Abstract
Background & Aims
Familial adenomatous polyposis is an autosomal dominant disorder characterized by development of hundreds of colorectal adenomas and eventually colorectal cancer. Oral administration of the spice curcumin has been followed by regression of polyps in patients with this disorder. We performed a double-blind, randomized trial to determine the safety and efficacy of curcumin in patients with familial adenomatous polyposis.
Methods
Our study comprised 44 patients with familial adenomatous polyposis (18 to 85 years old) who had either not undergone colectomy or had undergone colectomy with ileorectal anastomosis or ileal anal pouches and had 5 or more intestinal adenomatous polyps, enrolled in Puerto Rico or the United States from September 2011 through November 2016. Patients were randomly assigned (1:1) to groups given 100% pure curcumin (1500 mg orally, twice per day), or identical-appearing placebo capsules, for 12 months. The number and size of lower gastrointestinal tract polyps were evaluated every 4 months for 1 year. The primary outcome was the number of polyps in the curcumin and placebo groups at 12 months or at the time of withdrawal from the study according to the intention-to-treat principle.
Results
After 1 year of treatment the average rate of compliance was 83% in the curcumin group and 91% in the placebo group. After 12 weeks, there was no significant difference between the mean number of polyps in the placebo group (18.6; 95% CI, 9.3–27.8) vs the curcumin group (22.6; 95% CI, 12.1–33.1) (P=.58). We found no significant difference between mean polyp size in the curcumin group (2.3 mm; 95% CI, 1.8–2.8) vs the placebo group (2.1 mm; 95% CI, 1.5–2.7) (P=.76). Adverse events were few with no significant differences between the groups.
Conclusions
In a double-blind, randomized trial of patients with familial adenomatous polyposis, we found no difference in mean number or size of lower intestinal tract adenomas between groups of patients given 3000 mg/day curcumin vs placebo for 12 weeks. Clinicaltrials.gov no: NCT00641147
Keywords: FAP, herbal, cancer prevention, turmeric
INTRODUCTION
Familial adenomatous polyposis (FAP) is an autosomal dominant condition characterized by the development of hundreds of colorectal adenomas in teenagers and young adults (1,2). This condition is caused by a germline alteration in the APC gene on the long arm of chromosome 5 (3–6). Almost all patients with FAP will have colorectal cancer by the fifth decade of life if prophylactic colectomy is not performed (1).
The regression of intestinal adenomas in patients with familial adenomatous polyposis occurs with the use of some nonsteroidal anti-inflammatory drugs including sulindac and celecoxib (7). However, these compounds have a variety of side effects that can limit their long-term use (7).
Curcumin is the major yellow pigment extracted from turmeric, the powdered root of the herb Curcuma longa. This compound has long been used as a spice in Asia and is considered a safe food additive (8). Curcumin is a naturally occurring polyphenol composite with antioxidant and anti-inflammatory biological effects and can regulate numerous intracellular targets that control tumor progression (8). The regression of intestinal adenomas in five FAP patients was reported with the use of curcumin in combination with homeopathic doses of quercetin (9). However, no controlled trial of curcumin to reduce intestinal adenomas in patients has been reported.
METHODS
Study Population
The study was conducted from September 2011 to November 2016 at the Johns Hopkins Hospital and the University of Puerto Rico Medical Sciences Campus. The trial is registered at ClinicalTrials.gov (NCT00641147). Participants were identified and recruited from the Johns Hopkins Polyposis Registry or from the University of Puerto Rico Familial Colorectal Cancer Registry. Written informed consent was obtained from all participants. The protocol was approved by institutional review boards of the Johns Hopkins Hospital and the University of Puerto Rico Medical Sciences Campus.
Patients with FAP from 18 to 85 years old who had either not undergone colectomy or had undergone colectomy with ileorectal anastomosis or ileal anal pouches and had five or more intestinal adenomatous polyps were eligible for the study. The following were the reasons for exclusion from the study: absence of effective birth control (in women of childbearing age); pregnancy; a white-cell count of less than 3500 per ml; platelet count of less than 100,000 per ml; blood urea nitrogen level of more than 25 mg per deciliter (8.9 mmole per liter) or serum creatinine concentration of more than 1.5 mg per deciliter (132.6 umol per liter); malignancy; unwillingness to discontinue NSAIDs; active gastroesophageal reflux; history of peptic ulcer; active bacterial infection; use of warfarin or antiplatelet agents; or allergy to curcumin. There was a 3 month washout period for individuals taking NSAIDs, curcumin, turmeric, aspirin, calcium supplements, vitamin D, green tea, or polyphenol E supplements.
