Abstract
Introduction:
Despite 30 years of research on HIV, a vaccine to prevent infection and limit disease progression remains elusive. The RV144 trial showed moderate, but significant protection in humans and highlighted the contribution of antibody responses directed against HIV envelope as an important immune correlate for protection. Efforts to further build upon the progress include the use of a heterologous prime-boost regimen using DNA as the priming agent and the attenuated vaccinia virus, Modified Vaccinia Ankara (MVA), as a boosting vector for generating protective HIV-specific immunity.
Areas Covered:
In this review, we summarize the immunogenicity of DNA/MVA vaccines in non-human primate models and describe the efficacy seen in SIV infection models. We discuss immunological correlates of protection determined by these studies and potential approaches for improving the protective immunity. Additionally, we describe the current progress of DNA/MVA vaccines in human trials.
Expert Commentary:
Efforts over the past decade have provided the opportunity to better understand the dynamics of vaccine-induced immune responses and immune correlates of protection against HIV. Based on what we have learned, we outline multiple areas where the field will likely focus on in the next five years.
Keywords: AIDS, HIV vaccine, DNA, modified vaccinia ankara, MVA, heterologous prime-boost, antibody responses, T cell responses
1. Introduction
The global health community has been faced with the problem of human immunodeficiency virus (HIV) for over 30 years now, with nearly 35 million people in the world living with HIV in 2013 [201]. Development of highly active anti-retroviral therapy and educating individuals on HIV prevention strategies have contributed significantly to the drop in the number of new infections from 3.4 million in 2001 to 2 million in 2013 [202]. However, it is essential to have an efficacious vaccine as a long-term solution to control the epidemic. A vaccine should prevent new infections and control the virus replication in infected individuals to limit disease progression. There are many challenges facing the development of an HIV vaccine including an incomplete knowledge of the immunological correlates of protection, the high diversity of HIV within an infected individual (2–6% [1,2]) as well as in the population (about 15–20% within a clade; 25–35% between clades [3–5]), the ability of HIV to rapidly mutate in an infected host, and a complex glycan structure that helps protect the virus against neutralization. It is clear that overcoming these challenges for an effective HIV vaccine will require the induction of both humoral and cellular immunity against conserved viral targets. Neutralizing antibodies (NAb) to prevent infection and spread of the virus will be critical and non-neutralizing antibodies (non-NAb) to assist in antibody-dependent cellular cytotoxicity (ADCC) and other antibody-mediated clearance mechanisms also play a significant role [6,7]. Cytolytic CD8+ T cells will be crucial in the elimination of HIV-infected cells and CD4+ helper T cells will be required for the proper development and maturation of antibody and CD8+ T cell responses; in particular, CD4+ T follicular helper (Tfh) cells are needed for long-lived and high avidity antibodies [8,9]. Development of protective immune responses by vaccination will likely require a multi-faceted approach since other regimens have shown to be ineffectual.
Although there are many vaccines under preclinical development, to date only five have been tested for efficacy in humans. The AIDSVAX Phase III trials were the first two to undergo HIV vaccine efficacy studies [10–12]. These trials sought to engage humoral responses alone to protect from infection by utilizing a bivalent gp120 protein immunogen derived from subtype B isolates (AIDSVAX B/B) in one study or subtype B and E isolates (AIDSVAX B/E) in the second study. Both trials elicited binding antibody responses to HIV envelope (Env), did not elicit neutralizing antibody responses against primary HIV isolates and failed to provide any protection from HIV acquisition [10–12]. The third efficacy study was the Phase IIb Step trial which employed an adenovirus 5 (Ad5) vector expressing HIV Gag, Pol, and Nef that aimed to generate cell-mediated immunity to protect against HIV infection or change early plasma virus levels [13,14]. The regimen was shown to be immunogenic for inducing HIV-specific CD8+ T cells, but did not confer protection against HIV infection or diminish HIV replication. In addition, HIV incidence was higher in vaccinated men with pre-existing Ad5 immunity and/or without circumcision. The Step trial did not include an HIV Env immunogen and thus did not generate antibody responses against HIV Env. HVTN 505 was a Phase IIb trial that utilized a heterologous DNA prime/Ad5 boost regimen aimed at reducing HIV viral loads as well as preventing HIV acquisition [15]. Heterologous regimens refer to the use of two or more different vehicles for delivery of the antigens of interest such as DNA and Ad5. In contrast to Step trial, the HVTN 505 trial used immunogens that also expressed HIV Env gp140 in addition to Gag and Pol, and was conducted in participants that were circumcised with low/no Ad5-specific antibodies. The DNA prime/Ad5 boost was designed to induce HIV-specific, multifunctional CD4+ and CD8+ T cells and antibodies to envelopes of the major circulating strains. Vaccinations however were eventually halted for lack of efficacy when it was determined that the vaccine did not reduce the rate of HIV acquisition compared to the placebo arm nor viral load set points. In 2009, the RV144 Thai trial was the first Phase III HIV vaccine trial to achieve a modest vaccine efficacy of 31.2% (50–60% within the first year of vaccination) and was the first human trial to demonstrate that it is possible to prevent HIV infection by vaccination [16,17]. This was achieved by utilizing a heterologous prime-boost regimen, combining recombinant canarypox vector (ALVAC) immunizations with the AIDSVAX B/E protein immunogen to induce both arms of the adaptive immune system. These results provided a ray of hope to the HIV vaccine field and highlighted the contribution of anti-Env antibody responses as an important immune correlate of reduced risk of infection [18].
Heterologous prime/boost vaccination approaches have the power to induce a robust humoral and cellular immunity. One such approach is priming with DNA and boosting with modified vaccinia Ankara (referred to as DNA/MVA vaccine hereafter). Our group and a number of other researchers have developed the DNA/MVA vaccine approach for HIV for more than 15 years. Studies have been performed in mice [19–27], rhesus macaques [28–35] and humans [36–43]. Results thus far show that DNA/MVA vaccines induce strong anti-HIV immunity in animal models as well as humans. DNA/MVA vaccine-induced immunity has been shown to confer protection from acquisition of mucosal infections in preclinical non-human primate (NHP) models. Given the large amount of literature on DNA/MVA vaccines for HIV, it will be very hard to review all the incredible amount of work done by many investigators. Thus, in this review, we will primarily discuss the immunogenicity and efficacy of DNA/MVA vaccine studies in NHPs, as well as strategies to enhance them such as the use of immunomodulatory adjuvants and genetic modification of MVA.
2. DNA Vaccine: Ideal priming vector
DNA vaccines are DNA plasmids that transfect cells when injected in vivo and subsequently express the encoded antigen to induce an antigen-specific adaptive immune response. DNA vaccines have the potential for use in global health vaccines due to their excellent safety profile, rapidity of construction, generic manufacturing, and stability at room temperature. Preclinical models have proven DNA to be immunogenic and provide protection against infectious diseases, cancer, allergy, and autoimmunity. Though not licensed for human use yet, progress through numerous phase I and II clinical trials have shown that DNA vaccines can safely induce responses in vaccinated humans and may prove to be useful as prophylactic or therapeutic vaccines. Although DNA vaccines have been shown to be immunogenic and efficacious in small animal models, studies in NHPs and humans demonstrate that the immunogenicity of naked DNA is inconsistent and, oftentimes, below the limit of detection [44–49]. Studies in rhesus macaques (RMs) indicated that intradermal immunizations generate significantly higher CD8+ T cell responses compared to intramuscular immunizations [31]. The immunogenicity of DNA can be significantly enhanced by optimizing the delivery of DNA i.e. using a gene gun or via electroporation, combining DNA with genetic adjuvants to stimulate the immune response, or maximizing the expression of the antigen (reviewed in [50–52]). In particular, use of electroporation showed great promise in dramatically enhancing the immunogenicity of DNA in humans [53,54]. Additionally, adjuvanted DNA vaccines alone delivered intramuscularly with electroporation have been shown to be protective against mucosal SIV challenges in RMs [55]. Other methods to circumvent the low responses generated by DNA are heterologous boost immunizations utilizing protein or live viral vectors. In general, booster immunizations with proteins have yielded induction of strong humoral immune responses whereas booster immunizations with viral vectors yielded strong cellular and humoral immune response. One of the viral vectors we, and others in the field, use extensively with DNA in a prime-boost regimen is MVA.
