Abstract
Mitral regurgitation is the most common valve disorder in the Western world, and although surgery is the established therapeutic gold standard, percutaneous transcatheter mitral interventions are gaining acceptance in selected patients who are inoperable or at an exceedingly high surgical risk. For such patients, multidetector computed tomography (MDCT) can provide a wealth of valuable morphological and functional information in the preoperative setting. Our aim is to give an overview of the MDCT image acquisition protocols, post-processing techniques, and imaging findings with which radiologists should be familiar to convey all relevant information to the Heart Team for successful treatment planning.
Keywords: Mitral regurgitation, Transcatheter mitral valve repair, Transcatheter mitral valve replacement, Cardiac multidetector computed tomography
1. Introduction
Although surgery is the gold standard treatment for patients with symptomatic mitral regurgitation (MR), transcatheter mitral valve interventions have emerged over the last decade as a viable option in selected patients with unacceptably high surgical risk [1,2]. According to the 2017 ESC Guidelines, transcatheter mitral valve repair (TMVRep) may be considered for symptomatic patients with severe chronic MR who are at high surgical risk or are inoperable, and should be discussed by the Heart Team to avoid futile treatment [2]. Similarly, the 2017 update of the 2014 American College of Cardiology/American Heart Association (ACC/AHA) has stated that TMVRep may be considered for severely symptomatic patients (NYHA class III to IV) with chronic severe primary MR (stage D) who have favourable anatomy for the repair procedure and a reasonable life expectancy, but have a prohibitive surgical risk because of serious comorbidities and remain symptomatic despite optimal management and therapy for heart failure [3].
While surgery can be performed through direct or videoendoscopic guidance, transcatheter-based approaches cannot, making periprocedural imaging a key step for treatment planning. In this context, multidetector computed tomography (MDCT) has proved to be a robust imaging modality that can yield valuable information for accurate and safe treatment planning.
Our purpose is threefold:
-
•
to explain the rationale for the use of MDCT in the preoperative planning of transcatheter mitral valve interventions
-
•
to describe suitable MDCT image acquisition protocols and post-processing techniques for treatment planning
-
•
to discuss the main MDCT findings that radiologists need to assess and convey to the Heart Team.
2. Pathophysiology of MR
MR is defined as the abnormal backflow of blood from the left ventricle into the left atrium due to malfunction (either primary or secondary) of the mitral valve system, and is the most common manifestation of valve dysfunction in the Western world.
In organic (or primary) forms, MR is caused by anatomical changes affecting one or more components of the mitral valve complex. MR has most often a degenerative aetiology, including mitral prolapse, flail mitral leaflet, post-endocarditic or (once common, but now rare in industrialised countries) post-rheumatic sequelae. On the other hand, functional (or secondary) MR is characterised by the presence of mitral regurgitation in the absence of organic lesions. In this latter case, MR is secondary to both regional and global ventricular remodelling (usually secondary to ischaemic or dilated idiopathic cardiomyopathy) [[4], [5], [6], [7]].
The mechanism of MR has been classified by Carpentier et al. on the basis of the opening and closing motions of the mitral leaflets into the following four types:
-
•
Type I: normal leaflet motion (annular dilatation, leaflet perforation)
-
•
Type II: excessive leaflet motion (leaflet prolapse)
-
•
Type IIIa: restricted leaflet motion during both diastole and systole, often associated with leaflet thickening and commissural fusion (as typically found in rheumatic disease)
-
•
Type IIIb: restricted leaflet motion during systole (leaflet tethering due to left ventricular remodelling and displacement of papillary muscles) [8].
Under normal conditions, the mitral valve is hermetically closed during systole, thus preventing retrograde blood flow from the left ventricle into the left atrium. In contrast, in patients with MR, a fraction of the left ventricular blood volume flows back into the left atrium during systole and is returned into the left ventricle during diastole, progressively leading to left ventricular volume overload. As a consequence, left ventricular remodelling with dilation and compensatory hypertrophy will occur, and the amount of blood pumped into the aorta (effective cardiac output) will tend to decrease, resulting into a reduction in cardiac output. On the left atrial side, MR can lead to different alterations in relation to its severity, but above all depending on its onset being acute or chronic:
-
•
In acute MR (e.g. secondary to rupture of chordae tendineae or a papillary muscle due to acute myocardial infarction), there is no progressive adaptation of the left atrium to the sudden volume overload, and hence no left atrial dilation. This leads to a rapid increase of left atrial and pulmonary venous pressures that typically evolves towards acute pulmonary oedema.
-
•
Chronic MR is characterised by a progressive adaptation of the left atrium, which tends to expand. Over time, the left ventricle may develop a loss of contractile efficiency due to prolonged volume overload with an increase in end-diastolic pressure. Moreover, left ventricular dilation may lead to dilation of the mitral annulus, which further increases MR severity in a potential downward spiral towards chronic heart failure [4,5,9].
3. Transcatheter mitral valve procedures: when and how?
In the past years, several transcatheter mitral valve procedures have been developed building on existing surgical techniques as a conceptual framework.
The MitraClip NT® system (Abbott Vascular, Santa Clara, CA) is a mitral valve repair system that mimics the ‘edge-to-edge’ repair described by Maisano et al. [10]. As its name suggests, it consists of a clip that allows capturing both the anterior and posterior mitral leaflets with its two arms, resulting in shrinking of the regurgitant mitral valve orifice. TMVRep with Mitraclip® is performed using a trans-septal approach with 3D-transesophageal echocardiography as the gold standard for preprocedural planning and intraoperative guidance, although MDCT can provide incremental anatomical data such as for the severity assessment of mitral valve calcifications (which may interfere with or contraindicate device implantation) [6,11,12]. Among current percutaneous treatment options, Mitraclip®-based edge-to-edge TMVRep is the most commonly performed transcatheter mitral valve procedure, has gained both CE and FDA approval, and although the rate of residual MR up to 5 years is higher than with surgical repair, it is generally safe and can improve symptoms while inducing reverse left ventricular remodelling [13].
