Abstract
Background
Although it has been purported that HIV-positive individuals may experience a greater degree of intoxication than HIV-negative individuals following acute alcohol consumption, no research to date has empirically tested this supposition. The present investigation entailed a randomized controlled experiment to identify whether the administration of a weight-specified dose of alcohol would lead to differential blood alcohol concentrations (BACs) among HIV-positive versus HIV-negative men.
Methods
In a specialized barroom laboratory, 143 men (n=76 HIV-positive, n= 67 HIV-negative; Mean age=42.9) consumed beverages based on a formulation of 0.7g alcohol/kg body weight over a 15-minute timeframe. BAC was assessed via breathalyzer at two set time-points (10- and 13-minutes post-consumption) and then periodically until detoxification (BAC<.040%). Primary outcomes included 1) area under the curve (AUC), calculated based on all of one’s BAC readings; 2) “BAC-EXP,” defined as one’s BAC reading 13-minutes post-consumption; and 3) BAC-PEAK, defined as one’s highest recorded BAC reading.
Results
Contrary to predictions, AUC (t(141)=2.23, p=.027), BAC-EXP (t(141)=2.68, p=.008), and BAC-PEAK (t(141)=2.29, p=.023) were significantly lower among HIV-positive versus HIV-negative participants. These effects were sustained in multivariable models controlling for age, race, and AUDIT-based hazardous drinking classification. Among the HIV-positive sample, outcomes did not significantly differ based on HIV viral load detectability, antiretroviral therapy (ART) status, or ART adherence.
Conclusions
The administration of a controlled, weight-specified dose of alcohol led to lower BACs among HIV-positive versus HIV-negative participants. These differences might derive from decreased body fat percentage and delayed gastric emptying associated with HIV seropositivity; however, additional research is necessary to verify these mechanisms. Unique alcohol dosing formulas based on HIV serostatus may be required in future alcohol administration experiments involving HIV-positive samples.
Keywords: Alcohol, HIV, Blood Alcohol Concentration (BAC), Intoxication, Experiment
INTRODUCTION
Alcohol use and hazardous drinking are common among people living with HIV (PLWH) (Cook et al. 2001; Galvan et al. 2002; National Institute on Alcohol Abuse and Alcoholism 2010; Samet et al. 2004) and have been linked to a number of detriments in HIV treatment- and prevention-related outcomes. PLWH who consume alcohol are at an elevated risk for nonadherence to antiretroviral therapy (ART) (Hendershot et al. 2009; Rehm et al. 2017), attrition from HIV-related care (Monroe et al. 2016; Vagenas et al. 2015), treatment-related complications and treatment failure (Braithwaite et al. 2007; Miguez et al. 2003; Williams et al. 2016), and increased mortality (Braithwaite et al. 2007; Gmel et al. 2011; Justice et al. 2016; Neblett et al. 2011). Alcohol use has also been identified as a driver of PLWH’s engagement in condomless sexual activity and subsequent HIV transmission (Scott-Sheldon et al. 2013; Shuper et al. 2009).
Potentially compounding these adverse effects is that the intoxicating impact of alcohol may be more pronounced among PLWH versus those who are not infected with the virus. For instance, in a study of over 2,600 HIV-positive and HIV-negative men, McGuinnis et al. (McGinnis et al. 2015) found that men who were HIV-positive reported that they required significantly fewer alcoholic drinks to “feel a buzz” compared to their uninfected counterparts. This difference was exacerbated when taking into account HIV-positive participants’ HIV viral load, whereby those whose HIV virus was not suppressed (i.e., those with detectable viral loads) reported significantly fewer alcoholic drinks to “feel a buzz” than both uninfected participants and HIV-positive participants who had achieved viral suppression. This latter finding accords with previous experimental work conducted by McCance-Katz et al. (McCance-Katz et al. 2013; McCance-Katz et al. 2012), whose research entailed first administering a controlled dose of alcohol to a small sample of untreated PLWH, and then repeating alcohol administration procedures with the same set of participants following ART initiation. Results demonstrated that while subjective intoxication did not differ based on one’s ART status, blood alcohol concentration (BAC) was shown to be reduced by 10%–15% following ART initiation. McCance-Katz et al. speculated that having unsuppressed HIV may result in immune activation and inflammation as well as intestinal damage, which in turn could lead to a decrease in alcohol metabolism and an increase in alcohol absorption.
Despite these findings, however, there has been no research to date that has empirically evaluated alcohol’s impact among HIV-positive versus HIV-negative individuals, and it remains unknown whether being infected with HIV can in-and-of-itself affect one’s level of intoxication. Given the harmful role that alcohol plays in HIV treatment and prevention efforts among PLWH, it remains crucial to identify the extent to which intoxication may be influenced by HIV seropositivity. The present investigation therefore sought to address this gap by directly assessing potential differences between HIV-positive and HIV-negative individuals with respect to BACs attained following controlled alcohol administration. It was hypothesized that after receiving a body weight-specified dose of alcohol, HIV-positive individuals would demonstrate significantly higher BACs than their uninfected counterparts. Additionally, recognizing the marked reductions in BACs that have been shown among HIV-positive individuals who have been initiated on ART (McCance-Katz et al. 2012), the present investigation also entailed an exploration of differences in BACs attained among ART-treated versus untreated PLWH. As an extension of this, BAC differences were also explored between HIV-positive participants who had a detectable HIV viral load versus those who had achieved viral suppression, and between those who were optimally versus suboptimally adherent to ART.
