Abstract
Background & Aims
Screening of individuals who have a high risk of pancreatic ductal adenocarcinoma (PDAC), due to genetic factors, frequently leads to identification of pancreatic lesions. We investigated the incidence of PDAC and risk factors for neoplastic progression in individuals at high risk for PDAC enrolled in a long-term screening study.
Methods
We analyzed data from 354 individuals at high risk for PDAC (based on genetic factors of family history), enrolled in Cancer of the Pancreas Screening cohort studies at tertiary care academic centers from 1998 through 2014 (median follow-up time, 5.6 years). All subjects were evaluated at study entry (baseline) by endoscopic ultrasound and underwent surveillance with endoscopic ultrasound, magnetic resonance imaging, and/or computed tomography. The primary endpoint was the cumulative incidence of PDAC, pancreatic intraepithelial neoplasia grade 3, or intraductal papillary mucinous neoplasm with high-grade dysplasia (HGD) after baseline. We performed multivariate Cox regression and Kaplan-Meier analyses.
Results
During the follow-up period, pancreatic lesions with worrisome features (solid mass, multiple cysts, cyst size >3 cm, thickened/enhancing walls, mural nodule, dilated main pancreatic duct >5 mm, or abrupt change in duct caliber) or rapid cyst growth (>4 mm/year) were detected in 68 patients (19%). Overall, 24/354 patients (7%) had neoplastic progression (14 PDACs and 10 HGDs) over a 16-year period; the rate of progression was 1.6%/year and 93% had detectable lesions with worrisome features before diagnosis of the PDAC or HGD. Nine of the 10 PDACs detected during routine surveillance were resectable; a significantly higher proportion of patients with resectable PDACs survived 3 years (85%) compared with the 4 subjects with symptomatic, unresectable PDACs (25%), which developed outside surveillance (log rank P<.0001). Neoplastic progression occurred at a median age of 67 years; the median time from baseline screening until PDAC diagnosis was 4.8 years (inter-quartile range, 1.6–6.9 years).
Conclusions
In a long-term (16-year) follow-up study of individuals at high-risk for PDAC, we found most PDACs detected during surveillance (9/10) to be resectable, and 85% of these patients to survive for 3 years. We identified radiologic features associated with neoplastic progression.
Keywords: PanIN-3, IPMN, familial pancreatic cancer, early detection
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the third, and is projected to soon become the second leading cause of cancer deaths in the USA1. The estimated incidence rate of developing PDAC is 1.5%, with 53,670 new cases and 43,090 death expected in 20172, 3. The 5-year survival rates of patients with PDAC remains low (8.2%)2. Screening for pancreatic cancer is not recommended for the general population (U.S. Preventive Services Task Force, http://www.uspreventiveservicestaskforce.org/) but is being evaluated for patients with a significantly elevated risk. Risk can be estimated by considering the number of affected blood relatives who have had pancreatic cancer (familial pancreatic cancer or FPC kindred defined as having at least 2 first degree relatives with pancreatic cancer)4, 5 and knowing whether an individual carries a deleterious germline mutation in a pancreatic cancer susceptibility gene6. The latter include germline mutations in BRCA1, BRCA2, ATM, PALB2, CDKN2A (familial atypical multiple mole melanoma syndrome), mismatch repair genes MLH1, MSH2, MSH6, PMS2 (Lynch syndrome), STK11 (Peutz-Jeghers syndrome), and PRSS1 (hereditary pancreatitis)7–13. Several large academic centers have conducted screening programs for these asymptomatic high-risk individuals (HRIs)14–24. An international consortium of experts recommended pancreatic screening and surveillance be evaluated for HRI with an estimated lifetime risk of PDAC of > 5%4.
Pancreatic cancer surveillance programs utilizing a combination of endoscopic ultrasound, magnetic resonance imaging, and computed tomography scans have detected a high prevalence of asymptomatic pancreatic lesions, mostly cysts, that represent the major associated precursor lesions (pancreatic intraepithelial neoplasia, PanINs; intraductal papillary mucinous neoplasms, IPMNs), in HRI. Most of the pancreatic cysts identified in HRI are small (<1 cm) and often multiple; their prevalence is much more common than in the general population and increases with patient age18. Depending on the age and other characteristics of the study population and the imaging modalities, the prevalence of precursor lesions identified by screening has ranged from 6%-52%16, 18, 21, 22, 24–26. Pancreatic neuroendocrine tumors (PanNETs) have also been detected16, 18, 21, 22, 24–26. Most studies evaluating the diagnostic yield of pancreatic screening have only reported lesions detected at baseline screening12, 14,15, 16, 17, 18, 20, 21, 24, 26. Identification of screening abnormalities and other risk factors that predict neoplastic progression to PDAC or high grade precursor neoplasms might improve surveillance programs and guide management of detected lesions.
The aims of this study were to determine the cumulative incidence of PDAC and high grade precursor neoplasms (IPMN-HGD and PanIN-3) and identify risk factors that predict neoplastic progression in HRI undergoing long term surveillance after baseline screening. We also report the outcomes of HRI with detected neoplasms.
METHODS
Study Design and Patients
From 1998 to 2014, 581 asymptomatic HRI were prospectively enrolled into one of the Johns Hopkins Cancer of the Pancreas Screening studies (CAPS 117, CAPS 216, CAPS 318, or CAPS 4) at participating tertiary referral American academic medical centers with comprehensive multidisciplinary pancreas screening programs. The institutional review boards of The Johns Hopkins Medical Institutions and all participating sites approved the CAPS studies. All subjects provided informed consent for baseline screening and follow-up.
Inclusion criteria for HRI in the CAPS studies:
Patients who met clinical criteria for Peutz-Jeghers syndrome, or who had a mutation in the STK11 gene, and who were at least 30 years old;
Individuals from a familial pancreatic cancer (FPC) kindred who had at least one affected first-degree relationship to the HRI, and were at least 50 years old (CAPS studies 1-3, from 1998-2010) or at least 55 years (CAPS 4 study, from 2010) or 10 years younger than the youngest pancreatic cancer in the family.
Individuals with confirmed germline mutations in the BRCA1, BRCA2, PALB2, PRSS1, CDKN2A, or MLH1, MSH2, MSH6, PMS2 (Lynch syndrome), with at least 1 affected first or second degree relative, and at least 50 years old, or 10 years younger than the youngest pancreatic cancer in the family.