Alternative devices for percutaneous treatment of functional MR are available such as the Cardioband System® (Valtech Cardio, Or Yehuda, Israel) and the CARILLON Mitral Contour System® (Cardiac Dimensions, Inc., Kirkland, WA), which have been developed for direct and indirect transcatheter mitral annuloplasty procedures, respectively. The Cardioband® system is a catheter-based device that functions as a percutaneous annuloplasty band. Using a transvenous and trans-septal approach, the Cardioband® device is deployed via multiple screw fixation on the posterior mitral annulus from the anterolateral to the posteromedial commissures, with intraprocedural adjustment to reduce the septolateral diameter of the mitral annulus and restore leaflet coaptation, resulting in a direct ‘surgical-like’ annuloplasty. On the other hand, the CARILLON Mitral Contour System® consists of a proximal anchor and a distal anchor connected by a shaping ribbon, and is positioned in the coronary sinus and great cardiac vein using standard cardiac catheterisation techniques. The CARILLON implant is a fixed length, double anchor device designed to plicate the tissue next to the mitral annulus during the deployment process.
While the Mitraclip NT® system can be used for percutaneous repair of both primary (type II of Carpentier’s classification) and functional (type I-IIIb) MR, direct and indirect percutaneous mitral annuloplasty is confined to functional MR only (with mitral annulus dilation and apical displacement of papillary muscles).
The device armamentarium for percutaneous mitral valve interventions has significantly expanded over the last years with the introduction of new systems for transcatheter mitral valve replacement (TMVR), including the CardiAQ-Edwards® valve (Edwards Lifesciences; Irvine, CA), Tiara® valve (Neovasc Inc; Richmond, BC), Tendyne® valve (Abbott Vascular, Santa Clara, CA), Intrepid TMVR® system (Medtronic Inc, Redwood City, CA), Caisson TMVR® system (LivaNova PLC, Maple Grove, MN), MValve® (MValve Technologies Ltd, Herzliya, Israel), and HighLife valve® (HighLife Medical, Irvine, CA) as some examples. Such evolution has been driven by anatomical and pathophysiological factors related to the D-shape of the mitral annulus and the heterogeneous pathogenesis of MR, leading to prosthesis designs that differ mostly in the valve anchoring mechanisms (i.e. apical tether, native leaflet engagement, mitral annulus clamping, annular winglets, radial force, external anchor, subannular mitral ring or mitral annulus clamping). TMVR procedures may offer several advantages over TMVRep in primary (organic) forms of MR, but their role in treating secondary (functional) types of MR has not yet been established. Moreover, there is still uncertainty about the best implantation modality for mitral devices. In fact, the majority of percutaneous mitral valve prostheses are positioned via a transapical approach (i.e. through puncture of the left ventricular apex), whereas others are deployed through a trans-septal (venous) approach, which would be ideal in highest risk patients [11,12,14,15]. Encouraging data have recently been provided by a feasibility study (NCT02321514) about the effectiveness and safety of transapical TMVR using a self-expanding device in patients with native MR at high risk for cardiac surgery, resulting in NYHA functional class improvement with mild or no symptoms in 75% of patients and successful device implantation without cardiovascular mortality, stroke, and device malfunction in 86.6% of patients at 30-day follow-up [16].
4. MDCT image acquisition protocol
4.1. General considerations
While cardiac ultrasound is the gold standard for diagnosing, grading and monitoring mitral valve disease before and after treatment and (in combination with fluoroscopy and angiography) for intraoperative guidance, MDCT has gained widespread acceptance for interventional planning in patients candidate to transcatheter mitral valve procedures. Cardiac MDCT has several strengths, including:
-
•
fast imaging time (<10 s) and widespread availability
-
•
excellent spatial resolution (≃0.5 mm) with voxel isotropy, allowing for high quality 2D and 3D reconstructions of the mitral valve and subvalvular apparatus at any time point of the cardiac cycle
-
•
high reproducibility and relative operator-independence
-
•
direct visualisation and quantification of mitral calcifications
-
•
panoramic view of the whole heart (including the coronary arteries, the aortic root and the left ventricular outflow tract) and the chest wall [5,6,11,12,[17], [18], [19], [20]].
Image acquisition with electrocardiographic gating (ECG-gating) is mandatory to obtain motion-free images of the mitral valve and the surrounding structures at a given single phase, or multiple phases of the cardiac cycle. The higher the temporal resolution, the higher the heart rate at which motion-free images can be obtained and the ability to reject potential arrhythmia-related artefacts. In this setting, usage of a MDCT scanner with at least 64 detector rows is recommended as a minimum hardware requirement, as fewer heart beats are needed to scan the entire heart with increased z-axis coverage and shorter tube rotation time. At the other side of the spectrum, wide coverage MDCT scanners with up to 16 cm detector width allow whole heart imaging in a single heartbeat, whereas second and third generation dual source MDCT technology can yield a temporal resolution less than 80 ms, providing diagnostic quality images even in patients with atrial fibrillation and/or obviating the need for beta-blockers to lower heart rate prior to scanning [18,21,22].
4.2. Scan protocol
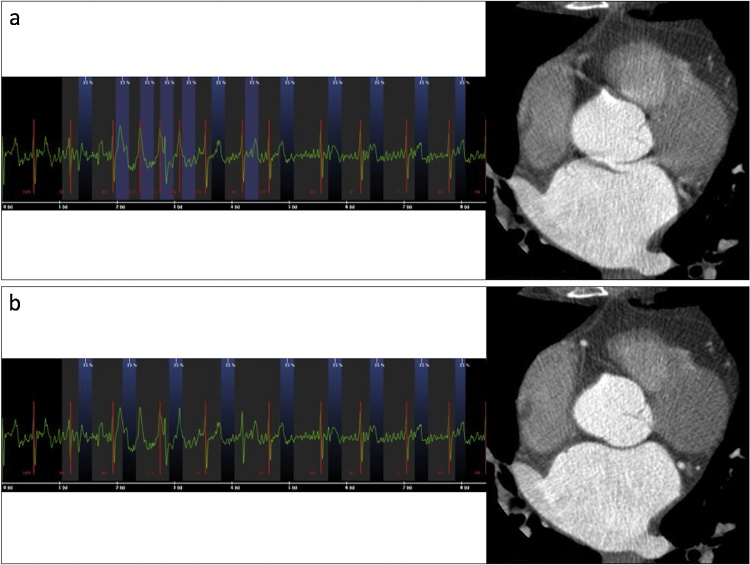
With other scanning parameters kept unchanged, retrospective ECG-gating is usually preferred over prospective ECG-gating owing to its higher temporal resolution and to the possibility to mitigate or eliminate arrhythmia-related artefacts by means of ECG-editing (Fig. 1). In order to accurately assess the mitral valve morphology and size during the various phases of the cardiac cycle, a recommended approach is to perform a retrospectively ECG-gated acquisition and reconstruct MDCT datasets every 5% or 10% of the R-R interval from 0% to 90% [20].
