Abstract
Purpose:
To describe surgical outcomes and structural characteristics of IOLs implanted with transconjunctival sutureless intrascleral (SIS) fixation in human eyes.
Design:
Retrospective interventional surgical case series involving live and cadaveric human eyes.
Methods:
In this study, we investigated the surgical outcomes and structural anatomy of secondary IOLs implanted with the SIS technique in human eyes. All cases involving SIS IOL fixation performed at a single academic center from January 1 2012 through July 30 2016 were reviewed to describe the surgical technique, common indications, clinical outcomes, and the rate of common operative complications. In order to investigate the structure of SIS-fixated IOLs in vivo, slit-lamp biomicroscopy, ultrasound biomiscroscopy (UBM), and intraoperative endoscopy were analyzed to describe anatomic outcomes. The primary anatomic outcomes were the optic pupillary centration and location of haptic externalization. Results were correlated with cadaveric human eyes that underwent the SIS-IOL technique. Cadaveric eyes were imaged and analyzed using high-resolution photography for centration, stress measurements at the haptic-optic junction, and qualitative descriptors of IOL optic and haptic position.
Results:
A total of 122 consecutive patients that underwent IOL placement using SIS technique were included in the study with mean follow-up of 1.52 years (range, 0.4–4.5 years). The majority (75%) of patients received a new 3-piece IOL for primary aphakia or after IOL exchange. The other patients (25%) had a dislocated 3-piece IOL that was rescued using the SIS technique. Preoperative mean Snellen visual acuity was 20/633 (logMAR=1.501). At the final visit, the mean best-corrected visual acuity was 20/83 (logMAR=0.6243) and final mean spherical equivalent was −0.57 diopters. The most common complications were vitreous hemorrhage (22% of eyes), which resolved spontaneously in majority of cases, and cystoid macular edema. The rates of IOL dislocation, IOL decentration, haptic erosion, IOL tilting, iris capture and endophthalmitis were low. Intraoperative endoscopy and UBM demonstrated a securely fixated IOL, and well-centered optic without iris or ciliary body touch. Structural study of cadaveric eyes confirmed IOL optic and haptic anatomy observed during live human surgery. The ab interno haptic insertion was the anterior pars plana, away from iris, ciliary processes and ora serrata. The degree of haptic externalization was correlated with the degree of strain on the haptic-optic junction. The angle of the haptic-optic junction in SIS-fixated IOLs (33.97°) was not significantly different compared to overlaid native non-fixated IOL (32.93°), but increased slightly with degree of haptic tip externalization (36.26 ° and 39.16° for 2mm and 3mm haptic externalizations, respectively).
Conclusion:
In this comprehensive study, we demonstrate the surgical outcomes achieved with SIS fixation of IOLs. Surgical and post-operative complications do occur, albeit at a low rate, and can effectively be managed with excellent anatomic and visual outcomes. The structural and anatomic data in this study may help guide SIS placement and optimize long-term surgical results.
Keywords: Secondary IOL implantation, sutureless intrascleral fixation, aphakia, pars plana vitrectomy
Introduction
Successful modern cataract surgery culminates in placement of the intraocular lens (IOL) “in-the bag,” or if the posterior capsule is compromised, in the ciliary sulcus. Eyes without adequate capsular support for either can have IOL implantation by placement of anterior chamber IOLs (ACIOLs),1 iris-fixated IOLs, or scleral-fixated IOLs.2,3 Each of these approaches has its unique advantages and disadvantages. A number of techniques for scleral fixation have been described, and the selection and the success of each of these techniques are largely predicated on the individual surgeon’s preference and experience with the particular technique.
We have recently reported a sutureless, trans-conjunctival approach to scleral fixation (SIS) with 25-gauge4 and 27-gauge5 trocars, as a modification of Prenner et. al.6 and Prasad techniques.7 The most common indication for this procedure include aphakia following complicated cataract surgery, aphakia following lensectomy during complex RD repair, IOL dislocation from multiple causes with either IOL rescue or exchange, and crystalline lens subluxation. The one-year clinical and refractive outcomes of this technique for a limited number of patients have been published.4 Although the rate of post-operative complications of SIS fixation was low in this study, the increasing use of this technique requires a more detailed understanding of the anatomy of scleral-fixated IOLs and their clinical outcomes.
A comprehensive review of the surgical outcomes of all patients who underwent scleral fixation using the SIS technique at our practice over a four-year period was completed. In this manuscript we retrospectively describe the structural anatomy of SIS-fixated IOLs in human eyes, using careful examination, ultrasound biomicroscopy, intraoperative endoscopy and detailed measurements of SIS fixation in post-mortem cadaveric human eyes. We report structural information, anatomic data and analysis of the haptic and haptic-optic junction strain during 3-piece IOL fixation in cadaveric eyes. We believe that this structure-outcomes analysis is important to refine our surgical approach and further improve our visual and anatomic outcomes with SIS technique.
Methods
The study was conducted with Institutional Review Board approval and was in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2000 and 2008.