Study Design
The participants were entered into a double-blinded, placebo-controlled trial. Participants, care providers and data analysists were blinded. Participants were randomly assigned to receive 100% pure curcumin in three 500 mg capsules or identical-appearing placebo capsules orally two times a day for 12 months in consecutively numbered bottles. After the investigator had obtained consent from participants, the research nurse contacted the independent study pharmacist. The study pharmacist then dispensed either the curcumin or placebo capsules according to a computer-generated randomization list with a 1:1 allocation using a randomization block sizes of 4.2.4. The curcumin and placebo were generously supplied by the National Cancer Institute. Compliance with treatment was assessed by pill counts and telephone calls.
Lower intestinal adenomatous polyps were assessed by flexible video sigmoidoscopy. Two investigators (MC, FG), who did not review the records of previous examinations, made all the assessments. Evaluations were performed before treatment with curcumin or placebo was begun (month 0) and every 4 months after treatment was initiated for a total of 12 months. At each examination, the endoscopist counted the total number of polyps in the circumference of the colorectum from 20 cm to the anal verge or in the entirety of the ileal pouch, and the examination was recorded on videotape. The diameter of up to five polyps just distal to 20 cm was measured in millimeters with a graduated scale passed through the biopsy channel of the sigmoidoscope. These measurements were averaged to determine the mean size of each participant’s polyps. There were no changes to the methods after the trial commenced.
Evaluation of Safety
Adverse events were monitored by means of telephone every two to four weeks and at each four-month visit. A complete blood count was obtained and levels of glucose, blood urea nitrogen, serum creatinine, serum electrolytes, and bilirubin were measured at 0, 4 and 12 months. Adverse events were graded in accordance with the Common Toxicity Criteria of the National Cancer Institute (10). On this scale a score of 0 indicates no adverse event and a score of 5 a fatal event.
Statistical Analysis
The primary outcome variable was the number of polyps in the curcumin and placebo groups at 12 months or at the time of withdrawal from the study according to the intention-to-treat principle. The primary analysis was a two sample t-test comparing the final mean polyp numbers in the two arms of the study. Analysis of covariance (ANCOVA) was used to confirm the primary treatment arm comparison by adjusting for baseline polyp number. Final polyp size was similarly analyzed with a two sample t-test followed by ANCOVA.
Adverse events and increases in polyp burden were compared between study arms with Fisher’s exact or Chi-square tests.
Correlation between pre-and post-treatment polyp number was assessed with Pearson’s correlation coefficient. All p-values reported are two-sided. The sample size was calculated to provide the study with 80 percent power to detect a difference of 1 SD in the number of polyps between the groups using a two-sided alpha of 0.05 level t-test. There were no changes to the trial outcomes after the trial commenced. All authors had access to the study data and reviewed and approved the final manuscript
RESULTS
Demographic Characteristics
Sixty-two individuals were assessed for eligibility. Of these 10 individuals did not meet inclusion criteria and 8 declined to participate.
Forty-four eligible participants were randomly assigned between the two treatment groups. Twenty-one patients received curcumin and 23 received placebo. There were no significant differences in demographic characteristics between the two groups (Table 1). At 12 months (end of intervention trial), 6 participants in the curcumin and 4 in the placebo group had been withdrawn. In the curcumin arm, one participant apiece was withdrawn for development of pruritus, desmoid tumor, diagnosis of thyroid cancer, noncompliance, to have their colon removed prophylactically and increasing polyp number and size. In the placebo arm, one participant apiece was withdrawn for increasing polyp number and referred to surgery, inability to make visits, thyroid cancer, and personal reasons.
Table 1.
