Abstract
In this cross-sectional study, we investigated host genetic factors and ethnic variation in circulating Plasmodium falciparum merozoite surface protein 2 (msp-2) clones among children with asymptomatic malaria.
Isolates from seventy two asymptomatic malaria children were used for genotyping block 3 of msp-2 gene by nested polymerase chain reaction (PCR). Sickle cell trait and glucose-6-phosphate dehydrogenase (G6PD) deficiency were analysed by restriction fragment length polymorphism of DNA products from PCR targeting codons 6 and 68 of the beta-globin (HBB) and G6PD genes respectively. ABO blood group was typed by agglutination method.
A total of forty two msp-2 genotypes (20 for 3D7 and 22 for FC27) were detected for an average (standard error of mean) multiplicity of infection (MOI) of 2.45 (0.16). The MOI was statistically the same among the five identified ethnic groups (P = 0.83). The overall prevalence of sickle cell trait and G6PD deficiency were 12.50 % and 22.22 % respectively. MOI was similar between children with Hb AA and Hb AS genotypes (P = 0.42). MOI was significantly high among children with a mutant G6PD genotype (P = 0.017). MOI was significantly higher in blood group O than group A (P = 0.03).
Our findings show that although ethnicity and sickle cell trait have no association with MOI, the association was observed with G6PD genotype and ABO group. The results suggest the need for extension and expansion of the current study in order to investigate the mechanisms involved.
Keywords: Infectious disease, Clinical genetics, Epidemiology, Pediatrics
1. Introduction
Malaria is a deadly parasitic disease caused by Plasmodium species transmitted to man by a mosquito bite of the Anopheles genus. Five species of Plasmodium are able to infect humans but P. falciparum has been recognized for the major cause of morbimortality throughout human history, especially in children under five years of age [1]. In countries where malaria is endemic, MOI defined as the number of different P. falciparum strains co-infecting a single host is frequent and can be a useful indicator of the transmission level as well as immune status [2]. In high transmission settings, majority of P. falciparum infections are asymptomatic or subclinical [3]. Asymptomatic parasite population detected by microscopy and/or other methods have been reported in several sites and is an important obstacle to controlling malaria [3, 4]. Previous studies conducted in Mozambique and other sub-Saharan African regions including Ghana, Kenya, Senegal, Gabon and Nigeria showed that a large proportion of individuals harbor asymptomatic infection [5, 6, 7, 8, 9, 10, 11].
In some malaria endemic areas, the clinical outcome of the infection and its progression to pathological complications depends on many factors involving the specific and dynamic combination of host and parasite properties [12, 13]. With respect to the host, it seems likely that the ability to establish an effective immune response against P. falciparum infection may involve genetic factors of the host. Today, it is well recognized that P. falciparum is a driving force of evolution has helped to shape the human genome and can select genes that contribute to resistance [14, 15].
G6PD deficiency and sickle cell trait are counted amongst the well-characterized human genetic defects [16, 17, 18]. Sickle-cell hemoglobin (HbS) is a structural variant of normal adult hemoglobin (HbA) caused by a point mutation: substitution of valine by glutamic acid at position 6 of the β-globin subunit (βS) in the beta-globin (HBB) gene [19]. G6PD deficiency characterized by reduced G6PD enzyme activity though a genetic disorder, but can remain asymptomatic [18]. Although these mutations are distributed globally, they are more prevalent in tropical and subtropical regions [20]. Sickle cell traits and a number of other human genetic traits, including G6PD deficiency and related hemoglobinopathies, are predominantly present in populations living in malaria endemic regions and have been suggested to confer some level of protection against severe forms of the disease [21, 22, 23, 24].
In addition, since the discovery of the ABO blood group, there is growing interest in their potential role in infectious diseases. Previous studies in patients with tumors [25] and viral infections [26] found ABO blood group associations. Singh et al. [27] observed a significant association between malaria and ABO blood groups where individuals with antigen O were the most susceptible. In Zimbabwe [28], Fischer and Boone found that Group O individuals were protected against cerebral malaria. In Cameroon, numerous studies on the pathophysiology of malaria have already been done [29, 30, 31, 32, 33]; however, very few have focused on the potential relationships between red blood cell polymorphisms and malaria infection [34, 35]. In this study, to investigate the influence of host factors on the infecting parasite population, we compared the number of merozoite surface protein 2 (msp-2) parasite clones per infection among asymptomatic isolates from Cameroonian children in relation to ethnicity, sickle cell trait, G6PD deficiency and ABO blood groups.
