Abstract
Background & Aims
It is not clear how age affects airway protective mechanisms. We investigated the effects of aging on upper esophageal sphincter (UES) and esophageal body pressure responses to slow and ultra-slow simulated reflux events and post-reflux residue.
Methods
We performed a prospective study of 11 elderly (74±9 years old) and 11 young (28±7 years old) healthy volunteers. Participants were placed in a supine position and evaluated by concurrent high-resolution impedance manometry and an esophageal infusion technique. Potential conditions of gastroesophageal reflux were simulated, via infusion of 0.1 N HCI and saline. UES and esophageal pressure responses were measured during: slow infusion (1 ml/sec) for 60 sec, 60 sec of post-infusion dwell period, ultra-slow infusion (0.05 ml/sec) for 60 sec, and 60 sec of a post-infusion dwell period. All infusions were repeated 3 times. We used the UES high pressure zone contractile integral (UES-CI) to determine responses of the UES.
Results
Young and elderly subjects each had a significant increase in the UES-CI during slow infusions and during entire passive dwell intervals compared to baseline (P<.01, both groups). Ultra-slow infusions were associated with a significant increase in UES-Cl in only the young group, in the late infusion period and into the dwell interval (P<.01). During the slow infusions and their associated dwell periods, young subjects had a higher frequency of secondary peristalsis than elderly subjects (P<.05). There was more secondary peristalsis during active infusions than dwell intervals. Secondary peristalsis was scarce during ultra-slow infusions in both groups.
Conclusions
UES and esophageal body pressure responses to low-volume ultra-slow reflux and associated post-reflux residue are reduced in elderly individuals. This deterioration could have negative effects on airway protection for people in this age group.
Keywords: reflux, upper esophageal sphincter, secondary peristalsis, post reflux period
Introduction
Mechanisms of airway protection against aspiration of gastric contents are complex and are not completely understood. The dual function of the pharynx as both the air and food passage allows luminal contiguity between the lung/airway and stomach, which provides the anatomical basis for aspiration of gastric contents. Our understanding of airway protection is evolving and there are currently two sets of mechanisms identified; the basal and reflex responses that can interrupt this anatomical contiguity and prevent pharyngeal transposition of gastric contents and aspiration.1–3 Basal mechanisms include the lower and upper esophageal sphincters that maintain sustained although fluctuating pressure barriers between the stomach and esophagus as well as the esophagus and pharynx, respectively. The reflex response mechanisms include several reflexes emanating from the esophagus, pharynx and larynx that result in either transient fortification of the UES pressure barrier and closure of the airway or clearance of the refluxate from the pharynx and esophagus.4–7
A number of studies have documented alterations of these reflex mechanisms in different conditions. For example, pharyngo-UES contractile, pharyngo-glottal closure and laryngo-UES contractile reflexes as well as the reflexive pharyngeal swallow were found to deteriorate with aging.8–10 The esophago-UES contractile reflex (EUCR), secondary esophageal peristalsis, and esophago-esophageal reflex have been reported to be abnormal and unable to prevent pharyngeal reflux of esophageal infusate in patients with reflux-attributed supraesophageal symptoms and regurgitation.11–12 An exaggerated esophago-UES relaxation reflex has been reported in similar patient groups.13
Theoretically, when gastric contents traverse the LES barrier and enter the esophagus, the EUCR and secondary esophageal peristalsis play a pivotal role in airway protection. The UES contractile reflex enhances the UES pressure barrier and secondary esophageal peristalsis clears the volume of refluxate from the esophagus, preventing entry of refluxate into the pharynx.2,14–16 Despite these observations, there remain significant gaps in our understanding of UES physiology as it relates clinically to gastroesophageal reflux. For example, although it is known that there is significant variability among reflux events in terms of volume, rate of entry into the esophagus, physical and chemical composition of refluxed material,16–17 and potential modulation of the reflex mechanisms through the life span,18,19 it is not known how the UES responds to slow and ultra-slow reflux events that can occur during decumbency and sleep when the airway is most vulnerable or whether aging affects these responses. Another important question about the relationship of gastroesophageal reflux and airway protection is how pharyngeal reflux is prevented if refluxate is not cleared from the esophageal lumen. Does the UES continue to remain contracted following cessation of reflux without clearance of refluxate and whether age affects the UES response under these conditions rendering the elderly more vulnerable to pharyngeal reflux.
Therefore, the aim of the present study was to 1) determine whether the UES continues to remain contracted following cessation of reflux without clearance of refluxate and 2) compare between healthy young and elderly the UES and esophageal pressure responses to well-defined slow and ultra-slow simulated acid and non-acid reflux events during active infusion and post-infusion dwell periods mimicking the post-reflux residue.
Methods
Subjects
We studied 11 elderly (74±9 yr) and 11 young (28±7 yr) healthy volunteers in supine position. The study was approved by the Medical College of Wisconsin institutional review board and all participants signed written informed consent prior to their studies. Participants had no history of known gastrointestinal disorders and were not taking any medications that might affect the function of the gastrointestinal tract. Participants with a history of neuromuscular or neurocognitive diseases such as stroke, dementia, Parkinson’s disease, myasthenia gravis, multiple sclerosis, as well as C-spine disorders, head or neck surgery, and substance abuse were excluded from the study.
Study protocol
For monitoring UES and esophageal pressure responses as well as the perfusate, we used a combined solid-state high resolution manometry and impedance catheter with 36 circumferential pressure sensors, spaced 1 cm apart measuring at a sample rate of 50 Hz and 18 impedance sensor couplets spaced 2 cm apart (Given imaging, Los Angeles, CA, USA).
For intra-esophageal slow and ultra-slow infusions, we used a catheter with a 3-mm outer diameter. To minimize the number of intubations, the infusion tube was affixed to the manometry catheter by a small band of thermoplastic, self-sealing laboratory film (Parafilm M, Pechiney Plastic Packaging, Menasha, WI, USA). The manometric catheter and infusion tube were arranged such that the infusion port was located about 5.5 cm proximal to the most distal pressure sensor.