MATERIALS AND METHODS
Overview
Data were collected through an HIV prevention-focused randomized controlled alcohol administration experiment involving a sample of HIV-positive and HIV-negative men. In a barroom laboratory, participants were randomly assigned to receive a weight-specified dose of alcohol (target BAC=.080%), placebo alcohol (target BAC=.000%), or water (control). Participants then proceeded through a computer-based sexual risk paradigm, and those who had received alcohol remained onsite and completed a series of breathalyzer tests until detoxification was achieved. Complete details regarding the protocol have been published (Shuper et al. 2016). Procedures were approved by the Research Ethics Board (REB) at the Centre for Addiction and Mental Health (Protocol# 034/2010).
Participants
Men were recruited from an outpatient clinic that specializes in care for HIV-positive individuals and MSM in Toronto, Canada. Eligibility criteria included the following: 1) ≥19 years of age (i.e., legal drinking age in the jurisdiction); 2) anal sex with a man in the past six months; 3) classification as a social drinker (i.e., consumed ≥5 drinks/week on average and ≥5 drinks in one episode during the past six months) (Davis et al. 2007); 4) no recent history of problematic alcohol or substance use; and 5) no contraindications for consuming alcohol to a BAC of approximately .10% as deemed by one’s physician (e.g., no Hepatitis C or other liver-related concerns).
Procedure
Experimental sessions were run separately for each participant, and all sessions took place in specialized laboratory facilities at an addictions and mental health hospital in Toronto, Canada. Prior to showing up for their session, participants were asked to fast for a minimum of three hours and to abstain from consuming alcohol and other drugs for a minimum of 24 hours. These requirements were verified upon participants’ arrival at their session, and this process included the administration of a breathalyzer test to ensure that BAC was .000%. Eligible individuals were asked to provide informed written consent. Participants were weighed, asked to indicate their height, and completed a computer-based self-administered questionnaire that queried socio-demographic factors, alcohol and substance use, sexual behavior, and personality traits.
Following the questionnaire, participants were brought into a barroom laboratory and randomly assigned to beverage consumption condition. Those assigned to the alcohol condition received 0.7g alcohol/kg body weight through beverages comprised of vodka and tonic water based on a 1:3 ratio. The total beverage volume was divided equally across three cups, with 5ml of lemon and lime juice was added to each one. Participants were asked to drink each cup over a five-minute period, resulting in a 15-minute beverage consumption timeframe (for details regarding procedures for placebo and control condition participants, see Shuper et al. 2016). Eight minutes after the consumption period, participants were asked to rinse their mouths with water to eliminate any residual mouth alcohol, and two minutes later (i.e., 10-minutes post-consumption), a breathalyzer test was administered. Another breathalyzer test was performed three minutes later (i.e., 13-minutes post-consumption), and participants were asked to complete a single-item subjective intoxication measure. These assessments occurred immediately prior to the commencement of the computer-based sexual risk paradigm (see Shuper et al. 2016) for details).
Once participants had finished going through the program (approximately 20 minutes), a breathalyzer test was performed, and participants were taken into a separate laboratory room where they were provided with a meal. Alcohol condition participants were administered intermittent breathalyzer tests until detoxification was achieved (i.e., BAC<.040%). Participants were not informed of any of their BACs during the study.
Measures
Sociodemographics and participant characteristics
A self-administered touchscreen-based survey included questions about age, race, employment, education, and sexual orientation. The survey also included the Alcohol use Disorders Identification Test (AUDIT) (Babor et al. 2001) to assess alcohol consumption patterns as well as hazardous drinking, defined as an AUDIT score ≥8; as well as the NIDA Drug Use Screening Tool (ASSIST) (National Institute on Drug Abuse 2012) to classify the consumption of a variety of recreational substances over the past three months. Measures specific to sexual risk taking were also included (see Shuper et al. 2016).
HIV-related factors
During the detoxification period, HIV-positive participants completed two validated measures that assessed adherence to antiretroviral therapy (ART) over the past four days (Chesney et al. 2000; Walsh et al. 2002). For each measure, consistent with previous research, optimal adherence was defined as taking ≥95% of one’s ART doses during the respective timeframe (Bock et al. 2016). Chart data were also extracted to identify HIV disease- and treatment-relevant factors, including HIV viral load, CD4 cell count, and one’s prescribed ART medications.
Primary outcome measures
BAC was measured using the Alco-Sensor IV breathalyzer from Intoximeters Inc. (St. Louis, MO). All participants completed breathalyzer tests at two defined time points, which occurred 10-minutes and 13-minutes after completing one’s last beverage. The latter was defined as “BAC-EXP,” as it occurred in conjunction with the commencement of the computer-based sexual risk paradigm experiment. Additional BAC readings were obtained following the completion of the paradigm and continued intermittently until detoxification.
BAC readings formed the basis of the study’s three primary outcome measures. First, reflective of a participant’s overall BAC trajectory, area under the curve (AUC) was calculated using the linear trapezoidal rule (Fairclough 2010) for each participant based on all of his BAC readings. Second, BAC-EXP was examined as it was obtained at a defined time-point consistent across participants. Third, BAC-PEAK (proportional to plasma Cmax) was examined; defined as the highest recorded BAC reading for each participant.
Secondary outcome measures
Three secondary outcomes were examined. Time to BAC-PEAK was based on the time elapsed between the commencement of beverage consumption and achievement of BAC-PEAK (Tmax). Time to detoxification was defined as the elapsed time between the commencement of beverage consumption and the time at which a BAC reading <.040% was recorded. Lastly, subjective intoxication was based on a self-report single-item that asked how intoxicated the participant felt at that moment in accordance with a 10-point Likert scale ranging from 1=“No Effect” to 10=“Extremely Intoxicated.” (Davis et al. 2009)
Statistical Analysis
Analyses and results that are presented focus only on those individuals who were randomly assigned to the alcohol condition.