Fig. 1.
Reduction of motion artefacts due to high R-R interval variability by means of ECG-editing (a, before ECG-editing; b, after ECG-editing).
The scan volume spans the entire heart from the level of the carina to 1 cm below the heart apex, and dose-saving techniques such as low kV scanning and iterative reconstruction algorithms can be used to reduce radiation exposure while filtering out image noise to preserve overall image quality. A suitable MDCT scan protocol is shown in Table 1.
Table 1.
Image acquisition protocol used at our Institution for MDCT-based planning of transcatheter mitral valve interventions (scanner type: Discovery CT750 HD, General Electric, Milwaukee, WI).
| Parameter | Value |
|---|---|
| Tube voltage | 100-120kV*° |
| Tube current | 400-600mA* |
| Slice thickness/reconstruction interval | 0.5 - 0.625 mm/0.4 mm |
| Scan field of view | As small as possible to include the heart |
| Iterative reconstruction algorithms | Yes (if available) |
| ECG-gating | Retrospective (no ECG modulation) |
| Reconstruction of ECG-gated datasets | Every 5-10% of the R-R cycle |
°Consider 80 kV in slim patients (body mass index <23 kg/m2 or <65 kg) without metallic valve prostheses or pacemaker leads, in combination with iterative image reconstruction algorithms.
Depending on patient size.
Depending on the available MDCT equipment and on a case-by-case basis, premedication with beta-blockers or ivabradine can be administered to reduce patients’ heart rate below 65–70 beats per minute so as to minimise potential cardiac motion artefacts.
4.3. Contrast injection protocol
A triphasic contrast medium injection should be performed to ensure optimal enhancement of the left atrium, left ventricle and coronary arteries [4]. A suitable protocol used at our Institution consists of a first bolus of 50–80 mL of contrast material at a high flow rate (4–6 mL/s), followed by a mix of 50–60 mL of contrast material and saline with 30%:70% dilution, and a final saline flush to maximise bolus compaction and avoid streaking artefacts due to pooling of hyperconcentrated contrast material in the right heart.
Contrast media with an iodine concentration between 300mgI/mL and 400mgI/mL are typically used for cardiac MDCT angiography, with higher concentrations allowing to reduce both contrast volume and flow rate compared with lower ones. Of course, a lower contrast volume can be administered using MDCT equipment with faster scan time, thereby reducing overall iodine load. Administration of the lowest possible amount of iodine (eventually combined with low kV settings and iterative reconstruction algorithms to maximise contrast enhancement and contrast-to-noise ratio) is recommended, especially in patients with impaired kidney function or severely reduced left ventricular ejection fraction [18,23].
Bolus tracking should be used for optimal timing of the cardiac MDCT angiography scan with contrast bolus arrival into the left atrium (e.g. with a 5-sec scan delay and a 100-150HU density threshold).
4.4. Image processing
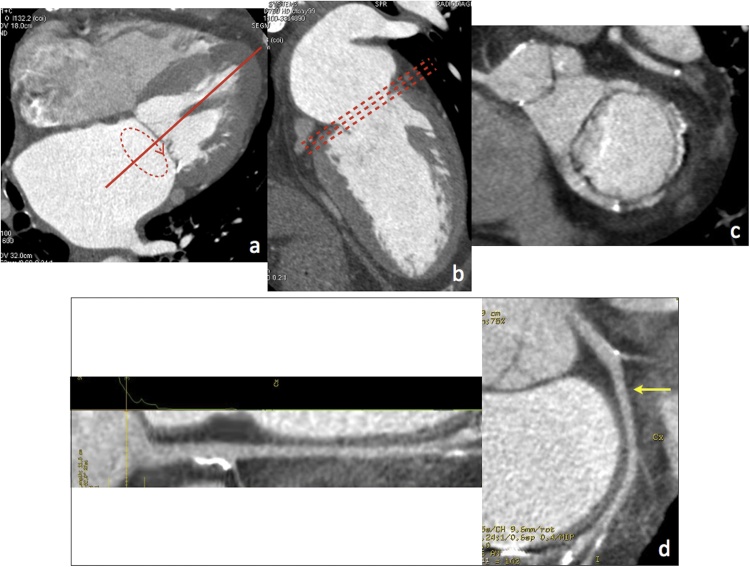
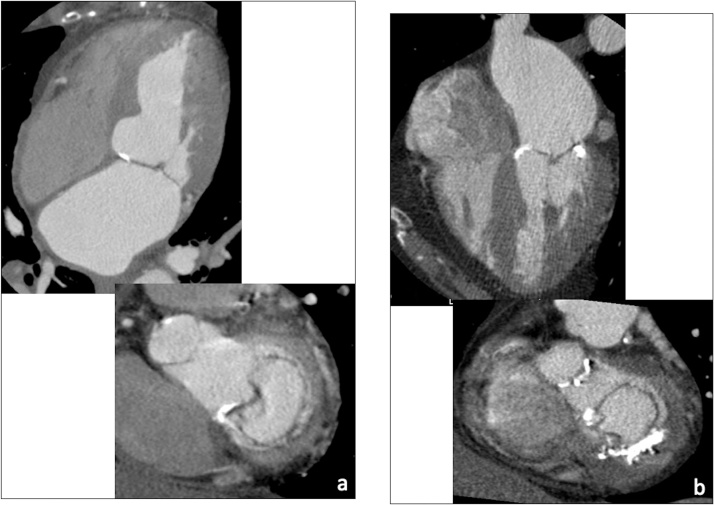
ECG-gated source axial images from each cardiac phase must be systematically reviewed on a workstation with time-resolved 4D image review capability. In particular, the ECG-gated dataset with the largest dimension of the mitral valve annulus (typically on mid- to end-diastolic phases) and the least motion artefacts must be chosen for sizing of the mitral annulus (Fig. 2a-c).