After at least 6 hours of fasting, all participants underwent trans-nasal placement of the manometric/impedance and infusion catheters following application of topical 2% lidocaine to the nasal cavity. The manometric catheter along with the infusion tube were then advanced through the esophagus until the most distal pressure sensor was marginally above the upper border of the lower esophageal sphincter (LES) high pressure zone. The catheter was then pulled back 2 cm and positioned such that the entire pharynx, UES, as well as the esophageal body except for the distal 2 cm could be recorded. A schematic representation of the position of the manometry / impedance catheter and perfusion port relative to the UES and LES is presented in Supplementary figure 1.All subjects were studied in the supine position following a ten-minute adaptation period.
Gastroesophageal reflux (GER) events were simulated by intra-esophageal injection of room temperature 0.1 N HCl (pH 1.2–1.4) and half normal saline (pH 5.8). UES and esophageal pressure responses were determined under four conditions while subjects refrained from swallowing to prevent refluxate clearance due to primary peristalsis: 1. Active slow infusions at the rate of 1 ml/sec for 60 seconds, 2. 60 seconds of post-infusion dwell period. 3. Active ultra-slow infusion at the rate of 0.05 ml/sec for 60 seconds and 4. 60 seconds of post-infusion dwell period. All infusions were repeated three times. Acid and saline infusion studies were performed on two different visits at least one week apart. A motor driven syringe pump (Model NE-1000, New Era Pump Systems, Inc, Farmington, NY, USA) was used to control the rate of infusions.
Volunteers were asked to report symptoms, including heartburn, regurgitation, cough and chest pain.
Manometric data analysis
We used the UES High Pressure Zone Contractile Integral (UES-CI) to determine the response of the UES to simulated reflux events. The UES-CI is the mean pressure (≥20 mmHg) within the prescribed space-time interval multiplied by duration (s) and span (cm) in units of mmHg-s-cm (Given imaging, Los Angeles, CA, USA). We measured 10 second epochs of UES-CI during the 10 seconds prior to the beginning of infusion and during 60 seconds of infusion as well as during 60 seconds post-infusion dwell period. If scarcely a swallow/primary peristalsis occurred during the infusion or dwell period, only the epochs 10 seconds before the swallow were included in the analysis. The epoch immediately prior to swallow/primary peristalsis was excluded from the analysis to avoid inclusion of swallow induced UES pressure changes. For example, if the swallow happened at 50 seconds into active infusion, only the first 40 seconds of the UES response during active infusion was included. With this approach, we were still able to use part of the data since we determined UES response for each 10 second epoch. However, the data after 40 seconds including the subsequent dwell interval were excluded from the data analysis.
Esophageal body response to simulated reflux events: The frequency of secondary peristalsis was counted during active infusion and post-infusion dwell periods. In addition, the UES pressure changes during secondary peristalsis were documented as an increase, decrease or no change. Non-peristaltic esophageal contraction was defined as non-peristaltic esophageal pressure activity encompassing a length of at least 5 cm exhibiting a pressure of more than 20 mmHg.
Statistical Analysis
All statistical analysis was conducted using StatsDirect Software (StatsDirect, Ltd, Altrincham, UK) and Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Descriptive statistics are reported as mean and standard deviation of the mean for UES-CI unless otherwise noted. Differences of UES-CI among baseline, active infusion and passive post-infusion dwell period were assessed using ANOVA with repeated-measures. To evaluate the age effect on UES-CI, Two-Way ANOVA was used with post-hoc t-test corrected for multiple comparisons (Tukey method). Fisher exact and chi-square tests were used for comparison of categorical variables. Two-tailed p-values <0.05 were considered statistically significant.
Results
UES response to slow acid and non-acid simulated reflux in young and elderly
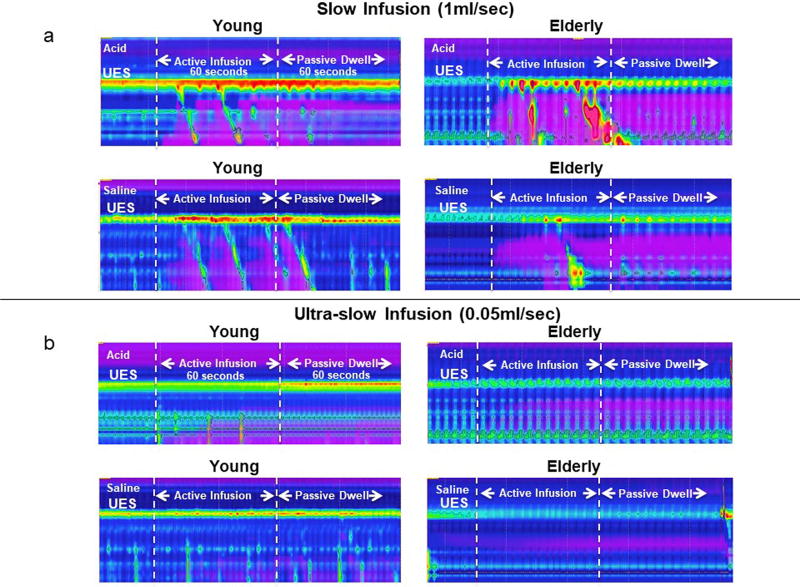
Representative examples of concurrent high-resolution manometry and intraluminal impedance color-contour plots showing the UES response to slow acid and saline infusions as well as their associated post-infusion dwell periods for young and elderly are presented in Figure 1a.
Figure 1.