Sample characteristics
Sociodemographic differences between HIV-positive and HIV-negative participants were evaluated through independent samples t-tests and chi-square tests for continuous and dichotomous measures, respectively. Fisher’s exact tests were employed for dichotomous measures when an expected cell size within a specific comparison was less than 5.
Primary outcome analyses
AUC, BAC-EXP, and BAC-PEAK were compared between HIV-positive and HIV-negative samples using independent samples t-tests. Three separate corresponding multivariable linear regression models were then tested to assess whether any associations between these outcomes and participant HIV serostatus remained significant when accounting for potential confounding factors that included age, race, and hazardous drinking (i.e., AUDIT ≥8).1 Participant weight and body mass index (BMI) were not included in multivariable models, because these factors had already been controlled for through the weight-specified alcohol dosing formula employed in the experiment.
Secondary outcome analyses
A series of independent samples t-tests were conducted to assess whether HIV-positive and HIV-negative participants differed with respect to 1) Tmax; 2) time to detoxification; and 3) subjective intoxication.
Exploratory analyses
An additional set of exploratory analyses focused solely on the subsample of HIV-positive participants. Among this group, independent samples t-tests were conducted to assess differences between 1) those with detectable HIV viral loads vs. undetectable HIV viral loads; 2) those on ART vs. those not on ART; and 3) those reporting optimal vs. suboptimal ART adherence; for all primary (i.e., AUC, BAC-EXP, BAC-PEAK) and secondary (i.e., Tmax, time to detoxification, subjective intoxication) outcomes.
RESULTS
Sample Characteristics
A total of 143 participants were randomly assigned to the alcohol condition, among whom 76 were HIV-positive and 67 were HIV-negative. Sociodemographic and other characteristics are presented in Table 1. As shown in the table, mean age was 42.9 (SD=10.3; Range=21–69), and approximately three quarters of participants (76.2%) identified their race/ethnicity as “white.” Compared to HIV-negative participants, those who were HIV-positive were significantly less likely to have a college/university education or to be employed. The difference in body weight between the two participant groups approached statistical significance, demonstrating a trend whereby HIV-positive participants weighed less than HIV-negative participants. Significant differences in body mass index (BMI) were demonstrated among the sample of participants for whom BMI could be calculated (n=127; n=75 HIV-positive, n=52 HIV-negative2), such that BMI was significantly lower among HIV-positive versus HIV-negative participants.
Table 1.
Characteristics of study participants.
| Characteristic | Total N=143 |
HIV-Negative n=67 |
HIV-Positive n=76 |
p* |
|---|---|---|---|---|
| Age: M (SD) | 42.9 (10.3) | 44.4 (10.0) | 41.6 (10.4) | .115 |
| Sexual orientation=Gay: n (%) | 141 (98.6) | 67 (100) | 74 (97.4) | .281 |
| Education=College diploma or higher: n (%) | 99 (69.2) | 57 (85.1) | 42 (55.3) | .000 |
| Employed full/part time: n (%) | 99 (69.2) | 57 (85.1) | 42 (55.3) | .000 |
| Weight, kg: M (SD) | 82.3 (15.2) | 84.9 (15.4) | 80.1 (14.7) | .061 |
| BMI†: M (SD) | 25.7 (4.5) | 26.9 (4.2) | 24.9 (4.4) | .011 |
| Race, n (%) | ||||
| White | 109 (76.2) | 54 (80.6) | 55 (72.4) | .249 |
| Latin American | 9 (6.3) | 3 (4.5) | 6 (7.9) | .314 |
| Black | 7 (4.9) | 0 (0) | 7 (9.2) | .010 |
| Chinese | 1 (0.7) | 1 (1.5) | 0 (0) | .469 |
| South-Asian | 2 (1.4) | 1 (1.5) | 1 (1.3) | .719 |
| Aboriginal | 0 (0) | 0 (0) | 0 (0) | n/a |
| Filipino | 2 (1.4) | 0 (0) | 2 (2.6) | .281 |
| Southeast Asian | 0 (0) | 0 (0) | 0 (0) | n/a |
| Arab | 1 (0.7) | 1 (1.5) | 0 (0) | .469 |
| West Asian | 0 (0) | 0 (0) | 0 (0) | n/a |
| Korean or Japanese | 0 (0) | 0 (0) | 0 (0) | n/a |
| Multi-Race | 12 (8.4) | 7 (10.4) | 5 (6.6) | .405 |
| Alcohol use | ||||
| AUDIT Total: M (SD) | 7.8 (3.5) | 7.6 (3.0) | 7.9 (3.9) | .580 |
| AUDIT ≥ 8: n (%) | 67 (46.9) | 31 (46.3) | 36 (47.4) | .895 |
| Substance use - past 3 months, n (%) | ||||
| Cannabis | 84 (58.7) | 36 (53.7) | 48 (63.2) | .253 |
| Cocaine | 36 (25.4) | 12 (18.2) | 24 (31.6) | .067 |
| Prescription stimulants | 6 (4.2) | 1 (1.5) | 5 (6.7) | .133 |
| Methamphetamine | 18 (12.6) | 3 (4.5) | 15 (19.7) | .006 |
| Inhalants | 21 (14.7) | 8 (11.9) | 13 (17.1) | .384 |
| Sedatives | 31 (21.7) | 15 (22.4) | 16 (21.1) | .847 |
| Hallucinogens | 17 (11.9) | 5 (7.5) | 12 (15.8) | .125 |
| Street opioids | 1 (0.7) | 0 (0) | 1 (1.3) | .531 |
| Prescription opioids | 9 (6.3) | 5 (7.5) | 4 (5.3) | .421 |
| HIV-related factors (HIV+ participants only) | ||||
| CD4 count (cells/mm3): M (SD) | 608.7 (200.3) | n/a | ||
| HIV viral load=undetectable: n (%) | 60 (80.0) | n/a | ||
| Currently on ART: n (%) | 62 (81.6) | n/a | ||
| Optimal (≥95%) ART adherence: 4 days | 54 (90.0) | n/a | ||
| Optimal (≥95%) ART adherence: 1 month | 47 (75.8) | n/a | ||
p values are based on t-tests, χ2 tests, or Fisher’s exact tests for differences between HIV- and HIV+ participants.
