Abstract
Objectives
Previous trials have demonstrated the efficacy and durability of computer-based cognitive behavioral therapy (CBT4CBT) as an add-on to standard outpatient care in a range of treatment-seeking populations. Aims of the present trial were to evaluate the efficacy and safety of CBT4CBT as a virtual stand-alone treatment, delivered with only minimal clinical monitoring, and clinician-delivered cognitive behavioral therapy (CBT) compared with treatment as usual (TAU) in a heterogeneous sample of treatment-seeking outpatients.
Methods
Randomized clinical trial in which 137 individuals meeting current DSM-IV-R criteria for substance abuse or dependence, were randomized to TAU, weekly individual CBT or CBT4CBT with brief weekly monitoring.
Results
Rates of treatment exposure differed by group, with best retention in the CBT4CBT+monitoring group and poorest in clinician CBT. The primary hypotheses were supported, with those receiving either delivery method of CBT (clinician or computer) reducing their frequency of substance use significantly more than those assigned to TAU. Six-month follow-up outcomes indicated continuing benefit of CBT4CBT+monitoring over TAU, but not for clinician-delivered CBT over TAU. Analysis of secondary outcomes indicated best learning of cognitive and behavioral concepts, as well as satisfaction with treatment, in CBT4CBT.
Conclusions
This first trial of computerized CBT as a ‘virtual standalone’ delivered in a clinical setting to a diverse sample of individuals with current substance use disorders indicated that it was safe, effective, and durable relative to standard treatment approaches and well-liked by participants. Clinician-delivered CBT, while efficacious within treatment period, was unexpectedly associated with higher drop-out and diminished effects at follow-up.
Clinicaltrials.gov ID number NCT01442597
Introduction
Drug and alcohol use remain one of the most costly public health problems in the US (1). Limited availability, uptake, and fidelity of evidence-based treatment has led to increased interest in web-based interventions due to greater accessibility, standardization, and potential cost-savings (2). Meta-analyses suggest a significant but modest effect of these approaches at decreasing substance use in varied populations (3, 4). However, interpretation is complex due to the varied level of rigor in the trials included, with common limitations including weak comparison conditions (wait-list or assessment-only), inadequate treatment exposure, and low rates of follow up (5). Moreover, evaluation of unguided ‘stand-alone’ web-based interventions are often conducted with less severe populations (non-clinical populations, risky drinkers); rarely with well-specified, rigorous comparisons with validated clinician-delivered versions of the same treatment (6).
We previously reported on the efficacy, durability, and cost-effectiveness of ‘Computer Based Training for Cognitive Behavioral Therapy’ (CBT4CBT), as an add-on to standard treatment for substance use in outpatient and methadone maintenance settings (7–10). However, these trials did not address the efficacy of CBT4CBT alone, an important step in establishing its efficacy and utility in the health care system. Herein we describe primary outcomes from a randomized clinical trial evaluating CBT4CBT as a virtual stand-alone as well as clinician-delivered CBT, each compared with standard outpatient treatment for a heterogeneous group of individuals seeking treatment for substance use disorders. The primary hypothesis was that individuals assigned to either form of CBT (clinician-delivered or CBT4CBT) would reduce their substance use relative to standard treatment. Based on previous work (8, 10, 11), we also hypothesized that the effects of either form of CBT would be durable relative to TAU through a six-month follow-up.
Methods
Participants
Participants were recruited from individuals seeking treatment at the Substance Abuse Treatment Unit of the Connecticut Mental Health Center in New Haven CT, between January 2012 and October 2016. Participants were English-speaking adults who met DSM-IV-R criteria for current (past 30 days) cocaine, marijuana, opioid or alcohol abuse or dependence. Exclusion criteria were minimized to facilitate recruitment of a broad and clinically representative outpatient sample; thus, individuals were excluded only if they (1) had an untreated or unstable psychotic disorder or had current suicidal/homicidal ideation, (2) could not read at a 6th grade level or (3) had a legal case pending resulting in inability to commit to 12 weeks of treatment.
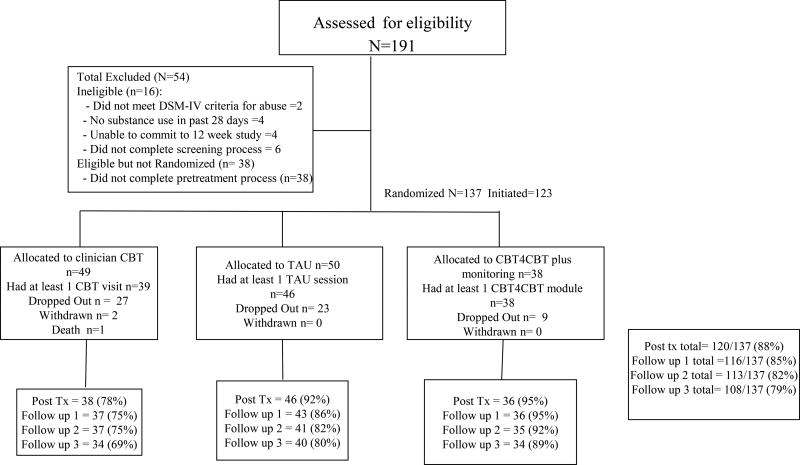
As shown in the CONSORT diagram (Figure 1), 137 of the 191 individuals screened were eligible for the study. Following provision of written informed consent approved by the Yale University Human Investigations Committee, participants were randomized in equal proportion to one of the three conditions described below, using a computerized urn randomization program (12) to balance treatment groups with respect to gender, ethnicity (minority, non-minority), education level (less than high school, high school graduate), primary drug (cocaine, marijuana, other), self-reported familiarity with computers (yes/no), and referral through the criminal justice system (yes/no).
Figure 1.