Examples of UES contractile response to slow 1ml/s (1a) and ultra-slow 0.05ml/s (1b) acid and saline intra-esophageal infusions in young and elderly. As seen in figure 1a, in both age groups and for both types of infusions, UES contraction occurred shortly after the start of infusions and continued during the entire period of infusion. In addition, UES contraction was sustained after the active infusion ceased through the passive dwell time in which acid and saline residue remained in the esophagus as evidenced by the impedance signature. In contrast, as seen in Figure 1b, in both acid and saline infusions, significant increase in UES pressure was only observed in the young group and at the late stage of infusion which was sustained into dwell interval (also see figure 2 and 3). As seen there was no significant UES response noted in the elderly either during active infusion or passive dwell interval.
Comparison of the baseline pre-infusion UES-CI between young and elderly showed a trend for higher values in the young. The difference, however, did not reach statistical significance. In both young and elderly, the slow infusion of both acid and saline resulted in significant increase in the UES contractile integral. The onset of end-expiratory UES pressure increase by 10 mmHg after the start of infusion as measured by E-sleeve averaged 11± 2 s and 10 ± 3 s in young for acid and saline infusion, respectively, and 12 ± 3 s and 13 ± 4 s in the elderly for acid and saline infusions, respectively. These intervals were not different between young and elderly as well as between acid and saline infusions. Compared to baseline pre-infusion, the increase in UES-CI in both young and elderly was statistically significant during the second epoch for both acid and saline infusions (p<0.05 for both groups). As seen in Figure 2 and 3, this pressure increase was maintained during the entire infusion period. In addition, although slightly diminished, this increase continued throughout the post-infusion dwell period before the refluxate was cleared at the end of this period by a swallow induced primary peristalsis (Figure 4, p<0.01 for both groups compared to baseline). Although there appeared to be a slight decrease in UES-CI during post-infusion dwell period compared to active infusion period, the differences only reached statistical significance for saline infusion in the elderly group (Figure 4).
Figure 2.
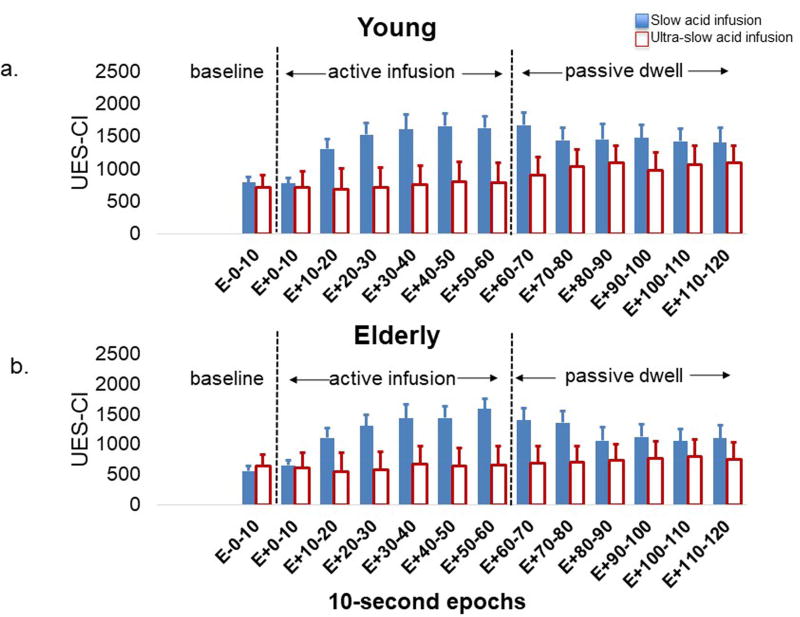
UES contractile response to slow (1ml/sec) and ultra-slow (0.05ml/sec) acid infusions in (2a) young and (2b) elderly. The X-axis displays each of the 10-second epoch before, during and after acid infusions. As seen in both young and elderly, the UES contractile response to slow acid infusion was significantly stronger than that of the ultra-slow infusion and the difference became notable starting at the second 10-second Epoch of infusion (p<0.001). UES-CI: UES high-pressure zone contractile integral
Figure 3.
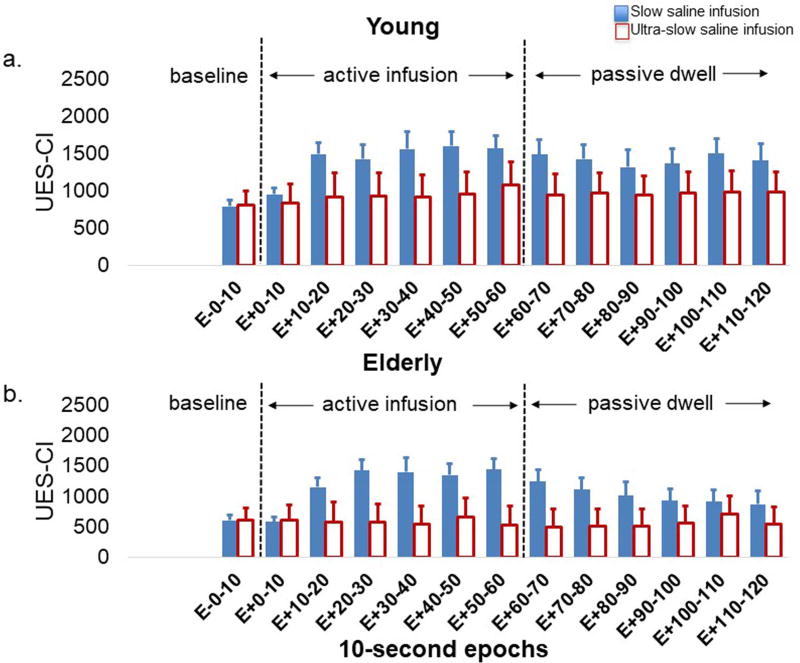
UES contractile response to slow (1ml/sec) and ultra-slow (0.05ml/sec) saline infusions in (3a) young and (3b) elderly. The X-axis displays each of the 10-second epoch before, during and after saline infusions. As seen in both young and elderly, similar to acid infusion, the UES contractile response to slow saline infusion was significantly stronger than that of the ultra-slow infusion and the difference became notable starting at the second 10-second Epoch of infusion (p<0.001). UES-CI: UES high-pressure zone contractile integral
Figure 4.