SIS surgical technique
Standard three-port small incision vitrectomy setup was utilized with 25g (or 27g) trocars (Alcon, Fort Worth, TX) for lens fixation. The trocars are placed at around 6 and 12 o’clock, at a fixed distance posterior to the limbus (most commonly at 2 mm), following an anti-parallel configuration in which haptics are externalized parallel to the limbus and projected in opposite direction following inverse “S” configuration. The toric marker was utilized to ensure that the fixating trocars were oriented 180-degree opposite of each other. Following completion of vitrectomy, a clear-corneal wound was created using a 2.75 mm surgical keratome and a three-piece MA60AC IOL (Alcon, Fort Worth, TX) was inserted into the eye. The trailing haptic was allowed to hang off the iris, while the leading haptic tip was grasped with MAXgrip forceps (GRIESHABER® MAXgrip™ forceps, Alcon, Fort Worth, TX) and externalized through the fixating sclerotomy. The same procedure was performed at the opposite end with the trailing haptic. For patients with dislocated 3-piece IOLs, the IOL was elevated to the anterior vitreous cavity either with forceps or soft tip under vacuum, and residual capsule was removed. The haptic tips were purchased with MAXgrip™ forceps and externalized sequentially in a similar fashion. The same SIS IOL fixation procedure was performed on cadaveric eyes during the wet lab portion of the study.
Intraoperative endoscopy and post-operative imaging
Endoscopy was performed on one patient who required removal of retained lens fragment with phaco fragmatome at the time of SIS-IOL placement. During this surgery, once both haptics were securely fixated in the scleral tunnel and the IOL appeared centered, the conjunctiva was opened superotemporally and a 19-gauge MVR blade was used to enlarge the superotemporal sclerotomy site and make an incision through the choroid. Through this incision, a curved 20g endo-illuminated endoscopy probe (Endo Optiks, Waltham, MA) was introduced, and used to inspect the nasal haptic entry site, peripheral retina and position of the optic in relation to the iris. Following completion, the sclerotomy and conjunctival peritomy were closed over the supero-temporal site, and the conjunctiva was repositioned over both fixating haptics to ensure that the haptic tips were completely covered (Figure 1, 2 and Video 1).
Figure 1.
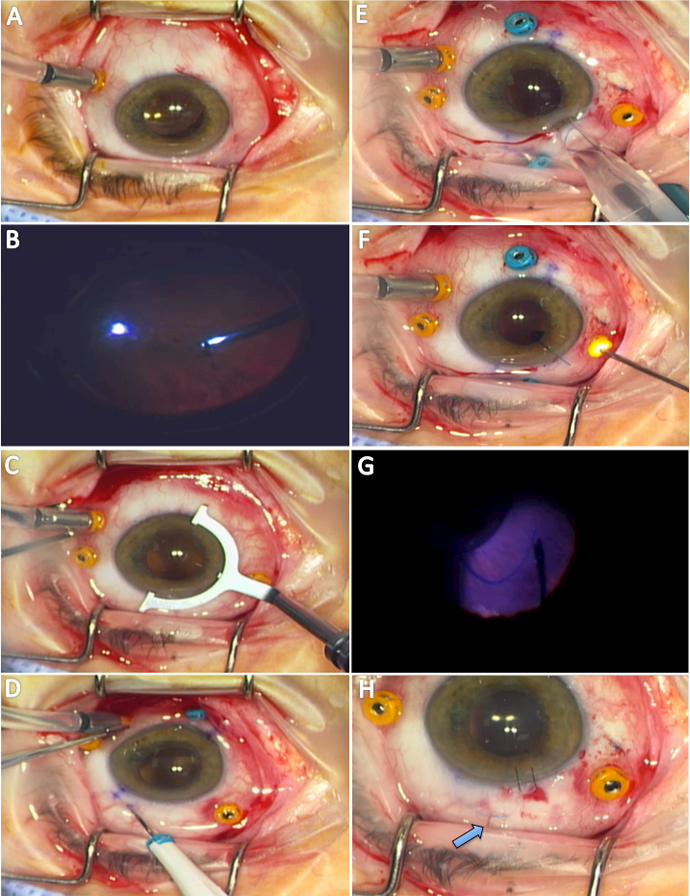
Intraoperative view of 23-gauge three-port pars plana vitrectomy (A, B). Using toric marker (C), the 25g fixating trocars were placed 180 degrees opposite each other in an antiparallel fashion 2 mm posterior to the limbus (D). Three piece MA60AC IOL was inserted (E) and “hung” off the iris (F). The 25g MAXgrip™ forceps were used to purchase the tip of the haptic with endoillumination (G) and externalized through the scleral tunnel. Higher magnification view demonstrates well-centered IOL with haptic (arrow) well covered by conjunctiva and tenons (H).
Figure 2.