CONSORT diagram, flow of participants through study
Treatments
Standard treatment-as-usual (TAU)
Participants were offered standard treatment at the clinic, which consisted of weekly group and/or individual therapy as determined by the clinical team. TAU was implemented by 22 members of the clinic staff (14 female, 8 male; 4 had doctoral degrees, 14 had masters degrees, and 4 had bachelor’s degrees). Topics discussed at each group or individual session were recorded by the clinicians immediately after each session; the most frequent topics reported were motivational interviewing (n=91), life skills (n=60), relapse prevention (n=22), harm reduction (n=6), mindfulness (n=6), or women and trauma, health and recovery, or Latino recovery (3 each). Participants in this and all conditions were offered standard ancillary services as needed, which included psychiatric, pharmacologic, and emergency services.
Clinician-delivered CBT
Participants assigned to this condition were offered 12 weekly, individual sessions of manual-guided CBT (13), delivered by 15 PhD-level clinicians or predoctoral level fellows (6 male, 9 female) who were trained via a didactic seminar and supervised training case as described in previous CBT trials (11, 14). All CBT sessions were recorded; 104 (52%) were rated using a validated adherence/competence monitoring tool (15), and ongoing feedback was provided to clinicians by an expert supervisor. Ratings indicated high adherence and competence; the mean adherence score (rating ranged from 1=did not occur to 7=covered extensively and in great depth) for the six core CBT items (functional analysis, coping skills training, reviewing practice exercises, explaining CBT concepts, assigning homework and agenda setting) was above 3 for all items and the mean quality score (where 1=very poor and 7=outstanding) was above 4 for all six items.
CBT4CBT plus monitoring
In this condition, participants were asked to complete one CBT4CBT module each week as their principal form of treatment, in conjunction with brief (~10 minute) in-person weekly clinical monitoring provided by a doctoral-level clinician. Monitoring sessions were manual guided (16) and followed guidelines for low-intensity interventions used in previous placebo-controlled trials (17, 18) and trials of internet-delivered treatment (19). These were intended to evaluate participants’ current functional status and safety, and review the participants’ use of the CBT4CBT program. Three clinicians conducted the monitoring sessions (1 male, 2 female; 2 PhD and 1 predoctoral fellow). As described previously (7, 10), participants accessed CBT4CBT through an ID/password system. The program contains seven core CBT skill topics (‘modules’) that include on-screen narration, graphic animation, quizzes and other interactive exercises to teach and model effective use of skills. Each module presents videos demonstrating use of a targeted CBT skill and concludes with printable take-home practice exercises (‘homework’).
Assessments
Participants were assessed before treatment, weekly during treatment, at the 12-week treatment termination point, and 1, 3, and 6 months after the termination point. Participants were administered the Structured Clinical Interview for DSM-IV-R (SCID) (20) prior to randomization to establish substance use and psychiatric diagnoses; the substance use section was re-administered at treatment termination to assess changes in rates of meeting diagnostic threshold over time (21). The Substance Use Calendar, similar to the Timeline Follow Back (22, 23), was administered weekly during treatment to collect day-by-day self-reports of drug and alcohol use for the 28-day period prior to randomization, as well as at each follow up interview. Self-reports of drug use were verified through urine toxicology screens that were obtained at every assessment visit. Breathalyzer samples were also collected at each visit. Participants were compensated for each assessment visits in gift cards ranging in value from $10 (weekly assessments) to $75 (final follow-up if all follow-up interviews were completed on time. Participants could earn up to $285 in gift cards if all interviews were completed.
Correspondence of self-reports of recent drug use and results from urine toxicology screens was excellent, but varied by drug type. Of 1378 urine samples collected during treatment (mean 10.2 per participant): 6.8% (n=94) indicated cocaine use when the participant denied recent use; 1.9% indicated opioid use when the participant denied recent use; 2.8% indicated benzodiazepine use when the participant denied recent use; and 10.5% indicated marijuana use when the participant denied use in the past 7–10 days, reflecting the longer half-life of cannabis and its detectability in urine. This rate is consistent with previous trials of marijuana-using individuals in this setting, where rates of discrepancy have been 13% (24) and 16% (25).
Data analyses
Power estimations were based on effect sizes of previous studies of CBT4CBT (26, 27) and clinician CBT (14), resulting in a target of 50 per condition (28). The primary outcome measure was change in self-reported frequency of substance use (operationalized as frequency of any drug or alcohol use by week from baseline through week 12), evaluated using random effects regression analyses, in SPSS Statistics 24 with a simple linear model and a single random intercept, and two contrasts testing the primary hypotheses (Contrast 1: clinician-delivered CBT versus TAU, Contrast 2: CBT4CBT plus monitoring versus TAU) for the 137 participants randomized to treatment. Primary drug (cocaine, marijuana or alcohol) was included as a cluster variable to account for different patterns of use associated with different drug types (e.g. regular daily use of marijuana or alcohol versus binge patterns for cocaine (30). Time was log transformed to account for the expectation of greater change early in treatment.
The six-month follow-up data were analyzed using the same contrasts, with piecewise random regression (31) to evaluate change from baseline through the 6-month follow-up by month and phase (within treatment versus follow-up). Analyses were repeated with the treatment-exposed sample (N=123) as well as those with adequate exposure to treatment (N=81). Results consistently paralleled the intent-to-treat analyses.
Because of the planned heterogeneity in drug and alcohol use in the sample, varying periods of detectability of different substances through urine monitoring (32, 33), and greater sensitivity to missing data (34), results of urine toxicology screens were secondary outcomes and analyzed via ANOVA models with the same contrasts as above; missing data were not imputed. Indicators of clinical significance (percentage of individuals who submitted urine specimens free of all drugs in the last two weeks of treatment, percentage who no longer met diagnostic threshold for abuse or dependence at the 12-week assessment) (21, 34, 35) were analyzed using chi-square models with the same contrasts; as were other secondary outcomes (CBT knowledge and satisfaction with treatment). The trial was not powered for a direct comparison of CBT4CBT to clinician delivered CBT (e.g., a non-inferiority analysis), as there were no prior direct comparisons of computer-versus clinician-delivered CBT upon which to base power calculations and estimations of confidence intervals (36, 37).
