CPeripheral white blood cell count (WBC, A), red blood cell count (RBC, B), and platelet count (PLT, C) of Lhcgr −/− and control mice at indicated ages. All data reflected mean ± SD (n = 6 mice/age/genotype from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance of differences between Lhcgr −/− and control mice (*P < 0.05, ***P < 0.001).
-
D–HPeripheral neutrophil (Ne, D), lymphocyte (Ly, E), monocyte (Mo, F), eosinophil (Eo, G), and basophil (Ba, H) counts of 8‐week‐old Lhcgr −/− and control mice. All data reflected mean ± SD (n = 6 mice/genotype from three independent experiments). Two‐tailed Student's t‐tests were used to assess the statistical significance of differences between Lhcgr −/− and control mice (***P < 0.001).
-
I–KCellularity of bone marrow (J) and HSC numbers in the bone marrow (K) of Lhcgr −/− and control mice that had been treated with one dose of 5‐FU 12 days before. 5‐FU treatment was performed as described in (I) (n = 6 mice/genotype from three independent experiments). Data represented mean ± SD. Two‐tailed Student's t‐tests were used to assess the statistical significance between Lhcgr −/− and control mice (*P < 0.05, ***P < 0.001).
We treated 8‐week‐old mice with 5‐FU to drive quiescent cells into cell cycle. At 12 days after the treatment, we observed significantly higher bone marrow cellularity and higher HSC frequency in the bone marrow of Lhcgr −/− mice (Fig 7I–K). Thus, Lhcgr regulates HSCs under normal condition and after replicative stress as well. This is consistent with a recent paper reporting that chemical suppression of LH promoted HSC regeneration after hematopoietic stresses (Velardi et al, 2018).
Lhcgr deletion accelerated leukemogenesis
To investigate whether the abnormally elevated hematopoiesis in Lhcgr‐deficient mice relates to leukemia development, we used a transplantable murine AML model driven by the MLL‐AF9 oncogene (Krivtsov et al, 2006). Lineage− hematopoietic stem and progenitor cells purified from 5‐fluorouracil‐pretreated Lhcgr −/− and Lhcgr +/+ mice were infected with MLL‐AF9 retroviruses, followed by the transplantation into lethally irradiated recipient mice. Four weeks after transplantation, the frequency of YFP+ Lhcgr −/− donor leukemic cells was significantly higher than that from Lhcgr +/+ mice (11.7% versus 32.5% Fig 8A and B). This AML model mainly expressed myeloid cell markers of Mac‐1 and Gr‐1, but rarely in lymphoid markers of B220 and CD3 (Fig 8C). Lhcgr −/− leukemic cells exhibited a notable decrease in differentiation as evidenced by the much lower frequency of Mac‐1+Gr‐1+ cells compared with Lhcgr +/+ counterparts (Gr‐1 level represents the extent of differentiation of leukemia cells, Fig 8C and D). More severe leukemic cell infiltration was also observed in the spleens and livers of the recipient mice receiving MLL‐AF9‐transduced Lhcgr −/− hematopoietic stem progenitor cells than the control ones (Fig 8E–G).
Figure 8. Lhcgr deletion accelerated leukemogenesis in MLL‐AF9‐induced AML model.