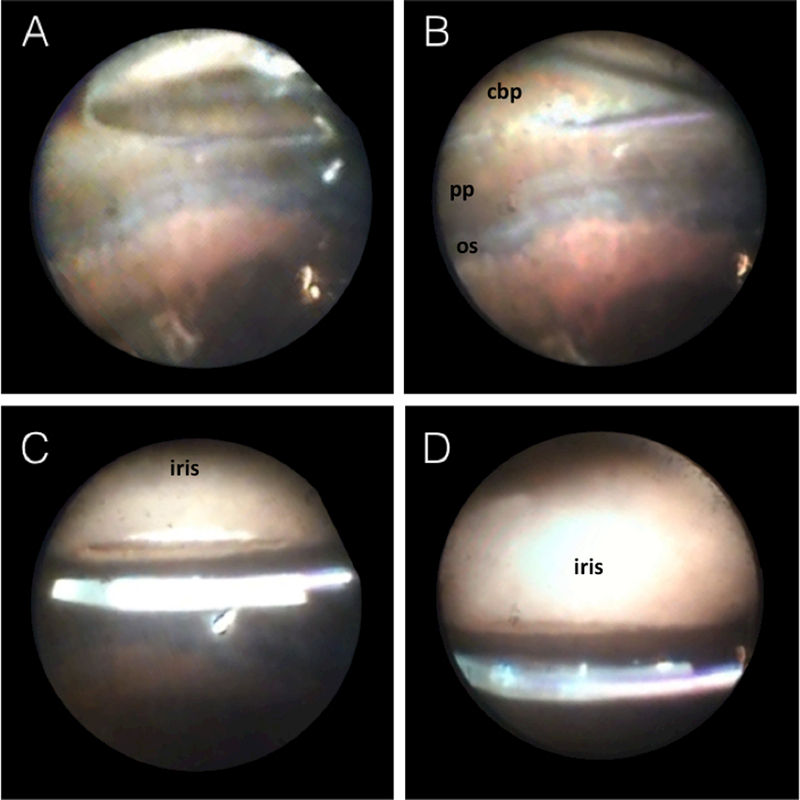
Intraoperative endoscopic view of the SIS-fixated IOL. Insertion of the 20g endoscope through the temporal pars plana allowed for viewing of the nasal aspect of the IOL and fixating haptic (A). The haptic is exiting the eye through the anterior pars plana, posterior to the ciliary processes (B). Anterior view demonstrates a well-positioned IOL just behind the pupil (C) with no IOL rotation or tilt (D).
The post-operative imaging was obtained at one month after surgery. The ultrasound biomicroscopy was performed using Ellex Eye Cubed system (Eden Prairie, MN). Both axial and radial scans were obtained to optimize viewing of IOL relationship with the iris and ciliary body structures (Figure 3).
Figure 3.
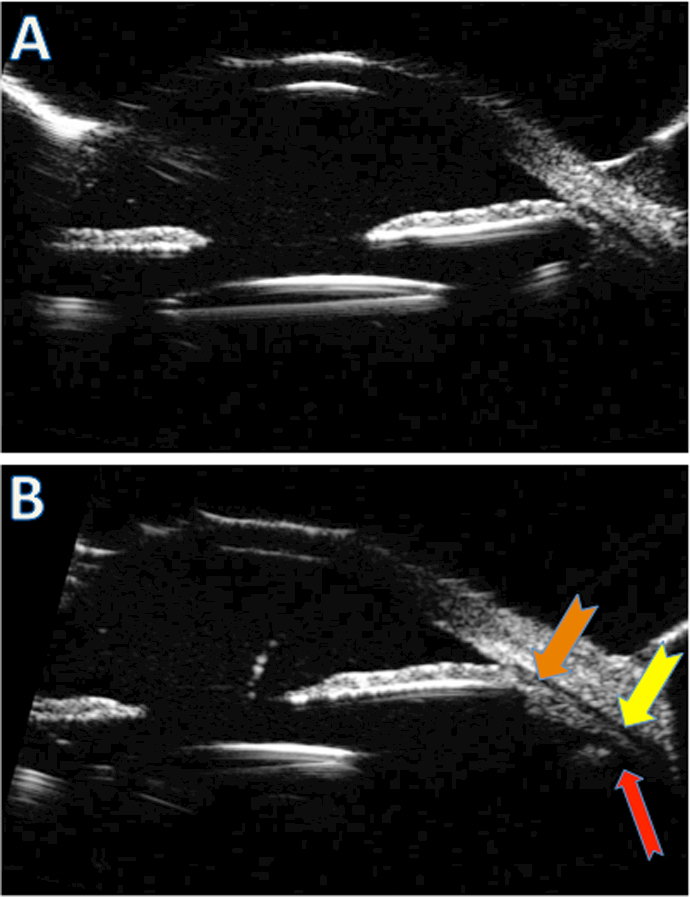
Post-operative appearance of SIS-fixated IOL. Ultrasound biomicroscopy (UBM) view of the IOL demonstrates well-centered optic with no iris touch on still (A) and kinetic axial scans (not shown). The radial view of ciliary body (thick arrows) shows haptic insertion to the eye wall just posterior to the ciliary body process (thin red arrow) (B).
Wetlab study
Post-mortem cadaveric eyes were obtained from the Eversight eye bank (Ann Arbor, MI). All eyes were harvested from respective donors using protocol mandated by Eversight. All cadaveric eyes underwent intracameral injection of 10% dextran solution to maintain corneal clarity and were transported in the dextran solution on ice. All were used for the wetlab within 24 hours of receipt from the eyebank. For each eye, axial length, horizontal and vertical white-to-white distance and haptic externalization measurements were obtained using surgical calipers.
