Abstract
Cytosolic phospholipase A2 (GIVA cPLA2) has attracted great interest as a medicinal target because it initiates the eicosanoid cascade and is involved in a number of inflammatory diseases. As a consequence, the development of potent synthetic inhibitors is of great importance. We have developed highly potent 2-oxoester inhibitors of GIVA cPLA2 presenting XI(50) values between 0.000019 and 0.000066. We demonstrate that the 2-oxoester functionality is essential for in vitro inhibitory activity, making these inhibitors useful research reagents. However, their high reactivity results in rapid degradation of the inhibitors in human plasma, limiting their pharmaceutical utility without further modification.
Introduction
Phospholipases A2 (PLA2s) have attracted great interest as medicinal targets for more than 20 years because they are involved in a number of inflammatory diseases.1 Among this superfamily of enzymes,2 cytosolic PLA2 (GIVA cPLA2) stands out because it exhibits a marked preference for the hydrolysis of arachidonic acid at the sn-2 position of phospholipid substrates releasing arachidonic acid and initiating the eicosanoid cascade.3 Both the physiological function and the role of cytosolic PLA2 have been recently summarized by Leslie.4 Due to the involvement of PLA2s in various inflammatory diseases, many synthetic inhibitors have been developed in both academia and pharmaceutical companies.1 Two recent review articles discuss the classes of PLA2 inhibitors and highlight the in vitro activities and selectivity and the in vivo studies in animal models.5,6
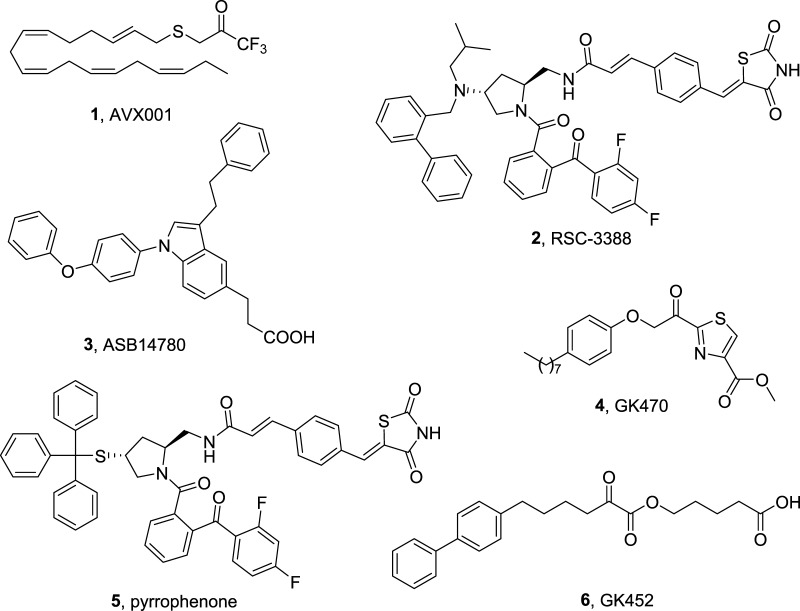
Most recently, a randomized double-blind placebo-controlled dose-escalation first-in-man study to assess the safety and efficacy of a topical cytosolic PLA2 inhibitor AVX001 (1, Figure 1) in patients with mild-to-moderate plaque psoriasis has demonstrated that treatment with AVX001 is well tolerated in doses up to 5%.7 Pharmacological inhibition of GIVA cPLA2 by inhibitor 2 (Figure 1) blocked Streptococcus pneumoniae-induced polymorphonuclear cells transepithelial migration in vitro, suggesting that this enzyme plays a crucial role in eliciting pulmonary inflammation during pneumococcal infection.8 The daily administration of the indole-based inhibitor ASB14780 (3, Figure 1), which had been developed by Tomoo and colleagues,9 markedly ameliorated liver injury and hepatic fibrosis following 6 weeks of treatment with CCl4, indicating that a GIVA cPLA2 inhibitor could be useful for the treatment of nonalcoholic fatty liver diseases, including fatty liver and hepatic fibrosis.10 A few years ago, we presented new thiazolyl ketones as inhibitors of GIVA cPLA2 and demonstrated the in vivo anti-inflammatory activity of inhibitor GK470 (4, Figure 1) in a collagen-induced arthritis model.11 The anti-angiogenic effects of this inhibitor (now named as AVX235) in a patient-derived triple-negative basal-like breast cancer model was evaluated and significant tumor growth inhibition was observed after 8 days of treatment.12 Most recent findings showed that blockage of GIVA cPLA2 by either inhibitor 2 or pyrrophenone (5, Figure 1) sensitized aggressive breast cancer to doxorubicin by suppressing ERK and mTOR kinases.13
Figure 1.
Structures of known GIVA cPLA2 inhibitors.
All of the above-described recent applications of synthetic GIVA cPLA2 inhibitors highlight the importance of identifying new highly potent inhibitors to regulate the activity of GIVA cPLA2. Last year, we reported the development of a novel class of GIVA cPLA2 inhibitors, namely, 2-oxoesters.14 2-Oxoester GK452 (6, Figure 1), containing a biphenyl system and a free carboxyl group, led to highly potent and selective GIVA cPLA2 in vitro inhibition (XI(50) 0.000078). The aim of the present work was to further understand the characteristics of 2-oxoesters and explore the possibility of producing more potent GIVA cPLA2 inhibitors.
Results and Discussion
Synthesis of Inhibitors
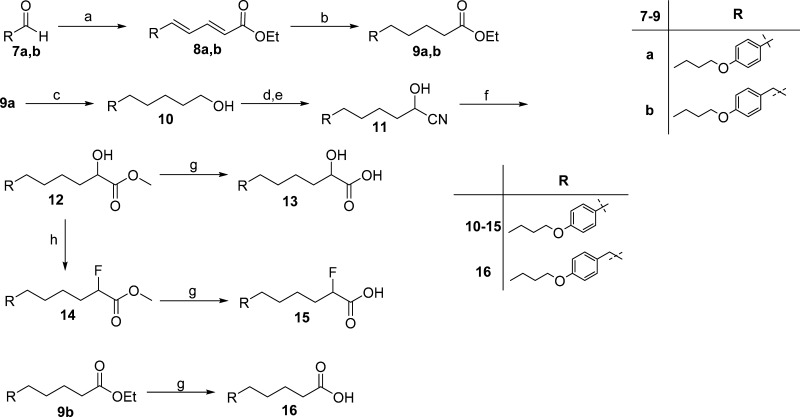
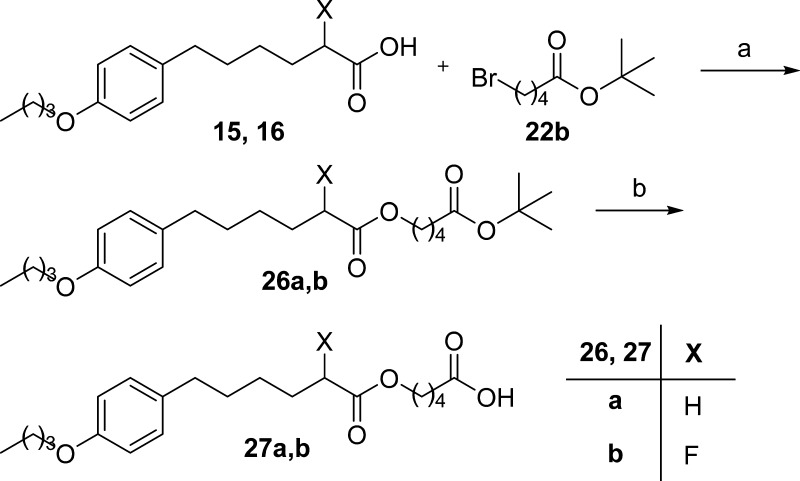
To extend the structure–activity relationship studies on 2-oxoester inhibitors, we (a) shortened the length between the activated carbonyl group and the biphenyl functionality, (b) replaced the biphenyl system with a para-alkoxy-substituted phenyl group, and (c) explored the importance of the activated carbonyl group. To this end, a variety of 2-substituted carboxylic acids, required for the target compounds, were synthesized as described in Scheme 1.
Scheme 1.