Results
Sample description
Table 1 presents baseline demographic characteristics and substance use and psychiatric diagnoses for the 137 randomized participants. The sample was predominantly male (75%); 49% identified themselves as African American, 34% as Caucasian, and 8% as Latino/a. Most were unemployed, 75% reported they had completed high school, and 35% were referred by the criminal justice system. In terms of self-reported primary drug type: 49% reported marijuana, 29% reported cocaine, 19% reported alcohol, with 2% reporting opioids and 1% reporting PCP. Most (81.8%) used both drugs and alcohol; 55% reported using at least two drugs in the prior month; 81% of participants submitted at least one urine sample prior to baseline assessment that was positive for at least one illicit drug.
Table 1.
Baseline demographics by treatment assignment
| Treatment group | ||||||||
|---|---|---|---|---|---|---|---|---|
| CBTb (N = 49) | TAUc (N=50) | CBT4CBT w/monitoringd (N=38) |
Total (N=137) | |||||
| Categorical variables | n | % | n | % | n | % | n | % |
|
| ||||||||
| Female | 12 | 24.5 | 13 | 26.0 | 10 | 26.3 | 35 | 25.5 |
| Hispanic ethnicity | 7 | 14.3 | 10 | 20.0 | 5 | 13.2 | 22 | 16.1 |
| Race | ||||||||
| Caucasian | 19 | 38.8 | 18 | 36.0 | 10 | 26.3 | 47 | 34.3 |
| African-American | 24 | 49.0 | 22 | 44.0 | 21 | 55.3 | 67 | 48.9 |
| Indicated Hispanic only | 3 | 6.1 | 6 | 12.0 | 2 | 5.3 | 11 | 8.0 |
| Multiracial/other | 3 | 6.1 | 4 | 8.0 | 5 | 13.2 | 12 | 8.7 |
| Completed high school | 33 | 67.3 | 39 | 78.0 | 31 | 81.6 | 103 | 75.2 |
| Unemployed | 35 | 71.4 | 36 | 72.0 | 23 | 60.5 | 94 | 68.6 |
| Referred by criminal justice system | 17 | 34.7 | 22 | 44.0 | 9 | 23.7 | 48 | 35.0 |
| On public assistance | 24 | 49.0 | 23 | 46.0 | 22 | 59.5 | 69 | 50.7 |
| Lifetime anxiety disordere | 4 | 8.2 | 4 | 8.0 | 0 | 0.0 | 8 | 5.9 |
| Lifetime major depression disorder | 8 | 16.3 | 20 | 40.0 | 9 | 23.7 | 37 | 27.0 |
| Current major depression disorder | 4 | 8.2 | 8 | 16.0 | 2 | 5.3 | 14 | 10.2 |
| Antisocial personality disorder | 12 | 25.0 | 13 | 27.1 | 7 | 19.4 | 32 | 24.0 |
| Principal substance used (self-report) | ||||||||
| Marijuana | 26 | 53.1 | 22 | 44.0 | 19 | 50.0 | 67 | 48.9 |
| Cocaine | 12 | 24.5 | 17 | 34.0 | 11 | 28.9 | 40 | 29.2 |
| Alcohol | 9 | 18.4 | 10 | 20.0 | 7 | 18.4 | 26 | 19.0 |
| Opioids | 0 | 0.0 | 1 | 2.0 | 1 | 2.6 | 2 | 1.5 |
| Hallucinogens | 2 | 4.1 | 0 | 0.0 | 0 | 0.0 | 2 | 1.5 |
| Concurrent alcohol+disorder | 16 | 32.7 | 24 | 48.0 | 10 | 26.3 | 50 | 36.5 |
| More than 1 drug use disorder | 8 | 16.3 | 10 | 20.0 | 2 | 5.3 | 20 | 14.6 |
| Using both alcohol and drugs at baseline | 37 | 75.5 | 42 | 84.0 | 33 | 86.8 | 112 | 81.8 |
|
| ||||||||
| Continuous variables (mean, SD) | mean | sd | mean | sd | mean | sd | mean | sd |
|
| ||||||||
| Age (mean +− SD) | 34.3 | 12.6 | 36.9 | 12.1 | 36.6 | 11.1 | 35.9 | 12.0 |
| Days of primary drug use past 28 | 15.0 | 10.1 | 12.3 | 9.6 | 14.3 | 10.1 | 13.8 | 9.9 |
| Age first used primary drug | 15.9 | 4.9 | 17.2 | 6.5 | 16.7 | 5.1 | 16.6 | 5.6 |
| Years of primary drug use | 8.7 | 9.7 | 10.4 | 9.1 | 10.5 | 11.0 | 9.8 | 9.8 |
| # of previous drug treatments | 1.6 | 2.6 | 2.2 | 3.8 | 1.7 | 2.6 | 1.8 | 3.1 |
| # of previous alcohol treatments | 1.2 | 3.3 | 1.6 | 3.8 | 0.8 | 2.1 | 1.2 | 3.2 |
| Times arrested, lifetime | 9.2 | 17.5 | 7.5 | 9.4 | 6.4 | 6.8 | 7.8 | 12.4 |
| Months incarcerated lifetime | 26.5 | 43.2 | 20.2 | 42.0 | 19.6 | 37.2 | 22.3 | 41.0 |
| Shipley IQ estimate-age and education corrected | 82.8 | 15.0 | 83.2 | 14.2 | 86.8 | 14.6 | 84.1 | 14.6 |
Indicates weekly clinician-delivered cognitive behavioral therapy
Indicates Treatment as Usual (TAU), weekly group and or individual counseling sessions
All psychiatric diagnoses from Structured Clinical Interview for DSM-IV-R (SCID)
Treatment adherence, retention and data availability by condition
Of the 137 individuals randomized, 123 completed at least 1 session of their assigned treatment (90%). Table 2 shows treatment retention was significantly higher in the CBT4CBT condition (mean 62 days of 84 completed), lowest in clinician CBT (43 days), with intermediate retention in TAU (55 days). Number of urine specimens collected also differed significantly by treatment (8.0 for clinician CBT, 10.5 for TAU, and 12.0 for CBT4CBT).
Table 2.
