Abstract
Posterior Cortical Atrophy (PCA) is a neurodegenerative syndrome that typically presents with predominant visual and spatial impairments. The early diagnostic criteria specify a relative sparing of functioning in other cognitive domains, including executive functions, language, and episodic memory, yet little is known of the cognitive profile of PCA as the disease progresses. Studies of healthy adults and other posterior cortical lesion patients implicate posterior parietal and temporal regions in executive functions of working memory and verbal fluency, both of which may impact episodic memory. Relatively little has been reported about these cognitive functions in PCA, and to our knowledge there has not yet been a study of the impact of such deficits on memory function in PCA. We sought to examine PCA patients’ performance on tests of executive function and the associations to verbal episodic memory encoding, storage, and delayed recall. Nineteen individuals with PCA underwent neuropsychological and neuroimaging evaluations as part of a comprehensive clinical assessment. We developed a novel consensus rating method—the Neuropsychological Assessment Rating (NAR) scale—to grade the severity of test performance impairments in selected cognitive domains and subdomains. Hypothesis-driven analyses demonstrated relative deficits in working memory and lexical-semantic retrieval. Preliminary analyses suggested associations between both deficits and atrophy in the left-hemisphere inferior parietal lobule. These executive deficits were also associated with impairments in verbal encoding and delayed recall, but not with recognition discriminability. We conclude that deficits in verbal executive functions impact verbal episodic memory in PCA. Our findings also support theories emphasizing the role of the posterior parietal cortex in supporting executive and lexical-semantic contributions to verbal episodic memory.
Keywords: Cognitive impairment, Working memory, Verbal episodic memory, Neurodegeneration, Posterior parietal atrophy
1. Introduction
Posterior cortical atrophy (PCA) is a focal neurodegenerative syndrome that primarily affects the parietal cortex, with or without involvement of occipital or posterior temporal cortex. Although PCA may infrequently arise due to non-Alzheimer’s disease (AD) pathologies (Mitchell et al., 2016), it is more commonly thought of as an atypical or “visual variant” of AD (Borruat, 2013; Kaeser, Ghika, & Borruat, 2015; Levine, Lee, & Fisher, 1993). Patients with PCA typically present with early visual/spatial dysfunction due to neurodegeneration in these posterior cortical regions, and exhibit a variety of visual and non-visual signs referable to posterior cortices including diminished ability to identify or reach for objects, deficits in numeracy, literacy, and praxis, and other elements of Balint’s and Gerstmann’s syndromes as initially described by Benson and colleagues (Benson, Davis, & Snyder, 1988). Current diagnostic criteria propose that executive functions, language, episodic memory, and comportment/insight are relatively preserved in the earlier stages of illness, although PCA usually evolves toward a multi-domain cognitive-behavioral-motor dementia syndrome (Crutch et al., 2017). We are just beginning to develop a framework for understanding cognitive dysfunction outside of the visuospatial domain as PCA progresses.
In clinical neuropsychological or neurological assessment, executive dysfunction is commonly attributed to frontal lobes or frontostriatal circuit damage. Yet a growing body of cognitive and imaging neuroscience literature has built a compelling case for the existence of large-scale distributed brain networks that subserve executive functions, including not only frontal cortical and striatal regions but also lateral and medial parietal cortical regions (Corbetta & Shulman, 2002; Daffner & Willment, 2014; Dosenbach et al., 2006; Margulies et al., 2009; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). Specifically, key nodes of the dorsal attention network (DAN) and frontoparietal network (FPN) include regions of the superior and inferior parietal lobules (SPL and IPL), and intraparietal sulcus (IPS). The SPL and IPL have been implicated in directing responses to goal-appropriate stimuli via top-down control of attention (Corbetta & Shulman, 2002; Fox et al., 2005; Vincent et al., 2008), and strategic memory retrieval efforts (Cabeza, 2008), while regions of the IPL/IPS have also been specifically associated with supporting working memory tasks of “mental manipulation” (Champod & Petrides, 2007, 2010), a cognitive ability that plays a critical role in episodic memory encoding (Buchsbaum & D’Esposito, 2008; Wolk, Dickerson, & Alzheimer’s Disease Neuroimaging, 2011). Activity within the IPL has been associated more directly with successful learning when encoding tasks involve memory search and retrieval as an opportunity for deeper encoding (Karpicke and Roediger, 2008), as is the case with many verbal list learning tests. In addition, the central dorsal precuneus is viewed by some investigators as a key node in the frontoparietal executive control network based on its connectivity pattern (Margulies et al., 2009). These observations raise the possibility that, when lateral and medial parietal cortical regions undergo neurodegeneration in PCA, critical components of the large-scale networks subserving complex attention and executive function are likely to be affected, leading to deficits in these functions that, in turn, impact memory encoding and retrieval. In contrast, memory storage loss seen in temporolimbic amnesias, such as typical AD, would not be expected.
Circumscribed cognitive impairment outside of the visuospatial domain has been described previously in a few studies of patients with PCA. Recent work has called attention to the frequency of impairment on memory tests in patients who otherwise meet diagnostic criteria for PCA (Ahmed et al., 2016). In a single case study of a PCA patient, deficits in autobiographical memory were reported, and thought to be associated with hypoperfusion in the precuneus and parahippocampal gyrus (Gardini et al., 2011). Deficits in episodic memory were recently linked with atrophy and tau deposition in the lateral parietal cortex in some PCA patients (Bejanin et al., 2017), while executive function deficits specific to controlled lexical retrieval processes (e.g., verbal fluency tasks) and/or length-dependent auditory-verbal working memory (e.g., reverse digit span tasks) have also been described in the literature (Crutch, Lehmann, Warren, & Rohrer, 2013; Magnin et al., 2013; Mitchell et al., 2016). Consistent with these findings in PCA, studies of other posterior cortical lesion patients have also suggested involvement of posterior parietal and temporal regions in executive functions of working memory (Berryhill & Olson, 2008; Koenigs, Barbey, Postle, & Grafman, 2009) and verbal fluency (Abraham, Beudt, Ott, & Yves von Cramon, 2012).