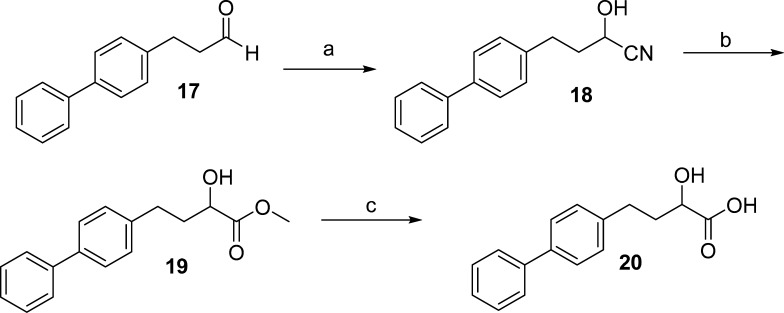
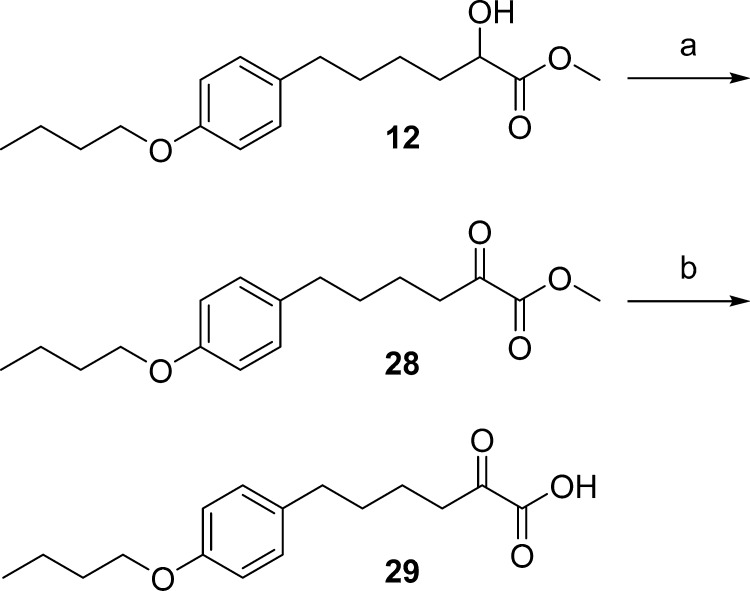
The synthesis of carboxylic acids 13, 15, and 16, bearing a 4-butoxy-phenyl group, started with the Wadsworth–Horner–Emmons olefination reaction of aldehydes 7a, 7b(15) with triethyl phosphonocrotonate to give the corresponding unsaturated esters 8a,b, which were hydrogenated to compounds 9a,b. The reduction of 9a led to alcohol 10, which subsequently provided cyanohydrin 11. Subsequent treatment with HCl in methanol gave 2-hydroxy methyl ester 12, which was then saponified to 2-hydroxy acid 13 by treatment with aqueous NaOH. Treatment of 12 with the fluorinating agent diethylaminosulfur trifluoride (DAST)16 resulted in 2-fluoro methyl ester 14, consequently providing 2-fluoro carboxylic acid 15 after saponification. The carboxylic acid 16 was also obtained by alkaline hydrolysis of 9b. The synthesis of 2-hydroxy acid 20 was accomplished by similar procedures starting from aldehyde 17,17 as depicted in Scheme 2.
Scheme 2.
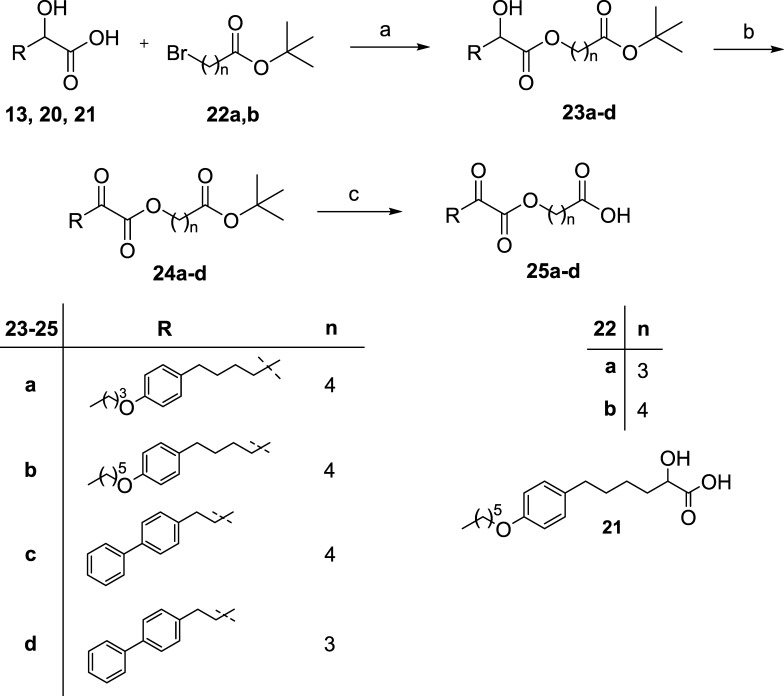
The key step in the synthesis of 2-oxoesters was the reaction between cesium salt of 2-hydroxy carboxylic acids with appropriate tert-butyl bromoalkanoate 22a,b(14) (Scheme 3). 2-Hydroxy esters 23a–d, obtained from 2-hydroxy carboxylic acids 13, 20, and the previously synthesized 21,14 were then oxidized to the corresponding 2-oxoesters 24a–d using the Dess–Martin periodinane reagent. Cleavage of tert-butyl protecting group led to the target compounds 25a–d (Scheme 3).
Scheme 3.
Likewise, reaction of 2-fluoro carboxylic acid 15 and acid 16 with tert-butyl bromopentanoate (22b) resulted in derivatives 26a,b, and subsequently, after the removal of tert-butyl group, to the desired products 27a,b (Scheme 4). Finally, the synthesis of 2-oxoacid 29 was accomplished as shown in Scheme 5 by saponification of the corresponding 2-oxoester 28 under mild conditions.
Scheme 4.
Scheme 5.
In Vitro Inhibition of GIVA cPLA2, GVIA iPLA2, and GV sPLA2
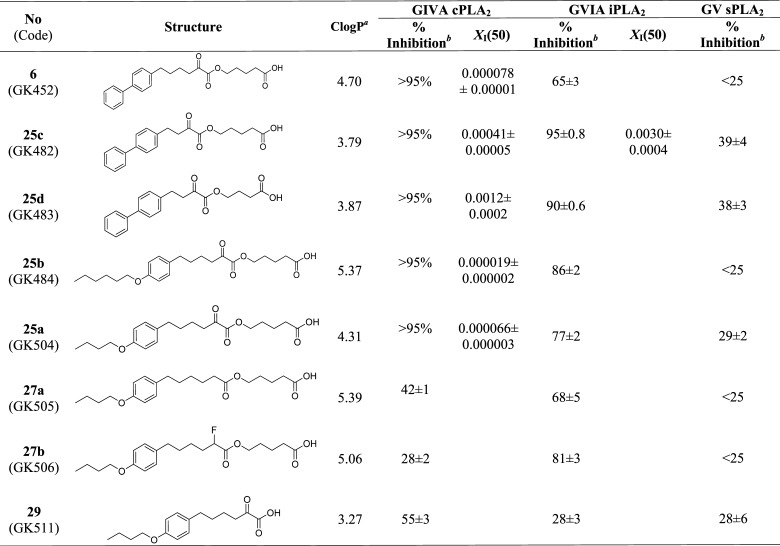
All of the new compounds synthesized were tested for in vitro inhibition of human GIVA cPLA2, calcium-independent phospholipase A2 (GVIA iPLA2), and secreted phospholipase A2 (GV sPLA2) using previously described mixed micelle-based assays.18−20 The inhibition results are presented in Table 1, either as percent inhibition or as XI(50) values. At first, the percent of inhibition for each PLA2 enzyme at a high mole fraction (0.091) of each inhibitor was determined. Then, the XI(50) values were measured for compounds that displayed more than 95% inhibition. The XI(50) is the mole fraction of the inhibitor in the total substrate interface required to inhibit the enzyme by 50%.
Table 1. In Vitro Inhibitory Potency and Selectivity of 2-Oxoesters.
Calculated with ChemDraw.
% Inhibition at 0.091 mol fraction of each inhibitor.
Because it is an important property of GIVA cPLA2 inhibitors, the Clog P values for all the compounds tested, calculated by ChemDraw, are also included in Table 1. The Clog P value is a measure of hydrophobicity and represents the calculated partition coefficient in octanol/water on a logarithmic scale.
Inhibitor 6 (GK452) (XI(50) 0.000078)14 is included in Table 1 for comparison purposes. When the distance between the oxoester functionality and the biphenyl system was decreased from four to two carbon atoms (compound 25c (GK482) and compound 25d (GK483)), the inhibitory potency against GIVA cPLA2 considerably decreased. This decrease was 1 order of magnitude in the case of a four-carbon linker (compound 25cXI(50) 0.00041), further decreasing (2 orders of magnitude) when the linker was further shortened (25dXI(50) 0.0012). These results are in accordance with our previous results14 and confirm that the optimum distance between the free carboxyl group and the oxoester functionality corresponds to four carbon atoms. In addition, compound 25c lost the selectivity because the inhibition of GVIA iPLA2 was observed (XI(50) 0.0030). Thus, the four-carbon linker was maintained for all the other derivatives. The optimum length of the linker may be correlated with stereochemical reasons, rather than hydrophobicity.
Interestingly, when one ring of the biphenyl system was replaced by a para-hexyloxy group, an increase in the inhibitor potency against GIVA cPLA2 was observed. Compound 25b (GK484) is the most potent inhibitor of GIVA cPLA2 ever reported with a XI(50) value of 0.000019, 4 times more potent than 6. However, the replacement of the phenyl ring by the hexyloxy group resulted in an increase in the Clog P value (from 4.70 for 6 to 5.37 for 25b). Thus, we shortened the hexyloxy chain to butyloxy one. Compound 25a (GK504) (XI(50) 0.000066) is 3 times less potent than 25b, but still slightly more potent than 6. In addition, its Clog P value (4.31) is lower than 5, which is favorable because Lipinski’s “rule of 5” predicts that poor absorption or permeation is more likely when CLog P is greater than 5.21 Neither 25b nor 25a presented significant inhibition of GVIA iPLA2 (86 and 77%, respectively, at a high mole fraction 0.091). In our experience, compounds that show inhibition of PLA2 less than 90% at the highest concentration tested (which corresponds to about 50 μM inhibitor) always exhibit XI(50) values greater than 0.01.11,23 The GIVA cPLA2 inhibitor 25b with a XI(50) 0.000019 is the most potent inhibitor of GIVA cPLA2 ever reported and is at least 500-fold selective over GVIA iPLA2.