The wetlab setup included VersaVIT 1.0 (Synergetics/Bausch and Lomb, St Louis, MO) with three-port 23g and 25g vitrectomy setup (Suppplemental Figure 1). Iris hooks were placed to maintain intraoperative mydriasis. The makeshift hand-held disposable BIOM wide-angle viewing lens (OCULUS, Port St. Lucie, FL) was used to perform the lensectomy, capsulectomy, core vitrectomy, and trim peripheral vitreous. The fixation of MA60AC IOLs was achieved with 25g trocars placed at 2 mm posterior to the limbus (Alcon, Fort Worth, TX) as described above. The standard cataract surgery instrumentation was used to assist in IOL insertion and positioning. Following IOL fixation, the eyes were cut at the equator using an inverted keratome blade and assisted by Westcott scissors. The residual vitreous skirt, peripheral retina and the choroid were removed using a vitrectomy cutter. Posterior view images were obtained using Nikon D700 camera (Melville, NY), and images saved as high-resolution jpeg files (Figure 4–6).
Figure 4.
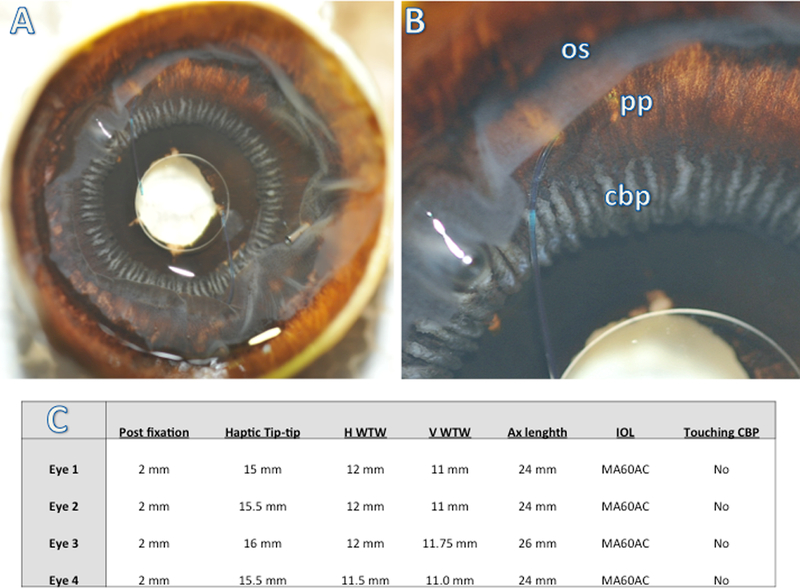
Posterior view of scleral-fixated IOL demonstrates well-centered IOL over the pupil with haptic insertion posterior to the ciliary processes on both ends (A). Image with higher magnification demonstrates haptic insertion posterior to the ciliary body processes (cbp) in the pars plana (pp), but anterior to the ora serrata (os) (B). The cut retina is reflected anteriorly over pars plana (B). Table summarizes four cadaveric eyebank eyes used in the study and their biometric measurements. The secondary, SIS-fixated MA60 IOL was implanted in all four eyes 2 mm posterior to the limbus, and in no case were the haptics in contact with the ciliary body processes (C).
Figure 6.
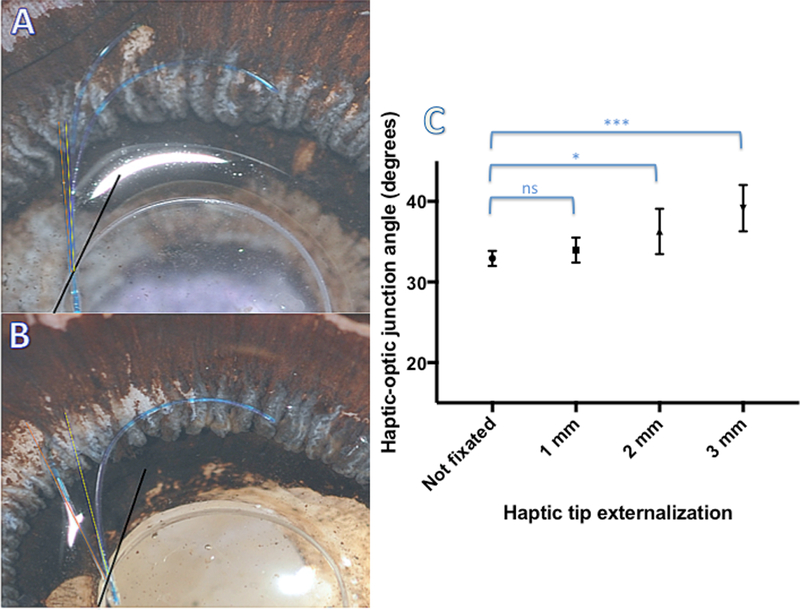
Haptic-optic junction strain in response to the degree of haptic externalization through the scleral tunnel. Scleral-fixated IOLs were externalized with increasing length of haptic tip showing. The angle between fixated (red) or non-fixated (yellow) IOL haptic and the tangent subtended to the rim of the optic at the haptic-optic junction was determined (A, B). Increasing haptic externalization results in an increased haptic-optic junction angle (ns=not significant, * significant p<0.05)
Image analysis
The images were processed with Adobe Photoshop and Image J software (National Institute of Health, Bethesda, MD). In order to determine the strain on the haptic-optic junction induced by the degree of haptic externalization, the angle between tangent at the haptic-optic junction at both superior and inferior haptics and the axis of the haptic were determined by a blinded investigator using Image J angle function. The mean haptic-optic angle for eyes with increasing haptic externalization was determined and compared using one-way ANOVA with multiple comparisons utilizing GraphPad Prism statistical software (GraphPad Prism, LaJolla, CA). For all statistical comparisons, significance cutoff was set at p<0.05.