BMI calculations were possible for 127/143 participants (88.8%) (n=52 HIV-negative; n=75 HIV-positive).
Alcohol and Substance Use
As shown in Table 1, mean AUDIT score was 7.8 (SD=3.5), and just under half of the sample (46.9%) met hazardous drinking criteria. Neither AUDIT scores nor the proportion of hazardous drinkers differed significantly between HIV-positive and HIV-negative participants. The use of substances in the past three months was also similar among HIV-positive and HIV-negative participants, with the exception of methamphetamine use, which was more common among those who were HIV-positive.
Primary Outcome Analyses: Tests of BAC Differences between HIV-Positive and HIV-Negative Participants
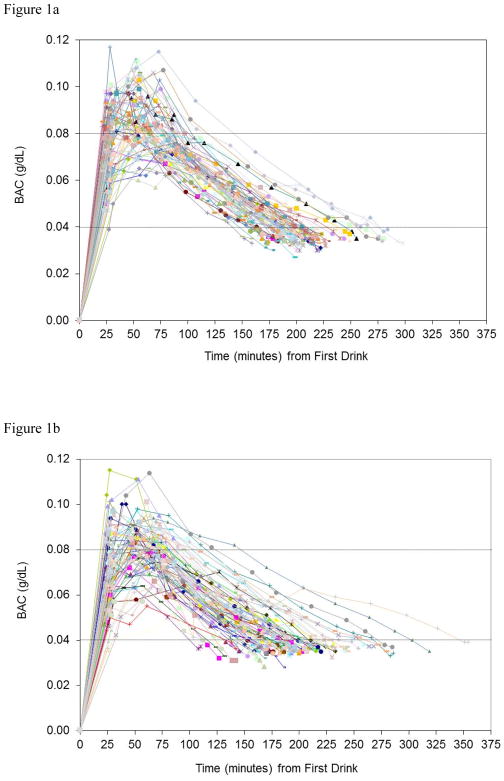
All BAC readings for each participant are plotted in Figure 1a (HIV-negative sample) and Figure 1b (HIV-positive sample). Tests of differences between HIV-positive and HIV-negative participants for primary outcomes are presented in Table 2. As shown in the table, contrary to predictions, AUCs were significantly smaller among the sample of HIV-positive participants than the sample of HIV-negative participants (t(141)=2.23, p=.027, d=0.38), indicating significantly lower BACs overall among the former group. Similarly, both BAC-EXP (t(141)=2.68, p=.008, d=0.43) and BAC-PEAK (t(141)=2.29, p=.023, d=0.40) were significantly lower among HIV-positive than HIV-negative participants.
Figure 1.
Figure 1a. BAC readings among HIV− participants (n=67)
Figure 1b. BAC readings among HIV+ participants (n=76)
Table 2.
Tests of differences between HIV-positive and HIV-negative participants with respect to primary and secondary alcohol-related outcomes.
| Outcome | Total (N=143) | HIV-Negative (n=67) | HIV-Positive (n=76) | t | p |
|---|---|---|---|---|---|
| Primary Outcomes | |||||
| AUC: M (SD) | 11.822 (2.943) | 12.399 (2.495) | 11.313 (3.218) | 2.233 | .027 |
| BAC-EXP: M (SD) | .076 (.015) | .079 (.013) | .073 (.015) | 2.680 | .008 |
| BAC-PEAK: M (SD) | .086 (.012) | .089 (.013) | .084 (.012) | 2.294 | .023 |
| Secondary Outcomes | |||||
| Time to BAC-PEAK (Tmax) (minutes): M (SD) | 53.4 (17.9) | 52.1 (18.7) | 54.5 (17.2) | −0.810 | .419 |
| Time to detoxification (minutes): M (SD) | 195.1 (39.0) | 200.9 (30.3) | 190.0 (44.9) | 1.712 | .089 |
| Subjective intoxication*: M (SD) | 6.6 (1.4) | 6.7 (1.5) | 6.5 (1.3) | 0.790 | .431 |
Assessed at the time of “BAC-EXP.” Scale ranges from 1=“No Effect” to 10=“Extremely intoxicated.”
Results from multivariable linear regression models that controlled for age, race, and AUDIT-based hazardous drinking classification are presented in Table S1, Table S2, and Table S3 of the online supplement for AUC, BAC-EXP, and BAC-PEAK, respectively. As shown in Table S1, the association between HIV serostatus and AUC remained significant when controlling for the above-mentioned factors, whereby AUC was significantly smaller among HIV-positive versus HIV-negative participants. Similarly, the association between HIV serostatus and BAC-EXP (Table S2), as well as the association between HIV serostatus and BAC-PEAK (Table S3), also remained significant in these multivariable models.