Demographic Characteristics of the Study Participants According to Treatment Group
| Characteristic | Curcumin | Placebo |
|---|---|---|
| N=21 | N=23 | |
| Age (yr) | 44.5± 15.4 | 38.7 ± 15.0 |
| Sex-no. (%) | ||
| Male | 7 (33) | 9 (39) |
| Female | 14 (67) | 14 (61) |
| Race-no. (%) | ||
| White | 10 (48) | 11 (48) |
| Hispanic | 11 (52) | 11 (48) |
| Black | 0 (0) | 1 (4) |
| Institution-no. (%) | ||
| Site 1 (JHH) | 11 (52) | 12 (52) |
| Site 2 (PR) | 10 (48) | 11 (48) |
| Surgical Status-no. (%) | ||
| Intact | 3 (14) | 4 (17) |
| Rectum (IRA) | 6 (29) | 12 (52) |
| Pouch (IAP) | 12 (57) | 7 (31) |
| Baseline no. of polyps | 23.3 ± 19.7 | 18.7 ± 13.1 |
| Baseline size of polyps (mm) | 3.1 ± 1.7 | 2.3 ± 0.6 |
JHH= Johns Hopkins site
PR= University of Puerto Rico site
IRA= Colectomy with ileorectal anastomosis
IAP= Protocolectomy with ileoanal pull through
Compliance and Adverse Events
The median (lower and upper quartile) rate of compliance (the proportion of total prescribed doses taken by a given participant) with treatment was 83 (70,94) percent among participants in the curcumin group and 91 (78,96) percent among those in the placebo group.
Treatment with curcumin for 12 months was well tolerated (Table 2). Few adverse events were reported, and the incidence of adverse events did not differ significantly between the curcumin and placebo groups. No perturbations of laboratory tests (complete blood count, glucose, blood urea nitrogen, serum creatinine, serum electrolytes, and bilirubin) were assessed to be related to the study agent. Six grade 2 or greater adverse events occurred in 4 participants taking curcumin and all were assessed as unrelated to the study agent. One participant experienced grade 1 pruritus with curcumin; this was the only adverse event evaluated as likely related to curcumin, and the participant was promptly withdrawn from the protocol.
Table 2.
Incidence and Severity of Adverse Events*
| Event | Curcumin | Placebo |
|---|---|---|
| N=21 (%) | N=23 (%) | |
| Abdominal pain | 3 (14)† | 3 (13) |
| Diarrhea | 2 (10) | 2 (9) |
| Pruritus | 1 (5)¶ | 0 |
| Thyroid cancer | 1 (5) | 1 (4) |
| Nausea | 0 | 2 (8) |
| Headache | 0 | 1 (4) |
| Sinusitis | 0 | 1 (4) |
| Cellulitis | 0 | 1 (4) |
| Herpes Zoster | 0 | 1 (4) |
| Facial abscess | 1 (5)‡ | 0 |
| Upper respiratory infection | 1 (5) | 0 |
| Influenza | 0 | 1 (4) |
| Urinary tract infection | 1 (5) | 0 |
| Chikungunya virus | 0 | 1 (4)‡ |
| Back strain | 1 (5)‡ | 0 |
| Plantar fasciitis | 0 | 1 (4) |
| Torn ligament | 0 | 1 (4) |
| Gout | 1 (5) | 0 |
| Depression | 0 | 1 (4)‡ |
| Back spasms | 0 | 1 (4) |
| Earache | 1 (5) | 0 |
| Vomiting | 0 | 1 (4) |
All adverse events were grade 1 (mild) unless specified below.
One participant had 3 separate episodes of abdominal pain from appendicitis (grade 3), cholecystitis (grade 2) and ovarian cyst (grade 2) and one participant had grade 3 abdominal pain from desmoid tumor – all events assessed as unrelated to curcumin
One participant with grade 1 pruritus assessed as likely related to curcumin
Grade 3 events assessed as unrelated to curcumin
Efficacy
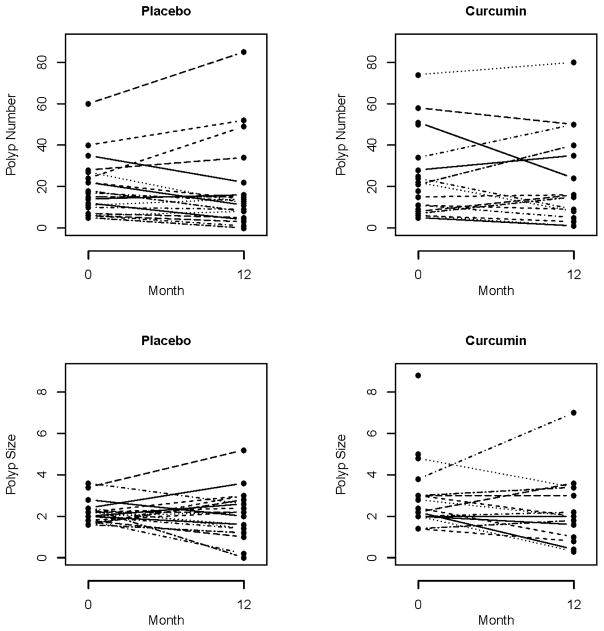
At the end of treatment, there was no significant difference between the mean number of polyps in the placebo group 18.6 (95% CI: 9.3, 27.8) compared to the curcumin group, 22.6 (95% CI: 12.1, 33.1), p=0.58 (Figure 1). Adjusting for baseline polyp number with ANCOVA provided a similar result. Also, the change in polyp number and percent change of polyp number from baseline were not significantly different between the treatment arms, p=0.85 and p=0.61 respectively (Table 3).
