Abstract
Although induction of CD8+ responses is widely accepted as critical in clearing viral infections and necessary for effective vaccines against viruses, much less is known regarding the role of these cells in bacterial and other infections, particularly those that enter the host via the gastrointestinal tract. In this commentary, I discuss the likelihood that CD8+ responses are also important in protection from intestinal Gram-negative bacteria, as well as the many factors that should be taken into consideration during the development of vaccines, based on eliciting long-term protection predominantly mediated by CD8+ responses against these organisms.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
It is widely accepted that vaccination is one of the most effective strategies ever developed to prevent and combat infections. Despite some stunning successes (e.g., eradication of smallpox, near eradication of polio) (Minor 2015), much remains to be learned regarding the basis of protective immunity (Levine and Sztein 2004). This is critical to accelerate the development of new-generation vaccines as well as to improve vaccines against organisms for which only moderately effective vaccines are available. One of the main problems in addressing this important issue is that protective immunity depends, to a large extent, on the interactions of each particular infectious organism with the human host. In other words, in this case, one size does not fit all. To complicate matters even further, for many infections that are human–host restricted, there are no reliable animal models that faithfully reproduce the human disease. In these cases, by necessity, we have to rely largely on human experimentation, which is limited not only by strict regulatory guidelines but also by accessibility to the human tissues that are the main targets for entry, as well as the persistence of individual pathogens. This is a major limitation, because circulating immune cells (blood is one of only a handful of tissues readily accessible in humans) do not necessarily reflect the responses of “resident” immune cells present in the local tissues (e.g., T resident [Tr]) (Park and Kupper 2015). In fact, each particular microenvironment is occupied by immune cell subsets, which have down-regulated or up-regulated defined sets of genes that allow them to remain in place (sometimes long-term, e.g., Tr), and interact with “nonimmune” cells types (e.g., intestinal epithelial cells [IECs], stromal cells), which are also critical for eliciting and maintaining appropriate immune responses (Hooper 2015). Equally complex are the cell subsets present in secondary lymphoid tissues (e.g., mesenteric lymph nodes, spleen), and their interactions, which, following exposure to antigens, result in the generation of specific effector responses and long-term B and T memory cells (Nera et al. 2015). For these reasons, among others, in this short perspective article, I will restrict my discussion to explore whether vaccines that elicit T-cell responses, particularly those mediated by CD8+ cells, are possible in humans by focusing on a single site and class of infectious agents, that is, bacteria that access the human host via the orogastric route. This discussion might provide a framework that can be extended to other organisms and tissues in which they take residence.
INORDINATE COMPLEXITY OF IMMUNE RESPONSES
Evidence uncovered during the past few decades, particularly in recent years, owing to remarkable technological advances, points to an extraordinarily complex immune system in which traditional innate as well as adaptive humoral and cellular immunity are unquestionably highly interrelated and interdependent. The explosion of information regarding the presence of multiple subsets of both effector and regulatory cells in every compartment paint a picture of great complexity. Major players include dendritic cells (DCs), macrophages, and other antigen-presenting cells (APCs) (Hammer and Ma 2013; Gao and Williams 2015; Pulendran 2015; Varol et al. 2015), B and T cells (Youngblood et al. 2013), granulocytes, as well as many cell types that defy their grouping into rigid classification schemes (e.g., mucosal-associated invariant T [MAIT] cells, natural killer (NK) cells, NK T cells, and innate lymphoid cells [ILCs]) (Gao and Williams 2015; Haeryfar and Mallevaey 2015; Howson et al. 2015; Van Kaer et al. 2015; Zajonc and Girardi 2015). Because these subsets are able to regulate each other through direct cell–cell interactions, as well as the production of cytokines and other soluble mediators, it is very important that they are all taken into consideration when trying to define the attributes of an effective vaccine that results in long-term protection.