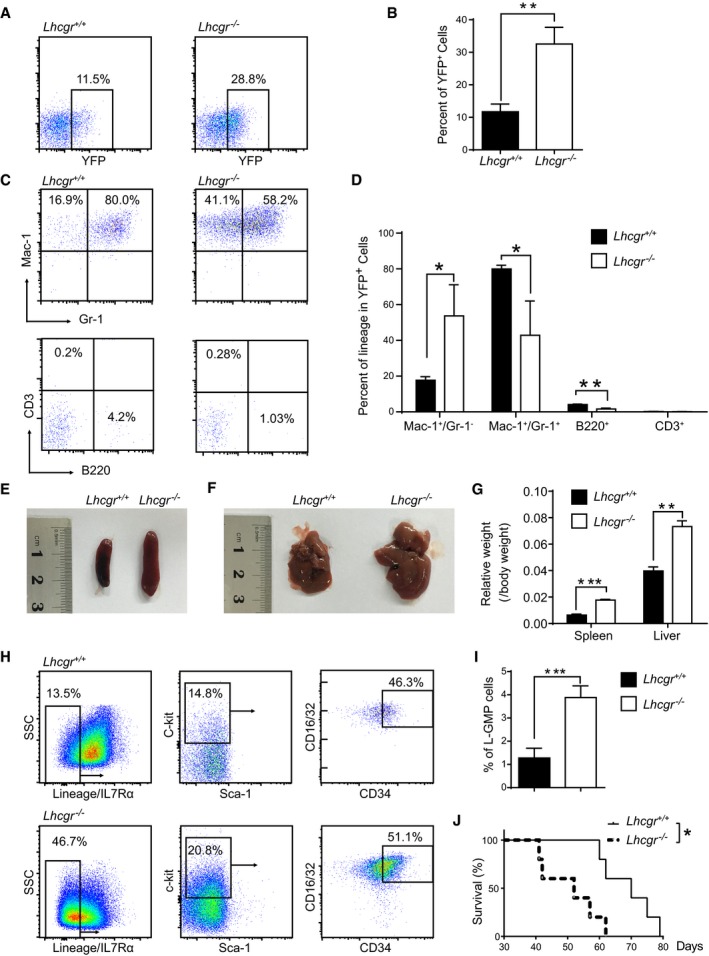
-
ARepresentative plots of YFP+ leukemia cells in peripheral blood of primary recipients transplanted with MLL‐AF9‐induced Lineage− hematopoietic stem/progenitor cells from Lhcgr −/− and control mice.
-
BQuantification data in panel (A) (n = 5 mice from three independent experiments).
-
CRepresentative plots of Mac‐1/Gr‐1 (marker of myeloid cells) and CD3/B220 (marker of lymphoid cells) in the peripheral blood of primary leukemic recipients.
-
DQuantification results in panel (C) (n = 5 mice from three independent experiments).
-
E, FRepresentative images of spleens (E) and livers (F) of the primary leukemic recipients.
-
GQuantification results of relative weight of spleens and livers in panels (E, F) (n = 5 mice from three independent experiments).
-
HRepresentative plots of Lineage−IL7Rα−Sca‐1−c‐kit+ CD34+ CD16/32+L‐GMP cells in the bone marrow of primary leukemic recipients.
-
IQuantification of the percentages of L‐GMP cells in panel (G) (n = 5 mice from three independent experiments).
-
JSurvival data for recipient mice receiving MLL‐AF9‐induced control and Lhcgr‐null hematopoietic stem/progenitor cells upon primary transplantation (n = 5 Lhcgr −/− and control).
We further examined how LH signaling affects the frequency of leukemia‐initiating cells (LICs) in the MLL‐AF9‐induced leukemia model. As shown in Fig 8H and I, the frequency of Lhcgr −/− immunophenotypic Lineage−IL7Rα−Sca‐1−c‐kit+CD34+FcγR+ L‐GMP cells, which were reported to be enriched for LICs (Somervaille & Cleary, 2006), was approximately threefold higher than Lhcgr +/+ ones (1.3% versus 3.9%). More strikingly, recipient mice that received MLL‐AF9‐transduced Lhcgr −/− donor cells had a significantly shortened overall survival than that of control recipients (66 days versus 52 days, Fig 8J). Taken together, these data indicate that Lhcgr inhibits the leukemia development in an MLL‐AF9‐induced AML model.
Discussion
Previous reports have identified a series of intrinsic factors that are involved in the regulation of HSC properties during development (Copley & Eaves, 2013). We hypothesized that extrinsic factors are important for integrating developmental cues into HSC properties, such as self‐renewal and differentiation. In this study, we provided evidence that LH signaling controls the HSC number during the period of mouse sexual maturation. We showed that deletion of Lhcgr caused an over twofold enlargement of the HSC pool in the bone marrow (Fig 4I, J and N), which was accompanied with significantly increased bone marrow cellularity (Fig 4H) and leukocytosis in the peripheral blood (Fig 7A–H). Clinically, leukocytosis is a potential risk factor of thrombosis (Carobbio et al, 2007; De Stefano et al, 2010) and many inflammatory diseases (Erlinger et al, 2003; Karthikeyan & Lip, 2006; Bartkeviciene et al, 2013; Jo et al, 2013). The abnormally elevated hematopoiesis in mice was also associated with faster leukemia development in an MLL‐AF9‐induced AML model (Fig 8). Thus, control of the HSC number during puberty is physiologically important for normal hematopoiesis.