Since this study evaluated predictors of progression during surveillance, we did not include HRI enrolled in the CAPS studies who had: 1) less than 6 months of follow-up after baseline screening, 2) surveillance at an outside institution without available medical records or clinical follow-up (lost to follow-up at John Hopkins Hospital), 3) prior surgery that prevented complete EUS examination of the pancreas. From 584 HRI enrolled in the CAPS 1-4 studies from 1998-2014, 104 patients were excluded from the current study because they continued surveillance at an outside institution, and 123 patients were excluded for less than six months of follow-up after baseline screening.
Surveillance Methods, Clinical Management, and Final Diagnosis
HRI were referred to the CAPS studies through the Johns Hopkins National Familial Pancreas Tumor Registry (NFPTR) (www.nfptr.org), physicians, genetic counselors, or by self-referral. After providing informed consent and completing a detailed questionnaire, all HRI were screened at baseline with EUS. Individuals who participated in the CAPS3 study also underwent baseline CT, individuals who enrolled in the CAPS 1 study (1998-2002, n = 38) underwent baseline EUS only17 and those in the CAPS 2 study (2002-2004, n = 78) underwent both EUS and CT16.
Since 2005, surveillance after baseline screening was performed on the CAPS 3 subjects (n = 216)18 continuing follow-up at Johns Hopkins and all CAPS 4 subjects enrolled at Hopkins (n = 249) using a combination of endoscopic ultrasound (EUS), magnetic resonance cholangiopancreatography (MRI/MRCP), and/or computed tomography (CT) at intervals dependent upon the presence or absence of neoplastic-type pancreatic lesions. HRI with a normal pancreas or EUS features of chronic pancreatitis were followed annually. Those with pancreatic cysts or indeterminate radiologic lesions underwent more frequent imaging with EUS and/or MRI or CT, according to published international guidelines4, 27, 28; every 6-12 months for those without a mural nodule or dilated pancreatic duct and every 3-6 months for larger cysts or cysts with worrisome features. Stable or improved appearance of pancreatic lesions resulted in decreased surveillance imaging frequency to every 12 months.
EUS-guided fine needle aspiration was not routinely performed on small cystic lesions < 1 cm.
Recommendations for pancreatic surveillance and surgical treatment were discussed at CAPS multidisciplinary clinical conferences and decision-making was individualized. After Sendai27 and Fukuoka28 international consensus guidelines for management of sporadic IPMNs became available, these were used as a guides for data collection and patient management, recognizing that these guidelines were not developed primarily for high risk individuals. Worrisome features were defined in this study according to the Sendai and Fukuoka International Consensus Guidelines for management of a mucinous cysts27, 28, included cyst size ≥ 3 cm, thickened/enhancing cyst walls, main pancreatic duct (MPD) dilation >5 mm, mural nodule in the cyst or main pancreatic duct, abrupt change in MPD caliber, or rapid cyst growth rate > 2 mm in 6 months or > 4 mm in 1 year. The latter was defined based upon studies reporting rapid cyst growth rate in sporadic IPMNs of > 2-5 mm per year associated with increased risk of malignancy29, 30 Suspicious cytology for pancreatic malignancy, when available, was also considered a worrisome feature27, 28, 31. For this study, any solid mass > 5 mm was considered a worrisome feature, if confirmed by repeat EUS, at least two imaging modalities, and/or increasing size.
Surgical intervention was offered to those suspected of having pancreatic cancer or high-grade dysplasia, based on imaging or cytology. In some cases, surgical resection was undertaken because of concern that it might be difficult to detect an early-stage pancreatic cancer in HRI with numerous pancreatic cysts. HRIs with pancreatic lesions with any worrisome feature were discussed in a multidisciplinary clinical conference consisting of a team of surgeons, gastroenterologists, radiologists, and pathologists. Decision-making was individualized, using clinical criteria and published guidelines for pancreatic cyst management. Most pancreatic resections were performed at The Johns Hopkins Hospital by highly experienced surgeons specializing in pancreaticobiliary diseases (CJY, RDS, CW, MW).
The final diagnoses were made by surgical pathology or cytology (percutaneous or EUS-guided fine needle aspirates). All pathological diagnoses were made by an expert pathologist (RHH) using standard and consensus international classification systems. Pathological reports and slides were reviewed for HRI who had surgery outside of Johns Hopkins. If the pathological specimen had multiple pancreatic lesions, the lesion with the highest pathologic grade was considered for endpoint analysis. Clinical findings and imaging characteristics were tracked over time to the last follow-up by prospective recording of imaging test results at Johns Hopkins Hospital and retrospective review of outside medical records and images, when applicable, up to December 2016.
Statistical Analysis
“Neoplastic progression” was defined the development of pathologically-proven PDAC and/or high grade PDAC precursor neoplasms (PanIN-3, IPMN-HGD). “Radiologic progression” was defined as the development of a lesion with one or more worrisome features after baseline imaging. Pancreatic neuroendocrine neoplasms (PanNET) were defined as well-differentiated neoplasms greater than 0.5 cm in size with predominantly neuroendocrine differentiation and low proliferation indices (2010, 2017 World Health Organization)32, 33 PanNETs were not included in the primary and secondary outcome variables but listed separately as one of the final pathological diagnoses.
Primary study outcome variables were: 1) the demographic clinical, and imaging factors associated with neoplastic progression (analyzed using Chi-square/Fisher’s exact test for categorical variables and t-test/Mann-Whitney test for continuous variables), 2) the rate of neoplastic progression stratified by age and adjusted for varying rates of radiologic progression(analyzed by Kaplan Meier and Cox regression analyses), and, 3) the hazard rates of neoplastic progression among all radiologic progressors and those with different types of lesions(solid mass, cyst or pancreatic duct lesions), adjusted for clinical factors and baseline pancreatic abnormalities (analyzed by Cox proportional hazards regression modeling).
Secondary study outcome variables were: 1) the cumulative incidence of pathologically proven PDAC and high grade PDAC precursors (PanIN-3, IPMN-HGD), 2) the resectability and tumor stage of clinically relevant pancreatic neoplasms detected by screening/surveillance, and 3) the median survival time, 3-year survival rate, and overall mortality of neoplastic progressors. All statistical analysis were performed using Stata® version 14.1 (Stata Corp, College Station, Texas).
RESULTS
Baseline Characteristics of Screened HRI
The baseline characteristics of the 354 HRI are summarized in Table 1. The majority of HRI (97%) were familial PC relatives, 16% of those screened had a known deleterious germline variant. About half the study population was male, predominantly Caucasian race, with the mean age at baseline of 56.4 years (range 22-81).
Table 1.