For example, when addressing a major population, for example CD8+, we have to take into account that it is composed of many subsets, including, among others, T naïve (TN), T effector/memory (TEM), T central/memory (TCM), and CD45RA+ TEM (TEMRA) (Sallusto et al. 2004; Newell et al. 2012), and that CD8+-mediated responses are restricted by highly polymorphic classical major histocompatibility complex (MHC) class-Ia molecules and nonpolymorphic MHC-like molecules such as HLA-E, an MHC class-Ib molecule (Heinzel et al. 2002; Salerno-Goncalves et al. 2004; Rodgers and Cook 2005), as well as by the MHC class-I-related protein 1 (MR1) used by MAIT cells, the majority of which are CD8+ (Walker et al. 2012). Each of these subsets is specialized in presenting particular classes of antigens. Because of this complexity, what constitutes a CD8+-mediated response has been controversial, with many investigators associating CD8+ responses to those restricted by MHC class-Ia molecules when, in reality, CD8+ cells represent a constellation of numerous effector cells, able to respond to a multiplicity of antigenic stimuli. Also, it is important to consider that the activation, differentiation, and maturation of CD8+ cells is highly dependent on the “support” provided by many subsets of APCs, which, in addition to processing and presenting antigens, release cytokines and other factors, which in aggregate with those produced by CD4+ cells (including T follicular/helper cells [TFH]) (Hale and Ahmed 2015), IECs (Hooper 2015), and other cell types in response to extracellular and intracellular bacteria have the ability to tilt the adaptive T-cell responses toward effector or regulatory phenotypes. These complex interactions ultimately lead to the induction of either protective or ineffective, or even reactogenic, responses following vaccination. Also, it should be noted that it is likely that, ultimately, an appropriate balance between effector and regulatory cells (e.g., regulatory T [Treg] cells [Burzyn et al. 2013; Panduro et al. 2016; Su et al. 2016], regulatory B [Breg] cells [Zhou et al. 2016]) might be a determining factor in eliciting long-lasting protective responses following immunization with minimal or, ideally, no reactogenicity to vaccination. Because of space limitations, it is not possible to address the role of each of these cell subsets and their complex interactions. A large number of extensive and excellent reviews have been published on this subject and a few of them are referenced above.
To address the central question of this commentary, it is my opinion that vaccines against enteric bacteria, which rely predominantly on CD8+ responses, are indeed possible. However, in all likelihood, this will be restricted to particular organisms with a “lifestyle” that is amenable to being controlled by CD8+-mediated responses. For example, when considering bacteria that spend most of the time intracellularly (e.g., the Gram-negative pathogenic bacteria Salmonella enterica serovar Typhi [S. Typhi]), it is reasonable to speculate that CD8+ cells able to kill infected cells and produce interferon γ (IFN-γ) and other cytokines/chemokines to activate the innate and other adaptive immune responses, will be the dominant effector mechanism, which will ultimately lead to eradication of this pathogen from the host. In contrast, it is unlikely that CD8+ cells will be the dominant effector response that will control other enteric bacteria, such as enterotoxigenic Escherichia coli (ETEC), which reside extracellularly (Bourgeois et al. 2016). Antibody responses in the gut microenvironment, supported by TFH and other cell types, will likely play the dominant roles in protection from enteric extracellular bacteria. As an example, I will briefly describe some of the lessons learned from more than two decades of studying immunity to S. Typhi, which suggest that vaccines that elicit strong CD8+ responses can be protective.