To confirm the importance of the oxoester functionality, we removed the activated carbonyl group. Compound 27a (GK505) abolished the inhibitory potency against GIVA cPLA2. Because the only structural difference between 27a and 25a is the lack of the carbonyl group, a comparison of the inhibitory potencies of 25a and 27a makes it clear that the carbonyl group of the oxoester functionality is essential for GIVA cPLA2 inhibition. To explore if an electron-withdrawing group at position 2 could contribute to the inhibitory potency, the fluoro derivative 27b (GK506) was studied. However, again, no inhibition of GIVA cPLA2 was observed. Finally, 2-oxoacid 29 (GK511) was evaluated in vitro and only a very weak inhibition of GIVA cPLA2 was recorded (55% at a high mole fraction 0.091). Because this 2-oxoacid is a fragment of 2-oxoester 25a, it is clear that the oxoester functionality is absolutely necessary for the inhibition.
Plasma Stability Studies
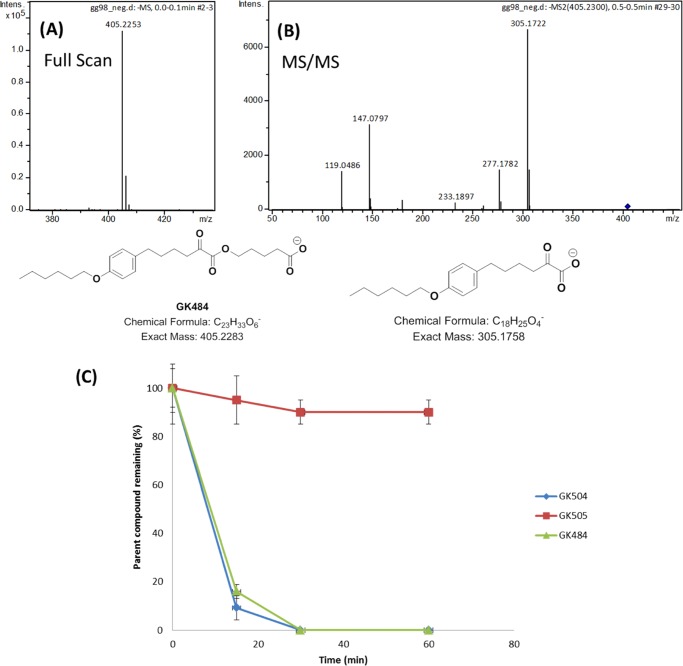
Determination of the stability of new chemical entities in plasma is important, as compounds (with the exception of pro-drugs), which rapidly degrade in plasma, generally show poor in vivo performance.22 The stability of compounds 25a, 25b, and 27a in human plasma was studied in a time-dependent manner. Our aim was to understand the difference between the two highly potent 2-oxoesters GIVA cPLA2 inhibitors 25a and 25b in comparison to compound 27a lacking the activated carbonyl group. A liquid chromatography-high resolution mass spectrometry (LC-HRMS) method was developed to measure the inhibitor levels in plasma. All of the HRMS spectra were obtained in electrospray ionization (ESI) negative ion mode. The molecular ion of inhibitor 25b (GK484) was recorded at m/z 405.2253 (Figure 2A), whereas in the MS/MS spectrum (Figure 2B), the most intense signal corresponds to the loss of C5H8O2 (−100.0524), indicating the fragmentation of the ester bond. The HRMS spectra of inhibitors 25a and 27a are shown in the Supporting Information. In all cases of 2-oxoester inhibitors, the fragmentation of the ester bond was observed. Plasma samples incubated with each inhibitor were taken at 0, 15, 30, and 60 min and the molecular ion of each inhibitor was used to estimate the inhibitor level. The stability results are presented in Figure 2C as percent parent compound remaining at each time point. Inhibitors 25a (GK504) and 25b (GK484), containing the 2-oxo group, are not detectable in the plasma samples after 30 min, in contrast to 27a (GK505), whose percentage remains close to 100% after 1 h. Apparently, the activated carbonyl group of the 2-oxoester functionality of inhibitors 25a and 25b increases the chemical reactivity of the ester bond toward hydrolysis in comparison to the less active simple ester bond of inhibitor 27a. These results indicate that the activated carbonyl group, which is essential for the enzyme inhibition, makes the compounds susceptible to rapid degradation in human plasma. A compound like 27a, lacking the activated carbonyl group, is rather stable in plasma, however lacking the inhibitory properties.
Figure 2.
Precursor ion (A) and MS/MS HRMS spectrum (B) of inhibitor GK484. Time-dependent degradation of inhibitors GK484, GK504, and GK505 in human plasma (C).
Conclusions
In conclusion, a number of new 2-oxoesters and analogues were synthesized. GK484 is the most potent inhibitor of GIVA cPLA2 ever reported with a XI(50) value of 0.000019. The study of the in vitro inhibitory activity highlighted the importance of the 2-oxoester group for the inhibitory potency against GIVA cPLA2. The stability studies of 2-oxoesters in human plasma indicate that a fine-tuning between the reactivity of the carbonyl group and its stability will be needed in order for 2-oxoesters to be used in vivo as pharmacological agents.
Experimental Section
General
Merck Silica Gel 60254 aluminum plates and Silica Gel 60 (70–230 or 230–400 mesh) were used for thin-layer chromatography and chromatographic purification of products, respectively. For visualizing spots, UV light and/or phosphomolybdic acid was employed. Melting points were estimated by a Büchi 530 apparatus and uncorrected. 1H, 13C, and 19F NMR spectra were recorded on Varian Mercury at 200, 50, and 188 MHz, respectively. CDCl3 was used as the solvent. Chemical shifts are given in ppm and coupling constants (J) in Hz. Peak multiplicities are typified as follows: s, singlet; d, doublet; dd, doublet of doublets; t, triplet; q, quartet; qt, quintet; and m, multiplet. Dichloromethane was dried by standard procedures and stored over molecular sieves. No further purification of other solvents and chemicals was needed. The HRMS spectra were recorded on a Bruker Maxis Impact QTOF Spectrometer.
Synthesis
Compound 7a was commercially available, and compounds 7b,1517,17 and 21(14) have been described elsewhere and their analytical data are in accordance with literature.
General Procedure for Synthesis of Unsaturated Esters 8a,b
To a flame-dried, round-bottomed flask containing powdered molecular sieves (1 g), LiOH·H2O (1.5 mmol, 36 mg) and a solution of aldehyde 7a,b (1 mmol) in dry THF (10 mL) were added under Ar atmosphere. Triethyl 4-phosphonocrotonate (1.5 mmol, 375 mg) was added and the mixture was left under reflux overnight. The reaction mixture was filtered through celite and the solvent was evaporated under reduced pressure. The products were obtained after trituration with MeOH.
Ethyl (2E,4E)-5-(4-Butoxyphenyl)penta-2,4-dienoate (8a)
Yield 60%; white solid; mp 57–60 °C; 1H NMR (200 MHz, CDCl3): δ 7.49–7.18 (m, 3H, arom, =CH), 6.89–6.50 (m, 4H, arom, 2 × =CH), 5.87 (d, J = 15.2 Hz, 1H, =CH), 4.17 (q, J = 7.1 Hz, 2H, CH2OCO), 3.87 (t, J = 6.4 Hz, 2H, CH2O), 1.82–1.60 (m, 2H, CH2), 1.47–1.33 (m, 2H, CH2), 1.25 (t, J = 7.1 Hz, 3H, CH3CH2OCO), 0.92 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3) δ 167.3, 160.2, 145.3, 140.4, 128.9, 128.7, 124.1, 120.1, 114.9, 67.9, 60.3, 31.5, 19.4, 14.6, 14.1; Anal. Calcd for C17H22O3: C, 74.42; H, 8.08; found: C, 74.06; H, 8.53.
Ethyl (2E,4E)-6-(4-Butoxyphenyl)hexa-2,4-dienoate (8b)
Yield 50%; white solid; mp 62–65 °C 1H NMR (200 MHz, CDCl3): δ 7.36–7.17 (m, 1H, =CH), 7.03 (d, J = 8.4 Hz, 2H, arom) 6.81 (d, J = 8.4 Hz, 2H, arom), 6.15 (d, J = 8.0 Hz, 2H, 2x=CH), 5.79 (d, J = 15.3 Hz, 1H, =CH), 4.16 (q, J = 7.1 Hz, 2H, CH2OCO), 3.89 (t, J = 6.3 Hz, 2H, CH2O), 3.59–3.30 (m, 2H, PhCH2), 1.81–1.63 (m, 2H, CH2), 1.45 (m, 2H, CH2), 1.25 (t, J = 7.1 Hz, 3H, CH3CH2OCO), 0.96 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 166.3, 157.2, 143.9, 142.2, 129.9, 128.9, 128.4, 119.5, 66.9, 59.5, 113.9, 37.8, 30.8, 18.7, 13.7, 13.3; Anal. Calcd for C18H24O3: C, 74.97; H, 8.39; found: C, 74.64; H, 8.72.
General Procedure for Hydrogenation
To a solution of unsaturated esters 8a,b (1 mmol) in EtOH (10 mL), 10% Pd/C was added and the mixture stirred overnight under H2 atmosphere. The reaction mixture was filtered through celite and concentrated in vacuo to isolate the saturated product 9a,b.