SIS surgical outcomes:
Clinical and operative notes of all patients who underwent secondary IOL implantation between January 1, 2012 through July 30, 2016 at Associated Retinal Consultants, Royal Oak, MI were reviewed. Only the patients who underwent SIS IOL fixation during this period and had at least 3 months of follow-up were included in the analysis (Table 1 and 2). Patients with incomplete medical records, duration of follow up shorter than the specified 3 months and patients undergoing scleral IOL fixation with other than SIS technique were excluded. Patients’ demographic data including age, gender, and race, primary diagnosis, ocular and systemic comorbidities, best corrected visual acuity, refractive error as determined by Snellen autorefractor (Topcon, Oakland, NJ), intraocular pressure, anterior and posterior exam findings were reviewed. Operative notes detailing surgical approach, gauge and position of the vitrectomy and fixating cannulas, and any noted intra-operative complications were reviewed.
Table 1.
Demographics and baseline characteristics of IOL scleral fixation using SIS technique.
| N (total) | 122 |
| Mean age (yrs) | 65.9 |
|
Male Female |
72 (59%) 50 (41%) |
| New SIS IOL fixation | 42.6% |
| IOL exchange with SIS fixation | 32.4% |
| IOL repositioning with SIS fixation | 25.0% |
| Indications | |
|
Subluxed or dislocated IOL |
|
|
Aphakia post-complex CE with/without retained lens fragments |
|
|
Subluxed or dislocated crystalline lens, not amenable to anterior approach Aphakia following trauma, including OGI |
|
|
Mean follow-up (years) |
1.52 |
|
Range follow-up (years) |
0.4 – 4.54 |
| 20g 3 (2.5%) | |
| 23g 5 (4.1%) | |
| Scleral fixation (gauge) | 25g 96 (78.8%) |
| 27g 18 (14.8%) | |
| Location of fixation sclerotomies | 1.5 mm 12 (9.8%) |
| 1.75 mm 1 (0.8%) | |
| 2.0 mm 108 (88.5%) | |
| 2.5 mm 1 (0.8%) |
Table 2.
Visual, refractive and surgical outcomes of patients undergoing sutureless scleral fixation.
| No. SIS- IOLs in place at last follow-up | 118/122 (96.7%) |
| - 3 pts IOL exch to ACIOL | |
| - 1 pt IOL exch to Akreos IOL | |
| Mean visual acuity | |
| - pre-operative (baseline) | 20/633 (logMAR 1.501) |
| - post-operative (last f/u visit) | 20/83 (logMAR 0.6243) |
| Mean spherical equivalent | − 0.565 diopters |
| Complications: | |
| • New vitreous hemorrhage | 27/122 (22.1%) |
|
• Vit hemorrhage resolved spontaneously |
18/122 (15.5%) |
|
• Vit hemorrhage req PPV (with or without IOL repo) |
8/122 (6.6%) |
| • CME | 26/122 (21.3%) |
| • Iris touch/capture | 4/122 (3.3%) |
| • Endophthalmitis (sterile) | 1/122 (0.8%) |
|
• Re-operation for IOL/haptic issues |
13/122 (10.7%) |
| - haptic dis-insertion | 5 |
| - haptic malposition | 2 |
| - haptic break | 2 |
| - optic iris capture | 2 |
| - haptic tip erosion | 1 |
| - IOL tilt | 1 |
Results
Demographics and surgical outcomes of IOL fixation using the SIS technique are summarized in Tables 1 and 2. A total of 122 SIS procedures were included in this study. All surgeries were performed between January 1, 2012 and July 30, 2016 at Associated Retina Consultants, with mean follow-up of 1.52 years (0.4–4.54). Patients’ average age was 65.9 years, and majority of them were male (62.8%). The main indications for surgery were subluxed or dislocated IOL, aphakia following complicated cataract surgery or trauma, and subluxed or dislocated crystalline lens where lensectomy was not amenable to anterior surgical approach. A majority of the patients received a new IOL for primary aphakia (42.6%) or during IOL exchange (32.4%), while in 25% of patients a dislocated three-piece IOL was rescued and sclerally fixated (Table 1). The SIS fixation was most-commonly performed with 25g trocars (78.8%), compared to 27g (14.8%), 20g (2.5%) and 23g (4.1%), respectively. The fixation trocars were most commonly inserted at 2.0 mm posterior to the limbus (88.5%, Table 1). At the last follow-up visit, all patients (122) had an IOL, however in four patients SIS IOL was exchanged for an ACIOL (3 patients) and Akreos AO lens (1 patient). At the last follow-up, the mean best-corrected visual acuity was 20/83 (logMAR=0.6243), improved from pre-operative mean 20/633 (logMAR=1.501), while the final refraction was mean −0.565 diopter spherical equivalent. The most common peri- and post-operative complication was vitreous hemorrhage. In vast majority of patients (18 out of 27), the hemorrhage resolved spontaneously, while 8 patients required repeat vitrectomy surgery (Table 2). 21.3% of patients had some cystoid macular edema observed on OCT, but a majority of those were effectively treated with topical steroid and/or non-steroidal anti-inflammatory drops. The incidence of IOL dislocation, decentration, haptic erosion, breakage and disinsertion were low. Three patients developed intraoperative retinal tears, which were treated with endolaser without long-term sequelae. Another patient developed retinal detachment, which was repaired successfully and one patient developed culture-negative endophthalmitis.