2. Materials and methods
2.1. Study design and data collection
Briefly, a cross-sectional survey was conducted in asymptomatic children living in the Mvan neighborhood located at the periphery of the Yaoundé city, central region (Cameroon), where year-round malaria transmission occurs. This area is characterized by the presence of several tribes; but the most numerous are Ewondo, Bulu and Eton. The study took place from March to April 2016 in 236 children aged 3–14 years. The inclusion criteria included i) obtaining consent from the parent or legal representative of the child, ii) living permanently in the area, iii) axillary body temperature of <37.5 °C, iv) absence of recent treatment received for malaria (at least in the last 7 days) and v) lack of symptoms suggestive of malaria or any other severe systemic illness. Blood samples were collected using Vacutainer® tubes (Nanjing Everich Medicare Co Ltd, China) and EDTA.K3 as anti-coagulant. A small aliquot of whole blood was blotted on to Whatman filter Paper number 1 (Maidstone, UK) following the manufacturer's instructions, for parasite genotyping and erythrocytes polymorphisms studies. The remaining blood was immediately used to determine malaria parasite status and density, as well as blood group. The study was approved by the Institutional Ethical Review Committee of University of Douala. Informed consent from the parent or guardian was required prior to inclusion in the study.
2.2. Determination of blood group
ABO blood groups were typed by agglutination using commercial antisera (Cypress Diagnostics, Langdorp, Belgium) as described previously [36]. Briefly, three drops of whole blood were added on a clean glass slide; then a drop of antiserum for blood groups A, B and AB was applied. The blood cells and the antigen were then homogenized for detection of eventual agglutination.
2.3. Parasite assessment
Parasitaemia was determined by microscopic examination of Giemsa-stained thick blood slides using WHO (2010) methods [37]. A blood smear was considered negative if parasites were not detected after examination of at least 200 oil-immersion fields of the thick smear. To estimate the approximate number of parasites per microliter of blood, the number of malaria parasites counted on the thick film against 200 leukocytes was multiplied by 40, based on the assumption that the average leukocyte count was 8,000/μl of blood. Molecular identification of parasites species and msp-2 block 3 genotyping detecting 3D7 and FC27 allelic types were carried out by nested polymerase chain reaction (PCR) as previously described [38, 39].
2.4. Multiplicity of msp-2 infection
Multiplicity of infection was defined as the number of different P. falciparum strains co-infecting a single host for the examined gene. Mean multiplicity of infection (MOI) was estimated by dividing the total number of distinct msp-2 genotypes detected by the number of positive samples [40].
2.5. Molecular identification of G6PD variants and sickle cell trait
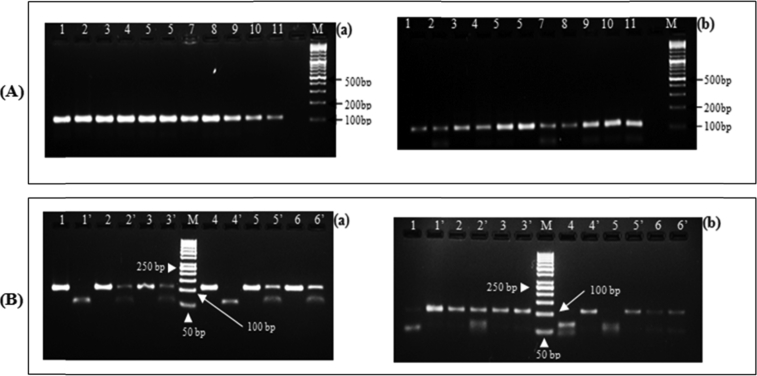
After DNA extraction (QIAamp DNA Mini Kit, Qiagen, Germany) of the dried blood spot samples, sickle cell trait and G6PD deficiency (due to G202A mutation) were determined by PCR-Restriction Fragment Length Polymorphism (RFLP). The 110 base pairs (bp) containing codon 6 of the HBB gene and the 109 bp containing codon 68 of the G6PD gene were amplified independently using Eppendorf Mastercycler Gradient (Eppendorf, Germany) according to protocols previously described [41, 42, 43, 44] with some modifications. Briefly, 4 μL of DNA extract were added to a final volume of 25 μL in a reaction mixture containing 1X Taq buffer with 15 mM MgCl2; 10 mM of dNTPs mix (2.5 mM each), 0.4 μM of each primer and 1.6 U of Taq polymerase enzyme (Table 1). The PCR reaction conditions included an initial denaturation at 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 30 sec, annealing at 57 °C (for sickle cell trait) or 63 °C (for G6PD) for 30 sec, extension at 72 °C for 30 sec and a final extension at 72 °C for 5 min. The PCR products were separated on 3 % agarose gel pre-stained with ethidium bromide and DNA bands were visualized under a UV transilluminator by using the Alpha Innotech system (Alpha Innotech-Genetic Technologies, Inc, USA).
Table 1.