Fig. 2.
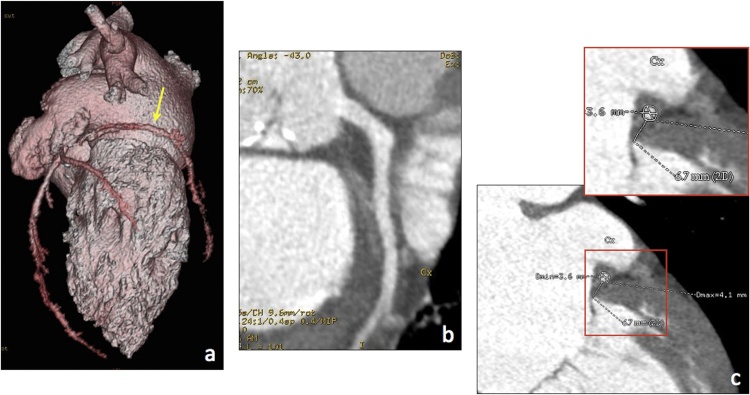
(a–c) MPR views for optimal evaluation of the mitral valve complex (a, 4-chamber view; b, 2-chamber view; c, short axis view at the level of the mitral annulus, aligned parallel to dashed lines in b). (d) Straightened and stretched CPR views of the circumflex artery (arrow).
Multiplanar reformations (MPR, including conventional two-, three-, four-chamber, and short axis views) are generated to obtain the best orientation for assessment of the mitral valve complex and the surrounding structures of interest. On the other hand, curved planar reformatted (CPR) views allow to assess the entire course of the coronary arteries (with particular reference to the circumflex artery, coursing along the left atrioventricular groove near the mitral annulus) and to accurately evaluate atherosclerotic plaques or vessel stenoses (Fig. 2d).
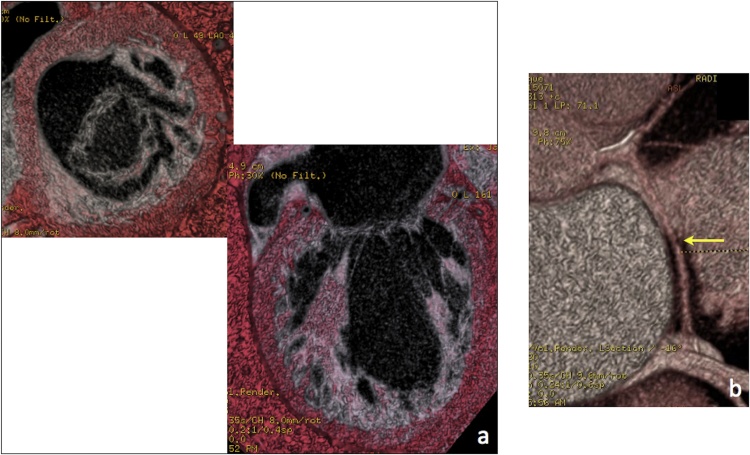
Finally, 3D and 4D Volume Rendering (VR) views may provide a comprehensive assessment of the spatial distribution of mitral calcifications and a panoramic depiction of mitral valve morphology and nearby structures (Fig. 3).
Fig. 3.
VR views of the mitral valve complex (a) and the circumflex artery coursing along the left atrio-ventricular groove (b, arrow).
5. MDCT findings - what the radiologist and interventional cardiologist need to know from each other
5.1. Assessment of mitral annulus morphology and device sizing
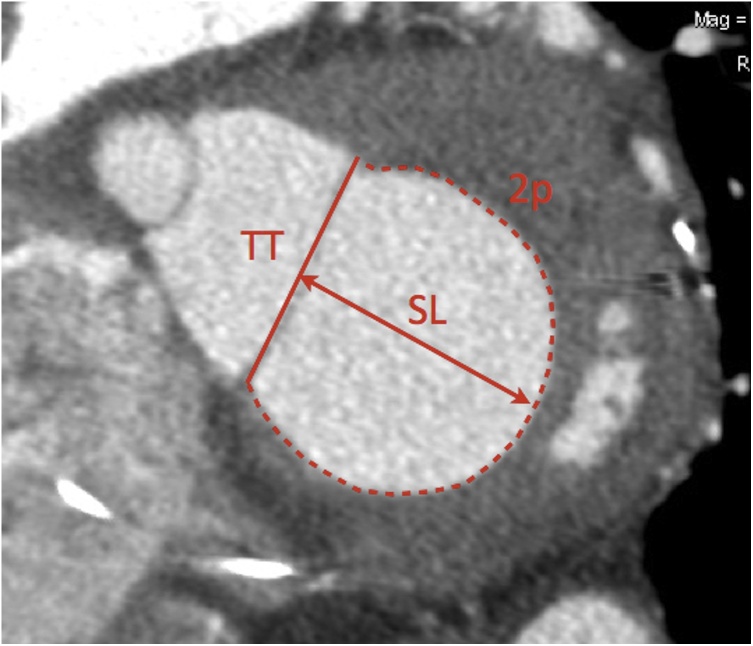
A direct approach to mitral valve segmentation consists of drawing the contours of the saddle-shaped mitral annulus on conventional four-, three-, two-chamber and short axis views by placing 16 seeding points along the posterior mitral leaflet insertion and the fibrous continuity. However, segmentation using this approach can be time consuming and challenging due to the non-planar shape of the mitral annulus, and the inclusion of the anterior horn of the mitral annulus for device sizing carries the risk of left ventricular outflow tract (LVOT) obstruction [11,12]. A simpler method devised by Blanke et al (simplified D-shape model) consists of assimilating the mitral annulus to a planar D-shape by connecting the two fibrous trigones along a virtual straight line, thereby excluding the anterior horn [11,12,24,25] (Fig. 4). The maximum and minimum diameter, perimeter and area of the mitral valve annulus, and the septal-to-lateral and trigone-to-trigone distance should be measured. It should be noted that different devices may require specific measurements, such as the CardiaQ® valve (Edwards Lifesciences; Irvine, CA) relying on the maximum diameter and the Tiara® valve (Neovasc Inc; Richmond, BC) on the intercommissural distance, respectively [12].