3. Modified Vaccinia Ankara: Ideal boosting vector
The RV144 trial highlighted the potential of poxvirus vectors as candidate HIV vaccines. Poxviruses, specifically vaccinia virus, have been utilized as expression vectors for foreign DNA for over 30 years now [56–59]. One of the major advantages of poxviruses for vaccine development is that they can stably accommodate at least 25 Kb of foreign DNA without loss of infectivity, allowing for insertion of large genes or an array of genes [60]. In addition, preexisting immunity to vaccinia in the population is low since its discontinued use in the smallpox vaccination campaign that ended in 1980. Unfortunately, the use of live virus as a vaccine for smallpox possessed some safety risks with 1–2 deaths and hundreds of cases with complications severe enough to require hospitalization for every million vaccine recipients [61]. As a result, development of next generation poxvirus vectors sought an increased safety profile.
Modified Vaccinia Ankara, or MVA, is an attenuated derivative of vaccinia that has proven to be safe and immunogenic in humans. Vaccinia underwent over 570 passages in chicken embryo fibroblast cells resulting in deletion of about 12% of its genome and rendering many immune modulatory genes, meant to counteract the immune response of the host, to be non-functional [62,63]. The deletions also limited the host-range of MVA leading it to be replication-incompetent in human and other mammalian cells [64]. MVA is unable to disseminate in the host and cause pathology, even in immune-compromised hosts [65]. The replication defect occurs at the late stage of virion assembly allowing for uncompromised late gene and immunogen expression. This property of MVA is thought to contribute significantly to its strong immunogenicity. Genetic instability of the inserted recombinant gene, such as HIV Env, in MVA has been reported to lead to truncation of the gene or exertion of negative selection pressure on viral growth [66–68]. The lab of B. Moss showed frame shift mutations and large genetic deletions flanking the insertion site of the recombinant gene contribute to the genetic instability in MVA [69]. However, moving the recombinant gene into a site between essential, conserved MVA genes led to enhanced stability of insert.
MVA has proven to be effective at inducing strong CD4+ and moderate CD8+ T cell responses and durable antibody responses. The safety profile and responses generated led MVA to be a leading candidate as a third generation smallpox vaccine [70]. However, the immunogenicity of MVA is limited in a homologous prime/boost approach due to the induction of strong anti-MVA immunity that will limit transgene expression. Accordingly studies have shown that MVA-only immunizations are less effective in protecting against neutralization resistant SIV challenges in RMs [35]. To avoid the problem of anti-vector immunity, a number of studies used MVA as a booster immunization in conjunction with multiple vectors (heterologous prime/boost) and observed a profound boost of both cellular and humoral immune responses. These priming vectors include DNA, protein, bacteria, adenoviruses, and other poxviruses.
4. DNA Prime/MVA Boost Regimen Elicits Potent Cellular and Humoral Immunity
As discussed above, we and others took advantage of the heterologous prime/boost regimen by employing DNA followed by MVA immunizations. Heterologous prime-boost approaches may refer to differences in the antigen inserts, vectors used, or both. The virtue of utilizing different vectors is in avoiding the generation of high vaccine vector-specific responses that develop after repeated immunizations that could diminish desired responses to the immunogen [71]. An advantage to use of different inserts in the prime and boost is the potential to increase the breadth and depth of the generated immune response. Using cross-clade sequences or multiple divergent sequences from a single clade may elicit a broad enough antibody response to be effective [72].
DNA primes induce low-level, but broad cellular responses that can be subsequently boosted by immunogenic viral vectors such as MVA. This regimen has the potential to stimulate optimal cellular and humoral responses against HIV/SIV [32,73–75]. Our DNA and MVA vaccines express three major proteins of HIV: Gag, Pol, and Env. The DNA and MVA vaccines are uniquely designed to produce VLPs that display a membrane-bound trimeric form of Env, a feature critical for induction of strong NAb responses [76–78]. Ongoing experiments in our laboratory confirmed the expression of Env trimers on the DNA transfected/MVA infected cell membranes based on strong binding of various broadly neutralizing antibodies that recognize only native trimeric form of Env and weak binding of non-neutralizing Abs (unpublished observations). The HIV genes encoded by our vaccines have been modified for safety via deletion of the long terminal repeats, inactivation of protease, and introduction of mutations in the zinc finger region of the Gag gene to inhibit packaging of viral RNA into the VLPs [77].
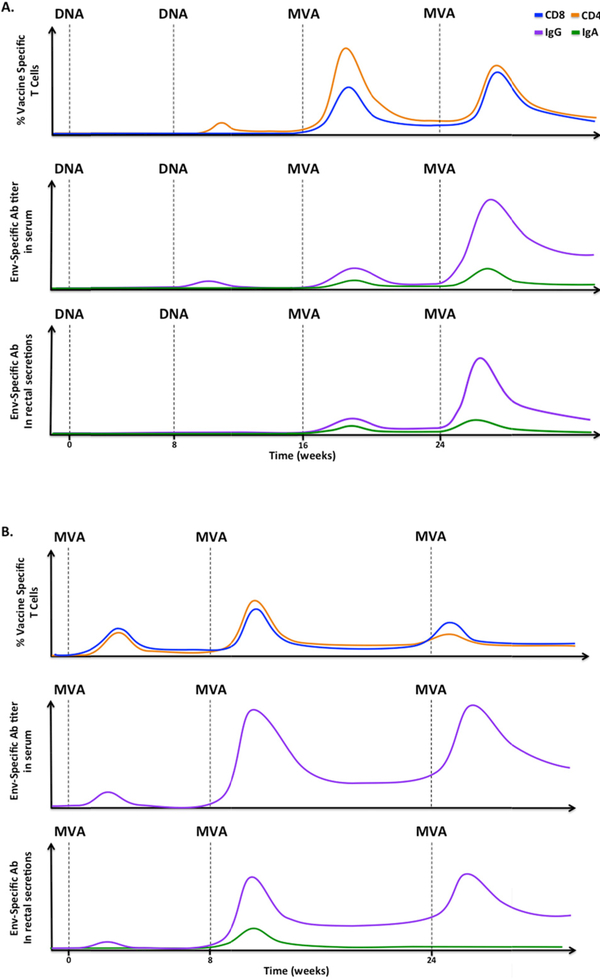
In rhesus macaques and humans, the DDMM regimen (two sequential DNA immunizations followed by two sequential MVA immunizations) has been shown to induce optimal cellular and humoral immunity (Figure 1). In general, two doses of non-codon optimized DNA delivered intramuscularly primed very low levels of cellular and antibody responses [31,79–81]. However, administration of the first MVA boost (MVA1) dramatically enhanced the CD4+ and CD8+ T cell responses and, to a lesser degree, antibody responses against Env. The addition of a second MVA boost (MVA2) did not increase CD4+ T cell response, but enhanced the CD8+ T cell response by 2–4 fold and dramatically enhanced Env-specific serum IgG by about 10–50 fold [82,83]. By comparison, in a three dose, MVA-only regimen (MMM), the antibody response was amplified after MVA2 and was further enhanced after the third MVA (MVA3). Cellular responses reached the peak after MVA2 and were not boosted following MVA3. The magnitude of CD4+ responses in the MMM regimen did not reach the height achieved by the heterologous regimen, though CD8+ T cell responses were either similar (in RMs that did not present an immunodominant CD8 epitope) or considerably lower (in RMs that did present an immunodominant CD8 epitope). In contrast to strong systemic immune responses, intramuscular DNA/MVA vaccinations induced weaker SIV-specific cellular and humoral responses in the rectal and genital mucosae [81,82,84]. Continued efforts to improve vaccine-induced mucosal responses will be key to preventing infection by controlling virus at mucosal sites.
Figure 1. Schematics representing the patterns of vaccine-induced T cells and antibodies after DNA/MVA and MVA only immunizations in rhesus macaques.
Vaccine-induced responses after A) two DNA immunizations followed by two MVA immunizations or B) three MVA immunizations only [81,83]. Top row: SIV-specific CD8+ (blue) and CD4+ (orange) T cell responses. Env-specific serum IgG (purple) and IgA (green) levels in the serum (middle) or mucosae (bottom). Data for Env-specific serum IgA levels are not available for MVA only immunized animals. Please note that these graphs are only models to reveal the patterns and do not depict absolute values.
Late boosting immunizations are likely important to sustain high levels of anti-HIV immunity. A recent study in RMs showed that a third MVA boost administered a year after the second MVA can recall serum and rectal antibody responses to levels similar to after the second MVA [84]. Also in a Phase I DDDM trial in Sweden, some volunteers received a second MVA booster approximately 3 years after the first MVA and showed recall of anti-HIV cellular response and improved ELISA binding antibody responses against Env and Gag [43]. Pre-existing antivaccinia antibody responses could pose a problem in subsequent MVA boosts, but a long waiting period between boosts indicate that these antibody responses do not interfere with anti-HIV recall responses.
