Abstract
Background
Alcohol use disorder (AUD) is prevalent among individuals diagnosed with human immunodeficiency virus (HIV), and both HIV and alcohol use have been shown to negatively affect the integrity of white matter pathways in the brain. Behavioral, functional, and anatomical impairments have been linked independently to HIV and alcohol use, and these impairments have bases in specific frontally-mediated pathways within the brain.
Methods
Magnetic Resonance Imaging (MRI) data were acquired for 37 HIV+ participants without dementia or hepatitis C. Imaging data were processed through the FreeSurfer and TraCULA pipelines to obtain four bilateral frontal white matter tracts for each participant. Diffusion metrics of white matter integrity along the highest probability pathway for each tract were analyzed with respect to demographics, disease-specific variables, and reported substance use.
Results
Significantly increased Axial Diffusivity (AD; decreased axonal integrity) and a trending increase in Mean Diffusivity (MD) were observed along the Anterior Thalamic Radiation (ATR) in participants with a history of AUD. A diagnosis of AUD explained over 36% of the variance in diffusivity along the Anterior Thalamic Radiation overall when accounting for clinical variables including Nadir CD4 and age-adjusted HIV infection length.
Conclusions
The present study provides evidence of HIV-related associations between alcohol use and indicators of axonal integrity loss along the ATR, a frontal pathway involved in the inhibition of addictive or unwanted behaviors. Reduced axonal integrity of this pathway was greatest in HIV+ participants with an AUD, even when considering the effect of age-adjusted disease length and severity (Nadir CD4). This finding implicates a potential biological mechanism linking reduced integrity of frontal white matter to the high prevalence of AUD in an HIV+ population without dementia or hepatitis C.
Keywords: Alcohol Use Disorder, HIV, tractography, white matter, neuroimaging
INTRODUCTION
Alcohol and HIV: Prevalence and Clinical Factors
The increased prevalence of alcohol and substance use disorders among individuals diagnosed with HIV is a well-documented phenomena (Galvan et al., 2002), with a relatively high number of individuals meeting criteria for alcoholism in comparison to the general population (Lefevre et al., 1995). Between one-third and two-thirds of individuals infected with HIV are diagnosed with an AUD at some point in their lifetime, a rate which is three times higher than that of the general population (Petry, 1999). One proposed mechanism for this association is that reduced treatment adherence associated with alcohol use is likely a strong factor that may contribute to the negative biological disease sequelae in HIV. This includes a number of negative behavioral consequences associated with alcohol abuse, such as reduced self-care and poor medication management (Meyerhoff, 2001), which are likely to interact with HIV-related impacts on the brain.
Alcohol Effects on the Brain
Alcohol use and HIV both have negative effects on brain volumes and characteristics, with many findings shared by the two disorders as well as independent phenomena associated with each (Rosenbloom et al., 2010). Alcohol use in general has been shown in structural MR studies to exhibit deleterious effects on numerous brain regions, including the frontal lobes (Fein et al., 2002; Jernigan et al., 1991; Kubota et al., 2001; Moselhy et al., 2001), as well as the thalamus (Harding et al., 2000; Mechtcheriakov et al., 2007). Increased functional MR responses in prefrontal and anterior thalamic regions have been demonstrated in those with alcohol use disorders (AUD) compared to those without AUD, suggesting involvement of these specific pathways involved in the regulation of emotion, attention, and satiety (George et al., 2001). Alcohol use has been shown in neuropathological studies to negatively affect the structural integrity of the largest white matter pathways of the brain in the general population (Wiggins et al., 1988), and there is also evidence of a specific interaction with advancing age (Sorg et al., 2015). Of particular interest, one diffusion MR study of middle-aged men found a number of frontally-projecting white matter regions to exhibit microstructural compromise in heavy drinkers compared to low or moderate drinkers (McEvoy et al., 2018).
HIV Effects on the Brain
HIV infection alone has also been linked to decreased neuronal integrity in the frontal cortex on neuropathological examination (Everall et al., 1991), including after progression to more severe infection (Ketzler et al., 1990). The association between the duration of HIV infection and neurodegeneration has been inconsistent, although there is increasing evidence that the expression and progression of neurodegenerative diseases may be facilitated by HIV (Brew et al., 2009). Duration of HIV infection has been shown to affect a number of white matter pathways, including the frontal projections (forceps minor) of the corpus callosum and the anterior thalamic radiation (ATR) (Zhu et al., 2013). The ATR is a white matter pathway which connects the mediodorsal thalamic nuclei to the frontal cortex (Kahle et al., 2003). It is part of a collection of frontal-subcortical circuits which are functionally involved in the inhibition and regulation of appetitive, attention, impulsive, and emotional responses and behaviors (Cummings, 1995). Due to its extensive interconnectivity with amygdalar, orbitofrontal, and dorsolateral prefrontal regions, it is behaviorally an important pathway for examination in a clinical population which struggles with addictive behaviors.
Combined Effects of HIV and Alcohol on the Brain
In addition to independent effects, there is evidence to suggest that comorbid HIV and alcohol use disorder has deleterious effects on the large white matter pathways of the brain (Pfefferbaum et al., 2007). Given that the specific neuropsychological impairments exhibited by individuals with both alcoholism (Chen et al., 2007; Lennane, 1988; Moselhy et al., 2001; Sullivan et al., 2003) and HIV (Sullivan, 2009) are often frontal-executive in nature, the white matter effects of comorbid disease have been hypothesized to extend into frontal white matter pathways of the brain (Rosenbloom et al., 2010). While the effects of disease duration on gray matter brain volumes (Cohen et al., 2010) and frontal white matter (Zhu et al., 2013) has been documented in MR studies, little attention has been given to the combinatory effect of alcohol use and HIV on microstructural changes to specific frontal white matter pathways. Additionally, our group has previously shown that frontal white matter integrity is reduced in hepatitis-C co-infection (Gongvatana et al., 2011), and exclusion of this population will be important for isolating specific HIV and alcohol related effects.