LESSONS LEARNED FROM STUDIES WITH ATTENUATED S. TYPHI VACCINES AND INFECTIONS WITH WILD-TYPE S. TYPHI IN A HUMAN CHALLENGE MODEL
Studies conducted during the past quarter century using peripheral blood mononuclear cells (PBMCs) obtained from volunteers orally immunized with the licensed Ty21a oral typhoid vaccine, as well as with several attenuated S. Typhi vaccine candidates, have shown that the responses elicited by oral immunization are extraordinarily complex, involving all arms of the immune response, including innate immunity, antibody responses, and B memory cells (BM) to lipopolysaccharide (LPS) and flagellar antigens, as well as a wide array of cell-mediated immunity (CMI). The latter include proliferative responses, cytokines, and other mediators produced by both CD4+ and CD8+ cells showing a dominance of type 1 (T helper 1 [TH1] and T cytotoxic 1 [Tc1]) proinflammatory cytokines, which include, among others, IFN-γ, tumor necrosis factor-α (TNF-α), and interleukin (IL)-17 (Sztein et al. 1994; Viret et al. 1999; Wyant et al. 1999; Salerno-Goncalves et al. 2002, 2003, 2004, 2010; Kirkpatrick et al. 2005; Salerno-Goncalves and Sztein 2009; McArthur and Sztein 2012). Moreover, we have shown that individuals vaccinated with Ty21a (Sztein et al. 1995; Salerno-Goncalves et al. 2004) and the vaccine candidate CVD 909 (Wahid et al. 2007) elicited CD8+ cytotoxic T lymphocytes (CTLs) able to kill S. Typhi–infected autologous targets mostly through a FAS-independent, granule-dependent pathway. Of note, these responses were found to be restricted by both classical class-Ia and nonclassical class-Ib HLA-E molecules, showing that multiple mechanisms might be involved in killing of S. Typhi–infected cells and S. Typhi–induced cytokine production (Salerno-Goncalves et al. 2002, 2003, 2004). Of great importance, these long-term (up to 3 years) responses were multiphasic and mediated predominantly by TEM and TEMRA, with a lesser TCM component, and the cells were multifunctional, that is, they showed more than one function simultaneously, a characteristic widely believed to be associated with protective immunity (Betts et al. 2003, 2006; Precopio et al. 2007; Qiu et al. 2012). These responses show that it is possible to elicit, through immunization, long-term strong CD8+ T memory responses. Also, we showed IL-10 production by PBMCs from volunteers immunized with attenuated typhoid vaccines to S. Typhi flagella, suggesting the induction of Treg (Wyant et al. 1999; Wahid et al. 2007). Interestingly, we and others have tried, and failed, to observe a correlation on a volunteer-by-volunteer basis between serum antibody titers to S. Typhi LPS and/or S. Typhi flagella and CMI in individuals immunized with various attenuated S. Typhi vaccine strains (Dham and Thompson 1982; Sztein et al. 1994; Tacket et al. 2000; Salerno-Goncalves et al. 2003). This panoply of responses, however, only indicate that the vaccines are immunogenic, and cannot be construed as an indication that they are the effector immune responses associated with protection.
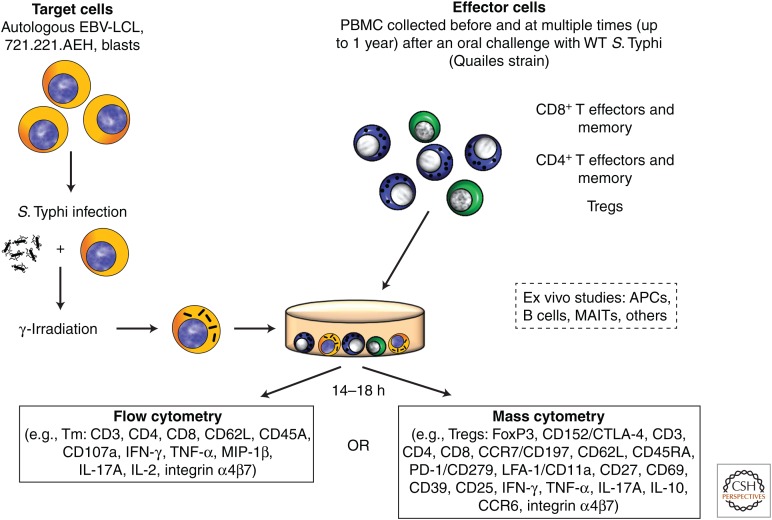
The only viable approach, however difficult, to show that a particular effector immune response(s) is(are) associated with protection in humans is through evaluation of immunity in human challenge models (Chakraborty et al. 2016; Chen et al. 2016). A wild-type S. Typhi challenge model in humans pioneered at the University of Maryland in the 1950s and 1960s (Hornick et al. 2007) was recently reestablished in Oxford by Dr. Pollard and his team (Waddington et al. 2014). The availability of PBMCs from these volunteers, some of whom developed typhoid disease (TD) and some who did not (NoTD), allowed us the unique opportunity to use advanced technologies to uncover responses that might represent mechanistic or nonmechanistic immunological correlates of protection (CoP) (Plotkin and Gilbert 2012). Participants who developed TD showed serological responses to flagellin and LPS antigens. In contrast, no changes were observed in these antibody levels in NoTD volunteers, suggesting that, rather than a CoP, these antibodies were the result of clinical disease involving local and systemic infection (Waddington et al. 2014). We