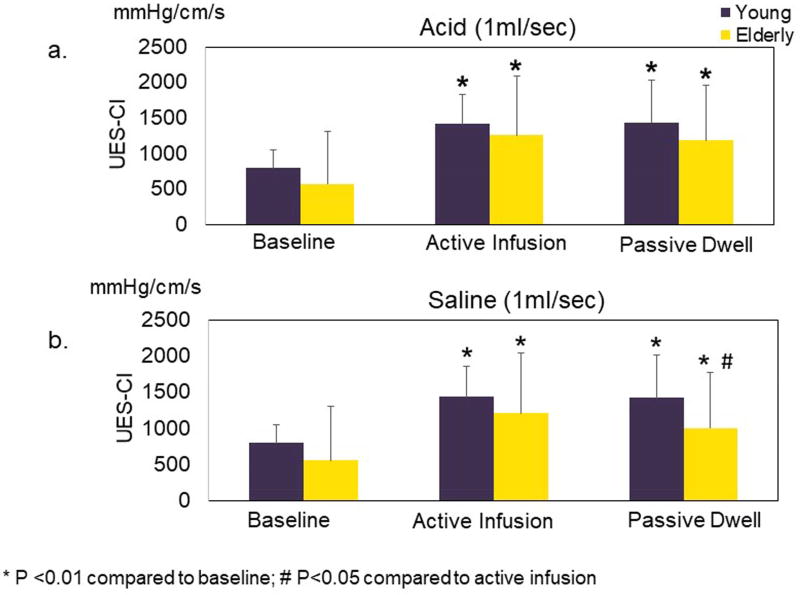
UES pressure response to slow (1ml/sec) (4a) acid and (4b) saline intra-esophageal infusion in young and elderly. The average of 10-second epochs of UES-CI is presented. In acid infusion, both young and elderly showed significant increase in UES pressure during active acid infusion as well as during the passive dwell interval when compared to baseline. Similar to acid infusion, in saline infusion, both young and elderly also showed significant increase in UES-CI during active infusion and passive dwell interval. UES-CI: UES high-pressure zone contractile integral
The magnitude of increase in mean UES-CI relative to baseline was similar between young and elderly for both active infusion and dwell period irrespective of the composition of infusate (p>0.05).
In both age groups, comparison of the UES pressure response to slow acid infusion with that of saline infusion as well as UES pressure response between acid and saline during post-infusion dwell periods did not show any statistical difference (Supplementary figure 2).
UES response to ultra-slow acid and non-acid simulated reflux in young and elderly
A representative example of UES pressure change in response to ultra-slow acid and saline infusion in young and elderly is shown in Figure 1b.
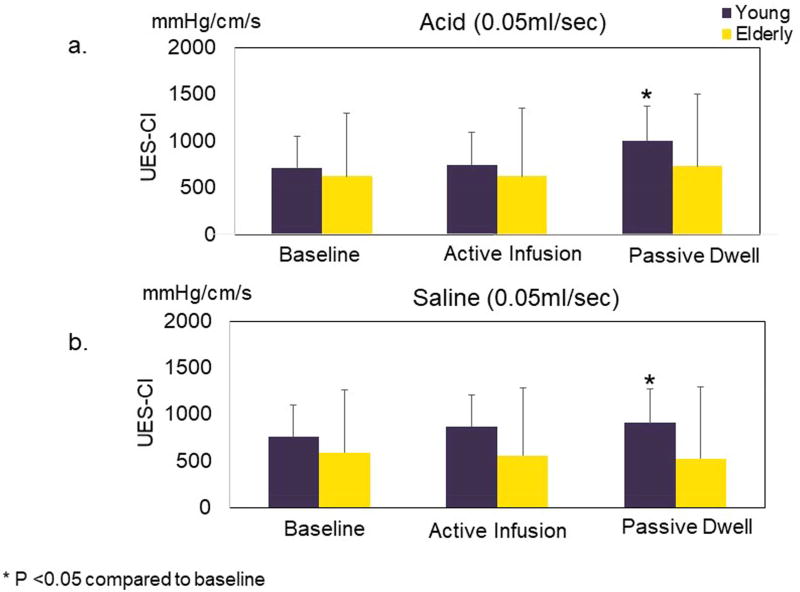
As seen for both acid and saline infusions, the UES pressure change in the young became perceptible toward the end of active infusion period and continued to enhance further during the post-infusion passive dwell time. In contrast, the elderly showed no appreciable UES-CI changes during or after the 0.05 ml/s infusions. Epoch-wise quantitative data about UES-CI during active infusion and passive post-infusion dwell period for both acid and saline infusions are presented in Figure 2 and 3. Average Epoch values for UES-CI for all groups and infusion types and periods are shown in Figure 5. As seen in the young group for both ultra-slow acid and saline infusions, UES-CI in the post infusion dwell time but not during active infusion period was significantly higher than the pre-infusion UES-CI values (p<0.05). In contrast, ultra-slow infusion of neither acid nor saline resulted in any significant increase in the UES-CI in any of the studied periods in the elderly.
Figure 5.
UES pressure response to ultra-slow (0.05ml/sec) (5a) acid and (5b) saline intra-esophageal infusion in young and elderly. Average of 10-second epoch UES-CI is presented. In both acid and saline, significant increase of UES-Cl was only observed in the young group and at the late stage of infusion which was sustained into dwell interval (also see figure 2 and 3). There was no significant UES response noted in the elderly either during active infusion or passive dwell interval. UES-CI: UES high-pressure zone contractile integral
The magnitude of increase in mean UES-CI relative to baseline was significantly higher in the young group during dwell interval for both acid and saline infusions (p<0.05) compared to the elderly. During active infusion, although the young group generally seemed to have a higher UES response compared to the elderly, the difference was only statistically significant for saline infusion.