The ability to examine characteristics of white matter with diffusion tensor Magnetic Resonance Imaging (DTI) in-vivo is well-established (Basser and Pierpaoli, 1996; Le Bihan, 2006; Pierpaoli et al., 2001). The DTI metric mean diffusivity (MD) is a non-specific representation of tissue integrity as a magnitude of overall diffusion at a voxel, and fractional anisotropy (FA) reflects axonal coherence as measured by directionally restricted water diffusion (Le Bihan et al., 2001; Sundgren et al., 2004). Axial diffusivity (AD) is a DTI metric which represents diffusion of water parallel to a white matter tract within a voxel and is sensitive to axonal degeneration, while radial diffusivity (RD) represents the diffusion of water perpendicular to a white matter pathway and is sensitive to demyelination (Beaulieu, 2002; Song et al., 2003). The specific validity of diffusion measures such as AD and RD as indicators of experimentally-produced damage and demyelination has also been established in recent years (Mac Donald et al., 2007; Song et al., 2005; Sun et al., 2006). These studies show that experimentally-induced damage results in decreased axial diffusivity values in the acute phase, whereas studies of chronic and neurodegenerative diseases show an increase in axial diffusivity associated with disease factors. For example, initial decreases in AD are seen in the acute phase of neuronal injury (Mac Donald et al., 2007; Sun et al., 2006), while increased AD is observed in chronic diseases including HIV (Chen et al., 2009; Pfefferbaum et al., 2009; Zhu et al., 2013) as well as other neurodegenerative disorders such as Alzheimer’s and Huntington’s disease (Agosta et al., 2011; Della Nave et al., 2011; Metwalli et al., 2010; Rosas et al., 2010).
The current investigation was implemented to examine the contribution of comorbid disease to the microstructural integrity of frontal white matter pathways in a sample of HIV+ individuals without dementia or Hepatitis-C co-infection. We hypothesize that one or more frontal white matter pathways are negatively affected by the effects of alcohol use and HIV disease length when examining common diffusion metrics.
MATERIALS AND METHODS
Study Design
Twenty-eight men and nine women (N = 37) were recruited from the Brown University Center for Aids Research (CFAR) as part of an NIAAA-sponsored study of the effects of alcohol use in HIV (P01AA019072). The study was approved by the Institutional Review Boards for the Miriam Hospital and Brown University and informed consent was obtained from each participant before enrollment. Participants were recruited with the overarching goal of obtaining comparatively equal samples of non-drinkers, moderate drinkers, and heavy drinkers as determined by current use. For more details regarding participant recruitment and materials, please see our previously published work (Woods et al., 2016).
Study Cohort
Participants were excluded for a history of neurological or psychiatric illness that would impact cognitive functioning as well as a history of traumatic brain injury (TBI) with loss of consciousness >10 minutes. Those participants meeting inclusion criteria were assessed with MRI, a battery of neuropsychological tests, a number of self-report measures, a clinical interview, as well as blood and urine collection. Of the total 91 HIV+ participants meeting inclusion criteria as part of the larger R01 study, 31 participants did not receive a diffusion MRI scan due to time constraints leaving a total of 60 participants for analysis. Of these 60 participants, 20 participants were excluded based on a history of Hepatitis-C co-infection, as this has been shown to have confounding effects on interpretation of HIV-related findings (Gongvatana et al., 2011; Hinkin et al., 2008; Operskalski and Kovacs, 2011). Three additional participants were excluded from the remaining 40 participants using an in-house visual rating scale for the presence and frequency of hyperintense “noise” within the post-processed DWI data. This yielded a final sample of 37 viable participants who were receiving a steady course of anti-retroviral treatment at the time of the study, with a current CD4 count above the 200 cell count threshold typically used to identify individuals with AIDS. The infection length variable utilized in this study is defined as the number of years elapsed since HIV diagnosis. All participants had Mini-Mental Status Exam (MMSE) scores of 25 or higher.
Self-report Measures
For the purposes of this study, participants were noted as having a diagnosable AUD if (1) the structured clinical interview (SCID-I for DSM-IV-TR) indicated that they met the criteria for a lifetime alcohol use disorder, and (2) they met the NIAAA criteria for heavy drinking based on the 90-day Timeline Follow-Back (TLFB; Fals-Stewart et al., 2000). Participants with active substance dependence for opiates or cocaine based on this structured interview were excluded from participation. Participants were noted as having no AUD if they (1) did not meet criteria for a lifetime alcohol use disorder on the structured clinical interview, and (2) they did not meet the NIAA criteria for heavy drinking on the 90-day TLFB. Additional measures of self-report for the present study included the Kreek-McHugh-Schluger-Kellogg (KMSK) scale, which provides information regarding the duration, frequency, and quantity of use for common substances of abuse at the period of heaviest lifetime use (Kellogg et al., 2003).
Neuroimaging Data Acquisition and Pre-processing
All neuroimaging data were collected on a Siemens Trio 3T MRI at Brown University (Siemens Corporation, 2013). Whole-brain T1 images were acquired in 255 interleaved axial 1mm slices (TR=1.90s, TE=2.98s, FOV=2562 mm), and whole-brain diffusion tensor images were acquired in 69 interleaved axial 2mm slices (TR= 1.10s, TE=103ms, FOV=1282 mm). Structural T1 data were examined for imaging artifacts, and subsequently processed through the FreeSurfer automated pipeline (Fischl et al., 2002). Diffusion data were combined into a dataset containing 64 DWI (b=1000 mm/s2), one b0, and 10 b=5 mm/s2 volumes for a total of 75 volumes per scan. Raw data were denoised using the LPCA filter (Manjón et al., 2013), and preprocessed as in prior work by Nir et al. (Nir et al., 2017). DTI measures have been shown to be sensitive to the effects of head motion (Jones et al., 2013; Jones and Cercignani, 2010; Yendiki et al., 2014), however all data met or exceeded the minimum criteria set forth in clinical studies of head motion (Knaus et al., 2010; Shukla et al., 2011).
Diffusion Imaging Data Processing
Partially pre-processed diffusion data were then processed through the remaining TraCULA processing pipeline steps (Yendiki, 2011). The eight bilateral pathways projecting to the frontal regions of the brain were retained for analyses, which includes the anterior thalamic radiation, forceps minor of the corpus callosum, cingulate gyrus bundle of the cingulum, and the uncinate fasciculus. The resulting pathways were visually inspected for abnormalities, and exclusion of frontal pathways due to aberrant anatomical characteristics was not necessitated in this sample. Given that there are no biological or pathological mechanisms inherent to HIV that would induce unilateral damage to frontal white matter pathways and to restrict the possibility of Type II error, each of the frontal tracts provided by TraCULA were summed with their opposing hemisphere counterpart and averaged to obtain four bilateral pathways. These white matter pathways were reconstructed using the probability distributions of voxel-based fiber orientations along each tract, producing Axial Diffusivity (AD), Radial Diffusivity (RD), Fractional Anisotropy (FA), and Mean Diffusivity (MD) values for each pathway. In order to further restrict the possibility of Type II error and increase the strength of the findings, only the following TraCULA output variables were examined along the four pathways for the highest probability pathway only (99% probability of connection; Average Center): AD, RD, MD, and FA. This process serves to increase confidence that the pathways examined are indeed representative of true neuroanatomy and do not include potentially aberrant or inaccurate pathways produced by the probabilistic model.