To our knowledge there are no studies directly examining the association between executive dysfunction in PCA and episodic memory test performance. We believe this is a particularly important topic to clarify, since episodic memory in PCA is currently conceptualized as a relatively spared domain. Our clinical observations of patients reinforce this idea, since many PCA patients are capable of discussing richly detailed memories of their own recent life or of current events, in contrast to patients with amnesic syndromes. Yet many PCA patients struggle to perform formal verbal memory tasks in the clinic (e.g., list learning). We undertook the present study of patients in our PCA cohort to test the hypotheses that (1) verbal executive function deficits are a common feature of PCA and have a neuroanatomical basis in the lateral parietal cortex, and (2) these executive deficits impact performance on verbal encoding and delayed recall in episodic memory tasks, but do not impact the recognition discriminability of previously learned information. We tested these hypotheses by examining neuropsychological performance in subdomains of attention/executive function and language, relating this performance to cortical thickness, and finally, examining the relationship of executive functions to verbal memory stages of encoding, delayed recall, and recognition discriminability.
2. Methods
2.1 Participant Characteristics
Nineteen individuals (16 females; mean age of 63 years, age range of 52-81 years, all were Caucasian) were included in this study (Table 1). These patients were referred to the Massachusetts General Hospital (MGH) Posterior Cortical Atrophy program, a collaboration between the MGH Frontotemporal Disorders Unit and Psychology Assessment Center (PAC), between 2006 and 2016. All patients underwent clinical neuropsychological evaluation and structural brain imaging as part of their diagnostic work-up and were included in this study only if diagnostic consensus was reached by their neurologist (BCD) and neuropsychologist (JCS) based on prior diagnostic criteria for PCA (Renner et al., 2004; Tang-Wai et al., 2004). In the process of retrospective review for the current study, all participants also meet current diagnostic criteria (Crutch et al., 2017). These evaluations were performed as part of the patients’ clinical care prior to the development of our uniform PCA neuropsychological battery, and therefore these patients received varied neuropsychological test batteries. The average time since symptom onset was 4.1 years, with a range of 2 to 12 years. Most of these patients represented earlier stages of the disorder (14 of these 19 patients had symptom durations of 4 years or less). Individuals were excluded from analysis if they had a severe mental illness (e.g., major depressive disorder, bipolar disorder, schizophrenia), seizure disorder, major stroke, brain tumor, hydrocephalus, substance use disorder, multiple sclerosis, HIV-associated cognitive impairment, or acute encephalopathy. This work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The Partners HealthCare Human Research Committee Institutional Review Board in Boston, Massachusetts provided approval for the secondary data analysis reported here.
Table 1.
Demographic and clinical characteristics of the PCA sample.
| Demographic (N=19) | Mean (SD) |
|---|---|
| Age (years) | 63.1 (7.4) |
| Sex (Male: Female) | 3M:16F |
| Education (years) | 16.6 (2.1) |
| Handedness (RH:LH) | 18:1 |
| Duration of symptoms (years) | 4.1 (2.4) |
| MMSE (out of 30) | 21.4 (6.9) |
| CDR | 0.5 (N=13); 1 (N=6) |
| CDR SOB | 3.4 (1.4) |
RH = Right-Handed; LH = Left-Handed;
MMSE = Mini Mental State Examination;
CDR= Clinical Dementia Rating;
SOB= Sum of Boxes
2.2. Neuropsychological Assessment Rating (NAR) scale
We developed the Neuropsychological Assessment Rating (NAR) scale to accomplish the goal of allowing for clinical judgment, together with normative data, to contextualize the severity of impairment on neuropsychological test performance within conceptually distinct cognitive domains, recognizing that many tests draw on multiple domains. The NAR approach was designed to address issues inherent in studying a group of patients who receive varied neuropsychological batteries or non-standardized test administration as part of routine clinical evaluation. Rather than exclude non-standardized test administration, as prospective protocolled approaches require, the NAR scale allows for a clinically meaningful severity rating to be generated within every cognitive domain using available test data and pertinent behavioral observations obtained from neuropsychology report. NAR scores focus solely on neuropsychological test performance, and do not capture clinical symptom severity as reported by patients or family members. The purpose of generating NAR scores for this cohort was to quantify impairment in selected pertinent cognitive domains: Attention/Executive Functions, Language, Memory, and Visuospatial Functions. In the current study, Attention/Executive Function and Language scores were used as independent variables to investigate possible associations with traditional neuropsychological memory test scores described further in the next section.
Test data from clinical neuropsychological evaluations were first rated on the NAR scale independently by two neuropsychologists (BW and DP) and a neurologist (SMM) on the four cognitive domains detailed above. Each domain was further divided into component subdomains. Specifically, the Attention/Executive Function domain was further divided into subdomains of basic/sustained attention, working memory, lexical-phonological controlled retrieval (hereafter referred to as “controlled retrieval”), and reasoning/problem-solving. The Language domain included subdomains of syntax/grammar, lexical-semantic retrieval, auditory comprehension, and single word comprehension, in line with previously published descriptions (Sapolsky, Domoto-Reilly, & Dickerson, 2014). To account for the combined executive and language contributions to verbal fluency (Shao, Janse, Visser, & Meyer, 2014), raters utilized clinical judgment to determine whether impairments in verbal fluency were driven primarily by executive dysfunction (i.e., generalized controlled search/retrieval processes manifesting as lexical-phonological retrieval impairment) or primarily reflecting impairment in language-specific lexical-semantic retrieval. This judgment was based not only on the relative performance on letter and category fluency tasks, but also on other measures of executive function and language detailed in the Supplemental Materials. Further, the lexical-semantic retrieval subdomain also captured word retrieval deficits in spontaneous conversation as reported in the neuropsychology report. The Memory domain included subdomains of episodic memory, temporal orientation, and semantic memory. Lastly, the Visuospatial domain included subdomains of visual attention, visual construction, face/object/color processing, and reading. Raters utilized clinical judgment to determine whether reading impairments were driven by higher visual processing dysfunction as opposed to attention/executive, language, or memory dysfunction. Because all these patients demonstrated some form of visual dysfunction, many neuropsychological tests that are typically visually presented were either not administered in a standardized fashion or discontinued prematurely and, thus, were rated based on raw scores and descriptions of performance.