Ethyl 5-(4-Butoxyphenyl)pentanoate (9a)
Yield 99%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.07 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 4.12 (q, J = 7.1 Hz, 2H, CH2OCO), 3.92 (t, J = 6.4 Hz, 1H, CH2O), 2.56 (t, J = 6.9 Hz, 2H, PhCH2), 2.31 (t, J = 7.0 Hz, 2H, CH2CO), 1.84–1.70 (m, 2H, CH2), 1.69–1.56 (m, 4H, CH2), 1.56–1.38 (m, 2H, CH2), 1.24 (t, J = 7.1 Hz, 3H, CH3CH2O), 0.97 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 173.8, 157.5, 134.2, 129.4, 114.5, 67.8, 60.4, 34.9, 34.4, 31.7, 31.4, 24.8, 19.5, 14.5, 14.1; Anal. Calcd for C17H26O3: C, 73.35; H, 9.41; found: C, 73.07; H, 9.69.
Ethyl 6-(4-Butoxyphenyl)hexanoate (9b)
Yield 99%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.08–6.94 (d, J = 8.3 Hz, 2H, arom), 6.85–6.67 (d, J = 8.6 Hz, 2H, arom), 4.43–4.10 (m, 2H, CH2OCO), 3.87 (t, J = 6.4 Hz, 2H, CH2O), 2.64–2.39 (m, 2H, PhCH2), 2.33–2.18 (m, 2H, CH2CO), 1.86–1.30 (m, 10H, 5 × CH2), 1.22 (t, J = 7.2 Hz, 3H, CH3CH2O), 0.94 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 173.5, 157.0, 134.1, 129.0, 114.3, 67.3, 59.9, 34.6, 34.0, 31.3, 29.6, 28.4, 24.6, 19.1, 14.0, 13.7; Anal. Calcd for C18H28O3: C, 73.93; H, 9.65; found: C, 73.72; H, 9.82.
5-(4-Butoxyphenyl)pentan-1-ol (10)
A solution of 9a (1 mmol, 0.27 g) in anhydrous Et2O (10 mL) under Ar was cooled at 0 °C. DIBALH (1.2 mmol, 1.2 mL) was added dropwise and the mixture was left at room temperature for 30 min. The reaction was quenched by adding ice at 0 °C and left to stir for 30 min. The mixture was filtered through celite and concentrated under reduced pressure. The residue was purified by column chromatography, using EtOAc/petroleum ether (bp 40–60 °C) 3:7 as eluent. Yield 90%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.07 (d, J = 8.5 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 3.60 (t, J = 6.5 Hz, 2H, CH2OH), 2.50 (t, J = 6.4 Hz, 2H, PhCH2), 2.08 (brs, 1H, OH), 1.86–1.30 (m, 10H), 0.97 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 157.4, 134.7, 129.4, 114.5, 67.9, 63.0, 35.2, 32.8, 31.8, 31.6, 25.6, 19.5, 14.1; Anal. Calcd for C15H24O2: C, 76.23; H, 10.24; found: C, 76.09; H, 10.32.
6-(4-Butoxyphenyl)-2-hydroxyhexanenitrile (11)
To a stirring solution of alcohol 10 (1 mmol, 0.24 g) in dry CH2Cl2 (10 mL), iodobenzene diacetate (1.2 mmol, 0.39 g) and a catalytic amount of TEMPO (10%, 0.024 g) were added and the reaction mixture was left stirring at room temperature for 1 h. The reaction mixture was washed consecutively with 10% aqueous Na2S2O3 (10 mL), 10% aqueous NaHCO3 (10 mL), and brine (10 mL), dried over Na2SO4, and concentrated in vacuo. The aldehyde was then dissolved in CH2Cl2 (1.5 mL) and 4 N aqueous NaHSO3 (2 mmol, 0.5 mL) and the mixture was left stirring for 30 min at room temperature. The organic solvent was removed under reduced pressure and H2O (1.5 mL) was added. The mixture was cooled down to 0 °C and 4 N aqueous KCN (0.5 mL) was added dropwise within 2 h under vigorous stirring, followed by stirring overnight at room temperature. Then, H2O (10 mL) was added and extracted with CH2Cl2 (3 × 10 mL). The combined organic layers were washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography with EtOAc/petroleum ether (bp 40–60 °C) 3:7 as eluent. Yield 71%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.08 (d, J = 8.3 Hz, 2H, arom), 6.83 (d, J = 8.3 Hz, 2H, arom), 4.53–4.38 (m, 1H, CH), 3.95 (t, J = 6.4 Hz, 2H, CH2O), 2.58 (t, J = 7.1 Hz, 2H, PhCH2), 1.93–1.38 (m, 10H, 5 × CH2), 0.98 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 157.5, 134.2, 129.5, 120.4, 114.7, 68.0, 61.4, 35.3, 35.0, 31.6, 31.3, 24.4, 19.5, 14.2; MS (ESI) m/z (%): 279 ([M + NH4]+, 100); Anal. Calcd for C16H23NO2: C, 73.53; H, 8.87; N, 5.36; found: C, 73.32; H, 9.03; N, 5.18.
4-([1,1′-Biphenyl]-4-yl)-2-hydroxybutanenitrile (18)
To a stirring solution of aldehyde 17 (1.0 mmol) in CH2Cl2 (1.5 mL), an aqueous solution of NaHSO3 6 M (0.25 mL, 1.5 mmol) was added and the mixture was left stirring for 30 min at room temperature. The organic solvent was evaporated under reduced pressure and H2O (5 mL) was added. The mixture was cooled down to 0 °C and an aqueous solution of KCN 6 M (0.25 mL, 1.5 mmol) was added dropwise under vigorous stirring. The reaction was left stirring for 18 h at room temperature and then the mixture was extracted with CH2Cl2 (2 × 20 mL), washed with brine, and dried over Na2SO4. The organic solvent was evaporated under reduced pressure and the residue was purified by flash column chromatography using EtOAc/petroleum ether (bp 40–60 °C) 2.5:7.5 as eluent. Yield 59%; white solid; mp 93–95 °C; 1H NMR (200 MHz, CDCl3): δ = 7.66–7.13 (m, 9H, arom), 4.16 (q, J = 7.2 Hz, 1H, CH), 3.76 (brs, 1H, OH), 2.90 (t, J = 7.4 Hz, 2H, PhCH2), 2.22–2.13 (m, 2H, CH2). 13C NMR (50 MHz, CDCl3): δ 140.6, 139.3, 138.6, 128.8, 128.7, 127.3, 127.1, 126.9, 119.9, 60.2, 36.4, 30.2; MS (ESI) m/z (%): 255 ([M + NH4]+, 100); Anal. Calcd for C16H15NO: C, 80.98; H, 6.37; N, 5.90; found: C, 80.64; H, 6.68; N, 5.73.
General Procedure for Synthesis of 2-Hydroxy Esters 12 and 19
Cyanohydrins 11 and 18 (1 mmol) were dissolved in 4 N HCl/MeOH (10 mL) and the reaction mixture stirred for 24 h at room temperature. The organic solvent was evaporated under reduced pressure. The residue was dissolved in Et2O (10 mL) and re-evaporated. Dilution and evaporation were repeated twice. The product was purified by flash column chromatography using EtOAc/petroleum ether (bp 40–60 °C) 2:8 as eluent.
Methyl 6-(4-Butoxyphenyl)-2-hydroxyhexanoate (12)
Yield 65%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.07 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 4.25–4.10 (m, 1H, CH), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 3.77 (s, 3H, OCH3), 2.83 (bs, 1H, OH), 2.63–2.48 (m, 2H, PhCH2), 1.93–1.37 (m, 10H, CH2), 0.97 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 176.0, 157.4, 134.5, 129.4, 114.5, 70.6, 67.9, 52.8, 35.3, 35.0, 34.5, 31.6, 24.6, 19.5, 14.1; MS (ESI) m/z (%): 312 ([M + NH4]+, 100); Anal. Calcd for C17H26O4: C, 69.36; H, 8.90; found: C, 69.18; H, 9.02.
Methyl 4-([1,1′-Biphenyl]-4-yl)-2-hydroxybutanoate (19)
Yield 64%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.64–7.22 (m, 9H, arom), 4.25 (q, J = 4.0 Hz, 1H, CH), 3.77 (s, 3H, OCH3), 2.84 (t, J = 8.2 Hz, 2H, PhCH2), 2.30–1.84 (m, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ 175.6, 140.9, 140.1, 138.9, 129.0, 128.7, 127.1, 127.0, 126.9, 69.6, 52.5, 35.8, 30.6; MS (ESI) m/z (%): 288 ([M + NH4]+, 100); Anal. Calcd for C17H18O3: C, 75.53; H, 6.71; found: C, 75.32; H, 6.84.
Methyl 6-(4-Butoxyphenyl)-2-fluorohexanoate (14)
A solution of diethylaminosulfur trifluoride (1.1 mmol, 0.15 mL) in CH2Cl2 (0.23 mL) was added to a flame-dried flask, under Ar atmosphere, and cooled down to −78 °C. A solution of ester 12 (1 mmol, 0.29 g) in anhydrous CH2Cl2 (0.3 mL) was added dropwise and the reaction stirred for 2 h at −78 °C and then overnight at room temperature. The reaction mixture was then concentrated under reduced pressure and the product was purified by column chromatography using EtOAc/petroleum ether (bp 40–60 °C) 2:8 as eluent. Yield 55%; yellowish oil; 1H NMR (200 MHz, CDCl3): δ 7.09 (d, J = 8.5 Hz, 2H, arom), 6.83 (d, J = 8.5 Hz, 2H, arom), 4.90 (dt, J = 49.0 Hz, J = 6.1 Hz, 1H, CHF), 3.94 (t, J = 6.4 Hz, 2H, CH2O), 3.77 (s, 3H, OCH3), 2.58 (t, J = 7.3 Hz, 2H, PhCH2), 1.94–1.40 (m, 10H, CH2), 0.99 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 170.3 (d, J = 23.9 Hz), 157.1, 133.7, 129.0, 114.1, 88.7 (d, J = 184.0 Hz), 67.4, 52.02, 34.5, 32.1 (d, J = 21.0 Hz), 31.2, 31.0, 23.8 (d, J = 3 Hz), 19.1, 13.7; 19F NMR (188 MHz, CDCl3): δ −150.03 (qt, J = 24.4 Hz). MS (ESI) m/z (%): 314 ([M + NH4]+, 100); Anal. Calcd for C17H25FO3: C, 68.89; H, 8.50; found: C, 68.66; H, 8.71.