Fig. 4.
Sizing of mitral annulus on MPR short axis view using Blanke's D-shape model. TT = trigone-to-trigone distance, SL = septal-to-lateral distance, 2p = perimeter. Mitral cross-sectional area can also be directly measured.
5.2. Extent and location of mitral annular calcifications
Mitral annular calcification (MAC) is a result of slowly progressive calcification of the fibrous component of the mitral annulus and more commonly affects the posterior portion of the annulus. Risk factors for MAC development include advanced age, female sex, chronic kidney disease, left ventricular hypertrophy (e.g. due to systemic hypertension and aortic stenosis), metabolic diseases, prior chest irradiation, and Barlow's disease.
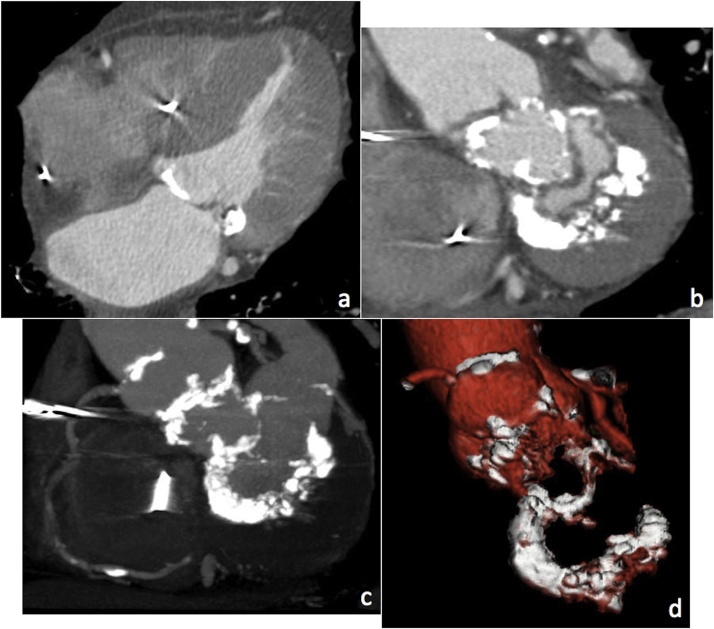
Although MAC is typically confined to the mitral annulus and the base of the leaflets, in some cases it can extend further into the leaflets down to the chordae tendineae, papillary muscles, and left ventricular myocardium. MDCT can directly determine the precise location and extent of MAC, which can be spotty or confluent in shape and characterised as protruding or non-protruding (Figs. 5 and 6 ).
Fig. 5.
(a) Mild and (b) moderate MAC as shown by 4-chamber (upper) and short axis MPR views (lower).
Fig. 6.
Severe MAC as shown by 4-chamber (a) and short axis MPR views (b). MIP (c) and VR (d) views show the overall extent of MAC reaching the mitral-aortic curtain and the aortic valve.
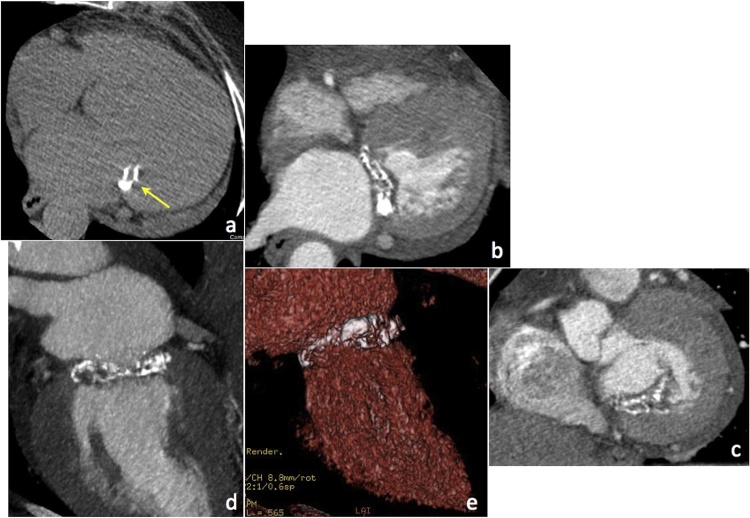
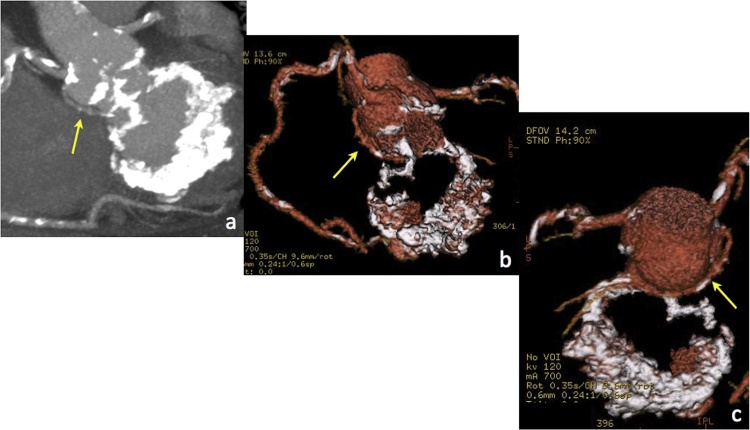
Another cause of MAC is caseous calcification of the mitral annulus (CCMA), a benign entity that can mimic a granuloma, abscess or cardiac mass. CCMA typically involves the periannular area adjacent to the posterior mitral leaflet and manifests as a well defined peripherally calcified mass with a central region of variable attenuation without contrast enhancement (this latter feature being a key criterion to differentiate CCMA from other disease conditions, such as tumours) (Fig. 7).