used PBMCs from these participants to perform exhaustive immunological studies, including the induction of T and BM and Treg responses (McArthur et al. 2015; Fresnay et al. 2016; Toapanta et al. 2016), as well as to study the effects of challenge on activation of circulating DCs and macrophages (Toapanta et al. 2015), and associate these responses with clinical outcome in an effort to define CoP. The generalized experimental design used for the performance of studies using PBMC obtained from participants orally challenged with wild-type S. Typhi is illustrated in Figure 1. We found, for the first time, that S. Typhi–specific CD8+ responses correlate with clinical outcome in humans challenged with wild-type S. Typhi. Particularly important was the finding that higher multifunctional S. Typhi–specific CD8+ baseline responses were associated with protection against typhoid and delayed disease onset and that following challenge there was a decrease in circulating S. Typhi–specific CD8+ TEM with homing potential to the gut as well as extraintestinal tissues, suggesting migration to the site(s) of infection (Fresnay et al. 2016). We also provided the first evidence that prechallenge up-regulation of the gut homing molecule integrin α4β7 in Treg, followed by a significant down-regulation postchallenge consistent with Treg homing to the gut, as well as up-regulation of activation molecules postchallenge, was associated with the development of TD (McArthur et al. 2015). These results suggest that Treg plays an important role in clinical outcome and, presumably, in immunity elicited by vaccination (McArthur et al. 2015). Thus, we learned not only that effector and memory CD8+ responses might indeed be the dominant effector mechanism of protection to a gut intracellular bacteria, but also that Treg appears to be involved in determining clinical outcome as well, suggesting that the concomitant evaluation of both responses might be important in determining who is protected and who is not.
Figure 1.
In vitro stimulation of peripheral blood mononuclear cells (PBMCs) isolated from wild-type (WT) S. Typhi–exposed participants with S. Typhi–infected targets and ex vivo studies: Experimental design. PBMCs obtained before and at various times after oral exposure to the WT S. Typhi Quailes strain were incubated in vitro with autologous Epstein–Barr virus transformed lymphoblastoid B-cell lines (EBV-LCL), 721.221.AEH EBV-LCL (to measure HLA-E-restricted responses) or blasts infected with WT Salmonella enterica serovar Typhi (S. Typhi). After an overnight incubation, cells were fixed and stained for flow cytometry (14-color) or mass cytometry (up to 34 metal-conjugated monoclonal antibodies) depending on whether CD4+ or CD8+ T effector and/or memory subsets (TEM), or regulatory (Tregs) T cells were evaluated. Activation and other characteristics of B cells, antigen-presenting cells (APCs), mucosal-associated invariant T cells (MAITs), or other populations were evaluated ex vivo without stimulation. IFN-γ, Interferon γ; TNF-α, tumor necrosis factor α, MIP-1β, macrophage inflammatory protein-1β, IL-17A, interleukin 17A; FoxP3, forkhead box P3; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; LFA-1/CD11a, lymphocyte function-associated antigen-1; CD279/PD1, programmed cell death-1; CCR6, C-C chemokine receptor type 6; CCR7, C-C chemokine receptor type 7.
Another lesson learned is that we have to pay particular attention to baseline responses. Until very recently, the existence of preimmunization immunity in humans, and how it might impact vaccination, was not rigorously studied. The almost ubiquitous and variable “responses” to specific antigens in unexposed/unvaccinated individuals observed before vaccination in humans were routinely dismissed with statements such as “we do not know why they are there but we see them all the time and this is something that we have to live with when doing human research.” However, our data and that of others (Su et al. 2013), whether because of previous exposure to the pathogen, cross-reactivity, or recognition of several ligands by some T cells, clearly indicate that baseline responses have to be taken into consideration during the development of novel vaccines.