Secondary Outcome Analyses: Tests of Differences in Other Alcohol-Related Indicators between HIV-Positive and HIV-Negative Participants
Tests of differences between HIV-positive and HIV-negative participants for Tmax, time to detoxification, and subjective intoxication are presented in Table 2. As shown in the Table, HIV-positive and HIV-negative participants did not significantly differ on any of these secondary outcomes.
Exploratory Analyses: Differences in BACs and Other Alcohol-Related Indicators based on HIV Treatment Factors
As shown in Table 3, HIV-positive participants with detectable HIV viral loads did not significantly differ from those with undetectable HIV viral loads on any primary or secondary outcome measure, and no significant differences were found based on ART status. Similarly, as demonstrated in Table 4, no significant differences were found based on ART adherence.
Table 3.
Tests of differences among the HIV-positive sample based on HIV viral load detectability and antiretroviral therapy (ART) status with respect to primary and secondary alcohol-related outcomes.
| Outcome | HIV Viral Load | t | p | Taking ART | t | p | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Detectable (n=15) | Undetectable (n=60) | No ART (n=14) | On ART (n=62) | |||||
| Primary Outcomes | ||||||||
| AUC: M (SD) | 11.02 (3.18) | 11.43 (3.25) | 0.436 | .664 | 10.78 (3.29) | 11.43 (3.22) | 0.685 | .496 |
| BAC-EXP: M (SD) | 0.068 (.014) | 0.074 (.016) | 1.203 | .233 | 0.068 (.014) | 0.074 (.015) | 1.219 | .227 |
| BAC-PEAK: M (SD) | 0.080 (.010) | 0.085 (.012) | 1.317 | .192 | 0.080 (.010) | 0.085 (.012) | 1.373 | .174 |
| Secondary Outcomes | ||||||||
| Time to BAC-PEAK (Tmax) (minutes): M (SD) | 60.9 (17.4) | 53.1 (17.0) | −1.579 | .119 | 62.2 (17.2) | 52.8 (16.8) | −1.881 | .064 |
| Time to detoxification (minutes): M (SD) | 189.8 (50.0) | 190.7 (44.1) | 0.066 | .947 | 186.6 (51.2) | 190.7 (43.8) | 0.307 | .760 |
| Subjective intoxication*: M (SD) | 6.1 (1.5) | 6.6 (1.3) | 1.370 | .175 | 6.2 (1.6) | 6.6 (1.3) | 0.876 | .384 |
Assessed at the time of “BAC-EXP.” Scale ranges from 1=“No effect” to 10=“Extremely intoxicated.”
Table 4.
Tests of differences among the HIV+ sample based on antiretroviral therapy (ART) adherence over past four days and past month with respect to primary and secondary alcohol-related outcomes.
| Outcome | 4-Day ART Adherence | t | p | 1-month ART Adherence | t | p | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Suboptimal (i.e., < 95%) Adherence (n=6) |
Optimal (i.e., ≥95%) Adherence (n=54) |
Suboptimal (i.e., < 95%) Adherence (n=15) |
Optimal (i.e., ≥95%) Adherence (n=47) |
|||||
| Primary Outcomes | ||||||||
| AUC: M (SD) | 10.43 (2.54) | 11.57 (3.33) | −0.806 | .424 | 12.20 (3.11) | 11.19 (3.24) | 1.060 | .293 |
| BAC-EXP: M (SD) | 0.077 (.025) | 0.073 (.015) | 0.552 | .583 | 0.075 (.019) | 0.073 (.014) | 0.426 | .672 |
| BAC-PEAK: M (SD) | 0.089 (.014) | 0.084 (.012) | 0.897 | .373 | 0.088 (.010) | 0.084 (.013) | 1.192 | .238 |
| Secondary Outcomes | ||||||||
| Time to BAC-PEAK (Tmax) (minutes): M (SD) | 43.5 (16.9) | 54.2 (16.8) | −1.481 | .144 | 51.6 (17.0) | 53.2 (16.9) | −0.317 | .753 |
| Time to detoxification (minutes): M (SD) | 168.3 (27.9) | 193.2 (45.4) | −1.310 | .195 | 201.0 (53.3) | 187.5 (40.3) | 1.044 | .301 |
| Subjective intoxication*: M (SD) | 6.0 (1.8) | 6.6 (1.3) | −1.108 | .272 | 6.4 (1.5) | 6.6 (1.3) | −0.445 | .658 |
Assessed at the time of “BAC-EXP.” Scale ranges from 1=“No effect” to 10=“Extremely intoxicated.”
DISCUSSION
The present investigation entailed the first controlled experiment to empirically test BAC differences between HIV-positive and HIV-negative samples. Contrary to predictions, HIV-positive participants attained significantly lower BACs compared to their HIV-uninfected counterparts following the receipt of a weight-specified dose of alcohol. These differences remained significant in multivariable models that controlled for age, race/ethnicity, and alcohol consumption patterns. In exploratory analyses involving the HIV-positive sample, BACs were not shown to significantly differ based on HIV viral load detectability, ART status, or ART adherence. The effect sizes for the associations between HIV serostatus and primary BAC outcomes were small to medium in magnitude. While these effect sizes are less robust than those typically shown for the association between biological sex and BAC (e.g., Dettling, Witte, Skopp, et al., 2009; Frezza, Padova, Pozzato, et al., 1990), the present findings nevertheless demonstrate a clear and consistent link between HIV seropositivity and the attainment of diminished BAC levels.