Fig. 7.
MDCT appearance of CCMA in a patient with atrial fibrillation and severe MR; (a) precontrast axial image, (b) 4-chamber and (c) short axis MPR views, (d) MIP and (e) VR views.
The presence of leaflet calcification must also be reported, since severe calcifications along the device grasping zone are at higher risk of embolisation into the blood stream, and especially bulky calcifications of the anterior mitral valve leaflet could be displaced into the LVOT, resulting in LVOT obstruction [8,26,27].
5.3. Landing zone and myocardial shelf
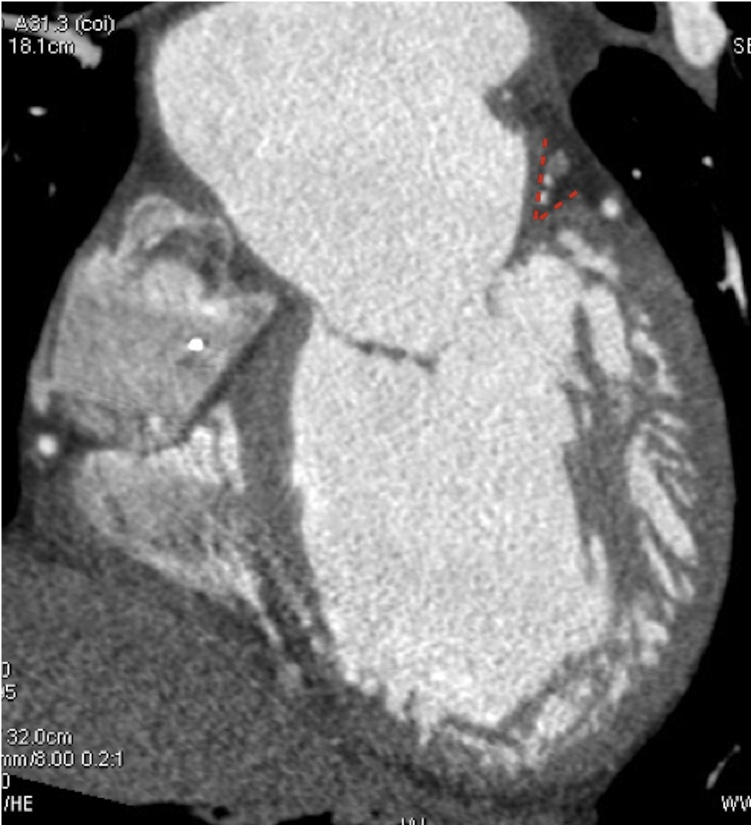
The anatomy of the landing zone (defined as the area where the mitral device is deployed) varies between functional MR and mitral prolapse. In functional MR, regional wall motion abnormalities and/or left ventricle dilation lead to outward displacement of the papillary muscle, resulting in tethering of mitral leaflets, annular dilation, and basal myocardium remodelling with formation of a so-called ‘myocardial shelf’, which can be identified at MDCT (Fig. 8).
Fig. 8.
2-chamber MPR view showing myocardial shelf (red dashed lines) in a patient with severe left ventricular dilation.
In degenerative mitral valve disease (DMVD), fibroelastic deficiency and myxomatous degeneration can lead to diffuse valvular thickening, redundant leaflets and chordal elongations. The insertion of posterior mitral valve leaflet and annulus may be displaced into the left atrium, which is referred to as mitral annular disjunction. A posterior myocardial shelf is typically not recognisable in DMVD, and the basal myocardium may bulge into the lumen with hyperdynamic and hypertrophic left ventricle [12,24,25]. Of note, the myocardial shelf can change its morphology and size over the cardiac cycle and disappear in systole [26]. Therefore, the use of TMVR devices anchoring to the infero-lateral basal myocardium requires that the posterior myocardial shelf be identified and sized dynamically both in systole and diastole to ensure proper device capture and positioning [25,26].
5.4. Assessment of the circumflex artery, coronary sinus, aorto-mitral angle, and prediction of fluoroscopic angulation
The patency of the circumflex artery and its course along the left atrio-ventricular groove must be evaluated and reported due to its close spatial relationship with the posterior mitral attachment. An extremely short distance between the mitral annulus and the circumflex artery is a contraindication to some transcatheter mitral annuloplasty procedures due to the risk of damaging the vessel during device fixation (Fig. 9, Fig. 10) [6,11].
Fig. 9.
Preprocedural assessment of the circumflex artery. (a) VR shows the vessel course along the left atrio-ventricular groove (arrow), whereas CPR (b) and CPR-derived cross-sections along the vessel centerline (c) provide detailed and reproducible information about its patency and distance from the mitral annulus.
Fig. 10.
MIP (a) and VR images (b, c) show retroaortic course of the circumflex artery (arrow) in a patient with bulky MAC extending to the mitral-aortic curtain and the aortic valve.
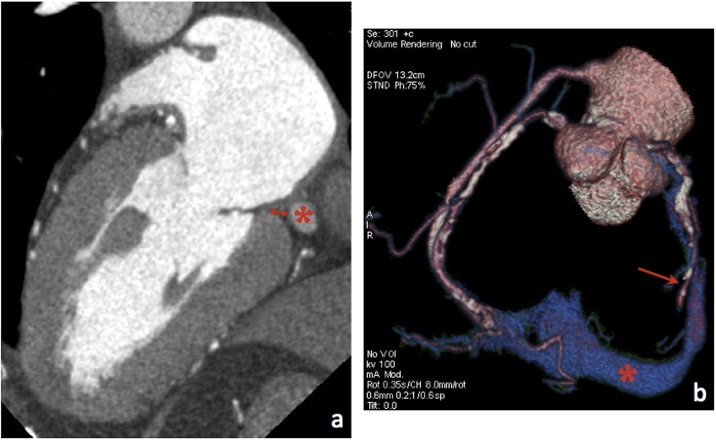
The spatial relationship between the mitral annulus and the coronary sinus (CS) should be evaluated and illustrated using MPR and VR reconstructions. The distance between them should also be measured on MPR and/or CPR views and reported to avoid CS perforation or dissection during or after the procedure (Fig. 11). Moreover, a wide angle between the CS and the mitral annulus is associated with poor force transmission to the mitral annulus, potentially resulting in procedure failure [5,6].