Adherence, Serious Adverse Events, Secondary and follow-up outcomes by treatment assignment
| CBT (N=49) | TAU (N=50) | CBT4CBT w/monitoring (N=38) |
Contrast 1: CBT v TAU |
Contrast 2: CBT4CBT vs TAU |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | f | P | p Eta2 | f | P | p Eta2 |
|
| ||||||||||||
| General protocol adherence | ||||||||||||
| Days in treatment (max=84) | 42.8 | 30.7 | 55.5 | 27.3 | 61.7 | 26.8 | 4.85 | .03 | 0.04 | 1.09 | .30 | 0.01 |
| Number of urine specimens collected | 8 | 6.6 | 10.5 | 5.9 | 12 | 6.6 | 2.3 | .13 | 0.02 | 1.1 | .30 | 0.01 |
| Total number of treatment sessionsa | 4.1 | 3.4 | 5.6 | 3.1 | 6.9 | 3.57 | 4.99 | .03 | 0.04 | 3.59 | .06 | 0.03 |
| Treatment-specific adherence | ||||||||||||
| Total individual sessions | 4.1 | 3.4 | 2.98 | 3.4 | NA | 2.53 | .12 | 0.03 | ||||
| Total group sessions | NA | 2.6 | 3.2 | NA | ||||||||
| Total monitoring sessions | NA | NA | 6.8 | 3.6 | ||||||||
| Total CBT4CBT modules | NA | NA | 5.5 | 2.3 | ||||||||
| Number of homework assignments completed | 2.8 | 2.5 | NA | 2.2 | 2.4 | 0.23 | .63 | 1.57 | 0.12 | .73 | 0.65 | |
|
| ||||||||||||
| Serious adverse eventsb | N | % | N | % | N | % | WaldX2 | P | Exp (b) | Wald X2 | p | Exp (b) |
|
| ||||||||||||
| # (%) Participants with 1 or more SAEs during treatment-substance use or psychiatric | 3 | 6.1 | 2 | 4.0 | 1 | 2.6 | .23 | .63 | 1.57 | .12 | .73 | .65 |
| Participants with 1 or more SAEs during treatment medical | 2 | 4.1 | 1 | 2 | 3 | 7.9 | .35 | .55 | 2.09 | 1.49 | .22 | 4.20 |
| Participants with 1 or more SAEs during follow-up-substance use or psychiatric | 1 | 2.7 | 1 | 2.3 | 3 | 8.6 | .01 | .91 | 1.17 | 1.35 | .25 | 3.94 |
| Participants with 1 or more SAEs during follow-up-medical | 4 | 10.8 | 3 | 7 | 3 | 8.9 | .36 | .55 | 1.62 | .07 | .79 | 1.25 |
|
| ||||||||||||
| Secondary substance use outcomes | Mean | SD | Mean | SD | Mean | SD | f | P | p Eta2 | f | p | p Eta2 |
|
| ||||||||||||
| Percent of urine specimens negative for all drugs | 33.1 | 43.3 | 34.3 | 39.7 | 37 | 41.1 | 0.02 | .89 | .000 | 0.33 | .57 | .003 |
| Percent cocaine-negative urine toxicology screens-all subjects | 86.3 | 30.2 | 74.9 | 36.8 | 90.7 | 21.9 | 2.98 | .09 | .024 | 5.45 | .02 | .043 |
| Percent cocaine-negative urine toxicology screens, cocaine users only (n's are 9, 17, 11) | 63.6 | 46.2 | 39.4 | 37.3 | 75.3 | 34.4 | 2.29 | .14 | .060 | 5.73 | .02 | .140 |
| Percent marijuana-negative urine toxicology screens-all subjects | 44.5 | 47.1 | 43.6 | 44.2 | 48.8 | 43.3 | 0.01 | .93 | .000 | 0.27 | .60 | .002 |
| Percent marijuana-negative urine toxicology screens, marijuana users only (n's are 23, 19, 18) | 14.7 | 32.0 | 22.3 | 34.5 | 17.7 | 29.4 | 0.57 | .45 | .010 | 0.19 | .66 | .003 |
|
| ||||||||||||
| Categorical outcomes, indicators of clinical significance | Number | % | Number | % | Number | % | Wald | p | Exp (b) | Wald | p | Exp(b) |
|
| ||||||||||||
| Provided no drug-positive urine specimens, last 2 weeks of treatment | 9 | 18.4 | 9 | 18 | 13 | 34.2 | 0.00 | .96 | 1.030 | 3.00 | .09 | 2.370 |
| Did not meet DSM-IV-R criteria for primary substance use diagnosis at 12 weeks (N's are 41, 47, 37) | 16 | 51.6 | 15 | 42.9 | 20 | 66.7 | 0.51 | .48 | .710 | 3.61 | .06 | .380 |
|
| ||||||||||||
| Follow-up outcomes | Mean | SD | Mean | SD | Mean | SD | f | p | p Eta2 | f | p | p Eta2 |
|
| ||||||||||||
| Percent days abstinent from drugs and alcohol (selfreport) Mean, SD | 61.4 | 35.7 | 67.3 | 34.3 | 75.2 | 30.9 | 0.55 | .46 | .005 | 1.00 | .32 | .009 |
|
| ||||||||||||
| Urine specimen negative for all drugs (# negative/# collected) | % | % | % | WaldX2 | p | Exp(b) | Wald X2 | p | Exp (b) | |||
|
| ||||||||||||
| One month follow-up (n=73) | 6/18 | 33.3 | 16/29 | 55.2 | 12/26 | 46.2 | 2.10 | .15 | 2.460 | 0.40 | .51 | 1.440 |
| Three month follow-up (n=79) | 7/23 | 30.4 | 11/33 | 33.3 | 14/23 | 60.9 | 0.10 | .82 | 1.140 | 4.00 | .04 | 3.210 |
| Six month follow-up (n=102) | 8/33 | 24.2 | 12/39 | 30.9 | 15/30 | 50.0 | 0.40 | .54 | 1.390 | 2.60 | .11 | .440 |
Clinician CBT offered up to 12 individual sessions, TAU offered up to 12 group sessions with individual sessions as needed. CBT4CBT offered 12 clinical monitoring sessions plus up to 7 CBT4CBT modules
SAE indicates any Serious Adverse Event resulting in death or leading to hospitalization.