HSCs are likely a direct target of LH. Previous work has detected Lhcgr expression in Sca‐1+Lineage−CD45+ hematopoietic cells (Mierzejewska et al, 2015). We showed that Lhcgr was highly expressed by CD150+/−CD48−Lineage−Sca‐1+c‐kit+ HSCs/MPPs, but not by HPCs, restricted progenitors or mature hematopoietic cells (Fig 2E–J). The timing of Lhcgr activation in HSCs correlated with the action of LH on HSCs (Fig 2G and H). Deletion of Lhcgr did not have a significant effect on the HSC‐supporting microenvironment (Fig 5A–J). Finally, transplantation of wild‐type bone marrow cells into lethally irradiated Lhcgr −/− mice and control mice had similar bone marrow cellularity and HSC number (Fig 5K–M).
The increased numbers of MPPs, B cells, and/or T cells in the bone marrow of Lhcgr −/− mice (Fig 4K and M) phenocopied those observed in ovariectomized female or castrated male mice (Fig 3D, F, K and M). Considering that CLPs or lymphocytes did not express Lhcgr (Fig 2E), the effects of LH on lymphocytes are likely attributed to downstream sex hormones.
We only observed increased counts in WBC but not RBC or PLT in Lhcgr −/− mice (Fig 7A–C). One explanation is that RBC and PLT counts are more tightly controlled than WBC counts. There might be a negative feedback by downstream progenitors that balanced the increased HSC number in erythrocyte and platelet formation in Lhcgr −/− mice. Second possibility is that Lhcgr signaling affects the myeloid/lymphoid differentiation but not erythroid/megakaryocytic differentiation of HSCs. The third possibility is that the Lhcgr+ HSCs may be myeloid‐/lymphoid‐primed HSCs. These are all interesting possibilities that will open many avenues for future research.
Materials and Methods
Mice
Wild‐type, Lhcgr +/+, and Lhcgr −/− mice (Zhang et al, 2001) were housed in the Animal Facility at Shanghai Institute of Biochemistry and Cell Biology (SIBCB), Chinese Academy of Sciences. For BrdU incorporation assays, mice were given an intraperitoneal injection of 1 mg BrdU (Sigma) per 6 g of body mass in PBS and maintained in 1 mg/ml of BrdU in the drinking water for indicated days. For castration in male mice, an incision was made in the scrotum and the testis and attached testicular fat pads were pulled out of the incision. Spermatochords were individually ligated with absorbable sutures (4‐0 chromic gut), then excised, and then, 1–3 non‐absorbable sutures (3‐0 Tevdek II) were used to close the skin. For ovariectomy in female mice, skin around the dorsal midline caudal to the posterior borders of the ribs was shaved and an incision was made to expose the ovaries on each side. The ovaries were isolated, ligated with absorbable sutures (4‐0 chromic gut), and excised, and then, three to four non‐absorbable sutures were used to close the skin. Sham‐treated mice underwent similar surgeries except that the gonads were left intact. For LH treatment, mice were injected with recombinant hLH (5 IU/mice/day) for 14 consecutive days. To induce replicative stress by 5‐FU, mice were intraperitoneally administered one dose of 5‐FU (8 days apart; 150 mg/kg/day). For all mouse experiments, no formal randomization techniques or blinding was used. All procedures were approved by the Institutional Animal Care and Use Committees of SIBCB.
Flow cytometry
Bone marrow cells were dissociated to a single‐cell suspension by passing through a 25‐G needle and then filtering through a 40‐μm nylon mesh. HSCs were identified by the following antibodies: anti‐CD150 (TC15‐12F12.2), anti‐CD48 (HM48‐1), anti‐Sca1 (E13‐161.7), anti‐c‐kit (2B8) and lineage antibody cocktail (anti‐Ter119 (TER‐119), anti‐B220 (6B2), anti‐Gr‐1 (8C5), anti‐CD2 (RM2‐5), anti‐CD3 (17A2), anti‐CD5 (53‐7.3), and anti‐CD8 (53‐6.7)). DAPI was used to exclude dead cells. Antibodies were from eBioscience or BioLegend. For the staining of anti‐Lhcgr antibody (Bioss, bs‐0984R), cells were pre‐fixed by 0.01% formaldehyde for 15 min and permeabilized by 0.5% Triton X‐100 in PBS.