Baseline Patient Characteristics
| Progressor (n = 24) |
Non-Progressor (n = 330) |
Total (n = 354) |
P-value | ||
|---|---|---|---|---|---|
| PDAC (n = 14) |
High Grade Precursor Neoplasm1 (n = 10) |
||||
| Mean age (range) | 62.5(45-75) | 64.4(47-78) | 55.8(29-81) | 56.4 (29-81) | <0.0001 |
| Age > 60 years | 9(70%) | 7(64%) | 117(35%) | 133(38%) | 0.009 |
| Male | 7 (50%) | 1(10%) | 160 (48%) | 168 (48%) | 0.052 |
| Race and Ethnicity | 1.0 | ||||
| Black | 0 | 0 | 8(2.4%) | 8 (2.2%) | |
| White | 14(100%) | 10(100%) | 308 (93%) | 332 (94%) | |
| Asian | 0 | 0 | 4(1%) | 4 (1.1%) | |
| More than one race | 0 | 0 | 9(3%) | 9 (2.5%) | |
| Hispanic | 0 | 0 | 1(0.3%) | 1 (0.2%) | |
| Familial PC | 14(100%) | 8(80%) | 322(94%) | 344 (97%) | 0.043 |
| 3 or more FDR with PDAC | 3(21%) | 0 | 26(8%) | 29(8%) | 0.167 |
| 3 or more relatives with PDAC | 11(79%) | 5(50%) | 179(54%) | 195(55%) | 0.178 |
| Mutation carrier | 1(7%) | 3(30%) | 53(16%) | 57 (16%) | 0.327 |
| Peutz-Jeghers syndrome | 0 | 2 (20%) | 8(2%) | 10(3%) | 0.043 |
| CDKN2A | 0 | 0 | 4(1%) | 4 (1%) | 1.0 |
| BRCA1/BRCA2/PALB2 | 1(7%) | 1(10%) | 39(12%) | 41(12%) | 1.0 |
| Lynch syndrome | 0 | 0 | 1(0.3%) | 1(0.3%) | 1.0 |
| PRSS1 | 0 | 0 | 1(0.3%) | 1(0.3%) | 1.0 |
| Current smoking | 1(7%) | 3(30%) | 25(86%) | 29(8%) | 0.07 |
| Ever smoked | 5(36%) | 5(50%) | 119(36%) | 129 (36%) | 0.702 |
| Personal history of other cancer | 4(29%) | 3(30%) | 64(19%) | 71 (20%) | 0.419 |
| Type II diabetes | 1(7%) | 0 | 27(8%) | 28 (8%) | 1.0 |
| BMI ≥30 | 2(14%) | 3(30%) | 82(24%) | 87(25%) | 0.694 |
| Dilated MPD at baseline2 | 9(64%) | 5(50%) | 62(19%) | 76 (21%) | <0.0001 |
| Three or more cysts at baseline | 5(36%) | 8(80%) | 36(11%) | 49(14%) | <0.0001 |
Familial PC (pancreatic cancer) = kindred with at least one pair of affected relatives with pancreatic ductal adenocarcinoma (PDAC)
FDR = first-degree relatives; MPD = main pancreatic duct
High grade precursor neoplasm = intraductal papillary mucinous neoplasm (IPMN) with high grade dysplasia or pancreatic intraepithelial neoplasia (PanIN)-3
Dilated MPD defined by Rosemont criteria (≥ 3.5 mm in the head, ≥ 2.5 mm in the body, and/or ≥ 1.5 mm in the tail), < 5 mm
One hundred eighty-five HRI (52% of 354) had no lesions detected at baseline. Fourteen HRI (4%) had solid hypoechoic masses > 1 cm or nodules < 1 cm at baseline, and 4 (1.1%) had both cysts and solid lesions. The remaining 151 (43% of 354) HRI had no solid lesions and one or more cystic lesions detected at baseline, and 14% (49/354) had 3 or more cysts. The mean size of the largest cyst at baseline was 8 mm (range 1.6 mm – 28 mm). At baseline, 76 (21.4%) HRI had a mildly dilated main pancreatic duct according to the Rosemont criteria34 (≥ 3.5 mm in the head, ≥ 2.5 mm in the body, and/or ≥ 1.5 mm in the tail) but < 5 mm (Table 1).
Cumulative Incidence of Neoplastic Progression and Types of Detected Neoplasms
The median follow-up time for the entire cohort was 5.6 years (interquartile range 3.9-8.9 years). Fifty-four (79%) of the 68 HRI with pancreatic lesions with worrisome features detected by surveillance had diagnostic pathology either by surgical resection (n = 44) and/or percutaneous or EUS-guided fine needle aspiration (n = 6). Thirty of these 54 asymptomatic HRI (8.5% of the entire cohort) were diagnosed with a clinically significant primary pancreatic neoplasm: 14 had PDAC (4% or the entire cohort), 10 had IPMN-HGD and/or PanIN-3 (3% of the cohort) and 6 had PanNET > 5 mm (1.7% of the entire cohort). The overall detection rate for PDAC or a high grade dysplasia in 354 HRI over the 16-year study period was 7%, including prevalent and incident neoplasms. Detailed information about the 24 patients with PDAC or high grade dysplasia is summarized in Table 2. The cumulative incidence for PDAC or a high grade precursor neoplasm during the study period (excluding 2 asymptomatic cancers detected at baseline) was 22/339 or 6.5%.
Table 2.