General Procedure for Saponification
To a stirring solution of methyl esters 9b, 12, 14, and 19 (1 mmol) in 1,4-dioxane (10 mL), 2 N aqueous NaOH (2.0 mL) was added and the mixture stirred at room temperature overnight. The solvent was evaporated under reduced pressure and the residue was dissolved in H2O (10 mL) and extracted with Et2O (3 × 10 mL). The aqueous layer was then acidified with 2 N HCl (4.0 mL) and extracted with EtOAc (3 × 10 mL) followed by drying over Na2SO4 and concentrating in vacuo.
6-(4-Butoxyphenyl)-2-hydroxyhexanoic Acid (13)
Yield 95%; yellowish solid; mp 88–90 °C; 1H NMR (200 MHz, CDCl3): δ 7.06 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 4.28–4.22 (m, 1H, CH), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 2.55 (t, J = 7.2 Hz, 2H, PhCH2), 1.95–1.37 (m, 10H, 5 × CH2), 0.97 (t, J = 6.4 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3) δ 180.0, 157.4, 134.5, 129.4, 114.6, 70.4, 68.0, 35.0, 34.2, 31.6, 24.7, 19.5, 14.1; MS (ESI) m/z (%): 279 ([M – H]−, 100); Anal. Calcd for C16H24O4: C, 68.55; H, 8.63; found: C, 68.32; H, 8.75.
6-(4-Butoxyphenyl)-2-fluorohexanoic Acid (15)
Yield 95%; yellowish solid; mp 65–67 °C; 1H NMR (200 MHz, CDCl3): δ 9.90 (s, 1H, COOH), 7.08 (d, J = 8.5 Hz, 2H, arom), 6.83 (d, J = 8.6 Hz, 2H, arom), 4.95 (dt, J = 48.6 Hz, 6.4 Hz, 1H, CHF), 3.95 (t, J = 6.5 Hz, 2H, CH2O), 2.58 (t, J = 7.2 Hz, 2H, PhCH2), 2.06–1.36 (m, 10H, 5 × CH2), 0.98 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 175.4 (d, J = 25 Hz), 157.1, 133.9, 129.1, 114.3, 88.2 (d, J = 185 Hz), 67.6, 34.6, 32.0 (d, J = 20.0 Hz), 31.3, 31.0, 23.9 (d, J = 2.5 Hz), 19.2, 13.8; 19F NMR (188 MHz, CDCl3): δ −150.4 (qt, J = 25.6 Hz). MS (ESI) m/z (%): 281 ([M – H]−, 100); Anal. Calcd for C16H23FO3: C, 68.06; H, 8.21; found: C, 67.93; H, 8.46.
6-(4-Butoxyphenyl)hexanoic Acid (16)
Yield 95%; yellowish solid; mp 198–200 °C; 1H NMR (200 MHz, CDCl3): δ 7.01 (d, J = 7.9 Hz, 2H, arom), 6.76 (d, J = 8.4 Hz, 2H, arom), 3.90 (m, 2H, CH2O), 2.70–2.33 (m, 2H, PhCH2), 2.15 (d, J = 6.6 Hz, 2H, CH2CO), 1.85–1.10 (m, 10H, CH2), 0.99 (t, J = 5.6 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 180.7, 156.9, 134.4, 128.9, 114.0, 67.5, 36.8, 34.7, 31.2, 29.1, 25.7, 19.0, 13.6; MS (ESI) m/z (%): 263 ([M – H]−, 100); Anal. Calcd for C16H24O3: C, 72.69; H, 9.15; found: C, 72.51; H, 9.29.
4-([1,1′-Biphenyl]-4-yl)-2-hydroxybutanoic Acid (20)
Yield 90%; white solid;. mp 189–191 °C; 1H NMR (200 MHz, CDCl3): δ 7.64–7.19 (m, 9H, arom), 4.18–4.10 (m, 1H, CH), 2.80 (t, J = 7.4 Hz, 2H, PhCH2), 2.23–1.83 (m, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ 177.8, 142.2, 141.7, 140.1, 138.7, 129.9, 129.7, 128.0, 127.7, 70.6, 37.2, 31.9; MS (ESI) m/z (%): 255 ([M – H]−, 100); Anal. Calcd for C16H16O3: C, 74.98; H, 6.29; found: C, 74.78; H, 6.42
General Procedure for Synthesis of 23a–d and 26a,b
To a stirred solution of carboxylic acid (1 mmol) in tetrahydrofuran (THF) (6 mL) and 20% aqueous solution CsCO3 (1.1 mmol, 1.8 mL) were added and left stirring for 10 min at 80 °C. The organic solvent was evaporated under reduced pressure and the residue was dissolved in N,N-dimethylformamide (DMF) (15 mL). Subsequently, the appropriate tert-butyl 5-bromoalkanoate, 22a or 22b (1.2 mmol), was added and the reaction mixture was left vigorously stirring under reflux for 72 h. The reaction mixture was concentrated in vacuo and then water (20 mL) was added and extracted with EtOAc (2 × 20 mL). The organic phase was dried (Na2SO4) and concentrated under reduced pressure. The residue was purified by flash column chromatography using EtOAc/petroleum ether (bp 40–60 °C) 3:7 or 2:8.
5-(tert-Butoxy)-5-oxopentyl 6-(4-butoxyphenyl)-2-hydroxyhexanoate (23a)
Yield 35%; yellowish oil; 1H NMR (200 MHz, CDCl3): δ 7.06 (d, J = 8.5 Hz, 2H, arom), 6.79 (d, J = 8.6 Hz, 2H, arom), 4.19–4.11 (m, 3H, CH, CH2OCO), 3.92 (t, J = 6.4 Hz, 2H, CH2O), 2.81 (d, J = 5.8 Hz, 1H, OH), 2.60–2.48 (m, 2H, PhCH2), 2.24 (t, J = 6.9 Hz, 2H, CH2CO), 1.86–1.36 [m, 23H, 7 × CH2, C(CH3)3], 0.95 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 175.6, 172.8, 157.4, 134.5, 129.4, 114.5, 80.6, 70.6, 67.9, 65.5, 35.1, 34.5, 31.7, 31.6, 28.3, 28.1, 24.7, 21.7, 19.5, 14.1; MS (ESI) m/z (%): 459 ([M + Na]+, 100); Anal. Calcd for C25H40O6: C, 68.78; H, 9.24; found: C, 68.51; H, 9.42.
5-(tert-Butoxy)-5-oxopentyl 6-(4-(hexyloxy)phenyl)-2-hydroxyhexanoate (23b)
Yield 49%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.05 (d, J = 8.7 Hz, 2H, arom), 6.79 (d, J = 8.7 Hz, 2H, arom), 4.23–4.04 (m, 3H, CHOH, CH2O), 3.91 (t, J = 6.5 Hz, 2H, OCH2), 2.82 (brs, 1H, OH), 2.54 (t, J = 7.0 Hz, 2H, PhCH2), 2.41 (t, J = 7.0 Hz, 2H, CH2CO), 1.89–1.10 [m, 27H, 9 × CH2, C(CH3)3], 0.89 (t, J = 6.6 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 175.3, 172.5, 157.1, 134.2, 129.1, 114.2, 80.3, 70.3, 67.9, 65.2, 34.8, 34.2, 31.5, 31.4, 29.2, 28.0, 27.8, 25.7, 24.4, 22.6, 21.4, 14.0; MS (ESI) m/z (%): 487 ([M + Na]+, 100); Anal. Calcd for C27H44O6: C, 69.79; H, 9.55; found: C, 69.62; H, 9.72.
tert-Butyl 5-((4-([1,1′-Biphenyl]-4-yl)-2-hydroxybutanoyl)oxy)pentanoate (23c)
Yield 36%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.62–7.22 (m, 9H, arom), 4.28–4.04 (m, 3H, OCH2, CH), 2.82–2.78 (m, 3H, PhCH2, OH), 2.33–1.94 (m, 4H, CH2), 1.77–1.57 (m, 4H, 2 × CH2), 1.44 (s, 9H, C(CH3)3); 13C NMR (50 MHz, CDCl3): δ 175.1, 172.5, 140.9, 140.2, 138.9, 129.0, 128.7, 127.1, 127.0, 126.9, 80.3, 69.6, 65.3, 35.9, 34.8, 30.6, 28.0, 27.8, 21.4; MS (ESI) m/z (%): 430 ([M + NH4]+, 100); Anal. Calcd for C25H32O5: C, 72.79; H, 7.82; found: C, 72.65; H, 7.98.