Statistical Analyses
Diffusion metric values extracted from the tractography data were normalized with Winsorization and Blom transformation (Blom, 1958; Jones et al., 2017; Templeton, 2011), as described in previous research in this area (Jones et al., 2017). All other variables of interest met the requirements of normality specified by the GLM model and thus did not require statistical normalization. Statistical analyses were performed with SPSS Statistics v24.0 (IBM Corporation, 2016) in the form of independent samples t-tests, chi-squared tests, linear regression, and analysis of variance. Tables and statistical figures were created using R 3.3.3 statistics package and ggplot 2.2.1 (R Development Core Team, 2016). The AD, RD, MD, and FA diffusion metrics along the 99% probability path (Average Center) for each bilateral tract were examined in relationship to the clinical and demographic variables of interest in a multiple analysis of variance (MANOVA) with Bonferroni adjustment for multiple comparisons applied where appropriate.
RESULTS
Sample Characteristics
Chi-square analyses and independent samples t-tests of demographic variables across the sample of 37 individuals yielded no significant differences between AUD+ and AUD- participants when comparing by age, gender, education, and race. It is noted that although no participants had a current CD4 cell count below the threshold of 200 used to identify AIDS, 27% of participants had Nadir CD4 values below this cutoff at one point in their disease. Descriptive data for demographic variables are shown below (Table 1).
Table 1.
Sample Demographics
| Variable | Total Mean (SD) | AUD+ Mean (SD) | AUD- Mean (SD) |
|---|---|---|---|
| N = 37 | N = 25 | N = 12 | |
| Age | 47.2 (9.0) | 46.3 (7.5) | 48.3 (9.4) |
| Gender (%) | |||
| Male | 75.7 | 70.8 | 81.8 |
| Female | 24.3 | 29.2 | 18.2 |
| Race (%) | |||
| Caucasian | 73.0 | 75.0 | 71.0 |
| African-American | 13.5 | 12.5 | 14.5 |
| Other | 13.5 | 12.5 | 14.5 |
| Ethnicity (%) | |||
| Hispanic | 10.8 | 12.5 | 9.1 |
| Non-Hispanic | 89.2 | 87.5 | 90.9 |
| Educational Level (%) | |||
| Did not finish high school | 21.6 | 16.7 | 27.3 |
| High school | 19.5 | 20.8 | 18.2 |
| Greater than high school | 56.8 | 62.5 | 54.5 |
| HIV Infection Length (yrs) | 16.1 (8.0) | 16.2 (8.8) | 15.6 (6.4) |
| Current CD4 | 637.7 (293.1) | 688.3 (316.6) | 628.4 (275.2) |
| Nadir CD4 | 282.4 (179.7) | 300.5 (185.3) | 221.4 (130.4) |
| KMSK Alcohol Sum | 10.2 (3.0) | 11.4 (1.4) | 7.4 (4.3) |
Note: AUD = Alcohol Use Disorder; HIV = Human Immunodeficiency Virus; KMSK Sum= Kreek-McHugh-Schluger-Kellogg scale summed frequency, quantity, and duration of lifetime alcohol use
Within-groups Substance Use Relationships
When comparing all HIV+ participants by history of Alcohol Use Disorder with independent samples t-test, those with a history of AUD reported significantly higher total frequency, use, and duration of alcohol consumption on the KMSK (t [36] = 2.92, p < .05). Alcohol use reporting on the KMSK was not significantly associated with HIV disease characteristics, including age-adjusted infection length (r2 [36] = −0., p = 0.81) or Nadir CD4 (r2 [36] = −0.06, p = .65).
Bivariate Relationship between Demographic, Clinical, and Diffusion Metrics
Bivariate correlations between demographic, clinical, and diffusion-related metrics are presented below (Figure 1a). Given the significant positive association between age and infection length (r2 = 0.51, p=.002), the unstandardized residual representing the unique effect of infection length was extracted from a linear regression for use in MANOVA analyses. This new variable, henceforth referred to as the “age-adjusted infection length”, has a zero-correlation with age (r2 = 0.00, p=1.00) and a strong relationship with infection length (r2 = 0.86, p<.001), indicating that it did indeed represent the unique effect of infection length alone. When examining the entire group of HIV+ participants, there was no association between age, age-adjusted infection length, or Nadir CD4 with any of the examined diffusion metrics. There was a positive association of infection length with RD along the ATR in the full cohort, as well as a stronger positive association of these variables in the AUD+ group specifically. However, this effect appears to be strongly driven by age, as there is no association of ATR diffusivity with the age-adjusted infection length variable in the cohort or its sub-groups. In general, it is noted that many diffusion metrics for the pathways examined were highly intercorrelated with one another (Figure 1b). The demographic and clinical factors examined in this study did not have a significant relationship with diffusion metrics when using a threshold of p<.01 comparing AUD+ to AUD- participants. There was, however, a significant negative bivariate association between age and FA within the ATR for the AUD- group alone (Figure 1c).
Figure 1a.