NAR scores were as follows: 0 = clinically normal, 0.5 = questionable/very mild impairment, 1= mild impairment, 2= moderate impairment, and 3= severe impairment. A rating of “9” was given if test data and observations were not deemed sufficient to rate a given domain with confidence and not included in global domain ratings; this occurred rarely. The most severely rated subdomain score within each cognitive domain was the overall domain severity score. NAR scoring guidelines for each cognitive domain and subdomain, including identification of domain-specific tests, are detailed in the Supplementary Materials. After independent rating, a consensus meeting was held, and each clinician’s independent ratings were discussed until consensus was reached for each patient. These consensus NAR scores were used in analysis. A one-sample analysis of variance (ANOVA) was conducted to determine the statistical significance between the different cognitive domain NAR scores (Figure 1), and between subdomain scores within each cognitive domain (Figure 3).
Figure 1. Neuropsychological assessment ratings (NAR) in PCA.
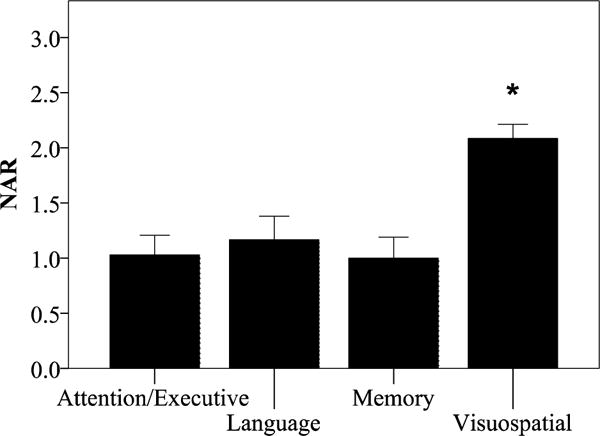
NAR scores demonstrate primary impairment on tests of Visuospatial functions, and relative sparing on tests of Attention/Executive Function, Language, and Memory. Group means of NAR scores ± 1 standard error of the mean are shown. F (3,15) = 15.4, p=10−6, partial eta2 = 0.75. * indicates statistical significance.
Figure 3. NAR scores indicate multi-domain cognitive deficits in PCA.
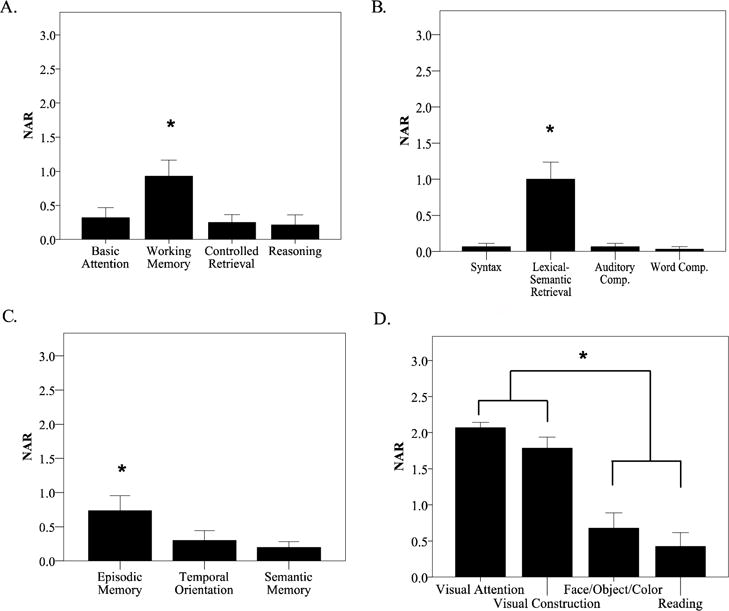
NAR scores in the (A) Attention/Executive Function, (B) Language domain (C) Memory domain, and (D) Visuospatial domains indicate relative cognitive impairment in working memory, word retrieval, and episodic memory, as well as a relatively greater impairment in dorsal stream functions of visual attention and visual construction, compared to ventral stream functions of face/object/color processing and reading. Group means of NAR scores ± 1 standard error of the mean are shown. * indicates statistical significance.
2.3 Memory test performance
In order to examine the cognitive contributions specific to the different stages of episodic memory (encoding, delayed recall, and recognition discriminability), performance on traditional neuropsychological memory tests were collected as continuous variables. Normative z-scores based on age, sex, and education were calculated from raw scores on each of these episodic memory stages across the three different verbal list learning tests patients received in the clinic: the California Verbal Learning Test, Second Edition (Delis, Kramer, Kaplan, & Ober, 2000), the California Verbal Learning Test, Second Edition- Short Form (Delis et al., 2000), and the Hopkins Verbal Learning Test- Revised (Brandt & Benedict, 2001). Encoding scores are reported as total number of words encoded across all learning trials. Delayed recall was reported as the number of words freely retrieved after a long (~10-20 minutes) delay. Recognition discriminability was defined as the number of target words correctly identified (hits) minus the number of false-positive errors.
2.4 Statistical analyses using NAR scores to predict memory test performance
Hierarchical linear regression analyses were conducted to investigate the association between NAR severity ratings and the three stages of verbal episodic memory. Guided from a priori hypotheses regarding contributions of working memory and verbal fluency to episodic memory, the NAR subdomain scores of Attention/Executive Function and Language domains were the independent variables, and z-scores of total encoding, delayed recall, and recognition discriminability were the dependent variables. All regression analyses controlled for age and education by entering them in Step 1 of the hierarchical linear regression, and the independent variables of interest were entered individually in Step 2. Post-hoc regression analyses were also conducted to investigate the association between performance on specific tests within each subdomain found to be a significant predictor of memory (e.g., digit span or verbal fluency tasks) and stages of episodic memory. For these analyses, as well as routine group comparison analyses described in results, p values <0.05 were considered statistically significant. Primary hypothesis-driven analyses were conducted with no corrections for multiple comparisons applied. Statistical analyses were run in IBM SPSS Version 24.0 (Armonk, NY).