PCR/RFLP conditions used to identify the sickle cell and G6PD genetic variants of individuals in the present study.
| Mutations types | Codons | Primer oligonucleotide sequence (5′–3′) | Amplicon size (bp) | RFLP pattern (fragment size in bp) |
||
|---|---|---|---|---|---|---|
| RE | Wild-type | Mutant | ||||
| (Val → Met) | 68 | GTG GCT GTT CCG GGA | 109 | Hin1 II | 109 | 63, 46 |
| TGG CCT TCT G | ||||||
| CTT GAA GAA GGG CTC | ||||||
| ACT CTG TTT G | ||||||
| (Glu → Val) | 6 | ACA CAA CTG TGT TCA | 110 | Bsu361 I | 54, 56 | 110 |
| CTA GC | ||||||
| CAA CTT CAT CCA CGT | ||||||
| TCA CC | ||||||
RFLP: Restriction Fragments Length Polymorphisms; RE: Restriction Enzyme; bp: base pairs.
Val: Valine; Met: Methionine; Glu: Glutamic acid.
2.6. RFLP analysis of PCR- amplified fragments
Seven microliters of the amplified fragment were treated with 0.3 μL of restriction endonuclease enzymes (Thermo Scientific, Waltham, USA) Bsu36 I (10 U/μL) in the case of the point mutation causing the sickle cell trait or Hin1 II (5U/μL) in case of G6PD deficiency; and digested at 37 °C for 16 hours in 10X buffer tango in a total volume of 20 μL. Subsequently, the digestion products pre-stained with ethidium bromide were separated on a 3% agarose gel and visualized under ultraviolet light and the sizes of the PCR products were estimated using a 50 bp DNA ladder marker (Thermo scientific, Waltham, USA). The Bsu36 I enzyme has a recognition site at codon 6 in the normal HBB gene cleaving it into two fragments (56 bp and 54 bp), while the fragment amplified from DNA of sickle cell mutation remained uncleaved (110 bp). For G6PD analysis, the sizes of the restriction fragment were 109 bp for the normal allele; while the mutant allele cleaved and gave two bands (63 bp and 46 bp) [Table 1].
2.7. Data management and analysis
The Fisher's exact test was used to compare the distribution of msp-2 allelic family with respect to different host genetic variants in asymptomatic P. falciparum children. Mann Whitney and Kruskal-Wallis tests were performed to compare the number of distinct parasite alleles per infected isolate in the subset of patients harboring parasites. All statistical analyses were performed using GraphPad Prism demo (version 6.05, GraphPad Software Inc) softwares. P values of <0.05 were considered to indicate statistical significance.
3. Results
3.1. Characteristics of the study population
A total of eighty three children with P. falciparum asymptomatic malaria met the inclusion criteria and were enrolled in the study. 7 participants were excluded because of the unsuccessfully genotyping of msp-2 gene and 4 due to the lack of ethnicity information. Finally, seventy two children from 3 to 14 years with asymptomatic malaria were admitted to participate in the study. The distribution of different ethnic groups was 41/72, 15/72, 5/72, 6/72 and 5/72 for the Ewondo, Bulu, Eton, Bassa and Haoussa respectively (Table 2). The mean age ± standard error of mean (SEM) of the children was 6.9 ± 0.3 years (95 % CI: 6.2–7.6) and 52.8 % (38/72) were female.
Table 2.
Genotyping of P. falciparum msp-2 polymorphic region block 3 according to the ethnicity.
| Ethnicity | Msp-2 allelic family types | No. of isolates | Allele frequency (%) | Allele size range (bp) | MOI |
|---|---|---|---|---|---|
| Ewondo | 3D7 | 24 | 58.6 | 450–1300 | 2.4 |
| FC27 | 6 | 14.6 | 200–750 | ||
| 3D7 + FC27 | 11 | 26.8 | |||
| Total | 41 | 100.0 | |||
| Eton | 3D7 | 1 | 20.0 | 600–700 | 3.0 |
| FC27 | 1 | 20.0 | 340 | ||
| 3D7 + FC27 | 3 | 60.0 | |||
| Total | 5 | 100.0 | |||
| Bassa | 3D7 | 5 | 83.3 | 480–1300 | 2.2 |
| FC27 | 0 | 0.0 | - | ||
| 3D7 + FC27 | 1 | 16.7 | |||
| Total | 6 | 100.0 | |||
| Haoussa | 3D7 | 4 | 80.0 | 500–1300 | 2.0 |
| FC27 | 0 | 0.0 | - | ||
| 3D7 + FC27 | 1 | 20.0 | |||
| Total | 5 | 100.0 | |||
| Bulu | 3D7 | 10 | 66.7 | 480–1200 | 2.7 |
| FC27 | 2 | 13.3 | 300–360 | ||
| 3D7 + FC27 | 3 | 20.0 | |||
| Total | 15 | 100.0 |
MOI: Multiplicity of infection.