Study treatments were comprised of different components (i.e. group and individual sessions, CBT4CBT modules) and differed across groups: treatment exposure varied from a mean of 4.1 individual CBT sessions in the clinician CBT group, 5.6 individual or group sessions in TAU, and 6.8 brief individual monitoring sessions in CBT4CBT. Participants assigned to CBT4CBT also completed a mean of 5.5 modules of the 7 modules offered, which is comparable to previous CBT4CBT studies (7, 10, 38, 39). The number of CBT homework assignments completed did not differ by CBT condition.
Rates of serious adverse events are also shown in Table 2. One patient assigned to clinician CBT committed suicide (institutional review concluded the suicide did not appear to be related to treatment received) and 2 were withdrawn (1 was hospitalized for 5 days for suicidal ideation; 1 was referred for a 30-day inpatient treatment stay for substance abuse). Rates of other serious adverse events did not differ by treatment condition, either within treatment or during the 6-month follow-up.
At treatment termination (12-week assessment), data was collected from 120 individuals (88% of the intention-to-treat sample; 90% of the treatment-exposed sample). Regarding rates of follow-up, 84% (115/137) of the intention to treat sample was reached for at least one follow-up, and 79% were reached for the 6-month follow-up. Rates of assessment completion at treatment termination significantly differed by treatment condition (X2=6.44, p=.04), with contrasts indicating lower rates for clinician CBT compared to TAU (Wald=3.72, p=.05), but were not significantly different for the 1, 3, or 6-month follow-up interviews. Overall level of data missingness was significantly higher for clinician CBT than the other two conditions (treatment condition (Wald=6.6, p=.04).
Effects of study treatment on substance use outcomes within treatment and through follow-up
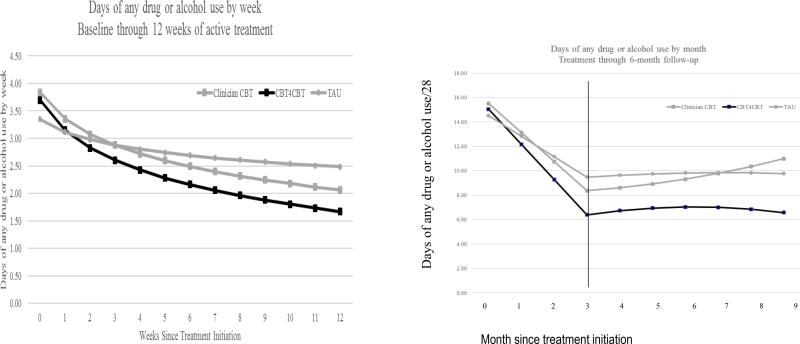
Results of random effects regression analyses for the primary outcome (days of any drug or alcohol use by week) are presented in Table 3 and illustrated in Figure 2. For the intent-to-treat sample, analyses of data collected during the active treatment phase indicated reduction in frequency of any substance use over time by week for the whole sample during the 12-week treatment period (effect for time, t=−4.61 (df 1, 999), p<.001), and also confirmed the two primary hypotheses: greater reductions in frequency of any drug or alcohol use over time for clinician CBT compared with TAU (t=−3.41 (df 1,1019), p<.01) and for CBT4CBT+monitoring compared with TAU (t=−2.26, df (1,996), p=.02). Results were similar regardless of sample (all randomized including data after dropout, treatment initiators, treatment exposed, or excluding participants whose primary drug was not marijuana, cocaine, or alcohol) and regardless of how primary drug was modeled (e.g., included as a random factor or ignored).
Table 3.
Results of random regression analyses Estimates for effects of contrasts on primary outcome (days of any drug or alcohol use by week)
| 95% Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | SE | df | t | p | Lower | Upper |
| Intention to treat sample (N==137, 1098 observations), within treatment (weeks 1–12) | |||||||
|
| |||||||
| Intercept | 3.39 | .33 | 179.99 | 10.32 | .00 | 2.74 | 4.04 |
| Contrast 1 main effecta | .40 | .47 | 188.10 | .85 | .40 | −.53 | 1.33 |
| Contrast 2 main effect | .20 | .50 | 179.96 | .40 | .69 | −.79 | 1.19 |
| Time (week) | −.45 | .10 | 999.74 | −4.61 | .00 | −.65 | −.26 |
| Contrast 1 by week | −.51 | .15 | 1019.23 | −3.41 | .00 | −.81 | −.22 |
| Contrast 2 by week. | −.33 | .14 | 996.71 | −2.26 | .02 | −.61 | −.04 |
|
| |||||||
| Subset of individuals who initiated treatment (N=123, 1084 observations), within treatment (weeks 1–12) | |||||||
|
| |||||||
| Intercept | 3.38 | .34 | 161.77 | 9.81 | .00 | 2.70 | 4.06 |
| Contrast 1 main effect | .54 | .51 | 168.00 | 1.06 | .29 | −.47 | 1.55 |
| Contrast 2 main effect | .22 | .51 | 164.18 | .43 | .67 | −.79 | 1.23 |
| Time (week) | −.45 | .10 | 981.46 | −4.55 | .00 | −.64 | −.26 |
| Contrast 1 by week | −.53 | .15 | 987.60 | −3.48 | .00 | −.83 | −.23 |
| Contrast 2 by week. | −.33 | .14 | 986.19 | −2.27 | .02 | −.61 | −.04 |
|
| |||||||
| Subset of individuals with adequate exposure to treatment (N=81, 881 observations), within treatment (weeks 1–12) | |||||||
|
| |||||||
| Intercept | 3.46 | .42 | 107.99 | 8.15 | .00 | 2.62 | 4.30 |
| Contrast 1 main effect | .73 | .67 | 108.18 | 1.09 | .28 | −.60 | 2.05 |
| Contrast 2 main effect | .10 | .61 | 107.84 | .17 | .87 | −1.11 | 1.31 |
| Time (week) | −.52 | .11 | 799.27 | −4.94 | .00 | −.73 | −.31 |
| Contrast 1 by week | −.61 | .17 | 798.97 | −3.65 | .00 | −.94 | −.28 |
| Contrast 2 by week. | −.30 | .15 | 799.32 | −1.99 | .05 | −.60 | .00 |
|
| |||||||
| Follow up data | |||||||
|
| |||||||
| Intention to treat sample, all data points (N=137, 1172 observations), results of piecewise regression with phase (within treatment versus follow-up months 1–6) | |||||||
|
| |||||||
| Intercept | 14.51 | 1.35 | 231.36 | 10.74 | .00 | 11.85 | 17.17 |
| Contrast 1 main effect | 1.00 | 1.93 | 233.67 | .52 | .60 | −2.79 | 4.80 |
| Contrast 2 main effect | .53 | 2.05 | 230.82 | .26 | .80 | −3.52 | 4.58 |
| Time (month) | −1.68 | .39 | 1044.99 | −4.26 | .00 | −2.45 | −.91 |
| Contrast 1 by month | −.70 | .58 | 1059.44 | −1.21 | .23 | −1.84 | .43 |