5. Efficacy of DNA/MVA vaccines against neutralization-sensitive and neutralization-resistant mucosal SIV infections
Over the past 17 years, we and others have tested the efficacy of DNA/MVA vaccines in the NHP model [31–33,79,81,82,85–92]. To test vaccine efficacy, the field initially used a single, high-dose, intrarectal SIV or SHIV challenges (Table 1). Protection from infection against high dose challenges was not observed but vaccinated animals showed 10–100 fold reductions in peak viral loads compared to controls. Subsequently, to better mimic human transmission events of HIV, the field used repeated, moderate-dose, intrarectal SIVsmE660, SHIV162P3, or SIVmac251 challenges [81,82,93–95]. SIVsmE660 is a relatively neutralization-susceptible virus and SIVmac251 is a neutralization-resistant virus, making it difficult to protect against. Interestingly, DNA/MVA vaccinations demonstrated a significant delay in acquisition of intrarectal SIVsmE660 infection with an estimated vaccine efficacy of around 60% per challenge exposure [81]. A similar protection against SIVsmE660 was also observed in TRIM5a permissive animals [96]. Similarly, Ellenberger et al. demonstrated that an HIV clade AG DNA/MVA vaccine (DDDM regimen) significantly delays acquisition of repeated heterologous intrarectal clade B SHIV (SHIV162P3) challenges with an estimated vaccine efficacy of 64% per challenge exposure [94,95]. The DNA/MVA vaccines also showed evidence for protection against intrarectal SIVmac251 infection in rhesus macaques. Barouch et al. showed that the DNA/MVA regimen can significantly delay the acquisition of intrarectal, repeat dose SIVmac251 infection in RMs with a per-exposure vaccine efficacy of 81% and this delay was associated with V2-directed antibodies [35].
Table 1.
Summary of DNA/MVA vaccine efficacy studies in rhesus macaques
| Challenge Virus |
Immunization Route |
Challenge Route |
Challenge Dose | Refs | Results | |
|---|---|---|---|---|---|---|
| SIVmac251 | 1 | Intramuscular | Rectal | Repeated, moderate dose | 35, 93 | Vaccine efficacy ranges from 50–81%. Reduction in peak viremia of vaccinated RMs compared to control animals range from 0–1000 fold reduction. |
| 2 | Nasal | Vaginal | Repeated, moderate dose | 73 | ||
| SIVE660 | 3 | Intramuscular | Rectal | Repeated, moderate dose | 82, 83 | |
| SF162P3 | 4 | Intramuscular | Rectal | Repeated, moderate dose | 94 | |
| SIVmac239 | 5 | Intradermal intra-rectal |
Rectal | Single, high dose | 32 | No effect on protection from infection was seen against high dose viral challenges. Reduction in peak viremia of vaccinated RMs compared to control animals range from 10–100 fold. |
| 6 | Intradermal | Rectal | Single, high dose | 91 | ||
| SHIV 89.6P | 7 | Intra-rectal | Rectal | Single, high dose | 89 | |
| 8 | Nasal | Rectal | Single, high dose | 88 | ||
| 9 | Intradermal or Intramuscular | Rectal | Single, high dose | 31 | ||
| SHIV−4 | 10 | Intramuscular/ intra-rectal + intra-oral | Intravenous |
Single, high dose | 92 |
6. CD40L as an adjuvant for DNA/MVA vaccines: enhancement of cellular and humoral immune responses and protection
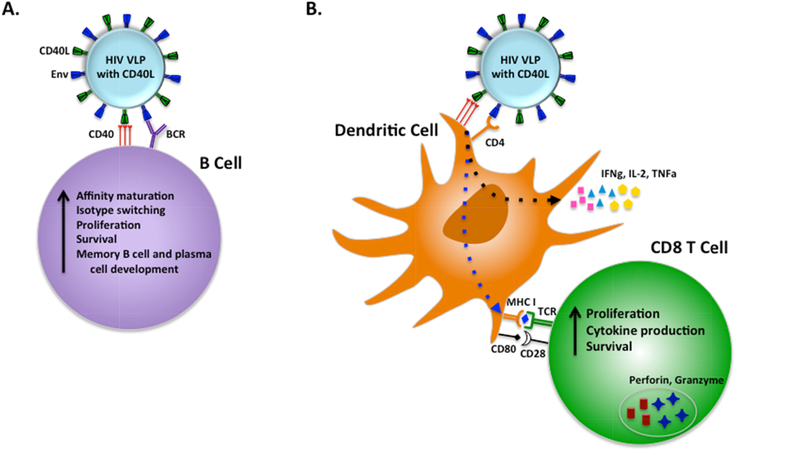
While the DDMM platform induces a combination of desired cellular and humoral responses, the quality and magnitude of these responses could still be greatly improved. A strategy developed by us is the use of the co-stimulatory molecule CD40L as an adjuvant during vaccination. CD40L is predominantly expressed on activated CD4+ T cells and binds its receptor, CD40, which is expressed on DCs and B cells. Engagement of CD40 with CD40L by immature DCs leads to DC activation and maturation in order to prime antigen-specific CD8+ T cell responses. CD40 signaling on B cells promotes proliferation and immunoglobulin class switching for a matured antibody response. We designed our DNA vaccines to co-express CD40L with Gag and Env such that the vaccine produces HIV or SIV VLPs that display CD40L and native Env trimers on the surface (Figure 2). Our studies have shown that these CD40L-containing VLPs can activate DCs in vitro [83].
Figure 2. Schematic representation of VLPs expressing HIV antigens and CD40L activating Env-specific B cells and dendritic cells for cross-presentation.
Virus-like particles (VLPs) are produced to express trimeric Env and CD40L on the surface. A) VLPs activate B cells through interactions between Env and B cell receptors (BCR) as well as between CD40L and CD40 on B cells. CD40L mediated activation of B cells could promote affinity maturation, isotype switching, proliferation and survival leading to development of long lived memory B cells and plasma cells. B) CD40-CD40L interactions between VLPs and dendritic cells (DCs) leads to DC activation by upregulating T cell co-stimulatory molecules and cytokine production resulting in generation of long lived memory CD8 T cells [83]. Env expression on VLPs increases their targeting to DCs by binding to CD4.
In a recent study, DNA co-expressing CD40L and SIVmac239 immunogens was tested for enhanced immunogenicity and protection against SIVsmE660 compared to unadjuvanted DNA in RMs [83]. The CD40L-adjuvanted DNA significantly enhanced titers and avidity of Env-specific IgG antibodies in the serum. Levels of NAb against a difficult to neutralize SIVsmE660 isolate were low, but present in most RMs in the CD40L group and were absent in the unadjuvanted group. Additionally, the breadth of cellular responses was improved in the CD40L-adjuvanted group [83]. Upon repeated heterologous SIVsmE660 challenges, the adjuvanted group demonstrated protection from infection with a vaccine efficacy of 76%. The high antibody avidity strongly correlated with protection. For infected RMs, the CD40L-adjuvanted animals showed control of virus replication in the blood and rectum at 2 weeks after infection compared to unimmunized controls. This study revealed that inclusion of CD40L during DNA primes of a DNA/MVA vaccine improves protection by enhancing the quality of anti-Env antibodies.
In RM models assessing SIV analogs of the vaccines used in the RV144 and HVTN505 trials, protection against SIVmac251 challenges was a better predictor of vaccine efficacy in humans compared to protection against SIVsmE660. A second study was conducted to investigate the ability of CD40L to protect against heterologous SIVmac251 rectal challenges by using CD40L-adjuvanted DNA as well as MVA vaccinations [93]. Impressively, the CD40L adjuvant enhanced protection from acquisition of SIVmac251 infection with a vaccine efficacy of 50% [93]. The adjuvanted animals showed control of virus replication by maintaining a 100-fold reduction in viremia compared to unimmunized controls during the set point phase. Unadjuvanted animals only showed a trend towards a 10-fold reduction. These results demonstrated that CD40L-adjuvanted DNA/MVA vaccines can enhance protection against both neutralization-sensitive and neutralization-resistant mucosal SIV infections.
7. Immune Correlates of Protection
Based on passive antibody transfer studies using broadly NAb in NHPs, it is clear that NAb can prevent infection when present in sufficient quantities following a mucosal challenge [97,98]. However, during a mucosal HIV infection, there is evidence that non-NAb can also significantly contribute to protection by detecting virally infected cells and targeting them for destruction by cytotoxic T cells and innate cells via Fc-mediated mechanisms such as ADCC, antibody-dependent cell-mediated virus inhibition (ADCVI), and phagocytosis (Box 1). As expected, the immune correlates for protection against neutralizable SIVsmE660 were different from neutralization resistant SIVmac251 infection.