2.3 Structural neuroimaging analysis
For the current study, we selected individuals who also received a structural T1-weighted scan at MGH. All scans were acquired using a Siemens Trio 3T or 1.5T scanner (Siemens Medical Systems). T1 image volumes were examined qualitatively by a cortical surface-based reconstruction and analysis of cortical thickness using FreeSurfer version 6.0 (http://surfer.nmr.mgh.harvard.edu). The general procedures for this processing method have been described in detail and applied and validated in a number of publications and presentations; the technical details can be found in select manuscripts (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl et al., 2002; Fischl et al., 2004). In order to visualize regions of cortical atrophy, a whole brain cortical thickness was contrasted (vertex-based t-test) between our PCA group and a group of age-matched healthy control participants (N= 56, mean age = 63.39 years) recruited for other studies. Results were thresholded at a significance level of p< 10−7 to illustrate areas of most prominent atrophy in PCA relative to controls.
To determine if NAR scores were related to atrophy in hypothesized posterior nodes of large-scale frontoparietal networks (i.e., SPL, IPL/IPS), statistical surface maps were generated by computing a general linear model for the effects of the cognitive performance variable of interest (NAR subdomain scores) on cortical thickness at each vertex point of the cortical surface model. This analysis was implemented in FreeSurfer version 6.0, using the mri-glmfit command with NAR scores as the independent variables of interest and cortical thickness as the dependent variable in the whole PCA cohort, as we have previously published (Dickerson et al., 2008). Given the relatively small number of patients and specific a priori hypotheses, an uncorrected statistical threshold of p < 0.01, one-tailed, was set for this preliminary analysis.
3. Results
3.1. Clinical characteristics of PCA sample
All PCA patients demonstrated: (1) primary visual and visuospatial impairment and relative sparing of anterograde memory, executive functions, and language skills based on history, neurological and neuropsychological examinations (Figure 1), and (2) evidence of posterior atrophy on structural imaging (Figure 2) consistent with the diagnostic criteria for PCA (Crutch et al., 2017). Means and standard deviations of NAR scores in each cognitive domain and subdomain are reported in Table 5 of the Supplementary Material. Globally, the PCA patients included in this study ranged from mild cognitive impairment to mild dementia (CDR either 0.5 or 1), with an average MMSE score of 23 out of 30. The scores within the individual CDR boxes highlight the presence of functional impairment with relative sparing of cognitive/behavioral domains, except, as expected, for orientation (Table 6 of the Supplementary Material). Duration of the patients’ illness (years since symptom onset) was not related to MMSE scores, or performance on any other neuropsychological measure (all p>0.2).
Figure 2. Cortical atrophy in PCA.
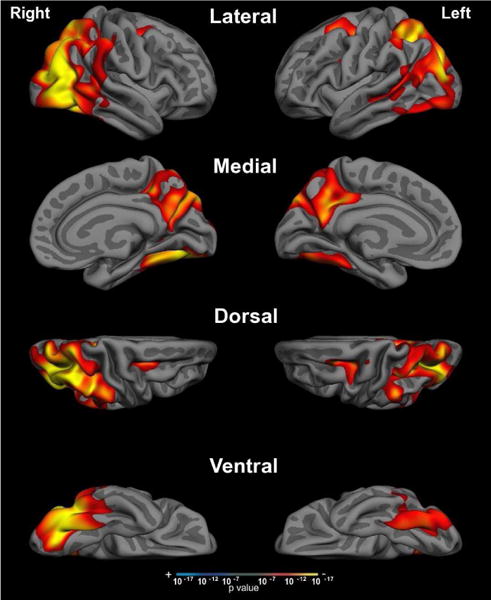
Compared to age-matched control participants, whole-brain cortical thickness analysis of PCA patients revealed significant bilateral occipital, lateral temporal, and parietal cortical atrophy with sparing of the medial temporal and frontal lobes. Threshold set at p <10−7.
3.2. Verbal executive function deficits in PCA
We observed a range of performance across tests of verbal executive functions and language, particularly in auditory-verbal working memory and lexical-semantic retrieval. Seventeen out of 19 patients (89%) were rated at some level of impairment (i.e., not “0” indicating absence of impairment or “9” indicating inability to rate) in the Attention/Executive Function domain. Fourteen out of 19 patients (74%) were likewise rated as impaired in the Language domain, and 15 out of 19 (79%) in the Memory domain. It should be noted that the vast majority of these impairments in non-visuospatial domains were at the level of “very mild” (0.5) or “mild” (1) impairment. As expected, all 19 patients (100%) were impaired in the Visuospatial domain, with the majority of ratings at either a “mild” (1) or “moderate” (2) level of impairment.
As a group within the Attention/Executive domain, working memory performance was rated as more impaired (NAR = 1.02 out of 3; F (1,13) = 13.3, p= 0.003, partial eta2 = 0.51)) than basic/sustained attention (0.4), controlled retrieval (0.4), and reasoning/problem solving (0.07; Figure 3A). Within the Language domain, lexical-semantic retrieval was rated as more impaired (1.2; F (1,14) = 15.6, p= 0.001, partial eta2 = 0.53) than syntax/grammar (0.06), auditory comprehension (0.1), and single word comprehension (0.02; Figure 3B). Of these Attention/Executive Function and Language subdomains, preliminary analysis revealed that auditory-verbal working memory and lexical-semantic retrieval were uniquely associated with atrophy in IPL/IPS (Figure 4). We did not see any significant pattern of association between our hypothesized regions of interest and NAR scores in any other subdomain. Within the Memory domain (Figure 3C; F (1,14) = 15.6, p< 0.001, partial eta2 = 0.53), we observed a relative deficit of verbal episodic memory rated as somewhat more impaired (0.78) than the other memory subdomains of temporal orientation (0.42) and semantic memory (0.2). Within the Visuospatial domain (Figure 3D; F (1,13) = 129.1, p< 0.001, partial eta2 = 0.91), dorsal visual stream functions of visual attention (2.03) and visual construction (1.61) were rated as more severely impaired relative to ventral stream functions of face/object/color processing (0.59) and reading (0.38).