| Contrast 2 by month | −1.20 | .59 | 1040.02 | −2.02 | .04 | −2.37 | −.04 |
| Phase (Treatment vs follow-up) | 1.93 | 1.17 | 1033.60 | 1.65 | .10 | −.37 | 4.22 |
| Contrast 1 by phase | .52 | 1.72 | 1036.08 | .31 | .76 | −2.84 | 3.89 |
| Contrast 2 by phase | 1.54 | 1.74 | 1031.67 | .88 | .38 | −1.88 | 4.96 |
| Time (month) by phase | −0.02 | 0.10 | 1030.85 | −0.21 | 0.83 | −0.22 | 0.18 |
| Contrast 1 by month by phase | 0.06 | 0.15 | 1030.39 | 0.42 | 0.68 | −0.23 | 0.36 |
| Contrast 2 by month by phase | −0.04 | 0.15 | 1029.81 | −0.26 | 0.79 | −0.34 | 0.26 |
Contrast 1=effect for clinician CBT versus TAU, Contrast 2=effect for CBT4CBT+monitoring versus TAU
Figure 2.
Change in primary outcome (change in frequency of days of any drug or alcohol use over time by treatment group), estimates from random effects regression analyses. Panel one: Within treatment, change over time by week. Panel 2: Results from piecewise regression, estimates from both treatment and follow-up phases by month.
Follow-up data are also illustrated in Figure 2 (panel 2). Analyses indicate an overall effect of time, as participants as a group reduced their frequency of drug or alcohol use from the start of treatment to the end of the follow-up by month (effect for time t=−4.26 (df 1, 1044), p<.01) but with the effect of phase (within treatment versus follow-up) significant only at a trend level (effect for phase t=1.65 (df 1, 1033), p=.10). The effect for the contrast of CBT4CBT plus monitoring versus TAU was significant, indicating sustained effects of CBT4CBT relative to TAU over time (t=−2.02 (df 1, 1040), p=.04), but the effect of clinician CBT versus TAU was not significant when including follow-up data.
Secondary substance use outcomes within treatment and follow-up
Secondary outcomes are presented in Table 2. In terms of urine toxicology screens negative from all drugs, among the participants who reported drug use at baseline (n = 132), the percentage of drug-free urine specimens was highest in CBT4CBT+monitoring (37%), lowest in clinician CBT (33.1%) and intermediate for TAU (34.3%), but differences were not statistically significant. Effects were significant for cocaine-negative urine specimens for both the sample as a whole, as well as only those who reported cocaine as their primary drug, with those assigned to CBT4CBT+monitoring submitting a significantly higher proportion of cocaine-negative urine specimens than those assigned to TAU. Rates of positive breathalyzer samples were low and did not differ by treatment condition.
In terms of indicators of clinical significance, the percentage of participants with no urine specimens testing positive for drugs in the last 2 weeks of treatment favored CBT4CBT+monitoring (34%) versus TAU and clinician CBT (both 18%), which reached trend level (p=.09). Rates of individuals no longer meeting DSM-IV diagnostic threshold for current substance dependence at treatment termination also favored CBT4CBT+monitoring (66.7%) over clinician CBT (51.6%) and TAU (42.9%), also at a trend level (p=.06).
Results evaluating the self-reported percentage of days abstinent during follow-up were largely consistent with the primary random effects regression analyses, indicating the highest percentage of days abstinent reported in CBT4CBT+monitoring, but these were not statistically significant. Results of urine toxicology screens collected at each follow-up indicated a significantly higher proportion of drug-negative urines for those assigned to the CBT4CBT+monitoring condition compared with TAU at the 3-month follow-up (CBT4CBT=60.9%, TAU=33.3%; Wald=4.0, p=.04); this effect was not significant at the final 6-month follow-up (CBT4CBT=50.0%, TAU=30.9%; Wald=2.6, p=.11).
Knowledge of CBT concepts and treatment satisfaction
A 40-item true/false test assessing basic knowledge of cognitive and behavioral concepts (“Everyone’s triggers are the same”, “It’s always best to trust your gut when thinking about a problem”) was added after the trial began. Fifty-two participants completed it at baseline and treatment termination. Participants as a whole increased their scores over time (time, F=8.04, p<.01); those assigned to CBT4CBT+monitoring had the largest gain in percent correct over time (mean scores at treatment termination, CBT=65%, TAU=72%, CBT4CBT=81%; group by time, F=4.32, p=.02).
A treatment satisfaction form validated in previous studies (7, 40) was administered at the treatment termination interview to assess satisfaction with treatment overall and with specific aspects. For the question “Overall, how satisfied are you with the treatment you received?”, more individuals assigned to CBT4CBT+monitoring responded with the highest possible level (“very satisfied”) (82.4%) compared with those assigned to clinician CBT (63.9%) or TAU (60.0%), although at a trend level (X2=4.8, p=.09). Similarly, for the question, “Overall, how would you describe your condition at present”, more individuals assigned to CBT4CBT+monitoring responded with the highest possible level “excellent” (44.1%) compared with those assigned to clinician CBT (19.4%) or TAU (28.9%), again at trend level (X2=5.1, p=.08). Satisfaction with amount of treatment received did not differ across treatment groups (“very satisfied” with amount of treatment: clinician CBT=55.6%, TAU=57.8%, CBT4CBT=58.8%, X2=0.08, p=.96); nor did reported satisfaction with their clinician (“very satisfied with clinician”: clinician CBT=72.2%, TAU=80.0%, CBT4CBT=88.2%, X2=2.8, p=.25).