12. Key Issues
The RV144 trial utilized a heterologous prime-boost vaccination regimen with a canarypox vector prime in conjunction with a gp120 protein boost, and demonstrated moderate protection from HIV acquisition of 31.2%. There is a critical need for the development of improved vaccine approaches that will build on immune correlates learned from RV144 trial.
Heterologous prime-boost vaccination regimens provide robust cellular and humoral immunity that confer protection from infection.
DNA serves as a good priming vector and the use of adjuvants and dedicated delivery methods further enhance induced responses.
MVA serves an excellent boosting vector for multiple other vectors for induction of potent memory antibody and cellular responses.
DNA/MVA vaccines induce strong cellular and humoral immune responses that can be augmented by combining with CD40L adjuvant to enhance protection against neutralization-sensitive and neutralization-resistant strains of SIV.
Additional approaches to enhancing DNA/MVA immune responses include incorporation of new Env immunogens as a protein boost, genetic modification of MVA and inclusion of molecular adjuvants.
7.1. Immune correlates of protection against SIVsmE660 (neutralizable SIV)
A higher magnitude of gp140-specific IgG in rectal secretions at the time of intrarectal challenge predicted slower acquisition of SIVsmE660 [96]. The avidity of serum IgG antibody against E660 Env gp160 also predicted slower acquisition of intrarectal SIVsmE660 challenge [81–83]. In an ongoing study, we observed a significant direct association between higher ADCC activity against virus-infected cells and protection against intravaginal SIVsmE660 infection (unpublished observations). Intriguingly in all of our SIVsmE660 challenge studies, despite the fact that vaccination induced a strong NAb response, we did not observe a clear association between NAb levels and protection from infection. In an analysis aimed at understanding NAb-mediated protection in RMs immunized with DDMM vaccine, Burton et al. tested the capacity of pre-challenge antibodies of infected RMs to neutralize the Env variant that broke through to establish each infection [99]. This analysis revealed that vaccine-elicited serum antibodies capable of neutralizing the autologous breakthrough Env variant were present prior to challenge at moderate to high titers, yet failed to protect against SIV infection. Furthermore, vaccinated RMs that were either infected with or protected from SIV had similar serum neutralizing capacities against breakthrough transmitter founder viruses of the infected animals, indicating that the protected animals did not have more potent neutralizing activity against viruses that established infection. These findings suggest that the relationship between serum NAb titers and protection from mucosal HIV/SIV challenge in the setting of active immunization is more complex than previously recognized. The results highlight an important yet often overlooked difference between passively transferred and vaccine-elicited antibody protection; the latter occurs in the presence of ongoing host immune responses. Thus, vaccination itself could shift the balance away from protection and towards acquisition [100,101]. This could result from the generation and homing of vaccine-elicited CD4+ T cells to the portal of entry, which in turn could mitigate the beneficial effects of vaccine-elicited humoral immune responses.
7.2. Immune correlates of protection against SIVmac251 (neutralization-resistant SIV)
Most vaccines studies thus far have not generated NAb responses against highly neutralization-resistant SIVmac251 infection. Despite this, binding antibody responses focused to the V2 region of SIV Env has been reported to associate with significant delay in acquisition of SIVmac251 infection [35,93]. Similar associations were also observed with Adenovirus-based and ALVAC-based vaccines [35,102,103]. Additionally, a recent study demonstrated that multiple non-neutralizing functions of vaccine-elicited antibody contribute to protection against SIVmac251 [104]. While the mechanisms by which these antibodies provide protection are not entirely clear, these studies highlight the role of Env-specific non-NAb in contributing to protection from viral acquisition.
8. Potential Ways to Enhance DNA/MVA Vaccine-Induced Protective Immunity
8.1. Incorporation of Env Protein Boost in DNA/MVA Vaccines
While DNA/MVA vaccines stimulate a robust antibody response, the quality and magnitude of mucosal antibody responses in particular could be improved for better protection. The incorporation of protein into a heterologous vaccination regimen has proven to augment antibody production. The basis of the RV144 trial was ALVAC with additional Env subunit protein boosts during the last two immunizations and the resulting antibody response was effective at providing modest protection in participants. Addition of Env protein booster immunizations to the DDMM regimen in RMs enhanced antibody response by increasing the magnitude of Env-specific antibody, breadth of neutralizing ability, and ability to blunt peak viremia after SHIV challenge by nearly a 100-fold change compared to controls [34]. In our recent study, addition of gp140 protein only during the last MVA immunization of the DDMM regimen increased gp140-specific IgG titers, avidity, and tier 1 neutralization titers [105]. These studies indicate that protein incorporation could further generate desired antibody responses. However, the key is to identify an ideal Env protein immunogen to boost DNA/MVA vaccine induced antibody responses. In this direction, SOSIP versions of Env trimers may be ideal as recent studies demonstrate that this form of Env trimers display native structures and induces autologous tier 2 NAb responses in rabbits and, to some extent, in RMs [106]. In addition, delivering the Env booster immunizations with novel adjuvants that induce robust and persisting antibody response could prove to be beneficial.
8.2. Genetic Modification of MVA
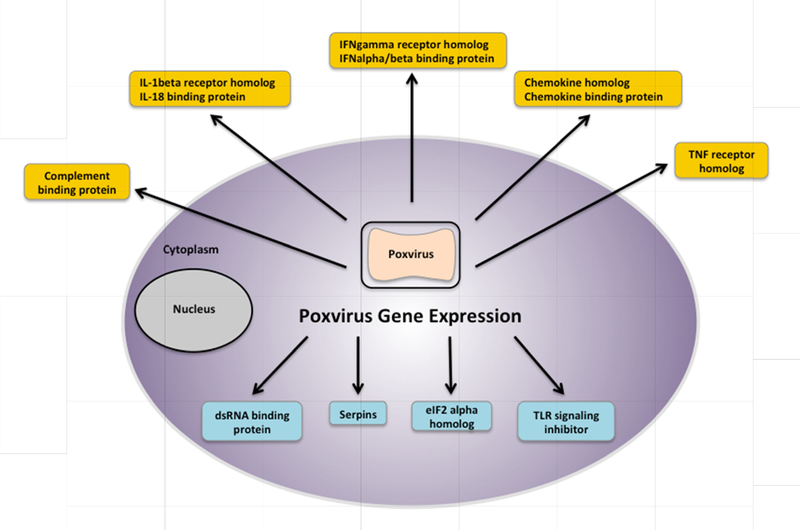
Poxviruses express a wide variety of immune modulatory genes such as cytokine binding proteins and inhibitors of interferon activity (Figure 3). Though MVA has lost a large portion of its genome, many modulatory genes remain functional. The directed disruption of these genes aims to enhance the virally stimulated, pro-inflammatory conditions required for a strong adaptive response [107–110]. MVA with a deletion of the N2L gene, an inhibitor of IRF3, led to increased beta interferon, proinflammatory cytokine, and chemokine expression. In mice, this vector showed improved magnitude and polyfunctionality of HIV-specific CD4+ and CD8+ T cells and enhanced Env-specific antibodies after the peak of response [111]. Deletion of the A35 gene in MVA increased virus-specific immunoglobulin production, class switching to IgG isotypes, and virus-specific ^γ secreting splenocytes [112]. Simultaneous deletion of four immune-modulatory genes in MVA (IL-18 binding protein, IL-1 beta receptor, a dominant negative Toll/IL-1 signaling adapter, and CC-chemokine binding protein) resulted in a 6-fold increase in HIV-specific cellular responses and 25-fold higher titers of Env gp120-specific antibodies in RMs [113]. Targeted modifications of the MVA genome has been shown to significantly influence the innate as well as adaptive responses in the host and provides a potential way forward for further enhancement of MVA immunogenicity.
Figure 3. Poxviruses express modulatory factors for cytokines, chemokines, and signaling pathways.
A poxvirus-infected cell is depicted here.
Another the viral genome modification approach is the reintroduction of poxvirus genes. Our lab is currently developing an MVA vector that expresses a vaccinia virus-derived, anti-apoptotic gene, B13R. Expression of B13R within an MVA infected cell delays the induction of apoptosis with the aim of enhancing infected cell survival (particularly of DCs) as well as immunogen production to lead to better antibody and T cell induction. There remains the potential for this strategy to lead to an increase in the replicative capacity of MVA, but in ongoing in vivo mouse models, this has not been observed. The reintroduction strategy has not yet been adopted in MVA to the degree gene disruption has been, but it certainly provides a novel approach to modifying the immunogenicity of an MVA vector.