Figure 1.
Polyp number and size at time 0 and 12 months in the Placebo and Curcumin Group.
Table 3.
Polyp Number: Final, Mean Change from Baseline, and Percent Change from Baseline Between Placebo and Curcumin Treatment Groups.
| Polyp number | Placebo | Curcumin | Mean difference | 95% CI | P-value* |
|---|---|---|---|---|---|
| Final polyp number | 18.57 | 22.62 | −4.05 | −18.76, 10.66 | 0.58 |
| Change in polyp number from baseline | −0.48 | −1.19 | 0.71 | −6.98, 8.40 | 0.85 |
| Percent change in polyp number from baseline | −15.46 | −5.46 | −10.00 | −49.98, 29.98 | 0.61 |
by t-test.
At the end of treatment, there was no significant difference between polyp size in the curcumin group, 2.3 mm (95% CI: 1.8, 2.8) compared to the placebo group, 2.1 mm (95% CI: 1.5, 2.7), p=0.76 (Figure 1). A videotaped evaluation at 12 months revealed no significant difference in decrease in polyp burden between treatment arms (p=0.85).
No significance difference was noted between the treatment arms for the primary and secondary outcome variables when a per-protocol analysis was conducted, with adjustment for study site, compliance, or surgical type, or when nonparametric analysis was done using Wilcoxon tests.
DISCUSSION
Prevention or delay in development of lower and upper tract adenomas with long term administration of a chemopreventive agent would be a significant therapeutic advance in the management of familial adenomatous polyposis patients. Such a discovery could be extrapolated to chemoprevention of sporadic and other hereditary forms of colorectal cancer. Regression of intestinal adenomas in FAP patients has been demonstrated with several NSAIDs and in combination with erlotinib (11), but the side effects of these agents limit prolonged use (7). Curcumin, a naturally occurring polyphenol compound used as a food spice, is viewed as a potential cancer chemopreventive agent due to its effect on multiple tumor progression pathways. Moreover, the limited oral bioavailability of curcumin results in only case reports of side effects, and yet this agent could be efficacious through luminal activity.
In this randomized, double-blinded, placebo-controlled study, utilizing 3 grams orally of pure curcumin daily, few adverse events occurred. Only one participant with pruritus had an adverse event attributable to the study agent. However, the FAP patients in this protocol did not have regression of lower intestinal adenomas. No difference was seen in adenoma regression between treatment arms with the intention to treat analyses or per protocol analyses adjusting for institution, compliance, or surgical status. Of note, there were no significant differences in the baseline characteristics between the curcumin and placebo groups.
There are several potential reasons for the lack of efficacy of curcumin in this trial. First, compliance with the study agent could have limited efficacy. However, the curcumin group took 83% of scheduled doses and the analysis was adjusted for compliance. Second, participants in the curcumin group had non-statistically significant differences in surgical status and ethnic background, but analysis revealed no benefit of curcumin to any subgroup. Third, there was a small sample size at the 12 month time point and the negative results could be explained in the light of the power with which the study was able to identify a positive result. However, there also was no statistically significant differences in the primary outcome variable at earlier study time points as well.
Finally, a lack of sufficient curcumin dosing in this trial may be a principle factor resulting in the lack of regression of adenomas. The participants in this study were deliberately administered 100% pure curcumin. The intention of the protocol was to test the ability of this agent alone to regress adenomas. In the previous pilot investigation that showed polyp regression, patients took half the dose of curcumin but in a formulation which contained piperine. Piperine, a component of black pepper, is a known inhibitor of hepatic glucuronidation that increases the absorption of curcumin in humans by 2000% and is reported to promote detectable serum concentrations at curcumin doses of 2 grams a day (12).