Discussion
This randomized clinical trial evaluating a web-based CBT intervention in a heterogeneous sample of treatment-seeking substance users found those assigned to either CBT4CBT with minimal clinical monitoring or clinician-delivered CBT had greater reductions in frequency of any drug or alcohol use compared with standard treatment. A six-month follow-up demonstrated continuing efficacy for CBT4CBT compared to TAU, but not for clinician CBT versus TAU. Multiple secondary outcomes favored CBT4CBT+monitoring, as well as indicators of clinical significance, such as a greater percentage of participants no longer meeting DSM-IV criteria for current substance dependence at the end of treatment.
This is the first randomized clinical trial to evaluate a web-based intervention delivered with minimal monitoring for individuals with DSM substance use disorders within a treatment-seeking clinical sample. These types of trials are rare (5), yet essential for validating web-based approaches as well as for realizing their promise to reduce the ‘treatment gap’ between the large proportion of individuals in need of evidence-based services and the limited number who actually receive them (41).
The results strongly support the safety, feasibility, and efficacy for CBT4CBT provided with minimal clinical monitoring. Participants assigned to this condition consistently achieved the best outcomes in terms of treatment retention, engagement, and substance use in comparison to an active control condition. Although a direct comparison (i.e., non-inferiority) was not tested here, CBT4CBT+monitoring appeared to outperform clinician-delivered CBT on all outcomes evaluated. There were no indications that CBT4CBT+monitoring was not ‘at least as good’ as clinician-delivered CBT; in addition to greater reductions in substance use and indicators of clinical significance, those assigned to CBT4CBT+monitoring showed the greatest increase in knowledge of CBT concepts and were most likely to report the highest levels satisfaction with treatment. This computerized version of CBT thus appears to be an engaging and attractive approach for those with substance use disorders (42).
While those assigned to clinician-delivered CBT did show greater reductions in substance use as compared to treatment as usual, it had the poorest level of treatment retention and engagement, as well as the lowest rates of abstinence during the follow-up period. This was unexpected, given one of the distinguishing features of CBT is its relative durability of effects (43, 44). Despite well-trained clinicians with high quality delivery, participants assigned to CBT dropped out of treatment sooner, had a greater number of withdrawals from treatment, and had the lowest rates of follow-up data collected. Reasons for this are not clear. It may be that weekly one-on-one CBT was too demanding for this population, many of which were referred to treatment by the criminal justice system.
Strengths of this trial include rigorous methodological features consistent with those for clinician-delivered therapies (45), including urn randomization, SCID-based diagnosis for inclusion, primary self-report outcome with biological verification, close monitoring of treatment delivery, and rates of follow-up data collection from >80% of intention to treat sample. Inclusion of a broad range of substance use, with most participants (82%) reporting both alcohol and drug use, enhances the generalizability of findings. However, despite being one of the first trials to include both a virtual stand-alone computerized CBT and clinician-delivered CBT, the study was not powered to directly contrast these two conditions; thus, it cannot be concluded that the effects of CBT4CBT+monitoring were equivalent or superior to clinician-CBT. This heterogeneous sample of ‘all comers’ were prescribed an array of medications (Supplemental Table 1), but these did not vary by treatment. The differential rate of attrition across treatment conditions limits the inferences that can be drawn regarding the secondary substance use outcomes, as these were evaluated using the intention-to-treat sample regardless of level of treatment exposure. In sum, this study provides strong support for CBT4CBT as an efficacious treatment for substance use, even when offered with limited clinical contact. Web-based CBT4CBT may not only broaden access to an evidence-based treatment, but may also be a more appealing option for many individuals.
Supplementary Material
Acknowledgments
Support was provided by National Institute on Drug Abuse grants R37-DA 015969 and P50-DA09241 and National Institute on Alcohol Abuse and Alcoholism grant R01 AA024122. Liz Vollono and Joanne Corvino provided critical support in implementing the trial; we also thank Drs. Elise DeVito and Sarah Yip for their helpful comments. We are deeply grateful for the creativity, skill, and resourcefulness of Melissa Gordon as well as Rick Leone, Craig Tomlin, and Doug Forbush of Yale ITS MedMedia.
Footnotes
Disclosures: Dr. Carroll is a member of CBT4CBT LLC, which makes CBT4CBT available to qualified clinical providers and organizations on a commercial basis. Dr. Carroll works with Yale University to manage any potential conflicts of interest.