9. Immunogenicity of DNA/MVA HIV vaccines in Humans
The clinical development of our DNA/MVA vaccines is led by Geovax Inc. and these vaccines have been tested in humans through the HIV Vaccine Trials Network. What we have discerned from human studies is that DNA/MVA HIV vaccine induces strong CD4+ T cell and antibody responses and lower CD8+ T cell responses. In Phase IIa human clinical trials for DDMM regimen, 65% of participants showed HIV-specific CD4+ T cell responses however, only 20% of the participants showed measurable HIV-specific CD8 T cell response; both were targeted mainly against epitopes of Gag, followed by Env, then Pol [116]. The low level of CD8+ T cell responders is typical of other vaccines in humans and remains an area in need of improvement, but the inclusion of DNA demonstrated enhanced CD4+ T cell responses over just MVA alone [117,118]. Serum IgG responses against gp140 and the immunodominant region of gp41 were developed in nearly all participants and impressively, the antibody responses to gp140 declined by only less than three-fold from the peak of response to 6 months post-last immunization. This is an improvement from what has been observed in the animal models, which is typically more than a 5-fold decline [105]. The durability of the gp140-specific humoral response was also better than that seen in the RV144 trial or homologous gp120 protein prime boost trials, which had a 10-fold decline in binding antibody levels in the same amount of time [119,120]. The durability of antibody levels is a key component of an HIV vaccine because they have the greatest potential for preventing infection [100,121].
Other groups have also tested DNA/MVA HIV vaccines in humans [39,41,43,95,122–125]. These studies differ with regard to number of doses of DNA primes and MVA boosts, the immunogens used, and their expression levels [95,122,123]. Hayes et al. utilized a DDDM regimen in healthy adults that exhibited enhanced breadth and magnitude of the cellular response and better viral inhibition in vitro compared to immunization with MVA alone [122]. A multi-gene, multi-clade DDDM Phase I study performed in Sweden demonstrated generation of HIV-specific CD4+ and CD8+ T cell responses, but poor development of Env antibody responses [39,41]. A subset of volunteers from that study received a late MVA booster, approximately 3 years after MVA1. MVA2 resulted in a continued strong cellular response, markedly improved binding antibody responses as detected by ELISA, more individuals with high avidity Env-specific antibodies, and low level of neutralization titers against the autologous Env in some of the vaccinees [43]. Phase I/II trials testing a multi-clade DDDMM vaccine for HIV in healthy adults in Tanzania demonstrated broad immunogenicity with HIV-specific IFNγ ELISpot and lymphoproliferative responses as well as potent ADCC responses in a high proportion of vaccinees [124,125]. Despite differences in immunogens, doses, and geographical location of these trials, DNA/MVA has demonstrated an ability to induce robust, durable responses that are promising for prevention and control of HIV. Further clinical development of DNA/MVA immunization regimens that provide high levels of cellular and antibody responses in humans currently remains in progress and only future large-scale trials will shed light on the efficacy of DNA/MVA to provide protection against HIV.
10. Expert Commentary
The field has made much progress in the last decade to improve vaccine efficacy against HIV resulting in evidence for modest protection against infection. In addition to the DNA/MVA vaccines discussed above, these efforts have led to the development of multiple viral vaccines for HIV including NYVAC, adenoviruses and rhesus cytomegalovirus. These vaccines are planned to be or are currently being tested in humans for immunogenicity and efficacy. Clinical studies will also test a combination of viral vectors and protein boosts that elicit strong cellular and humoral immunity. The efforts over the decade have also provided the opportunity to better understand immune correlates of protection and dynamics of vaccine-induced immune responses. Based on what we have learned, we believe the following are areas where the field will likely focus on in the next five years.
11. Five-Year View
11.1. Enhancing Tfh cells for long-lived antibody responses
One of the key findings of the RV144 trial was that vaccine protection was primarily restricted to the first year after vaccination and this was likely attributed to waning vaccine-induced antibody responses [126]. Thus, it is critical for vaccines to induce durable, high affinity antibodies and Tfh cells are integral to their development by providing help to cognate B cells in germinal centers via cytokines and costimulatory molecules [127]. However, as discussed above, it is important to note that in the case of HIV, CD4+ T cell help could also provide more target cells for the virus. In our recent study, DDMM plus gp140 protein (alum adjuvanted) vaccinated RMs generated Tfh cells that preferentially expressed the HIV co-receptor CCR5 and showed an association with peak viremia after SIV infection [105]. It is important to note that within the Tfh cell population, multiple subsets can be identified based on their chemokine receptor expression and each of these subsets may have different susceptibility to HIV infection. More work is required to fully understand the impact of Tfh cells on protection from HIV/SIV infection and how different vaccination regimens influence the quality of Tfh cells generated.
11.2. Generating HIV-resistant CD4+ helper T cells
Other approaches to improve DNA/MVA vaccinations aim to generate CD4+ T cells that are resistant to infection. Cytomegalovirus (CMV)-specific CD4+ T cells make more chemoattractants such as MIP-1 β and RANTES after stimulation compared to HIV-specific CD4+ T cells. CMV-specific CD4+ T cells are also more resistant to HIV infection, possibly due to the binding of CCR5 to its ligands, thus blocking CCR5 from HIV [128]. Altering the cytokine production profile of HIV-specific CD4+ T cells may contribute to inhibiting HIV acquisition. Rapamycin is a drug we have tested as an adjuvant for DNA/MVA vaccines and observed better generation of CD8+ T cell responses and a CD4+ T cell recall response that does not re-express CCR5, leading to the possibility that rapamycin, as an adjuvant, may reduce CD4+ T cell infection by HIV/SIV (unpublished observations). These qualitative changes may be desirable for maintaining strong cellular immunity while reducing the level of target cells.
11.3. Understanding vaccine-induced non-NAb subclass and effector functions
After HIV infection, innate immune cells appear to contribute to protection and viral control by mediating non-NAb activities such as ADCC and phagocytosis as recently demonstrated by Barouch et al. [104] (Box 1). Data suggest that non-NAb are comprised of different IgG subclasses with differential non-neutralizing activities. These IgG subclasses also vary in glycosylation patterns that have been shown to influence their affinity to Fc receptors, resulting in changes in non-NAb activity levels. For example, IgG1 with low levels of fucose exhibit higher ADCC activity compared to fucose-rich antibodies [129,130]. Determining which subclasses are beneficial to protection as well as the glycosylation signature of vaccine-elicited antibodies that mediate protection still remains to be deciphered.
11.4. Enhancing vaccine-induced mucosal immune responses
Most HIV vaccines currently under development use intramuscular immunizations, which are relatively poor at generating potent, long-lived mucosal immune responses. Immunization through mucosal routes has typically elicited better responses in mucosal tissues than systemic routes [131–133]. For example, oral immunizations in RMs have demonstrated strong immunity in the gut [134]. However, oral administration in humans is not practical because vaccines would not withstand the hostile acidic environment of the stomach. Similarly, it is also critical to induce strong immune responses in the genital mucosa as the majority of HIV infections worldwide occur through this route. An additional goal of an HIV vaccine is to prevent rapid depletion of CD4+ T cells in the gut that occurs within days after infection. Despite administration of anti-retroviral therapy, this depletion is only partially reversible and can contribute to disease progression. Thus, there is a great need for development of HIV vaccines that induce potent anti-viral immunity in the mucosae to prevent infection, control replication, and prevent rapid loss of CD4+ T cells. Mucosal routes of immunization are not the only approach to accomplish this as use of viral vectors such as Ad5 and adjuvanted, systemic DNA immunizations have recently been demonstrated to promote mucosal homing of vaccine-induced immune responses [55,135]. In conclusion, enhancing the quality and quantity of vaccine responses against HIV has progressed significantly in the last decade, but a complex pathogen such as HIV will require more focus on tailoring those responses to attack the most susceptible aspects of the virus. DNA and MVA vectors serve as an immunogenic, safe, and easy-to-modify platform to further advance the effort to control and provide protection from HIV.