Description of 24 High Risk Individuals with Neoplastic Progression
| Patient No. | Age at Diagnosis (years)/Gender | HRI Category | Time to Detection of Tumor (years) | Tumor Type/Size (mm) | Management | TNM stage | Outcome/Survival time from Diagnosis (years) | Cause of Death |
|---|---|---|---|---|---|---|---|---|
| PDACs Detected and Operated During Surveillance | ||||||||
| 1 | 72/F | FPC | 0.2 | PDAC/2 | Distal pancreatectomy | T1N1M1 | Alive/10.9 | NA |
| 2 | 46/F | FPC | 0.6 | PDAC/28 | Whipple | T2N1M0 | Died/12.3 | Gastric cancer surgery complications |
| 3 | 66/M | FPC | 2.2 | PDAC/28 | Distal pancreatectomy | T2N1M0 | Died/1.1 | PDAC |
| 4 | 79/F | FPC | 4.4 | PDAC/25 | Whipple | T3N1M0 | Alive/1.8 | NA |
| 5 | 73/F | FPC | 4.4 | PDAC/15 | Whipple | T3N0M0 | Alive/3.9 | NA |
| 6 | 70/M | FPC | 5.2 | PDAC/45 | No surgery; metachronous PDAC 5 years after Whipple formain duct IPMN | T4N1M1 | Died/0.2 | PDAC |
| 7 | 51/F | FPC | 6.8 | PDAC/35 | Distal pancreatectomy | T2N1M0 | Alive/7.2 | NA |
| 8 | 55/M | FPC | 9.7 | PDAC/27 | Distal pancreatectomy 8 years after Whipple for IPMN-adenoma | T3N0M0 | Died/1.1 | PDAC |
| 9 | 70/M | FPC | 9.1 | PDAC/7 | Total pancreatectomy | T1NM0 | Alive/3.7 | NA |
| 10 | 74/M | FPC | 9.9 | PDAC/36 | Distal pancreatectomy | T3N1M0 | Died/3.7 | PDAC |
| High Grade Precursor Neoplasms Detected and Treated During Surveillance | ||||||||
| 11 | 67/F | FPC | 0.3 | PanIN3 | Total pancreatectomy | NA | Alive/10.2 | NA |
| 12 | 56/F | FPC | 0.3 | Combined IPMN-HGD | Total pancreatectomy | NA | Alive/8.6 | NA |
| 13 | 53/F | FPC | 0.4 | PanIN3 | Total pancreatectomy | NA | Alive/4.4 | NA |
| 14 | 67/F | FPC | 2.0 | IPMN-HGD/10; IPMN-HGD/5 | Distal pancreatectomy followed by completion Whipple | NA | Alive/4.8 | NA |
| 15 | 58/F | FPC | 2.5 | IPMN-HGD; PanIN-3 | Whipple | NA | Alive/9.9 | NA |
| 16 | 66/F | PJS | 2.5 | IPMN-HGD | Distal pancreatectomy1 | NA | Alive/4.1 | NA |
| 17 | 76/F | FPC | 2.0 | PanIN3 | Whipple | NA | Alive/7.2 | NA |
| 18 | 56/F | FPC | 1.5 | PanIN3 in MPD | Whipple | NA | Alive/8.1 | NA |
| 19 | 72/M | FPC | 1.9 | IPMN-HGD | Distal pancreatectomy2 | NA | Alive/7.2 | NA |
| 20 | 47/M | PJS | 0.3 | IPMN-HGD | Whipple | NA | Alive/14.7 | NA |
| Tumors Detected Outside Surveillance (Late or Stopped Surveillance) | ||||||||
| 21 | 77/M | FPC | 1.6 | PDAC/25 | No surgery | T2N1M1 | Dead/2.2 | PDAC |
| 22 | 68/M | FPC | 4.5 | PDAC/22 | No surgery | T2N1M1 | Dead/0.3 | PDAC |
| 23 | 59/M | FPC | 9.0 | PDAC/25 | Total pancreatectomy | T2N1M0 | Dead/4.0 | PDAC |
| 24 | 82/F | FPC | 6.9 | PDAC/* | No surgery | TxNxM1 | Dead/0.5 | PDAC |
PDAC = pancreatic ductal adenocarcinoma,
first-degree relatives diagnosed with PDAC at <55 years
Patient reported diagnosis of an unresectable cancer, tumor size unknown.
One patient with multiple pancreatic lesions and abdominal lymphadenopathy was diagnosed with a metastatic pancreatic B cell lymphoma by EUS-guided fine needle aspiration. The remaining 23 of the 54 HRI (43%) with a pathologic diagnoses had lower grade dysplasia or non-dysplastic lesions in their resection specimens (IPMN-LGD/MGD, PanIN-1/2, benign PanNET microadenomas <0.5 cm, serous cyst adenoma). Most of these patients underwent resection in the early years of the CAPS program.
Outcomes of Non-Operated HRI with Worrisome Features
Of the 68 HRI who had lesions with worrisome radiological features at any time during the study period, 24 (35%) did not undergo surgery; and were followed for an average of 5.8 years (range 1-12 years). Five of these 24 HRI had cyst growth during follow-up but otherwise no evidence of neoplastic progression. Fourteen HRI with a dilated MPD had stable or improved duct diameter. Five non-operated HRI developed a solid mass diagnosed by EUS-FNA: 3 HRI had advanced PDAC (stopped or late for surveillance, Table 2), one HRI had the lymphoma mentioned above, and one HRI had a PanNET confirmed by EUS-FNA but the patient declined surgery.
Factors Associated with Neoplastic Progression
The mean age of HRI who developed PDAC or high grade dysplasia in this cohort was significantly greater than non-progressors (p < 0.0001, Table 1). Age > 60 years at baseline was associated with radiologic progression (HR 3.1, adjusted model, Supplementary Table 2). However, age was not a significant predictor of neoplastic progression in the multivariate analyses (hazard ratio or HR 1.64, 95% CI 0.65-4.14, Table 3). A greater proportion of HRI who developed PDAC or a high grade precursor neoplasm had a dilated main pancreatic duct at baseline compared to non-progressors (64% and 50% versus 19%, respectively, p <0.0001, Table 1) but the hazard ratio for a dilated main pancreatic duct was not significant in the multivariate analysis (HR 1.68, 95% CI 0.70-4.06, Table 3).
Table 3.
Cox Proportional Hazards Regression Model for Neoplastic Progression after Adjusting for Time Varying Radiologic Progression and the Type of Radiological Progression
| Adjusted Model | |||
|---|---|---|---|
| Hazard Ratio | p-value | 95% CI | |
| Any radiologic progression | 23.96 | <0.0001 | 9.43, 60.87 |
|
| |||
| Type of radiologic progression | |||
| Cyst or duct changes | 41.20 | <0.0001 | 12.52, 135.53 |
| Solid mass | 422.60 | <0.0001 | 102.42, 1743.74 |
| Age at baseline > 60 years | 1.64 | 0.29 | 0.65, 4.14 |
| Mutation positive | 0.66 | 0.53 | 0.18, 2.41 |
| Total lesions at baseline ≥ 3 | 4.85 | <0.0001 | 2.02, 11.64 |
| Dilated MPD at baseline1 | 1.68 | 0.25 | 0.70, 4.06 |
Dilated MPD defined by Rosemont criteria1 (≥ 3.5 mm in the head, ≥ 2.5 mm in the body, and/or ≥ 1.5 mm in the tail), < 5 mm
Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009;69:1251-61.