Fig. 11.
MPR (a) and VR images (b) allow assessment of the CS (asterisk) course, patency, and distance to the mitral annulus (double headed arrow). The single headed arrow in b) points to the circumflex artery. As shown in b), the patient has a bypass graft (saphenous vein) to the distal right coronary artery.
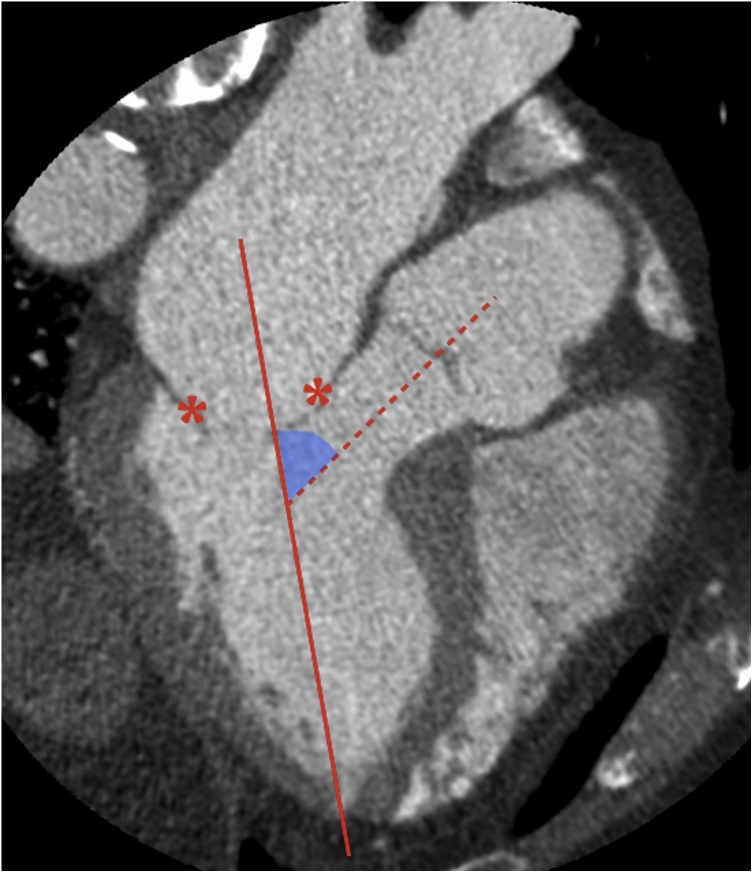
Finally, the aorto-mitral angle (i.e. the angle between the mitral annulus trajectory line and the LVOT long axis, which is directly related to the risk of TMVR-related LVOT obstruction) can be readily measured on 3-chamber MPR views (Fig. 12), and the neo-LVOT geometry after TMVR can be predicted on a patient- and device-specific basis by simulating device deployment via stereolithographic file integration [12,26,28]. Dedicated software plugins also allow prediction of the best fluoroscopic angulation for device placement based on preprocedural MDCT data [12,29].
Fig. 12.
Measurement of the aorto-mitral angle (light blue) from the intersection between the mitral annulus trajectory (straight line) and the LVOT long axis (dashed line). Asterisks indicate mitral leaflets.
5.5. Incidental findings
Last but not least, all images should be carefully scrutinised for any incidental cardiac and extracardiac findings that may delay treatment or affect overall patient’s prognosis and management (Fig. 13).
Fig. 13.
Some incidental findings that could occur upon systematic review of preprocedural MDCT studies. (a) 71-year-old male with bilateral pleural effusion (red asterisks) and lung nodule (arrow). (b) 78-year-old male with history of renal clear cell carcinoma previously treated with left nephrectomy and MDCT finding of hypervascular left subdiaphragmatic nodule (blue asterisk). (c) 82-year-old female with unexpected MDCT finding of pulmonary embolism (dashed arrows).
6. Conclusions
Transcatheter mitral valve procedures are gaining increasing acceptance as an alternative to surgery in patients at prohibitively high surgical risk, with the rapid development of new devices and growing operators’ experience fuelling the evolution of the percutaneous approach.
In this setting, MDCT has emerged as a key imaging modality for procedural planning, owing to its widespread availability, fast imaging time, excellent spatial resolution, and the ability to provide a comprehensive set of information to the interventional cardiologist.
Radiologists should become familiar with the imaging findings of patients candidate to transcatheter mitral valve procedures, as well as with the indications and requisites for such interventions. To this purpose, a tight interaction between radiologists and interventional cardiologists is essential to optimise imaging acquisition protocols and streamline reporting, in order to convey all relevant information for successful treatment planning and improve workflow.
Conflict of interest
None
Acknowlegment
Prof. Anna Sonia Petronio is consultant to Abbott (Santa Clara, CA), Boston Scientific (Marlborough, MA) and Medtronic (Redwood City, CA). No funding was received for this article by any of the authors.
Contributor Information
Lorenzo Faggioni, Email: lfaggioni@sirm.org.
Michela Gabelloni, Email: mgabelloni@sirm.org.
Sandra Accogli, Email: sandra.accogli@gmail.com.
Marco Angelillis, Email: angelillismarco@gmail.com.
Giulia Costa, Email: g-costa@virgilio.it.
Paolo Spontoni, Email: paolo.spontoni@gmail.com.
Anna Sonia Petronio, Email: as.petronio@gmail.com.
Davide Caramella, Email: davide.caramella@med.unipi.it.