3.2. Multiplicity of infection and ethnicity
A total of forty two different alleles were detected in msp-2 family typing. These alleles comprised 20 alleles of 3D7 and 22 of FC27 allelic families. The 3D7 allelic family was present in all ethnic groups and predominated among the Ewondo (58.6%), Bassa (83.3%), Haoussa (80.0%) and Bulu (66.7%). Polyclonal infections with both allelic families (3D7 + FC27) were observed in all populations (Table 2). The overall average (SEM) number of clones per infection in the population was 2.45 (0.16). Among the Ewondo, Bassa, Haoussa, Bulu and Eton, the mean multiplicity of infection was 2.4, 2.2, 2.0, 2.7 and 3.0 respectively and no significant difference was observed between these values using the Kruskal-Wallis test (P = 0.83) (Table 2).
3.3. Relation between multiplicity of msp-2 genotypes and erythrocyte variants
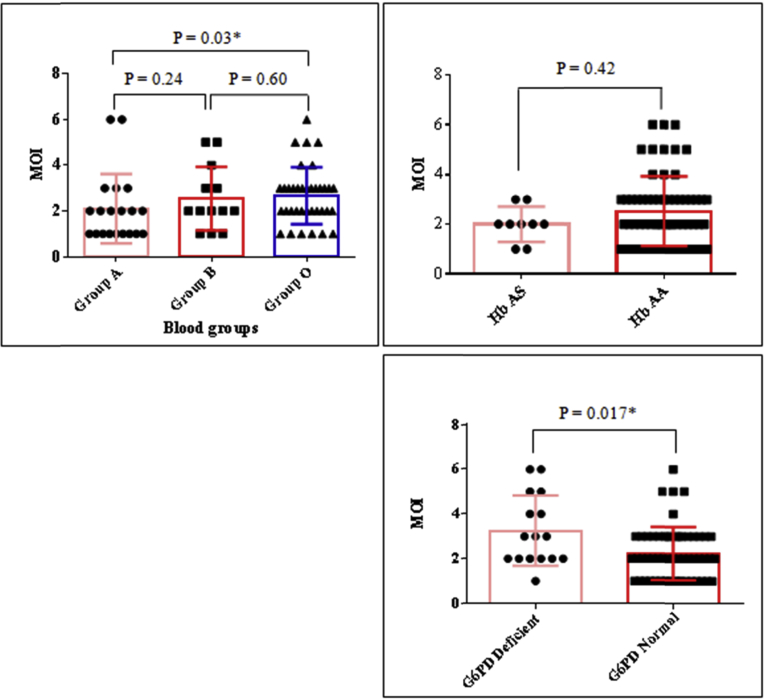
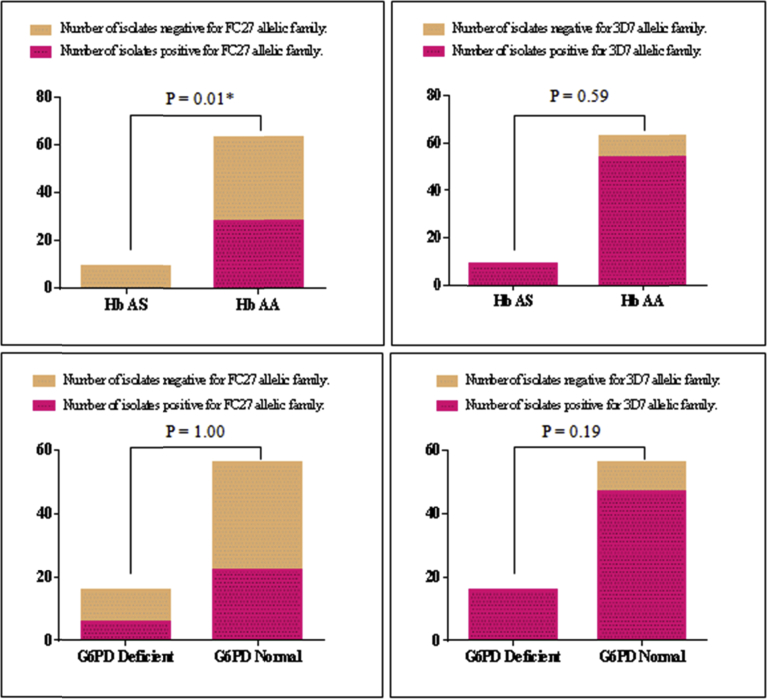
Gel profile illustrating the PCR amplicons as well as the RFLP fragments that we obtained is given in Fig. 1. The proportion of individuals having sickle cell trait and G6PD deficiency in the study population was 9/72 (12.50 %) and 16/72 (22.22 %) respectively. In order to determine the effect of erythrocyte polymorphism on multiplicity of Plasmodium infection, we compared the mean values of the number of the parasite strains co-infecting the children carrying normal genotypes with those carrying mutants (Fig. 2). The results indicate that participants with G6PD deficiency (mean ± SEM = 3.25 ± 0.39) had a significantly higher mean multiplicity of infection compared to normal individuals (mean ± SEM = 2.24 ± 0.16) [P = 0.017] (Fig. 2). Similarly, the analysis of the relationship between the ABO blood group system revealed that the multiplicity of the infection was on average significantly higher in group O (mean ± SEM = 2.66 ± 0.20) compared to A (mean ± SEM = 2.10 ± 0.34) (P = 0.03); but statistically equal to group B (mean ± SEM = 2.5 ± 0.40) [P > 0.05] (Fig. 2). The distribution of the clones of the msp-2 gene (FC27 and 3D7) according to the erythrocyte variants is given by Fig. 3. Compared to the children with Hb AS genotype, children with normal genotype were significantly more infected with FC27 allelic family (P = 0.01).