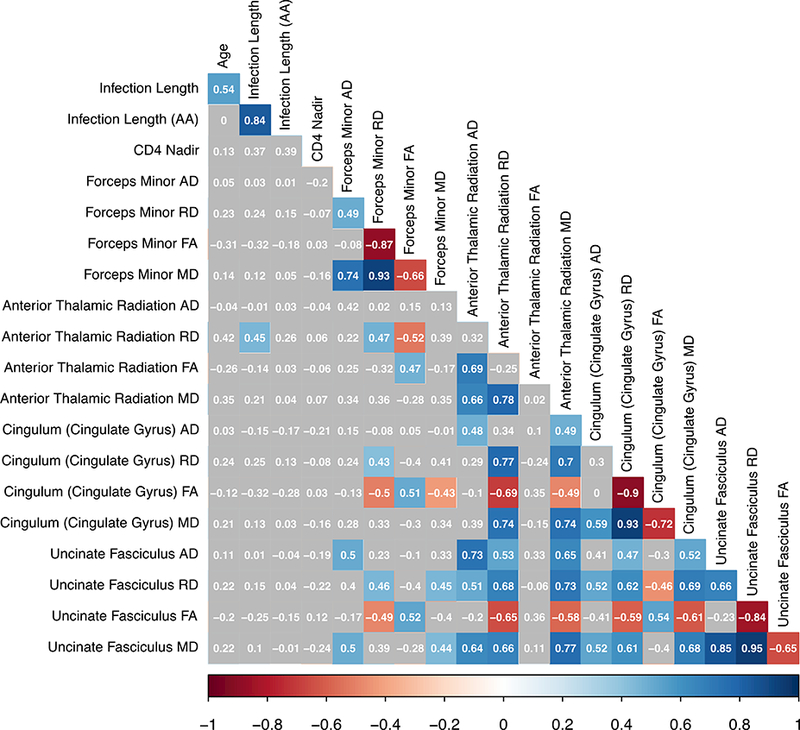
Bivariate correlations for all HIV+ participants (N=37). Note: colored boxes indicate correlation reached a significance level of p<.01.
Figure 1b.
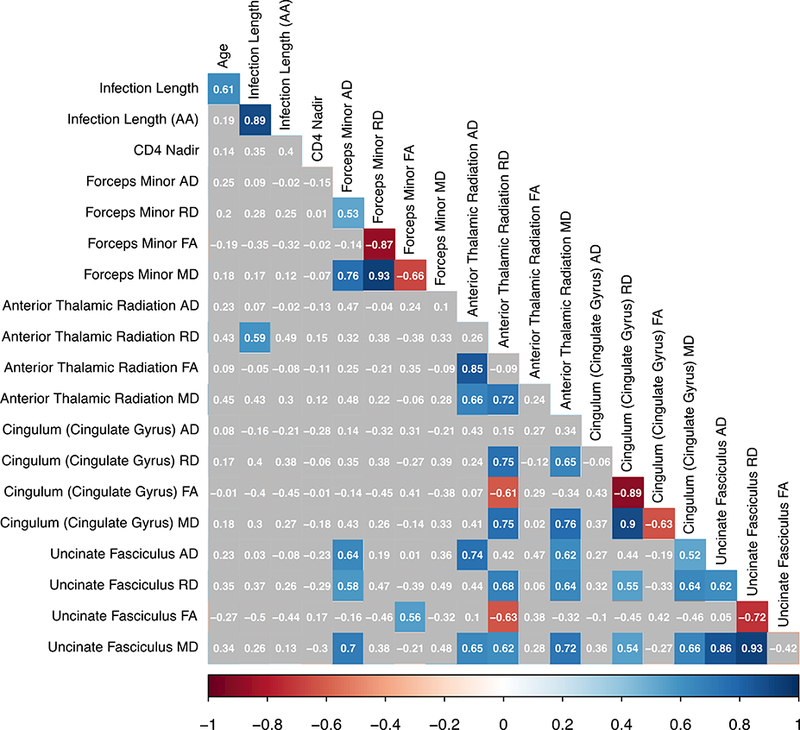
Bivariate correlations for AUD+ participants (N= 25). Note: colored boxes indicate correlation reached a significance level of p<.01.
Figure 1c.
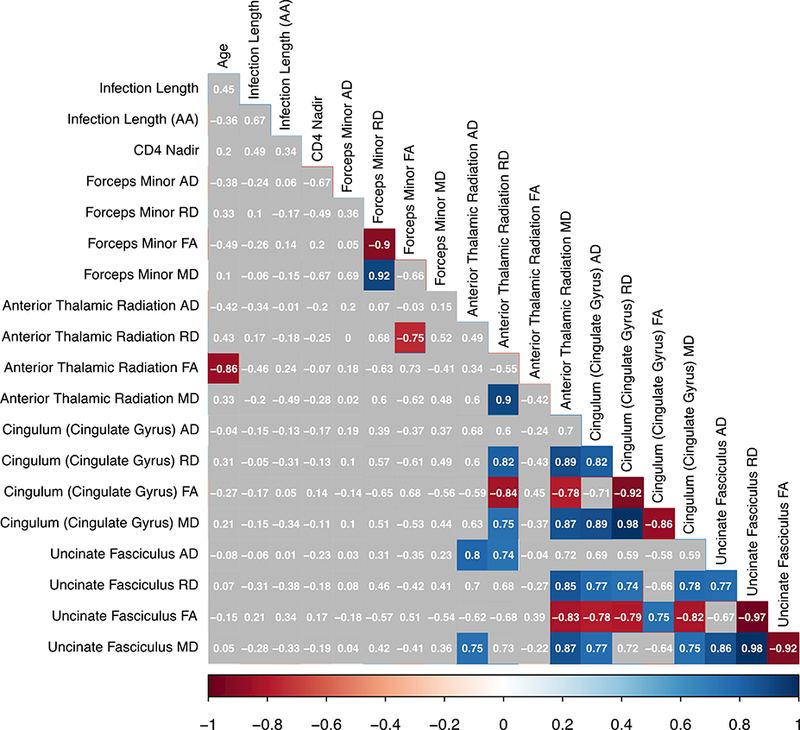
Bivariate correlations for AUD- participants (N = 12). Note: colored boxes indicate correlation reached a significance level of p<.01
AUD Group Comparisons of Diffusion Metrics
Group comparisons revealed that participants in the AUD+ group had significantly higher AD along the ATR pathway (M = 1.03e-3, SD = 5.5e-5) than the AUD- participants (M = 9.82e-4, SD =5.3e-5; Figure 2). A diagnosis of AUD explained 24.5% of the variance in ATR Axial Diffusivity and 15.0% of the variance in ATR Mean Diffusivity when accounting for differences in age, age-adjusted infection length, and Nadir CD4 (Table 2). In fact, a diagnosis of AUD explained 36.3% of the variance in all Anterior Thalamic Radiation diffusivity metrics. No effect of AUD was found for the remaining frontal white matter pathways and their respective diffusion metrics. Diffusion characteristics for each pathway comparing AUD+ and AUD- groups are displayed in Figure 2 below.
Figure 2.