4-(tert-Butoxy)-4-oxobutyl 4-([1,1′-biphenyl]-4-yl)-2-hydroxybutanoate (23d)
Yield 29%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.64–7.22 (m, 9H, arom), 4.27–4.05 (m, 3H, OCH2, CH), 2.83–2.78 (m, 3H, PhCH2, OH), 2.30 (t, J = 7.2 Hz, 2H, CH2CO), 2.09–1.86 (m, 4H, 2 × CH2), 1.44 [m, 9H, C(CH3)3]; 13C NMR (50 MHz, CDCl3): δ 175.0, 172.4, 141.0, 140.4, 138.7, 129.1, 128.6, 127.2, 127.1, 126.8, 80.4, 69.7, 65.2, 35.7, 34.6, 31.6, 28.2, 21.3; MS (ESI) m/z (%): 421 ([M + Na]+, 100); Anal. Calcd for C24H30O5: C, 72.34; H, 7.59; found: C, 72.16; H, 7.75.
5-(tert-Butoxy)-5-oxopentyl 6-(4-butoxyphenyl)-2-fluorohexanoate (26a)
Yield 41%; yellowish oil; 1H NMR (200 MHz, CDCl3): δ 7.06 (d, J = 8.6 Hz, 2H, arom), 6.80 (d, J = 8.7 Hz, 2H, arom), 5.03–4.71 (dt, J = 48.0 Hz, J = 6.4 Hz, 1H, CHF), 4.20–4.06 (m, 2H, CH2OCO), 3.92 (t, J = 6.5 Hz, 1H, CH2O), 2.60–2.45 (m, 1H, PhCH2), 2.24 (t, J = 6.6 Hz, 2H, CH2CO), 2.01–1.23 [m, 23H, 7 × CH2, C(CH3)3], 0.96 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 172.4, 169.9 (d, J = 23.9 Hz), 157.2, 133.8, 129.1, 114.19, 88.8 (d, J = 184.1 Hz), 80.2, 67.5, 64.9, 34.8, 34.6, 32.2 (d, J = 20.9 Hz), 31.3, 31.1, 28.0, 27.8, 23.9, 21.3, 19.2, 13.8; 19F NMR (188 MHz, CDCl3): δ −149.9 (qt, J = 24.4 Hz); MS (ESI) m/z (%): 456 ([M + NH4]+, 100); Anal. Calcd for C25H39FO5: C, 68.47; H, 8.96; found: C, 68.31; H, 9.12.
5-(tert-Butoxy)-5-oxopentyl 6-(4-butoxyphenyl)hexanoate (26b)
Yield 52%; yellowish oil; 1H NMR (200 MHz, CDCl3): δ 7.03 (d, J = 8.5 Hz, 2H, arom), 6.78 (d, J = 8.6 Hz, 2H, arom), 4.05 (m, 2H, CH2OCO), 3.89 (t, J = 6.4 Hz, 2H, CH2O), 2.51 (t, J = 7.5 Hz, 2H, PhCH2), 2.33–2.10 (m, 4H, 2 × CH2CO), 1.81–1.16 [m, 23H, 7 × CH2, C(CH3)3], 0.94 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (50 MHz, CDCl3): δ 173.4, 172.3, 157.0, 134.0, 128.9, 114.0, 79.8, 67.3, 63.5, 34.7, 34.5, 33.9, 31.2, 31.1, 28.4, 27.8, 27.8, 24.6, 21.3, 19.0, 13.6; MS (ESI) m/z (%): 443 ([M + Na]+, 100); Anal. Calcd for C25H40O5: C, 71.39; H, 9.59; found: C, 71.19; H, 9.71.
General Procedure for Oxidation of 2-Hydroxy Esters
To a stirring solution of 2-hydroxy esters 12 and 23a–d (1 mmol) in dry CH2Cl2 (10 mL), Dess–Martin periodinane (1.1 mmol, 0.47 g) was added and the reaction mixture was stirred for 1.5 h at room temperature. The organic phase was washed with a mixture of Na2S2O3 10% and NaHCO3 10% (15 mL, 1:1, v/v) and then with brine, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography using EtOAc–petroleum ether (bp 40–60 °C) 2:8 as eluent.
5-(tert-Butoxy)-5-oxopentyl 6-(4-butoxyphenyl)-2-oxohexanoate (24a)
Yield 75%; yellowish oil; 1H NMR (200 MHz, CDCl3): δ 7.05 (d, J = 8.6 Hz, 2H, arom), 6.79 (d, J = 8.6 Hz, 2H, arom), 4.23 (t, J = 6.1 Hz, 2H, CH2OCOCO), 3.91 (t, J = 6.4 Hz, 2H, CH2O), 2.83 (t, J = 6.5 Hz, 2H, CH2COCO), 2.55 (t, J = 6.6 Hz, 2H, PhCH2), 2.25 (t, J = 6.8 Hz, 2H, CH2COO), 1.82–1.23 [m, 21H, 6 × CH2, C(CH3)3], 0.95 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 194.6, 172.7, 161.4, 157.5, 134.0, 129.4, 114.5, 80.6, 67.8, 66.1, 39.4, 35.0, 34.9, 31.6, 31.1, 28.3, 28.0, 22.7, 21.6, 19.5, 14.1; MS (ESI) m/z (%): 452 ([M + NH4]+, 100); Anal. Calcd for C25H38O6: C, 69.10; H, 8.81; found: C, 68.97; H, 8.99.
5-(tert-Butoxy)-5-oxopentyl 6-(4-(hexyloxy)phenyl)-2-oxohexanoate (24b)
Yield 79%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.05 (d, J = 8.6 Hz, 2H, arom), 6.79 (d, J = 8.6 Hz, 2H, arom), 4.23 (t, J = 6.2 Hz, 2H, CH2OCO), 3.91 (t, J = 6.5 Hz, 2H, OCH2), 2.83 (t, J = 6.0 Hz, 2H, CH2COCO), 2.55 (t, J = 6.2 Hz, 2H, PhCH2), 2.25 (t, J = 7.1 Hz, 2H, CH2COO), 1.84–1.53 (m, 10H, CH2), 1.43 [s, 9H, C(CH3)3], 1.38–1.19 (m, 6H, CH2), 0.89 (t, J = 6.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 194.3, 172.4, 161.1, 157.2, 133.7, 129.1, 114.3, 80.3, 67.9, 65.9, 39.1, 34.8, 34.6, 31.6, 30.8, 29.2, 28.0, 27.7, 25.7, 22.6, 22.4, 21.3, 14.0; MS (ESI) m/z (%): 480 ([M + NH4]+, 100); Anal. Calcd for C27H42O6: C, 70.10; H, 9.15; found: C, 69.91; H, 9.32.
tert-Butyl 5-((4-([1,1′-Biphenyl]-4-yl)-2-oxobutanoyl)oxy)pentanoate (24c)
Yield 70%; yellowish oil; 1H NMR (200 MHz, CDCl3): δ 7.67–7.22 (m, 9H, arom), 4.25 (t, J = 6.2 Hz, 2H, CH2OCOCO), 3.22 (t, J = 6.9 Hz, 2H, CH2COCO), 3.00 (t, J = 7.1 Hz, 2H, PhCH2), 2.26 (t, J = 7.1 Hz, 2H, CH2CO), 1.84–1.57 (m, 4H, 2 × CH2), 1.44 [s, 9H, C(CH3)3]; 13C NMR (50 MHz, CDCl3): δ 193.3, 172.4, 160.8, 140.8, 139.3, 139.1, 128.8, 128.7, 127.2, 127.1, 126.9, 80.3, 66.0, 40.9, 34.8, 28.5, 28.0, 27.7, 21.3; MS (ESI) m/z (%): 428 ([M + NH4]+, 100); Anal. Calcd for C25H30O5: C, 73.15; H, 7.37; found: C, 73.01; H, 7.59.
4-(tert-Butoxy)-4-oxobutyl 4-([1,1′-biphenyl]-4-yl)-2-oxobutanoate (24d)
Yield 73%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.65–7.21 (m, 9H, arom), 4.28 (t, J = 6.4 Hz, 2H, CH2OCOCO), 3.22 (t, J = 6.9 Hz, 2H, CH2COCO), 2.99 (t, J = 7.2 Hz, 2H, PhCH2), 2.33 (t, J = 7.2 Hz, 2H, CH2CO), 2.09–1.91 (m, 2H, CH2), 1.43 [s, 9H, 3 × CH3]; 13C NMR (50 MHz, CDCl3): δ 194.5, 172.8, 159.8, 140.4, 139.4, 139.1, 128.9, 128.7, 127.4, 127.2, 126.8, 80.2, 65.1, 39.9, 34.6, 28.4, 28.2, 21.5; MS (ESI) m/z (%): 414 ([M + NH4]+, 100); Anal. Calcd for C24H28O5: C, 72.71; H, 7.12; found: C, 72.50; H, 7.34.
Methyl 6-(4-Butoxyphenyl)-2-oxohexanoate (28)
Yield 60%; colorless oil; 1H NMR (200 MHz, CDCl3): δ 7.07 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 3.85 (s, 3H, CH3O), 2.86 (t, J = 6.9 Hz, 2H, CH2CO), 2.57 (t, J = 6.9 Hz, 2H, PhCH2), 1.82–1.40 (m, 8H, 4 × CH2), 0.97 (t, J = 7.3 Hz, 3H); 13C NMR (50 MHz, CDCl3): δ 194.4, 161.7, 157.5, 134.0, 129.4, 114.5, 67.8, 53.2, 39.4, 34.9, 31.6, 31.1, 22.7, 19.5, 14.1; MS (ESI) m/z (%): 310 ([M + NH4]+, 100); Anal. Calcd for C17H24O4: C, 69.84; H, 8.27; found: C, 69.61; H, 8.38.