Long‐term competitive reconstitution assay
Eight‐ to 12‐week‐old recipient mice were irradiated using a RS2000 X‐ray irradiator with two doses of 540 rad (total 1,080 rad) delivered at least 2 h apart. Cells were transplanted intravenously into the retro‐orbital venous sinus of anesthetized mice. Recipient mice were periodically bled to assess the percentages of donor‐derived blood cells. Antibodies including anti‐CD45.2 (104), anti‐CD45.1 (A20), anti‐Ter119 (TER‐119), anti‐Gr‐1 (8C5), anti‐Mac‐1 (M1/70), anti‐B220 (6B2), and anti‐CD3 (KT31.1) were used to stain cells for analysis by flow cytometry.
Quantitative real‐time PCR
Cells were sorted directly into TRIzol (Life Technologies). RNA was reverse‐transcribed using SuperScript III Reverse Transcriptase (Life Technologies). Quantitative real‐time PCR was performed using SYBR green on a LightCycler 96 system (Roche). Primer sequences were listed in Appendix Table S1.
Bone sectioning, immunostaining, and confocal imaging
Freshly dissected bones were fixed in 4% paraformaldehyde overnight followed by 3‐day decalcification in 10% EDTA. Bones were sectioned using the CryoJane tape‐transfer system (Instrumedics). Sections were stained overnight with anti‐CD41‐APC (eBioscience, clone eBioMWReg30, 1:200), goat‐anti‐c‐kit (R&D, AF1356, 1:400), rabbit‐anti‐Lhcgr (Bioss, bs‐0984R, 1:50), and/or rabbit‐anti‐laminin (Abcam, ab11575, 1:400) antibodies. Donkey‐anti‐goat Alexa Fluor 488 and/or Donkey‐anti‐rabbit Alexa Fluor 555 were used as secondary antibodies (Life Technologies, 1:400). Slides were mounted with Anti‐fade Prolong Gold (Life Technologies), and images were acquired with a Leica SP8 confocal microscope.
Establishment and analysis for murine acute myeloid leukemia model
MSCV‐MLL‐AF9‐IRES‐YFP encoding plasmid was co‐transfected with a pCL‐ECO packaging plasmid (2:1) into 293T cells to produce retroviruses. Lineage− cells were isolated from 5‐fluorouracil‐pretreated Lhcgr −/− and Lhcgr +/+ mice and infected with MLL‐AF9 retroviruses. 2 × 105 infected cells were transplanted into lethally irradiated recipient mice by retro‐orbital injection. To evaluate leukemia development, myeloid or lymphoid lineages were analyzed by staining with Mac‐1, Gr‐1, CD3, and/or B220 antibodies. To examine the leukemia‐initiating cell frequency, Lineage−IL7Rα−Sca‐1−c‐kit+CD34+CD16/32+ immunophenotypic L‐GMP cells were determined by flow cytometric analysis. Overall survival was recorded and analyzed using log‐rank test.
Author contributions
YJP and HY performed most of the experiments in normal mice. XH performed experiments that illustrated the roles of Lhcgr in leukemia development. WD, XY, and ML maintained mouse colonies and completed all genotyping work. JZ and BOZ designed the experiments and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Review Process File
Acknowledgements
We thank H. Cheng for his kind discussion and suggestions, W. Bian for technical support in flow cytometry, and Y. Chen for technical support in confocal imaging. This work was supported by the National Key Program on Stem Cell and Translational Research (2017YFA0106400), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16020202), the National Natural Science Foundation of China (31771637, 81730006, 81570093), and the SKLEH‐Pilot Research Grant (ZK17‐04).
The EMBO Journal (2018) 37: e98984
Contributor Information
Junke Zheng, Email: zhengjunke@shsmu.edu.cn.
Bo O Zhou, Email: bo.zhou@sibcb.ac.cn.