Fig. 1.
Gel picture showing PCR amplicons and RFLP fragments. (A): represents PCR products, lines 1 – 11 are samples for sickle cell (a) and G6PD studies (b); line M represents 100 bp ladder molecular size marker. (B): presents restrictions fragments after digestion with enzyme. Lines 1, 2, 3, 4, 5 and 6 shows bands before digestion and lines 1’, 2’, 3’ 4’ 5’ and 6’ after digestion for the same isolates. Line M represents 50 bp ladder molecular size marker. G6PD: Glucose-6-phosphate dehydrogenase.
Fig. 2.
Multiplicity of infection (MOI) according to erythrocyte polymorphism in asymptomatic malaria children (*: significant difference at 0.05 level). Hb AS: Children with sickle cell trait, Hb AA: Children with normal haemoglobin; G6PD Deficient: children with the genotype resulting in a deficiency in the enzymatic activity of glucose-6-phosphate dehydrogenase (G6PD), G6PD Normal: children with the genotype conferring a normal enzymatic activity of G6PD; MOI: Multiplicity of infection.
Fig. 3.
Allele frequencies and erythrocyte polymorphism (*: statistical significant with Fisher's exact test) Hb AS: Children with sickle cell trait, Hb AA: Children with normal haemoglobin; G6PD Deficient: children with the genotype resulting in a deficiency in the enzymatic activity of Glucose-6-phosphate dehydrogenase (G6PD), G6PD Normal: children with the genotype conferring a normal enzymatic activity of G6PD; MOI: Multiplicity of infection.
4. Discussion
This study was undertaken to assess the influence of the number of msp-2 parasite clones per infection in P. falciparum asymptomatic children in relation to ethnicity, sickle cell trait, G6PD deficiency and ABO blood groups. Seventy two asymptomatic malaria children were evaluated for erythrocytic variants along with MOI. In Cameroon very few studies have focused on the relationship between malaria and erythrocytic variants [34, 35]. This is the one of the recent study to provide data on the erythrocytic variants and multiplicity of P. falciparum isolates in asymptomatic phenotype in Yaoundé, Cameroon.
No significant association was observed between the MOI and ethnicity. Although this study does not discuss the factors that make it possible to understand this result, the most likely explanation could lie in the intrinsic characteristics of the immune responses of the population studied. Moreover, the candidate alleles of the genes determining the resistance to P. falciparum infection, such as the IL4-524T gene, are found in a part of the Cameroonian population [45]. The non significant variation in the average number of clones observed in our comparative analysis would then be consistent with the hypothesis that ethnic groups living in this area would probably control the parasitic infection in the same way, certainly by means of immunomodulatory factors. Our observation is contrary to that obtained by Paganotti et al. in Burkina Faso, which found the number of clones per infection, was lower in the Fulani ethnic group compared to Mossi; with a greater difference in children under one year old and those over five years old [46].
Influence of MOI with erythrocytic variants was examined in the current study. MOI was significantly higher in group O compared to group A but not in group B; suggesting a protective effect of O antigen against clinical forms of malaria. Unlike the blood group, no effect of Hb AS on the multiplicity of infection was noted in this study. This corresponds to the reports of Senegal [47] but contrary to what was shown in Gabon [48]. This disagreement could be explained by differences in age ranges of the study population. However, the mean values we obtained revealed interesting trends suggesting that the multiplicity of infection tends to be lower in individuals of Hb AS genotype. Futhermore, the alleles of the FC27 family were significantly more abundant in the Hb AA children than in the carriers of the sickle cell trait. Some studies have shown the influence of hemoglobin status on the distribution of allelic families of the msp-2 gene [47]. Such an effect was not observed by Mockenhaupt et al. and Kiwanuka et al. [49, 50]. Therefore, further studies are needed to clarify this issue. MOI was significantly high in children with G6PD deficiency compared to those with normal gene. This observation is not in agreement with that of other reports [51]. A plausible explanation is the geographical variation of the genetic diversity of P. falciparum i.e. that the clones identified in our study area are probably different from those observed by these authors. Thus, there is probably a mechanism by which the parasite clones identified in this study would resist the G6PD deficient cell environment.