The detailed steps of the sutureless intrascleral fixation technique are summarized in Figure 1 and Video 1 and described in detail in Methods section. To confirm the fixation of the haptic post-SIS IOL fixation in vivo, we performed 20g endoscopy by enlarging one of the temporal sclerotomy sites. Figure 2 summarized the endoscopic view of the fixated IOL with 20g curved illuminated endoprobe passed through the supero-temporal sclerotomy. Herein, the nasal aspect of the IOL and nasal haptic are visible. As in this case, for IOL fixated 2 mm posterior to the limbus, the haptic inserts into the anterior pars plana, just posterior to the ciliary body processes without ciliary process touch or rubbing (Figure 2A, B). Good centration of the optic is visible over the pupil underside without iris-optic or iris-haptic capture or touch (Figure 2C, D). This intraoperative view is reinforced by the post-operative UBM. Axial static and kinetic scans (Figure 3A) demonstrated well-centered optic over the pupil, well away from the iris and without optic-iris or haptic-iris touch. Radial view (Figure 3B) demonstrated haptic insertion (red arrow) just posterior to the ciliary processes (orange and yellow arrows). The anterior segment photos demonstrated externalized haptic termini flat with the sclera and well covered by the conjunctiva and tenons without protrusion or extrusion (Figure 3C, D).
To further investigate the anatomy of human eyes post-SIS IOL fixation ex vivo, we obtained post-mortem donated human cadaveric eyes from the eye bank. Wetlab setup utilizing an operating microscope, VersaVIT vitrectomy machine (Synergetics, St Louis, MO) with 23g and 25g platform, and standard vitreoretinal and cataract surgical instrumentation is summarized in supplemental Figure 1. Posterior view of SIS-IOL fixated in cadaveric eyes demonstrated well-centered IOL over the pupil with haptic insertion posterior to the ciliary processes on both ends (Figure 4A). Higher magnification demonstrated haptic insertion at the anterior pars plana (pp), just posterior to the ciliary body processes (cbp) and anterior to the ora serrata (os) (Figure 4B). Similar anatomic profile was observed in all four cadaveric eyes with SIS-IOL fixation, with no ciliary body-haptic touch in any of the fixated eyes (Figure 4C). The measured biometric data for all eyes is summarized in Figure 4C. The anatomic profile of IOL haptic insertion relative to the anatomic structures (i.e. iris, ciliary body, ora serrata) as a function of axial length was not studied.
To investigate strain on the IOL haptic-optic junction produced by the scleral fixation, another non-fixated MA60AC IOL was overlaid with the SIS-fixated IOL in cadaveric eyes, aligning the optic and haptic-optic junctions (Figure 5). The configuration and the angle of the haptic-optic junction of SIS-fixated IOLs were then compared qualitatively and quantitatively to the non-fixated IOLs. Figure 5A demonstrates a posterior view of SIS-IOL in a 24 mm cadaveric eye with 1 mm externalization of the haptic tips. (Figure 5A). Comparing the contour of the proximal portion of the haptic and haptic-optic junction demonstrates similar angle of haptic-optic junction of both fixated and non-fixated IOLs in this eye (Figure 5B). To quantify the amount of haptic-optic junction strain, the haptic-optic junction angle was calculated using Image J software at different extent of haptic externalization (Figure 6A). The mean haptic-optic angle in non-fixated IOLs (32.93°) was not significantly different from SIS-fixed IOL with 1 mm haptic externalization (33.97 °). However, both 2mm and 3mm haptic externalizations produced a very small, but statistically significant increase in mean haptic-optic angle (36.26 ° and 39.16,° respectively) compared to non-fixated IOL (Figure 6C).
Figure 5.
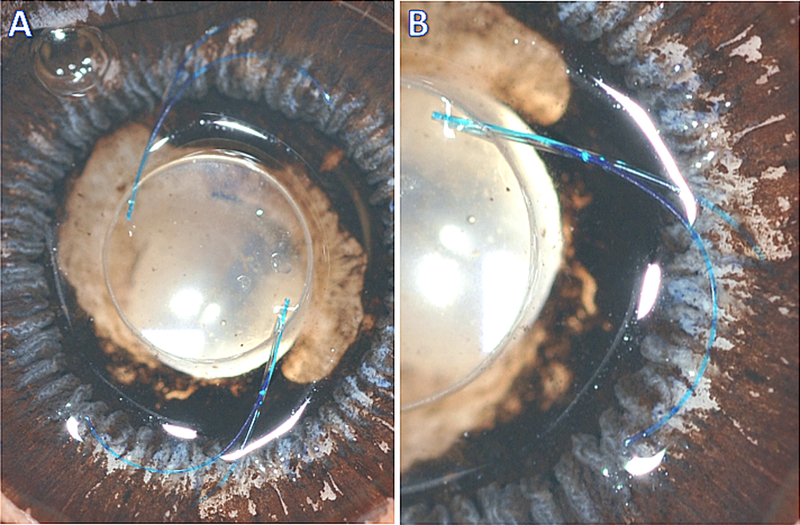
Posterior view of sclerally-fixated MA60AC IOL with 1 mm externalization of the haptic tips. Another free, non-fixated MA60AC IOL was overlaid over the fixated IOL with aligned optic and haptic-optic junctions (A). Comparing the contour of the proximal portion of the haptic and haptic-optic junction demonstrates similar angle of haptic-optic junction of both fixated and non-fixated IOLs (B).