Of note, a recent study reveals that familial adenomatous polyposis patients harbor colonic biofilms containing tumorigenic bacteria (13). In a murine model bacteria from these biofilms increased DNA damage in colonic epithelium and promoted faster tumor onset. One potential mechanism of curcumin chemoprevention may be directly altering the colonic microbiome. In mice, curcumin can increase bacterial richness, expand the native Lactobacilli and decrease the Coriobacteriales order. Similarly Bifidobacteria are also increased which appear to reduce aberrant crypt foci in models of murine colorectal carcinogenesis (14). Curcumin may also effect colonic bile acids, compounds associated with tumor promoting activity as evidenced in experimental studies in rodents and human epidemiologic data. Turmeric, of which curcumin is a component, can alter bile acid secretion and deconjugation through interplay with the microbiome (15). The interaction of chemopreventive agents with the microbiome is likely an important area of future research.
In summary, our results do not provide support for the use of pure curcumin at an oral dose of 3000 mg a day for regression of intestinal adenomas in FAP patients.
Acknowledgments
Supported in part by the John G Rangos Sr. Charitable Foundation; The Clayton Fund; and NIH grants CA P50 CA 62924-17, 1R01CA134620, U54CA096297, U54MD007587 and 1R01CA204345. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are indebted to Linda Welch for technical help. We thank Laura C. Wachter, M.A., R.Ph for pharmacy support.
Footnotes
Disclosures: The authors have no conflicts to disclose.
Author Contributions:
Cruz-Correa- A. B. C. D. E. F. G. H. I. K.; Hylind – A. B. C. D. E. F. G. H. I. K.; Hernandez-Marrero- D. F. G. H. K.; Zahurak- B. E. F. G. H.; Murray-Stewart- C. D. F. G. H. J; Casero-C. D. F. G. H. J.; Montgomery C. D. F. G. H. J.; Iacobuzio-Donahue- C. D. F. G. H. I. J.; Brosens-C. F. G. H. J.; Offerhaus- C. F. G. H. J.; Umar- C. F. H. J. K.; Rodriquez- C. F. H. J. K. Giardiello - A. B. C. D E. F. G. H. I. K
A: literature search, B: figures, C: study design, D: data collection, E: data analysis, F: data interpretation, G: writing, H: critical revision of the manuscript for important intellectual content, I: obtained funding; J: technical or material support; K: study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bussey HJR. Familial polyposis coli: family studies, histopathology, differential diagnosis, and results of treatment. Baltimore: Johns Hopkins University Press; 1975. [Google Scholar]
- 2.Giardiello FM, Burt RW, Jarvinen HJ, Offerhaus GJA. Familial adenomatous polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive Tract. Lyon France: IARC; 2010. [Google Scholar]
- 3.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 4.Kinzler KW, Nilbert MC, Su L-K, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 5.Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 6.Joslyn G, Carlson M, Thliveris A, et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Practice and Research Clinical Gastroenterology. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G. New insights into therapeutic activity and anticancer properties of curcumin. J Exp Pharmacol. 2017 Mar 31;9:31–45. doi: 10.2147/JEP.S70568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quescetin of adenomas in familial adenomatous polyposis. Clinical Gastroenterology and Hepatology. 2006;4:1035–8. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Common Terminology Criteria for Adverse Events (CTCAE) v4.0. https://evs.nci.nih.gov/ftp1/CTCAE/Documentation/CTCAE_Governance_2010-03-11.pdf. [PubMed]
- 11.Samadder NJ, Neklason DW, Boucher KM, Byrne KR4, Kanth P, Samowitz W, Jones D, Tavtigian SV, Done MW, Berry T, Jasperson K, Pappas L, Smith L, Sample D, Davis R, Topham MK, Lynch P, Strait E, McKinnon W, Burt RW, Kuwada SK. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: A randomized clinical trial. JAMA. 2016;315(12):1266–75. doi: 10.1001/jama.2016.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on The pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 13.Dejea C, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears C. Patient with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018:592–97. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFadden RT, Larmonier CB, Shehab KW, Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH, Caporaso G, Jobin C, Ghishan Fk, Kiela PR. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis. 2015;21:2483–2494. doi: 10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, Haque R, Ahmebn T, Gordon JI. Regulators of gut motility revealed by gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]