Chemical composition of the infusate did not affect the UES response to ultra-slow infusion. When comparing UES response to ultra-slow saline infusion with that of acid infusion in both young and elderly, the magnitude of UES pressure change in response to two types of infusions was not significantly different from each other in either active infusion or passive dwell interval.
Esophageal body pressure response to slow and ultra-slow acid and non-acid simulated reflux in young and elderly
Development of secondary peristalsis in slow infusion
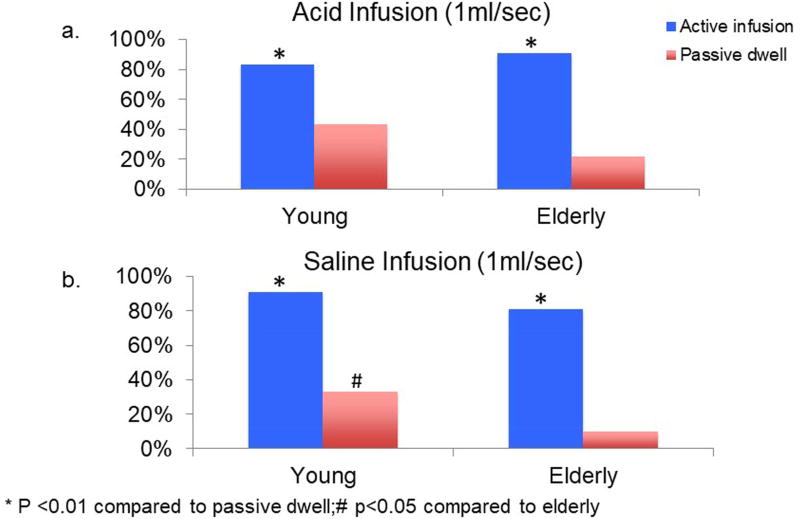
Slow infusion of both acid and saline resulted in development of at least one secondary peristaltic wave during 80–90% of infusions, respectively. The percent of post-slow infusion dwell periods during which any secondary peristalsis was developed was significantly lower than that of active infusion periods in both young and elderly and for both acid and saline infusions (p<0.01 Figure 6).
Figure 6.
Comparison of development of secondary peristalsis during slow (1ml/sec) infusion and passive dwell interval in (6a) acid infusion and (6b) saline infusion. In both acid and saline, active infusions resulted in more secondary peristalsis than those observed in the dwell intervals. During the dwell period, young subjects exhibited higher percentage of infusions resulting in secondary peristalsis compared to the elderly.
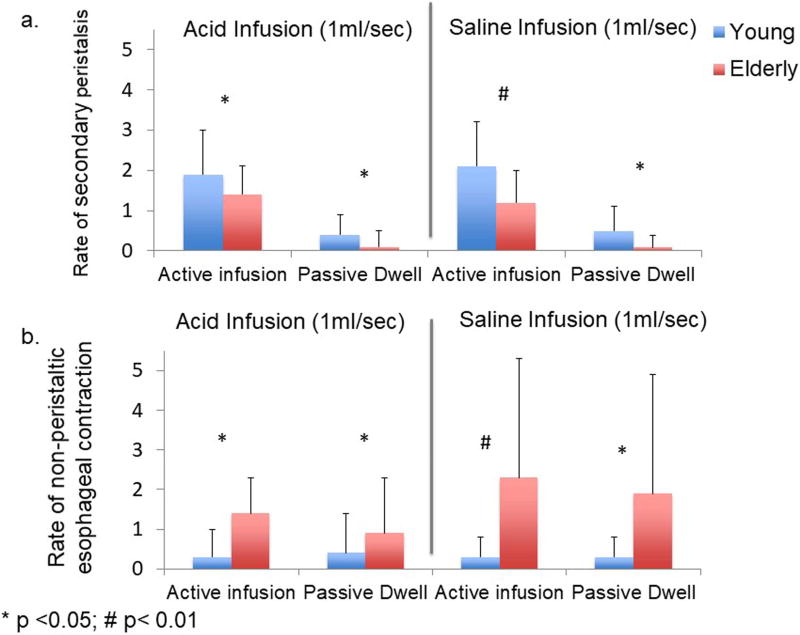
During slow infusions, although number of infusions resulting in at least one secondary peristaltic wave was similar between young and elderly, young subjects generated more frequent secondary peristalsis per infusion period than the elderly subjects (Figure 7a 1.9 vs 1.4, p<0.05 for acid; 2.1 vs 1.2, p<0.01 for saline). During post infusion dwell period, young subjects also had higher percentage of periods with secondary peristalsis compared to the elderly (figure 6, 43% vs 22%, p=0.08 for acid, 42% vs 10%, p<0.05 for saline). Similarly, young subjects showed a higher rate of secondary peristalsis per dwell period compared to the elderly (figure 7a, 0.4 vs 0.1, p<0.05 for acid; 0.5 vs 0.09, p<0.05 for saline).
Figure 7.
Rate of secondary peristalsis (7a) and rate of esophageal non-peristaltic contraction (7b) during slow (1ml/sec) acid and saline infusion as well as during their associated dwell interval (Mean ± SD). As seen in 7a, young subjects showed a higher frequency of secondary peristalsis compared to the elderly in both active infusion and passive dwell interval. As seen in figure 7b, the elderly exhibited more frequent non-peristaltic esophageal contraction during both slow infusion and passive dwell compared to young subjects.
There was no significant difference between acid and saline in frequency stimulation of secondary peristalsis during slow infusion and its subsequent dwell period.
The majority of secondary peristaltic waves were associated with a transient additional increase above the ongoing infusion-induced increase in UES-CI at the onset of secondary peristalsis regardless of infusion rate in both young (100%) and elderly (97%).
Development of secondary peristalsis in ultra-slow infusion
For both acid and saline, ultra-slow infusions resulted in much lower numbers of secondary peristalsis than slow infusion. Only scarce secondary peristalsis was observed during ultra-slow infusions in both age groups. There was no significant difference in the number of secondary peristalsis generated during ultra-slow infusions between young and elderly.