References
- Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197 [DOI] [PubMed] [Google Scholar]
- Bartkeviciene D, Pilypiene I, Drasutiene G, Bausyte R, Mauricas M, Silkunas M, Dumalakiene I (2013) Leukocytosis as a prognostic marker in the development of fetal inflammatory response syndrome. Libyan J Med 8: 21674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ (2006) Hematopoietic stem cells proliferate until after birth and show a reversible phase‐specific engraftment defect. J Clin Invest 116: 2808–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ (2007) Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci USA 104: 5878–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, Frenette PS (2014) Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20: 1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846 [DOI] [PubMed] [Google Scholar]
- Carobbio A, Finazzi G, Guerini V, Spinelli O, Delaini F, Marchioli R, Borrelli G, Rambaldi A, Barbui T (2007) Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood 109: 2310–2313 [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez‐Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS (2011) Bone marrow CD169 + macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208: 261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC (2011) Expression of the G‐CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G‐CSF in mice. J Exp Med 208: 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley MR, Eaves CJ (2013) Developmental changes in hematopoietic stem cell properties. Exp Mol Med 45: e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, Mico C, Tieghi A, Cacciola RR, Santoro C, Gerli G, Guglielmelli P, Pieri L, Scognamiglio F, Rodeghiero F, Pogliani EM, Finazzi G, Gugliotta L, Leone G, Barbui T et al (2010) Leukocytosis is a risk factor for recurrent arterial thrombosis in young patients with polycythemia vera and essential thrombocythemia. Am J Hematol 85: 97–100 [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED (2001) Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol 13: 553–558 [DOI] [PubMed] [Google Scholar]
- Erben RG, Raith S, Eberle J, Stangassinger M (1998) Ovariectomy augments B lymphopoiesis and generation of monocyte‐macrophage precursors in rat bone marrow. Am J Physiol 274: E476–E483 [DOI] [PubMed] [Google Scholar]
- Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H (2001) Role of oestrogen receptors alpha and beta in immune organ development and in oestrogen‐mediated effects on thymus. Immunology 103: 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlinger TP, Tarver‐Carr ME, Powe NR, Appel LJ, Coresh J, Eberhardt MS, Brancati FL (2003) Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am J Kidney Dis 42: 256–263 [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco‐Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A (2009) IFNalpha activates dormant haematopoietic stem cells in vivo . Nature 458: 904–908 [DOI] [PubMed] [Google Scholar]
- Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen‐Kiang S, Boyd RL, van den Brink MR (2009) Luteinizing hormone‐releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol 182: 5846–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem‐cell maintenance. Nature 495: 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Kim I, Lim MS, Morrison SJ (2011) Sox17 expression confers self‐renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev 25: 1613–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH (2004a) Gfi‐1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431: 1002–1007 [DOI] [PubMed] [Google Scholar]
- Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, Orkin SH (2004b) Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev 18: 2336–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I, Zhang FP, Kero J, Hamalainen T, Poutanen M (2002) Transgenic and knockout mouse models for the study of luteinizing hormone and luteinizing hormone receptor function. Mol Cell Endocrinol 187: 49–56 [DOI] [PubMed] [Google Scholar]
- Jo JY, Lee MY, Lee JW, Rho BH, Choi WI (2013) Leukocytes and systemic inflammatory response syndrome as prognostic factors in pulmonary embolism patients. BMC Pulm Med 13: 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan VJ, Lip GY (2006) White blood cell count and hypertension. J Hum Hypertens 20: 310–312 [DOI] [PubMed] [Google Scholar]
- Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal‐Estape A, Pinho S, Ciero P, Nakahara F, Ma'ayan A, Bergman A, Merad M, Frenette PS (2016) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351: 176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong DM, Dudakov JA, Hammett MV, Jurblum MI, Khong SM, Goldberg GL, Ueno T, Spyroglou L, Young LF, van den Brink MR, Boyd RL, Chidgey AP (2015) Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Rep 4: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121 [DOI] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ (2007) Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130: 470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91: 661–672 [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, Golub TR, Armstrong SA (2006) Transformation from committed progenitor to leukaemia stem cell initiated by MLL‐AF9. Nature 442: 818–822 [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV (2001) Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol 15: 184–200 [DOI] [PubMed] [Google Scholar]