5. Conclusions
The number of clones per infection is not influenced by ethnicity in our study area. Inherited blood disorders including sickle cell trait and G6PD deficiency are present and have some associations with MOI in asymptomatic malaria. However, further longitudinal studies with large sample size are needed to better understand the mechanisms involved.
Declarations
Author contribution statement
Dongang Nana Rodrigue Roman: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Vineeta Singh: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ngono Ngane Rosalie Anne, Koanga Mogtomo Martin Luther, Ngonde Essome Marie Chantal: Contributed reagents, materials, analysis tools or data.
Mouelle Sone Albert: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to all the participants of this study as well as to our field staff. We thank Mrs Monique Ngue and Amede Motsebo for technical assistance. We would like to thank the NAM & ST centre, India for NAM fellowship to Mr. Dongang Nana Rodrigue Roman for setting the basis of this study.
References
- 1.White N.J., Pukrittayakamee S., Hien T.T., Faiz M.A., Mokuolu O.A., Dondorp A.M. Malaria. Lancet. 2014 Feb;383(9918):723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 2.Babiker H.A., Ranford-Cartwright L.C., Walliker D. Genetic structure and dynamics of Plasmodium falciparum infections in the Kilombero region of Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1999 Feb;93(Suppl 1):11–14. doi: 10.1016/s0035-9203(99)90321-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang B., Han S.-S., Cho C., Han J.-H., Cheng Y., Lee S.-K. Comparison of microscopy, nested-PCR, and Real-Time-PCR assays using high-throughput screening of pooled samples for diagnosis of malaria in asymptomatic carriers from areas of endemicity in Myanmar. J. Clin. Microbiol. 2014 Jun;52(6):1838–1845. doi: 10.1128/JCM.03615-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laishram D.D., Sutton P.L., Nanda N., Sharma V.L., Sobti R.C., Carlton J.M. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar. J. 2012 Jan;11:29. doi: 10.1186/1475-2875-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudo E.S., Prista A., Jani I.V. Impact of asymptomatic Plasmodium falciparum parasitemia on the imunohematological indices among school children and adolescents in a rural area highly endemic for Malaria in southern Mozambique. BMC Infect. Dis. 2013 May;13:244. doi: 10.1186/1471-2334-13-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabunda S., Aponte J.J., Tiago A., Alonso P. A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malar. J. 2009 Apr;8:74. doi: 10.1186/1475-2875-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crookston B.T., Alder S.C., Boakye I., Merrill R.M., Amuasi J.H., Porucznik C.A. Exploring the relationship between chronic undernutrition and asymptomatic malaria in Ghanaian children. Malar. J. 2010 Feb;9:39. doi: 10.1186/1475-2875-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousema J.T., Gouagna L.C., Drakeley C.J., Meutstege A.M., Okech B.A., Akim I.N.J. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar. J. 2004 Jun;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Port A., Cot M., Etard J.-F., Gaye O., Migot-Nabias F., Garcia A. Relation between Plasmodium falciparum asymptomatic infection and malaria attacks in a cohort of Senegalese children. Malar. J. 2008 Sep;7:193. doi: 10.1186/1475-2875-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nkoghe D., Akue J.-P., Gonzalez J.-P., Leroy E.M. Prevalence of Plasmodium falciparum infection in asymptomatic rural Gabonese populations. Malar. J. 2011 Feb;10:33. doi: 10.1186/1475-2875-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eke R.A., Chigbu L.N., Nwachukwu W. High prevalence of asymptomatic Plasmodium infection in a suburb of Aba Town, Nigeria. Ann. Afr. Med. 2006 March;5:42–45. [Google Scholar]