One possible explanation behind the demonstrated lower BACs among HIV-positive participants relates to changes in body fat distribution. Because the proportion of body fat is inversely associated with total body water (Kalant & Roschlau 1998; Marshall et al. 1983), and because BAC is a function of total body water (Kalant 1996; Kalant & Roschlau 1998; Marshall et al. 1983; Norberg et al. 2003), changes in fat distribution should result in changes in BAC levels. A leaner individual should attain a lower BAC compared to an individual with a higher fat content, despite having the same overall body weight. Corroborating this supposition, Mills and Bisgrove (Mills & Bisgrove 1983) demonstrated that by adjusting alcohol doses to percent body fat, individual differences in BACs disappeared, and female participants who would normally achieve higher BACs had values comparable to their male counterparts.
Lipodystrophy, entailing changes in the distribution of fat, has been shown to occur among HIV-positive individuals, regardless of ART treatment (Guaraldi et al. 2014; Souza et al. 2013; Willig & Overton 2016). Grunfeld et al. (Grunfeld et al. 2010), for example, compared regional (i.e., leg, upper and lower truck, and arm) subcutaneous adipose tissue volumes between HIV-positive and HIV-negative participants upon initial assessment and over the course of a five-year follow-up using magnetic resonance imaging (MRI). Compared to HIV-negative participants, those who were HIV-positive had significantly lower adipose tissue volumes, indicating lipoatrophy, at baseline and after five years regardless of changes in HIV treatment. This difference remained significant even after controlling for covariates such as age, race, and weight. Furthermore, although both HIV-positive and HIV-negative participants gained fat over time, HIV-positive participants gained at a significantly lower rate compared to HIV-negative participants. Therefore, because alcohol is more soluble in water than in fat, it is likely that the redistribution of fat, and specifically fat loss, can change the absorption and distribution of alcohol in general, and as a result, the same amount of alcohol per body weight may yield lower BACs in HIV-positive individuals who likely have less body fat compared to HIV-negative individuals of similar body weight.
A second possible explanation underlying the significantly lower BACs among HIV-positive versus HIV-negative participants stems from potential differences in gastric emptying between the two groups. Gastric emptying determines the rate of absorption of orally administered ethanol into the blood stream (Jones 2011; Kalant 1996; Kalant & Roschlau 1998), such that faster gastric emptying results in more rapid absorption of alcohol, and vice versa. Thus, when gastric emptying slows down, alcohol absorption is reduced and lower BACs are achieved (Finch et al. 1974; Holt 1981; Holt et al. 1980). Of particular relevance is that gastric emptying has been shown to be significantly delayed among HIV-positive individuals compared to those not infected with HIV (Neild et al. 2000). Therefore, possible delayed gastric emptying among the present HIV-positive sample could have reduced alcohol absorption as well as increased exposure of alcohol to gastric alcohol dehydrogenase (ADH) enzymes responsible for the breakdown of alcohol, thereby resulting in lower BAC levels.
Interestingly, while the present findings consistently showed lower BACs among HIV-positive versus HIV-negative participants, the self-report subjective intoxication data accord with results from McGinnis et al., (McGinnis et al. 2015), in which their HIV-positive participants reported needing fewer drinks to feel a buzz than their HIV-negative participants. Specifically, our findings showed that perceived level of intoxication was the same for both HIV-positive and HIV-negative men, even though the former were significantly lower in their objective level of intoxication. It therefore appears that HIV-positive individuals may indeed experience subjective alcohol impairment at a lower level of actual alcohol intoxication compared to those who are not infected with the virus. Given this disconnect between subjective and objective intoxication, a more thorough examination of this occurrence, particularly one involving comprehensive measures to assess the subcomponents that underlie subjective intoxication (e.g., Courtney et al. 2013; Miranda, Jr. et al. 2014; Morean et al. 2013), would be required to gain a better understanding of these serostatus-derived differences.
Limitations
Findings should be viewed in terms of study limitations. First, the sample was comprised solely of men, most of whom were white. Furthermore, those who were deemed as alcohol abusers were excluded from participation. It would therefore be important to replicate the findings among a more diverse spectrum of individuals with respect to these characteristics. Second, for ethical reasons, individuals experiencing liver-related concerns did not receive medical clearance to take part in this study. Because PLWH experience such issues at a disproportionate level, exclusion based on this criterion may have resulted in a sample of PLWH who potentially had better liver functioning compared to the overall PLWH population; and this may have made the HIV-positive and HIV-negative samples artificially more similar within this regard. Although not ethically feasible within an experimental setting, employing a sample with greater variability in liver functioning could potentially result in greater variability in BACs attained. Third, the samples of HIV-positive participants who were not receiving ART, who were suboptimally adherent to ART, and who had detectable HIV viral loads were relatively small. Given the statistical power limitations inherent in the exploratory analyses involving these small samples, the ability to detect statistically significant differences in BACs based on ART- and viral load-related categorizations may have been hindered. Fourth, although disparities between HIV-positive and HIV-negative individuals with respect to body fat distribution and gastric emptying are suggested as possible mechanisms that explain the study findings, these aspects were not evaluated in the present experiment and therefore cannot unequivocally be deemed as drivers of HIV serostatus-based BAC differences. Finally, breathalyzer tests were not administered during the approximately 20-minute timeframe in which participants completed the computer-based sexual risk paradigm. An actual peak in BAC that may have occurred specifically during this period would therefore not have been identified, thus resulting in a small degree of variability in the designation of one’s BAC-PEAK and time to BAC-PEAK for such individuals. Future investigations in this domain would benefit from the inclusion of frequent breathalyzer tests during both ascending and descending BAC limbs that occur in close temporal proximity to one’s anticipated BAC-PEAK.