Finally, it is also important to be mindful of the fact that owing to migration of circulating antigen-specific cells to the appropriate microenvironment (the gut and secondary lymphoid tissues in the case of S. Typhi), it is possible that no responses are seen in circulation at particular times because the specific cells have already migrated to the appropriate site. This observation advocates for the importance of obtaining specimens at different time points to define the kinetics of the responses and to ensure that the immunity elicited by immunization is captured in circulation.
CONCLUDING REMARKS
Given the complexity of the infectious organisms/host interactions outlined above, it might not be possible to rationally design a long-term protective vaccine, mediated primarily by CD8+ and/or other mechanisms, until we have a much deeper understanding on the wide-ranging host responses, which include, among others, (1) traditional immunological effectors (e.g., innate, humoral, CMI), (2) regulatory cells, (3) contribution of the gut microbiome in shaping these interactions (Ferreira et al. 2010; Macia et al. 2012; Belkaid and Hand 2014), (4) the role of cells traditionally not considered part of the immune response (e.g., IECs, which produce cytokines and are critical in controlling the barrier that keeps the infectious bacteria from gaining access to systemic sites), and (5) the pathogenesis of each particular organism. We should also be mindful that it is possible that the CoP are different in endemic regions because of many host and environmental factors that are dissimilar between developed and underdeveloped regions of the world (e.g., differences in genetic makeup, microbiota, malnutrition, zinc deficiency). The relatively recent resurgence of interest in the use of human challenge studies holds great potential to dramatically accelerate our understanding of these complex responses. Also, the advent of extremely powerful technologies that allow for relatively simple and inexpensive analyses of, for example, the T- and B-cell repertoires (Jiang et al. 2013; Han et al. 2014; Newell and Davis 2014), gene expression of the complete or large portions of the human genome (e.g., transcriptomics, proteomics) (Hagan et al. 2015), epigenetic regulation (Valensisi et al. 2015), single-cell transcriptomics, extremely granular phenotypic and functional characterization of cell subsets by conventional flow cytometry and mass cytometry (CyTOF) (Newell et al. 2012), as well as the use of systems biology approaches (Nakaya et al. 2015), have the potential to provide critical information to dramatically accelerate the rational design of long-term protective vaccines. Equally important to achieve this goal is a better understanding of the mechanisms of action of adjuvants, including oral adjuvants such as double-mutant LT (dmLT) (El-Kamary et al. 2013), which have the potential not only to enhance immunity, but also to tilt the immune response toward the desired effector mechanism(s), as well as minimize the number of doses required for vaccination, leading to increased compliance and acceptance of vaccines as a critical public health tool. Finally, as discussed, we should be aware that despite the fact that a defined effector response is dominant in a particular context (e.g., CD8+ responses to S. Typhi), it is unlikely that this is the only effector mechanism involved in protection. In fact, a cluster of CD8+ responses in conjunction with many other immunological components (e.g., antibodies, CD4+ effector, memory, and TFH) and other biomarkers are all likely to play a role in protection as part of a constellation of host immune responses (which I refer to as “immunological clusters of protection” [iCoP]) that, acting in concert, result in the elimination of the invading pathogen.