References
- 1.Regueiro A., Granada J.F., Dagenais F., Rodés-Cabau J. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J. Am. Coll. Cardiol. 2017;69:2175–2192. doi: 10.1016/j.jacc.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H., Falk V., Bax J.J. ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura R.A., Otto C.M., Bonow R.O. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017;2017(70):252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Morris M.F., Maleszewski J.J., Suri R.M. CT and MR imaging of the mitral valve: radiologic-pathologic correlation. Radiographics. 2010;30:1603–1620. doi: 10.1148/rg.306105518. [DOI] [PubMed] [Google Scholar]
- 5.Delgado V., Tops L.F., Schuijf J.D. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc. Imaging. 2009;2:556–565. doi: 10.1016/j.jcmg.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan N., Patel P., Bartel T. Peri-procedural imaging for transcatheter mitral valve replacement. Cardiovasc. Diagn. Ther. 2016;6:144–159. doi: 10.21037/cdt.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers J.H., Franzen O. Percutaneous edge-to-edge MitraClip therapy in the management of mitral regurgitation. Eur. Heart J. 2011;32:2350–2357. doi: 10.1093/eurheartj/ehr101. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A.F., Pellerin M., Fuzellier J.F., Relland J.Y. Extensive calcification of the mitral valve anulus: pathology and surgical management. J. Thorac. Cardiovasc. Surg. 1996;111:718–729. doi: 10.1016/s0022-5223(96)70332-x. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke R.A., Crawford M.H. Mitral valve regurgitation. Curr. Probl. Cardiol. 1984;9:1–52. doi: 10.1016/0146-2806(84)90021-5. [DOI] [PubMed] [Google Scholar]
- 10.Maisano F., Torracca L., Oppizzi M. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur. J. Cardiothorac. Surg. 1998;13:240–245. doi: 10.1016/s1010-7940(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 11.Storz C., Mangold S., Mueller K.A. Cardiac CT for guiding mitral valve interventions. Curr. Cardiovasc. Imaging Rep. 2017;10:31. [Google Scholar]
- 12.Yu W.L., Omid-Fard N., Arepalli C. Role of computed tomography in pre- procedural planning of transcatheter mitral valve replacement. Struct. Heart. 2018;2:23–29. [Google Scholar]
- 13.Giannini C., Petronio A.S., De Carlo M. Integrated reverse left and right ventricular remodelling after MitraClip implantation in functional mitral regurgitation: an echocardiographic study. Eur. Heart J. Cardiovasc. Imaging. 2014;15:95–103. doi: 10.1093/ehjci/jet141. [DOI] [PubMed] [Google Scholar]
- 14.Maisano F., Alfieri O., Banai S. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. replacement? Eur. Heart J. 2015;36:1651–1659. doi: 10.1093/eurheartj/ehv123. [DOI] [PubMed] [Google Scholar]
- 15.Kuwata S., Taramasso M., Guidotti A., Nietlispach F., Maisano F. Ongoing and future directions in percutaneous treatment of mitral regurgitation. Expert Rev. Cardiovasc. Ther. 2017;15:441–446. doi: 10.1080/14779072.2017.1327349. [DOI] [PubMed] [Google Scholar]
- 16.Muller D.W.M., Farivar R.S., Jansz P. Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J. Am. Coll. Cardiol. 2017;69:381–391. doi: 10.1016/j.jacc.2016.10.068. [DOI] [PubMed] [Google Scholar]
- 17.Delgado V., Kapadia S., Marsan N.A., Schalij M.J., Tuzcu E.M., Bax J.J. Multimodality imaging before, during, and after percutaneous mitral valve repair. Heart. 2011;97:1704–1714. doi: 10.1136/hrt.2011.227785. [DOI] [PubMed] [Google Scholar]
- 18.Budoff M.J., Shinbane J.S., editors. Cardiac CT Imaging. Diagnosis of Cardiovascular Disease. Springer International Publishing; 2016. ISBN 978-3-319-28217-6. [Google Scholar]
- 19.Mahesh M., Cody D.D. Physics of cardiac imaging with multiple-row detector CT. Radiographics. 2007;27:1495–1509. doi: 10.1148/rg.275075045. [DOI] [PubMed] [Google Scholar]
- 20.Koo H.J., Yang D.H., Oh S.Y. Demonstration of mitral valve prolapse with CT for planning of mitral valve repair. Radiographics. 2014;34:1537–1552. doi: 10.1148/rg.346130146. [DOI] [PubMed] [Google Scholar]
- 21.Kalisz K., Buethe J., Saboo S.S., Abbara S., Halliburton S., Rajiah P. Artifacts at cardiac CT: physics and solutions. Radiographics. 2016;36:2064–2083. doi: 10.1148/rg.2016160079. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z., Choo G.H., Ng K.H. Coronary CT angiography: current status and continuing challenges. Br. J. Radiol. 2012;85:495–510. doi: 10.1259/bjr/15296170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faggioni L., Gabelloni M. Iodine concentration and optimization in computed tomography angiography: current issues. Invest. Radiol. 2016;51:816–822. doi: 10.1097/RLI.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 24.Blanke P., Dvir D., Cheung A. A simplified D-shaped model of the mitral annulus to facilitate CT-based sizing before transcatheter mitral valve implantation. J. Cardiovasc. Comput. Tomogr. 2014;8:459–467. doi: 10.1016/j.jcct.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanke P., Naoum C., Webb J. Multimodality imaging in the context of transcatheter mitral valve replacement: establishing consensus among modalities and disciplines. JACC Cardiovasc. Imaging. 2015;8:1191–1208. doi: 10.1016/j.jcmg.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Naoum C., Leipsic J., Cheung A. Mitral annular dimensions and geometry in patients with functional mitral regurgitation and mitral valve prolapse: implications for transcatheter mitral valve implantation. JACC Cardiovasc. Imaging. 2016;9:269–280. doi: 10.1016/j.jcmg.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Eleid M.F., Foley T.A., Said S.M., Pislaru S.V., Rihal C.S. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc. Imaging. 2016;9:1318–1337. doi: 10.1016/j.jcmg.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Blanke P., Naoum C., Dvir D. Predicting LVOT obstruction in transcatheter mitral valve implantation: concept of the neo-LVOT. JACC Cardiovasc. Imaging. 2017;10:482–485. doi: 10.1016/j.jcmg.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Biaggi P., Fernandez-Golfín C., Hahn R., Corti R. Hybrid imaging during transcatheter structural heart interventions. Curr. Cardiovasc. Imaging Rep. 2015;8(33) doi: 10.1007/s12410-015-9349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]