Discussion
In this manuscript we describe in vivo and ex vivo structural and anatomic characterization of human eyes with secondary IOLs implanted by sutureless intrascleral fixation (SIS). To the best of our knowledge, this is the largest and most comprehensive study on the surgical outcomes and anatomy of eyes with SIS-fixated IOLs. With the SIS technique, the scleral fixation of the haptics can be achieved with any gauge cannulas, although in our experience smaller gauge cannulas (such as 25g and 27g) are best suited as they create smaller and tighter scleral tunnels, produce less tissue injury and a decreased risk of post-operative hypotony.5 Over the course of this study the technique has evolved; we have moved toward smaller gauge fixation cannulas, and now only exclusively utilize either 27g or 25g fixation trocars. We use vitreoretinal forceps (such as Alcon’s MaxGrip forceps) to externalize haptics through the fixation sclerotomies. Other techniques of haptic externalization were reported using 25g, 27g or 30g needles in which the needles were used to externalize haptic tips producing near-identical scleral fixation, and offer potentially cheaper alternative to trocars.8–10 However, haptic loading into the needles, their simultaneous externalization and manipulation can be technically more challenging. The cannula-based technique uses a setup that is familiar and comfortable to retina surgeons. Furthermore, risks of posterior IOL dislocation, haptic amputation and iatrogenic vitreous hemorrhage with large needles are at least theoretically higher with the needle techniques. The other advantages of the SIS technique over the traditional Prenner and Agarwal scleral fixation are that it requires no conjunctival peritomy, avoids large sclerotomies, and does not require sutures. As a result, patients may have faster visual rehabilitation, experience less pain, and have less post-operative inflammation.
The goal of the sutureless scleral fixation is a well-centered IOL, with the optic at a distance away from iris to avoid capture, and haptics away from ciliary processes, iris and ora serrata. Therefore, placement of fixating cannulas is the critical step of SIS technique. We usually insert the cannulas at a fixed distance posterior to the limbus, in an anti-parallel fashion following the “inverse S” configuration. Placement of the cannulas 180 degrees opposite of each other is important to ensure proper IOL centration in the pupillary axis. Placement of cannula at the same distance posteriorly from the limbus on each end prevents horizontal IOL tilting, so careful intraoperative measurements of both fixating sclerotomy sites are critical. For most eyes with average axial length, including the majority of eyes in this study, fixating cannulas are placed 2 mm posterior to the limbus. Fixation of the lens too anteriorly can cause iris touch with chaffing, leading to persistent inflammation and uveitis-glaucoma-hyphema (UGH) syndrome. On the other hand, too posterior placement of fixation trocars may cause iatrogenic breaks and retinal detachment. Thus in majority of cases (eyes of normal axial length), we perform fixation at 2 mm posterior to the limbus. The cadaveric portion of the study reinforced that this establishes the insertion of haptics just at the posterior tip of the ciliary processes. In the eyes with high myopia or with prior scleral fixation with recurrent vitreous hemorrhages (i.e. UGH-type of clinical picture) where we displace fixation posteriorly to 2.5 mm or rarely 3 mm. Pre-operative UBM can be very helpful in those cases. In this study, endoscopic visualization of the SIS-fixated IOL at 2mm demonstrated haptic entry just posterior to the ciliary processes, and well anterior to the ora serrata (Figure 2). This is further reinforced by post-operative ultrasound biomicroscopy, which demonstrated IOL centration and absence of iris or ciliary body touch (Figure 3). The IOL anatomy was even better visualized in the cadaveric study, and in none of the four eyes did the haptics touch the ciliary body processes or placed too close to the ora serrata (Figure 4). In addition, endoscopic examination and UBM views demonstrated that the fixated IOL is parallel to the pupillary plane without rotation or tilting. The optic was well away from the iris, confirming good centration and adequate antero-posterior placement of the fixation. In our series, the most common complication of SIS fixation was vitreous hemorrhage, which in vast majority of patients was self-limiting. However, eight patients required repeat vitrectomy and IOL repositioning with more posterior placement of haptic fixation. Thus, any sign of IOL-iris touch may be an indication for a more posterior scleral haptic repositioning or placement of ACIOL.