Figure 4. Verbal working memory and lexical-semantic retrieval are associated with IPL/IPS atrophy in PCA.
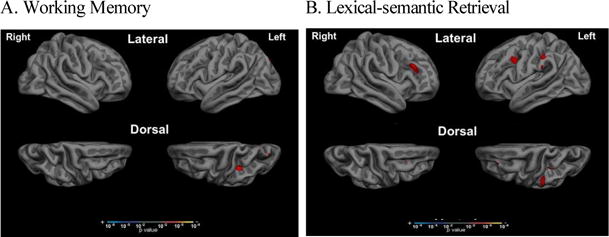
Whole cortical surface general linear modeling analysis revealed an association between atrophy in IPL/IPS and NAR scores of (A) working memory and (B) lexical-semantic retrieval within the group of PCA patients. A significance threshold was set at p<0.01 one-tailed, for this exploratory analysis.
3.3. Verbal episodic memory deficits in PCA
Performance on verbal list learning tests, our dependent variable of interest, reflected primary deficits in delayed recall (mean of z=−1.9) and total encoding (mean of z=−1.6), with relatively spared recognition discrimination (mean of z=−1.2; Figure 5; F (2,13) = 4.11, p=0.04, partial eta2 = 0.38). As a group, 65% of our sample performed more than 1.5 standard deviations below the normative mean on encoding or delayed recall tests, and 33% of our sample performed more than 1.5 standard deviations below the normative mean on recognition discriminability. Of note, performance on encoding and delayed recall were highly correlated in our sample (r=0.89, p=0.000001), both likely relying on similar strategic encoding/search processes. Recognition memory was also correlated with delayed recall (r=0.61, p=0.02) and encoding (r=0.52, p=0.05), though to a lesser extent.
Figure 5. Encoding and Delayed Recall are impaired in PCA.
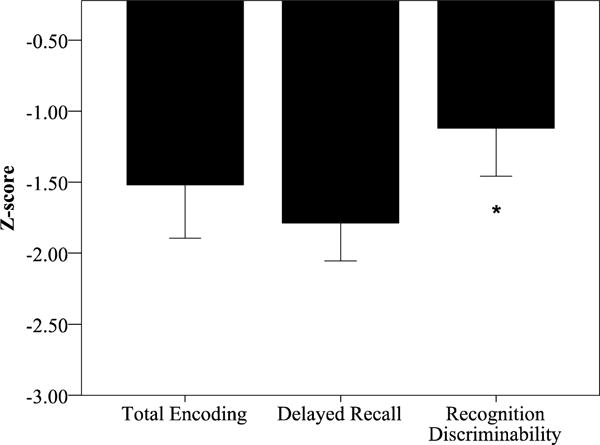
Verbal episodic memory performance (z-scores) indicate more severely impaired encoding and delayed recall, with relative sparing of recognition memory. Group means of z-scores ± 1 standard error of the mean are shown.
3.4. Verbal executive dysfunction and lexical-semantic retrieval predict encoding and delayed recall, but not recognition memory
After controlling for the effects of age and education, we found that NAR scores in executive function and language predicted encoding and delayed recall, but not recognition memory (Table 2). Specifically, NAR ratings of auditory-verbal working memory (beta = −0.84, p=0.001), controlled retrieval (beta = −0.60, p=0.03), and lexical-semantic retrieval (beta= −0.83, p<0.001) were all predictive of encoding performance. Similarly, auditory-verbal working memory (beta = −0.86, p=0.001), controlled retrieval (beta = −0.56, p=0.04), and lexical-semantic retrieval (beta = −0.77, p=0.003) were predictive of delayed recall performance. These subdomain NAR scores were not related to recognition discriminability scores (all p’s > 0.2). No other subdomains within Attention/Executive Function (basic/sustained attention, reasoning/problem-solving) and Language (syntax/grammar, auditory comprehension, and single word comprehension) were predictive of any stage of verbal episodic memory (all p’s >0.1). As a representative demonstration of these results, we show the association between performance on Memory Encoding (Figure 6, left y axis) Delayed Recall (Figure 6, right y axis) and performance on Digit Span Backward (Figure 6A) and Animal Fluency (Figure 6C), as well as the lack of association between performance on these tests and recognition discriminability (Figure 6B, 6D).
Table 2. Executive dysfunction predicts encoding and delayed recall, but not recognition memory.
Results of a hierarchical linear regression, controlling for age and education, showing predictors of performance on encoding (total learning), delayed recall, and retention across verbal list learning tests.
| Predictors of Encoding | Beta |
|---|---|
| Working Memory | −0.84* |
| Lexical-phonological controlled retrieval | −0.60* |
| Lexical-semantic retrieval | −0.83* |
|
| |
| Predictors of Delayed Recall | Beta |
|
| |
| Working Memory | −0.86* |
| Lexical-phonological controlled retrieval | −0.56* |
| Lexical-semantic retrieval | −0.77* |
|
| |
| Predictors of Recognition | Beta |
|
| |
| Working Memory | −0.36 |
| Lexical-phonological controlled retrieval | −0.28 |
| Lexical-semantic retrieval | −0.21 |
indicates statistical significance.
Figure 6. Digit Span Backward and Animal fluency performance are related to Encoding and Delayed recall, but not Recognition.