Development of non-peristaltic esophageal contraction
In both age groups, the frequency of esophageal non-peristaltic contractions was similar between active infusion and passive dwell periods for both slow infusion and ultra-slow infusions. However, the elderly tended to exhibit significantly more frequent non-peristaltic esophageal contraction during both periods compared to young subjects (Figure 7b). This difference was observed for both acid (1.4 vs 0.3, p<0.05 for active infusion; 0.9 vs 0.4, p<0.05 for passive dwell) and saline slow infusions (2.3 vs 0.3, p<0.01 for active infusion; 1.9 vs 0.3, p<0.05 for passive dwell). The differences among age groups were not significant for ultra-slow infusions.
Discussion
In this study, we determined the effect of aging on the esophago-UES contractile (EUCR) reflex and esophageal body pressure response to slow and ultra-slow acid and saline infusions. Study findings indicate that (1) for slow rate of fluid infusion, UES shows a similar contractile response in young and elderly. UES contracts not only during active infusion, but also continues its contractile response during post infusion dwelling of infusate; (2) for ultra-slow fluid infusion, there is a significant deterioration of EUCR in the elderly compared to young; (3) compared to young, in the elderly, there is a decreased frequency of secondary esophageal peristalsis, but increased frequency of non-peristaltic esophageal contraction in response to slow fluid infusions; (4) saline and acid infusions evoke similar EUCR and esophageal responses. These findings have significant clinical and physiologic ramifications due to the fact that the prevalence and complications of reflux disease increase with aging20,21 and a number of other complementary airway protective reflexes are found to deteriorate in the elderly.8–10
Mechanisms for prevention of pharyngeal reflux of gastric contents are not completely understood. This information is crucial to improve the diagnosis and management of a large number of aerodigestive tract disorders, ranging from dysphonia to aspiration pneumonia. These disorders depict the deleterious effects of gastric refluxate crossing the UES, the only anatomical barrier providing a high-pressure zone between the esophagus and pharynx,4 and coming in contact with airway mucosa.22, 23 A better understanding of the physiology of the UES relative to retrograde/orad movement of the gastric contents in the esophagus is critical for better defining the pathophysiology for airway protective mechanisms.
Although the existence of a high-pressure zone between the esophagus and pharynx was first described in 1955,24 understanding of the UES function as it relates to airway protection has progressed slowly compared to other fields of upper gut motility. In the mid 1950’s, the pressure enhancement of the UES in response to distension of the esophagus25 documented the existence of a EUCR, a vago-vagal reflex26 with progressively increasing sensitivity and stimulation frequency from distal to proximal esophagus.17,27 Subsequent studies showed that the receptors of this reflex are slowly adapting mechanoreceptors residing in the muscularis propria of the esophagus;28,29 with the afferent limb projecting to the medulla and its efferent limb projecting to the cricopharyngeus muscle, the effector organ of this reflex.28 More recently, other reflexes involving UES contraction that enhance the UES pressure barrier have been described; these include pharyngo-UES contractile reflex (PUCR) and laryngo-UES contractile reflex (LUCR),7,8 which are postulated to be provoked by refluxate in the pharynx and therefore, help prevent further entry of the gastric contents into the pharynx.
The significant variability of the physiologic triggers of the UES reflexes creates a major barrier in better defining and characterization of normal function of the UES as it relates to prevention of pharyngeal reflux. Hypothetically, while a large volume reflux may evoke the EUCR, smaller volumes of refluxate, especially in conditions such as aging that are associated with decreased sensitivity, may not elicit this reflex. The effect of aging on a number of airway protective reflexes8–10, such as PUCR, LUCR and reflexive pharyngeal swallow, has been previously studied. These studies have uniformly shown an increase in the threshold for stimulation, suggesting desensitization of these reflexes in the elderly. The effect of aging on EUCR triggered by balloon distention technique has been previously studied and has failed to show significant differences between young and elderly.30 However, it is noteworthy to mention that segmental distension of the esophagus, rarely, if at all, occurs during a gastroesophageal reflux event, therefore, although this technique provides a method for provocation of UES contraction, it is highly non-physiologic and may not be able to reveal the physiologic differences in the EUCR between young and elderly.30,31
Findings of the present study show that the UES remains contracted as long as the distending fluid remains in the esophageal lumen, even without any additional reflux episodes. This is of particular importance in situations when the refluxate has not been cleared from the esophagus by primary or secondary peristalsis, especially if the reflux events occur during sleep when airways are most vulnerable to aspiration. Experimental data suggest that a threshold volume ranging between 5 and 30 ml is required to stimulate secondary peristalsis in healthy individuals.32 This may explain the rarity of secondary peristalsis observed during ultra-slow infusion. In the absence of efficient secondary peristalsis to clear the esophageal stasis; a known risk factor for pharyngeal reflux,33 EUCR is the only remaining protective mechanism to prevent gastric contents from entering the pharynx. Earlier studies34,35 have shown that EUCR and secondary esophageal peristalsis can be stimulated in stage II and REM sleep, but is preempted by arousal in stages III and IV slow-wave sleep, providing airway protection during this physiologic unconscious state. These studies also showed that although UES pressure progressively declines with deeper stages of sleep, it could still reflexively contract during REM sleep, despite generalized hypotonia. An additional finding was that the threshold volume at the infusion rate of 2.7 ml/min for elicitation of EUCR and secondary peristalsis was significantly lower in REM compared with the stage II sleep and “awake” state. The infusion rate of 2.7 ml/min in these studies compares favorably with the ultra-slow rate of 0.05 ml/sec (3 ml/min) used in the present study in our awake volunteers. The findings of the current study indicate that young subjects can elicit EUCR in the late phase of fluid infusion at ultra-slow rate when the volume of infusate approximates 3 ml, and the response is sustained during the entire dwell period. In contrast to ultra-slow infusion, EUCR is triggered much earlier during slow infusion at the rate of 1 ml/sec. The present study indicates that the effect of aging on EUCR becomes evident during ultra-slow simulated reflux events and may potentially have special ramifications for airway protection in the elderly population during nocturnal reflux events.