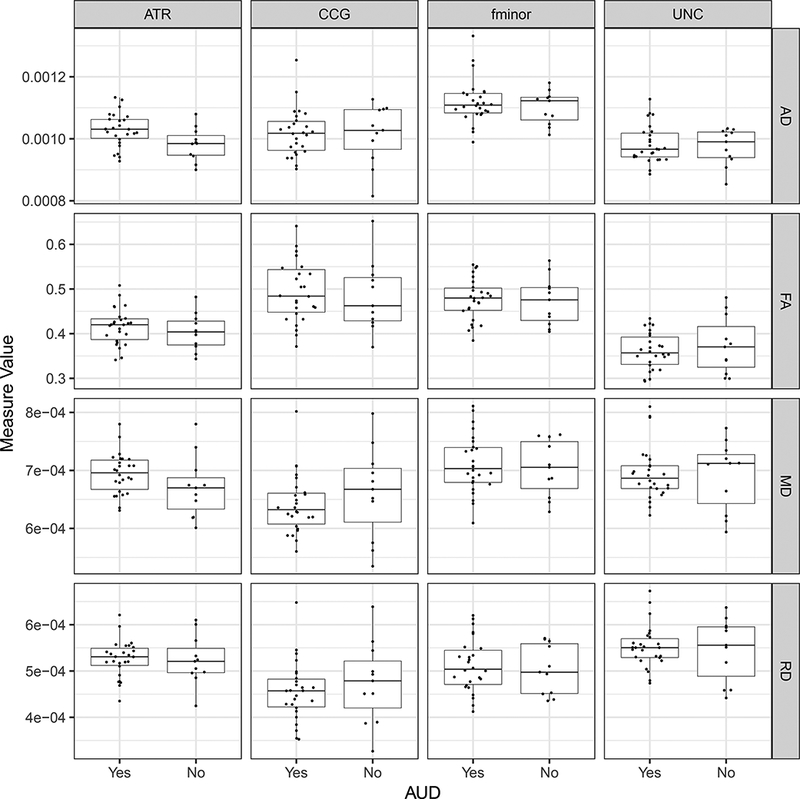
Box-notch plots displaying diffusion metrics for each frontal pathway by AUD group. Note: AUD: Alcohol Use Disorder; ATR: Anterior Thalamic Radiation; CCG: Cingulum (Cingulate Gyrus); fminor: Forceps Minor, Corpus Callosum; UNC: Uncinate Fasiculus; MD: Mean Diffusivity, FA: Fractional Anisotropy, RD: Radial Diffusivity, AD: Axial Diffusivity
Table 2.
MANOVA – Alcohol Use Disorder Diagnosis Predicting Frontal Diffusion Metrics (N = 37)
| Variable | AUD+ Mean (SD) N = 25 |
AUD- Mean (SD) N = 12 |
Wilks’ λ | F | df | p | Partial Eta Squared |
|---|---|---|---|---|---|---|---|
| Forceps Minor (Corpus Callosum) |
.889 | 0.75 | 4 | .570 | .111 | ||
| Axial Diffusivity | 1.11e-3 (7.3e-5) | 1.10e-3 (5.4e-5) | - | 1.70 | 1 | .203 | .002 |
| Radial Diffusivity | 5.12e-4 (5.8e-5) | 5.03e-4 (5.4e-5) | - | 0.75 | 1 | .393 | .027 |
| Fractional Anisotropy | 0.48 (0.05) | 0.47 (0.05) | - | 0.04 | 1 | .839 | .002 |
| Mean Diffusivity | 7.11e-4 (5.1e-5) | 7.0e-4 (4.8e-5) | - | 1.00 | 1 | .325 | .036 |
| Anterior Thalamic Radiation* | .637 | 3.42 | 4 | .024 | .363 | ||
| Axial Diffusivity** | 1.03e-3 (5.5e-5) | 9.82e-4 (5.3e-5) | - | 8.72 | 1 | .006 | .245 |
| Radial Diffusivity | 5.25e-4 (4.1e-5) | 5.23e-4 (5.3e-5) | - | 0.5 | 1 | .469 | .020 |
| Fractional Anisotropy | 0.42 (0.04) | 0.40 (0.04) | - | 1.03 | 1 | .319 | .037 |
| Mean Diffusivity* | 6.94e-4 (3.6e-5) | 6.71e-4 (5.3e-5) | - | 0.78 | 1 | .038 | .150 |
| Cingulum (Cingulate Gyrus) |
.740 | 2.11 | 4 | .110 | .260 | ||
| Axial Diffusivity | 1.02e-3 (7.7e-5) | 1.01e-3 (9.6e-5) | - | 1.24 | 1 | .276 | .044 |
| Radial Diffusivity | 4.57e-4 (6.6e-5) | 4.74e-4 (8.8e-5) | - | 0.02 | 1 | .904 | .001 |
| Fractional Anisotropy | 0.49 (0.07) | 0.48 (0.08) | - | 0.06 | 1 | .812 | .002 |
| Mean Diffusivity | 6.41e-4 (5.2e-5) | 6.61e-4 (8.0e-5) | - | 0.01 | 1 | .932 | .001 |
| Uncinate Fasciculus | .855 | 1.02 | 4 | .417 | .145 | ||
| Axial Diffusivity | 9.85e-4 (6.2e-5) | 9.74e-4 (5.9e-5) | - | 1.58 | 1 | .219 | .055 |
| Radial Diffusivity | 5.52e-4 (4.8e-5) | 5.46e-4 (6.8e-5) | - | 0.91 | 1 | .348 | .033 |
| Fractional Anisotropy | 0.36 (0.04) | 0.37 (0.06) | - | 0.96 | 1 | .335 | .035 |
| Mean Diffusivity | 6.96e-4 (4.7e-5) | 6.91e-4 (6.0e-5) | - | 1.12 | 1 | .299 | .040 |
Main effects of AUD group on diffusion metrics along the four frontal lobe pathways controlling for age, age-adjusted infection length, and Nadir CD4. Note:
indicates Bonferroni-adjusted significance level of p < .01 comparing AUD+ to AUD-;
indicates Bonferroni-adjusted significance level of p < .05 comparing AUD+ to AUD-; MD: Mean Diffusivity, FA: Fractional Anisotropy, RD: Radial Diffusivity, AD: Axial Diffusivity
Multivariate Relationship between HIV and Demographic Variables, Alcohol Use, and Diffusion Metrics
Bonferroni-corrected pairwise comparisons revealed significantly increased AD was prevalent along the ATR pathway in those HIV+ participants with a history of AUD compared to those without an AUD diagnosis (t[36] = 5.81, p < .01; Table 2). A MANOVA was conducted at a threshold of p<.01 with AUD diagnosis as a fixed factor, diffusion metrics of the frontal pathways as dependent variables, and age, age-adjusted infection length, and Nadir CD4 as covariates. The MANOVA was overall significant (F [4, 27] = 3.42, p = 0.024; Wilk’s Λ = .637, partial η2 = .363) and showed a univariate effect of AUD diagnosis on Axial Diffusivity of the ATR pathway (F = 8.72, p = 0.006; partial η2 = .245). A trending univariate effect of AUD diagnosis on Mean Diffusivity of the ATR pathway was also present (F = 4.78, p = .038; partial η2 = .150).