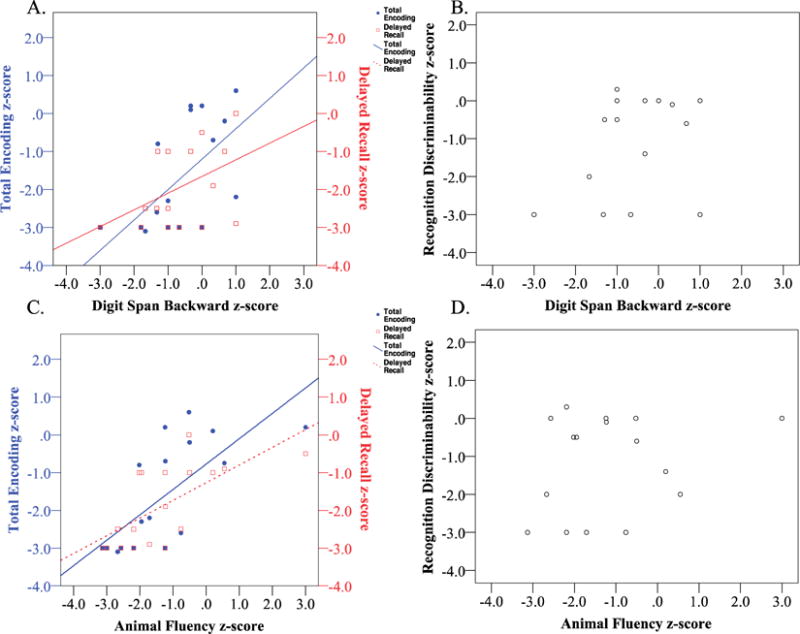
Digit Span Backward, one component of the working memory subdomain, is correlated with (A) Encoding (r= 0.59, p=0.01) and at a trend-level with Delayed Recall (r= 0.45, p= 0.07), but is not associated with (B) Recognition Discriminability (r= 0.3, p=0.2). Animal Fluency, one component of the lexical-semantic retrieval subdomain, is correlated with (A) Encoding (r = 0.71, p=0.001) and Delayed Recall (r = 0.67, p=0.002), but not to (B) Recognition Discriminability (r=0.2, p=0.4).
4. Discussion
Patients with PCA typically exhibit predominant cortical visual processing deficits with relative preservation of episodic memory, though a dominant hemisphere variant of PCA with less prominent visual symptoms has been described (Crutch et al., 2017). Though the diagnostic criteria refer to the cognitive profile at initial symptom onset, little is known about how the cognitive profile of PCA develops as disease progresses. Executive function, particularly in the verbal domain, has received little investigation in PCA. The purpose of this study was to test hypotheses generated from our clinical observations that patients with predominantly visual, biparietal, and ventral occipital variants of PCA (Crutch et al., 2017) frequently exhibit impairments in verbal executive function, and that these impairments contribute to difficulties on tests of verbal encoding and delayed recall. We focused on tests in the verbal modality to avoid confounding this investigation with the visual processing impairments these patients have. Because verbal working memory and goal-directed lexical retrieval skills are critical contributors to verbal episodic memory function, we hypothesized—and found— that relative deficits on working memory and lexical retrieval tasks would relate to impaired performance on encoding and delayed recall stages of verbal list learning tasks. In contrast, we found, as predicted, that these verbal executive deficits were not related to recognition memory performance.
Although much of the literature examining clinical deficits in PCA has focused on visual and visuospatial deficits, a few reports have included descriptions of working memory, executive function, and episodic memory deficits in this population (Ahmed et al., 2016; Crutch et al., 2013; Gardini et al., 2011; Migliaccio et al., 2012; Mitchell et al., 2016). Consistent with these reports, we observed a range of performance on verbal executive function and language tests, with some but not all patients demonstrating deficits relative to the normative population specifically in working memory, lexical-phonological controlled retrieval (e.g., initiation of responses, letter fluency), and lexical-semantic retrieval (e.g., naming, category fluency, conversational word-finding difficulty). Indeed, a “logopenic syndrome” has been described in PCA patients, and characterized by anomia, verbal fluency impairment, slowed speech rate, and length-dependent auditory-verbal working memory deficits (Crutch et al., 2013; Magnin et al., 2013; Mitchell et al., 2016). As hypothesized, these executive deficits in working memory and lexical retrieval were strongly related to impairments in verbal encoding and delayed recall, but not related to recognition memory. A diagnosis of PCA requires “relatively spared anterograde memory” (Crutch et al., 2017), and many patients are indeed able to report on richly detailed episodes from daily life and were scored on the NAR as relatively spared in comparison to visuospatial functioning. However, we confirm here what we have observed anecdotally, that while not all PCA patients demonstrate these deficits, many PCA patients have encoding/retrieval verbal memory impairments on list learning tests (65% in our sample) that are driven by verbal executive dysfunction. As expected, the retention of newly acquired information (recognition discriminability) was more often spared. In sum, we provide evidence here that deficits in episodic memory are specific to encoding and retrieval failures as have been documented in patients with executive dysfunction in other neurodegenerative syndromes (Cerciello, Isella, Proserpi, & Papagno, 2017; Consonni et al., 2017).
Although further study is necessary to understand the mechanisms of encoding and retrieval failure in PCA, previous work has suggested that successful performance of a supra-span verbal list learning task requires a combination of lexical-phonological processing (Buchsbaum & D’Esposito, 2008; Savill, Ellis, & Jefferies, 2017) and executive control skills including maintenance of attention on task-relevant stimuli (Cabeza, 2008), working memory skills of manipulation and strategic organization (Champod & Petrides, 2010; Longenecker et al., 2010), and goal-directed retrieval skills (Cabeza, 2008; Cabeza, Ciaramelli, & Moscovitch, 2012). Furthermore, auditory working memory and verbal list learning tasks may also rely on visualization/mental imagery strategies to enhance “deep encoding” (Hoshi et al., 2000) and support episodic retrieval (Baddeley, 1992; Greenberg & Rubin, 2003; Huijbers, Pennartz, Rubin, & Daselaar, 2011). Building on these findings, potential mechanisms of encoding failure to more directly investigate in the future include inefficient organization of information (e.g., serial versus semantic clustering) and working memory deficits in encoding serial position order. Potential mechanisms at the stage of retrieval may include impaired salience for retrieval goals, consistent with Cabeza’s Attention to Memory model (Cabeza et al., 2012), or deficits in visualization/imagery thought to guide retrieval success (Huijbers et al., 2011). Indeed, many of the items included on word list learning tests include a high proportion of concrete items (e.g., vegetables, tools, pieces of clothing), and as such visual imagery-based strategies can be commonly employed in encoding and retrieval of such information. Future investigation of the specific episodic memory processes that are relatively preserved in PCA compared to those that are impaired by the posterior cortical degeneration in these patients will add to our knowledge of the causal role of posterior cortices in memory mechanisms.