ACKNOWLEDGMENTS
This work is supported, in part, by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), and Department of Health and Human Services (DHHS) Grants R01-AI036525, U19-AI082655 (Cooperative Center for Human Immunology, CCHI), and U19-AI109776 (Center of Excellence for Translational Research, CETR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or NIH.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157: 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 281: 65–78. [DOI] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois AL, Wierzba TF, Walker RI. 2016. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 34: 2880–2886. [DOI] [PubMed] [Google Scholar]
- Burzyn D, Benoist C, Mathis D. 2013. Regulatory T cells in nonlymphoid tissues. Nat Immunol 14: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Harro C, DeNearing B, Ram M, Feller A, Cage A, Bauers N, Bourgeois AL, Walker R, Sack DA. 2016. Characterization of mucosal immune responses to enterotoxigenic Escherichia coli vaccine antigens in a human challenge model: Response profiles after primary infection and homologous rechallenge with strain H10407. Clin Vaccine Immunol 23: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, Hall RH, Kessler RA, Lock M, Haney D, et al. 2016. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis 62: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dham SK, Thompson RA. 1982. Studies of cellular and humoral immunity in typhoid fever and TAB vaccinated subjects. Clin Exp Immunol 48: 389–395. [PMC free article] [PubMed] [Google Scholar]
- El-Kamary SS, Cohen MB, Bourgeois AL, Van De Verg L, Bauers N, Reymann M, Pasetti MF, Chen WH. 2013. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin Vaccine Immunol 20: 1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RB, Antunes LC, Finlay BB. 2010. Should the human microbiome be considered when developing vaccines? PLoS Pathog 6: e1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresnay S, McArthur MA, Magder L, Darton TC, Jones C, Waddington CS, Blohmke CJ, Angus B, Levine MM, Pollard AJ, et al. 2016. Salmonella Typhi–specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J Transl Med 14: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Williams AP. 2015. Role of innate T cells in anti-bacterial immunity. Front Immunol 6: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeryfar SM, Mallevaey T. 2015. Editorial: CD1- and MR1-restricted T cells in antimicrobial immunity. Front Immunol 6: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan T, Nakaya HI, Subramaniam S, Pulendran B. 2015. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine 33: 5294–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Ahmed R. 2015. Memory T follicular helper CD4 T cells. Front Immunol 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GE, Ma A. 2013. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol 31: 743–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Glanville J, Hansmann L, Davis MM. 2014. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol 32: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. 2002. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med 196: 1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV. 2015. Epithelial cell contributions to intestinal immunity. Adv Immunol 126: 129–172. [DOI] [PubMed] [Google Scholar]
- Hornick RB, Woodward WE, Greisman SE. 2007. Doctor T. E. Woodward’s legacy: From typhus to typhoid fever. Clin Infect Dis 45: S6–S8. [DOI] [PubMed] [Google Scholar]
- Howson LJ, Salio M, Cerundolo V. 2015. MR1-restricted mucosal-associated invariant T cells and their activation during infectious diseases. Front Immunol 6: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He XS, Dekker CL, Zheng NY, Huang M, Sullivan M, et al. 2013. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med 5: 171ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick BD, Tenney KM, Larsson CJ, O’Neill JP, Ventrone C, Bentley M, Upton A, Hindle Z, Fidler C, Kutzko D, et al. 2005. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J Infect Dis 192: 360–366. [DOI] [PubMed] [Google Scholar]
- Levine MM, Sztein MB. 2004. Vaccine development strategies for improving immunization: The role of modern immunology. Nat Immunol 5: 460–464. [DOI] [PubMed] [Google Scholar]
- Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, Vieira AT, Kranich J, Mackay CR. 2012. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev 245: 164–176. [DOI] [PubMed] [Google Scholar]
- McArthur MA, Sztein MB. 2012. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PloS ONE 7: e38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur MA, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, Blohmke CJ, Dougan G, Angus B, Levine MM, et al. 2015. Activation of Salmonella Typhi–specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog 11: e1004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor PD. 2015. Live attenuated vaccines: Historical successes and current challenges. Virology 479–480: 379–392. [DOI] [PubMed] [Google Scholar]
- Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta AK, Gaujoux R, et al. 2015. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity 43: 1186–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nera KP, Kylaniemi MK, Lassila O. 2015. Regulation of B cell to plasma cell transition within the follicular B cell response. Scand J Immunol 82: 225–234. [DOI] [PubMed] [Google Scholar]