Box 1. Effect of Non-Neutralizing Antibodies on HIV Protection and Control
Neutralizing antibodies (NAb) specifically bind to pathogens to abrogate the pathogen’s ability to infect host cells. Non-neutralizing antibodies (non-NAb) are pathogen-specific antibodies that do not inhibit infectivity, but serve to tag pathogens or infected cells for destruction by innate effector cells. Binding of the non-NAb Fc (crystallizable fragment) region to Fc receptors (FcR) on innate cells signals the cell to clear the opsonized target. IgG-specific FcR are differentially expressed on innate cells and lead to different pathogen clearance mechanisms. Phagocytes mediate antibody-dependent cellular phagocytosis, while natural killer cells perform antibody-dependent cell-mediated cytotoxicity (ADCC) by releasing granules and utilizing Fas/FasL interactions to induce cell death. [136,137]. ADCVI, or antibody-dependent cell-mediated virus inhibition, is a metric of antibody-mediated antiviral activity of FcR-bearing cells that measures ADCC, beta chemokine production, and phagocytosis. The role of non-NAb in HIV still remains to be completely elucidated, though some investigators have demonstrated the potential for non-NAb to contribute to protection and control of SIV [138, 139]. In the RV144 trial, for participants with low serum IgA titers, IgG avidity and ADCC activity inversely correlated with infection indicating that these antibodies could have contributed to the observed protection [18]. In a study with rhesus macaques (RMs) that mimicked the RV144 trial, the few animals that were protected from SIVmac251 challenge were also found to have higher avidity to gp120 compared to those that became infected [103]. A study utilizing an adenovirus 5 host-range mutant and gp140 protein boost demonstrated that ADCC and ADCVI levels of vaccine-elicited non-NAb to Env correlated with reduced acute and chronic viremia following challenge with a pathogenic SHIV89.6P virus in RMs [140]. Passive transfer experiments with non-neutralizing monoclonal antibodies like F240 that recognizes the gp41 immunodominant region provided partial protection against a vaginal challenge [141]
Acknowledgments
Funding
Our work is supported in part by National Institute of Allergy and Infectious Diseases grants U19 AI109633, U19 AI096187.
Abbreviations
- HIV
Human immunodeficiency virus
- NAb
Neutralizing antibodies
- non-NAb
Non-neutralizing antibodies
- ADCC
Antibody-dependent cellular cytotoxicity
- Tfh cells
T follicular helper cells
- Env
Envelope
- Ad5
Adenovirus 5
- MVA
Modified vaccinia Ankara
- VLP
Virus-like particle
- DCs
Dendritic cells
- NHP
Non-human primate
- RMs
Rhesus macaques
- D
DNA
- M
MVA
- ADCVI
Antibody-dependent cell-mediated virus inhibition
- CMV
Cytomegalovirus
Footnotes
Declaration of Interest
RR Amara is listed as co-inventor of DNA/MVA vaccine technology that has been licensed to Geovax Inc by Emory University. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Shankarappa R, Margolick JB, Gange SJ et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. Journal of virology, 73(12), 10489–10502 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemelaar J The origin and diversity of the HIV-1 pandemic. Trends in molecular medicine, 18(3), 182–192 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary HIV-1 variation. British medical bulletin, 58, 19–42 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Excler JL, Robb ML, Kim JH. HIV-1 vaccines: challenges and new perspectives. Human vaccines & immunotherapeutics, 10(6), 1734–1746 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phanuphak N, Lo YR, Shao Y et al. HIV Epidemic in Asia: Implications for HIV Vaccine and Other Prevention Trials. AIDS research and human retroviruses, 31(11), 1060–1076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Excler JL, Ake J, Robb ML, Kim JH, Plotkin SA. Nonneutralizing functional antibodies: a new “old” paradigm for HIV vaccines. Clinical and vaccine immunology: CVI, 21(8), 1023–1036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su B, Moog C. Which Antibody Functions are Important for an HIV Vaccine? Frontiers in immunology, 5, 289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton DR, Ahmed R, Barouch DH et al. A Blueprint for HIV Vaccine Discovery. Cell host & microbe, 12(4), 396–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovas C, Yamamoto T, Gerner MY et al. CD4 T follicular helper cell dynamics during SIV infection. The Journal of clinical investigation, 122(9), 3281–3294 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn NM, Forthal DN, Harro CD et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. The Journal of infectious diseases, 191(5), 654–665 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Gilbert PB, Peterson ML, Follmann D et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. The Journal of infectious diseases, 191(5), 666–677 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Pitisuttithum P, Gilbert P, Gurwith M et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. The Journal of infectious diseases, 194(12), 16611671 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Buchbinder SP, Mehrotra DV, Duerr A et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebocontrolled, test-of-concept trial. Lancet, 372(9653), 1881–1893 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElrath MJ, De Rosa SC, Moodie Z et al. HIV-1 vaccine-induced immunity in the test- of-concept Step Study: a case-cohort analysis. Lancet, 372(9653), 1894–1905 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammer SM, Sobieszczyk ME, Janes H et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. The New England journal of medicine, 369(22), 2083–2092 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.**.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine, 361(23), 2209–2220 (2009).Describes the RV144 Thai trial results - the first HIV vaccine clinical trial to demonstrate efficacy in humans [DOI] [PubMed] [Google Scholar]
- 17.Vaccari M, Poonam P, Franchini G. Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV. Expert review of vaccines, 9(9), 997–1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes BF, Gilbert PB, McElrath MJ et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine, 366(14), 1275–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanke T, Blanchard TJ, Schneider J et al. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine, 16(5), 439–445 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Gherardi MM, Perez-Jimenez E, Najera JL, Esteban M. Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. Journal of immunology, 172(10), 6209–6220 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Gomez CE, Abaitua F, Rodriguez D, Esteban M. Efficient CD8+ T cell response to the HIV-env V3 loop epitope from multiple virus isolates by a DNA prime/vaccinia virus boost (rWR and rMVA strains) immunization regime and enhancement by the cytokine IFN-gamma. Virus research, 105(1), 11–22 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Abaitua F, Rodriguez JR, Garzon A, Rodriguez D, Esteban M. Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma. Virus research, 116(1–2), 11–20 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Liu J HM, McDonnel M, Amara RR, Wyatt LS, Moss B, Robinson HL. Dose-response studies for the elicitation of CD8 T cells by a DNA vaccine, used alone or as the prime for a modified vaccinia Ankara boost. Vaccine, 25(15), 2951–2958 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Brave A, Boberg A, Gudmundsdotter L et al. A new multi-clade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1-specific CD8(+) T-cell and humoral responses in mice. Molecular therapy: the journal of the American Society of Gene Therapy, 15(9), 1724–1733 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Belyakov IM, Ahlers JD, Nabel GJ, Moss B, Berzofsky JA. Generation of functionally active HIV-1 specific CD8+ CTL in intestinal mucosa following mucosal, systemic or mixed prime-boost immunization. Virology, 381(1), 106–115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallengard D, Applequist SE, Nystrom S et al. Immunization with multiple vaccine modalities induce strong HIV-specific cellular and humoral immune responses. Viral immunology, 25(5), 423–432 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mothe B, Hu X, Llano A et al. A human immune data-informed vaccine concept elicits strong and broad T-cell specificities associated with HIV-1 control in mice and macaques. J Transl Med, 13, 60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuller DH, Simpson L, Cole KS et al. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunology and cell biology, 75(4), 389396 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Robinson HL, Montefiori DC, Johnson RP et al. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nature medicine, 5(5), 526–534 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Allen TM, Vogel TU, Fuller DH et al. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. Journal of immunology, 164(9), 4968–4978 (2000). [DOI] [PubMed] [Google Scholar]
- 31.**.Amara RR, Villinger F, Altman JD et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science, 292(5514), 69–74 (2001).Demonstrated role of vaccine-induced memory T cell responses in controlling pathogenic SIV infection in macaques [DOI] [PubMed] [Google Scholar]
- 32.Horton H, Vogel TU, Carter DK et al. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. Journal of virology, 76(14), 7187–7202 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manrique M, Kozlowski PA, Wang SW et al. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal immunology, 2(6), 536–550 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Cox JH, Ferrari MG, Earl P et al. Inclusion of a CRF01_AE HIV envelope protein boost with a DNA/MVA prime-boost vaccine: Impact on humoral and cellular immunogenicity and viral load reduction after SHIV-E challenge. Vaccine, 30(10), 1830–1840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*.Barouch DH, Liu J, Li H et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature, 482(7383), 89–93 (2012).Reports vaccination of non-human primates mediating significant delay in acquisition from neutralization resistant SIV and suggests a role for nonneutralizing antibodies in protection from infection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cebere I, Dorrell L, McShane H et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine, 24(4), 417–425 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Goonetilleke N, Moore S, Dally L et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. Journal of virology, 80(10), 4717–4728 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanke T, Goonetilleke N, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. The Journal of general virology, 88(Pt 1), 1–12 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Sandstrom E, Nilsson C, Hejdeman B et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. The Journal of infectious diseases, 198(10), 1482–1490 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guimaraes-Walker A, Mackie N, McCormack S et al. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine, 26(51), 6671–6677 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Aboud S, Nilsson C, Karlen K et al. Strong HIV-specific CD4+ and CD8+ T-lymphocyte proliferative responses in healthy individuals immunized with an HIV-1 DNA vaccine and boosted with recombinant modified vaccinia virus ankara expressing HIV-1 genes. Clinical and vaccine immunology: CVI, 17(7), 1124–1131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goepfert PA, Elizaga ML, Sato A et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. The Journal of infectious diseases, 203(5), 610–619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson C, Godoy-Ramirez K, Hejdeman B et al. Broad and potent cellular and humoral immune responses after a second late HIV-modified vaccinia virus ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccinees. AIDS research and human retroviruses, 30(3), 299–311 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang ZQ, Spitalnik S, Tran M, Wunner WH, Cheng J, Ertl HC. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology, 199(1), 132–140 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Robinson HL, Hunt LA, Webster RG. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine, 11(9), 957–960 (1993). [DOI] [PubMed] [Google Scholar]