- 12.Pasvol G. How many pathways for invasion of the red blood cell by the malaria parasite? Trends Parasitol. 2003 Oct;19(10):430–432. doi: 10.1016/j.pt.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Miller L.H., Baruch D.I., Marsh K., Doumbo O.K. The pathogenic basis of malaria. Nature. 2002 Feb;415(6872):673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 14.Kwiatkowski D.P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 2005 Aug;77(2):171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson E.K., Kwiatkowski D.P., Sabeti P.C. Natural selection and infectious disease in human populations. Nat. Rev. Genet. 2014 Jun;15(6):379–393. doi: 10.1038/nrg3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravorty S., Williams T.N. Sickle cell disease: a neglected chronic disease of increasing global health importance. Arch. Dis. Child. 2015 Jan;100(1):48–53. doi: 10.1136/archdischild-2013-303773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappellini M.D., Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008 Jan;371(9606):64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 18.Mason P.J., Bautista J.M., Gilsanz F. G6PD deficiency: the genotype-phenotype association. Blood Rev. 2007 Sep;21(5):267–283. doi: 10.1016/j.blre.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Bunn H.F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997 Sep;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 20.Piel F.B., Patil A.P., Howes R.E., Nyangiri O.A., Gething P.W., Williams T.N. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 2010 Nov;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackinnon M.J., Mwangi T.W., Snow R.W., Marsh K., Williams T.N. Heritability of malaria in Africa. PLoS Med. 2005 Dec;2(12):e340. doi: 10.1371/journal.pmed.0020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams T.N. Human red blood cell polymorphisms and malaria. Curr. Opin. Microbiol. 2006 Aug;9(4):388–394. doi: 10.1016/j.mib.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Taylor S.M., Cerami C., Fairhurst R.M. Hemoglobinopathies: slicing the Gordian knot of Plasmodium falciparum malaria pathogenesis. PLoS Pathog. 2013;9(5) doi: 10.1371/journal.ppat.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwiatkowski D.P., Luoni G. Host genetic factors in resistance and susceptibility to malaria. Parassitologia. 2006 Dec;48(4):450–467. [PubMed] [Google Scholar]
- 25.Yuzhalin A.E., Kutikhin A.G. ABO and Rh blood groups in relation to ovarian, endometrial and cervical cancer risk among the population of south-east Siberia. Asian Pac. J. Cancer Prev. APJCP. 2012;13(10):5091–5096. doi: 10.7314/apjcp.2012.13.10.5091. [DOI] [PubMed] [Google Scholar]
- 26.Kumar N.C., Nadimpalli M., Vardhan V.R., Gopal S.D. Association of ABO blood groups with Chikungunya virus. Virol. J. 2010 Jun;7:140. doi: 10.1186/1743-422X-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S.K., Singh A.P., Pandey S., Yazdani S.S., Chitnis C.E., Sharma A. Definition of structural elements in Plasmodium vivax and P. knowlesi Duffy-binding domains necessary for erythrocyte invasion. Biochem. J. 2003 Aug;374(1):193–198. doi: 10.1042/BJ20030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer P.R., Boone P. Short report: severe malaria associated with blood group. Am. J. Trop. Med. Hyg. 1998;58:122–123. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]
- 29.Pankoui M.J.B., Gouado I., Fotso K.H., Zambou O., Amvam Zollo P.H., Grau G.E.R. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One. 2010 Oct;5(10) doi: 10.1371/journal.pone.0013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyasa R.B., Kimbi H.K., Zofou D., DeBarry J.D., Kissinger J.C., Titanji V.P.K. An evolutionary approach to identify potentially protective B cell epitopes involved in naturally acquired immunity to malaria and the role of EBA-175 in protection amongst denizens of Bolifamba, Cameroon. Malar. J. 2016 May;15:281. doi: 10.1186/s12936-016-1337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apinjoh T.O., Anchang-Kimbi J.K., Njua-Yafi C., Mugri R.N., Ngwai A.N., Rockett K.A. Association of cytokine and toll-like receptor gene polymorphisms with severe malaria in three regions of Cameroon. PLoS One. 2013 Nov;8(11) doi: 10.1371/journal.pone.0081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimbi H.K., Sumbele I.U., Nweboh M., Anchang-Kimbi J.K., Lum E., Nana Y. Malaria and haematologic parameters of pupils at different altitudes along the slope of Mount Cameroon: a cross-sectional study. Malar. J. 2013 Jun;12:193. doi: 10.1186/1475-2875-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Megnekou R., Lissom A., Bigoga J.D., Djontu J.C. Effect of pregnancy-associated malaria on T cell cytokines in Cameroonian women. Scand. J. Immunol. 2015 Jun;81(6):508–514. doi: 10.1111/sji.12286. [DOI] [PubMed] [Google Scholar]
- 34.Nkuo-Akenji T.K., Wepngong P., Akoachere J.F. Effects of ABO/Rh blood groups, G-6-P-D enzyme activity and haemoglobin genotypes on malaria parasitaemia and parasite density. Afr. J. Health Sci. Jul–Dec 2004;11:93–97. [PubMed] [Google Scholar]