- Newell EW, Davis MM. 2014. Beyond model antigens: High-dimensional methods for the analysis of antigen-specific T cells. Nat Biotechnol 32: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. 2012. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 36: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panduro M, Benoist C, Mathis D. 2016. Tissue tregs. Annu Rev Immunol 34: 609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CO, Kupper TS. 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, Gilbert PB. 2012. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 54: 1615–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med 204: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B. 2015. The varieties of immunological experience: Of pathogens, stress, and dendritic cells. Annu Rev Immunol 33: 563–606. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Zhang M, Zhu Y, Zheng F, Lu P, Liu H, Graner MW, Zhou B, Chen X. 2012. Multifunctional CD4 T cell responses in patients with active tuberculosis. Sci Rep 2: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JR, Cook RG. 2005. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol 5: 459–471. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Sztein MB. 2009. Priming of Salmonella enterica serovar Typhi-specific CD8+ T cells by suicide dendritic cell cross-presentation in humans. PLoS ONE 4: e5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Pasetti MF, Sztein MB. 2002. Characterization of CD8+ effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 169: 2196–2203. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM, Sztein MB. 2003. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain CVD 908-htrA. J Immunol 170: 2734–2741. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. 2004. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol 173: 5852–5862. [DOI] [PubMed] [Google Scholar]
- Salerno-Goncalves R, Wahid R, Sztein MB. 2010. Ex vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol 17: 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol 22: 745–763. [DOI] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. 2013. Virus-specific CD4+ memory-phenotype T cells are abundant in unexposed adults. Immunity 38: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LF, Del Alcazar D, Stelekati E, Wherry EJ, Davis MM. 2016. Antigen exposure shapes the ratio between antigen-specific Tregs and conventional T cells in human peripheral blood. Proc Natl Acad Sci 113: E6192–E6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, Levine MM. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis 170: 1508–1517. [DOI] [PubMed] [Google Scholar]
- Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM. 1995. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol 155: 3987–3993. [PubMed] [Google Scholar]
- Tacket CO, Sztein MB, Wasserman SS, Losonsky G, Kotloff KL, Wyant TL, Nataro JP, Edelman R, Perry J, Bedford P, et al. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun 68: 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toapanta FR, Bernal PJ, Fresnay S, Darton TC, Jones C, Waddington CS, Blohmke CJ, Dougan G, Angus B, Levine MM, et al. 2015. Oral wild-type Salmonella Typhi challenge induces activation of circulating monocytes and dendritic cells in individuals who develop typhoid disease. PLoS Negl Trop Dis 9: e0003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toapanta FR, Bernal PJ, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, Blohmke CJ, Angus B, Levine MM, et al. 2016. Oral challenge with wild-type Salmonella Typhi induces distinct changes in B cell subsets in individuals who develop typhoid disease. PLoS Negl Trop Dis 10: e0004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valensisi C, Liao JL, Andrus C, Battle SL, Hawkins RD. 2015. cChIP-seq: A robust small-scale method for investigation of histone modifications. BMC Genomics 16: 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L, Parekh VV, Wu L. 2015. The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front Immunol 6: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Mildner A, Jung S. 2015. Macrophages: Development and tissue specialization. Annu Rev Immunol 33: 643–675. [DOI] [PubMed] [Google Scholar]
- Viret JF, Favre D, Wegmuller B, Herzog C, Que JU, Cryz SJ Jr, Lang AB. 1999. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect Immun 67: 3680–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, Thompson BA, Kerridge SA, Kingsley RA, Zhou L, et al. 2014. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 58: 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. 2007. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine 25: 1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, Sahgal N, Leslie A, Oo Y, Geremia A, et al. 2012. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 119: 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyant TL, Tanner MK, Sztein MB. 1999. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun 67: 3619–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Ahmed R. 2013. T-cell memory differentiation: Insights from transcriptional signatures and epigenetics. Immunology 139: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc DM, Girardi E. 2015. Recognition of microbial glycolipids by natural killer T cells. Front Immunol 6: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Gong L, Wang X, Hu Z, Wu G, Tang X, Peng X, Tang S, Meng M, Feng H. 2016. The role of regulatory B cells in digestive system diseases. Inflammation Res 66: 303–309. [DOI] [PubMed] [Google Scholar]