Similar to previous findings using air injection to provoke secondary peristalsis,31 frequency stimulation of secondary peristalsis in response to intra-esophageal slow liquid infusion (both active infusion and dwell interval) in the elderly was found to be significantly lower compared to the young. The exact pathophysiologic mechanism for this difference is currently unknown. The initiation of secondary peristalsis is mediated by a vagal afferent pathway, with rapidly adapting mucosal and slowly adapting muscular mechanoreceptors both being required in such responses.28 The diminished secondary peristalsis in the elderly may be due to a defect of the afferent pathway, and possibly a decrease in the number of tension sensitive receptors.
The current study also indicates development of significantly more frequent non-peristaltic esophageal contractions among healthy elderly compared to young during both active infusion as well as post-infusion dwell period. We have previously reported12 a higher frequency of non-peristaltic esophageal contractions during intra-esophageal infusion in patients with gastroesophageal reflux disease and those with supra-esophageal complications of reflux disease compared to healthy controls. However, this study included elderly patients with supraesophageal complications of GERD. Considering the result of the current study, it is possible that the observed increase in non-peristaltic contraction in the previous study could also have been due to the effect of advanced age of the patients independent of their disease state.
In the present study, we simulated reflux events with two different chemical compositions; acid and saline. We did not detect any qualitative or quantitative difference in UES contractile response or elicitation of secondary peristalsis between acid and saline in either of the infusion rates. This finding is in concordance with a previous study that did not show any difference between UES responses to esophageal acid and water infusions.16
The results of the present study complement the existing knowledge on the physiology and pathophysiology of the UES and esophagus as they relate to gastroesophageal reflux and its supraesophageal complications. The findings of the present study need to be considered before attributing an observed abnormal UES function to a disease condition. In addition, they can inform the design of future studies evaluating patients with supraesophageal complications of reflux disease.
There are several limitations in the current study: by limiting our study subjects to healthy volunteers, we are unable to prospectively evaluate the clinical outcomes related to our physiologic findings, such as whether elderly with poorest EUCR or fewest secondary peristalsis are at higher risk for aspiration compared to young. Further studies are needed to determine this clinical correlation. In the present study, only two infusion rates / volumes were tested; 1ml/s and 0.05/s. It will be highly informative to test additional simulated reflux conditions to map the UES and esophageal responses to a wider range of refluxate volumes and rates that can potentially occur during actual reflux events.
In summary, both UES and esophageal body pressure responses to reflux events are deteriorated in the elderly compared to young. This deterioration includes lack of UES contractile response to ultra-slow intra-esophageal infusion as well as diminished incidence of secondary peristalsis in response to slow intra-esophageal infusion.
Supplementary Material
Acknowledgments
Supported in part by NIH P01DK068051 and T32DK061923
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial or personal conflict to disclose
Dr. Shaker had a major role in the study concept and design, acquisition and interpretation of the data, funding and supervision of the project and development of the manuscript. Dr. Mei had a major role in study concept, data acquisition, and preparation of the manuscript. Dr. Sanvanson had a major role to the study concept, data acquisition, interpretation of data, and preparation of the manuscript. Arshish Dua had a major role in study recruitment, data analysis, manuscript preparation. Dr. Dua had a major role in manuscript preparation. Dr. Edeani and Dr. Lynch had a major role in data acquisition, interpretation of data and preparation of manuscript. Dr. Siyuan Gao had a major role in data acquisition and interpretation of data. Amy Wilson had a major role in study recruitment.
References
- 1.Shaker R. Airway protective mechanisms: current concepts. Dysphagia. 1995;10:216–227. doi: 10.1007/BF00431413. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Lang I. Reflex mediated airway protective mechanisms against retrograde aspiration. Am J. Med. 1997;103(5A):64S–73S. doi: 10.1016/s0002-9343(97)00326-4. [DOI] [PubMed] [Google Scholar]
- 3.Shaker R, Hogan WJ. Reflex-mediated enhancement of airway protective mechanisms. Am J Med. 2000;108(suppl 4a):8S–14S. doi: 10.1016/s0002-9343(99)00289-2. [DOI] [PubMed] [Google Scholar]
- 4.Lang I. Development, Anatomy, and Physiology of the Upper Esophageal Sphincter and Pharyngoesophageal Junction. In: Shaker R, Belafsky PC, Postma GN, Eastering C, editors. Principe of Deglution. Springer; New York: pp. 235–256. [Google Scholar]
- 5.Shaker R, Dodds WJ, Ren J, et al. Esophago- glottal closure reflex: a mechanism of airway protection. Gastroenterology. 1992;102:857–861. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 6.Shaker R, Dodds WJ, Dantas EO, et al. Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology. 1990;98:1478–1484. doi: 10.1016/0016-5085(90)91078-k. [DOI] [PubMed] [Google Scholar]