Anatomically, we observed atrophy in bilateral posterior parietal cortices, medial parietal regions including the precuneus and retrosplenial cortices, and lateral occipital and temporal cortex in our PCA cohort compared to age-matched control participants. We did not observe group-level atrophy in the medial temporal lobes, consistent with the relatively intact recognition memory performance observed in this group. Our investigation of associations between cortical atrophy in posterior nodes of large-scale neurocognitive networks and executive dysfunction revealed that impairment in verbal-auditory working memory tasks and lexical-semantic retrieval tasks specifically were correlated with atrophy in the left-hemisphere IPL/IPS. This region is an important posterior node in several large-scale neurocognitive networks (DAN, FPN, DMN), and may have special significance as a nidus of degeneration and pathological protein deposition in Alzheimer’s disease across typical and atypical phenotypes perhaps owing to its status as part of a “cortical hub” subject to high metabolic demands throughout the lifespan (Buckner et al., 2009; Warren, Fletcher, & Golden, 2012). This region has previously been linked extensively to working memory and executive dysfunction in the literature (Cabeza et al., 2012; Corbetta & Shulman, 2002; Fox et al., 2005; Vasconcelos et al., 2014; Vincent et al., 2008; Wolk et al., 2011).
Specifically, previous work in healthy young adults has highlighted the role of lateral parietal cortical regions in supporting working memory (Champod & Petrides, 2007, 2010) and goal-directed controlled retrieval processes (Cabeza, 2008; Cabeza et al., 2012), both critical contributors to episodic memory performance. Cortical atrophy of the lateral parietal cortices has been directly linked to executive dysfunction in healthy older adults (Dickerson et al., 2008), and typical AD patients (Dickerson, Wolk, & Alzheimer’s Disease Neuroimaging, 2011; Vasconcelos et al., 2014; Wolk, Dickerson, & Alzheimer’s Disease Neuroimaging, 2010). Some studies have gone further to directly link atrophy in the angular gyrus to poorer performance on the first trial of a word list learning test in mild AD (Wolk et al., 2010, 2011), suggesting that the first trial of encoding is particularly susceptible to working memory deficits arising from angular gyrus atrophy. Taken together, these findings suggest that encoding and retrieval aspects of verbal episodic memory may be selectively targeted in PCA secondary to executive and lexical retrieval deficits arising from posterior parietal and lateral temporal atrophy, in contrast to the storage loss more consistent with temporolimbic amnesias such as in typical AD. This dissociation between posteromedial and temporal networks supporting distinct contributions to memory which ultimately interact (e.g., “Posterior Medial Anterior Temporal” framework) has indeed been a topic of great interest in recent years (Ranganath & Ritchey, 2012; Ritchey, Libby, & Ranganath, 2015). Further work is needed to clarify the specific neuroanatomical substrates underlying the cognitive deficits reported in this study.
It is important to consider one possible limitation of this study, which is the wide range of illness severity included in our patient cohort. Though we attempted to homogenize the group as much as possible by including only patients who met specified consensus diagnostic criteria (Crutch et al., 2017) at the level of MCI or mild dementia (CDR 0.5 or 1), our patients still varied with regard to time since symptom onset (2 to 12 years, though symptom duration for most patients fell between 2 to 4 years) and with regard to global cognitive performance (MMSE ranged from 11 to 29; CDR SOB ranged from 1.5 to 6.5). We may expect the extent of parietal and temporal cortical involvement would likely impact the cognitive profile. Longitudinal assessment will ultimately be critical for determining when in the trajectory of PCA non-visual executive function deficits arise, and the specific mechanisms by which they give rise to encoding/retrieval impairments.
Clinically, this study highlights two important points: 1) as previous work by Champod and Petrides (Champod & Petrides, 2007, 2010) indicates, working memory and executive function deficits can arise from posterior parietal dysfunction, and thus should be considered to be indicative of frontoparietal systems dysfunction rather than a frontal lobe lesion; 2) we need to improve the methods by which we demonstrate relative preservation of memory since it is considered an element of not only the diagnostic criteria for PCA, but also Primary Progressive Aphasia and behavioral variant Frontotemporal Dementia, and thus should be given a clearer operational definition. Patients with these disorders highlight the need to continue to refine our armamentarium of tests of memory function, taking into account the variety of attentional, executive, language, and visual functions that contribute to the ability to remember the episodes of our lives. Furthermore, the CDR and NAR data from the patients in this cohort demonstrate the critical need for an integrative visual/spatial clinical rating of signs and symptoms; such an instrument will likely be a fundamental need for clinical therapeutic trials targeting the PCA population.
Supplementary Material
Acknowledgments
The authors thank the patients and families who participated in this research, without whose partnership this research would not have been possible.