Neoplastic progressors were more likely to have multiple cysts (3 or more) at baseline, compared to non-progressors (PDAC 36% and high grade precursor neoplasm 80%, versus others 11%, p <0.0001), even after adjusting for other factors (HR 4.85, 95% CI 2.02-11.64, Table 3). Specific EUS and radiologic features (worrisome features) were associated with neoplastic progression (univariate and multivariate analysis, Supplementary Tables 1 and 2). In particular, the presence of a solid mass, mural nodule, thickened cyst wall, rapid cyst growth rate, and a MPD dilated to > 5 mm at any time during surveillance, were associated with the development of PDAC or high grade precursor neoplasm.
After adjusting for time varying rates of radiologic progression, Cox-proportional multivariate regression analysis showed that any radiologic progression was the strongest predictor for developing PDAC or a high grade precursor neoplasm (23-fold, p < 0.0001, Table 3; Figure 2). Specifically, the adjusted risk for neoplastic progression was 41-fold higher (95% CI 12.52-135.53, Table 3) for HRI with radiologic progression in a pancreatic cyst or in the main pancreatic duct, and 423-fold higher for HRI with a solid mass (95% CI 102.42-1743.74).
Figure 2.
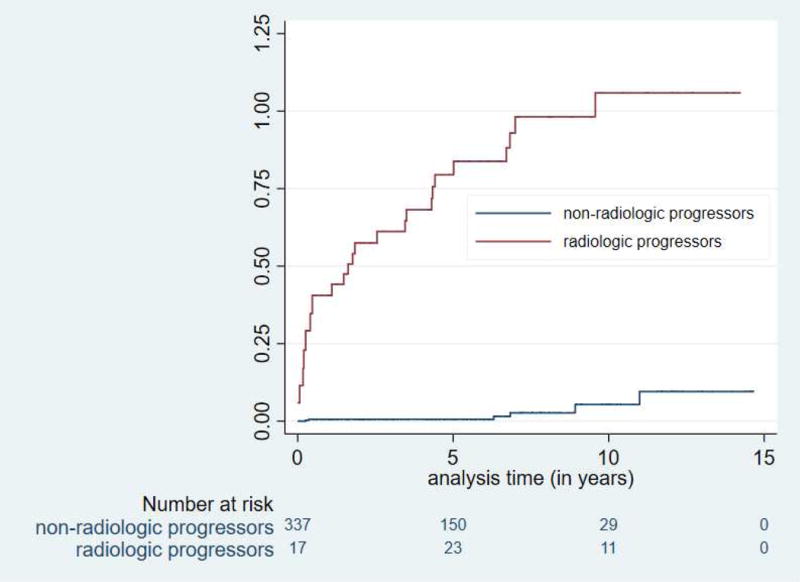
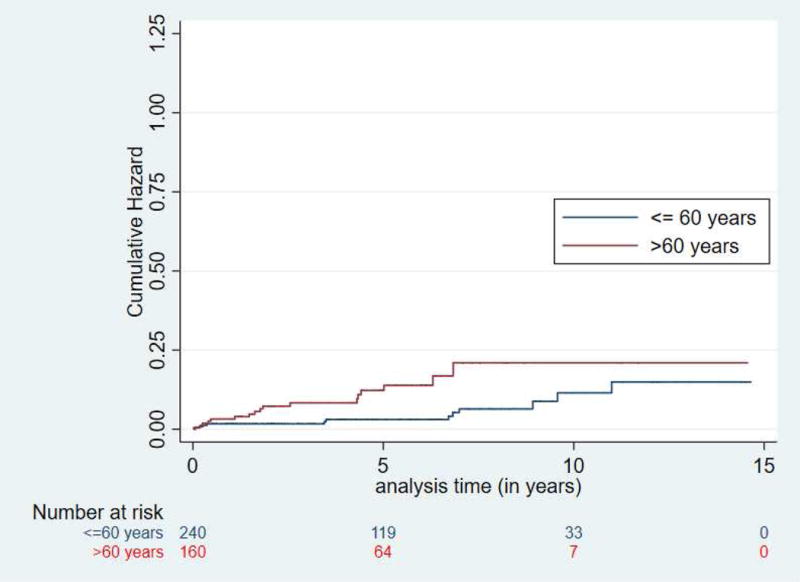
Risk for neoplastic progression was significantly increased in HRI with worrisome features (radiologic progression) (p < 0.0001) (2A) and those beginning screening at age > 60 (2B).
Rates of Progression and Time to Radiologic and Neoplastic Progression
The estimated rate of radiological progression after baseline screening, after excluding the 17 HRI that had a solid mass or cyst with worrisome features at baseline, was 4.3% per year (Supplementary Figure 1A). Progression rates were higher among those HRI > 60 years old at baseline screening (Supplementary Figure 1B). The median time for any radiologic worrisome feature to occur in HRI after baseline was 13.1 months (IQ range 0.2 – 52 months). The median time for radiologic progression in HRI who developed PDAC was 4.3 years (IQ range 1.0-6.5 years).
Neoplastic progression occurred at a rate of 1.6% per year (Figure 1) and at a much higher and faster rate in HRI with a lesion with a worrisome feature (Figure 2A). Twelve of the 14 HRI with PDACs and 8 of the 10 with high grade precursor neoplasms (IPMN-HGD and PanIN-3) resected were incident lesions detected during follow-up (Table 2). The median time to neoplastic progression from baseline screening for the 14 HRI who developed invasive PDAC after baseline screening was 4.8 years (IQ range 1.6-6.9 years), but was significantly shorter in those beginning screening at 60 years or older (median 1.7 years, IQ range 0.5-4.4 years) compared to younger HRI (median 5.2 years, IQ range 0.4-8 years) (Figure 2B). The mean age for HRI who developed PDAC was 67.6 years, (IQ range 59-74). Twelve of the 14 PDAC patients and 7 of the 10 HRI with HGD were > 60 years old at the time of diagnosis.
Figure 1.