- 7.Shaker R, Ren J, Xie P, et al. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol. 1997;273:G854–G858. doi: 10.1152/ajpgi.1997.273.4.G854. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura O, Easterling C, Aslam M, et al. Laryngo-upper esophageal sphincter contractile reflex in humans deteriorates with age. Gastroenterology. 2004;127(1):57–64. doi: 10.1053/j.gastro.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 9.Ren J, Xie P, Lang IM, et al. Deterioration of the pharyngo-UES contractile reflex in the elderly. Laryngoscope. 2000;110(9):1563–6. doi: 10.1097/00005537-200009000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Dua KS, Surapaneni SN, Kuribayashi S, et al. Effect of aging on hypopharyngeal safe volume and the aerodigestive reflexes protecting the airways. Laryngoscope. 2014;124(8):1862–8. doi: 10.1002/lary.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulualp SO, Toohill RJ, Kern M, et al. Pharyngo-UES contractile reflex in patients with posterior laryngitis. Laryngoscope. 1998;108(9):1354–7. doi: 10.1097/00005537-199809000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Babaei A, Venu M, Naini SR, et al. Impaired upper esophageal sphincter reflexes in patients with supraesophageal reflux disease. Gastroenterology. 2015;149(6):1381–91. doi: 10.1053/j.gastro.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szczesniak MM, Williams RB, Brake HM, et al. Upregulation of the esophago-UES relaxation response: a possible pathophysiological mechanism in suspected reflux laryngitis. Neurogastroenterol Motil. 2010 Apr;22(4):381–6. e89. doi: 10.1111/j.1365-2982.2009.01452.x. [DOI] [PubMed] [Google Scholar]
- 14.Andreollo NA, Thompson DG, Kendall GP, et al. Motor responses of the upper esophageal sphincter and body to intraluminal acid. Braz J Med Biol Res. 1989;22:51–60. [PubMed] [Google Scholar]
- 15.Wallin L, Boesby S, Madsen T. The effect of HCl infusion in the lower part of the oesophagus on the pharyngo-oesophageal sphincter pressure in normal subjects. Scand J Gastroenterol. 1978;13:821–826. doi: 10.3109/00365527809182197. [DOI] [PubMed] [Google Scholar]
- 16.Babaei A, Dua K, Naini SR, et al. Response of the upper esophageal sphincter to esophageal distension is affected by posture, velocity, volume, and composition of the infusate. Gastroenterology. 2012;142(4):734–743.e7. doi: 10.1053/j.gastro.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhardt DC, Shuck TJ, Bordeaux RA, et al. Human upper esophageal sphincter. Response to volume, osmotic, and acid stimuli. Gastroenterology. 1978;75:268–274. [PubMed] [Google Scholar]
- 18.Gupta A, Gulati P, Kim W, et al. Effect of postnatal maturation on the mechanisms of esophageal propulsion in preterm human neonates: primary and secondary peristalsis. Am J Gastroenterol. 2009;104(2):411–9. doi: 10.1038/ajg.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Duong HQ, Hoffmann RG, et al. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–38. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 20.Collen MJ, Abdulian JD, Chen YK. Gastroesophageal reflux disease in the elderly: more severe disease that requires aggressive therapy. Am J Gastroenterol. 1995;90:1053–1057. [PubMed] [Google Scholar]
- 21.Chait MM. Gastroesophageal reflux disease: Important considerations for the older patients. World J Gastrointest Endosc. 2010;2(12):388–396. doi: 10.4253/wjge.v2.i12.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciorba A, Bianchini C, Pelucchi S, et al. Gastroesophageal reflux and its possible role in the pathogenesis of upper aerodigestive tract disorders. Minerva Gastroenterol Dietol. 2007;53:171–180. [PubMed] [Google Scholar]
- 23.Toohill RJ, Kuhn JC. Role of refluxed acid in pathogenesis of laryngeal disorders. Am J Med. 1997;103:100S–106S. doi: 10.1016/s0002-9343(97)00333-1. [DOI] [PubMed] [Google Scholar]
- 24.Fyke FE, Jr, Code CF. Resting and deglutition pressure in the pharyngoesophageal region. Gastroenterology. 1955;29:24–34. [PubMed] [Google Scholar]
- 25.Creamer B, Schlegel J. Motor responses of the esophagus to distention. J Appl Physiol. 1957;10:498–504. doi: 10.1152/jappl.1957.10.3.498. [DOI] [PubMed] [Google Scholar]
- 26.Freiman JM, El-Sharkawy TY, Diamant NE. Effect of bilateral vagosympathetic nerve blockade on response of the dog upper esophageal sphincter (UES) to intraesophageal distention and acid. Gastroenterology. 1981;81:78–84. [PubMed] [Google Scholar]
- 27.Aslam M, Kern M, Shaker R. Modulation of oesophago-UOS contractile reflex: effect of proximal and distal esophageal distension and swallowing. Neurogastroenterol Motil. 2003;15:323–329. doi: 10.1046/j.1365-2982.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 28.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1246–G1263. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 29.Szczesniak MM, Fuentealba SE, Burnett A, et al. Differential relaxation and contractile responses of the human upper esophageal sphincter mediated by interplay of mucosal and deep mechanoreceptor activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G982–G988. doi: 10.1152/ajpgi.00496.2007. [DOI] [PubMed] [Google Scholar]
- 30.Shaker R, Ren J, Podvrsan B, et al. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol. 1993;264(3 Pt 1):G427–32. doi: 10.1152/ajpgi.1993.264.3.G427. [DOI] [PubMed] [Google Scholar]
- 31.Ren J, Shaker R, Kusano M, et al. Effect of aging on the secondary esophageal peristalsis: presbyesophagus revisited. Am J Physiol. 1995;268(5 Pt 1):G772–9. doi: 10.1152/ajpgi.1995.268.5.G772. [DOI] [PubMed] [Google Scholar]
- 32.Ulualp SO, Toohill RJ, Shaker R. Loss of secondary esophageal peristalsis is not a contributory pathogenetic factor in posterior laryngitis. Ann Otol Rhinol Laryngol. 2001;110:152–157. doi: 10.1177/000348940111000211. [DOI] [PubMed] [Google Scholar]
- 33.Torrico S, Corazziari E, Habib FI. Barium studies for detecting esophagopharyngeal reflux events. The American journal of medicine. 2003;115(Suppl 3A):124S–129S. doi: 10.1016/s0002-9343(03)00210-9. [DOI] [PubMed] [Google Scholar]
- 34.Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–267. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj JS, Bajaj S, Dua KS, et al. Influence of sleep stages on esophago-upper esophageal sphincter contractile reflex and secondary esophageal peristalsis. Gastroenterology. 2006;130(1):17–25. doi: 10.1053/j.gastro.2005.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.