General Procedure for Cleavage of tert-Butyl Protecting Group
A solution of tert-butyl esters 24a–d, 26a,b (1 mmol) in CH2Cl2 (5 mL), and trifluoroacetic acid (TFA) (5 mL) was stirred for 1 h at room temperature. The organic solvent was evaporated under reduced pressure and then toluene (5 mL) was added and re-evaporated twice. The product was purified by precipitation with a mixture of EtOAc and petroleum ether (bp 40–60 °C) (5:95, v/v) or by column chromatography (CH2Cl2–MeOH, 95:5).
5-((6-(4-Butoxyphenyl)-2-oxohexanoyl)oxy)pentanoic Acid (25a)
Yield 72%; white solid; mp 55–58 °C. 1H NMR (200 MHz, CDCl3): δ 7.07 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 4.25 (t, J = 6.0 Hz, 2H, CH2OCO), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 2.84 (t, J = 6.8 Hz, 2H, CH2COCO), 2.57 (t, J = 6.8 Hz, 2H, PhCH2), 2.41 (t, J = 6.8 Hz, 2H, CH2COOH), 1.83–1.38 (m, 12H, 6 × CH2), 0.96 (t, J = 7.3 Hz, 3H); 13C NMR (50 MHz, CDCl3): δ 194.2, 179.3, 161.1, 157.2, 133.7, 129.1, 114.3, 67.6, 65.7, 39.1, 34.6, 33.3, 31.3, 30.8, 27.6, 22.4, 20.9, 19.2, 13.8; HRMS (ESI) m/z: [M – H]− Calcd for C21H29O6– 377.1970; found 377.1961; Anal. Calcd for C21H30O6: C, 66.65; H, 7.99; found: C, 66.37; H, 8.16.
5-((6-(4-(Hexyloxy)phenyl)-2-oxohexanoyl)oxy)pentanoic Acid (25b)
Yield 84%; white solid; mp 69–71 °C; 1H NMR (200 MHz, CDCl3): δ 7.06 (d, J = 8.3 Hz, 2H, arom), 6.80 (d, J = 8.6 Hz, 2H, arom), 4.25 (t, J = 5.6 Hz, 2H, CH2OCO), 3.91 (t, J = 6.5 Hz, 2H, OCH2), 2.84 (t, J = 5.7 Hz, 2H, CH2COCO), 2.55 (t, J = 6.7 Hz, 2H, PhCH2), 2.40 (t, J = 6.6 Hz, 2H, CH2COOH), 1.94–1.09 (m, 16H, CH2), 0.89 (t, J = 5.6 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3): δ 194.3, 179.2, 161.1, 157.2, 133.7, 129.2, 114.3, 67.9, 65.7, 39.1, 34.6, 33.3, 31.6, 30.8, 29.2, 27.6, 25.7, 22.6, 22.4, 20.9, 14.0; HRMS (ESI) m/z: [M – H]− Calcd for C23H33O6– 405.2283; found: 405.2253; Anal. Calcd for C23H34O6: C, 67.96; H, 8.43; found: C, 67.79; H, 8.67.
5-((4-([1,1′-Biphenyl]-4-yl)-2-oxobutanoyl)oxy)pentanoic Acid (25c)
Yield 79%; white solid; mp 114–116 °C; 1H NMR (200 MHz, CDCl3): δ 7.65–7.14 (m, 9H, arom), 4.26 (t, J = 6.1 Hz, 2H, CH2OCOCO), 3.22 (t, J = 6.5 Hz, 2H, CH2CO), 3.00 (t, J = 7.2 Hz, 2H, PhCH2), 2.40 (t, J = 6.9 Hz, 2H, CH2COOH), 1.90–1.59 (m, 4H, CH2); 13C NMR (50 MHz, CDCl3): δ 193.3, 179.2, 160.8, 140.8, 139.3, 139.1, 128.8, 128.7, 127.3, 127.1, 126.9, 65.8, 40.9, 33.3, 28.5, 27.6, 20.9; HRMS (ESI) m/z: [M – H]− Calcd for C21H21O5– 353.1394; found: 353.1388; Anal. Calcd for C21H22O5: C, 71.17; H, 6.26; found: C, 70.95; H, 6.44.
4-((4-([1,1′-Biphenyl]-4-yl)-2-oxobutanoyl)oxy)butanoic Acid (25d)
Yield 85%; white solid; mp 137–139 °C; 1H NMR (200 MHz, CDCl3): δ 7.63–7.20 (m, 9H, arom), 4.30 (t, J = 6.1 Hz, 2H, CH2OCOCO), 3.20 (t, J = 7.4 Hz, 2H, CH2CO), 2.99 (t, J = 7.2 Hz, 2H, PhCH2), 2.48 (t, J = 7.2 Hz, 2H, CH2COOH), 2.14–1.96 (m, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ 193.8, 179.7, 161.3, 141.2, 139.8, 139.6, 129.3, 129.2, 127.8, 127.6, 127.5, 65.2, 40.9, 29.7, 28.5, 23.4; HRMS (ESI) m/z: [M – H]− Calcd for C20H19O5– 339.1238; found: 339.1234; Anal. Calcd for C20H20O5: C, 70.58; H, 5.92; found: C, 70.41; H, 6.15.
5-((6-(4-Butoxyphenyl)-2-fluorohexanoyl)oxy)pentanoic Acid (27a)
Yield 72%; low-melting-point yellow solid; 1H NMR (200 MHz, CDCl3): δ 7.07 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 4.88 (dt, J = 48.0 Hz, J = 6.4 Hz, 1H, CHF), 4.20 (t, J = 5.6 Hz, 2H, CH2OCO), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 2.56 (t, J = 7.3 Hz, 2H, PhCH2), 2.40 (t, J = 6.6 Hz, 2H, CH2COOH), 2.07–1.35 (m, 14H, 7 × CH2), 0.97 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3) δ 179.3, 170.1 (d, J = 23.9 Hz), 157.2, 133.9, 129.1, 114.2, 88.8 (d, J = 184.1 Hz), 67.6, 64.8, 34.6, 33.3, 32.2 (d, J = 21.0 Hz), 31.3, 31.1, 27.7, 23.9 (d, J = 3 Hz), 20.9, 19.2, 13.8; 19F NMR (188 MHz, CDCl3) δ −149.9 (qt, J = 24.4 Hz); HRMS (ESI) m/z: [M – H]− Calcd for C21H30FO5– 381.2083; found: 381.2080; Anal. Calcd for C21H31FO5: C, 65.95; H, 8.17; found: C, 65.76; H, 8.31.
5-((6-(4-Butoxyphenyl)hexanoyl)oxy)pentanoic Acid (27b)
Yield 99%; low-melting-point yellowish solid; 1H NMR (200 MHz, CDCl3): δ 11.05 (s, 1H, COOH), 7.07 (d, J = 8.6 Hz, 2H, arom), 6.81 (d, J = 8.6 Hz, 2H, arom), 4.08 (t, J = 5.7 Hz, 2H, CH2OCO), 3.93 (t, J = 6.5 Hz, 2H, CH2O), 2.56 (t, J = 6.0 Hz, 2H, PhCH2), 2.45–2.22 (m, 4H, 2 × CH2), 1.85–1.20 (m, 14H, 7 × CH2), 0.97 (t, J = 7.3 Hz, 3H, CH3); 13C NMR (50 MHz, CDCl3) δ 179.4, 173.9, 157.1, 134.2, 129.1, 114.1, 67.5, 63.6, 34.7, 34.1, 33.4, 31.3, 31.2, 28.6, 27.8, 24.7, 21.1, 19.2, 13.8; HRMS (ESI) m/z: [M – H]− Calcd for C21H31O5– 363.2177; found: 363.2174; Anal. Calcd for C21H32O5: C, 69.20; H, 8.85; found: C, 69.01; H, 8.99.
6-(4-Butoxyphenyl)-2-oxohexanoic Acid (29)
To a stirring solution of methyl ester 28 (1 mmol) in MeOH (10 mL), 20% aqueous solution of Cs2CO3 (2 mmol, 3.3 mL) was added and the mixture was stirred at room temperature overnight. The solvent was evaporated under reduced pressure and the residue was dissolved in H2O (10 mL) and extracted with Et2O (3 × 10 mL). The aqueous layer was then acidified with 2 N HCl (4.0 mL) and extracted with EtOAc (3 × 10 mL), followed by drying over Na2SO4 and concentrating in vacuo. Yield 94%; white solid; mp 102–105 °C; 1H NMR (200 MHz, CDCl3): δ 8.44 (s, 1H), 7.07 (d, J = 8.4 Hz, 2H, arom), 6.82 (d, J = 8.5 Hz, 2H, arom), 3.94 (t, J = 6.5 Hz, 2H, CH2O), 2.94–2.61 (m, 1H, CHH), 2.57–2.21 (m, 3H, PhCH2, CHH), 1.83–1.18 (m, 8H, 4 × CH2), 0.97 (t, J = 7.3 Hz, 3H); 13C NMR (50 MHz, CDCl3): δ 195.4, 157.3, 133.6, 129.2, 114.3, 114.3, 67.6, 37.6, 34.5, 31.3, 30.8, 22.4, 19.2, 13.9; HRMS (ESI) m/z: [M – H] – Calcd for C16H21O4– 277.1445; found: 277.1444; Anal. Calcd for C16H22O4: C, 69.04; H, 7.97; found: C, 68.89; H, 8.21.