- 46.Ulmer JB, Donnelly JJ, Parker SE et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science, 259(5102), 1745–1749 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Roy MJ, Wu MS, Barr LJ et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine, 19(7–8), 764–778 (2000). [DOI] [PubMed] [Google Scholar]
- 48.MacGregor RR, Boyer JD, Ugen KE et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. The Journal of infectious diseases, 178(1), 92–100 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Graham BS, Koup RA, Roederer M et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. The Journal of infectious diseases, 194(12), 1650–1660 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert review of vaccines, 11(2), 189–209 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Frontiers in immunology, 4, 354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Expert review of vaccines, 12(5), 537–554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low L, Mander A, McCann K et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Human gene therapy, 20(11), 1269–1278 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Vasan S, Hurley A, Schlesinger SJ et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PloS one, 6(5), e19252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kutzler MA, Wise MC, Hutnick NA et al. Chemokine-adjuvanted electroporated DNA vaccine induces substantial protection from simian immunodeficiency virus vaginal challenge. Mucosal immunology, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panicali D, Davis SW, Weinberg RL, Paoletti E. Construction of live vaccines by using genetically engineered poxviruses: biological activity of recombinant vaccinia virus expressing influenza virus hemagglutinin. Proceedings of the National Academy of Sciences of the United States of America, 80(17), 5364–5368 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith GL, Mackett M, Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature, 302(5908), 490–495 (1983). [DOI] [PubMed] [Google Scholar]
- 58.Smith GL, Murphy BR, Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proceedings of the National Academy of Sciences of the United States of America, 80(23), 7155–7159 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moss B Vaccinia virus: a tool for research and vaccine development. Science, 252(5013), 1662–1667 (1991). [DOI] [PubMed] [Google Scholar]
- 60.Smith GL, Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene, 25(1), 21–28 (1983). [DOI] [PubMed] [Google Scholar]
- 61.Kennedy RB, Ovsyannikova I, Poland GA. Smallpox vaccines for biodefense. Vaccine, 27 Suppl 4, D73–79 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. The Journal of general virology, 72 ( Pt 5), 1031–1038 (1991). [DOI] [PubMed] [Google Scholar]
- 63.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. The Journal of general virology, 79 ( Pt 5), 1159–1167 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Carroll MW, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology, 238(2), 198–211 (1997). [DOI] [PubMed] [Google Scholar]
- 65.Stittelaar KJ, Kuiken T, de Swart RL et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine, 19(27), 3700–3709 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Wyatt LS, Belyakov IM, Earl PL, Berzofsky JA, Moss B. Enhanced cell surface expression, immunogenicity and genetic stability resulting from a spontaneous truncation of HIV Env expressed by a recombinant MVA. Virology, 372(2), 260–272 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burgers WA, Shephard E, Monroe JE et al. Construction, characterization, and immunogenicity of a multigene modified vaccinia Ankara (MVA) vaccine based on HIV type 1 subtype C. AIDS research and human retroviruses, 24(2), 195–206 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Cottingham MG, Carroll MW. Recombinant MVA vaccines: dispelling the myths. Vaccine, 31(39), 4247–4251 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Wyatt LS, Earl PL, Xiao W et al. Elucidating and minimizing the loss by recombinant vaccinia virus of human immunodeficiency virus gene expression resulting from spontaneous mutations and positive selection. Journal of virology, 83(14), 7176–7184 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Krempelhuber A, Vollmar J, Pokorny R et al. A randomized, double-blind, dosefinding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine, 28(5), 1209–1216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. Journal of virology, 77(1), 799–803 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ratto-Kim S, Currier JR, Cox JH et al. Heterologous prime-boost regimens using rAd35 and rMVA vectors elicit stronger cellular immune responses to HIV proteins than X homologous regimens. PloS one, 7(9), e45840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manrique M, Kozlowski PA, Cobo-Molinos A et al. Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara. Journal of immunology, 186(6), 35813593 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Manrique M, Kozlowski PA, Cobo-Molinos A et al. Resistance to infection, early and persistent suppression of simian immunodeficiency virus SIVmac251 viremia, and significant reduction of tissue viral burden after mucosal vaccination in female rhesus macaques. Journal of virology, 88(1), 212–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogel TU, Reynolds MR, Fuller DH et al. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. Journal of virology, 77(24), 13348–13360 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith JM AR, Campbell D, Xu Y, Patel M, Sharma S, Butera ST, Ellenberger DL, Yi H, Chennareddi L, Herndon JG, Wyatt LS, Montefiori D, Moss B, McClure HM, Robinson HL. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS research and human retroviruses, 20(12), 1335–1347 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Smith JM AR, McClure HM, Patel M, Sharma S, Yi H, Chennareddi L, Herndon JG, Butera ST, Heneine W, Ellenberger DL, Parekh B, Earl PL, Wyatt LS, Moss B, Robinson HL. Multiprotein HIV type 1 clade B DNA/MVA vaccine: construction, safety, and immunogenicity in Macaques. AIDS research and human retroviruses, 20(6), 654–665 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Wyatt LS, Earl PL, Liu JY et al. Multiprotein HIV type 1 clade B DNA and MVA vaccines: construction, expression, and immunogenicity in rodents of the MVA component. AIDS research and human retroviruses, 20(6), 645–653 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Amara RR VF, Staprans SI, Altman JD, Montefiori DC, Kozyr NL, Xu Y, Wyatt LS, Earl PL, Herndon JG, McClure HM, Moss B, Robinson HL. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. Journal of virology, 76(15), 7625–7631 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson HL, Sharma S, Zhao J et al. Immunogenicity in macaques of the clinical product for a clade B DNA/MVA HIV vaccine: elicitation of IFN-gamma, IL-2, and TNF-alpha coproducing CD4 and CD8 T cells. AIDS research and human retroviruses, 23(12), 1555–1562 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Lai L, Kwa SF, Kozlowski PA et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine, 30(9), 1737–1745 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lai L, Kwa S, Kozlowski PA et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. The Journal of infectious diseases, 204(1), 164–173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwa S, Lai L, Gangadhara S et al. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. Journal of virology, 88(17), 9579–9589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kannanganant S, Gangadhara S, Lai L et al. Local control of repeated-dose rectal challenges in DNA/MVA-vaccinated macaques protected against a first series of simian immunodeficiency virus challenges. Journal of virology, 88(10), 5864–5869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amara RR, Smith JM, Staprans SI et al. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. Journal of virology, 76(12), 6138–6146 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson HL, Montefiori DC, Villinger F et al. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology, 352(2), 285–294 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Lai L, Vodros D, Kozlowski PA et al. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology, 369(1), 153–167 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bertley FM, Kozlowski PA, Wang SW et al. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. Journal of immunology, 172(6), 37453757 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Wang SW, Bertley FM, Kozlowski PA et al. An SHIV DNA/MVA rectal vaccination in macaques provides systemic and mucosal virus-specific responses and protection against AIDS. AIDS research and human retroviruses, 20(8), 846–859 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Kannanganat S, Nigam P, Velu V et al. Preexisting vaccinia virus immunity decreases SIV-specific cellular immunity but does not diminish humoral immunity and efficacy of a DNA/MVA vaccine. Journal of immunology, 185(12), 7262–7273 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allen TM, Jing P, Calore B et al. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. Journal of virology, 76(20), 10507–10511 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Makitalo B, Lundholm P, Hinkula J et al. Enhanced cellular immunity and systemic control of SHIV infection by combined parenteral and mucosal administration of a DNA prime MVA boost vaccine regimen. The Journal of general virology, 85(Pt 8), 2407–2419 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Kwa S, Sadagopal S, Shen X et al. CD40L-Adjuvanted DNA/Modified Vaccinia Virus Ankara Simian Immunodeficiency Virus (SIV) Vaccine Enhances Protection against Neutralization-Resistant Mucosal SIV Infection. Journal of virology, 89(8), 4690–4695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellenberger D, Otten RA, Li B et al. HIV-1 DNA/MVA vaccination reduces the per exposure probability of infection during repeated mucosal SHIV challenges. Virology, 352(1), 216–225 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Ellenberger D, Wyatt L, Li B et al. Comparative immunogenicity in rhesus monkeys of multi-protein HIV-1 (CRF02_AG) DNA/MVA vaccines expressing mature and immature VLPs. Virology, 340(1), 21–32 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Chamcha V, Kannanganat S, Gangadhara S et al. Strong, but Age-Dependent, Protection Elicited by a Deoxyribonucleic Acid/Modified Vaccinia Ankara Simian Immunodeficiency Virus Vaccine. Open Forum Infect Dis, 3(1), ofw034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.**.Shibata R, Igarashi T, Haigwood N et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nature medicine, 5(2), 204–210 (1999).Demonstrates that passive transfer of neutralizing antibodies can prevent infection in non-human primates [DOI] [PubMed] [Google Scholar]