Follow-up voxel-wise MANOVA analysis of between-group AD values along the ATR revealed that 32% of voxels (16/50) in the bilateral pathway were significantly higher in the AUD+ group after controlling for infection length and Nadir CD4 with Bonferroni correction at a significance threshold of p<.01 to account for multiple comparisons (Figure 3).
Figure 3.
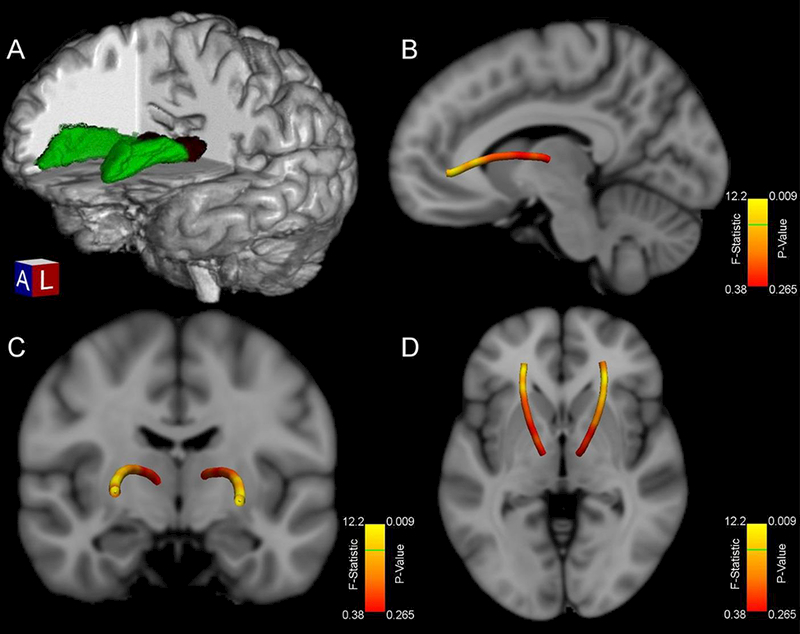
(A) 3-D depiction of a representative participant’s Anterior Thalamic Radiation pathway (green) originating from the thalamus (dark red) in native space (B-D) Anterior aspects of the ATR pathway show significantly higher Axial Diffusivity in AUD+ for 32% of voxels in the mean tract. Voxelwise f-statistic and p-values comparing participants’ Axial Diffusivity values by history of AUD after controlling for age-adjusted infection length and Nadir CD4 are displayed. Note: Green line on color bar indicates all values above that position are significant at p<.01 after Bonferroni correction.
DISCUSSION
The present study demonstrates alcohol-related microstructural compromise to frontal white matter in a non-demented HIV+ population without a history of hepatitis C who are currently receiving steady antiretroviral therapy. These results extend the past findings in this area and provide specific anatomical support to suggest that frontal white matter can be negatively affected in the least compromised HIV+ patients with alcohol use histories. Findings in the current study are provided in the context of a stringent neuroimaging and statistical approach in which only the statistically normalized data extracted from the pathways of 99% probabilistic certainty were examined at a significance threshold of p <.01. We believe this approach increases confidence in the observed relationships linking alcohol use in the context of HIV disease with axonal integrity compromise along a pathway functionally implicated in the suppression of risky decision-making behaviors.
The greatest degree of reduced axonal integrity as measured by axial diffusivity (AD) along the anterior thalamic radiation was seen in those with a history of alcohol use disorders. This significant positive relationship was observed mainly along the frontal portion rather than the thalamic portion of the pathway in a voxel-wise analysis (Figure 3), and has a number of potential implications. First, AD has been shown to reflect white matter axon integrity and increases in this metric suggest damage associated with chronic disease state mechanisms. The fact that the greatest association between white matter integrity and AUD was seen at the more anterior (frontal) portions of the ATR pathway suggests Wallerian degeneration of the pathways emanating from the thalamus, especially given that disruptions in axial diffusivity are thought to reflect loss of axonal integrity. The alcohol use literature to date suggests that frontal and thalamic white matter is particularly susceptible to damage with increasing heavy drinking, though this is the first study to demonstrate specific and selective damage to a functionally important pathway implicated in the maintenance of addictive and appetitive behaviors (Cummings, 1995).
As seen in Figure 2, MD was elevated for the AUD+ group; a difference which was not statistically significant likely due to the high variability within the AUD- group. There were trending associations for additional diffusion metrics including MD and FA within the ATR when comparing AUD+ to AUD-. Although the difference is less distinct and non-significant, there was generally higher FA observed in the AUD+ group in comparison to the AUD- group. The directionality of this finding is the opposite of that which one might expect, as the more diseased population typically has lower FA values in the region of interest. However, it is possible that heavy and/or chronic alcohol use results in disruption of crossing fibers within this region resulting in an over-inflation of mean FA. This phenomenon has been observed in neurodegenerative disease populations, such as Huntington’s and Alzheimer’s disease (Douaud et al., 2011, 2009). Future studies may reduce the occurrence of this finding by utilizing additional diffusion metrics which are more sensitive to multiple fiber orientations, such as Generalized Anisotropy (Cohen-Adad et al., 2008; Özarslan et al., 2005).
The strong association of age and increasing infection length was at least partially mitigated in the present study by removing the effect of age from infection length for further comparisons to diffusion characteristics. In our prior work, we found age-related reductions in white matter integrity (Seider et al., 2016; Zhu et al., 2013) while this association was not present in the current study. This difference is likely a result of cohort effects, given that our prior work recruited participants for their advanced age in the context of HIV while the present study recruited participants for their alcohol use histories. However, when taking the current findings into consideration within the context of aging, future investigations may warrant the analysis of an age and alcohol interaction effect on the integrity of the frontal white matter pathways implicated here.