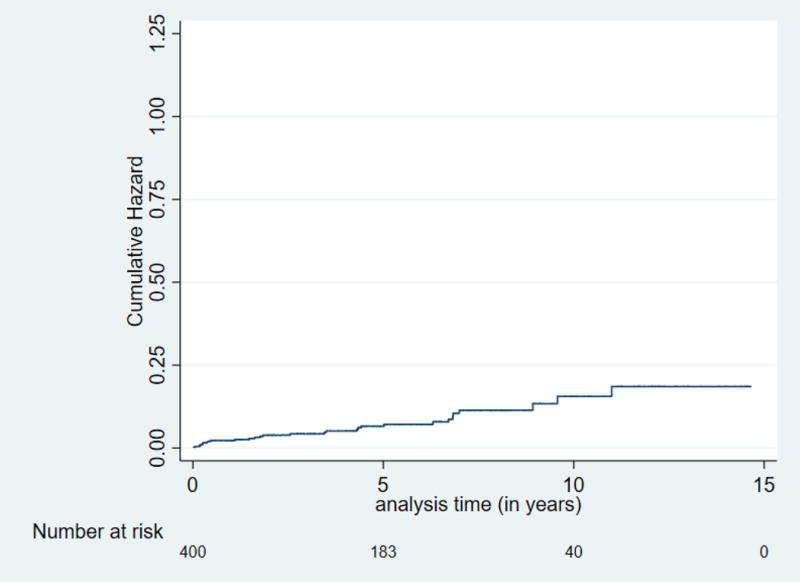
Cumulative risk (hazard) for neoplastic progression (PDAC, IPMN-HGD, or PanIN-3) for high risk individuals after baseline screening. Overall neoplastic progression rate was 1.6% per year.
Outcomes of Surveillance and Treatment
Detailed information on the HRI with pancreatic neoplasia is summarized in Table 2. The mean diameter of the screening-detected PDACs was 24.8 mm (range 7-45 mm). The two stage 1 pancreatic cancers were detected by EUS and not visualized by preoperative MRI or CT. Two of the resected PDACs were TNM stage IA (size 5 and 7 mm) (Figure 3), two were stage IIA (size 15 and 27 mm), and 7 were state IIB (size range 13-36 mm). Nine of the 10 invasive PDACs detected in patients followed according to the CAPS surveillance schedule were asymptomatic and resectable, whereas only 1 of the 4 patients presenting with symptoms had resectable disease. The latter had either stopped or were late for surveillance (Patients 21-24 Table 2) by a median of 37 months (IQ range 10.8-66 months). The other advanced metastatic PDAC developed in the remnant pancreas of Patient 6 five years after a Whipple operation for a main duct IPMN, despite annual CT surveillance.
Figure 3.
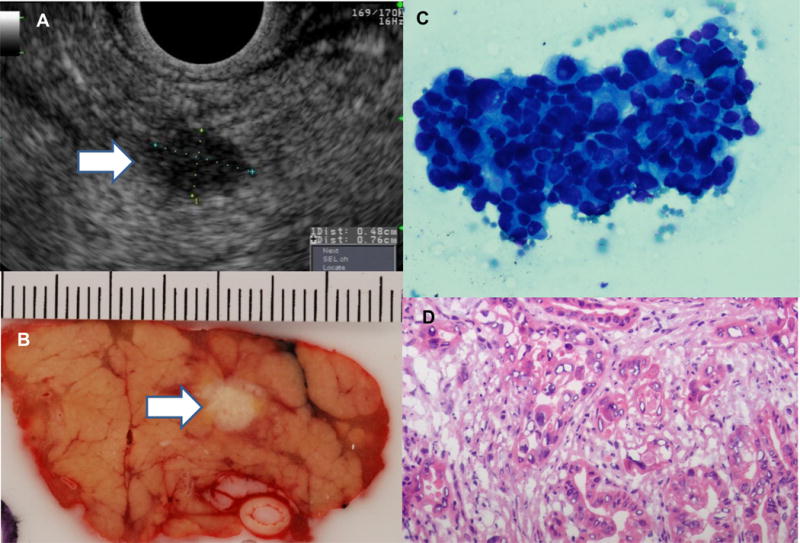
Incident asymptomatic 7 mm pancreatic cancer detected after 10 years of surveillance, shown by arrows in endoscopic ultrasound (EUS) image (A) and gross pathology section of the pancreatic body (B). Final pathologic diagnosis was stage T1N0 moderately differentiated adenocarcinoma with negative margins (cytology smear from EUS-guided fine needle aspiration) C; hematoxylin and eosin stain, venous and perineural invasion were not identified (D).
As of last follow-up, the overall mortality for HRI with PDAC was 64% (9/14). PDAC-specific mortality was 8/14 (57%), with one patient surviving 13 years before passing from complications related to a metachronous early gastric cancer surgery (Patient 2, Table 2). None of the 10 HRI with IPMN-HGD/PanIN3, and 6 with PanNet have died as of last follow-up.
The median survival time for the 20 HRI with PDAC or high grade precursor neoplasm (targets of screening) diagnosed during surveillance was significantly greater than that for 4 HRI who were late or stopped surveillance-5.3 years (IQ range 1.2 – 11.1 years) versus 1.4 years (IQ range 0.39-3.5 years, p < 0.0001. The overall 3-year survival rate was 57% for the 14 PDAC patients but the survival was significantly greater for the 10 asymptomatic HRI diagnosed during surveillance, compared to that for 4 HRI presenting with symptomatic advanced disease developing outside surveillance (85% versus 25%, respectively, log rank, p<0.0001, Figure 3).
DISCUSSION
Pancreatic lesions are frequently detected in HRI by EUS and/or MRI in pancreatic cancer screening programs18, 14, 16, 20, 21, 23–25. The majority of detected lesions are small cysts15, 18, 20, 25. In this study, we found that neoplastic progression is more common in HRI older than age 60, and those with multiple cysts, and/or a mildly dilated MPD at baseline. Although having multiple cysts (≥ 3) at baseline was a robust predictor of radiologic and neoplastic progression, it has not been identified as a significant predictor of progression in cohorts of sporadic IPMN35, 36.
Close to 19% of HRI developed evidence of a worrisome radiologic feature while undergoing surveillance, corresponding to an average rate of radiological progression of 4.3% per year. One important question for pancreatic cancer screening programs is the age to begin screening. There was no consensus among experts on this question in the 2012 CAPS Consensus Summit4. In the CAPS 1-3 studies (1998-2010), age 50 was selected as the age to initiate pancreatic screening for FPC relatives. After 2010, this age was raised to 55 years, but in all studies, screening would start at age 10 years less than that of the youngest PDAC relative. Two of the 14 PDAC patients and 3 of the 24 with PDAC or high grade precursor neoplasms in our cohort were younger than age 55 at the time of diagnosis, but both of these patients had young-onset pancreatic cancers in their family that made them eligible for screening using the age 55 or 10 year rule. Determining the optimal age to begin PDAC screening requires additional studies.