In Vitro PLA2 Activity Assays
Group-specific mixed micelle modified Dole assays were employed to determine the activity of human recombinant GIVA cPLA2, GVIA iPLA2, and GV sPLA2.18−20 To achieve optimum activity, the substrate was prepared using slightly different conditions for each enzyme: (i) GIVA cPLA2 mixed micelle substrate consisted of 400 μM Triton X-100, 95.3 μM PAPC, 1.7 μM arachidonyl-1-14C PAPC, and 3 μM PIP2 in a buffer containing 100 mM N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (HEPES) pH 7.5, 90 μM CaCl2, 2 mM dithiothreitol (DTT), and 0.1 mg/mL bovine serum albumin; (ii) GVIA iPLA2 mixed micelle substrate consisted of 400 μM Triton X-100, 98.3 μM PAPC, and 1.7 μM arachidonyl-1-14C PAPC in a buffer containing 100 mM HEPES pH 7.5, 2 mM adenosine 5′-triphosphate, and 4 mM DTT; and (iii) GV sPLA2 mixed micelles substrate consisted of 400 μM Triton X-100, 98.3 μM PAPC, and 1.7 μM arachidonyl-1-14C PAPC in a buffer containing 50 mM Tris–HCl pH 8.0, and 5 mM CaCl2. Initially, the compounds were screened at 0.091 mol fraction (5 μL of 5 mM inhibitor in dimethyl sulfoxide) in substrate (495 μL). XI(50) values were determined for compounds exhibiting more than 95% inhibition. Inhibition curves were generated using GraphPad Prism 5.0 and the nonlinear regression by plotting percentage of inhibition vs log (mole fraction) to calculate the reported XI(50) and its associated error.
Plasma Stability Studies
The reactions were initiated by the addition of test compound to 200 μL of preheated (37 °C) human plasma to yield a final concentration of 1 mg/mL. Samples (50 μL) were taken at 0, 15, 30, and 60 min and acetonitrile (200 μL) was added. The samples were subjected to vortex mixing and then centrifugation for 5 min. The clear supernatants were analyzed by LC-HRMS/MS using an AB Sciex 4600 Triple TOF combined with a micro-LC Eksigent and an autosampler. Electrospray ionization (ESI)—negative mode—was used for the MS experiments. Halo C18 2.7 μm, 90 Å, 0.5 × 50 mm2 from Eksigent was used as a column and the mobile phase consisted of a gradient (A: acetonitrile/0.01% formic acid—isopropanol 80/20 v/v; B: H2O/0.01% formic acid). The data acquisition was carried out with MultiQuant from AB SCIEX (version 3.0). Each sample was studied in triplicate. The plot of the percentage of the remaining compound in comparison to the initial concentration vs time was designed.
Acknowledgments
This research has been co-financed by the European Union (European Regional Development Fund-ERDF) and Greek national funds through the Operational Program “Competitiveness and Entrepreneurship” of the National Strategic Reference Framework (NSRF)—Research Funding Program: “Phospholipases A2 inhibitors: Developing a drug pipeline for the treatment of inflammatory neurological disorders” (G.K.) and by NIH Grant GM20501-42 (E.A.D.). M.G.K. would like to thank the National Scholarship Foundation (IKY) for a fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01214.
HRMS spectra of inhibitors 25a (GK504) and 27a (GK505) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Dennis E. A.; Cao J.; Hsu Y. H.; Magrioti V.; Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez A. M.; Mouchlis D. V.; Dennis E. A. Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 2018, 67, 212–218. 10.1016/j.jbior.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. A.; Norris P. C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie C. C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotou M. G.; Limnios D.; Nikolaou A.; Psarra A.; Kokotos G. Inhibitors of phospholipase A2 and their therapeutic potential: An update on patents (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 217–225. 10.1080/13543776.2017.1246540. [DOI] [PubMed] [Google Scholar]
- Ong W. Y.; Farooqui T.; Kokotos G.; Farooqui A. A. Synthetic and natural inhibitors of phospholipases A2: Their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 2015, 6, 814–831. 10.1021/acschemneuro.5b00073. [DOI] [PubMed] [Google Scholar]
- Omland S. H.; Habicht A.; Damsbo P.; Wilms J.; Johansen B.; Gniadecki R. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1161–1167. 10.1111/jdv.14128. [DOI] [PubMed] [Google Scholar]
- Bhowmick R.; Clark S.; Bonventre J. V.; Leong J. M.; McCormick B. A. Cytosolic phospholipase A2α promotes pulmonary inflammation and systemic disease during Streptococcus pneumoniae infection. Infect. Immun. 2017, 85, 280–317. 10.1128/IAI.00280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoo T.; Nakatsuka T.; Katayama T.; Hayashi Y.; Fujieda Y.; Terakawa M.; Nagahira K. Design, synthesis, and biological evaluation of 3-(1-aryl-1H-indol-5-yl)propanoic acids as new indole-based cytosolic phospholipase A2α inhibitors. J. Med. Chem. 2014, 57, 7244–7262. 10.1021/jm500494y. [DOI] [PubMed] [Google Scholar]
- Kanai S.; Ishihara K.; Kawashita E.; Tomoo T.; Nagahira K.; Hayashi Y.; Akiba S. ASB14780, an orally active inhibitor of group IVA phospholipase A2, is a pharmacotherapeutic candidate for nonalcoholic fatty liver disease. J. Pharmacol. Exp. Ther. 2016, 356, 604–614. 10.1124/jpet.115.229906. [DOI] [PubMed] [Google Scholar]
- Kokotos G.; Feuerherm A. J.; Barbayianni E.; Shah I.; Sæther M.; Magrioti V.; Nguyen T.; Constantinou-Kokotou V.; Dennis E. A.; Johansen B. Inhibition of group IVA cytosolic phospholipase A2 by thiazolyl ketones in vitro, ex vivo, and in vivo. J. Med. Chem. 2014, 57, 7523–7535. 10.1021/jm500192s. [DOI] [PubMed] [Google Scholar]
- Kim E.; Tunset H. M.; Cebulla J.; Vettukattil R.; Helgesen H.; Feuerherm A. J.; Engebraten O.; Mælandsmo G. M.; Johansen B.; Moestue S. A. Anti-vascular effects of the cytosolic phospholipase A2 inhibitor AVX235 in a patient-derived basal-like breast cancer model. BMC Cancer 2016, 16, 191. 10.1186/s12885-016-2225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Qu M.; Sun Y.; Wan H.; Chai F.; Liu L.; Zhang P. Blockage of cytosolic phospholipase A2 alpha sensitizes aggressive breast cancer to doxorubicin through suppressing ERK and mTOR kinases. Biochem. Biophys. Res. Commun. 2018, 496, 153–158. 10.1016/j.bbrc.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Kokotou M. G.; Galiatsatou G.; Magrioti V.; Koutoulogenis G.; Barbayianni E.; Limnios D.; Mouchlis V. D.; Satpathy B.; Navratil A.; Dennis E. A.; Kokotos G. 2-Oxoesters: A novel class of potent and selective inhibitors of cytosolic group IVA phospholipase A2. Sci. Rep. 2017, 7, 7025 10.1038/s41598-017-07330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew A. P.; Berlin M.; Dong H.; Qian Y.. Alanine-Based Modulators of Proteolysis and Associated Methods of Use. WO2017011590A1, 2017.
- Middleton W. J. New fluorinating reagents. Dialkylaminosulfur fluorides. J. Org. Chem. 1975, 40, 574–578. 10.1021/jo00893a007. [DOI] [Google Scholar]
- Mceachern E. J.; Sun J.; Vocadlo D. J.; Zhou Y.; Zhu Y.; Selnick H. G.. Glycosidase Inhibitors and Uses Thereof. WO201432188A1, 2014.
- Kokotos G.; Six D. A.; Loukas V.; Smith T.; Constantinou-Kokotou V.; Hadjipavlou-Litina D.; Kotsovolou S.; Chiou A.; Beltzner C. C.; Dennis E. A. Inhibition of group IVA cytosolic phospholipase A2 by novel 2-oxoamides in vitro, in cells, and in vivo. J. Med. Chem. 2004, 47, 3615–3628. 10.1021/jm030485c. [DOI] [PubMed] [Google Scholar]
- Stephens D.; Barbayianni E.; Constantinou-Kokotou V.; Peristeraki A.; Six D. A.; Cooper J.; Harkewicz R.; Deems R. A.; Dennis E. A.; Kokotos G. Differential inhibition of group IVA and group VIA phospholipases A2 by 2-oxoamides. J. Med. Chem. 2006, 49, 2821–8. 10.1021/jm050993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six D. A.; Barbayianni E.; Loukas V.; Constantinou-Kokotou V.; Hadjipavlou-Litina D.; Stephens D.; Wong A. C.; Magrioti V.; Moutevelis-Minakakis P.; Baker S. F.; Dennis E. A.; Kokotos G. Structure-activity relationship of 2-oxoamide inhibition of group IVA cytosolic phospholipase A2 and group V secreted phospholipase A2. J. Med. Chem. 2007, 50, 4222–35. 10.1021/jm0613673. [DOI] [PubMed] [Google Scholar]
- Lipinski C.; Lombardo F.; Dominy B.; Feeney P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Kokotos G.; Hsu Y.-H.; Burke J. E.; Baskakis C.; Kokotos C. G.; Magrioti V.; Dennis E. A. Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2. J. Med. Chem. 2010, 53, 3602–3610. 10.1021/jm901872v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L.; Kerns E. H.; Hong Y.; Chen H. Development and application of high throughput plasma stability assay for drug discovery. Int. J. Pharm. 2005, 297, 110–119. 10.1016/j.ijpharm.2005.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.