Studies have shown that initial decreases in AD and RD are seen in the acute phase of neuronal injury (Mac Donald et al., 2007; Sun et al., 2006), but increases in these metrics are seen in chronic disease states including HIV (Chen et al., 2009; Pfefferbaum et al., 2009; Zhu et al., 2013) as well as other neurodegenerative disorders (Agosta et al., 2011; Della Nave et al., 2011; Metwalli et al., 2010; Rosas et al., 2010). The present findings suggest that alcohol use to the degree sufficient for a diagnosable AUD results in damage to at least one major frontal pathway (ATR), and trends for reduced integrity across the remaining frontal lobe pathways as a function of alcohol use. This introduces a potential circularity problem in that damage may result in difficulty inhibiting recurrent problematic drinking, given that studies show the function of ATR to be primarily one of inhibition of problematic social behaviors. It is also possible that white matter compromise along the ATR is a result of HIV-specific neurochemical processes or an inherent predisposition to deterioration only apparent in those individuals who reported using the highest amounts of alcohol. The potential link between white matter integrity of the ATR and comorbid HIV and AUD which may result in behavioral inhibition difficulties should be investigated in future studies in terms of neuropsychological, behavioral, and biological associations. Specifically, neuropsychological studies may focus on the association between reduced integrity of the ATR and executive functioning deficits as a behavioral manifestation of inhibitory control difficulties.
A number of white matter pathways have been associated with HIV disease and alcohol use in past studies (McEvoy et al., 2018; Pfefferbaum et al., 2009, 2007; Sorg et al., 2015; Zhu et al., 2013) that were investigated yet not significant in the present study, such as the corpus callosum. We believe this difference lies in the high degree of specificity utilized in the neuroimaging data analysis for the present study. This includes a restriction of participants to those without cognitive impairment or HCV co-infection, and a restriction of data analysis to the statistically-normalized, Bonferroni corrected data extracted from the 99% probability pathway thresholded to a significance level of p<.01.
With regard to HIV-related clinical factors, it is possible that negative behaviors associated with heavy alcohol use interferes with adherence to antiretroviral treatment, which in turn worsens the effects of HIV on the central nervous system by producing more frequent and severe periods of infection and CD4 level fluctuations. While the present study is limited in sample size and thus restricted in its ability to comment on a potential interaction, there is likely a strong interplay of alcohol use history, clinical HIV factors, and length of infection as it relates to frontal white matter integrity that should be investigated in larger samples.
Limitations:
The cross-sectional design of this study limits the ability to interpret the causal relationship of many of the findings in regard to degradation of white matter tracts as a function of disease. As such, it is not possible to determine whether the disease itself resulted in deterioration of the pathways leading to more problematic drinking, or whether problematic drinking which may predate the onset of the illness results in reduced integrity of these pathways further exacerbated by the illness. As discussed above, HIV+ individuals may be predisposed to develop both white matter deterioration and alcohol use disorders. To overcome this limitation, future studies should evaluate longitudinal changes in the white matter pathways as a function of drinking behaviors to determine potential causation in this regard. Limitations in regard to the neuroimaging method include the use of averaged diffusion characteristics over an entire pathway for initial investigation of associations with disease characteristics, although follow-up analyses were voxel-wise in nature and provided an increased degree of regional specificity. Lastly, due to a limited number of females in the AUD- cell (only two females), the present study is statistically limited in its ability to interpret meaningful differences as they relate to gender differences.
Acknowledgments
Supported by the National Institute on Alcohol Abuse and Alcoholism (P01AA019072)
Footnotes
Conflict of Interest: The authors of this manuscript declare that they have no conflict of interest.
REFERENCES
- Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, Filippi M (2011) White Matter Damage in Alzheimer Disease and Its Relationship to Gray Matter Atrophy. Radiology 258:853–863. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson—Ser B 111:209–219. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - A technical review. NMR Biomed. [DOI] [PubMed] [Google Scholar]
- Blom G (1958) Statistical Estimates and Transformed Beta Variables. New York, John Wiley. [Google Scholar]
- Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G (2009) Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. [DOI] [PubMed] [Google Scholar]
- Chen ACH, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, Chorlian DB, Stimus AT, Begleiter H (2007) Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res 31:156–165. [DOI] [PubMed] [Google Scholar]
- Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, Bullitt E, Shen D, Lin W (2009) White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage 47:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Adad J, Descoteaux M, Rossignol S, Hoge R (2008) Detection of multiple pathways in the spinal cord white matter using q-ball imaging. Biomed Imaging From Nano to Macro. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B (2010) Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 16:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL (1995) Anatomic and Behavioral Aspects of Frontal‐Subcortical Circuits. Ann N Y Acad Sci 769:1–14. [DOI] [PubMed] [Google Scholar]
- Della Nave R, Ginestroni A, Diciotti S, Salvatore E, Soricelli A, Mascalchi M (2011) Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich’s ataxia. Neuroradiology 53:367–372. [DOI] [PubMed] [Google Scholar]
- Douaud G, Behrens TE, Poupon C, Cointepas Y, Jbabdi S, Gaura V, Golestani N, Krystkowiak P, Verny C, Damier P, Bachoud-Lévi AC, Hantraye P, Remy P (2009) In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage 46:958–966. [DOI] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TEJ, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Kindlmann G, Matthews PM, Smith S (2011) DTI measures in crossing-fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage 55:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL (1991) Neuronal loss in the frontal cortex in HIV infection. Lancet (London, England) 337:1119–1121. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell T, Freitas T, McFarlin S, Rutigliano P (2000) The Timeline Followback Reports of Psychoactive Substance Use by Drug-Abusing Patients: Psychometric Properties. J Consult Clin Psychol 68:134–144. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ (2002) Cortical gray matter loss in treatment-naïve alcohol dependent individuals. Alcohol Clin Exp Res 26:558–64. [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M (2002) The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol 63:179–186. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ (2001) Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 58:345–52. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Cohen RA, Correia S, Devlin KN, Miles J, Kang H, Ombao H, Navia B, Laidlaw DH, Tashima KT (2011) Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 17:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J (2000) Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123:141–154. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SSA, Levine AJ, Barclay TR, Singer EJ (2008) Neurocognition in individuals co-infected with HIV and hepatitis C. J Addict Dis 27:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS (1991) Reduced Cerebral Grey Matter Observed in Alcoholics Using Magnetic Resonance Imaging. Alcohol Clin Exp Res 15:418–427. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M (2010) Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Jones JD, Kuhn T, Mahmood Z, Singer EJ, Hinkin CH, April D, Jones JD, Kuhn T, Hinkin CH, Thames AD (2017) Neuropsychology Longitudinal Intra-Individual Variability in Neuropsychological Performance Relates to White Matter Changes in HIV Longitudinal Intra-Individual Variability in Neuropsychological Performance Relates to White Matter Changes in HIV. Neuropsychology 11. [Google Scholar]