Funding:
This research was supported by NIH grants R21 AG051987 and P01 AG005134 and by the David Mooney Family Fund for PCA Research. This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant number(s) S10RR021110, S10RR023043, S10RR023401.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: None
References
- Abraham A, Beudt S, Ott DV, Yves von Cramon D. Creative cognition and the brain: dissociations between frontal, parietal-temporal and basal ganglia groups. Brain Res. 2012;1482:55–70. doi: 10.1016/j.brainres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Baker I, Husain M, Thompson S, Kipps C, Hornberger M, Hodges JR, Butler CR. Memory Impairment at Initial Clinical Presentation in Posterior Cortical Atrophy. J Alzheimers Dis. 2016;52:1245–1250. doi: 10.3233/JAD-160018. [DOI] [PubMed] [Google Scholar]
- Baddeley A. What is Autobiographical Memory? Theoretical Perspectives on Autobiographical Memory. 1992:13–29. [Google Scholar]
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, Ayakta N, Cantwell A, Janabi M, Lauriola M, O’Neil JP, Gorno-Tempini ML, Miller ZA, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain. 2017 doi: 10.1093/brain/awx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia. 2008;46:1775–1786. doi: 10.1016/j.neuropsychologia.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruat FX. Posterior cortical atrophy: review of the recent literature. Curr Neurol Neurosci Rep. 2013;13:406. doi: 10.1007/s11910-013-0406-8. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test- Revised. Professional manual. Lutz, FL: Psychological Assessment Resouces, Inc.; 2001. [Google Scholar]
- Buchsbaum BR, D’Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerciello M, Isella V, Proserpi A, Papagno C. Assessment of free and cued recall in Alzheimer’s disease and vascular and frontotemporal dementia with 24-item Grober and Buschke test. Neurol Sci. 2017;38:115–122. doi: 10.1007/s10072-016-2722-7. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007;104:14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: a parametric functional magnetic resonance imaging study. J Neurosci. 2010;30:3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni M, Rossi S, Cerami C, Marcone A, Iannaccone S, Francesco Cappa S, Perani D. Executive dysfunction affects word list recall performance: Evidence from amyotrophic lateral sclerosis and other neurodegenerative diseases. J Neuropsychol. 2017;11:74–90. doi: 10.1111/jnp.12072. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013;84:460–466. doi: 10.1136/jnnp-2012-303309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, de Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suarez Gonzalez A, Tang-Wai DF, Yong KXX, Carrillo M, Fox NC, on behalf of the Alzheimer’s Association ISTAART Atypical Alzheimer’s Disease and Associated Syndromes Professional Interest Area Consensus classification of posterior cortical atrophy. Alzheimer’s & Dementia. 2017;13:870–884. doi: 10.1016/j.jalz.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Willment KC. Executive control, the regulation of goal-directed behaviors, and the impact of dementing illness. In: Dickerson BC, Atri A, editors. Dementia: Comprehensive principles and practice. Oxford University Press; 2014. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test: Adult version (CVLT-II): Manual, Second Edition. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA, Alzheimer’s Disease Neuroimaging, I Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82:45–51. doi: 10.1136/jnnp.2009.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardini S, Concari L, Pagliara S, Ghetti C, Venneri A, Caffarra P. Visuo-spatial imagery impairment in posterior cortical atrophy: a cognitive and SPECT study. Behav Neurol. 2011;24:123–132. doi: 10.3233/BEN-2011-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Oda I, Wada Y, Ito Y, Yutaka Y, Oda M, Ohta K, Yamada Y, Mamoru T. Visuospatial imagery is a fruitful strategy for the digit span backward task: a study with near-infrared optical tomography. Brain Res Cogn Brain Res. 2000;9:339–342. doi: 10.1016/s0926-6410(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Rubin DC, Daselaar SM. Imagery and retrieval of auditory and visual information: neural correlates of successful and unsuccessful performance. Neuropsychologia. 2011;49:1730–1740. doi: 10.1016/j.neuropsychologia.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Kaeser PF, Ghika J, Borruat FX. Visual signs and symptoms in patients with the visual variant of Alzheimer disease. BMC Ophthalmol. 2015;15:65. doi: 10.1186/s12886-015-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29:14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DN, Lee JM, Fisher CM. The visual variant of Alzheimer’s disease: a clinicopathologic case study. Neurology. 1993;43:305–313. doi: 10.1212/wnl.43.2.305. [DOI] [PubMed] [Google Scholar]
- Longenecker J, Kohn P, Liu S, Zoltick B, Weinberger DR, Elvevag B. Data-driven methodology illustrating mechanisms underlying word list recall: applications to clinical research. Neuropsychology. 2010;24:625–636. doi: 10.1037/a0019368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin E, Sylvestre G, Lenoir F, Dariel E, Bonnet L, Chopard G, Tio G, Hidalgo J, Ferreira S, Mertz C, Binetruy M, Chamard L, Haffen S, Ryff I, Laurent E, Moulin T, Vandel P, Rumbach L. Logopenic syndrome in posterior cortical atrophy. J Neurol. 2013;260:528–533. doi: 10.1007/s00415-012-6671-7. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Agosta F, Toba MN, Samri D, Corlier F, de Souza LC, Chupin M, Sharman M, Gorno-Tempini ML, Dubois B, Filippi M, Bartolomeo P. Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex. 2012;48:1298–1309. doi: 10.1016/j.cortex.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SB, Lucente D, Larvie M, Cobos MI, Frosch M, Dickerson BC. A 63-Year-Old Man With Progressive Visual Symptoms. JAMA Neurol. 2016;74:114–118. doi: 10.1001/jamaneurol.2016.2210. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63:1175–1180. doi: 10.1212/01.wnl.0000140290.80962.bf. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Libby LA, Ranganath C. Cortico-hippocampal systems involved in memory and cognition: the PMAT framework. Prog Brain Res. 2015;219:45–64. doi: 10.1016/bs.pbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Sapolsky D, Domoto-Reilly K, Dickerson BC. Use of the Progressive Aphasia Severity Scale (PASS) in monitoring speech and language status in PPA. Aphasiology. 2014;28:993–1003. doi: 10.1080/02687038.2014.931563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill N, Ellis AW, Jefferies E. Newly-acquired words are more phonologically robust in verbal short-term memory when they have associated semantic representations. Neuropsychologia. 2017;98:85–97. doi: 10.1016/j.neuropsychologia.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Vasconcelos LG, Jackowski AP, Oliveira MO, Ribeiro Flor YM, Souza AA, Bueno OF, Brucki SM. The thickness of posterior cortical areas is related to executive dysfunction in Alzheimer’s disease. Clinics. 2014;69:28–37. doi: 10.6061/clinics/2014(01)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8:451–464. doi: 10.1038/nrneurol.2012.135. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC, Alzheimer’s Disease Neuroimaging Initiative Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC, Alzheimer’s Disease Neuroimaging Initiative Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54:1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