The SIS technique requires the force of the IOL suspension to be transmitted onto the haptics and haptic-optic junction. Due to the nature of three-piece IOL design, the weakest and most vulnerable portion of the IOL is the haptic-optic junction. To gain insight whether this may be a long-term vulnerability for SIS-fixation, we quantified the amount of strain produced by the IOL fixation in cadaveric human eyes. Our results show that most of the haptic stretching during SIS scleral fixation appears to occur primarily at the curvature of distal haptics. Initially, flexible curve of the distal haptic stretches to accommodate passage through the fixating sclerotomies and externalization of haptic tips (Figure 5). This may explain why most of the IOL repositioning were for haptic tip disinsertion (Table 1). One way this problem can be mitigated is by flanging the haptic tips in the scleral tunnel with low temp cautery, creating a button at the haptic tip that acts as a safety to prevent haptic retraction in the vitreous cavity. We used the angle subtended by the axis of the haptic root and the tangent to the optic rim at the haptic optic junction as a surrogate marker of the fixation strain at the haptic-optic junction. We found a very small, but significant increase in haptic-optic junction angle that is proportional to the degree of haptic tip externalization. Although these structural changes appear minimal and are re-assuring, their clinical importance in long-term stability of SIS-fixated IOLs requires further study.
The limitations of this study include its retrospective design, and variability in surgical technique among multiple surgeons in our group. Furthermore, a small number of cadaveric eyes were included in the wetlab analysis with most eyes having average axial length is another limitation. It would be important to extend this analysis to highly myopic and hyperopic eyes as the anatomic and structural parameters in these eyes may be different. Additionally, in this study we only used a single type of IOL (Alcon’s MA60AC) as this is the IOL that we most commonly use in live human surgery. However, the impact of IOLs with larger optic diameter (i.e. MA50BM IOL) or IOLs with larger haptic tip-tip diameter on haptic and haptic-optic junction strain with SIS technique should be studied.
Supplementary Material
Summary statement:
Clinical outcomes from 122 patients who underwent sutureless intrascleral fixation (SIS) of secondary intraocular lenses (IOL) were assessed and correlated with anatomic and structural analysis in cadaveric human eyes.
Acknowledgements:
The authors wish to thank Beth Brauer, Synergetics/Bausch and Lomb (St Louis, MO) for technical assistance during wetlab portion of this study. The authors wish to thank Laurie Lau-Sickon for technical assistance with UBMs. The authors also wish to acknowledge Eversight for their help in acquiring the cadaveric eyes used in this study, and thank Eversight’s donors and their families who made the generous decision to donate the eyes.
Sources of support: Bozho Todorich (Fellow’s Forum Research Award, American Society of Retina Specialists) and Maria Woodward (receives grant funding from the National Eye Institute NEI, K23EY023596); otherwise none.
Abbreviations:
- SIS
(sutureless intrascleral fixation)
- PPV
(pars plana vitrectomy)
- PPL
(pars-plana lensectomy)
- IOL
(intraocular lens)
- UBM
(ultrasound bio-microscopy)
Footnotes
Financial Disclosures: Bozho Todorich (none), Maxwell Stem (none), Aristomenis Thanos (none), Lisa Faia (none), George Williams (consultant for Alcon), Jeremy Wolfe (none) and Maria Woodward (none)
Data Access Responsibility and Analysis: Dr. Wolfe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References:
- 1.Bellucci R, Pucci V, Morselli S, Bonomi L. Secondary implantation of angle-supported anterior chamber and scleral-fixated posterior chamber intraocular lenses. Journal of Cataract & Refractive Surgery 22, 247–252 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Scharioth GB1 PS, Georgalas I, Tataru C, Pavlidis M. Intermediate results of sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg 36, 254–259 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Hirashima DESE, Meirelles RL, Alberti GN, Nosé W. Outcomes of iris-claw anterior chamber versus iris-fixated foldable intraocular lens in subluxated lens secondary to Marfan syndrome. Ophthalmology 117, 1479–1485 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Abbey AM et al. Sutureless scleral fixation of intraocular lenses: outcomes of two approaches. The 2014 Yasuo Tano Memorial Lecture. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 253, 1–5, 10.1007/s00417-014-2834-9 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Todorich B, Thanos A, Woodward MA & Wolfe JD Sutureless Intrascleral Fixation of Secondary Intraocular Lens Using 27-Gauge Vitrectomy System. Ophthalmic Surg Lasers Imaging Retina 47, 376–379, 10.3928/23258160-20160324-14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prenner JL, Feiner L, Wheatley HM & Connors D A novel approach for posterior chamber intraocular lens placement or rescue via a sutureless scleral fixation technique. Retina 32, 853–855, 10.1097/IAE.0b013e3182479b61 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Prasad S Transconjunctival sutureless haptic fixation of posterior chamber IOL: a minimally traumatic approach for IOL rescue or secondary implantation. Retina 33, 657–660, 10.1097/IAE.0b013e31827b6499 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Yamane S, Inoue M, Arakawa A & Kadonosono K Sutureless 27-gauge needle-guided intrascleral intraocular lens implantation with lamellar scleral dissection. Ophthalmology 121, 61–66, 10.1016/j.ophtha.2013.08.043 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Agirretxe I, Acera-Osa A & Ubeda-Erviti M Needle-guided intrascleral fixation of posterior chamber intraocular lens for aphakia correction. J Cataract Refract Surg 35, 2051–2053, 10.1016/j.jcrs.2009.06.044 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Maruko I, Koizumi H, Kogure-Katakura A & Iida T Extraocular Technique of Intrascleral Intraocular Lens Fixation Using a Pair of the Shaft-Bended 27-Gauge Needles. Retina 37, 191–193, 10.1097/IAE.0000000000001257 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