Implications and Conclusions
Given that the present experiment was the first of its kind, and recognizing the uniqueness of the findings, it would be imperative to replicate the study to ensure that the same patterns emerge prior to making recommendations pertaining to PLWH-specific, alcohol-focused clinical treatment guidelines as well as public health messaging. Furthermore, subsequent research is necessary to objectively evaluate the extent to which any BAC differences based on HIV serostatus are attributable to the purported differences in body composition and gastric emptying.
Nevertheless, the current findings suggest that PLWH, regardless of ART status or HIV viral load suppression, attain significantly lower BACs following the consumption of a controlled, weight-specified dose of alcohol. While biological sequelae of HIV seropositivity may diminish PLWH’s objective level of intoxication, their perceived feeling of intoxication tends to match that of their more objectively intoxicated HIV-negative counterparts. Taken together, these findings have implications for research investigating the impact of alcohol consumption among PLWH. For example, it may be necessary to devise and employ unique alcohol dosing formulas specific to PLWH when including such individuals in future alcohol administration experiments, whereby the dose administered to attain a targeted BAC level would not only need to be based on one’s weight, but also on one’s HIV serostatus. Relatedly, experiments focused on eliciting a targeted level of subjective intoxication may require different alcohol dosing formulations for HIV-positive and HIV-negative individuals in order to achieve the same degree of perceived alcohol-induced impairment among the two groups. Identifying what may be driving the disparity between HIV-positive and HIV-negative individuals with respect to their objective versus subjective level intoxication would also be of key importance, as both constructs can independently impact HIV prevention-related behaviors (Davis et al. 2009; Rehm et al. 2017; Shuper et al. 2017). Overall, such initiatives, in conjunction with the present findings, could ultimately influence alcohol consumption guidelines for PLWH.
Supplementary Material
Acknowledgments
Funding for this work was provided by the National Institutes of Health (NIH)/National Institute on Alcohol Abuse and Alcoholism (NIAAA) (4R21AA020236-02, PI: Shuper) and the Canadian Institutes of Health Research (HHP111404, PI: Shuper). We would like to thank Professor Emeritus Harold Kalant and Professor Anh Dzung Lê for their input regarding our findings. We would also like to thank study participants, research team members, and physicians and staff at the Maple Leaf Medical Clinic. The authors declare no conflicts of interest.
Footnotes
Additional multivariable models were conducted in which methamphetamine use and cocaine use during the past three months were included as factors. Neither of these two factors was significantly associated with any of the three primary BAC outcomes (i.e., AUC, BAC-EXP, or BAC-PEAK). Furthermore, these additional models produced the same significant findings as those yielded through the multivariable models presented in this manuscript, whereby participant HIV serostatus remained significantly associated with all three primary BAC outcomes.
Although all participants were weighed, height was not assessed for the first 16 study participants.
Reference List
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG World Health Organization. Dept. of Mental Health and Substance Dependence. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. 2. World Health Organization; Geneva: 2001. [Google Scholar]
- Bock P, James A, Nikuze A, Peton N, Sabapathy K, Mills E, et al. Baseline CD4 Count and Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis. J Acquir Immune Defic Syndr. 2016;73:514–521. doi: 10.1097/QAI.0000000000001092. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, et al. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ashenhurst J, Bacio G, Moallem N, Bujarski S, Hartwell E, et al. Craving and subjective responses to alcohol administration: validation of the desires for alcohol questionnaire in the human laboratory. J Stud Alcohol Drugs. 2013;74:797–802. doi: 10.15288/jsad.2013.74.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KC, George WH, Norris J, Schacht RL, Stoner SA, Hendershot CS, et al. Effects of alcohol and blood alcohol concentration limb on sexual risk-taking intentions. J Stud Alcohol Drugs. 2009;70:499–507. doi: 10.15288/jsad.2009.70.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KC, Hendershot CS, George WH, Norris J, Heiman JR. Alcohol’s effects on sexual decision making: an integration of alcohol myopia and individual differences. J Stud Alcohol Drugs. 2007;68:843–851. doi: 10.15288/jsad.2007.68.843. [DOI] [PubMed] [Google Scholar]
- Dettling A, Witte S, Skopp G, Graw M, Haffner HT. A regression model applied to gender-specific ethanol elimination rates from blood and breath measurements in non-alcoholics. Int J Legal Med. 2009;123(5):381–5. doi: 10.1007/s00414-008-0282-y. [DOI] [PubMed] [Google Scholar]
- Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials. 2. Chapman and Hall/CRC; Boca Raton, FL: 2010. [Google Scholar]
- Finch JE, Kendall MJ, Mitchard M. An assessment of gastric emptying by breathalyser. Br J Clin Pharmacol. 1974;1:233–236. doi: 10.1111/j.1365-2125.1974.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322(2):95–9. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Gmel G, Shield KD, Rehm J. Developing a method to derive alcohol-attributable fractions for HIV/AIDS mortality based on alcohol’s impact on adherence to antiretroviral medication. Popul Health Metr. 2011;9:5. doi: 10.1186/1478-7954-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C, Saag M, Cofrancesco J, Jr, Lewis CE, Kronmal R, Heymsfield S, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24:1717–1726. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Stentarelli C, Zona S, Santoro A, Beghetto B, Carli F, et al. The natural history of HIV-associated lipodystrophy in the changing scenario of HIV infection. HIV Med. 2014;15:587–594. doi: 10.1111/hiv.12159. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. Observations on the relation between alcohol absorption and the rate of gastric emptying. Can Med Assoc J. 1981;124:267–77. 297. [PMC free article] [PubMed] [Google Scholar]