After initial screening, the cumulative incidence of invasive PDAC in our cohort was 3.4% (12/354 HRI). Our overall PDAC detection rate is higher than reported in other screening programs of FPC relatives, most of which are closer to 1% or less14, 15, 20, 25, 26. This probably reflects the longer period of surveillance, and the older average age of our cohort. In our study consisting mostly of familial PDAC relatives, the number of individuals needed to undergo regular screening and surveillance (using EUS and MRI) to detect a PDAC or high-grade precursor neoplasm was 23. Recently, three European centers performing prospective screening with MRI and EUS in 411 HRI reported a detection of incident PDAC in 13 (7.3%) of 178 CDKN2A mutation carriers and 3(1.4%) of 214 familial PC relatives25. In patients with sporadic IPMNs, the cumulative incidence for PDAC over 5 years is generally lower but varies considerably depending upon the cohort studied35, 37, 38. The National Cancer Institute estimates the lifetime risk for PDAC is 1.6%, based on 2012-2014 data2(http://seer.cancer.gov/statfacts/html/pancreas.html).
The majority (71%) of PDACs detected during surveillance in our high risk cohort were asymptomatic resectable TNM stage I and II cancers, which compares favorably with the tumor stage and resectability rate (15-20%) of symptomatic PDAC2. A similar down staging was found in the European study that followed mostly CKDN2A/p16-Leiden mutation carriers; 75% of screening-detected PDACs were resectable25. Furthermore, the overall 3-year survival rate of our HRI with PDAC was substantially higher than that of PDAC patients in the United States (57% vs. 8.9%, Surveillance, Epidemiology, and End Results Program or SEER data, http://seer.cancer.gov/statfacts/html/pancreas.html)). The difference is even greater when considering only screening-detected PDAC in our cohort (3-year survival 90%). Similarly, the 5-year survival rate for 13 CDKN2A/p16 Leiden mutation carriers with screening-detected PDAC (24%) was higher than that for symptomatic mutation carriers (15%)25 or sporadic PDAC (4-7%)2. Even with the limited number of progressors in large high risk cohorts, these accumulated data of increased resectability and improved survival rates suggest a potential benefit of surveillance for HRI.
Our screening program using EUS and MRI also identified IPMN-HGD and PanIN-3 in resection specimens of 3.1% of the cohort (10/354). Other high risk surveillance programs have detected and treated a low number of patients with high grade precursor neoplasms, ranging from none to 1.9%; this lower rate may result from the demographic profile of the patients undergoing screening20,25,26. Precursor neoplasms with high grade dysplasia have been recommended by the International Cancer of the Pancreas Screening Consortium as ideal targets for detection and treatment4.
The selection of asymptomatic HRI for pancreatic resection to treat PDAC or to remove concerning pancreatic lesions is challenging. Currently, selection of cases for surgery relies on the detection of specific concerning radiologic and EUS features that are predictive of high-grade pancreatic neoplasia or PDAC. The criteria for surgical resection have evolved over the 16 year study period with improved understanding of the natural history of pancreatic cysts and other lesions identified in both HRI and those with incidentally detected pancreatic abnormalities. The selection of patients for surgery is still far from perfect and reflects the limitations of pancreatic imaging to detect high grade precursor neoplasms, particularly PanIN-3. Better biomarkers than can detect high grade pancreatic neoplasia in secretin-stimulated pancreatic juice, pancreatic cyst fluid, and blood are needed to improve the selection of patients for surgery39–41, 42, 43, 44.
The current study has several limitations. First, the methods for surveillance and management of pancreatic precursor lesions evolved thanks to improvements in imaging and better understanding of the natural history of these lesions. Second, we were unable to track the outcomes of HRI who chose to continue surveillance at other centers. In addition, the gene mutation status for the pancreatic cancer risk in most HRI in our cohort was not known. Furthermore, the estimation of the prevalence of high grade precursor neoplasms in our cohort is limited by the inability of standard pancreatic imaging tests to detect PanIN-3. Our results are derived from a predominantly FPC cohort followed at a single institution, which may limit generalizability. Finally, we do not have a concurrent control group.
In conclusion, the results of our long-term pancreatic cancer screening program show that continued follow-up of HRI can successfully detect resectable PDAC and high-grade precursor neoplasms. Among individuals undergoing pancreatic surveillance, specific detectable lesions with worrisome features predicted neoplastic progression. The short-term outcomes of patients with screening-detected PDAC s are improved. Large prospective multicenter studies are needed to further evaluate the potential benefits of PDAC screening.
Supplementary Material
Supplementary Figure 1 Cumulative risk (hazard) for radiologic progression over the 16-year study period for high risk individuals after baseline screening.
Supplementary Figure 2 Cumulative risk (hazard) for radiologic progression stratified by baseline age > 60 years (red upper curve) and ≤60 years (blue lower curve) (p = 0.0001).
Figure 4.
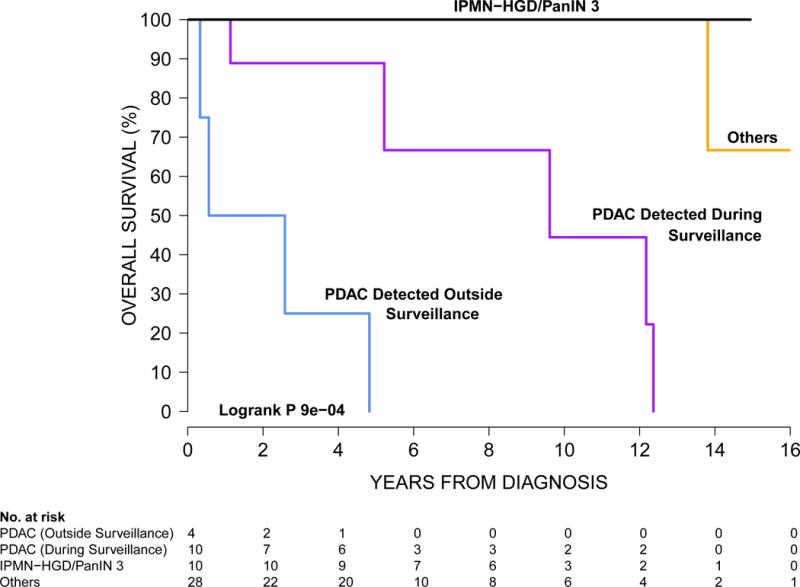
Kaplan-Meier curves for overall survival for high risk individuals diagnosed with pancreatic neoplasms diagnosed by surgery or endoscopic ultrasound guided fine needle aspiration. The group “Others” includes pathologically-proven lower grade pancreatic neoplasms that were not PDAC, IPMN-HGD, or PanIN-3 (IPMN with LGD or MGD, PanIN-2, PanNET, serous cystadenoma, pseudocyst).