References
- 1.U.S. Department of Health and Human Services (HHS) Office of the Surgeon General: Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health. Washington, DC: HHS; 2016. [PubMed] [Google Scholar]
- 2.Marsch LA, Carroll KM, Kiluk BD. Technology based interventions for the treatment and recovery management of substance use disorders: A JSAT special issue. J Subst Abuse Treat. 2014;46:1–4. doi: 10.1016/j.jsat.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boumparis N, Karyotaki E, Schaub MP, Cuijpers P, Riper H. Internet interventions for adult illicit substance users: a meta-analysis. Addiction. 2017;112:1521–1532. doi: 10.1111/add.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tait RJ, Spijkerman R, Riper H. Internet and computer based interventions for cannabis use: a meta-analysis. Drug Alcohol Depend. 2013;133:295–304. doi: 10.1016/j.drugalcdep.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Kiluk BD, Sugarman DE, Nich C, Gibbons CJ, Martino S, Rounsaville BJ, Carroll KM. A methodological analysis of randomized clinical trials of computer-assisted therapies for psychiatric disorders: toward improved standards for an emerging field. The American journal of psychiatry. 2011;168:790–799. doi: 10.1176/appi.ajp.2011.10101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13:288–295. doi: 10.1002/wps.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll KM, Ball SA, Martino S, Nich C, Babuscio T, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted cognitive-behavioral therapy for addiction. A randomized clinical trial of 'CBT4CBT'. Am J Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: A 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100:178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olmstead TA, Ostrow CD, Carroll KM. Cost-effectiveness of computer-assisted training in cognitive-behavioral therapy as an adjunct to standard care for addiction. Drug and alcohol dependence. 2010;110:200–207. doi: 10.1016/j.drugalcdep.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy G, Marino D, Ball SA. Computer-assisted delivery of cognitive-behavioral therapy: Efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171:436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- 12.Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;(Supplement 12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 13.Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, Maryland: NIDA; 1998. [Google Scholar]
- 14.Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Fenton L, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alc Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 16.Carroll KM, Nuro KF, Rounsaville BJ, Petrakis I. Unpublished manual. Yale University Psychotherapy Development Center; 1998. Compliance Enhancement: A Clinician's Manual for Pharmacotherapy Trials in the Addictions. [Google Scholar]
- 17.Volpicelli JR, Pettinati HM, McLellan AT, O'Brien CP. Combining medication and psychosocial treatments for addictions: The BRENDA approach. New York, Guilford: 2001. [Google Scholar]
- 18.Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud. Alcohol Supplement. 2005:170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- 19.Kenwright M, Marks IM, Graham C, Franses A, Mataix-Cols D. Brief scheduled phone support from a clinician to enhance computer-aided self-help for obsessive-compulsive disorder: Randomized clinical trial. J Clinical Psychology. 2005;61:1499–1508. doi: 10.1002/jclp.20204. [DOI] [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 21.Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. J Consult Clin Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 23.Hersh D, Mulgrew CL, Van Kirk J, Kranzler HR. The validity of self-reported cocaine use in two groups of cocaine abusers. J Consult Clin Psychology. 1999;67:37–42. doi: 10.1037//0022-006x.67.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivational/skillsbuilding therapy to treat young adults with marijuana dependence. J Consult Clin Psychology. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107:1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll KM, Kiluk BD, Nich C, Gordon MA, Portnoy GA, Marino DR, Ball SA. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171:436–444. doi: 10.1176/appi.ajp.2013.13070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh F. SSIZE: A sample size program for clinical and epidemiological studies. American Statistician. 1991;45 [Google Scholar]
- 29.Nursing Times open learning programme. M4: assertiveness. Part (II): How assertive are you? [continuing education credit] Nurs Times. 1992;88:i–viii. [PubMed] [Google Scholar]
- 30.Rounsaville BJ, Petry NM, Carroll KM. Single versus multiple drug focus in substance abuse clinical trials research. Drug and alcohol dependence. 2003;70:117–125. doi: 10.1016/s0376-8716(03)00033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singer JD, Willett JB. Applying Longitudinal Data Analysis: Modeling Change and Event Occurence. New York: Oxford University Press; 2003. [Google Scholar]
- 32.Schwartz RH. Urine testing in the detection of drugs of abuse. Archives of internal medicine. 1988;148:2407–2412. [PubMed] [Google Scholar]
- 33.Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92:717–727. [PubMed] [Google Scholar]
- 34.Carroll KM, Kiluk BD, Nich C, DeVito EE, Decker S, LaPaglia D, Duffey D, Babuscio TA, Ball SA. Towards empirical identification of a clinically meaningful indicator of treatment outcome for drug addiction: Features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year cocaine use follow-up outcomes. Drug Alcohol Depend. 2014;137:3–19. doi: 10.1016/j.drugalcdep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiluk BD, Carroll KM, Duhig A, Falk DE, Kampman K, Lai S, Litten RZ, McCann DJ, Montoya ID, Preston KL, Skolnick P, Weisner C, Woody G, Chandler R, Detke MJ, Dunn K, Dworkin RH, Fertig J, Gewandter J, Moeller FG, Ramey T, Ryan M, Silverman K, Strain EC. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug and alcohol dependence. 2016;158:1–7. doi: 10.1016/j.drugalcdep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeHenanff A, Giraudeau B, Baron G, Ravaud P. Quality of reporting of non-inferiority and equivalence randomized trials. JAMA : the journal of the American Medical Association. 2006;295:1147–1151. doi: 10.1001/jama.295.10.1147. [DOI] [PubMed] [Google Scholar]
- 37.Scott IA. Non-inferiority trials: determining whether alternative treatments are good enough. Medical Journal of Australia. 2009;190:326–330. doi: 10.5694/j.1326-5377.2009.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 38.Carroll KM, Devito EE, Shi JM, Nich C, Sofuoglu M. A randomized trial of galantamine and computerized cognitive behavioral therapy for methadone maintained individuals with cocaine use disorder. Journal of Clinical Psychiatry. doi: 10.4088/JCP.17m11669. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiluk BD, Devore KA, Buck MB, Nich C, Frankforter TL, LaPaglia DM, Yates BT, Gordon MA, Carroll KM. Randomized Trial of Computerized Cognitive Behavioral Therapy for Alcohol Use Disorders: Efficacy as a Virtual Stand-Alone and Treatment Add-On Compared with Standard Outpatient Treatment. Alcoholism, clinical and experimental research. 2016;40:1991–2000. doi: 10.1111/acer.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donovan DM, Kadden RM, DiClemente CC, Carroll KM. Client satisfaction with three therapies in the treatment of alcohol dependence: results from project MATCH. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2002;11:291–307. doi: 10.1080/10550490290088090. [DOI] [PubMed] [Google Scholar]
- 41.Kazdin AE. Addressing the treatment gap: A key challenge for extending evidence-based psychosocial interventions. Behaviour Research and Therapy. 2017;88:7–18. doi: 10.1016/j.brat.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 42.McKay JR. Making the hard work of recovery more attractive for those with substance use disorders. Addiction. 2017;112:751–757. doi: 10.1111/add.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll KM, Nich C, Ball SA, McCance-Katz EF, Frankforter TF, Rounsaville BJ. One year follow-up of disulfiram and psychotherapy for cocaine-alcohol abusers: Sustained effects of treatment. Addiction. 2000;95:1335–1349. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- 44.Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- 45.Chambless DL, Hollon SD. Defining empirically supported therapies. Journal of consulting and clinical psychology. 1998;66:7–18. doi: 10.1037//0022-006x.66.1.7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.