- Kahle W, Leonhardt H, Platzer W (2003) Color Atlas and Textbook of Human Anatomy In Three Volumes, Thieme flexibook. Thieme. [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ (2003) The Kreek-McHugh-Schluger-Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend 69:137–150. [DOI] [PubMed] [Google Scholar]
- Ketzler S, Weis S, Haug H, Budka H (1990) Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol 80:92–94. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, Tager-Flusberg H (2010) Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tensor MRI study. Brain Lang 112:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T (2001) Alcohol consumption and frontal lobe shrinkage: Study of 1432 non-alcoholic subjects. J Neurol Neurosurg Psychiatry 71:104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D (2006) Looking into the functional architecture of the brain with diffusion MRI. Int Congr Ser 1290:1–24. [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark C a, Pappata S, Molko N, Chabriat H (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546. [DOI] [PubMed] [Google Scholar]
- Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J (1995) Alcohol consumption among HIV-infected patients. J Gen Intern Med 10:458–460. [DOI] [PubMed] [Google Scholar]
- Lennane KJ (1988) Patients with Alcohol-Related Brain Damage: Therapy and Outcome. Aust Drug Alcohol Rev 7:89–92. [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D (2007) Diffusion Tensor Imaging Reliably Detects Experimental Traumatic Axonal Injury and Indicates Approximate Time of Injury. J Neurosci 27:11869–11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón JV, Coupé P, Concha L, Buades A, Collins DL, Robles M (2013) Diffusion Weighted Image Denoising Using Overcomplete Local PCA. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Elman JA, Eyler LT, Franz CE, Hagler DJ, Hatton SN, Lyons MJ, Panizzon MS, Dale AM, Kremen WS (2018) Alcohol intake and brain white matter in middle aged men: Microscopic and macroscopic differences. NeuroImage Clin 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J (2007) A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry 78:610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD (2010) Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 1348:156–164. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ (2001) Effects of alcohol and HIV infection on the central nervous system. Alcohol Res Heal J Natl Inst Alcohol Abus Alcohol 25:288–98. [PMC free article] [PubMed] [Google Scholar]
- Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36:357–368. [DOI] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Villalon-Reina JE, Isaev D, Zavaliangos-Petropulu A, Zhan L, Leow AD, Jack CR, Weiner MW, Thompson PM (2017) Fractional anisotropy derived from the diffusion tensor distribution function boosts power to detect Alzheimer’s disease deficits. Magn Reson Med 78:2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operskalski EA, Kovacs A (2011) HIV/HCV co-infection: Pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özarslan E, Vemuri BC, Mareci TH (2005) Generalized scalar measures for diffusion MRI using trace, variance, and entropy. Magn Reson Med 53:866–876. [DOI] [PubMed] [Google Scholar]
- Petry NM (1999) Alcohol use in HIV patients: what we don’t know may hurt us. Int J STD AIDS 10:561–570. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: Synergistic white matter damage. Brain 130:48–64. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV (2009) Frontostriatal fiber bundle compromise in HIV infection without dementia. Aids 23:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (2001) Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage 13:1174–1185. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2016) R: A Language and Environment for Statistical Computing. R Found Stat Comput Vienna Austria 0:{ISBN} 3–900051-07–0. [Google Scholar]
- Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, Fischl B, Pappu V, Onorato C, Cha JH, Salat DH, Hersch SM (2010) Altered white matter microstructure in the corpus callosum in Huntington’s disease: Implications for cortical “disconnection.” Neuroimage 49:2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan E V, Pfefferbaum A (2010) Focus on the brain: HIV infection and alcoholism: comorbidity effects on brain structure and function. Alcohol Res Health 33:247–57. [PMC free article] [PubMed] [Google Scholar]
- Sanford Ryan, Ances Beau M, Meyerhoff Dieter J, Price Richard W, Fuchs Dietmar, Zetterberg Henrik, Serena Spudich DLC (2018) Longitudinal Trajectories of Brain Volume and Cortical Thickness in Treated and Untreated Primary HIV Infection. Clin Infect Dis 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, Correia S, Tashima K, Cohen RA (2016) Age exacerbates HIV-associated white matter abnormalities. J Neurovirol 22:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller RA (2011) Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry Allied Discip 52:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140. [DOI] [PubMed] [Google Scholar]
- Sorg SF, Squeglia LM, Taylor MJ, Alhassoon OM, Delano-Wood LM, Grant I (2015) Effects of aging on frontal white matter microstructure in alcohol use disorder and associations with processing speed. J Stud Alcohol Drugs 76:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV (2009) Special section of neuropsychology review on HIV/NeuroAIDS. Neuropsychol Rev 19:143. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SHA, Pryor MR, De Rosa E, Pfefferbaum A (2003) Disruption of frontocerebellar circuitry and function in alcoholism In: Alcoholism: Clinical and Experimental Research, pp 301–309. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308. [DOI] [PubMed] [Google Scholar]
- Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R (2004) Diffusion tensor imaging of the brain: Review of clinical applications. Neuroradiology. [DOI] [PubMed] [Google Scholar]
- Szabo G (1999) Consequences of alcohol consumption on host defence. Alcohol Alcohol 34:830–841. [DOI] [PubMed] [Google Scholar]
- Templeton GF (2011) A two-step approach for transforming continuous variables to normal: Implications and recommendations for IS research. Commun Assoc Inf Syst 28:41–58. [Google Scholar]
- Wiggins RC, Gorman A, Rolsten C, Samorajski T, Bailinger WE, Freund G (1988) Effects of aging and alcohol on the biochemical composition of histologically normal human brain. Metab Brain Dis 3:67–80. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Porges EC, Bryant VE, Seider T, Gongvatana A, Kahler CW, de la Monte S, Monti PM, Cohen RA (2016) Current Heavy Alcohol Consumption is Associated with Greater Cognitive Impairment in Older Adults. Alcohol Clin Exp Res 40:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A (2011) Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B (2014) Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Zhong J, Hu R, Tivarus M, Ekholm S, Harezlak J, Ombao H, Navia B, Cohen R, Schifitto G (2013) Patterns of white matter injury in HIV infection after partial immune reconstitution: A DTI tract-based spatial statistics study. J Neurovirol 19:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