Acknowledgments
Grant Support: This work was supported by NIH grants (CA62924, CA176828, CA154823, CA132829), Susan Wojcicki and Dennis Troper, The Pancreatic Cancer Action Network, The Lustgarten Foundation for Pancreatic Cancer Research, The John and Peter Hooven Memorial Endowment, Hugh and Rachel Victor, ChiRhoClin, Inc.
Abbreviations used in this paper
- PDAC
pancreatic ductal adenocarcinoma
- IPMN
intraductal papillary mucinous neoplasm
- PanIN
pancreatic intraepithelial neoplasia
- PanNET
pancreatic neuroendocrine tumor
- PJS
Peutz-Jeghers syndrome
- CT
computed tomography
- EUS
endoscopic ultrasonography
- FNA
fine-needle aspiration
- MRCP
magnetic resonance cholangiopancreatography
- CAPS
Cancer of the Pancreas Screening
- FPC
familial pancreatic cancer
- HGD
high grade dysplasia
- MGD
moderate grade dysplasia
- LGD
low grade dysplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Conceived and designed the study: MC, MG; acquisition of data: JAA, MC, AML, EJS, EKF, IRK, RDS, CJY, CW, MW; analysis and interpretation of data: JAA, MC, MG, RHH, AK; drafted the manuscript: JAA, MC; statistical analysis: MC, AB, AS, GW; revised the manuscript and agreed with the manuscript’s results and conclusions: all the authors; study support: MC, MG; obtained funding: MC, RHH, MG; study supervision: MC, MG
Disclosures: MG, APK and RHH have received royalties for the licensing of PALB2 as a pancreatic cancer susceptibility gene. All other authors have no relevant financial, personal, or professional conflicts.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Instittute NC. In: SEER Cancer Statistics Review, 1975-2014. Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. Bethesda, Maryland: National Cancer Institute; 2017. [Google Scholar]
- 3.Ibrahim IS, Bonsing BA, Swijnenburg RJ, et al. Dilemmas in the management of screen-detected lesions in patients at high risk for pancreatic cancer. Fam Cancer. 2017;16:111–115. doi: 10.1007/s10689-016-9915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–47. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 6.Klein AP, Beaty TH, Bailey-Wilson JE, et al. Evidence for a major gene influencing risk of pancreatic cancer. Genet Epidemiol. 2002;23:133–49. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- 7.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–64. doi: 10.1038/ajg.2009.725. author reply 1265. [DOI] [PubMed] [Google Scholar]
- 9.Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17:569–77. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebours V, Boutron-Ruault MC, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol. 2008;103:111–9. doi: 10.1111/j.1572-0241.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Sukhni W, Rothenmund H, Borgida AE, et al. Germline BRCA1 mutations predispose to pancreatic adenocarcinoma. Hum Genet. 2008;124:271–8. doi: 10.1007/s00439-008-0554-0. [DOI] [PubMed] [Google Scholar]
- 12.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. Jama. 2009;302:1790–5. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;2:41–6. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg. 2012;16:771–83. doi: 10.1007/s11605-011-1781-6. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch DK, Dietzel K, Bargello M, et al. Multiple small “imaging” branch-duct type intraductal papillary mucinous neoplasms (IPMNs) in familial pancreatic cancer: indicator for concomitant high grade pancreatic intraepithelial neoplasia? Fam Cancer. 2013;12:89–96. doi: 10.1007/s10689-012-9582-y. [DOI] [PubMed] [Google Scholar]
- 16.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 17.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 18.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmey MB, Bronner MP, Byrd DR, et al. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc. 2002;56:S82–6. doi: 10.1016/s0016-5107(02)70092-8. [DOI] [PubMed] [Google Scholar]
- 20.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–8. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–54. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider R, Slater EP, Sina M, et al. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer. doi: 10.1007/s10689-010-9414-x. [DOI] [PubMed] [Google Scholar]
- 23.Vasen HF, Wasser M, van Mil A, et al. Magnetic Resonance Imaging Surveillance Detects Early-Stage Pancreatic Cancer in Carriers of a p16-Leiden Mutation. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 24.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–81. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 25.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol. 2016;34:2010–9. doi: 10.1200/JCO.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 26.Harinck F, Konings IC, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505–13. doi: 10.1136/gutjnl-2014-308008. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.1016/j.cgh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Kwong WT, Lawson RD, Hunt G, et al. Rapid Growth Rates of Suspected Pancreatic Cyst Branch Duct Intraductal Papillary Mucinous Neoplasms Predict Malignancy. Dig Dis Sci. 2015;60:2800–6. doi: 10.1007/s10620-015-3679-8. [DOI] [PubMed] [Google Scholar]
- 31.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. quize12-3. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO) WHO Classification of Tumours of the Digestive System. Geneva: WHO Press; 2010. [Google Scholar]
- 33.World Health Organization (WHO) WHO Classification of Tumors of the Endocrine Organs. Fourth. Geneva: WHO Press; 2017. [Google Scholar]
- 34.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251–61. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 35.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364–70. doi: 10.1097/MPA.0b013e31820a5975. [DOI] [PubMed] [Google Scholar]
- 36.Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4:1265–70. doi: 10.1016/j.cgh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Hisada Y, Nagata N, Imbe K, et al. Natural history of intraductal papillary mucinous neoplasm and non-neoplastic cyst: long-term imaging follow-up study. J Hepatobiliary Pancreat Sci. 2017 doi: 10.1002/jhbp.463. [DOI] [PubMed] [Google Scholar]
- 38.Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014;46:22–9. doi: 10.1055/s-0033-1353603. [DOI] [PubMed] [Google Scholar]
- 39.Eshleman JR, Norris AL, Sadakari Y, et al. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol. 2015;13:963–9 e4. doi: 10.1016/j.cgh.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–33. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol. 2013;11:719–30 e5. doi: 10.1016/j.cgh.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Sadakari Y, Shindo K, et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66:1677–1687. doi: 10.1136/gutjnl-2015-311166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114:10202–10207. doi: 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suenega M, Yu J, Shindo K, et al. Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clinical Cancer Research. 2018 doi: 10.1158/1078-0432.CCR-17-2463. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Cumulative risk (hazard) for radiologic progression over the 16-year study period for high risk individuals after baseline screening.
Supplementary Figure 2 Cumulative risk (hazard) for radiologic progression stratified by baseline age > 60 years (red upper curve) and ≤60 years (blue lower curve) (p = 0.0001).