- 98.Shingai M, Donau OK, Plishka RJ et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. The Journal of experimental medicine, 211(10), 2061–2074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.**.Burton SL, Kilgore KM, Smith SA et al. Breakthrough of SIV strain smE660 challenge in SIV strain mac239-vaccinated rhesus macaques despite potent autologous neutralizing antibody responses. Proceedings of the National Academy of Sciences of the United States of America, 112(34), 10780–10785 (2015).Demonstrates failure of vaccination to prevent infection despite induction of potent autologous serum neutralizing antibody response against the transmitted virus [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis GK, DeVico AL, Gallo RC. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proceedings of the National Academy of Sciences of the United States of America, 111(44), 15614–15621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fouts TR, Bagley K, Prado IJ et al. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proceedings of the National Academy of Sciences of the United States of America, 112(9), E992–999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gordon SN, Doster MN, Kines RC et al. Antibody to the gp120 V1/V2 loops and CD4+ and CD8+ T cell responses in protection from SIVmac251 vaginal acquisition and persistent viremia. Journal of immunology, 193(12), 6172–6183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pegu P, Vaccari M, Gordon S et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. Journal of virology, 87(3), 17081719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barouch DH, Alter G, Broge T et al. HIV-1 vaccines. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science, 349(6245), 320–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iyer SS, Gangadhara S, Victor B et al. Codelivery of Envelope Protein in Alum with MVA Vaccine Induces CXCR3-Biased CXCR5+ and CXCR5-CD4 T Cell Responses in Rhesus Macaques. Journal of immunology, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.*.Sanders RW, van Gils MJ, Derking R et al. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science, 349(6244), aac4223 (2015).Describes design and production of soluble HIV Env protein that resembles the native, membrane-bound trimer [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia-Arriaza J, Najera JL, Gomez CE et al. A candidate HIV/AIDS vaccine (MVA-B) lacking vaccinia virus gene C6L enhances memory HIV-1-specific T-cell responses. PloS one, 6(8), e24244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Falivene J, Del Medico Zajac MP, Pascutti MF et al. Improving the MVA vaccine potential by deleting the viral gene coding for the IL-18 binding protein. PloS one, 7(2), e32220 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perdiguero B, Gomez CE, Najera JL et al. Deletion of the viral anti-apoptotic gene F1L in the HIV/AIDS vaccine candidate MVA-C enhances immune responses against HIV-1 antigens. PloS one, 7(10), e48524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Arriaza J, Najera JL, Gomez CE, Sorzano CO, Esteban M. Immunogenic profiling in mice of a HIV/AIDS vaccine candidate (MVA-B) expressing four HIV-1 antigens and potentiation by specific gene deletions. PloS one, 5(8), e12395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Arriaza J, Gomez CE, Sorzano CO, Esteban M. Deletion of the vaccinia virus N2L gene encoding an inhibitor of IRF3 improves the immunogenicity of modified vaccinia virus Ankara expressing HIV-1 antigens. Journal of virology, 88(6), 3392–3410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rehm KE, Roper RL. Deletion of the A35 gene from Modified Vaccinia Virus Ankara increases immunogenicity and isotype switching. Vaccine, 29(17), 3276–3283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garber DA, O’Mara LA, Gangadhara S et al. Deletion of specific immune-modulatory genes from modified vaccinia virus Ankara-based HIV vaccines engenders improved immunogenicity in rhesus macaques. Journal of virology, 86(23), 12605–12615 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- .Excler JL, Robb ML, Kim JH. Prospects for a globally effective HIV-1 vaccine. Vaccine, 33 Suppl 4, D4–12 (2015). [DOI] [PubMed] [Google Scholar]
- .Fischer W, Perkins S, Theiler J et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nature medicine, 13(1), 100–106 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Goepfert PA, Elizaga ML, Seaton K et al. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. The Journal of infectious diseases, 210(1), 99–110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehendale S, Thakar M, Sahay S et al. Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: a phase I randomised Trial in HIV-uninfected Indian volunteers. PloS one, 8(2), e55831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramanathan VD, Kumar M, Mahalingam J et al. A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS research and human retroviruses, 25(11), 1107–1116 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Karasavvas N, Billings E, Rao M et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS research and human retroviruses, 28(11), 1444–1457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goepfert PA, Tomaras GD, Horton H et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine, 25(3), 510–518 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunological reviews, 236, 125–138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hayes P, Gilmour J, von Lieven A et al. Safety and immunogenicity of DNA prime and modified vaccinia ankara virus-HIV subtype C vaccine boost in healthy adults. Clinical and vaccine immunology: CVI, 20(3), 397–408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Munseri PJ, Kroidl A, Nilsson C et al. Priming with a simplified intradermal HIV-1 DNA vaccine regimen followed by boosting with recombinant HIV-1 MVA vaccine is safe and immunogenic: a phase IIa randomized clinical trial. PloS one, 10(4), e0119629 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bakari M, Aboud S, Nilsson C et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine, 29(46), 8417–8428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Joachim A, Nilsson C, Aboud S et al. Potent Functional Antibody Responses Elicited by HIV-I DNA Priming and Boosting with Heterologous HIV-1 Recombinant MVA in Healthy Tanzanian Adults. PloS one, 10(4), e0118486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim JH, Excler JL, Michael NL. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annual review of medicine, 66, 423–437 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Crotty S Follicular helper CD4 T cells (TFH). Annual review of immunology, 29, 621–663 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Casazza JP, Brenchley JM, Hill BJ et al. Autocrine production of beta-chemokines protects CMV-Specific CD4 T cells from HIV infection. PLoS pathogens, 5(10), e1000646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shinkawa T, Nakamura K, Yamane N et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. The Journal of biological chemistry, 278(5), 3466–3473 (2003). [DOI] [PubMed] [Google Scholar]
- 130.Shields RL, Lai J, Keck R et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. The Journal of biological chemistry, 277(30), 26733–26740 (2002). [DOI] [PubMed] [Google Scholar]
- 131.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine, 11(4 Suppl), S45–53 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Belyakov IM, Berzofsky JA. Immunobiology of Mucosal HIV Infection and the Basis for Development of a New Generation of Mucosal AIDS Vaccines. Immunity, 20(3), 247–253 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Demberg T, Robert-Guroff M. Mucosal Immunity and Protection Against HIV/SIV Infection: Strategies and Challenges for Vaccine Design. International reviews of immunology, 28(1–2), 20–48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou Q, Hidajat R, Peng B et al. Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIVmac251. Vaccine, 25(47), 8021–8035 (2007). [DOI] [PubMed] [Google Scholar]
- 135.Ganguly S, Manicassamy S, Blackwell J, Pulendran B, Amara RR. Adenovirus type 5 induces vitamin A-metabolizing enzymes in dendritic cells and enhances priming of guthoming CD8 T cells. Mucosal immunology, 4(5), 528–538 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huber M, Trkola A. Humoral immunity to HIV-1: neutralization and beyond. Journal of internal medicine, 262(1), 5–25 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Robinson HL. Non-neutralizing antibodies in prevention of HIV infection. Expert opinion on biological therapy, 13(2), 197–207 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Florese RH, Demberg T, Xiao P et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. Journal of immunology, 182(6), 3718–3727 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alpert MD, Harvey JD, Lauer WA et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS pathogens, 8(8), e1002890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xiao P, Zhao J, Patterson LJ et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. Journal of virology, 84(14), 7161–7173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.*.Burton DR, Hessell AJ, Keele BF et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proceedings of the National Academy of Sciences of the United States of America, 108(27), 11181–11186 (2011).Demonstrates that passive transfer of non-neutralizing antibody provides limited protection against mucosal SIV challenge [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.HIV/AIDS. http://www.who.int/mediacentre/factsheets/fs360/en/
- 202.UNAIDS Fact Sheet. http://www.unaids.org/en/resources/campaigns/globalreport2013/factsheet.