- Holt S, Stewart MJ, Adam RD, Heading RC. Alcohol absorption, gastric emptying and a breathalyser. Br J Clin Pharmacol. 1980;9:205–208. doi: 10.1111/j.1365-2125.1980.tb05834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW. Pharmacokinetics of Ethanol - Issues of Forensic Importance. Forensic Sci Rev. 2011;23:91–136. [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H. Pharmacokinetics of Ethanol: Absorption, Distribution, and Elimination. In: Begleiter H, Kissin B, editors. The Pharmacology of Alcohol and Alcohol Dependence. Oxford University Press; New York: 1996. pp. 15–58. [Google Scholar]
- Kalant H, Roschlau WHE. Principles of Medical Pharmacology. 6. Oxford University Press; New York: 1998. [Google Scholar]
- Marshall AW, Kingstone D, Boss M, Morgan MY. Ethanol elimination in males and females: relationship to menstrual cycle and body composition. Hepatology. 1983;3:701–706. doi: 10.1002/hep.1840030513. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Gruber VA, Beatty G, Lum PJ, Rainey PM. Interactions between alcohol and the antiretroviral medications ritonavir or efavirenz. J Addict Med. 2013;7:264–270. doi: 10.1097/ADM.0b013e318293655a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Lum PJ, Beatty G, Gruber VA, Peters M, Rainey PM. Untreated HIV infection is associated with higher blood alcohol levels. JAIDS. 2012;60:282–288. doi: 10.1097/QAI.0b013e318256625f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis KA, Fiellin DA, Tate JP, Cook RL, Braithwaite RS, Bryant KJ, et al. Number of Drinks to “Feel a Buzz” by HIV Status and Viral Load in Men. AIDS Behav. 2015 doi: 10.1007/s10461-015-1053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Mills KC, Bisgrove EZ. Body sway and divided attention performance under the influence of alcohol: dose-response differences between males and females. Alcohol Clin Exp Res. 1983;7:393–397. doi: 10.1111/j.1530-0277.1983.tb05492.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, et al. Characterizing subjective responses to alcohol among adolescent problem drinkers. J Abnorm Psychol. 2014;123:117–129. doi: 10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, et al. Heavy Alcohol Use Is Associated With Worse Retention in HIV Care. JAIDS. 2016;73:419–425. doi: 10.1097/QAI.0000000000001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Treat TA. The Subjective Effects of Alcohol Scale: development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychol Assess. 2013;25:780–795. doi: 10.1037/a0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. [Accessed 7 June 2017];Alcohol Alert. 2010 Available at: https://pubs.niaaa.nih.gov/publications/AA80/AA80.htm.
- National Institute on Drug Abuse. [Accessed 13 April 2017];Resource Guide: Screening for Drug Use in General Medical Settings. 2012 Available at: https://www.drugabuse.gov/publications/resource-guide.
- Neblett RC, Hutton HE, Lau B, McCaul ME, Moore RD, Chander G. Alcohol consumption among HIV-infected women: impact on time to antiretroviral therapy and survival. J Womens Health (Larchmt ) 2011;20:279–286. doi: 10.1089/jwh.2010.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neild PJ, Nijran KS, Yazaki E, Evans DF, Wingate DL, Jewkes R, et al. Delayed gastric emptying in human immunodeficiency virus infection: correlation with symptoms, autonomic function, and intestinal motility. Dig Dis Sci. 2000;45:1491–1499. doi: 10.1023/A:1005587922517. [DOI] [PubMed] [Google Scholar]
- Norberg A, Jones AW, Hahn RG, Gabrielsson JL. Role of variability in explaining ethanol pharmacokinetics: research and forensic applications. Clin Pharmacokinet. 2003;42:1–31. doi: 10.2165/00003088-200342010-00001. [DOI] [PubMed] [Google Scholar]
- Rehm J, Probst C, Shield KD, Shuper PA. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul Health Metr. 2017;15:4. doi: 10.1186/s12963-017-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Walstrom P, Carey KB, Johnson BT, Carey MP. Alcohol use and sexual risk behaviors among individuals infected with HIV: a systematic review and meta-analysis 2012 to early 2013. Curr HIV/AIDS Rep. 2013;10:314–323. doi: 10.1007/s11904-013-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuper PA, Joharchi N, Irving H, Rehm J. Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: review and meta-analysis. AIDS Behav. 2009;13:1021–1036. doi: 10.1007/s10461-009-9589-z. [DOI] [PubMed] [Google Scholar]
- Shuper PA, Joharchi N, Monti PM, Loutfy M, Rehm J. Acute Alcohol Consumption Directly Increases HIV Transmission Risk: A Randomized Controlled Experiment. JAIDS. 2017;76:493–500. doi: 10.1097/QAI.0000000000001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuper PA, Joharchi N, Rehm J. Protocol for a Controlled Experiment to Identify the Causal Role of Acute Alcohol Consumption in Condomless Sex among HIV-Positive MSM: Study Procedures, Ethical Considerations, and Implications for HIV Prevention. AIDS Behav. 2016;20(Suppl 1):S173–S184. doi: 10.1007/s10461-015-1128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza SJ, Luzia LA, Santos SS, Rondo PH. Lipid profile of HIV-infected patients in relation to antiretroviral therapy: a review. Rev Assoc Med Bras (1992 ) 2013;59:186–198. doi: 10.1016/j.ramb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: a Systematic Review : Alcohol and the HIV Continuum of Care. Curr HIV/AIDS Rep. 2015;12:421–436. doi: 10.1007/s11904-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res. 2016;40:2056–2072. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig AL, Overton ET. Metabolic Complications and Glucose Metabolism in HIV Infection: A Review of the Evidence. Curr HIV/AIDS Rep. 2016;13:289–296. doi: 10.1007/s11904-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.