- Lessard J, Faubert A, Sauvageau G (2004) Genetic programs regulating HSC specification, maintenance and expansion. Oncogene 23: 7199–7209 [DOI] [PubMed] [Google Scholar]
- Li JY, Adams J, Calvi LM, Lane TF, Weitzmann MN, Pacifici R (2013) Ovariectomy expands murine short‐term hemopoietic stem cell function through T cell expressed CD40L and Wnt10B. Blood 122: 2346–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DR, Ansari AA, Akinbami MA, Wallen K, Gould KG, McClure HM (1994) Neonatal treatment with luteinizing hormone‐releasing hormone analogs alters peripheral lymphocyte subsets and cellular and humorally mediated immune responses in juvenile and adult male monkeys. J Clin Endocrinol Metab 78: 292–298 [DOI] [PubMed] [Google Scholar]
- Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW (2001) Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol 2: 718–724 [DOI] [PubMed] [Google Scholar]
- Mierzejewska K, Borkowska S, Suszynska E, Suszynska M, Poniewierska‐Baran A, Maj M, Pedziwiatr D, Adamiak M, Abdel‐Latif A, Kakar SS, Ratajczak J, Kucia M, Ratajczak MZ (2015) Hematopoietic stem/progenitor cells express several functional sex hormone receptors‐novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells Dev 24: 927–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola HK, Orkin SH (2006) The journey of developing hematopoietic stem cells. Development 133: 3733–3744 [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT (2014) The bone marrow niche for haematopoietic stem cells. Nature 505: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ (2014) Oestrogen increases haematopoietic stem‐cell self‐renewal in females and during pregnancy. Nature 505: 555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF (2003) Bmi‐1 is required for maintenance of adult self‐renewing haematopoietic stem cells. Nature 423: 302–305 [DOI] [PubMed] [Google Scholar]
- Sanchez‐Aguilera A, Arranz L, Martin‐Perez D, Garcia‐Garcia A, Stavropoulou V, Kubovcakova L, Isern J, Martin‐Salamanca S, Langa X, Skoda RC, Schwaller J, Mendez‐Ferrer S (2014) Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady‐state hematopoiesis. Cell Stem Cell 15: 791–804 [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML (2006) Identification and characterization of leukemia stem cells in murine MLL‐AF9 acute myeloid leukemia. Cancer Cell 10: 257–268 [DOI] [PubMed] [Google Scholar]
- Thurmond TS, Murante FG, Staples JE, Silverstone AE, Korach KS, Gasiewicz TA (2000) Role of estrogen receptor alpha in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology 141: 2309–2318 [DOI] [PubMed] [Google Scholar]
- Velardi E, Tsai JJ, Radtke S, Cooper K, Argyropoulos KV, Jae‐Hung S, Young LF, Lazrak A, Smith OM, Lieberman S, Kreines F, Shono Y, Wertheimer T, Jenq RR, Hanash AM, Narayan P, Lei Z, Moore MA, Kiem HP, van den Brink MRM et al (2018) Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat Med 24: 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmson AS, Stubelius A, Borjesson AE, Wu J, Stern A, Malin S, Martensson IL, Ohlsson C, Carlsten H, Tivesten A (2015) Androgens regulate bone marrow B lymphopoiesis in male mice by targeting osteoblast‐lineage cells. Endocrinology 156: 1228–1236 [DOI] [PubMed] [Google Scholar]
- Wilson CA, Mrose SA, Thomas DW (1995) Enhanced production of B lymphocytes after castration. Blood 85: 1535–1539 [PubMed] [Google Scholar]
- Wilson A, Trumpp A (2006) Bone‐marrow haematopoietic‐stem‐cell niches. Nat Rev Immunol 6: 93–106 [DOI] [PubMed] [Google Scholar]
- Yarram SJ, Perry MJ, Christopher TJ, Westby K, Brown NL, Lamminen T, Rulli SB, Zhang FP, Huhtaniemi I, Sandy JR, Mansell JP (2003) Luteinizing hormone receptor knockout (LuRKO) mice and transgenic human chorionic gonadotropin (hCG)‐overexpressing mice (hCG alphabeta+) have bone phenotypes. Endocrinology 144: 3555–3564 [DOI] [PubMed] [Google Scholar]
- Ye M, Zhang H, Amabile G, Yang H, Staber PB, Zhang P, Levantini E, Alberich‐Jorda M, Zhang J, Kawasaki A, Tenen DG (2013) C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat Cell Biol 15: 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I (2001) Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 15: 172–183 [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841 [DOI] [PubMed] [Google Scholar]
- Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L (2014) Megakaryocytes maintain homeostatic quiescence and promote post‐injury regeneration of hematopoietic stem cells. Nat Med 20: 1321–1326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Review Process File