- 35.Quakyi I.A., Leke R.G., Befidi-Mengue R., Tsafack M., Bomba-Nkolo D., Manga L. The epidemiology of Plasmodium falciparum malaria in two Cameroonian villages: Simbok and Etoa. Am. J. Trop. Med. Hyg. 2000 Nov-Dec;63(5-6):222–230. [PubMed] [Google Scholar]
- 36.Enosolease M.E., Bazuaye G.N. Distribution of ABO and Rh-D blood groups in the Benin area of Niger-Delta: Implication for regional blood transfusion. Asian J. Transfus. Sci. 2008 Jan;2(1):3–5. doi: 10.4103/0973-6247.39502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . secondth ed. World Health Organisation; Switzerland: 2010. Basic Malaria Microscopy.http://who.int/iris/bitstream/10665/44208/1/9789241547826_eng.pdf [Google Scholar]
- 38.Snounou G., Viriyakosol S., Jarra W., Thaithong S., Brown K.N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 1993 Apr;58(2):283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 39.Snounou G., Zhu X., Siripoon N., Jarra W., Thaithong S., Brown K.N. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1999 Aug;93(4):369–374. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- 40.Ibara-Okabande R., Koukouikila-Koussounda F., Ndounga M., Vouvoungui J., Malonga V., Casimiro P.N. Reduction of multiplicity of infections but no change in msp2 genetic diversity in Plasmodium falciparum isolates from Congolese children after introduction of artemisinin-combination therapy. Malar. J. 2012 Dec;11:410. doi: 10.1186/1475-2875-11-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saiki R.K., Scharf S., Faloona F., Mullis K.B., Horn G.T., Erlich H.A. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 42.Ayatollahi M., Zakerinia M., Haghshenas M. Molecular analysis of Iranian families with sickle cell disease. J. Trop. Pediatr. 2005 Jun;51(3):136–140. doi: 10.1093/tropej/fmh101. [DOI] [PubMed] [Google Scholar]
- 43.Vizzi E., Bastidas G., Hidalgo M., Colman L., Pérez H.A. Prevalence and molecular characterization of G6PD deficiency in two Plasmodium vivax endemic areas in Venezuela: predominance of the African A-(202A/376G) variant. Malar. J. 2016 Jan;15:19. doi: 10.1186/s12936-015-1069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirono A., Beutler E. Molecular cloning and nucleotide sequence of cDNA for human glucose-6-phosphate dehydrogenase variant A(-) Proc. Natl. Acad. Sci. U.S.A. 1988 Jun;85(11):3951–3954. doi: 10.1073/pnas.85.11.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockman M.V., Hahn M.W., Soranzo N., Goldstein D.B., Wray G.A. Positive selection on a human specific transcription factor binding site regulating IL4 expression. Curr. Biol. 2003 Dec;13:2118–2123. doi: 10.1016/j.cub.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 46.Paganotti G.M., Babiker H.A., Modiano D., Sirima B.S., Verra F., Konaté A., Ouédraogo A.L., Diarra A., Mackinnon M.J., Coluzzi M., Walliker D. Genetic complexity of Plasmodium falciparum in two ethnic groups of Burkina Faso with marked differences in susceptibility to malaria. Am. J. Trop. Med. Hyg. 2004 Aug;71(2):173–178. [PubMed] [Google Scholar]
- 47.Konaté L., Zwetyenga J., Rogier C., Bischoff E., Fontenille D., Tall A. Variation of Plasmodium falciparum msp1 block 2 and msp2 allele prevalence and of infection complexity in two neighbouring Senegalese villages with different transmission conditions. Trans. R. Soc. Trop. Med. Hyg. 1999 Feb;93(Suppl 1):21–28. doi: 10.1016/s0035-9203(99)90323-1. [DOI] [PubMed] [Google Scholar]
- 48.Ntoumi F., Mercereau-Puijalon O., Ossari S., Luty A., Reltien J., Georges A. Plasmodium falciparum: sickle-cell trait is associated with higher prevalence of multiple infections in Gabonese children with asymptomatic infections. Exp. Parasitol. 1997 Sep;87(1):39–46. doi: 10.1006/expr.1997.4173. [DOI] [PubMed] [Google Scholar]
- 49.Mockenhaupt F.P., Ehrhardt S., Otchwemah R., Eggelte T.A., Anemana S.D., Stark K. Limited influence of haemoglobin variants on Plasmodium falciparum msp1 and msp2 alleles in symptomatic malaria. Trans. R. Soc. Trop. Med. Hyg. 2004 May;98:302–310. doi: 10.1016/j.trstmh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Kiwanuka G.N., Joshi H., Isharaza W.K., Eschrich K. Dynamics of Plasmodium falciparum alleles in children with normal haemoglobin and with sickle cell trait in western Uganda. Trans. R. Soc. Trop. Med. Hyg. 2009 Jan;103:87–94. doi: 10.1016/j.trstmh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 51.Vafa M., Troye-Blomberg M., Anchang J., Garcia A., Migot-Nabias F. Multiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variants. Malar. J. 2008 Jan;7:17. doi: 10.1186/1475-2875-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]